Composition of Sea Water
So far, the discussion of the composition of sea water has been based mainly on the results of the fundamental investigations of Dittmar. Since his time our knowledge of the composition of sea water has increased tremendously. Improved methods of analysis have been developed and consequently more accurate values can be obtained. Tests have also been developed for the detection and determination of elements other than those previously discussed. Particular efforts have been devoted to the study of the so-called plant nutrients—that is, those elements
| Designated substance | Abbreviation | Units (p = preferred, a = alternative) | |||
|---|---|---|---|---|---|
|
|
| ‰ | ||
| Ammonia-nitrogen | Ammonia-N | p | |||
| Argon | Argon | p | |||
| Arsenate-arsenic | Arsenate-As | p | |||
| Arsenite-arsenic | Arsenite-As | p | |||
| Borate-boron | Borate-B | p | |||
| Calcium | Ca | p | a | ||
| Carbon dioxide | Carbon dioxide-C | p | |||
| CO2 | a | ||||
| Chlorinity | Cl | p | |||
| Copper | Cu | p | |||
| Iron | Fe | p | |||
| Magnesium | Mg | p | a | ||
| Manganese | Mn | p | |||
| Nitrate-nitrogen | Nitrate-N | p | |||
| Nitrite-nitrogen | Nitrite-N | p | |||
| Nitrogen (gas) | N2 | p | a | ||
| Oxygen (gas) | O2 | p | a | ||
| Phosphate-phosphorus | Phosphate-P | p | |||
| Potassium | K | p | a | ||
| Radioactive substances | p | ||||
| Salinity | S | p | |||
| Silicate-silicon | Silicate-Si | p | |||
| Sodium | Na | p | a | ||
| Sulphate | Sulphate-S | p | |||
| SO4 | a | ||||
| Hydrogen sulphide | Sulphide-S | p | |||
| H2S | a | ||||
Hence, in order to prepare a tabulation of the composition of sea water it is necessary to combine the results of numerous workers who have examined samples from different sources. All available data were collected by Thompson and Robinson (1932), and additional references will be found in the following discussion. In some cases the information is extensive, but for other elements only a few determinations have been made on water from a single locality. We shall first examine the quantities of the major elements—that is, those which bear a virtually constant relationship to the chlorinity.
In table 35 is given a compilation of the major ions that make up over 99.9 per cent of the known dissolved solid constituents of sea water. The sources of these data have been discussed by Lyman and Fleming (1940). The concentrations of the various ions are shown for water of 19.00 ‰ chlorinity, and also the Cl-ratios. The quantities are also expressed in terms of chemical equivalents per kilogram for water of 19.00 ‰ chlorinity and as milligram-atoms per 20° liter. Chlorosity factors are given for units of milligram-atoms. The carbon dioxide has been reported as bicarbonate. This method is not strictly accurate, because the bound carbon dioxide content of sea water is variable, but, as will be shown in the discussion of the carbon dioxide system, the sum of the chemical equivalents of carbonate and bicarbonate is virtually constant for any chlorinity.
It is immediately seen that the sum of the halides (chloride, bromide, and fluoride) by weight is greater than the chlorinity. The amount of iodide is negligible. Even if the bromide is calculated as chloride, and if the fluoride is disregarded because it does not take part in the chlorinity determination, the chloride equivalent is 1.00045 times greater than the chlorinity. The reasons for this apparent discrepancy have been discussed on page 52.
Lyman and Fleming (1940) obtained the following empirical equation for the dissolved solids as represented in table 35:
From this it will be seen that in water of 19.00 ‰ chlorinity the total dissolved solids are 34.4816 ‰, but, according to the equation used to calculate the salinity from the chlorinity (p. 51), the salinity is 34.325 ‰. Thus, the total amount of dissolved solids is greater than the salinity. If, on the other hand, the salinity is calculated from the total solids, using the definition for the former quantity—that is, by converting the bicarbonate to oxide and converting the bromide to chloride—we obtain the salinity “by definition” as 34.324 ‰. This agreement must be considered as more or less accidental, as there are many uncertainties in the analytical data. Confidence in the values is strengthened, however, by the fact that the sodium: chlorinity ratio as reported by Robinson and Knapman (1941) agrees exactly with the value that Lyman and Fleming (1940) found by difference. Although the table represents the most probable figures for the composition of the major dissolved constituents, it is subject to change as additional data become available.
| Ion | ‰ | Cl-ratio, g per unit Cl | Equivalent per kg of sea water | mg-atoms per liter | Chlorosity factor, mg-atoms per unit Cl | Authority |
|---|---|---|---|---|---|---|
Total dissolved solids = 34.4816 ‰ Sum of constituents (HCO3− as O−, and Br− as Cl−) = 34.324 ‰ Salinity (S ‰ = 0.030 + 1.805 Cl ‰) = 34.325 ‰ | ||||||
| Chloride, Cl− | 18.9799 | 0.99894 | 0.5353 | 548.30 | 28.173 | Dittmar (1884), Jacobsen and Knudsen (1940) |
| Sulphate, SO4− | 2.6486 | 0.1394 | 0.0551 | (SO4-S) 38.24 | 1.451 | Thompson, Johnston, and Wirth (1931) |
| Bicarbonate, HC03− | 0.1397 | 0.00735[a] | 0.0023 | (HCO3−C) 2.34 | 0.120 | Revelle (1936) |
| Bromide, Br− | 0.0646 | 0.00340 | 0.0008 | 0.83 | 0.0426 | Dittmar (1884) |
| Fluoride, F− | 0.0013 | 0.00007 | 0.0001 | 0.07 | 0.0036 | Thompson and Taylor (1933) |
| Boric acid,c H3BO3 | 0.0260 | 0.00137[b] | [c] | (H3BO3-B)0.43 | 0.0221 | Harding and Moberg (1934), Igelsrud, Thompson, and Zwicker (1938) |
| _______ | ||||||
| Total | 0.5936 | |||||
| Sodium,[d] Na+ | 10.5561 | 0.5556 | 0.4590 | 470.15 | 24.153 | By difference, and Robinson and Knapman (1941) |
| Magnesium, Mg++ | 1.2720 | 0.06695 | 0.1046 | 53.57 | 2.752 | Thompson and Wright (1930) |
| Calcium, Ca++ | 0.4001 | 0.02106 | 0.0200 | 10.24 | 0.5262 | Kirk and Moberg (1933); Thompson and Wright (1930) |
| Potassium, K+ | 0.3800 | 0.02000 | 0.0097 | 9.96 | 0.5113 | Thompson and Robinson (1932) |
| Strontium, Sr++ | 0.0133 | 0.00070 | 0.0003 | 0.15 | 0.0077 | Webb (1938) |
| _______ | ||||||
| Total | 0.5936 | |||||
The data in table 35 apply more specifically to surface water than to deep water. Both bicarbonate ion and calcium will be slightly higher in deeper water. Furthermore, some of the other compounds not included in this tabulation, such as nitrate and silicate, may be present in sufficient quantities to disturb the balance of the anions and cations shown in the table. The Cl-ratios should therefore be considered more as indices than as absolute values. However, in no case will the ratios vary by more than a unit or two in the last decimal place when the water under investigation is from the open sea. Under abnormal conditions, as in highly diluted water, larger departures may be found. By definition the salinity is not zero at zero chlorinity; hence the ratios of certain elements would be expected to approach infinity at very high dilutions when the diluting water contained substances other than halides. Therefore, in studies in areas of highly diluted water the character of the river water should be taken into account. As pollution problems frequently occur in such areas, it will be necessary to determine the normal ratios for different dilutions for a specific zone before any conclusions can be drawn as to the nature or extent of the pollution.
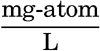 [
[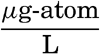 [
[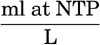 [
[