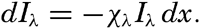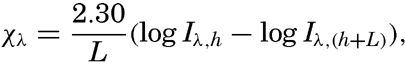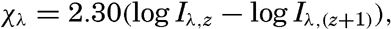Absorption of Radiation
Absorption Coefficients of Distilled Water and of Pure Sea Water. In water the intensity of parallel beams of radiation of wave length λ decreases in the direction of the beams, the decrease in a layer of infinitesimal thickness being proportional to the energy, I, and to the thickness of the layer:
The coefficient of proportionality, x, is called the absorption coefficient. Integrating this equation between the limits x = h and x = h + L, one obtains where the factor 2.30 enters because base-10 logarithms are used instead of natural logarithms, and where L is the thickness of the layer within which the energy of the radiation is reduced from Iλ,h to Iλ,(h+L) The latter equation also serves as a definition of the absorption coefficient. The numerical value of the absorption coefficient depends upon the unit of length which L is expressed. In physics the unit is 1 cm, but in oceanography it has become common practice to use 1 m as the unit of length. Therefore the numerical values of the coefficients that will be given here are one hundred times larger than the corresponding values given in textbooks of physics.The decrease of intensity of radiation passing through a layer of water depends not only upon the amount of radiation that is truly absorbed—that is, converted into another form of energy—but also upon the amount that is scattered laterally. In “pure” water the scattering takes place against the water molecules, and the effect of scattering is related to the molecular structure of the water (p. 47). However, when the absorption in pure water is measured, the effect of scattering is not separated but is included in the absorption coefficient, which varies greatly with wave length.
A great number of measurements of the absorption coefficients in pure water have been conducted, but the results by different investigators do not agree (Dorsey, 1940). Thus, at a wave length of .48 μ
| Hüfner and Albrecht, 1891.................................0.048 |
| Ewan, 1895...............................................0.030 |
| Aschkinass, 1895..........................................0.020 |
| Sawyer, 1931.............................................0.015 |
From the table it is evident that water is very transparent for radiation of wave lengths between 0.4 μ and 0.6 μ; that is, for visible rays in the violet, blue, green, and yellow parts of the spectrum. It is less transparent for orange and red light, and in the infrared the transparency is practically nil (fig. 21), because, if the absorption coefficient per meter equals 100, 99.5 per cent of the radiation is absorbed in a layer of thickness 5.3 cm.
| Wave length in μ | Absorption coefficient per meter | Wave length in μ | Absorption coefficient per meter | Wave length in μ | Absorption coefficient per meter | Wave length in μ | Absorption coefficient per meter |
|---|---|---|---|---|---|---|---|
| .32 | 0.58 | .52 | 0.019 | .85 | 4.12 | 1.60 | 800 |
| .34 | 0.38 | .54 | 0.024 | .90 | 6.55 | 1.70 | 730 |
| .36 | 0.28 | .56 | 0.030 | .95 | 28.8 | 1.80 | 1700 |
| .38 | 0.148 | .58 | 0.055 | 1.00 | 39.7 | 1.90 | 7300 |
| .40 | 0.072 | .60 | 0.125 | 1.05 | 17.7 | 2.00 | 8500 |
| .42 | 0.041 | .62 | 0.178 | 1.10 | 20.3 | 2.10 | 3900 |
| .44 | 0.023 | .65 | 0.210 | 1.20 | 123.2 | 2.20 | 2100 |
| .46 | 0.015 | .70 | 0.84 | 1.30 | 150 | 2.30 | 2400 |
| .48 | 0.015 | .75 | 2.72 | 1.40 | 1600 | 2.40 | 4200 |
| .50 | 0.016 | .80 | 2.40 | 1.60 | 1940 | 2.50 | 8500 |
Collins has compared the absorption in distilled water to that in salt solutions, and from his results it can be concluded that the dissolved salts in concentrations occurring in sea water exert a negligible effect on the absorption coefficient. The maximum effect appears to be about 1.3 per cent, and the uncertainty of the observed values is greater than
It has also been concluded that the effect of temperature on absorption, which has been established in the case of distilled water, is applicable to uncontaminated sea water. The effect of temperature changes is to increase the absorption in certain parts of the infrared by about 0.5 per cent for every temperature increase of 1°C, but over a large part of the spectrum the temperature effect is much smaller. When dealing with sea water the effect can be neglected.
Extinction Coefficients in the Sea. In oceanography the greater interest is attached to the rate at which downward-traveling radiation decreases. The rate of decrease can be defined by means of a coefficient similar to the absorption coefficient:
where Iλ,s and Iλ,(z+1) represent the radiation intensities of wave length λ on horizontal surfaces at the depths z and (z + 1) m. Different names have been proposed for this coefficient, such as transmissive exponent (Clarke, 1933) or extinction coefficient (Pettersson, 1936a). The latter name has been widely used and will be employed here, although the process by which the intensity of radiation is reduced will be called absorption. The absorption of radiation in the sea is complicated by the increased scattering due to suspended particles and by the presence of dissolved colored substances. The extinction coefficient of radiation of a given wave length therefore varies within wide limits from one locality to another, and in a given locality it varies with depth and time.The first crude measurements of absorption in the visible part of the spectrum were made by lowering a white disc of standard size (30 cm), the Secchi disc, and observing the depth at which the disc disappeared from sight. Comparisons with recent exact measurements by other methods have shown that in the English Channel the extinction coefficient of visible rays can roughly be attained from the formula κ = 1.7/D, where D is the maximum depth of visibility in meters, as determined by the Secchi disc (Poole and Atkins, 1929).
The next step in the investigation of the absorption of radiation in sea water was made by subsurface exposure of photographic plates enclosed in watertight containers. Such experiments, which were conducted by Helland-Hansen (1912a) on the Michael Sars Expedition by exposing panchromatic plates at different depths in the vicinity of the Azores, showed that photographic plates were blackened at very great depths.
In other experiments colored filters were used, which showed that the red portion of the spectrum was rapidly absorbed, whereas the green and blue rays penetrated to much greater depths. Quantitative results as to the absorption at different wave lengths were obtained by using spectro- photometers (Knudsen, 1922), but the methods were laborious and not sensitive enough to be used at great depths.
The introduction in recent years of photoelectric cells has made possible rapid and accurate determinations of extinction coefficients in different parts of the spectrum. A number of different instruments have been and still are in use, but a standardized technique has been proposed by a committee of the International Council for the Exploration of the Sea (Atkins et al, 1938). Because of the wide variation in absorption at different wave lengths, efforts have been directed toward measuring exactly the absorption in narrow spectral bands. The determinations are accomplished by lowering stepwise a photoelectric or photronic cell enclosed in a watertight container and provided with suitably colored filters, and by observing on deck the photoelectric current by means of a sensitive galvanometer or a suitable bridge circuit. The measurements must be made at constant incident light either on clear, sunny days or on days when the sky is uniformly overcast, because the rapid variations in incident light that occur on days with scattered clouds will naturally lead to erroneous results as to the absorption. To determine the percentage amount of radiation that reaches a certain depth, it is necessary to make simultaneous readings of the incident radiation on board ship. For the different precautions that have to be taken, reference is made to papers listed in the bibliography, particularly to Atkins et al (1938).
These methods give information as to the absorption in layers of definite thickness. Instruments for measurements of the transparency of sea water at given depths and of the scattering of light have been designed by H. Pettersson (1936b) and have been used for determining relative values. It has been demonstrated, particularly, that at boundary surfaces sharp variations in transparency and scattering occur. The study of the absorption of radiation in the sea is in rapid progress, and several of the following generalizations are therefore presented with reservations.
The main results as to the character of the extinction coefficient in the sea of radiation of different wave lengths can be well illustrated
| Type of water | Wave length in μ | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| .46 | .48 | .515 | .53 | .565 | .60 | .66 | .80 | 1.00 | |
| Pure water | .015 | .015 | .018 | .021 | .033 | .125 | .280 | 2.40 | 39.7 |
| Oceanic water lowest | .038 | .026 | .035 | .038 | .074 | .199 | |||
| Oceanic water average | 086 | .076 | .078 | .084 | .l08 | .272 | |||
| Oceanic water highest | .160 | .154 | .143 | .140 | .167 | .333 | |||
| Coastal water lowest | .224 | .230 | .192 | .169 | .375 | .477 | |||
| Coastal water average | .362 | .334 | .276 | .269 | .437 | .623 | |||
| Coastal water highest | .510 | .454 | .398 | .348 | .489 | .760 | |||
The transparency of the water for radiation of different wave lengths can be expressed by means of the percentage amounts of radiation which penetrate a 1-m layer. These percentage amounts are given in table 23, from which it is seen that the greatest transparency of the clearest oceanic water is at a wave length of 0.48 μ—that is, in the blue part of the spectrum—whereas the greatest transparency of coastal water is at wave lengths 0.53 μ or higher—that is, in the green or green-yellow part of the spectrum. It is also seen that 97.5 per cent of radiation of wave length 0.48 μ passes through 1 m of the clearest oceanic water, but only 63.5 per cent of radiation of the same wave length passes through 1 m of turbid coastal water.
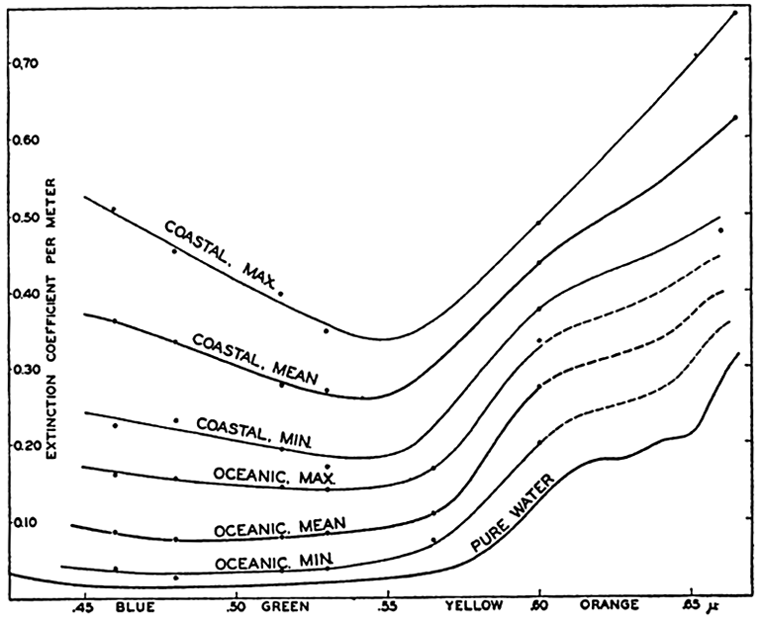
Extinction coefficients of radiation of different wave lengths in pure water and in different types of sea water.
[Full Size]
The great difference between the mean and the maximum values of the extinction coefficients shows that the absorption of sea water varies within very wide limits. In the example presented in table 22 the percentage variations are about the same in coastal and oceanic water, and the maximum values in the oceanic water approach the minimum values in the coastal water. In any given locality, great variations also occur in a vertical direction, layers of low absorption alternating with layers of high absorption, and this feature further complicates the actual conditions.
Similar results have been obtained by other investigators from such widely different areas as in the English Channel (Poole and Atkins, 1929;
| Type of water | Wave length (μ) | ||||||
|---|---|---|---|---|---|---|---|
| .46 | .48 | .515 | .53 | .565 | .60 | .66 | |
| Pure water | 98.5 | 98.5 | 98.2 | 97.9 | 96.8 | 88.3 | 75.9 |
| Oceanic water lowest | 96.4 | 97.5 | 96.6 | 96.3 | 92.9 | 81.8 | |
| Oceanic water average | 91.8 | 92.7 | 92.5 | 91.8 | 89.8 | 75.9 | |
| Oceanic water highest | 85.1 | 85.7 | 86.7 | 86.9 | 84.5 | 71.6 | |
| Coastal water lowest | 80.0 | 79.4 | 82.6 | 84.5 | 68.7 | 62.0 | |
| Coastal water average | 69.7 | 71.6 | 75.9 | 76.4 | 64.6 | 53.6 | |
| Coastal water highest | 60.0 | 63.5 | 67.1 | 70.6 | 61.4 | 46.7 | |
Influence of the Altitude of the Sun Upon the Extinction Coefficient. The extinction coefficient is a measure of the reduction of intensity on a, vertical distance and depends, therefore, upon the obliquity of the rays. The obliquity of the incident rays is reduced, however, by refraction when entering the water from the air and by the effect of scattering. When the sun's rays pass the water surface, the angle of refraction increases from zero with the sun in zenith to 48.5 degrees with the sun at the horizon, and therefore the most oblique rays penetrating into the water form an angle of less than 48 degrees with the vertical. Owing to the scattering and the sifting out by absorption of the most oblique rays, the radiation that penetrates to moderate depths will become nearly vertical, and the measured extinction coefficients will be independent, within wide limits, of the altitude of the sun. The reduction of the obliquity of the incident radiation has been directly demonstrated by Johnson and Liljequist (1938). Conditions at very. low sun have not been examined, but it is probable that at low sun the extinction coefficients are increased, and this increase may have bearing
The Scattering of Radiation in the Sea The Scattering of Radiation has been examined both directly by means of Pettersson's scattering meter (p. 83) and by measuring the relative intensities of downward- and upward-traveling radiation or vertical and horizontal radiation. Jorgensen and Utterback (1939) found that in coastal waters the intensity of the upward-traveling radiation ranged for the short wave lengths from 1 to 3 per cent of that of the downward-traveling radiation, and for the long wave lengths from 0.5 to 2 per cent. In oceanic water Utterback (1936) found ratios between 1 and 2 per cent at the shorter wave lengths. Clarke (1936) found considerably higher values in shallow coastal waters, but similar values in the deep basin of the Gulf of Maine.
The relative intensities of horizontal and vertical radiation have been measured by Clarke off the east coast of the United States and by Poole and Atkins in the English Channel. The greatest value found by Clarke was 17 per cent, but Poole and Atkins (1929) have reported an average value of 50 per cent for the horizontal radiation down to a depth of 25 m in the English Channel. The conclusion that can be drawn from these experiments is that the subsurface illumination becomes more and more diffuse with increasing depth, particularly in coastal waters, but that the directional character of the radiation is lost only slowly. This conclusion is particularly true in clear oceanic water, where Helland-Hansen (1912a) found that the vertical radiation was distinctly more intense than the horizontal at a depth of 500 m. (p. 82).
Cause of the Great Extinction Coefficients in the Sea. The great extinction coefficients in the sea as compared to those of absolutely pure water are as a rule ascribed to the presence of minute particles which cause scattering and reflection of the radiation and which themselves absorb radiation. If such particles are small compared to the wave length, λ, of the radiation, the scattering will be proportional, according to Lord Rayleigh, to λ−4, and the effect therefore at wave length, say, .46 μ will be 2.86 times greater than at wave length .60 μ. This selective effect leads to a shift toward longer wave lengths in the region of minimum absorption.
Clarke and James (1939) found that the increased absorption in oceanic water was chiefly caused by suspensoids that could be removed by means of a “fine” Berkefeld filter, and that these suspensoids were largely nonselective in their effect. Utterback's data (1936) indicated, on the other hand, that the increased absorption in oceanic water is at least in part due to selective scattering, because at short wave lengths the extinction coefficients were increased more, above those of pure water, than at longer wave lengths (table 22). Kalle (1938) is of the
The increase of the absorption coefficients in coastal waters appears to be due in part to another process. Clarke and James (1939) conclude from their examination that in coastal water both suspensoid and “filter-passing” materials are effective in increasing the absorption, and that each exerts a highly selective action, with greatest absorption at the shorter wave lengths. These great absorptions at the shorter wave lengths are demonstrated by Utterback's measurements (table 22). Clarke does not discuss the nature of the “ filter-passing” material, but Kalle (1938) has shown that in sea water water-soluble pigments of yellow color are present. These pigments appear to be related to the humic acids, but their chemical composition has not been thoroughly examined, for which reason Kalle calls them “yellow substance.” This yellow substance seems to occur in greatest abundance in coastal areas, but Kalle has demonstrated its presence in the open ocean as well and believes that it represents a fairly stable metabolic product related to the phytoplankton of the sea. The selective absorption 'of this yellow substance may then be responsible, in part, for the character of the absorption in coastal water and for shift of the band of minimum absorption toward longer wave lengths.
It has not been possible anywhere to demonstrate any direct influence of phytoplankton populations on the absorption, but very dense populations may cut down the transparency. At present it appears that the major increase of absorption of sea water over that of pure water is due to two factors: the presence of minute suspended particles, and the presence of dissolved “yellow substance.” The former factor dominates in oceanic waters, and the latter is particularly important in coastal waters.
The Color of the Sea. The color of the sea, as it appears to an observer ashore or on board a vessel, varies from a deep blue to an intense green, and is in certain circumstances brown or brown-red. The blue waters are typical of the open oceans, particularly in middle and lower latitudes, whereas the green water is more common in coastal areas, and the brown or “red” water is observed in coastal regions only.
The color of the sea has been examined by means of a Secchi disk (p. 82) by observing the color that the water appears to have when seen against the white submerged surface of the disc. This color is recorded according to a specially prepared color scale, the “ Forel scale” (Krümmel, 1907). The method is a rough one and the scale is not adapted for
Kalle has critically reviewed earlier theories as to the causes of the color of the sea and arrives at conclusions that appear to be consistent with all available observations. The blue color is explained, in agreement with earlier theories, as a result of scattering against the water molecules themselves, or against suspended minute particles smaller than the shortest visible wave lengths. The blue color of the water is therefore comparable to the blue color of the sky. The transition from blue to green cannot be explained, however, as a result of scattering, and Kalle concludes that this transition is due to the above-mentioned “ yellow substance,” pointing out that the combination of the yellow color and the “natural” blue of the water leads to a scale of green colors as observed at sea. Fluorescence may contribute to the coloring but appears to be of minor importance.
The color of suspended larger particles, if present in great abundance, can give color to the sea. In this case the color is not determined by the optical properties of the water or by dissolved matter, but by the colors of the suspended inorganic or organic particles, and the water is appropriately called “ discolored.” Discoloration can be observed when large quantities of finely suspended mineral particles are carried into the sea after heavy rainfall, or when very large populations, several million cells per liter, of certain species of algae or dinoflagellates are present very near the surface. Thus, the “red water” (often more brown than red) which is quite frequently observed in many areas and after which the Red Sea and the Vermilion Sea (Gulf of California) have been named is due to abundance of certain algae (in the Red Sea, Trichodesmium erythraeum) or dinoflagellates. The discoloration, beautiful examples of which have been given by Gunther (1936), is, however, a phenomenon of the typical coastal waters, the green colors being frequent in waters near the coast or at sea in high latitudes, and the blues charac teristic of the open ocean in middle and lower latitudes (fig. 214, p, 784).