XVIII. Interrelations of Marine Organisms
In the preceding chapters we have dealt at some length with the external conditions affecting the lives of organisms of the sea. Except for incidental references, the discussion has been concerned mainly with factors of the inorganic environment. But it must be borne in mind that the organisms themselves as a group form a vital portion of the environment, being a part of the whole series of factors that control the lives of the individuals. This complete aggregation of organisms of all kinds, together with their activities, constitute what is known as the organic environment. Its study is concerned with the interrelations within the whole marine populations, both plants and animals.
In the final analysis, many of the inorganic factors operate indirectly through the organic factors which they foster. To use the most general illustration, animals can be abundant only where inorganic surroundings permit an abundant growth of plants or where currents carry in food from regions so supplied.
It has been noted (p. 275) that the inanimate environment may be classified into a number of more or less sharply defined divisions or provinces. Of these, the biotope is the primary topographic unit (p. 279). In the sea it represents a portion of the marine environment in which the conditions are sufficiently uniform to foster a characteristic community of organisms. It follows that other biotopes with different sets of environmental conditions will be populated by somewhat different communities although many organisms with wide tolerance may be common to two or more communities. Because terminology used in evaluating communities is in an unsettled state the reader is referred to ecological studies dealing specifically with the subject (Petersen, 1913, Allee, 1934, Shelford, Weese, et al, 1935, Pearse, 1939).
NATURAL ASSOCIATIONS OF ORGANISMS
Depending upon their degree of intimacy, four types of associations may be recognized among organisms. The four types are: (1) association arising from similar requirements as to environment; (2) commensalism; (3) symbiosis; and (4) parasitism.
Environmental Association. Animals and plants of different species will be found associated together in ecological communities or biocoenoses provided their environmental requirements with respect to physical and chemical conditions are much the same. The food relationships or predatory habits of the members must be such that their habits do not interfere seriously with each other. Much of the food may be brought to the community incidentally by currents and other water movements, but usually a restricted food cycle is established and many carnivorous animals prey upon each other. One species may dominate numerically owing to better means of protection or greater powers of survival or proliferation.
It should be noted that in a natural community the inorganic forces function to select a general type of fauna. All species that have not become adapted to the physiographic conditions, the range of salinity or temperature, will be promptly eliminated from the community. For instance, only organisms suited to live on muddy bottoms can become established in such habitats, and the same is true of rocky shores (Ricketts and Calvin, 1939, Pearse, 1939). However, since there is a great array of animals capable of living within the extremes of chemical-physical conditions of these environments, a competition for food and space is established among the members. It is here that some of the biotic forces come into play in determining the species or groups of species that will make up any given community. It has been found that certain species live most successfully only in certain combinations of associates (Petersen, 1913). The principal characteristic animal or animals supply the name to the community; thus there may be a Venus community or a Macoma community, depending upon the importance of these bivalves and the combination of animals associated with them. Communities are also sometimes named for the habitat, for example, they may be estuarine communities, exposed rocky coast communities, or wharf-pile communities.
The forces operating to mold the character of a fauna are thus a combination of the organic and inorganic factors, but the communities are more or less unstable since the forces so acting do not remain unchanged. Changes resulting from erosion of the substratum or from the degree or character of terrigenous deposition on a shore, for example, will result in a gradual succession of communities. It is obvious also that the organisms themselves, plants and animals, both pelagic and benthic, slowly bring about fundamental changes in the nature of the bottom, its macro- and microscopic structure, even to great ocean depths. Alterations may result from (1) burrowing activities of many forms such as worms, molluscs, crustaceans, and others that live in or upon the mud and sand, grinding and intermixing the sediments to a depth of a third of a meter or more; (2) boring into and eroding such harder structures as
Commensalism. A closer association than that above discussed is commensalism. Here two organisms live together, one at least being benefited and the other neither injured nor benefited. Certain small decapods are frequently found thus associated with bivalves, living within the mantle cavity. Scaleworms (polynoids) inhabit similar positions with limpets and chitons, and are also found between the rays or in the ambulacral grooves of starfish. Certain small fishes (gobies) live in the burrows of burrowing worms and crustaceans, and many small pelagic fish seek refuge among the stinging tentacles of large jellyfish and siphonophores. According to Wilson (1932) at least 80 per cent of the copepods belonging to the suborder Notodelphyoidea with 41 genera are commensals within ascidians. Certain barnacles (Coronula) and also diatoms (Cocconeis coticola) grow upon the skin of whales. The relative abundance of this diatom on the skin of whales is correlated with the length of time the animals have been in Antarctic waters, since this diatom does not thrive in the warm waters of lower latitudes. The yellow color imparted by these organisms to the ventral surface of the whale is thus an index of the fat condition, for it is in these waters that fatting takes place (Hart, 1935). Commensalism is very common among marine animals.
Symbiosis. Another type of yet closer association is found in the relationship known as symbiosis, wherein two or more organisms live together, this usually being obligatory and mutually beneficial. As illustrative of this mode of life may be mentioned the association between certain one-celled yellow (Zooxanthella) or green (Zoochlorella) algal plants and such animals as radiolaria, sponges, corals, sea anemones, and even bivalves and echinoderms. The algal partner, which in the sea usually is one of the Zooxanthellae, may live within the cytoplasm of the single cell as in the radiolaria; or within the cells intracellularly, or within the body cavities of multicellular forms. This type of relationship is common in the sea but its significance in the economy of the sea is not
It is probable that the algae symbiotic with such tropical planktonic animals as the Radiolaria represent an important flora which compensates in part for the scarcity of the free phytoplankton diatoms generally reported from high seas of tropical waters where the plant nutrients are low in the surface waters (Hardy, 1936).
Parasitism. In this type of close association one organism lives at the expense of another, known as its host. There are large numbers of both external and internal parasites in the sea. Even the diatoms do not escape; Chaetoceros, for instance, is host to Paulsenella, an unarmored dinoflagellate. Among the temporary planktonic parasites are unarmored dinoflagellates, many copepods, and the cercaria larvae of the flukes.
NUTRITIONAL RELATIONSHIPS
In the sea, as in the terrestrial environment, the prime relationship between organisms is that associated with nutrition, and, indeed, the relationships leading to the above classification of associations are, in the final analysis, nutritional.
Other relationships are expressed in associations for protection and, within species, for reasons of propagative or social instincts. But the association for protection is only a reaction against rapacious enemies seeking food and is therefore part of a nutritional relationship between predator and prey.
Allen (1934) has reviewed pertinent literature dealing with the microscopic plants as the primary food of the sea. In the sea as on land the nutritional relationships result in a food cycle of producers and consumers in which solar energy and the regeneration of inorganic nutrients utilized by plants in photosynthesis form vital links. But the relation
The Significance of Micro-plants
It has been shown that sea water possesses in solution all of the necessary inorganic elements, phosphorus, nitrogen, iron, and so on, for the manufacture of plant substance with the aid of light. In other words, the sea as a whole possesses the potentialities of sustaining such autotrophic organisms as are capable of capitalizing upon this tremendous supply of nutrient material held in solution. Although the sea offers these possibilities to plant growth, at the same time it also sets up certain serious physical difficulties and barriers by reason of the magnitude, depth, and fluidity of the oceans. We know that it is only the merest rim of the sea that has sufficient light and, at the same time, suitable substratum for attached and other bottom-living plants, and that in the open sea plant life is restricted by the factor of illumination to only the upper few meters represented by the euphotic zone. This relative restriction of living space makes it of vital importance to plants that they overcome or minimize materially the effect of gravity that might lead to their destruction below the euphotic zone. This serious challenge of the pelagic environment has been successfully met by the plants, and the method by which it has been conquered has determined largely also the kind of animal life that the sea can support.
In order that the vast stretches of the ocean may be populated and its nutrient resources used by plants beyond a depth of about 40 m, special types of plants adapted to a floating existence have evolved. To accomplish a pelagic habit, two methods appear to be open. (1) The development of buoyant forms with air bladders or similar features to keep them at the surface. This is the means evolved by the macroscopic alga Sargassum. This method, however, is subject to serious handicaps and can hardly be considered a successful one; moreover, although plants of this nature float in large masses and reproduce vegetatively in restricted places such as the Sargasso Sea, yet their natural home is along the shore attached to rocks from which they have been torn. Such buoyant plants can extract nutrients only from a shallow layer at the immediate surface and unless maintained in large eddies of relatively quiet water, they are subject to being rapidly driven by the wind and currents into coastal areas, there to be destroyed by stranding and by beating of the surf. The contribution of organic material by such plants is relatively small. (2) The really successful method by which plants have been able to
The Significance of Micro-animals
One may ask what influence this type of development of the plants has had on the animal population of the sea. Along what lines has the animal economy evolved to exploit this vast supply of microscopic plant food in the sea? A plant population consisting mainly of scattered individuals that are microscopic in size must impose certain restrictions and requirements upon the animal grazers that depend upon it for nourishment. It is significant that in the sea the chief grazers, representing the main bulk of the zooplankton, are also microscopic or semimicroscopic in size and vast in numbers. Foremost among the grazers of the pelagic region are placed the copepods, the diet of which has been shown by direct analysis to consist mainly of diatoms, dinoflagellates, and other micro-plants, but many other small herbivores, especially protozoans, euphausiids, and larval stages of larger invertebrates also graze directly upon the phytoplankton.
Through all the tiny grazers of the sea nature accomplishes two important ends: first, complete utilization of even the minutest particles of primary food; and, second, transformation of the organic material of these plants into animal substance of size sufficiently large to be caught and utilized by carnivorous forms. The large number of carnivorous animals that occur in the sea is abundant evidence that the numbers of microscopic grazers must indeed be great (p. 896). Much economy would result if the larger animals could feed directly upon the plants in the manner of the large terrestrial animals, but the nature of the environment prohibits the growth of large plants except in a narrow fringe along shore and, except for a small amount in the eel grasses, no seeds with concentrated nutriments are produced as on land for immediate use or as a store to be drawn upon during periods of low vegetative growth. It is true that some fishes, particularly the herrings, feed to some extent directly upon minute plants, especially during their larval stages (Lebour, 1924a), and an exceptional few, such as the menhaden with their notably fine filtering apparatus, feed partly on diatoms and dinoflagellates throughout life (Bigelow, 1926).
In our concept of plant-animal relations, the plants are to be looked upon chiefly as autotrophic organisms capable of converting inorganic material to organic material, thus making it available to animals as particulate food. Lohmann found in the Bay of Kiel that for every multicellular animal there were one thousand protozoan and seven thousand protophytan forms. It is extremely difficult to obtain reliable measurements of the relative volume of plant and animal substance in the sea, but, since only through the endothermic process of photosynthesis can the solar energy be bound and become available for the building of animal substance, it is obvious that the mass of plant material produced must be greater over a long period of time than the mass of animal material. It appears that even at periods of only moderate production the very rapid rate of reproduction in unicellular plants is sufficient to maintain the zooplankton population, even though the bulk of plants actually present at any moment may be less than that of the animals. It should be emphasized that the rapidity of reproduction of the nanno- and microplankton is as important as the bulk present in the water as food at any given time. Lohmann calculated that even though the mass of plants may fall below that of animals in the Bay of Kiel, yet plant production usually exceeds animal consumption during the summer months. The excess may be 29 mm3/100 l of sea water. During winter both production and consumption fall off, but during midwinter, January and February, there is a production deficiency of −0.8 mm3/100 l of sea water.
It must be pointed out that some of the organic material produced by plants is lost to the animals through solution in the sea water; the content of dissolved organic matter in the sea runs as high as six or more milligrams per liter of sea water (up to three milligrams of carbon per liter; p. 250). This represents much more organic material in the sea in solution than exists there as particulate food at any one time, and any organisms that are capable of reclaiming this dissolved organic material have an important role in the economy of the sea. Bacteria doubtless serve this purpose (p. 911), but other saprophytic forms may also play a part. Some dinoflagellates are believed to be saprophytic, but their utilization of dissolved organic matter from the water in such dilute concentrations has not been demonstrated. Attention has already been called to certain “olive-green cells” regularly collected in deep water from the South Atlantic by the Meteor. These have a maximum distribution below the euphotic zone and Hentschel (1936) believes them to live heterotrophically. If this is true, they are important in reclaiming dissolved organic matter and building it into bodies of suitable size for use by filter-feeding animals at mid-depths.
In the following analysis of food relationships our purpose is not so much to learn the habits of individual animals, though to do so is extremely important and indispensable in understanding the biology of
In studying the food relationships of the marine organisms, it is perhaps most convenient for our purpose to group the animals according to the kind or source of food upon which they subsist and according to general methods of feeding, remembering at the same time that the feeding habits of many are unknown and that many have habits not clearly confined to any one of these categories but overlapping more or less into others. The larval and adult stages may also differ both in food required and in the method of procuring it. The first two groups given below are based on the source and kind of food used and, incidentally, on the method of feeding. The last two groups are based mainly on methods of feeding, but this feature itself results largely from the nature of the food.
Plankton and Filter Feeders
Under this heading are included the forms that feed upon microscopic or semimicroscopic organisms and suspended detritus floating or swimming freely in the water. It is here that the uniqueness of the food cycle of the aquatic environment is most clearly manifest. It is not practicable to segregate strictly the true plankton feeders as a group from the feeders on finely divided, suspended organic detritus because most plankton feeders include detritus in their diet, owing in part to the method of gathering food. They may also be designated as “suspension feeders,” after Hirsch. Many of the plankton feeders may be called filter feeders because of the method in which they collect their food. Most of them are provided with some type of screening device through which the water is passed while the small organisms are retained as food. A few examples will serve as illustrations.
In filter-feeding copepods, Calanus finmarchicus for example, the head appendages known as the second maxillae are provided with a number of curved setae or spines each covered by numerous fine hairlike processes. The appendages are paired and together form the main part of a filtering net or chamber just posterior to the mouth. The head appendages lying between the first antennae and the second maxillae,
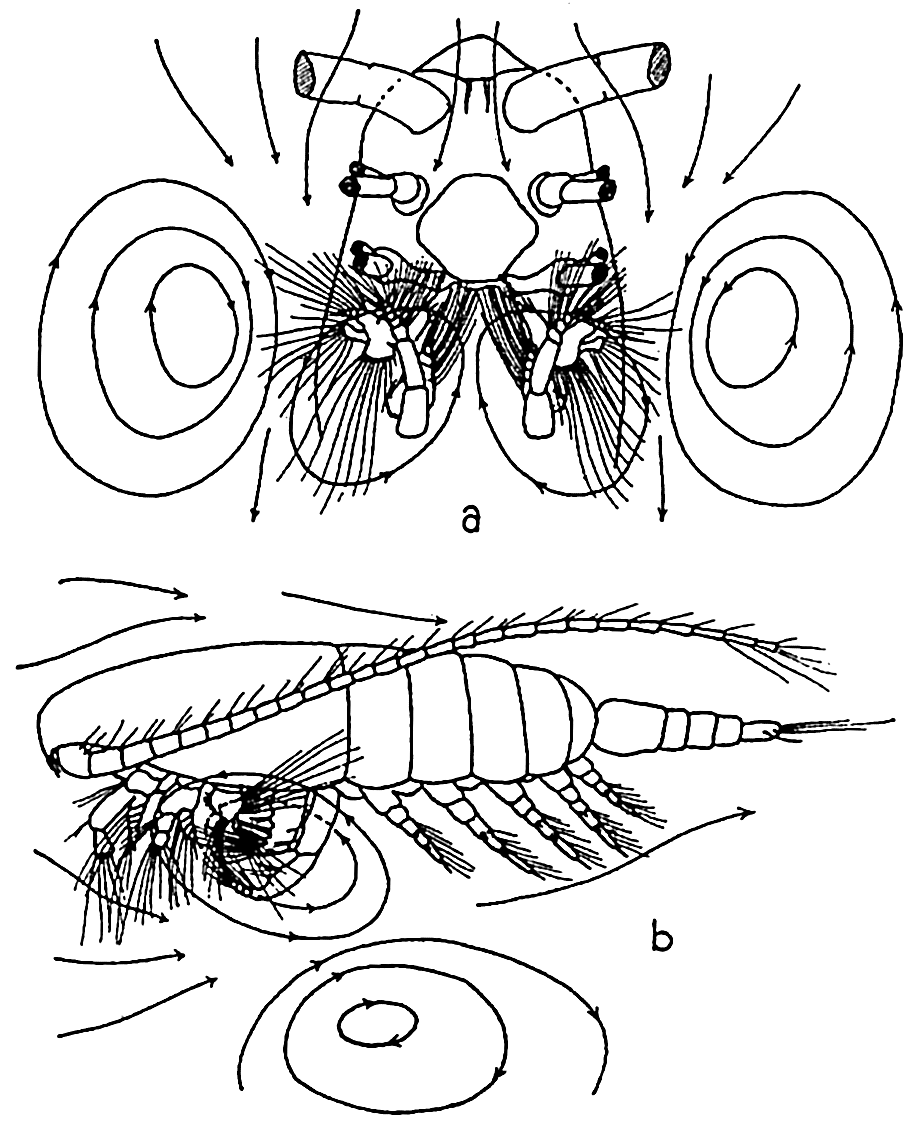
The filter-feeding apparatus of Calanus finmarchicus: (a) ventral view, anterior portion of animal, distal parts of first and second antennae and mandibles removed; (b) lateral view of entire animal. The lines with arrows indicate the vortices set up. (According to Cannon.)
The great abundance of euphausiid crustaceans makes them highly important plankton feeders because they share largely with the copepods the distinction of being grazers upon diatoms and, owing to their habit of living in large swarms below the euphotic zone, they must be of great significance in intercepting and utilizing the slowly sinking plant material produced above in the lighted zone. Euphausiids are known to be quite omnivorous, feeding on a wide variety of floating material, plants, animals, and detritus. This they comb out of the water with their long thoracic limbs which together form a basket through which water is pumped by the swimming legs (Bigelow, 1926, Lebour, 1924b).
The pelagic tunicate Oikopleura is a most remarkable type of filter feeder. Its food consists of the minutest of drifting organisms, the nannoplankton such as coccolithophores, bacteria, small diatoms and dinoflagellates, and other minute forms, which are filtered from the water by means of gratings in the animal's temporary vestments or “houses.” This portion of the plankton may constitute as much as a third or more of the total mass of plankton at some seasons and localities. The house of Oikopleura, in which it lives while drifting about in the plankton (fig. 239), is a gelationous investing structure secreted by the animal. Water is drawn into the house through funnel-like structures guarded by a set of fine mesh gratings (outer filter) capable of excluding organisms of size greater than about 0.127 mm × 0.0345 mm. In the house the water circulates through another set of filters (inner filter) that retain organisms about 0.030 mm in diameter. The water is circulated by undulatory movements of the animal's “tail” and is expelled through a second opening in the house, thus propelling the house through the water. The extremely fine material collected on the inner filter is drawn into the animal's mouth by means of ciliar action. After a few hours the screening devices become clogged and the animal then escapes from the structure through a third separate opening (exit). Having freed itself from the old useless vestment, it secretes a new one with all the complicated structures for gathering the type of food upon which it is dependent.
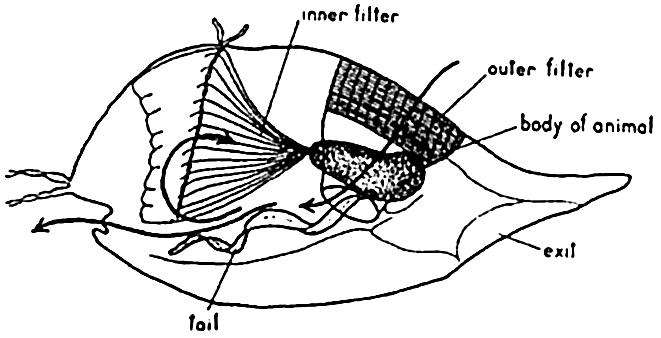
The filter-feeding apparatus of Oikopleura.
There are also many plankton filter feeders among the sedentary or burrowing animals. Indeed, the permanently attached forms so characteristic of the marine fauna (and by comparison so conspicuously wanting in land fauna) can exist as such only because the water carries to them sufficient nourishment in the form of suspended particulate food,
A familiar example of the filter-feeding habit is that of the simple sponge, wherein flagellated cells lining internal cavities propel the water into the sponge by way of the numerous incurrent pores covering the surface of the body. After passing through the more or less complicated canal system, the water is then expelled through a common opening, the osculum, but enroute the individual flagellated cells select out the fine particles of food carried by the water.
Many plankton feeders may be better classified as preying animals, although in some respects they combine this habit with filter feeding. Any attempt to distinguish between such categories is based largely on the relative degree of selectivity exercised in feeding. Few animals are wholly indiscriminate in feeding, and even filter feeders exercise some degree of selection, either by a mechanical segregation of size dependent upon apertures of the screening apparatus, as in Oikopleura or sponges, or by rejecting through ciliary or other action certain particles unpalatable for chemical or physical reasons.
We have mentioned copepods chiefly as herbivorous plankton filter feeders but not all copepods feed upon diatoms and other phytoplankton organisms. The free-living types like Tortanus and Candace that may be considered largely carnivorous and rapacious have very strongly built mouth appendages for catching and holding their prey.
Jellyfishes and ctenophores are highly predaceous in habit, feeding voraciously upon other plankton animals that drift within their reach. The former paralyzes its prey by means of batteries of nettle cells which cover the tentacles. The latter (Pleurobrachia) when in swarms are very destructive to large numbers of other small organisms, of which they sweep the waters quite clean. The prey is entangled in the trailing tentacles which are provided with sticky adhesive cells. In his study of food relationships in the Gulf of Maine, Bigelow states that “of all the members of the plankton, the most destructive to smaller or weaker animals are the several coelenterates, and especially the ctenophore genus Pleurobrachia, a pirate to which no living creature small enough for it to capture and swallow comes amiss.”
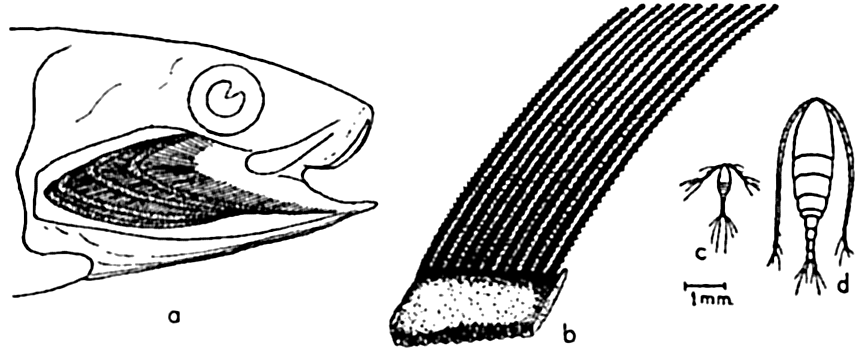
The filter-feeding apparatus of the California sardine: (a) gill cover and gills removed to show one side of branchial sieve formed by gill rakers; (b) enlarged camera lucida drawing of section of branchial sieve; (c) Oithona plumifera, a small copepod drawn to the same scale as (b); d, Calanus finmarchicus, a medium-sized copepod drawn to same scale as (b). Compare with fig. 90, p. 377.
The formidable jaws of the arrow worm Sagitta attest that it is also a highly rapacious plankton feeder; being able to snatch individual organisms like Calanus and larval fish despite the fact that it possesses only light-sensitive “eye spots” instead of true eyes (fig. 228a).
Among smaller important plankton forms, mention should also be made of the tintinnids, radiolarians, foraminifera, Noctiluca, and other planktonic Protozoa that engulf such small organisms as chance carries within their reah. These are plankton feeders but not filter feeders. That some may exercise a degree of selective feeding is indicated by the tintinnids, some of which are found regularly to contain only the shells of silicoflagellates, while others select certain coccolithophores, the coccoliths or armor of which they use in building their shells.
Among the more or less obvious preying plankton feeders may be placed many fishes, notably herring, mackerel, sardines, and others of this type (p. 896) which either select out individual animals of the plankton or filter quite indiscriminately by the aid of the gill rakers, which form a net through which water entering the mouth must pass in its course over the gills and out under the gill coverings (fig. 240). The fineness of the net or branchial sieves formed by the gill rakers varies with different types of fishes, and in unclogged condition determines the minimum size of the planktonic organisms that can be sieved out for food. Even the menhaden, Brevoortia tyrannus, with a notably fine branchial sieve, is unable to retain organisms as minute as coccolithophores and small diatoms and infusoria. In the herring the sieve is much coarser, and though these fish are known to select out organisms individually the gill rakers must assist materially in retaining many of the smaller Crustacea. The stomach of a single herring has been found to contain more than 60,000 copepods. It should be noted that though much of plankton feeding may appear indiscriminate, yet a good deal of selection does occur, since swarms of specific prey may be selected and followed, as is evidenced in the herring and in the filter-feeding whalebone whales. The plankton-feeding fishes are swift swimmers but the usefulness of this ability must be in large measure to escape their enemies, the typical large predators (see below), although most plankton feeders do in part also prey upon other smaller fishes.
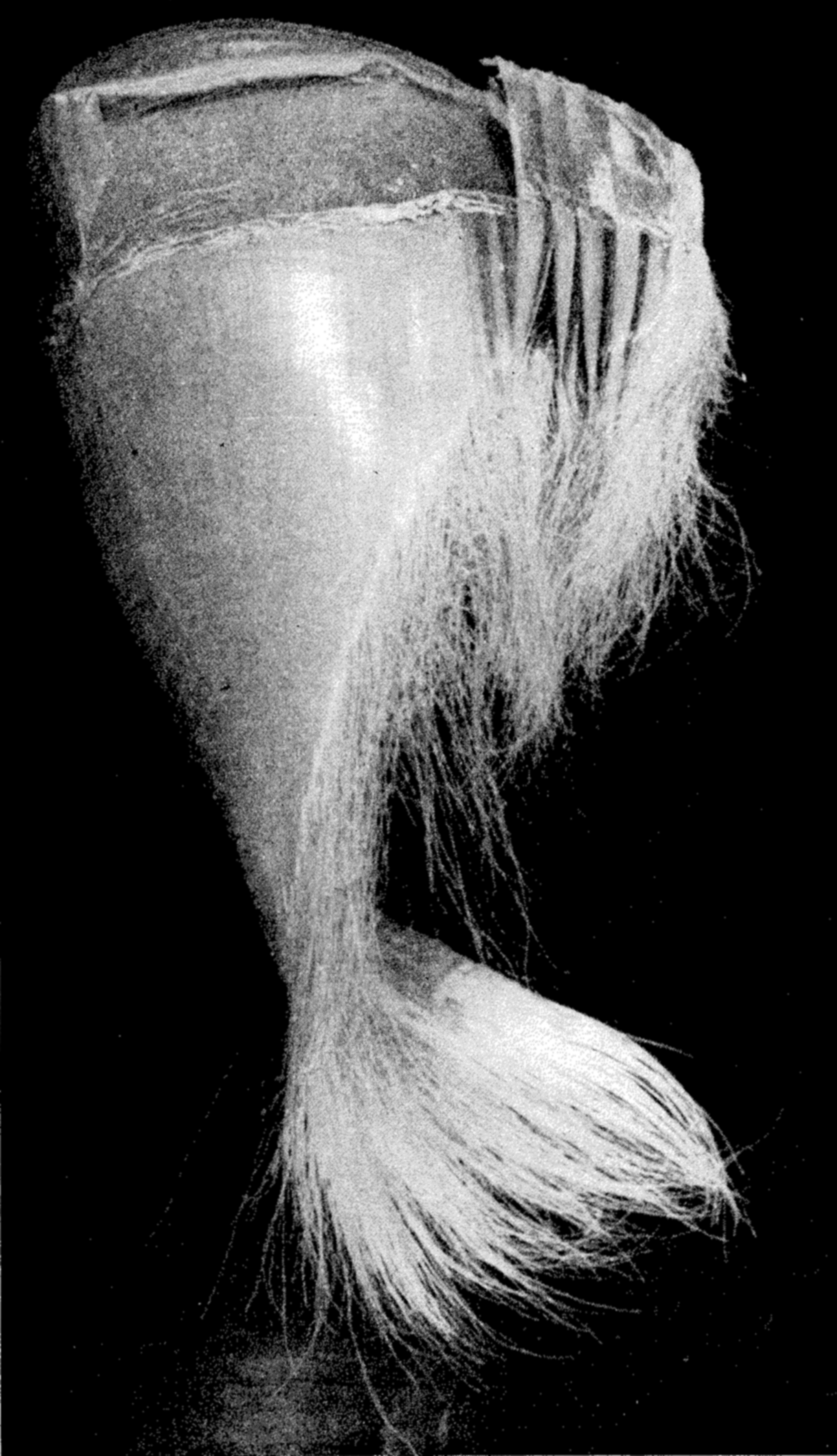
A portion of the frayed baleen plates forming the filter-feeding apparatus of the whalebone whale.
It is a strange fact that the largest animals, that is, certain of the whales, are plankton feeders, living upon great masses of very small animals. These are the Mystacoceti or whalebone whales, of which the blue whale or sulphur bottom, the largest of all living animals, is an example (fig. 76 a, p. 314). In the mouth of this type of whale are suspended the closely set plates of whalebone (fig. 241) through the frayed ends of which water is passed while the planktonic euphausiids, copepods, pteropods, and so forth, which make up the principal diet, are filtered out. Whales are most abundant in waters rich in planktonic life and, as indicated in fig. 244, p. 904, their numbers may be correlated with the abundance of planktonic food of their preference.
Numerous other examples could be given from diverse animal groups to illustrate the manner in which nature exploits the supply of microscopic but vastly numerous and scattered particulate food floating freely in the water. Any considerable fluctuations in the abundance or distribution of the planktonic food must quickly affect the plankton feeders and, in due time of course, other types of feeders as well.
More will be said later (p. 901) about general problems involving filter feeding, production, and population density.
Detritus Feeders and Scavengers
We have learned that much of the organic material produced in the pelagic zone is precipitated to the bottom in the form of living or dead bodies of the planktonic and nektonic organisms and their excreta. Added to this is detrital material resulting from disintegration of benthic plants and animals and also from influx of terrestrial material. In
The organic material available in this mixture on the bottom is fed upon by bacteria and other microorganisms such as Protozoa, nematodes, and rotifers, and the whole mixture in turn is consumed by larger detritus feeders. Bottom organic detritus is sometimes considered the main source of nourishment for most benthic invertebrates. In a survey of Danish waters Blegvad (1914) concluded that of 90 or more species of invertebrates investigated, 69 (the most common animals) were some form of detritus eaters, while 5 were herbivorous and carnivorous and 16 were purely carnivorous. In the strictest sense only those organisms subsisting solely on detrital organic remains are detritus feeders, but it is not practicable in a general survey to draw the line so closely. So in a broader sense, we see that some detritus feeders may be nourished in part by living organisms and are not necessarily entirely scavengers in habit. In the littoral zone, especially in the eulittoral zone, part of the detrital mixture consists of photosynthetic organisms such as littoral diatoms growing naturally on the bottom and thus contributing to the organic material available in the detritus.
The concentration of detritus feeders is, of course, dependent upon the extent of production of plants and nonscavenger animals. Where this production is great, there also the scavengers must be numerous. With increasing depth, the detrital food on the bottom becomes less. The plant material diminishes until in the great depths not even sinking pelagic plants produced in the euphotic zone above ever reach the bottom, being disintegrated through bacterial action or autolysis, or eaten and converted into bodies of animals. Such mid-depth and abyssal pelagic animals serve as links in a series of changes that convey sufficient organic material, as animal detritus, from the euphotic zone downward to the bottom to support at least a sparse benthic population of detritus-feeders, and the animals that in turn live upon them. It is hardly conceivable that any plant material as such ever reaches the bottom to enrich the detritus of abyssal depths. The rate of sinking of Chaetoceros diatoms in still water is only about 1 m in 4 3/4 hours. Plankton animals such as salps sink at the rate of 4000 m in 2 days 7 hours (Hesse, Allee, and Schmidt, 1937). The low temperature of great depths is important in delaying bacterial action, thus allowing more time for sinking before complete disintegration takes place, while the rate of sinking must
Various types of animals may be mentioned as detritus feeders. Their habits of feeding are varied and may combine several methods. Such burrowing worms as the lugworm Arenicola and others, and the protochordate Balanoglossus, swallow the mud indiscriminately in the process of digging and are nourished by whatever digestible material may be present in the mud and sand.
The clam Macoma nasuta, lying buried in the mud and sand of shallow water, extends its long inhalant siphon to suck up slime that has accumulated on the surface of the mud. The digestible material thus taken in is entangled in mucus and propelled by cilia to the labial palps and the mouth. The bivalves Nacula and Yoldia gather detrital material by directly extending the unusually long labial palps to pick up the food.
Among the echinoderm detrital feeders the sea cucumbers, illustrated by Stichopus, suck up large quantities of mud and detritus that has come to rest on the bottom. It has been calculated (Crozier, 1918) that in certain shallow coastal areas of Bermuda, these animals pass 6 or 7 kg (dry weight) of mud per square meter per year through their digestive tracts; in a certain enclosed area of 1.7 mi2 the mud eaten annually may be from 500 to 1000 tons. The stomach fluids are somewhat acid and may dissolve calcium carbonate. Feeding of this type is important in the biological “working over” of bottom deposits. The sea urchins, Strongylocentrotus spp., also subsist on plant and animal detritus. The mud-dwelling brittle star lies buried below the surface of the mud with several arms extended out over the surface in contact with the top slime which they collect and carry to the mouth.
Mud-dwelling tube worms collect the nutrient-rich detritus by means of extended cirri along which food material can be carried in ciliated grooves. Detritus feeders living in the mud also have their quota of filter and mucus feeders, although the material consumed doubtless also consists of typical planktonic organisms as well as suspended organic detritus stirred off the immediate bottom by currents or, in some instances, purposely stirred into suspension by the animals, for example, by Callianassa and other Crustacea.
The echiuroid worm Urechis, which inhabits U-shaped burrows in muddy sand, has a remarkable method of obtaining food through a combination of mucus secretion and filtration. Mucous glands at the anterior end of the worm secrete a funnel-shaped mass of mucus 5 to 20 cm long lining the upper end of the burrow in front of the animal. The broad end of the funnel fits against the walls of the burrow, while the narrow end fits as a snug collar around the anterior end of the animal. Water is then forcibly pumped through the burrow from front to back and in its passage through the mucous tube small particles of detritus,
An interesting method of detrital feeding is shown by the sand crab Emerita, which inhabits wave-washed sandy beaches especially in tropical and subtropical waters. Living in sand burrows in the lower part of the intertidal region, the animals face seaward while their long feathery antennae protrude from the sand to intercept fine detrital material that is washed out with the receding waves.
Littoral Browsers
In the littoral zone where large quantities of attached benthic plants are produced, many herbivorous or omnivorous animals also are found that feed directly upon the growing plants and are therefore to be considered a complement to the small but numerous planktonic grazers and the plant detrital feeders so vital in converting plants into animal substance. The large benthic algae have their greatest significance as a source of animal food in the temperate and boreal regions (p. 293).
Numbered among these littoral algal grazers are many gastropods, crabs, shrimps, and amphipod and isopod crustaceans. The devices used in mincing the plants consist of horny rasplike radulae in the gastropods, and claws, pinchers, and mandibles of heavy chitin in the crustaceans. A number of fishes, for example the rudder fish (Kyphosus) and the butterfly fish (Chaetodon) as well as other reef and littoral fishes, also browse on the attached algae.
It is not feasible to separate sharply the browsers from the detritus feeders and scavengers, since they perhaps all feed more or less indiscriminately upon growing plants or upon living or dead fragments, some of which may be washed far to sea. Indeed, most plants are eaten after they have become detached from their moorings and while in the process of breaking up mechanically or through decay. This was also the conclusion of Hewatt (1937) in special observations on food relations of intertidal animals at Monterey Bay. Petersen (1918) reports that eel grass is utilized mainly as detritus and that it may either be spread over the bottom or carried as fine particles in the water.
The relation of marine wood-boring organisms to their food supply constitutes an unusual one in the sea and may be arbitrarily mentioned under this presnent heading.
Much organic material is washed or blown into the sea from land which incidentally becomes available as food for marine life. Few instances can be cited wherein typical organisms of the sea are directly dependent upon organic products from the land. Such dependence is
Preying Animals
On the whole, it is animals of this type that are best known to the layman, for they are usually relatively large and their feeding habits tend towards the spectacular. We have already had occasion to mention a few of the preying animals such as the jellyfish, arrow worm, certain copepods, plankton-feeding fishes, and whalebone whales because of their role in utilizing the food offered by the micro- and macroplankton. The fishes and whales of this type were considered, in part, also as filter feeders.
Fast-swimming predators, among which are the surface fishes tuna, barracuda, and salmon, are provided with well-developed eyes and efficient teeth to aid in capture of their prey, which consists largely of plankton-feeding fishes. It should be noted here that such plankton-feeding fishes as the herring, sardine, menhaden, mackerel, anchovy, and alewife are fishes of exceptionally great abundance and are eagerly sought as food by the larger preying animals of the pelagic region. Some idea of their numbers may be gleaned from the fact that over 500,000 tons of sardines have been taken in in one year from California waters alone (Scofield, 1937), and the menhaden yield of the east American coast may reach 400,000 tons in a year (Tressler, 1923). Such fishes must therefore stand as an important link between the animal plankton and the larger piscine predators unprovided with direct means of gaining sustenance from the small planktonic life. High in this complex food pyramid are also the toothed whales and other marine mammals.
The pelagic fishes of great depths are also predators but they are of relatively small size, usually ranging upward to only a few centimeters. This small size may well be correlated with the scarcity of food at these depths. The diminutive sizes not only represent a conservation of organic material in growth, but also, owing to the increased ratio of
Among the nonplankton-feeding predator mammals of the sea are the toothed whales, for example the sperm whale (fig. 76b, p. 314), which is provided with teeth only in the lower jaw and which dives to great depths for its favorite food the squids, including the giant squid, the largest of all invertebrates. Other cetacean predators are the killer whales, porpoises, and dolphins, animals adapted to swimming with incredible speed and provided with teeth in both upper and lower jaws. To these must be added the seals, the sea lions, and the walruses. The first two catch their prey (fish and crustaceans) with very well-developed but ordinary teeth, while the last are specialized with long tusks with which to dig shellfish from the bottom.
Many benthic animals are predaceous, living upon each other and upon other bottom animals already discussed as users of detrital and finely divided food occurring on the bottom. The list includes many bottom-living fish called “bottom feeders” or “ground fish.” The best known among these are the plaice, flounders, halibut, croakers, cods, and rays which live on the crustaceans, shellfish, worms, and coelenterates of the bottom community.
Sea stars are notably voracious feeders on bivalve molluscs, a single specimen having been known to devour five or six clams in a day. Predaceous snails, distinguished by their long siphons, are also enemies of bivalves and other molluscs, drilling a neat hole through the shell and eating the soft contents.
The interrelations of the organisms of the sea are diagrammatically summarized in fig. 242. The volumes indicated are not based on computations and should be considered as being only very roughly proportional
The marine bacteria are a vital link in the nutritional relationships of all marine organisms, but it is more convenient to discuss these later under a special heading (p. 908).
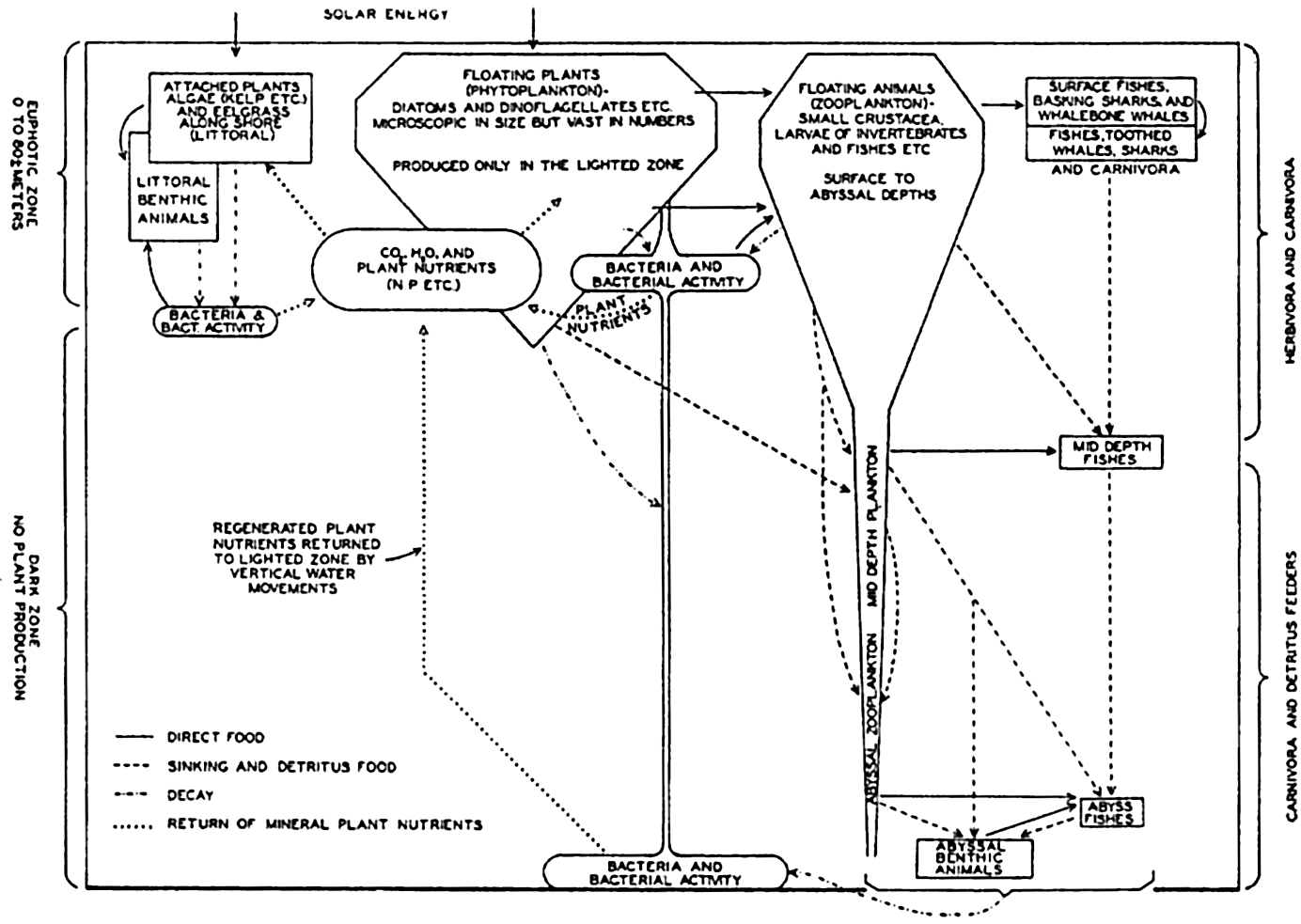
The main features of the interrelations of marine organisms.
BIOLOGICAL FACTORS INFLUENCING MOVEMENTS AND CONCENTRATION OF ORGANISMS
From what has just been said concerning the interrelations of organisms, it is obvious that in a study of the combination of factors controlling the whole or a given portion of the population it is necessary to consider seriously not only physical and chemical factors but also biological factors that have direct bearing on the lives of the individuals of the population. These factors are associated chiefly with feeding but there may be other less obvious influences. We have already alluded to the abundance of plant production in the coastal waters and to the inanimate factors making for high productivity. The direct corollary is the abundant animal life of the coastal regions as opposed to the more sparse population of the waters far from shore.
Phytoplankton—Zooplankton
In attempting to evaluate the importance of the ecological relationships of natural biological groups in the sea as a whole, we are forced to
Organisms making up the plankton are usually short-lived, particularly the photosynthetic organisms since these are notably sensitive to changes in the physical-chemical living conditions. Seasonal and sometimes interseasonal periods prevail, therefore, when but little phytoplankton can be found; but, at least in temperate regions, a vernal production followed by subsidiary increases of phytoplankton can be depended upon each year. This vernal production of phytoplankton must be considered as an event of great significance, for it comes at a time coinciding with abundant production of pelagic larvae, especially of invertebrates, that feed upon it (see below). Any factor, whether it be in reference to phytoplankton food or to inorganic living conditions, that hampers the success of these swarms of larvae will function immediately to the disadvantage of higher plankton feeders that utilize the larvae directly or the adult population resulting therefrom. Studies have indicated that among these feeders may be placed many commercial fishes, especially the young, but also the adults. Any degree of failure of the spawning or development of larvae of the permanent plankton (of which copepods may be considered typical) must also be reflected only a few weeks later in the adult stock available in any area, for the adults are short-lived and apparently die after the spring and summer propagative periods and therefore depend upon the success of these broods to maintain the adult stock at a high numerical level. Copepods, we know, feed upon the phytoplankton and in turn constitute an important item in the diet of many fishes. Assuming that phytoplankton production may be correlated with abundance of light, an example of the far-reaching effect of phytoplankton production on the mackerel is offered in a study by Allen (1909), which shows a direct correlation between the abundance of fish caught during May and the total hours of sunshine for the preceding February and March, over a period of seven years (fig. 243). Official figures for the mackerel fisheries as a whole show a marked drop in 1906, and Bullen (1909) has shown a
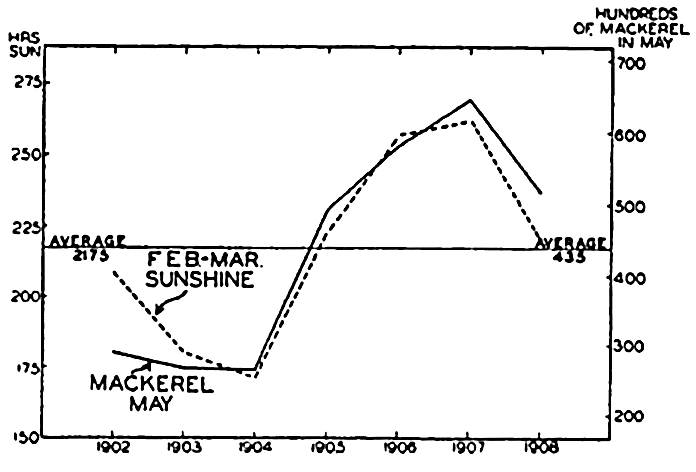
Correlation between sunshine and the abundance of mackerel. (From Allen).
Many investigators have observed that during the growing season when diatoms occur in abundance there frequently appears to be a scarcity of zooplankton. A combination of causes doubtless operates to produce this effect. Two main hypotheses have been advanced in explanation of the phenomenon of inverse relations. The least complicated explanation is found in the hypothesis of grazing (Harvey, 1934), which holds that diatom numbers are controlled through consumption by animals. The alternate hypothesis is that of animal exclusion, advanced by Hardy (Hardy and Gunther, 1935; Hardy 1936).
Animal Exclusion. According to this view, which involves a consideration of the regular vertical diurnal migrations of the zooplankton, certain of the animals during a part of the day swim upward into the layer of water where diatoms are being produced. The duration of their sojourn in the upper layer is inversely related to the concentration of the phytoplankton. Thus they are excluded vertically for a considerable period of time when diatom production has resulted in a dense swarm of diatoms. It should be noted that this exclusion is primarily a vertical one; nevertheless it may result in lateral exclusion when there is a difference in the speed or direction of flow of the upper euphotic layer and of the deeper layer into which the animals have descended and in which, when the diatoms are very abundant, they may spend a disproportionately long time. A lateral displacement may therefore be more or less complete and the greatest concentration of animals will finally occur in areas where diatoms are relatively scarce. The implications of this hypothesis on the habit of diurnal migrations should be noted (p. 836).
As evidence in support of this hypothesis, it was found during the Discovery investigations in the Antarctic that the greatest concentration of zooplankton occurs in areas low in phytoplankton content and relatively high in phosphate content, which indicated that phytoplankton production had been low for some time. Correlated with this disparity was the fact that blue and fin whales, which are known to seek out and feed upon zooplankton, were present in greatest numbers in the areas rich in phosphates. Some support is also given through experiments which indicate that some animals are more strongly negatively phototropic in the presence of many diatoms (Lucas, 1936). Plankton animals exhibiting the most pronounced activity in diurnal vertical migrations are the ones most likely to be excluded according to the hypothesis. Young stages are believed to show less tendency to exclusion. In the hypothesis of animal exclusion, which is considered as a tentative one, the emphasis is placed on the inimical effect (presumably chemical) of plants on the animals.
Grazing. The concept in this hypothesis shifts the emphasis to the effect of the grazing zooplankton as a control of phytoplankton production. That grazing is a highly important factor in the control of phytoplankton is given much support by various investigators (Harvey et al, 1935, Fuller, 1937).
According to this point of view, large phytoplankton and zooplankton populations cannot exist simultaneously in the same area for long, since the extensive grazing by the zooplankton prevents the phytoplankton from building up or maintaining dense growth. As a result of investigations of Harvey et al (1935) it is indicated that in general “a change in diatom population is brought about by a change in one or both of two opposing factors—the rate of growth of the diatoms (depending upon illumination and probably on concentration of nutrient salts) and the rate at which the diatoms are eaten (depending upon the number and kind of herbivorous animals).” Therefore, in any area a dense phytoplankton population is the result of optimum growing conditions combined with a relative scarcity of grazing animals, the yield of plant cells having been greater per unit time than the consumption by animals plus the loss that may result through other causes. Since dense plant growth is favored in the absence of grazers it appears as if the animals have been excluded because they avoid a dense diatom population. Evidence of grazing is found in the fact that phytoplankton may “disappear” when nutrients and other growing conditions are good.
In this connection it is significant to note that computations indicate that during maximum grazing plankton animals may consume somewhat less than half their own weight per day. The copepod Eurytemora hirundoides is said to eat as many as 120,000 small diatoms (Nitzschia closterium) in a day (Harvey et al, 1935). Lohmann (1908), in an exhaustive study of plankton at Kiel, assumed that each metazoan animal requires a daily ration of one tenth its own volume. A 30 per cent daily increase in plants was assumed to take place, and only this much could then be removed without reducing the initial plant stock by overgrazing. As stated elsewhere (p. 885), calculations on this basis showed a plant deficiency during the seasons of low plant production. As a matter of fact, the rate of increase in numbers of plants cannot be so simply stated. It varies greatly and so also does the rate of feeding in grazing animals, and widely different combinations of plants and grazers must occur.
Evidence has been brought to indicate that some plankton filter-feeding animals, that is, copepods and mysids, filter the water at a rather constant rate regardless of the concentration of the microscopic particulate food that is present (Lucas, 1936, Fuller and Clarke, 1936, Fuller, 1937, Fleming, 1939). Other factors, such as light, temperature, and size of particles, function to vary the filtering rate (Fuller, 1937). This
| Time in days | Population (cells per liter) | ||
|---|---|---|---|
| Grazing element doubled | Grazing element increased fivefold | ||
| 0 | 1,000,000 | 1,000,000 | |
| 1 | 487,000 | 62,000 | |
| 2 | 237,000 | 3,900 | |
| 3 | 106,000 | 240 | |
| 4 | 56,000 | 15 | |
| 5 | 27,000 | <1 | |
When plants have been reduced through grazing or when conditions for growth of the plants become less favorable and the division rate diminishes to a point where production of new cells is less than the number consumed by the grazers, animals again dominate the field. The progressively diminishing efficiency in catching plants as they become scarce is important to the survival of the diatoms since as a result there is likely always to be some “seed” left in the water. It is important to note that observations taken at fixed places, in areas where an exchange of water masses occurs, may show an apparent succession of phyto- and zooplankton populations. However, under such conditions no true alternation has occurred but only an apparent one owing to the exchange of water within the area under observation. Investigations of food relations in the plankton can, of course, be most reliably carried on in bodies of water such as bays or other closed systems that are sufficiently isolated to experience little or no influence from inflow or outflow from adjacent waters and therefore support an endemic self-contained population. In open coastal areas where much exchange of water occurs, the details
Some investigations do, nevertheless, show a clear relation between phytoplankton increases and the dependent plankton animals. For example, in Loch Striven, a semienclosed area, Marshall, Nicholls, and Orr (1934) were able to correlate directly three main successful broods of Calanus finmarchicus with diatom increases in (1) March-April, (2) May, and (3) July and August-September, the main spawning having occurred in February-March, May, and July. A fourth period of spawning occurred but was abortive owing to scarcity of food for the early stages of development. There were also indications that a storage of fat can take place during periods of plentiful phytoplankton.
Nielsen (1937) found that in the open coastal waters of Iceland the phytoplankton maximum occurred in May. In these waters the zooplankton was poor and was represented mainly by juvenile individuals, while at the same time in the protected fjords, where diatom maximum came a month earlier, there was an abundance of animal plankton with numerous full-grown individuals.
Mention should here be made of the mutually beneficial relationship derived by the phytoplankton and the zooplankton through an exchange of oxygen and carbon dioxide in solution. We know that during photosynthesis by the plants much oxygen is produced in the waters of the euphotic zone. It is not clear, however, to what extent this means of aeration is a necessary supplement to that which results from diffusion at the contact zone with the atmosphere. In isolated quiet waters it must be an important item. Waters of great depths have a sufficient supply of dissolved oxygen to support their characteristic types of animal life and this must have been transported directly from the surface or euphotic zone through diffusion and the action of water currents. It is probable, however, that a greater rate of metabolism in the more abundant animals of shallower depths, where temperatures are higher and food more abundant, sets up a requirement which could not readily be met by diffusion of oxygen from the surface alone. Plankton animals do occur in the oxygen minimum layer below the euphotic zone of the Pacific, and in the Gulf of California they are found at mid-depth layers where oxygen is near zero. The numbers of animals in the oxygen minimum layer are nevertheless small, and this may have resulted in part from the low oxygen content in the absence of photosynthesis or rapid diffusion from better aerated waters.
Plants in the lighted zone must derive some benefit through the carbon dioxide produced by animals living in the same waters; but here also the significance of this source, though certainly not negligible, has not been established.
The interrelations of the plankton animals have not been studied in such detail as those between the plants and animals. That many of them are carnivorous or omnivorous has been mentioned, and among these the medusae and ctenophores are notably influential in sweeping the waters clean of other planktonic animals upon which they feed (p. 890).
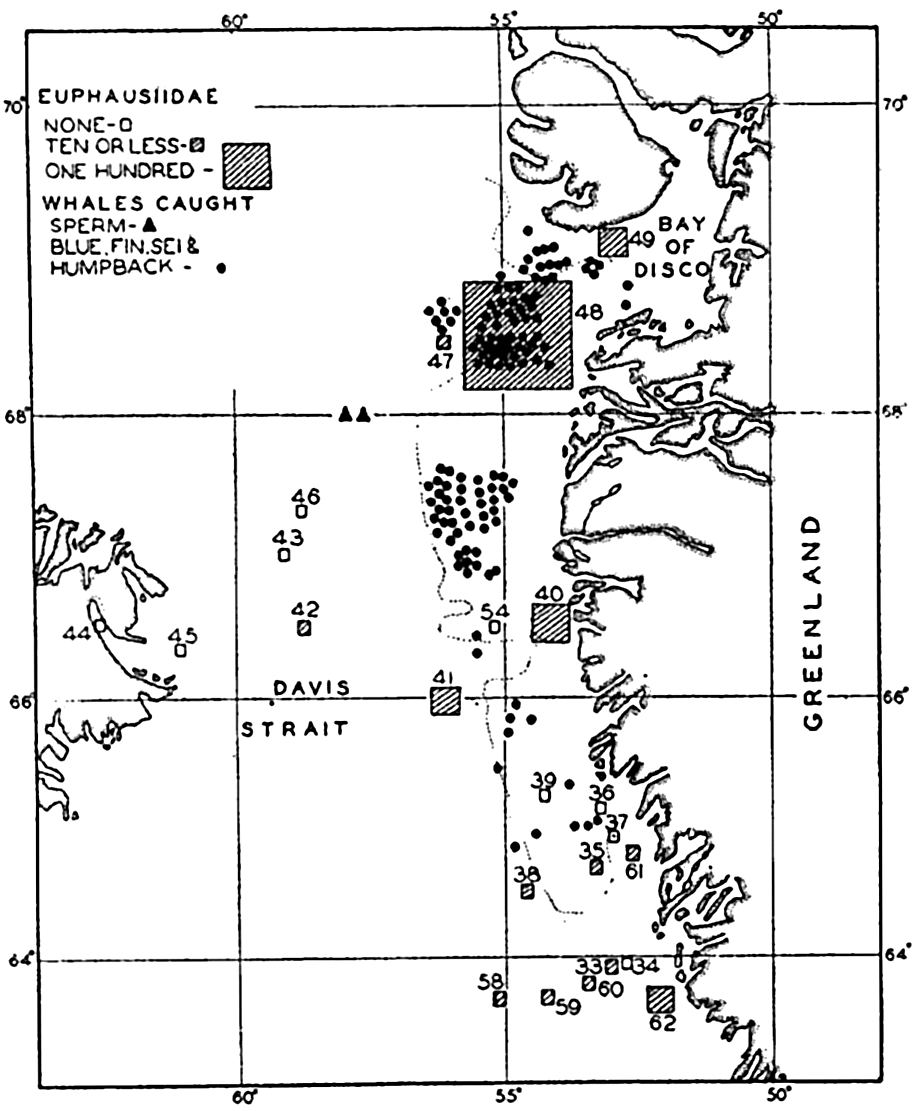
Correlation of the catches of whales with abundance of euphausiids in Davis Strait. (After Hjort and Ruud.)
Nekton
The migrations and concentrations of nektonic animals are governed largely by two biologic factors, reproduction and search of food. Mention need only be made of such truly phenomenal alternating wanderings as are witnessed in the salmon and the eel. Many other fishes also migrate, although the reasons for the migration of some of them have not been ascertained even in a general way. As a matter of fact, even in the
Among the great wanderers of the seas are the marine mammals. The habits of the Alaskan fur seal in returning to the Bering Sea to breed are widely known, and some whales migrate regularly to warm waters for breeding and between breeding times to the feeding grounds of the polar regions for food.
It will be realized from the above that any study of migratory animals and their habits as related to these biologic factors is complicated to the extent to which breeding and feeding grounds do not coincide.
It is well known among whalers and scientists that blue, fin, sei, and humpback whales feed wholly or partly upon planktonic life. Scientific investigations carried on in cooperation with whaling companies have shown, as might be expected, that the occurrence of plankton-feeding whales is correlated with the distribution of the planktonic life upon which they are known to subsist. In Davis Strait it was shown by Hjort and Ruud (1929) that young euphausiid crustaceans (the young are considered to reflect numbers of old specimens not so readily caught with vertically hauled nets) had their maximum concentration over certain coastal banks, and it is precisely in these waters that the maximum numbers of whales also occurred (fig. 244). The euphausiids tend to collect in swarms during breeding and the appearance of blue whales coincides with the periods of spawning of the dominant species in the region. Sperm whales, which feed largely on squids and not directly on plankton, are found to be present farther offshore, as indicated in the figure. The same authors compare the numbers of sei whales (Balaneoptera borealis) with the concentration of Calanus finmarchicus during the same period from the coastal banks. Figure 245 shows a striking correlation in the abundance of these animals, with the increase in Calanus preceding somewhat the appearance of the whales. Data also
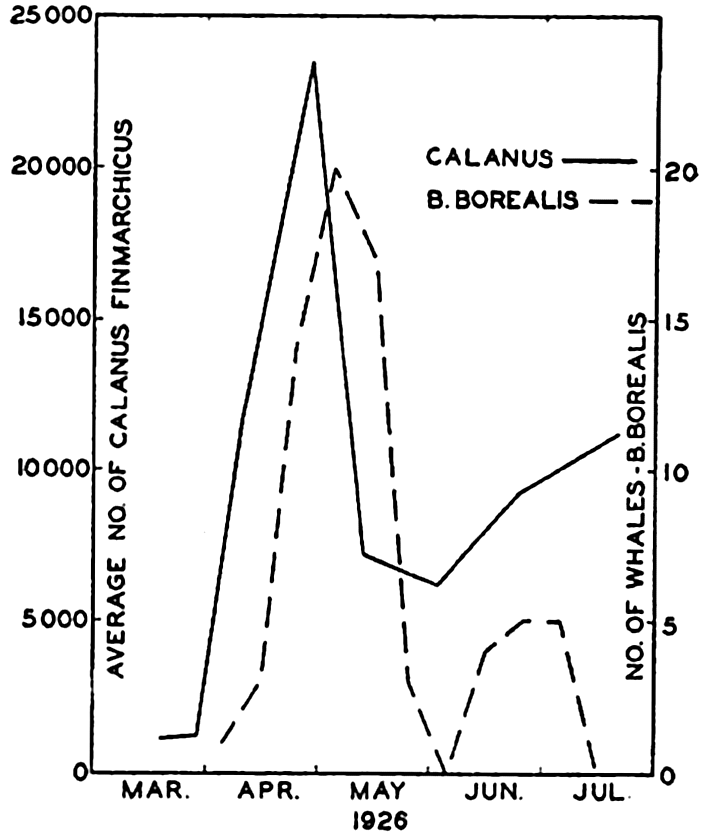
Correlation of the catch of sei whales (Balaneoptera borealis) with the number of copepods. (After Ruud.)
Investigations by the Discovery likewise showed a positive correlation between distribution of blue and fin whales and abundance of zooplankton, especially with their favorite food Euphausia superba (Mackintosh. 1934, Hardy and Gunther, 1935).
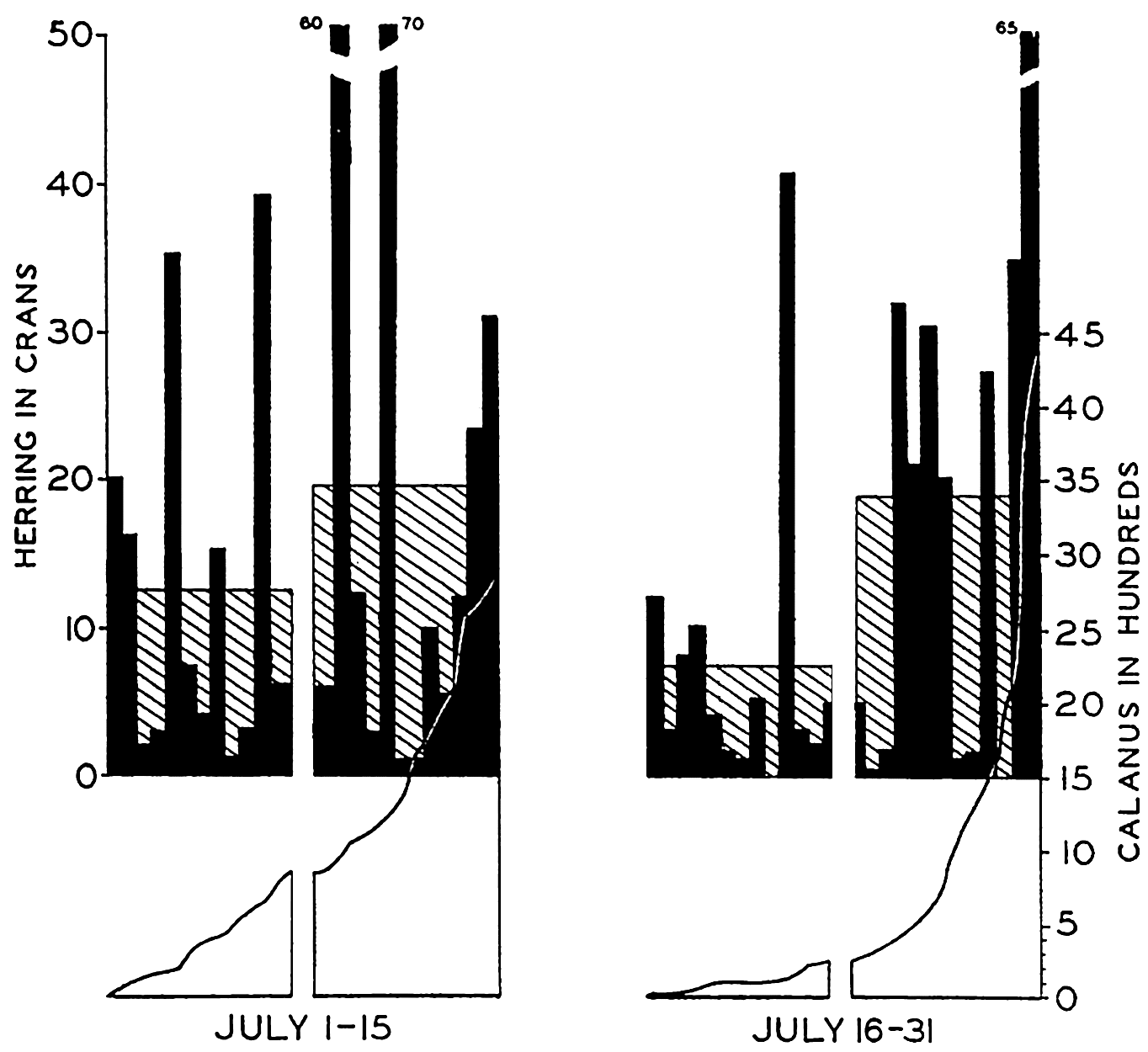
Histograms showing (in black) the individual catches of herring in half-monthly periods arranged in each period from left to right in the order of ascending values of Calanus in the associated plankton samples. The average catches of herring are shown as shaded histograms. The left half of each series represents catches in the poorer, and the right half in the richer Calanus water as indicated in the graph for associated Calanus values. (From Lucas.)
Numerous other examples might be given showing the concentration of whalebone whales in coastal areas rich in plankton food, and their instinctive migrations into these waters at the swarming season of such forms as euphausiids is truly phenomenal. One is impressed with the efficiency of the metabolism of some of the large animals which feed upon the animal plankton. For instance, at birth the blue whales are about 7 m long and weigh about 2000 kg (4400 lb), and at weaning, seven
Among the pelagic fishes the best material illustrative of correlation with plankton comes from studies of the herring. It has long been believed that the movements and concentrations of these fish are associated with the relative concentrations of the zooplankton upon which they feed. Hardy, Lucas, Henderson, and Fraser (1936) have recently investigated the plankton by means of the plankton indicator (a quick-sampling device) and, in correlating the numbers of Calanus copepods of the plankton with the amount of herring caught by fishermen in the same areas, they have shown in most instances that the greatest number of adult herring are caught in Calanus-rich waters. Theoretically the gains derived through fishing exclusively in Calanus-rich waters may be as high as 21 per cent. In Fig. 246, from Lucas, is shown the general trend of the relation that exists when the Calanus series are arranged in ascending order of numbers and each half of the series compared with the average catch of herring for the period.
A negative correlation is found between heavy phytoplankton and herring. North Sea fishermen have observed this in practice, and designate the heavily diatom-populated waters as “weedy water” or “stinking water” and consider it a bad omen to fishing.
Benthos
Among the benthic organisms the animate factors of the environment are manifest not only in predacity and competition for food but also in competition for favorable space for attachment or burrowing. The competition for space is most obvious in sessile forms such as barnacles and mussels of the intertidal zone, where overcrowding must lead to elimination as growth proceeds, and many pelagic larvae of sessile forms perish by failure to find attachment at all. A foremost problem in oyster culture is the curtailment of oyster production that results in beds in which the slipper shell Crepidula is a serious competitor with the oyster for food and space. In English waters as much as twenty tons of Crepidula have been removed from certain beds daily, and some beds there and elsewhere have been completely abandoned for culture because of this pest.
Many good examples of the influence of biological factors on the benthic populations are also offered by studies of other pests on oyster beds (Orton, 1937). Among these we may mention the common drill Urosalpinx cinerea, which may destroy 50 per cent of the young oysters settling on a bed. In Australia whole beds have been destroyed in a few days by yet another predator, the mangrove crab Scylla serrata. The extent to which sea stars are a factor in reducing the yield of oysters in Long Island Sound is indicated by reports that as much as 500,000 bushels are annually killed by these pests in that small area alone (Galtsoff and Loosanoff, 1939).
In the above, attention has been directed to the effect of predacity on the prey, but we may shift the emphasis and consider an instance wherein the predator is the object of economic interest.
According to quantitative investigations of benthic invertebrates with reference to their utilization as food for the bottom-feeding plaice, it has been calculated by Blegvad (1930) that over an area of 3445 square miles in the Kattegat there is produced, as a minimal figure, about 230,000 tons of “first-class” plaice food. (The food is considered first-class when it is of a type available to young as well as adult animals, and second-class when it can be eaten only by the grown animals.) This amount of food is believed sufficient to produce 12,800 tons of plaice. Animals known to compete for the food of the plaice take about one tenth of the total.
These few examples selected from a field of study intensively pursued for economic reasons are doubtless illustrative also of similar biological aspects that enter into the control of any population in the sea (see also fig. 249, p. 937).
MARINE BACTERIA AND THEIR ROLE IN THE BIOLOGICAL AND CHEMICAL CYCLES IN THE SEA
Because of the specific role of bacteria in the nutritional cycle of the sea, it is fitting at this point to consider in some detail the general biology and activities of these microorganisms.
Although the existence of marine bacteria has long been recognized and was early verified even in depths of over 1000 m in the Gulf of Naples (Russell, 1892), yet it is only in recent years that the study of bacteria in the metabolism of the sea has gotten well under way. For a synoptic review of works especially relevant to the development of the subject the student is referred to Benecke (1933).
Structure and Reproduction
Bacteria are unicellular organisms although some of them form chains or groups of cells. Morphologically there are the following three forms:
In their structure and activities they form a rather well-defined group of organisms, although some possess animal characteristics, especially in chemical composition, while others approach more nearly the plants, particularly the blue-green algae, with which they share the characteristic of having scattered nuclear material. Some authors consider them as occupying an intermediate position between the plants and animals (Jordan, 1931). A distinct plant characteristic is the ability of some to synthesize such complex substances as amino acids with only ammonia as a source of nitrogen. It is important to note that none possess chlorophyll, nor are their cell walls composed of cellulose. For convenience, however, they are all placed with the plants; but owing to the absence of chlorophyll in their structure they must be regarded as fungi, and since their common method of multiplication is by simple fission, they are named fission fungi or Schizomycetes.
Structurally, the marine bacteria are represented mostly by motile rods and various types of vibrios or comma-shaped forms, and there are fewer spore-formers in the sea than on land (Waksman, 1934). About 70 per cent of marine bacteria are colored as opposed to 15 per cent of the terrestrial forms.
Characteristically, bacteria are the smallest of all organisms, some measuring only 0.0005 mm in diameter. This extreme minuteness has a profound bearing on the activities of bacteria and also introduces very special problems and difficulties in the technique of collecting and in the estimation of actual numbers and mass.
Characteristic also of bacteria is their rapid rate of reproduction. This is accomplished by vegetative cell division. It may again be emphasized here that in nearly all instances where swarms of organisms—bacteria, diatoms, dinoflagellates—occur in such large numbers that the water is discolored by the accumulation of their bodies, the method of reproduction has been one of binary fission. Assuming an uninterrupted physiological state and optimum growing conditions, the accumulation of individuals can be extremely rapid since the increase is by geometric progression. The rate of division of bacteria may be as frequent as once every twenty or thirty minutes. Hence, biologically, there is a tremendous potentiality to build up an excessively large mass. However, large masses do not accumulate because of natural checks which hold the tendency to mass production within bounds. Among such checks may be mentioned availability of food supply, formation of toxic metabolic products, physical-chemical conditions of the water, nature of substratum, and consumption by other organisms. An understanding of these checks or controlling factors is one of the ends sought in the study of marine bacteriology, for such an understanding provides an explanation
Associated with bacterial reproduction is the faculty of spore formation, whereby the individual bacterium of certain species is enabled to withstand adverse conditions while remaining in a state of quiescence for long periods of time. How significant this faculty is in the marine environment, where physical and chemical conditions are relatively uniform, at least with respect to the open water, is not clear. Viable bacteria have been taken from strata of marine mud where they must have remained buried for thousands of years (p. 920). However, the functions of these buried forms as effective transformers in the water or immediate bottom have apparently ceased, and the likelihood of their revival in the sea bottom is remote. Those not too deeply buried may serve to some extent as food for burrowing detritus-feeding animals.
Bacterial Modes of Life
For our immediate study of the relationship of bacteria to other organisms and to the chemical cycles of the sea, it is necessary to take into consideration the vital implications of their activities as a part of the dynamic energy cycle within the marine population of which they are a part. The investigations of marine bacteria are therefore concerned chiefly with their physiological processes as applied to the sea and its chemical and biological problems. Their indispensable function in the economy of the sea is primarily one concerned with transformation of organized substances and not with accumulation or storage of organic matter.
In order to understand better the activities of bacteria, it is necessary to distinguish between certain of the different modes of life. These are concerned especially with the source of nutrition and the oxygen supply.
Autotrophic Bacteria. These resemble green plants in their ability to build carbohydrates and proteins out of the simple substances carbon dioxide and inorganic salts. Some of these, known as photosynthetic, possess coloring material, or bacteriochlorin, and use radiant energy in building up protoplasm, while others, known as chemosynthetic, derive their energy from the oxidation of various inorganic compounds such as H2S, S, or NH4.
The amount of organic material synthesized in the sea by bacteria is small when compared to that produced by the chlorophyll-bearing plants. It is not fully known to what extent the chemosynthetic forms contribute to organic material on the sea bottom where depths are so great that there is insufficient light for photosynthesis, but the concentration of benthic animals suggests correlation mainly with the pelagic and benthic algae as the source of primary food and not with the bacteria.
However, autotrophic purple sulphur bacteria have been on several occasions reported as being so abundant in isolated inshore situations as to impart a red tint to the water, to the surface of algae, or to the bottom (Benecke, 1933).
Heterotrophic Bacteria. These obtain their energy by the oxidation of organic compounds. Hence they live as saprophytes or parasites. Most bacteria of the sea are of this type.
We have learned that in the cycle of organic material in the sea it falls to the phytoplankton, in particular, but also to other algae to synthesize organic substance from such inorganic raw materials as carbon dioxide, nitrates, phosphates, and others. But the store of certain of these materials may be exhausted in the euphotic layer and become bound as part of the substance of organisms. However, over a long period of time organisms die at the same rate as they are born, and thus a continuous return of the raw material is possible if a transforming agency is provided. Such an agency exists in the heterotrophic bacteria, whose enormous task is to perpetuate this phase of the cycle through mineralization of organic matter. Animals take part in the general cycle, but owing to the fact that as a group they are neither producers nor transformers (in the sense that plants and bacteria are), a reduced cycle could go on without them; indeed, considerable quantities of plants must at times by-pass the animals and be directly reduced to inorganic state by bacteria (fig. 248, p. 926). The interposition of animals no doubt functions to smooth out the cycle so that bacterial activity goes on at a more even rate throughout the year. There appears to be no evidence that the number or kind of bacteria show any marked seasonal cycles (Lloyd, 1930, ZoBell, 1938). During seasons of plant production, rapidly growing animals incorporate large quantities of plant substance into their own structure and, owing to the longer life cycles of many animals, much of this material may become more gradually available to bacteria at periods of minimum plant production. Illustrative of this is Lohmann's study of the cycle of plankton organisms over the whole year at Kiel. According to his observations the plants for the year averaged 56 per cent of the volume of the total pxsankton However, it was found that from December to February plants formed scarcely a third of the total plankton. At Plymouth the winter production of plants is set at about one fourth of the summer rate. These rates apply only to calculations based on given diatom populations during twenty-four hour periods of production during these seasons and may vary with other species and conditions (Harvey et al, 1935).
Utilization of Dissolved Organic Matter. In discussing the chemistry of the sea, it was pointed out (p. 248) that an appreciable quantity of organic matter is present in solution in sea water. The concentrations per unit volume of water are very much smaller than those
It was believed by Pütter (1907) that many marine animals are able to absorb dissolved organic matter through their gills and integuments and may thus obtain a portion of their food through utilization of the organic matter in solution in the sea. There is little evidence that other forms than bacteria make any considerable use of this supply of nutriment (Krogh, 1934b, Bond, 1933).
According to some authorities the low concentration and uniform distribution of dissolved organic matter in the sea may result from the action of heterotrophic bacteria. Sea water may be looked upon as a dilute culture medium in which the upper limit of concentration of dissolved organic substance is a threshold value maintained by the bacteria at a level below 10 mg/l. In apparent contradiction it must be mentioned that this may appear too dilute for bacterial growth, since for some non-marine bacteria 10 mg/l of different carbon compounds is the minimum concentration in which successful growth occurs (Stephenson, 1939). This may also be true for marine bacteria suspended free in the water. But when the bacteria and other organic material are adsorbed to solid surfaces a greater efficiency in utilization of dilute organic matter is possible, and growth occurs although concentrations may be much lower than 10 mg/l.
Experimentally it has been shown by ZoBell and Anderson (1936a) that in normal filtered sea water bacteria may flourish but do so best when grown in receptacles providing the greatest solid surface area per unit volume of sea water. Increased surfaces were obtained by introducing sterile beads, siliceous sand, and so forth. Increasing the ratio of solid surface to water leads to increased bacterial activity only when the nutrient medium is of low concentration such as that prevailing in the sea. It is explained that the increased surfaces offer places for attachment of greater numbers of periphytic bacteria (that is, those which grow attached to solid surfaces). Clean glass microscope slides submerged in the sea also quickly show a concentration of bacteria following adsorption of organic food and provision of solid surface.
The efficiency of the activities of periphytic bacteria is believed to be enhanced owing to adsorption of organic matter to solid surfaces and to the reduction of diffusion at the immediate surfaces of bodies and within the interstices at the tangent of the bacterial cell and the solid surfaces. Thus these micro-volumes serve as concentrating foci for bacterial
In nature the solid surfaces are provided by all types of particulate matter, animate or inanimate, on the bottom, in the plankton, and on the nekton.
Oxygen Relations. It is convenient to classify the bacteria further on the basis of their different oxygen requirements or tolerances. There are obligate aerobes which use free oxygen, obligate anaerobes which function in the total absence of free oxygen, facultative forms which may live in either type of environment, and microaerophiles which require a reduction of free oxygen but not to the point of anaerobic conditions. Most marine bacteria are said to be of this type.
Bacterial Chemical Transformations. To facilitate study of the cycle of substances in the sea, it is necessary further to classify the marine bacteria into several convenient general groups, namely nitrifying, denitrifying, nitrogen fixing, sulphur, and iron bacteria, and so on, based upon their metabolic activities in the transformation of these substances. To describe these groups is to describe the role of bacteria in the chemical and biological cycles of the sea.
The Nitrogen Cycle
The main features of the nitrogen cycle must be considered because of the outstanding importance of nitrogen to all forms of life and because of the great general interest it holds in illustrating the successive ecological implications with respect to bacteria.
It has been pointed out (chapter VI) that nitrogen exists in the sea in combination with other elements, for example, in ammonia (NH3), and as oxides of nitrogen in nitrite ion (NO2), and nitrate ion (NO3). Nitrogen enters into the composition of all living things. It is one of the building blocks (nutrients) used by plants in forming the complex protein molecules of their bodies from which animals must derive their nitrogen. However, not all forms of nitrogen can be used by plants, hence the complex nitrogenous compounds found in both plants and animals must, upon death of the organisms, be decomposed, along with their products of excretion, to chemically simpler compounds utilizable by the plants. This decomposition, we know, is accomplished mainly by the activity of proteolytic bacteria.
The process of decomposition involves a series of steps in which specifically different bacteria are concerned. The early stages of the transformations are not fully known, but amino acids result and the nitrogen compounds—ammonia, nitrites and nitrates—are a part of this process. It is these inorganic compounds and also, to some extent, the amino acids that can be used directly by the plants for their supply of nitrogen.
The main store of nitrogen in the sea has been considered elsewhere (pp. 181 and 242). We need to be concerned here only with the main features of the chemical circulation of this element as activated by organisms including the plants, animals, and bacteria living within the sea. We may begin with the large and complex protein molecule of plant or animal tissue which is broken down into more simple products containing nitrogen. The cycle is composed of six transitions known as ammonification, nitrification, nitrogen assimilation, denitrification, and nitrogen fixation.
Ammonification. It is well known that ammonia is a product associated with decay of organic material. There are a number of stages and different products involved in this simplification of the molecule, among which products are the amino acids, considered present in the sea only by inference (Cooper, 1937). The bacterial process of deaminization results in splitting off the NH2 group in the amino acids, and there are many types of bacteria in the sea which are endowed with the ability to carry on this process. In weak solutions the ammonia may be intercepted at this point and the nitrogen assimilated directly by diatoms, as is known to occur also with higher plants. ZoBell (1935) and Cooper (1937) review the literature, in which there is much evidence that ammonia nitrogen constitutes one of the important sources of nitrogen utilized by unicellular algae. Ammonia is present usually only in small quantities and its distribution and concentration appear to indicate the place and intensity of organic decomposition (Redfield and Keys, 1938).
Nitrification. It was formerly suggested by some investigators that the sea water must derive its nitrates through drainage from land or through electrical discharges and photochemical processes. The nitrifying bacteria occurring near shore were thought to have washed in from land and not to live normally in the sea. In more recent years investigations have concluded from bacteriological and chemical studies that this type of bacteria is also beyond doubt truly marine. Carey (1938) summarizes briefly the development of our knowledge of these microorganisms in the sea.
The complete process of nitrification includes the formation of nitrates from ammonia and nitrites (NH3 → NO2 → NO3). The organisms responsible for converting the ammonia to nitrite are called Nitrosomonas and Nitrosococcus. They may live in the absence of organic material, obtaining their carbon through assimilation of carbon dioxide and their energy to carry on life processes through the oxidation of ammonia to nitrites. Investigations by Carey indicate that this takes place mainly in two regions, on or near the bottom in coastal areas and in the water in association with plankton at moderate depths, though some nitrites may also be formed at mid-depths. Oxidation of ammonia to nitrite may also occur photochemically (p. 256).
Following the conversion to nitrites, another group of bacteria oxidizes the nitrites to nitrates. The source of carbon for the nitrateformers is apparently also the carbon dioxide, and the energy is derived through oxidation of nitrities.
Nitrogen Assimilation. The process of nitrogen assimilation is primarily a function of the plants, the phytoplankton and benthic algae, but bacteria also assimilate nitrogen. Nitrogenous nutrients such as amino acids, ammonia, nitrates, or nitrites are the available sources of the nitrogen used by the plants in building up the amino-nitrogen of the protoplasm. It is not clear which of the last three mineralized compounds are preferred by phytoplankton as a source of nitrogen, but field observations provide evidence that they may be used simultaneously. During periods of low plant production the process of nitrification results in the storage of nitrogen as nitrates, and subsequent outbursts of diatoms then draw heavily upon this supply. After assimilation these plants may die and be dissociated directly by bacteria, or what is perhaps the most usual occurrence, they may be eaten by animals which require organic nitrogen from the plants directly or indirectly. In any event, a return to the inorganic nitrogen compounds is eventually effected by the bacteria, although animals preying directly upon each other may keep the organic nitrogen within their own cycle for some time. However, due to constant losses, this cycle cannot be self-perpetuating, and there must therefore be a large group of herbivorous animals to serve as food for the carnivorous forms.
Nitrate Reduction and Denitrification. The nitrogen cycle in the sea is more involved than is indicated by the above discussion. Gran (1901) and later Baur (1902) showed that denitrifying bacteria also exist in the sea. These denitrifiers and nitrate-reducers produce the effect just opposite to nitrification. Accordingly, nitrates are reduced to nitrites by splitting off a part of the oxygen, and other bacteria, the true denitrifiers, may carry the process even further with complete reduction of the nitrites and evolution of free nitrogen (NO3 → NO2 → N2). This last step, which is apparently not of great importance in the sea, constitutes a loss to the cycle in the sea unless the elementary nitrogen can be reclaimed by nitrogen-fixing bacteria (see below). The relatively uniform distribution of dissolved nitrogen in the sea also suggests that true denitrifiers and nitrogen fixers are not important in the general economy of the sea (Hamm and Thompson, 1941).
It has been held that the process of reduction of nitrates and nitrites by bacteria is characteristic of an environment lacking or poor in oxygen. Under these anaerobic conditions and in the presence of organic matter the bacteria find their source of needed oxygen in the molecules of these compounds. Rather little is known about the extent of denitrification in the sea, but since most sea waters have at least a small supply of
Nitrogen Fixation. In the terrestrial environment, the fixation of free nitrogen by bacteria is an important factor. To what extent similar nitrogen fixation occurs in the sea through the activity of bacteria is not well known. However, nitrogen fixers are reported from coastal areas, some in symbiosis with algae, and the extent to which they function in fixing free nitrogen depends upon environmental conditions involving the amount of available nitrogen compounds at their disposal, since nitrogen fixation is not an obligatory process (Benecke, 1933).
Phosphorus, Carbon, and Sulphur Cycles
Though they are allotted relatively less space for discussion, it is not intended to minimize the importance of bacterial activity in the transformation of these elements.
Phosphorus is another of the important plant nutrients which has a biologically activated cycle involving alternation of organic and inorganic phases. Whereas carbon dioxide is always present in sufficient quantity for the needs of marine plants, the phosphorus, like the nitrogen, may be depleted to the extent of interfering with the fullest growth. In laboratory experiments phosphate is apparently quickly regenerated by bacteria and other agencies following the death of plants and animals. In studies on stored sea water, Renn (1937) and Waksman et al (1938) found that phosphates are assimilated by bacteria in the growth of their cell substance but that bacterial competition with diatoms for this element is not serious under these conditions, since upon death and autolysis of the bacteria the phosphates are returned in a few days in mineralized form. When a supply of decomposing diatoms is at hand, the phosphates are regenerated from these more rapidly than they are consumed by the bacteria. Under these experimental conditions, approximately two thirds of the total phosphorus present in the diatoms was liberated within 132 hours through bacterial activities. Cooper (1935) found a more rapid regeneration of phosphates from animal plankton than from diatoms.
It must be emphasized, however, that these quick regenerations of phosphates in laboratory experiments are at variance with findings relative to the rate of regeneration in the sea, where renewal in the water is much slower, requiring three to four months (p. 260).
The complex carbon compounds built up by marine plants and animals are decomposed through bacterial action with the formation of carbon dioxide. This formation supplements the carbon dioxide that is produced through respiration by all other organisms and helps maintain the
Sulphur is one of the essential constituents of living matter, and its compounds, like those of other elements of protoplasm, are acted upon by bacteria. Transformations wrought by bacteria in the chemical compounds of sulphur may have far-reaching effects on the associated plant and animal populations and also upon chemical and geological phenomena. First, it may be mentioned that plants utilize a small quantity of sulphur in their metabolism; the compound used, namely sulphate, is produced by chemical or biological oxidation. Second, in the decomposition of organic compounds containing sulphur, hydrogen sulphide is produced as a disintegration product which, when present in large quantities, is inimical to plant and animal life. The odor of hydrogen sulphide is frequently noticeable at low tide in the organically rich muds of protected bays and in muds brought up from deeper water.
This hydrogen sulphide may be formed by splitting off the H2S group of the protein molecule or through a process of reduction of the proteins by heterotrophic bacteria. The inorganic compounds of sulphur, such as sulphates and sulphites, may also be reduced to hydrogen sulphide by heterotrophic bacteria under anaerobic conditions in the presence of organic material. Hence it is important to note that through intramolecular respiration oxidation of organic matter can continue even though all free oxygen has been removed. In areas with little or no circulation of water near the bottom and with an accumulation of organic detritus these heterotrophs as well as true sulphur bacteria abound, but the hydrogen sulphide evolved inhibits any other forms of life. In the Black Sea hydrogen sulphide is found from 180 m to the bottom; and from a depth of 300 m to 1500 m it increases from 1.48 cm3/l to 6.17 cm3/l (fig. 237, p. 872, Nikitin, 1931). Other classical examples are found in the oyster pools of threshold fjords (p. 802).
Not all bacteria that are involved in the sulphur cycle produce hydrogen sulphide. The metabolic requirements of the true sulphur bacteria produce the opposite effect through the process of oxidation. During the assimilation of carbon dioxide by autotrophic sulphur bacteria in the presence of free oxygen, the hydrogen sulphide is oxidized according to the following chemical equation:
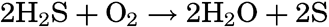
In some types of bacteria, including many purple bacteria, the sulphur is deposited as reserve material within the bacterial cell. The nitrogen needed is obtained from ammonium salts.
Bacteria and Bottom Deposits
Only brief mention can be made of the activities of bacteria in the processes of sedimentation on the ocean bottom. Further consideration
The activities of the various types of bacteria that occur in great abundance in the bottom sediments are believed to have a significant role in determining the character of the bottom deposits and the diagenesis of rock strata. Some of the results of chemical transformations wrought by these microorganisms are:
Formation of humus, a very stable organic end product of decomposition with a characteristic carbon-nitrogen ratio varying from 8:1 to 12:1 (Jensen, 1914).
Calcium precipitation in the presence of calcium salts and high pH.
It is believed by some investigators—Drew (1914), Bavendamm (1932), and others reviewed by Benecke—that bacteria in the sea are important agents in the precipitation of calcium carbonate in marine sediments and may therefore be of special geological importance. Many bacteria are known experimentally to produce an alkaline reaction in the presence of calcium and organic material, yielding ammonia. Such organisms may encrust themselves with areolas of calcium carbonate. The extent to which this type of precipitation can occur is still a moot question. Lipman (1929) was of the opinion that in the sea there can be no calcium precipitation because sea water does not contain sufficient concentration of calcium, but the general opinion seems to be that under certain natural conditions bacterial precipitation of calcium can and does occur in the sea, especially in bays in the tropics where there is an abundance of organic material.
Iron and manganese may be precipitated by bacteria that form sheaths of compounds of these metals (Harder, 1919). For example, they obtain energy from the oxidation of the soluble ferrous bicarbonate using the carbon dioxide liberated and precipitating ferric hydroxide.
Distribution of Bacteria in the Sea
The greatest numbers of marine bacteria have been found in the coastal waters where the greatest abundance of plant and animal life is also produced. In vertical distribution we find two main centers, on the bottom a few millimeters below the mud-water interface, and in the pelagic zone attached to the floating plants and animals and other particulate matter (fig. 247). Reference to table 100, from ZoBell and Anderson (1936b), will illustrate typical vertical distribution off the California coast. It should be noted that even though one center of distribution is in the pelagic zone, associated with the plankton, the existence of bacteria is apparently not truly planktonic but sessile upon organisms, and must therefore also closely coincide vertically and horizontally with the maximum distribution of these organisms.
It has been indicated by Carey and Waksman (1934) and others that bacteria are also present in relatively small numbers in the water even to a depth of 5000 m, and to that extent must be considered planktonic. But since the favorite habitat is one of attachment, it is not possible at present to know how normal the true planktonic existence is. Many individuals may be there through accident and be able to survive and multiply only to the degree that they are capable of utilizing the dilute organic matter that is in solution in the water. The advantages of a periphytic habit were discussed elsewhere (p. 912). A planktonic distribution must, however, be of great significance in the dispersal of these microorganisms.
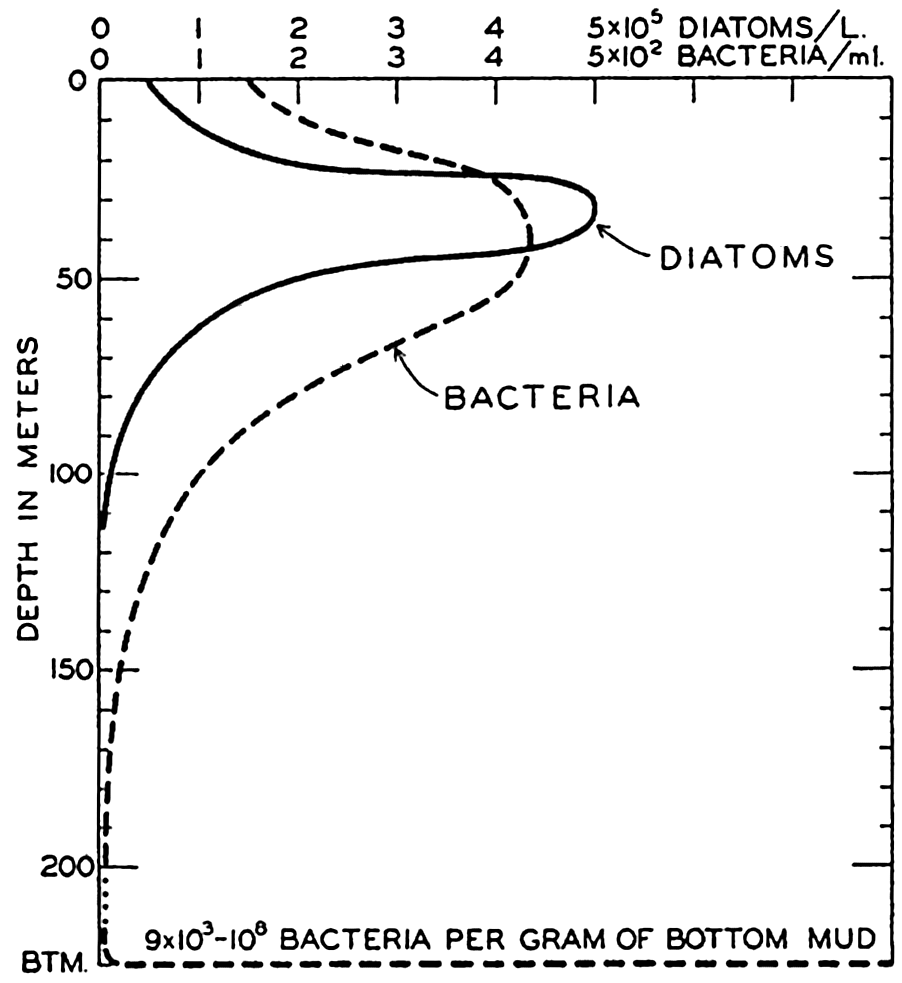
The vertical distribution of bacteria in the sea.
The greatest density by far of bacterial populations is found on the bottom, where as many as 420,000,000 cells per gram of wet mud may occur (ZoBell and Anderson, 1936b). Such dense populations are possible mainly because in a thin layer representing an interface between mud and water there is concentrated a large portion of the organic detritus, dead bodies of plants and animals, which is constantly sinking and forming an abundant rich food supply for bacteria as well as for bottom-dwelling animals which compete with them. Most bacteria occur in the first few millimeters of bottom ooze and there is a gradual diminution of numbers with the increasing thickness of the bottom deposits. Viable
In fig. 247 is shown the vertical distribution of bacteria and its relation to the diatom population. It will be noted that the large bacterial population of the pelagic region coincides roughly with the vertical range of floating plants and animals. This apparently results from the periphytic habit of many bacteria. The plankton organisms—diatoms, copepods, and so forth—living most abundantly in the euphotic zone, serve as surfaces for attachment and offer a favorable environment for multiplication (p. 912). The presence of a large periphytic bacterial population in the plankton results in prompt bacterial decomposition of large quantities of dead organisms before they have sunk to great depths; thus a large portion of mineralized plant nutrients is regenerated within or only a little below the euphotic zone.
| Sample | Depth (m) | Number of bacteria | ||
|---|---|---|---|---|
| Water | 1 | 147 | 344 | 261 |
| Water | 10 | 238 | 400 | 360 |
| Water | 20 | 292 | 528 | 395 |
| Water | 50 | 86 | 620 | 208 |
| Water | 100 | 14 | 17 | 53 |
| Water | 200 | 3 | 2 | 0 |
| Water | 500 | 2 | 0 | 0 |
| Sediment | Bottom | 2,170,000 | 768,000 | 16,200,000 |
| E. W. Scripps Station No. | 32A1 | 32B2 | 33A3 | |
| Water depth | 704 m | 610 m | 1287 m | |
Bibliography
Allee, W. C.1934. “Concerning the organization of marine coastal communities” . Ecological Monographs, v. 4, p. 541–554, 1934.
Allen, E. J.1909. “Mackerel and sunshine” . Marine Biol. Assn. U. K., Jour., v. 8, p. 394–406, 1909.
Allen, W. E.1934. “The primary food supply of the sea” . Quart. Rev. Biol., v. 9, p. 161–180, 1934.
Bauer, E.1902. “Ueber zweid enitrificirende Bakterien aus der Ostsee” . Komm. z. Wissensch. Untersuch. der Deutschen Meere in Kiel und d. Biologischen Anstalt auf Helgoland. Wissensch. Meeresuntersuch., N. F., Abt. Kiel, Bd. VI. p. 11–21, 1902.
Bavendamm, W.1932. “Die microbiologische Kalkfällung in der tropischen See” . Archiv. f. Mikrobiol., v. 3, p. 205–276, 1932.
Benecke, W.1933. “Bacteriologie des Meeres” . In Abderhalden's Handbuch d. Biol. Arbeitsmethoden, v. 9, p. 717–854, 1933.
Bigelow, H. B.1925. “Plankton of the offshore waters of the Gulf of Maine” . U. S. Bur. Fisheries, Bull., v. 40 (for 1924), pt. 2, 509 pp., 1925.
Blegvad, H.1914. “Food and conditions of nourishment among the communities of invertebrate animals found on or in the sea bottom of Danish waters” . Danish Biol. Sta., Repts., v. 22, p. 41–78, 1914.
Blegvad, H.1930. “Quantitative investigation of bottom invertebrates in the Kattegat with special reference to the plaice food” . Danish Biol. Sta., Repts., v. 36, p. 3–55, 1930.
Bond, R. M.1933. “A contribution to the study of the natural food-cycle in aquatic environment” . Bingham Oceanogr. Coll., Bull., v. 4, art. 4, 89 pp., 1933.
Boynton, L. C., and R. C. Miller. 1927. “The occurrence of a cellulase in the shipworm” . Jour. Biol. Chem., v. 75, p. 613–618, 1927.
Braarud, T., and A. Klem. 1931. “Hydrographical and chemical investigations in the coastal waters off Möre and in the Romsdalsfjord” . Hvalrådets Skrifter, no. 1, 88 pp., 1931.
Bullen, G. E.1909. “Plankton studies in relation to western mackerel fishery” . Marine Biol. Assn. U. K., Jour., v. 8, p. 269–302, 1909.
California, Div. Fish and Game. 1937. “California's sardine catch” . Fish Bull. no. 49, p. 11–15, 1937.
Cannon, H. G.1928. “On the feeding mechanism of the copepods, Calanus finmarchicus, and Diaptomus gracilis.” British Jour. Exper. Biol., v. 6, p. 131–144, 1928.
Carey, C. L.1938. “The occurrence and distribution of nitrifying bacteria in the sea” . Jour. Marine Research, v. 1, p. 291–304, 1938.
Carey, C. L., and S. A. Waksman. 1934. “The presence of nitrifying bacteria in deep seas” . Science, v. 79, p. 349–350, 1934.
Cooper, L. H. N.1935. “The rate of liberation of phosphate in sea water by the breakdown of plankton organisms” . Marine Biol. Assn. U. K., Jour., v. 20, p. 197–200, 1935.
Cooper, L. H. N.1937. “The nitrogen cycle in the sea” . Marine Biol. Assn. U. K., Jour., v. 22, p. 183–204, 1937.
Crozier, W. J.1918. “The amount of bottom material ingested by holothurians (Stichopus)” . Jour. Exper. Zool., v. 26, p. 379–389, 1918.
Drew, G. H.1914. “On the precipitation of calcium carbonate in the sea by marine bacteia and on the action of denitrifying bacteria in tropical and temperate seas” . Carnegie Inst. Washington, Pub. no. 182, p. 7–45, 1914.
Fisher, W. K., and G. E. MacGinitie. 1928. “Natural history of an echiuroid worm” . Ann. Mag. Nat. Hist., ser. 10, p. 204–213, 1928.
Fleming, Richard H.1939. “The control of diatom populations by grazing. Conseil Perm” . Internat. p. l'Explor. de la Mer, Jour. du Conseil, v. 14, no. 2, p. 210–227, 1939.
Fox, D. L., (ed.). 1936. “The habitat and food of the California sea mussel” . Scripps Inst. Oceanogr., Bull., v. 4, p. 1–64, 1936.
Fuller, J. L.1937. “Feeding rate of Calanus finmarchicus in relation to environmental conditons” . Biol. Bull., v. 72, p. 233–246, 1937.
Fuller, J. L., and G. L. Clarke. 1936. “Further experiments on the feeding of Calanus finmarchicus” . Biol. Bull., v. 70, p. 308–320, 1936.
Galtsoff, P. S., and V. L. Loosanoff. 1939. “Natural history and method of controlling the starfish (Asterias forbesi Desor)” . U. S. Bur. Fisheries, Bull., v. 49, p. 75–132, 1939.
Gran, H. H.1901. “Studien über Meeresebakterien” . Bergens Mus. Aarbog, no. 10, p. 1–23, 1901.
Hamm, Randall E., and T. G. Thompson. 1941. “Dissolved nitrogen in the sea water of the Northeast Pacific with notes on the total carbon dioxide and the dissolved oxygen” . Jour. Marine Research, v. 4, p. 11–27, 1941.
Harder, E. C.1919. “Iron–depositing bacteria and their geologic relations” . U. S. Geol. Surv., Prof. Paper, no. 113, 89 pp., 1919.
Hardy, A. C.1936. “Plankton ecology and the hypothesis of animal exclusion” . Linn. Soc. London, Proc., 148th Session, p. 64–70, 1936.
Hardy, A. C., and E. R. Gunther. 1935. “The plankton of the South Georgia whaling grounds and adjacent waters, 1926–1927” . Discovery Repts., v. 11, p. 1–456, 1935.
Hardy, A. C., C. E. Lucas, G. T. D. Henderson, and J. H. Fraser. 1936. “The ecological relations between the herring and the plankton investigated with the plankton indicator” . Marine Biol. Assn. U. K., Jour., v. 21, p. 147–291, 1936.
Hart, T. J.1935. “On the diatoms of the skin film of whales, and their possible bearing on problems of whale movements” . Discovery Repts., v. 10, p. 249–282, 1935.
Harvey, H. W.1934. “Annual variation of planktonic vegetation, 1933” . Marine Biol. Assn. U. K., Jour., v. 19, p. 775–792, 1934.
Harvey, H. W., L. H. N. Cooper, M. V. Lebour, and F. S. Russell. 1935. “Plankton production and its control” . Marine Biol. Assn. U. K., Jour., v. 20, p. 407–442, 1935.
Hentschel, E.1936. “Allgemeine Biologie des Südatlantischen Ozeans” . Deutsche Atlantische Exped. Meteor, 1925–1927, Wiss. Erg., Bd. 11, 343 pp., 1936.
Hesse, R., W. C. Allee, and K. P. Schmidt. 1937. Ecological animal geography. New York, John Wiley & Sons, 597 pp., 1937.
Hewatt, Willis G.1937. “Ecological studies on selected marine intertidal communities of Monterey Bay, California” . Amer. Midland Naturalist, v. 18, p. 161–206, 1937.
Hjort, Johan, and Johan T. Ruud. 1929. “Whaling and fishing in the North Atlantic” . Conseil Perm. Internat. p. l'Explor. de la Mer, Rapp. et Proc.-Verb., v. 56, no. 1, 123 pp., 1929.
Jensen, P. B.1914. “Studies concerning the organic matter of the sea bottom” . Danish Biol. Sta., Repts., v. 22, p. 1–39, 1914.
Jordan, E. O.1931. Textbook of general bacteriology. Philadelphia and London, W. B. Saunders, 819 pp., 1931.
Krogh, August. 1934a. “Physiology of the blue whale” . Nature, v. 133, p. 635–637, 1934.
Krogh, August. 1934b. “Conditions of life at great depths in the ocean” . Ecological Monographs, v. 4, p. 430–439, 1934.
Lebour, Marie V.1924a. “The food of young herring” . Marine Biol. Assn. U. K., Jour., v. 13, p. 325–330, 1924.
Lebour, Marie V.1924b. “The Euphausiidae in the neighborhood of Plymouth” . II. Nyctiphanes couchii and Meganyctiphanes norvegica. Marine Biol. Assn. U. K., Jour., v. 13, p. 810–847, 1924.
Lipman, Charles B.1929. “Further study on marine bacteria with reference to the Drew hypothesis on CaCO3 precipitation in the sea” . Carnegie Inst.
Lloyd, Blodwen. 1930. “Bacteria of the Clyde Sea area: a quantitative investigation” . Marine Biol. Assn. U. K., Jour., v. 16, p. 879–907, 1930.
Lohmann, H.1908. “Untersuchungen zur Feststellung des vollständigen Gehaltes des Meeres an Plankton” . Komm. z. Wissensch. Untersuch. d. Deutschen Meere in Kiel und d. Biologischen Anstalt auf Helgoland. Wissensch. Meeresuntersuch., N.F., Abt. Kiel, Bd. 10, p. 131–370, 1908.
Lucas, C. E.1936. “On certain interrelations between phytoplankton and zooplankton under experimental conditions” . Conseil Perm. Internat. p. l'Explor. de la Mer, Jour. du Conseil, v. 11, p. 343–362, 1936.
MacGinitie, G. E.1939. “The methods of feeding of tunicates” . Biol. Bull, v. 77, p. 443–447, 1939.
Mackintosh, N. A.1934. “Distribution of the macroplankton in the Atlantic sector of the Antarctic” . Discovery Repts., v. 9, p. 65–160, 1934.
Marshall, S. M., A. G. Nicholls, and A. P. Orr. 1934. “On the biology of Calanus finmarchicus. V. Seasonal distribution, size, weight and chemical composition in Loch Striven in 1933 and their relation to phytoplankton” . Marine Biol. Assn. U. K., Jour., v. 19, p. 793–828, 1934.
Nielsen, S. E.1937. “On the relation between the quantities of phytoplankton and zooplankton in the sea” . Conseil Perm. Internat. p. l'Explor. de la Mer. Jour. du Conseil, v. 12, p. 147–154, 1937.
Nikitin, W. N.1931. “Die untere Planktongrenze und deren Verteilung im Schwarzen Meer” . Internat. Rev. d. ges. Hydrobiol. u. Hydrogr., Bd. 25, p. 102–130, 1931.
Orton, J. H.1937. Oyster biology and oyster culture. London, Edward Arnold & Co., 211 pp., 1937.
Parr, A. E.1934. “Report on experimental use of a triangular trawl for bathypelagic collecting. With an account of the fishes obtained and a revision of the family Cetomimidae” . Bingham Oceanogr. Coll., Bull., v. 4, art. 6, 59 pp., 1934.
Pearse, A. S.1939. Animal ecology. New York, McGraw-Hill Book Co., 642 pp., 1939.
Petersen, C. G. J.1913. “Valuation of the sea. II. The animal communities of the sea bottom and their importance for marine zoogeography” . Danish Biol. Sta., Repts., v. 21, p. 1–44, 1913.
Petersen, C. G. J.1918. “The sea bottom and its production of fish food” . Danish Biol. Sta., Repts., v. 25, 62 pp., 1918.
Pütter, A.1907. “Der Stoffhaushalt des Meeres” . Zeitschr. f. allg. Physiol., v. 7, p. 321–368, 1907.
Redfield, A. C., and A. B. Keys. 1938. “The distribution of ammonia in waters of the Gulf of Maine” . Biol. Bull., v. 74, p. 83–92, 1938.
Renn, C. E.1937. “Bacteria and the phosphorus cycle in the sea” . Biol. Bull., v. 72, p. 190–195, 1937.
Ricketts, E. F., and J. Calvin. 1939. Between Pacific tides. Stanford Univ. Press, 320 pp., 1939.
Rittenberg, Sydney C.1940. “Bacteriological analysis of some long cores of marine sediments” . Jour. Marine Research, v. 3, p. 191–201, 1940.
Russell, H. L.1892. “Untersuchungen über in Golf von Neapel lebende Bacterien. Zeitschr” . Hyg. Infk., Bd. 11, p. 165–206, 1892.
Scofield, W. L.See: California, Div. Fish and Game.
Seiwell, H. R., and G. E. Seiwell. 1938. “The sinking of decomposing plankton in sea water and its relationship to oxygen consumption and phosphorus liberation” . Amer. Philos. Soc., Proc., v. 78, p. 465–481, 1938.
Shelford, V. E., A. O. Weese, et al.1935. Some marine biotic communities of the Pacific coast of North America. Ecological Monographs, v. 5, p. 251–354, 1935.
Stephenson, M.1939. Bacterial metabolism. New York, Longmans, Green & Co., 2nd ed., 391 pp., 1939.
Tressler, Donald K.1923. Marine products of commerce. New York, Chemical Catalog Co., 762 pp., 1923.
Vaughan, T. Wayland. 1930. The oceanographic point of view. p. 40–56 in Contributions to marine biology. Stanford Univ. Press, 277 pp., 1930.
Waksman, S. A.1934. “The role of bacteria in the cycle of life in the sea” . Scientific Monthly, v. 38, p. 35–49, 1934.
Waksman, S. A., M. Hotchkiss, C. Carey, and Y. Hardman. 1938. “Decomposition of nitrogenous substances in sea water by bacteria” . Jour. Bact., v. 35, p. 477–486, 1938.
Wilson, C. B.1932. “Copepods of the Woods Hole region, Massachusetts” . U. S. Nat. Mus., Bull. 158, 623 pp., 1932.
Yonge, C. M.1928. “Feeding mechanisms in the invertebrates” . Biol. Reviews. v. 3, p. 21–76, 1928.
Yonge, C. M.1931. “Digestive processes in marine invertebrates and fishes” . Conseil Perm. Internat. p. l'Explor. de la Mer, Jour. du Conseil, v. 6, p. 175–212, 1931.
Yonge, C. M.1936. “Mode of life, feeding, digestion, and symbiosis with Zooxanthellae in the Tridacnidae” . Great Barrier Reef Exped. 1928–29, Repts., v. 1, p. 283–321, 1936.
Yonge, C. M.1940. “Observations on the biology of coral reefs” . Sixth Pacific Sci. Congr., California, 1939, Proc., v. 3, p. 605–613, 1940.
ZoBell, Claude E.1935. “The assimilation of ammonium nitrogen by Nitzschia closterium and other marine phytoplankton” . Nat. Acad. Sci., Proc., v. 21, p. 517–522, 1935.
ZoBell, Claude E.1938. “Studies on the bacterial flora of marine bottom sediments” . Jour. Sedimentary Petrol., v. 8, p. 10–18, 1938.
ZoBell, Claude E., and D. Q. Anderson. 1936a. “Observations on the multiplication of bacteria in different volumes of stored sea water and the influence of oxygen tension and solid surfaces” . Biol. Bull., v. 71, p. 324–342, 1936.
ZoBell, Claude E., and D. Q. Anderson. 1936b. “Vertical distribution of bacteria in marine sediments” . Amer. Assn. Petrol. Geol., Bull., v. 20, p. 258–269, 1936.