CALCIUM CARBONATE
Factors Which Determine Accumulation and Deposition of Calcareous Material
Interest in geological problems concerning the conditions which led to the formation of limestones has greatly stimulated the study of the calcium carbonate content of marine deposits, because most calcareous formations were recognized as having a marine origin although in certain cases no fossil remains of marine organisms could be identified. Questions therefore arose regarding the environmental factors which determine the carbonate content of sediments laid down in different regions and regarding the possibility of inorganic precipitation of calcium carbonate in the sea. As the calcareous material in marine deposits is predominantly composed of skeletal structures of plants and animals, the marine biologist is directly concerned with the problem of the formation of such sediments. The precipitated carbonates are formed from materials in
The terms “calcareous” and “calcium carbonate” are not strictly synonymous. According to analyses (p. 231 and p. 991), calcareous skeletal structures contain variable proportions of other substances, such as magnesium carbonate. However, the percentage of materials other than calcium carbonate is generally small and for this reason is commonly neglected. Most of the calcium carbonate found in marine deposits is in the crystalline form known as calcite, whereas the calcareous structures of live organisms apparently contain at least some in the form of aragonite. Whether or not there is a selective solution of the aragonite or whether there is a transformation of aragonite to calcite after the death of the organisms is not known. As was pointed out in the discussion of globigerina ooze, much of the fine material is composed of minute calcite crystals which apparently arise from the mechanical disintegration of the foraminiferal skeletons and are not due to any process of reprecipitation. Finely divided calcium carbonate is found on certain shallow banks in low latitudes, such as the Bahama Banks. This is in the form of aragonite and is thought to have been precipitated directly from sea water without the immediate participation of organic agencies (Vaughan, 1924, Smith, 1940). The presence of small amounts of authigenic dolomite, that is, the form of carbonate containing equal proportions of calcium and magnesium, has been reported by Correns (1937, 1939) for the pelagic deposits of the Equatorial Atlantic. No satisfactory explanation has yet been advanced to account for the existence of calcium carbonate in the form of aragonite under one set of conditions and as calcite under another.
The precipitation or solution of calcium carbonate is controlled by the degree of saturation of the water under a given set of conditions. Although the conditions which favor either precipitation or solution of calcium carbonate are known, it is impossible to state definitely for a given set of conditions whether the water is saturated with calcium carbonate or whether precipitation or solution may take place. The supply of calcium carbonate by rivers is relatively large and therefore it is reasonable to assume that conditions closely approaching saturation must prevail.
In most discussions of calcareous sediments the emphasis is placed upon the percentage of calcium carbonate rather than upon the character
In chapter VI (p. 207) it was shown that conditions favorable for the precipitation of calcium carbonate are most likely to occur in water having high temperature and high salinity and where the activity of plants has reduced the carbon dioxide content of the water. Such conditions are found in low latitudes over shallow bottoms, but apparently in a few localities only does such precipitation occur. Smith (1940) has described the precipitation of aragonite that occurs on the Bahama Banks (see also Vaughan, 1924) and has pointed out that there the process is facilitated by seeding with the minute aragonite crystals stirred up from the bottom.
Precipitation through organic agencies does not require that the sea water be saturated with calcium carbonate. However, deposits high in calcareous skeletal structures are generally restricted to areas where the physical-chemical environment is such that the ionic product [Ca++] X [CO3−] is large (see p. 205). The photosynthetic activity of plants, which removes carbon dioxide from solution and hence tends to increase the concentration of CO3−ions, offers a simple mechanism for
Transportation is of less importance to the distribution of calcareous material than to that of certain other constituents of the sediments. The benthic forms are large and can be moved only by the action of waves or extremely strong currents. However, wave action, boring organisms, and organisms which feed on the bottom mud tend to break down the larger fragments and, in this way, coral sands and muds are formed. Such a breakdown may favor a certain amount of solution. Agencies of lateral transportation are not very important even for those pelagic forms which live in the upper layers, since their skeletons sink quite rapidly and are not transported far from the region of development.
The potential supply of calcareous material to the bottom is dependent upon the production of calcareous forms either in the plankton of the overlying water or among the benthic organisms. However, a certain amount of solution may take place after the death of the organism and may occur either while settling through the water or on the sea bottom. Whether or not solution will take place depends upon the physical-chemical conditions prevailing in the water but, as the exact value for the solubility product is as yet unknown, it is impossible to state whether or not the waters of the oceans are saturated with calcium carbonate. Earlier studies lay particular emphasis upon the solution which takes place while the calcareous material is settling through the water. Examinations of the vertical distribution of calcium and alkalinity in the Pacific and Atlantic Oceans show that usually the lowest values are found in the surface layer where a certain amount of precipitation, probably through organic agencies, has taken place. Within this surface layer the concentrations are approximately the same in both the Atlantic and the Pacific Oceans, but at greater depths the existing data indicate that the waters of the Pacific contain somewhat more calcium and have a higher alkalinity: chlorosity ratio than those of the Atlantic. The larger values in the Pacific can probably be correlated with the lower oxygen content of the intermediate and deep water. The consumption of oxygen at subsurface levels is accompanied by an increase in the total carbon dioxide and therefore tends to favor the solution of any calcareous material which may settle through the water. However, it should be remembered that the consumption of oxygen, hence the production of carbon dioxide in deeper waters, is independent of the supply of calcium carbonate settling from above. Therefore we may expect that in certain localities the subsurface waters are undersaturated, whereas in other areas where there has been an adequate supply of calcareous material the deeper waters may be completely saturated with calcium carbonate.
Calcareous material on the sea bottom is exposed to the water, and if the water is undersaturated must pass into solution. In the presence of calcareous material there must be a zone within which the water is saturated with calcium carbonate and additional solution can take place only when this water is replaced by currents or by eddy diffusion. The rate at which calcium carbonate can be dissolved therefore depends upon the character of the flow and the gradients which are established and which for a given set of conditions cannot exceed a certain upper limit. If the rate of supply of calcium carbonate is greater than the rate at which the dissolved material can be carried away there will be a net gain in the amount of calcareous material and, consequently, accumulation will take place. If the supply is less than the rate at which it can be dissolved there will be no accumulation of calcareous material although considerable amounts may fall to the sea bottom in that locality. Besides the solution which may take place if the overlying waters are undersaturated, the production of carbon dioxide on the sea bottom, a result of the decomposition of organic matter, will favor the solution of calcareous material. Even if the overlying water is saturated with calcium carbonate this process will lead to a certain amount of solution. The number of benthic organisms is largely determined by the supply of decomposable organic matter and, in addition to the production of carbon dioxide, their activities in burrowing and as mud eaters will favor the breakdown of calcareous material. Extensive observations by Wattenberg in the Atlantic Ocean show a characteristic increase in the alkalinity: chlorosity ratio immediately over the bottom, which indicates a certain amount of solution of calcareous material. Furthermore, Wattenberg's data show a decrease in the dissolved oxygen content immediately adjacent to the bottom, indicating active production of carbon dioxide.
Calcium carbonate is most likely to dissolve in water which has come from high latitudes and in which the carbon dioxide content has been increased by oxidative processes. Water from high latitudes fills the great ocean basins below depths of a thousand meters or more. In some parts of the South Atlantic Ocean there is a fairly well-defined northward flow of water immediately over the bottom and, according to the results of the Meteor expedition, such areas are poor in calcium carbonate. As the water moves toward the Equator it undoubtedly takes up more calcium carbonate and can therefore dissolve less and less, for which reason alone we may expect to find calcareous sediments in low latitudes. In areas of large organic production where there is an abundant supply of organic matter to the sea floor, as along coasts where there is upwelling, and in areas of diverging currents and active winter mixing in high latitudes, we may expect to find sediments rather low in calcium carbonate because of the large amount of solution which can take place on the bottom.
The Distribution of Calcium Carbonate
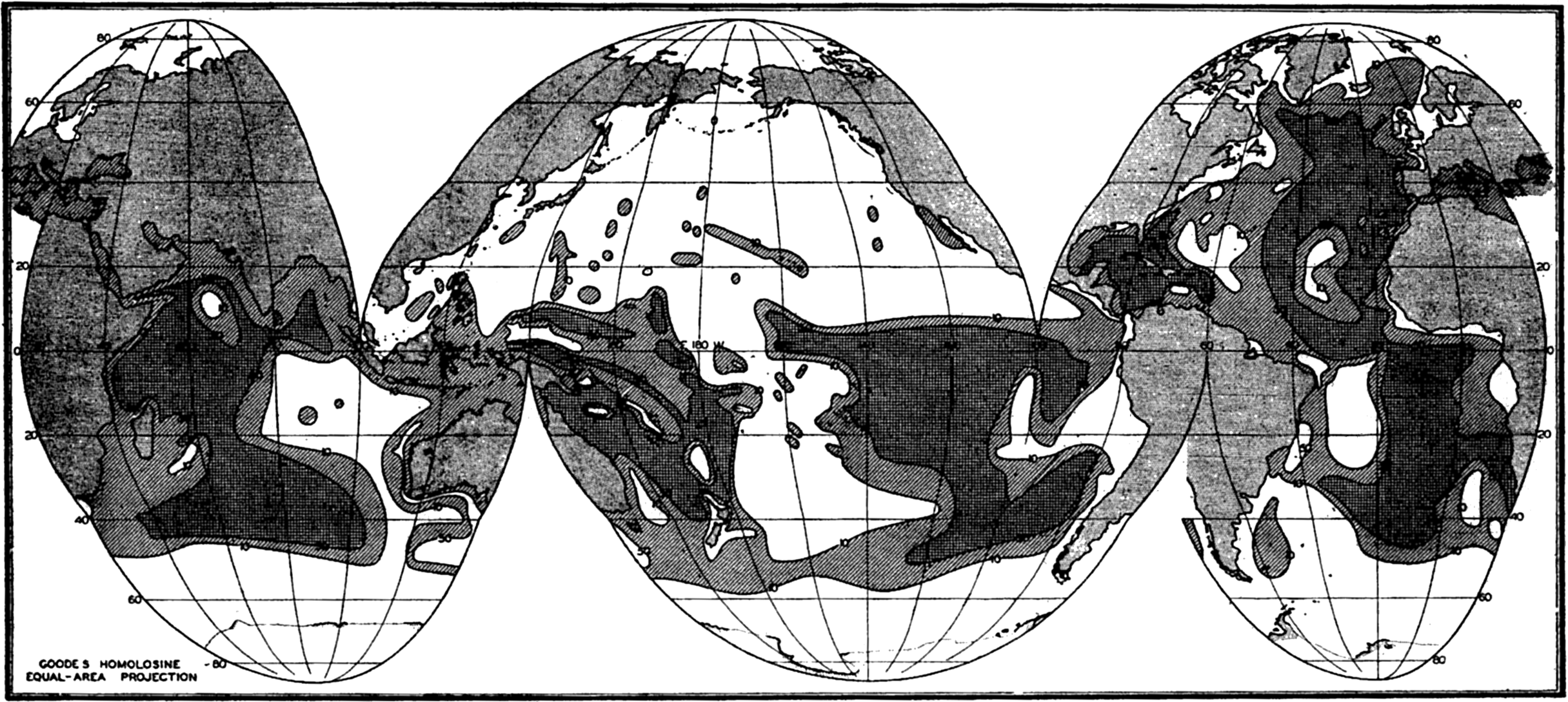
The distribution of calcium carbonate in pelagic deposits has already been discussed in some detail. In fig. 258 the percentage of calcium carbonate in the superficial layers of the sediments is shown without regard to the type of deposit or to the depth. This map, which is based on that of Trask (1937), shows only the 10 per cent and 50 per cent contours. However, as pointed out before, the transition from sediments low in carbonate to those high in calcareous material usually occurs in a relatively short distance. The 30 per cent contour which limits the pelagic calcareous deposits from the red clay and siliceous types is shown in fig. 253. Trask's map has been revised to take into account more recent data, from Schott (1939a) for the Indian Ocean, Revelle (1936) for the Pacific Ocean, and Correns et al (1937) and Pratje (1939) for the Atlantic Ocean. Figure 258 shows that sediments containing more than 50 per cent calcium carbonate are restricted to latitudes lower than 50 degrees except in the North Atlantic, where calcareous sediments extend considerably further north. A comparison of figs. 253 and 258 shows that there is no abrupt change in calcium carbonate content with distance from shore that would correspond to the transition from terrigenous to pelagic deposits. The sediments near shore and in enclosed areas are by definition described as “terrigenous,” but as can be seen from fig. 258 no such demarcation exists in the distribution of calcium carbonate. Off most continental coasts within low latitudes there is an increase in calcium carbonate content with increasing distance from the coast. This is particularly marked in regions where there is a transport of cold water from high latitudes and upwelling, as off the west coast of South America. Furthermore, in regions where there is an abundant supply of inorganic material as along coasts of heavy rainfall and off the mouths of rivers, the carbonate content is generally low. One of the striking features of the carbonate distribution is the very low values found in the North Pacific. A possible explanation for this anomalous distribution will be discussed below. It will also be noted that the carbonate content of the deeper parts of the ocean basins is generally low.
Trask (1937) has studied the distribution of the percentage of calcium carbonate in marine sediments to determine the relationships between surface temperature, surface salinity, depth, distance from shore, and the calcium carbonate content of the underlying sediments. This statistical study showed a positive correlation between the carbonate content and the salinity of the overlying surface water. When salinities were less than 34 ‰ the carbonate content was generally less than 5 per cent, whereas in regions where the surface salinity exceeded 36 ‰ the calcium carbonate was generally greater than 50 per cent. The calcium carbonate content also increased with increasing surface temperature but decreased with depth in deeper water. In general, nearshore sediments have considerably smaller contents of calcium carbonate than the pelagic deposits although this relation will not necessarily hold in low latitudes. The correlation obtained by Trask between the carbonate content of the sediments and certain conditions in the surface layers indicates the importance of the potential supply of calcareous material. Surface conditions are, in general, no indication of those which prevail at lower levels and which would determine whether or not the calcareous material would redissolve while sinking or after reaching the bottom. That is, the agreement which Trask obtained between the carbonate content of the sediments and the temperature and salinity conditions in the surface waters merely emphasizes the fact that calcareous deposits will accumulate in areas where the conditions favor the development of carbonate-secreting organisms.
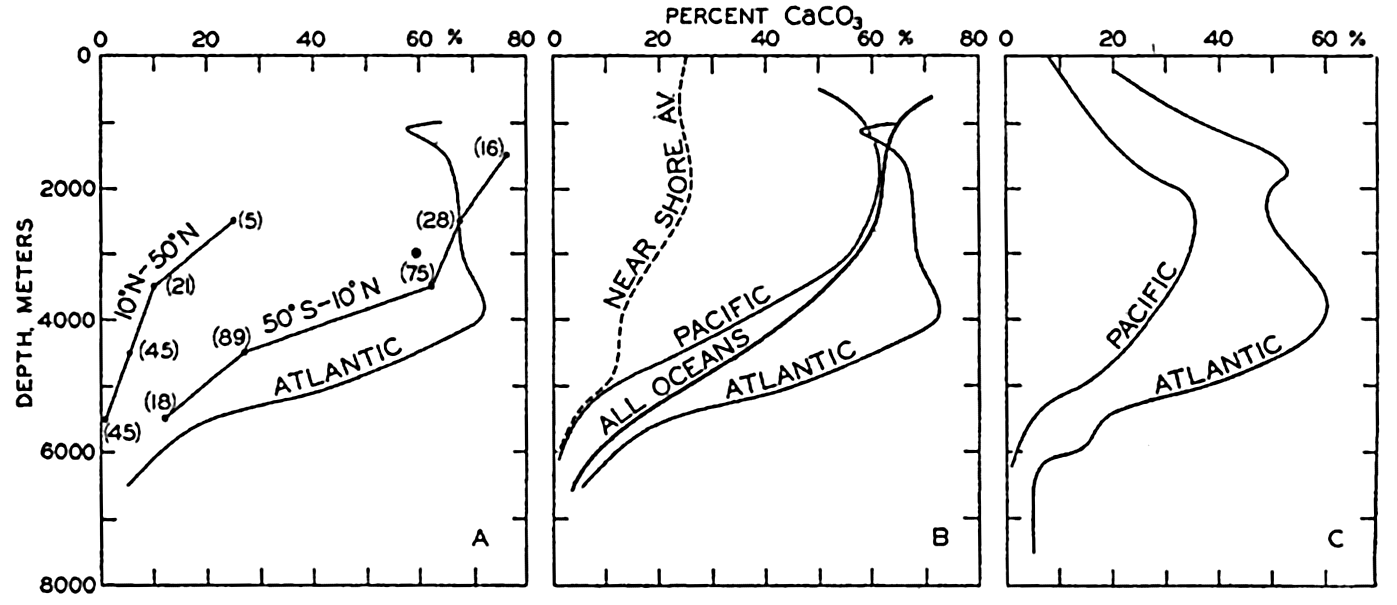
Percentage of calcium carbonate in marine sediments as a function of depth. (A) Averages for pelagic sediments from the North Pacific (10°N to 50°N), South Pacific (50°S–10°N), and from the Atlantic Ocean. (B) Averages for pelagic sediments from the Atlantic and Pacific Oceans and from all oceans, and the average curve for nearshore deposits. (C) Averages for all types of sediments from the Atlantic and the Pacific Ocean.
[Full Size]
Revelle (1936) examined the distribution of calcium carbonate in the sediments of the Pacific Ocean with reference to the variations with latitude and depth. The average values arranged according to latitude and depth for the pelagic deposits are shown in table 114. The decrease in carbonate with increasing latitude, that is, decreasing surface temperatures, and the small percentages found at greater depths are readily seen. It will also be noted that the calcium carbonate content of sediments from the North Pacific is less than that of material from the corresponding range in latitude and depth from the South Pacific. The marked decrease does not take place exactly at the Equator, but apparently at about 10°N. The curves obtained when these data are averaged in two groups, one for the North Pacific between 10°N and 50°N, and
| Depth range (meters) | Latitude | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50°–40°S | 40°–30°S | 30°–20°S | 20°–10°S | 10°S–0 | 0–10°N | 10°–20°N | 20°–30°N | 30°–40°N | 40°–50°N | |
| 0–1000 | 41.0 | |||||||||
| 1000–2000 | 94.0 | 74.4 | 75.0 | 38.4 | ||||||
| 2000–3000 | 82.0 | 74.7 | 63.5 | 65.9 | 68.0 | 57.8 | 32.5 | 18.0 | 22.0 | |
| 3000–4000 | 63.6 | 53.4 | 72.3 | 54.0 | 49.4 | 0.6 | 11.7 | 56.0 | 6.0 | |
| 4000–5000 | 37.5 | 18.8 | 17.9 | 19.9 | 47.5 | 55.9 | 17.6 | 1.9 | 1.8 | |
| >5000 | 0.0 | 16.0 | 10.7 | 24.7 | 1.0 | 0.8 | 0.6 | 0.5 | ||
The data in fig. 259A are presented to emphasize the differences between the average calcium carbonate content as a function of depth in the North and South Pacific and in the Atlantic Ocean. In fig. 259B the curve for the pelagic deposits of the Atlantic Ocean is repeated and the average for the Pacific Ocean as a whole is shown. Also included in this diagram are Trask's data for pelagic deposits which represent an average for all oceans. In addition, a curve based on Trask's average for nearshore sediments is included to show the lower calcareous content of terrigenous deposits. It is immediately noticed that the Atlantic Ocean is high in percentage of calcium carbonate compared to the Pacific. In fig. 259C are shown the vertical distribution of the percentage calcium carbonate where all samples, both terrigenous and pelagic, are combined. The data for the Pacific are from Revelle (1936) and those for the Atlantic are from Pia (1933). Unfortunately, Trask's data have not been combined to give a grand average for both nearshore and pelagic sediments. The differences between nearshore and pelagic
The extreme and the average carbonate contents of the various types of marine sediments have been given in table 108. Vaughan (1924) calculated the average calcium carbonate content of marine sediments from the Challenger values for the different types of deposits and the area covered by each type. In this way the following values were obtained:
| Average, all deposits | 32.2% CaCO3 |
| Average, pelagic deposits | 33.4% CaCO3 |
| Average, terrigenous deposits | 24.6% CaCO3 |
More complete data concerning the calcium carbonate content and the areal extent of the different types of deposits would modify these values somewhat. The data presented in fig. 259 can be used to estimate the average calcium carbonate content when the area represented by the different depth intervals is known (table 5, p. 21). Trask's figures for the calcium carbonate content of the pelagic deposits when treated in this way give an average of 37 per cent CaCO3. The data for the Pacific and the Atlantic (fig. 259B) give averages of 27 per cent and 54 per cent, respectively. When all types of sediments are combined (fig. 259C), we obtain an average of 18.8 per cent CaCO3 for the Pacific Ocean and 41 per cent for the Atlantic Ocean. The available data therefore indicate that the average calcium carbonate content of pelagic deposits is about 37 per cent and for terrigenous sediments about 25 per cent. Owing to the greater frequency of sampling in intermediate and low latitudes, the values for the terrigenous sediments may be somewhat high. The data in Clarke (1924, p. 34) indicate that the mean value for fossil-bearing shallow-water deposits is probably about one half of this, namely 12.5 per cent.
It should be pointed out that the averages given above are those for a superficial layer of the marine deposits and represent the averages for a certain thickness, regardless of the rate of deposition. The average obtained in this way, therefore, does not correspond to the ratio between the calcareous and noncalcareous material which may be carried to the sea. In order to obtain a balance sheet for the supply and deposition it is necessary to take into account the relationship between the rate of deposition and the calcium carbonate content. As shown on p. 1036, the terrigenous sediments accumulate much more rapidly than the pelagic deposits and, because of their lower calcium carbonate content, the ratio of the rates of accumulation of calcareous and non-calcareous material is 1:5.2, that is, the percentage of calcium carbonate in the total annual sedimentation is 19 per cent.
It is impossible to discuss the pattern of distribution of percentage of calcium carbonate in detail, and only a few of the major features can be considered. These are (1) the higher calcium carbonate content of the sediments of intermediate and low latitudes and the somewhat anomalous northward extension of calcareous sediments in the North Atlantic, (2) the decrease in calcium carbonate content with increasing depth in pelagic deposits, (3) the possible reasons for the difference between the carbonate content of the sediments in the Atlantic and the Pacific Oceans and between those in the North and South Pacific. Owing to the incomplete understanding of the processes involved, only the general character of the controlling agencies can be pointed out.
The contrast between the calcium carbonate content of the sediments in intermediate and low latitudes on one hand and in high latitudes on the other is undoubtedly related to the higher production of calcareous forms in low latitudes. In fact, there are virtually no calcareous planktonic forms found in high latitudes. Another factor which may contribute to the difference is that of solution of the calcium carbonate either in the water or after it has reached the bottom. As shown elsewhere, conditions favorable for the solution of calcium carbonate are characteristic of high latitudes in contrast to those in low latitudes. The relative rate of accumulation of noncalcareous material may play some part in determining the general distribution, but it is considered as secondary compared with the absolute rate of supply of calcareous material.
The decrease in the percentage of calcium carbonate with depth in pelagic deposits may be attributed to a number of factors. Possibly the most important is the effect which topography will have upon the deposition of extremely fine-grained inorganic debris. Such debris will tend to accumulate in depressions where it will be deposited more rapidly than upon topographic highs, and hence will tend to dilute the calcium carbonate. Two other factors must be considered. The first of these, namely the amount of solution during sinking, has been considered to be an important factor since the material which is deposited at great depths has to pass through a longer column of water and is therefore exposed to the solvent action for a longer period. The second factor is that of solution on the bottom. If there is a greater supply of decomposable organic material to the deep basins, the larger production of carbon dioxide may tend to dissolve more of the calcium carbonate which reaches the bottom. All of these factors may be effective in determining the decrease in the percentage of calcium carbonate with increasing depth in the open ocean.
In order to present any explanation for the difference in character between the sediments in the Atlantic and Pacific Oceans and the sediments of the North and South Pacific, it is helpful to return to the concept of the stationary distribution of properties in the water. As
It is more difficult to offer any definite reason for the difference between the North and the South Pacific. Most of the supply by rivers is actually in the North Pacific, therefore there must be a net transport of calcium across the Equator. The lower calcium carbonate content of the sediments may be due to the greater relative rate of accumulation of noncalcareous material or to the slower relative rate of deposition of calcareous material. Conditions within the water itself may also play a part. The deep-water circulation of the Pacific is such that the water flowing in from the south must ultimately turn around and flow south again (p. 752). There is a general decrease in the oxygen content and, hence, an increase in the carbon dioxide content from south to north. Therefore, conditions in the North Pacific may be more favorable for the solution of calcium carbonate both in the water and on the bottom. Further studies of the chemistry of the waters and more adequate knowledge of the solubility of calcium carbonate will make possible a more exact analysis of these various factors.