Oxygen
Oxygen is indispensable to the maintenance of the life processes of all organisms. It is available for normal metabolic activities of nearly all organisms only when it is in solution in a free state. A very few forms, notably the anaerobic bacteria, are able to carry on intermolecular respiration whereby the oxygen bound in the complex molecule of organic substance is made available as a source of energy. Therefore, biologically, free oxygen is comparable to carbon dioxide in being one of the two most important dissolved gases in the sea.
There are about 200 ml of oxygen in a liter of air as opposed to a maximum of about 9 ml in a liter of sea water. This is a great boon to air-breathing life, but with this advantage go also some disadvantages resulting from the need of maintaining moist respiratory surfaces in a desiccating environment. Aquatic and atmospheric respiration are similar in that the oxygen requires water as a respiratory medium; but in the former it should be noted that the oxygen-laden water is typically passed freely over the surface of relatively exposed respiratory organs or surfaces. Hence, although the concentration of oxygen reaching the respiratory surface is small, there is some compensation in its being rapidly replenished with complete flushing and aeration of the surfaces.
More oxygen can be dissolved in fresh water than in sea water, which should be advantageous to fresh-water animals; but, on the other hand,
The rate of metabolism is roughly doubled by a 10° increase in temperature. Therefore, during winter or in deep cold water, owing to the decreased rate of metabolism, much less food is required for repair following this slowing up of the katabolic processes. This reduced requirement must have an important bearing on the survival of many forms during winter when little food is being produced by the plants. The amount of oxygen used is somewhat different for different marine animals.
The order of magnitude of oxygen consumption in milliliters per gram of wet weight per hour within a temperature range of 17° to 25°C for various groups of marine invertebrates is as follows (averages from data of Fox, 1936, and compilations of Heilbrunn, 1937): Protozoa (Colpidium) 2.0; Coelenterates (jellyfish and ctenophores) 0.005; Echinoderms (various) 0.026; Annelids (various) 0.017; Crustacea (shrimps) 0.181.
For various marine bacteria the rate of oxygen consumption is very high compared to the above figures, being of the order of about 110 ml/g/hr at 22°C and depending upon nutrient conditions (ZoBell, 1940).
The great differences in the amounts of oxygen used per gram of wet weight are related to the great diversity in the water content of the living organisms. For example, low values found in coelenterates are correlated with the small percentage of solid organic material. Norris (in Hyman, 1940), for instance, found that the organic matter constituted only 0.85 per cent of the jellyfish Aequorea. The stage of development of some animals is also a factor in the rate of respiration. At 10°C Stage V of Calanus finmarchicus was shown to consume 0.25 ml of oxygen per thousand individuals per hour while adult females consumed 0.40 ml of oxygen per thousand individuals per hour (Marshall, Nicholls, and Orr, 1935). In recalculating the data of these investigators so as to present the oxygen consumption per hour in relation to weight in grams—using the average dry weight 27.35 mg/100 individuals of Stage V (Marshall, Nicholls, and Orr, 1935, p. 805), and considering the dry weight at 25 per cent of wet weight as Heilbrunn has done in his compilations—we arrive at an oxygen consumption of 0.228 ml/g/hr. If these figures present an accurate picture, we must assume that the metabolic rate of copepods is relatively high compared to other invertebrates, but this is in keeping with their very active lives. The figures arrived at for oxygen consumption by this species at Woods Hole are even considerably higher, namely 0.896 ml/g/hr at 15.5°C (see below). These values are higher than the ones found for certain marine fishes (Wells, 1935), the maximum oxygen consumption for Fundulus at 16°C being 0.220 ml/g/hr and for Girella at 20°C 0.242 ml/g/hr.
The concentration of oxygen in the sea is not only very much less per unit volume than in the air but there is also very much greater irregularity in its distribution and in some instances very sharp gradients may exist. The range of oxygen may be from 0 to 6.4 ml/l over a depth range of only 10 m, and in such rather isolated instances as in stagnant fjords the oxygen deficiencies are markedly reflected in the fauna.
The physical factors involved in the concentration, renewal, and distribution of oxygen in the sea are discussed elsewhere, and from these we note that on the whole the ocean, even in abyssal depths, is well supplied with oxygen for aquatic breathing organisms. We may therefore consider that oxygen is not, as a rule, a determining factor in the distribution and movements of most marine life. However, the irregularities in oxygen content and distribution referred to above are none the less highly important and, in many restricted instances, the low supply of oxygen and the hydrogen sulphide associated with this condition exclude all but anaerobic organisms on or near the bottom. Under these circumstances there may be not only an exclusion of animal life but a wholesale destruction of aerobic forms living at higher levels when temporary disturbances resulting from storms or surface cooling cause an upward displacement of these poorly aerated waters. This is witnessed periodically in certain Norwegian “threshold fjords” where a shallow sill prevents free water exchange with the sea and where summer heating and influx of fresh water have established a strong stratification of the water (pp. 147 and 802).
The Black Sea, with a maximum depth of 2104 m, being isolated from free circulation with the Mediterranean by the Bosporus Ridge, which extends upward to a depth of about 40 m, is illustrative of a condition where a more permanent biological climax has been reached. This high ridge precludes all opportunity of renewal of bottom water by oxygen-laden water from an outside source, and the accumulation of fresh water together with thermal conditions in the upper layers preclude any very deep aeration through convection currents. Decomposition of the rich organic material accumulating on the bottom from the productive surface layers has used up all of the free oxygen so that hydrogen sulphide extends upward from the bottom forming a layer of toxic water over 1800 m thick so that the lower limit of animal life is about 130 to 190 m (fig. 237). Only anaerobic bacteria can exist in the deeper portion of this environment, which constitutes about five times the volume of the upper portion capable of being inhabited by other organisms. These rigorous conditions, coupled with a reduced salinity, have led to an impoverished fauna with elimination of stenohaline and stenothermic species.
Very little is known regarding the relationship that exists between the oxygen-deficient or minimum layer (pp. 686–728) characteristic of
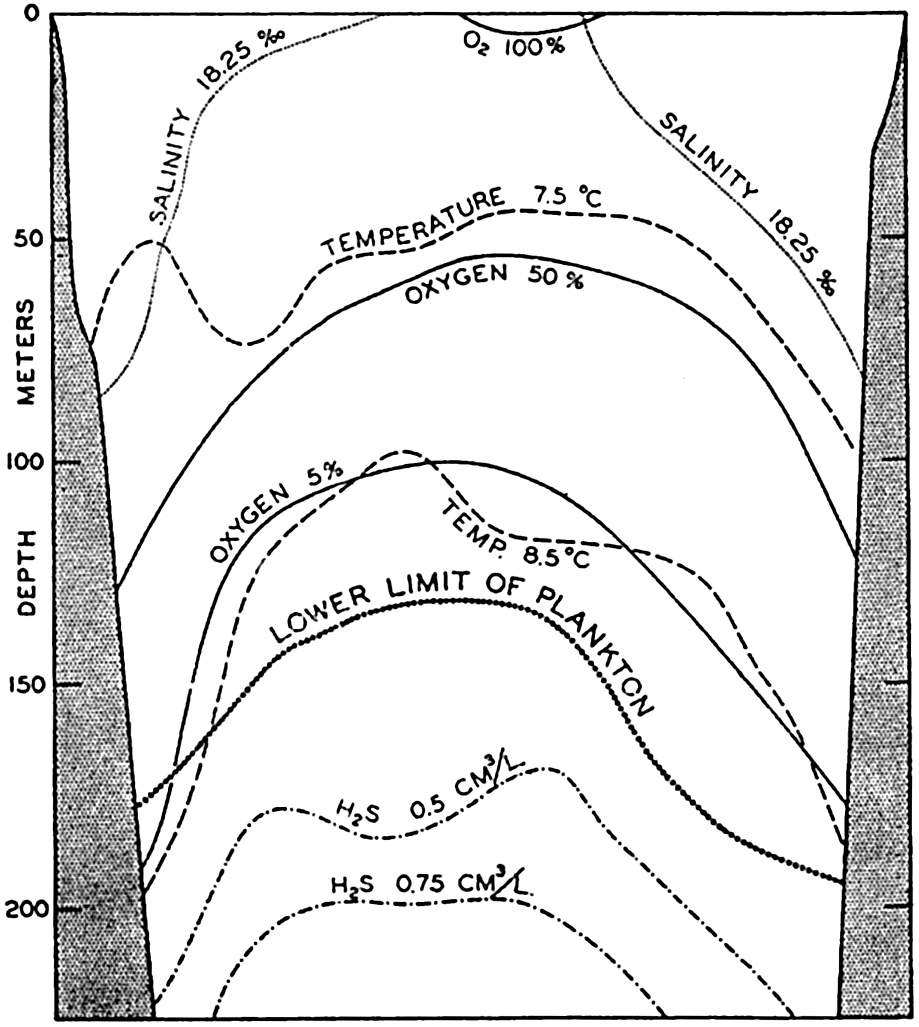
A section through the Black Sea to show hydrographic and biological conditions. (Modified from Nikitin.)
Schmidt (1925b) found that at a depth of 300 m in the Gulf of Panama, where there was only a 2 per cent oxygen saturation of water, plankton was about ten times more abundant than at the same depth in the Caribbean Sea with a saturation of 50 per cent. Oxygen determinations given in per cent may be misleading since they are a function of temperature and do not necessarily show the respiratory value associated with diminished metabolic rate in cold waters. The oxygen requirements of typically deep-sea plankton animals have apparently not been investigated.