Benthos, Animals of the Sea Floor
Wherever successful dredging operations have been conducted, the ocean floors have been found to be inhabited by benthic animals from the Arctic to the Antarctic and from shore to the greatest depth. However, the number of animals (that is, the concentration per unit area) varies greatly; moreover, the kinds of animals that make up the major portion of the population differ, especially the species, genera and families. Such differences are apparent in the populations of such small topographic units as biotopes, as well as in the larger environmental divisions, and they are the biological criteria for establishing the vertical zones, littoral, archibenthic, and abyssal-benthic, as well as the horizontal faunal areas to be discussed later. It is mainly with the fauna of these larger divisions that we shall deal. For greater detail on the zoogeography of the seas the reader is referred especially to Ekman's text (1935), in which an extensive bibliography is also included.
Animals of the Littoral Zone. The outstanding feature of the littoral zone, especially of the upper or eulittoral zone extending to depths of about 40 to 60 m, is the great diversity and variability of the physical-chemical conditions of habitats. The substratum varies from clean firm rocks to shifting sands and soft muds. Marked salinity gradients sometimes exist, and seasonal and diurnal fluctuations add variety to the life of animals of this zone. Wave and tide actions are highly important, particularly in the shallower portions. Morphologically, the animals are variously modified along special lines associated, for instance, with the type of bottom, degree of exposure, depth, feeding habits. Many of the sessile forms, such as the limpets and chitons of the intertidal zones, are flattened and streamlined the better to withstand the wash and impact of rushing waters. Mussels are securely attached by strong and flexible byssus threads, while adult barnacles, corals, tube worms, and encrusting Bryozoa are rigidly and permanently cemented to rocks, shells, or other solid objects. Less rigidly attached are the hydroids, sponges, and anemones. The sessile or immobile habit so conspicuous in vast numbers of adult marine organisms is highly characteristic of life in the sea. This mode of life is made possible only by the continuous supply of floating microscopic food and the water movements necessary for its production and dispersal (see following chapters).
Among the free-moving bottom forms we find adaptations and habits so varied that all conceivable habitat facies are used. The sea urchin, Strongylocentrotus purpuratus, for example, is able to bore into rocks for protection on exposed coasts. The shells of molluscs are frequently of more sturdy structure when grown on exposed, wave-washed shores. Corals on exposed reefs are massive and compact in comparison to the more fragile and branched types found in lagoons (Vaughan, 1919).
On muddy or soft bottom environments other adaptive modifications result. The shelled animals of these environments build relatively thin, fragile shells as compared with those of animals in exposed or rocky situations. Burrowing bivalves of muddy, sandy bottoms commonly possess an enlarged “foot” useful in digging, and the siphons are elongated to extend above the substratum for intake of water providing food and oxygen. In contrast, the bivalves of hard bottoms may have these structures much reduced, and in more active forms like the scallops, tactile organs and even eyes are developed on the mantle edge. Creeping snails possess a broad foot to aid in gliding over soft mud. Burrowing worms are able to maintain permanent or temporary tubes by means of a mucous or fibrous lining secreted by the animals. Many mud-inhabiting animals are detritus feeders, eating the mud for the organic material it contains or sucking up the detritus that has settled at the mud-and-water interface.
In the littoral zone there is an abundant supply of food for animals. This results directly from favorable conditions for the production of plants, both attached and floating, and from the availability of these plants directly or indirectly to the benthic animals. An appreciable amount of organic material of terrigenous or fresh-water origin must supplement the great quantities produced in this zone. Due to this ready supply of food, the littoral zone produces benthic animals in abundance. The actual concentration is variable, of course, depending upon such local conditions as type of bottom, rate of flow of overlying water, river outflow, and upon meteorological conditions. The last is especially pronounced in intertidal situations, where seasonal rains and freshets may dilute tide pools and exposed flats with devastating results to the more sensitive species. Unusual temperatures during exposure on the foreshore result in great losses (p. 844). Most animals of this zone have a wide range of tolerance to changing conditions, but the selective action of the environment in certain areas may produce a great concentration of the species most suited to the conditions. For example,
Most littoral areas are provided with good circulation, owing to irregular bottom configuration, to the effect of tidal actions and winds, and to seasonal or diurnal convection. However, in some bays the exchange of waters may be sluggish, with the result that the free oxygen is used up by decomposing organic matter—usually abundant in such bays—and that hydrogen sulphide is produced, making for precarious if not fatal living conditions. Extreme cases of this nature are found in certain threshold fjords of Norway, where the mouth of the fjord is partially cut off from the sea by a sill of shallower depth than the inner portion of the fjord and where fresh water from adjacent land drainage may form a thin top layer. These situations offer an excellent example of the effect of physical-chemical circumstances on the success of animal life. Normally in these pools there can be no exchange of water between the ocean and the deeper water of the fjord below the sill depth because the water flowing over the sill is of lower density than the deep water of the fjord. Much of the organic material resulting from the plankton and its dependent life in the upper layers sinks to the bottom and, in decomposing, depletes the oxygen supply of the bottom water. Highly toxic conditions, resulting particularly from production of hydrogen sulphide, make it impossible for benthic aerobic life to inhabit the bottoms of these fjords. In some fjords oysters are cultivated, but they must be kept suspended in racks above the hydrogen-sulphide-charged bottom water. The layer of fresh water insulates the lower water and may make for tropical submarine climate with temperatures rising to 30°C (p. 871). Occasionally unusual circumstances, such as continuous offshore winds, build up an offshore gradient that forces upward the heavier deeper oceanic water outside the sill to a height sufficient for it to flow over the sill into the pool, where it lifts the lighter toxic hydrogen-sulphide-laden waters toward the surface. This water is lethal to the fjord animals, fishes, and invertebrates, which upon death sink to the already organically rich muds of the bottom. These or similar catastrophic circumstances lead to mass fossilization of littoral marine life; and,
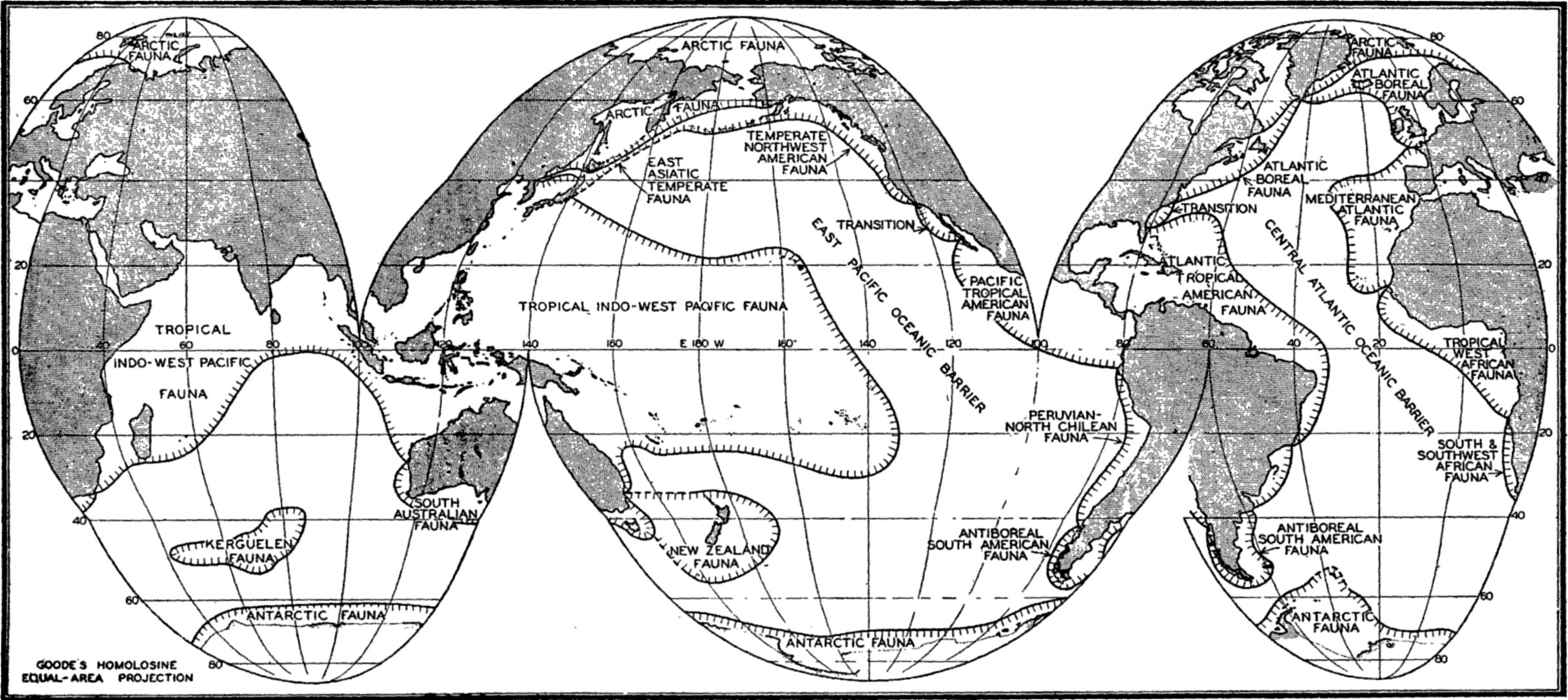
Horizontal Distribution of the Littoral Fauna. As a result of faunal studies and compilation of the work of many specialists, Ekman in his Tiergeographie des Meeres (1935) has divided the seas into faunal zones characterized by the species, genera, and families of animals found within the littoral zone of these regions (fig. 220). The animals thus employed include not only the littoral benthos but also pelagic forms that are bound by their life histories to the coastal zones. In such an analysis, the assemblage of species and genera of different animal groups that are confined to or are characteristic of the population of an area is the criterion for establishing the faunal regions. Obviously the number of endemic genera or of higher taxonomic orders is often of greater significance than the number of endemic species in distinguishing a faunal region, for the species are biologically of more recent origin. For example, the Pacific and the Atlantic tropical faunas of America, though now separated by the isthmus of Panama and having only relatively few species in common, show by numerous common genera (33 for certain crabs) and by geminate (closely related) species that these faunas were a continuous fauna in past geologic ages when the two great oceans were connected in this tropical region.
The faunal areas are not sharply defined, of course, and the boundaries are to be considered transition zones the width of which is determined largely by hydrographic features, water temperature being a cardinal determining factor. But other factors also determine the geographical extent of a faunal area. These are especially continental land barriers or broad expanses of deep water such as the East Pacific oceanic barrier (fig. 220). This, owing to its depth, precludes spreading of adult littoral forms through the abyssal zone, and, because of the vast horizontal extent of water between the American shores and the easternmost Polynesian Islands, prevents transport of pelagic larval stages of littoral forms except when these stages are of specially long duration.
Broadly speaking, the littoral fauna may be divided clearly into arctic, tropical, and antarctic. Between these there are gradations giving rise to such divisions as boreal, temperate, or antiboreal Kerguelen fauna. Some of the faunal divisions may also be subdivided into east-west regions; for instance, the tropical fauna, though homogenous in many characteristics—for example, in the formation of coral reefs—may be recognized as consisting of four parts, namely Indo-West Pacific, Pacific Tropical American, Atlantic Tropical American, and the Tropical West African.
Other faunal areas of the sea are given in fig. 220. These areas are again subdivided as the classification is made narrower to meet more local conditions. The Atlantic Boreal is, for example, divided into east and west sections and the Arctic into High and Low Arctic. For additional details and reference to original data, the student is referred to Ekman (1935). Many animals of eurythermic nature may be quite cosmopolitan and the sublittoral fauna may extend beyond the boundaries shown by the eulittoral animals. See also p. 845 for comparison of thermic boundaries to those of certain faunal regions.
Deep-sea Benthos. In 1843 Edward Forbes, pioneer in marine biology, observed the diminution of the number of animals with increasing depth of water beyond the littoral zone and he established with some hesitation what he called an “azoic zone” covering the deep ocean floor from depths below 300 to 700 m. Before this time, however, in 1819, Sir John Ross reported having found worms in mud brought up from a depth of 1800 m in Baffin Bay, but the idea of life existing in great depths was then so untenable that these and other findings were discredited. It was not until 1860 that positive proof of the existence of animal life in the deep sea was first provided by a broken submarine cable that was brought up for repair from a depth of over 2000 m in the Mediterranean with various bivalve molluscs, gastropods, hydroids, alcyonarians, and worms attached. The dredging operations of the Challenger and other expeditions have definitely shown that benthic animals do live in very great depths, probably in smaller numbers even at the greatest depths, since pressure and cold seem not to be excluding factors. During the Challenger Expedition more than 1500 animal species were discovered below 1000 m and a dredge haul at 6250 m yielded 20 specimens belonging to 10 species. Bottom deposits brought up in sounding tubes from the great deeps contained remains of foraminifera and sponges that probably live at these depths.
The summary in table 94, compiled from Murray (1895) and based on the Challenger observations, will serve to illustrate the vertical distribution of bottom fauna of size sufficiently large to be taken by the apparatus used. The specimens were collected with both dredge and bottom trawl and may therefore include a few not strictly benthic.
All of the animal world below the littoral zone may be spoken of collectively as the deep-sea fauna. Although not many deep-sea collections have been made, the endemic fauna appears to be divisible vertically into two parts, an upper archibenthic fauna (continental deep-sea fauna) and a lower-abyssal fauna, the dividing line between these two being placed at about 1000 m depth.
There is no well-defined border line, of course, between littoral and deep-sea fauna; the border is even less clearly defined between the archibenthic and the abyssal. The natural boundary between faunas is the region of most distinct faunal change. This boundary, however, is influenced by outside factors such as temperature and light, with their
| Zone | Number of stations | Average yield of species at each station | Average yield of individuals at each station |
|---|---|---|---|
| 180 m | 70 | 62.8 | |
| 180 to 900 m | 40 | 51.2 | 150 |
| 900 to 1800 m | 23 | 30.9 | 87 |
| 1800 to 2700 m | 25 | 24.0 | 80 |
| 2700 to 3600 m | 32 | 15.6 | 39 |
| 3600 to 4500 m | 32 | 10.6 | 25.6 |
| 4500 m | 25 | 9.4 | 24 |
Outstanding among the eurybathic forms given by Ekman are:
| Pennatularia | Kophobelemnon setelliferum | 36 to 3600 m |
| Polychaetes | Amphictera gunneri | littoral to 5000 m |
| Cirripeds | Verruca stroemia | littoral to 3000 m |
| Cumacea | Diastylis laevis | 9 to 3980 m |
| Bivalves | Scrobicularia longicallus | 36 to 4400 m |
| Snails | Neptunea islandica | 30 to 3000 m |
| Starfish | Henricia sanguinolenta | 0 to 2450 m |
| Brittle stars | Ophiocten sericeum | 5 to 4500 m |
| Sea urchins | Echinocardium australe | 0 to 4900 m |
| Sea cucumber | Mesothuria intestinalis | 20 to 2000 m |
That much of the animal life in the deep sea is truly endemic, not merely a downward extension of eurybathic forms, is shown by the presence of vast numbers of species and many genera and higher taxonomic orders that are found consistently only in these deeper zones. Important among the characteristically deep-sea forms are the glass sponges, Hexactinellida, with 15 families, 80 genera, and about 400 species; seven families of Pennatularia; the deep-sea Holothuroidea, of the order Elasipoda with four families, over 20 genera, and many species.
Tables 95 and 96, based on summaries of Ekman, depict further the arrangement of certain elements of the faunas of the three major bathymetric zones.
A striking characteristic of the deep-sea fauna is the relatively smaller number of species in proportion to the number of genera. On the basis of the Challenger data Murray (1913) concluded that the ratio of species to genera decreases regularly from coastal to offshore deep water, so that in the deepest zone the ratio of species to genera is 5 to 4, whereas in the shallow coastal water it is 3 to 1. It is a significant fact that whole orders and numerous families of various taxonomic groups are confined to the deep sea or are characteristic of its population.
| Animals | Zones | Number of species |
|---|---|---|
| Littoral, or littoral and archibenthic | 31 | |
| Crinoidea | Littoral to abyssal | 10 |
| (North Atlantic) | Archibenthic | 11 |
| Archibenthic and abyssal, or purely abyssal | 35 | |
| Littoral or mostly so | 13 | |
| Starfish | Littoral to abyssal | 28 |
| (North Atlantic) | Archibenthic or abyssal | 97 |
| Animals | Zones | Number of genera |
|---|---|---|
| Purely littoral | 62 | |
| Littoral and archibenthic | 14 | |
| Tunicata | Littoral to abyssal | 11 |
| Purely archibenthic. | 3 | |
| Archibenthic and abyssal | 4 | |
| Purely abyssal | 13 |
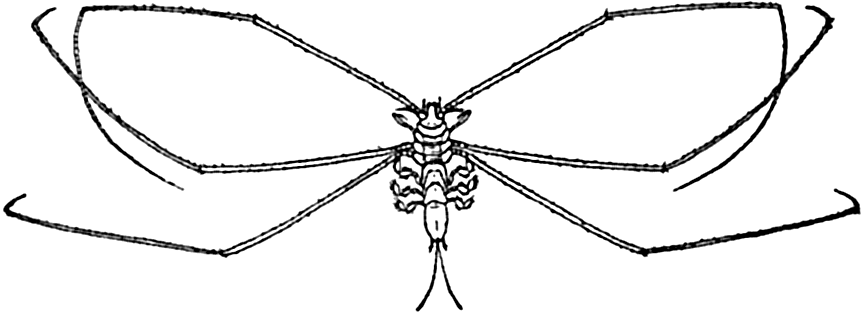
Deep-sea animals of the benthic region are in the main mud-dwelling forms adapted in various ways to this mode of life. A considerable number, typified by the isopod genus Munnopsis (fig. 221) and the shrimp Nematocarcinus, are adapted by elongated appendages to the quiet water and the softest of muds; sponges and hydroids are provided with
As previously stated, the population of the benthic region of the deep sea is relatively sparse, the animals decreasing numerically with increasing depth, also with distance from shore. The one factor most operative in limiting the abundance of animal life in the deep sea is undoubtedly food. Abyssal animals do indeed live a precarious life with regard to food. It is not surprising that only relatively few animals have been found on bottoms covered with red clay, for most deep-sea animals depend upon the nourishment obtainable directly or indirectly from the bottom oozes and tests show that the red clay is of all bottom deposits the poorest in organic material. Yet even it is not without its quota of marine metazoan life. It has been pointed out that with the intensified adversity of living conditions in abyssal regions, the animals adapted to survive the physical conditions there would be numerous were it not for a shortage of food. Since plants can live only in the lighted upper strata of the sea, it follows that deep-sea animals are either carnivores or detritus feeders, a population utterly dependent upon plant and animal production in the upper water layers and upon the ultimate sinking of dead bodies of these plants and animals to greater depths. Much of the surface production is broken down, however, by bacterial or autolytic action within the upper or intermediate layers and is thus lost to the dependent abyssal life. The direct food of abyssal forms
It is instructive to note that the poverty of animals in deep-sea zones is apparently not a direct result of depth of water but is intimately connected with distance from continental shores. Murray reports that collections made in depths between 1800 and 3650 m near shore yielded per haul an average of 121 specimens belonging to 39 species, whereas collections made in comparable depths further than about 500 km from shore yielded an average of only 21 specimens and 10 species per haul. This strongly indicates a relative shortage of food for benthic animals in the offshore localities. In the preceding chapter it was emphasized that conditions for phytoplankton production are enhanced in coastal waters where the supply of mineral nutrients in the lighted zone can be enriched through vertical circulation extending to a depth sufficient to tap the store of nutrients that accumulate there through the sinking of organisms produced in surface layers. Owing to the movements of such nutrient-enriched surface water, much of its plankton load becomes deposited in the deep inshore waters and thus supplies more food to the deep-sea benthic animals living there than is possible far from shore. Any offshore hydrographic condition that leads to enrichment of the plant nutrients in the surface layers will produce similar results, reflected in the abundance of benthic fauna that can be supported under such waters. This is illustrated by the surprisingly rich deep-sea benthos encountered by the Challenger Expedition in the deep offshore waters of the Antarctic, especially in the Kerguelen region. Murray believed that this exceptional abundance of deep-sea life resulted from offshore extension of coastal conditions owing to floating ice or to greater destruction of plankton life at the junction of waters of separate origin, but study of the now better-known hydrographic features of the Antarctic reveals that these rich accumulations lie under the region of the great Antarctic Convergence (fig. 158, p. 606) and, according to the investigations carried out by the Discovery, the surface waters of this region show possibilities of a rich supply of nutrients and of a great production of phyto- and zooplankton.
From this dependent relationship it becomes obvious that the deep-sea and abyssal fauna could have come into being only after the pelagic life of the sea had become established, or simultaneously with it. Both the pelagic animals and the deep-sea forms are believed to have been derived from the littoral fauna. The many structural modifications and the numerous endemic genera give evidence that the fauna of great depths is, indeed, an ancient one. But in generalizing, we may say that although the deep-sea fauna possesses many bizarre and unusual forms, nevertheless the structural adaptations found in these animals are only
Horizontal Distribution of Deep-sea Benthic Animals. As a rule deep-sea animals in general are widely distributed, but not to such an extent as was formerly believed would be the case owing to the uniform conditions of the deep-sea environment. The abyssal fauna, however, is the most widely distributed of benthic life, the archibenthic being next, and the littoral fauna the least widely distributed. In other words, the horizontal distribution of marine benthic animals increases directly with increasing depth. Benthic deep-sea genera are usually cosmopolitan, although the species may belong within the limits of certain oceans. Geographical submarine barriers are also influential in limiting distribution. A classical example is the Wyville Thomson Ridge, which forms a barrier between the deep-sea faunas of the Atlantic and the Norwegian Sea, only 12 per cent of the faunas being common to both seas. The reason for this will be discussed further on p. 849.