PLANT GROUPS OF THE SEA
In the sea, as on land, the plants are the real producers—that is, the organisms that are capable of elaborating complex organic substances from the simple inorganic compounds dissolved in the water. Without marine plants as synthesizers of primary food, development of marine animal life would be impossible beyond a negligible quantity that might be supported alongshore and in estuaries where particulate organic material of terrestrial origin would find its way into the sea.
A notable feature of marine vegetation is its dearth of variety when compared to the multiplicity of forms characterizing the terrestrial vegetation. Also, the types of plants most important in the production of primary food in the sea are in striking contrast to those constituting the chief synthesizers on land. This marked difference is readily explained, as we shall see, since it is dependent upon the radically different demands made on the plants by the marine environment. The poverty of plant variety in the sea is also in striking contrast to the abundant diversity of marine animal life. It may truly be said that the animal kingdom belongs mainly to the sea, while the terrestrial environment fosters the plants, although the most primitive of the plant groups, the algae, are wonderfully developed in the sea.
Light is of prime importance to all photosynthetic plants, and the possibility for attachment to the substratum is of secondary importance. More will be said about this later, but we must point out here that only in a very small portion of the sea are the two factors, light and suitable substratum for attachment, at the same time operative. This small portion of the sea wherein there may be sufficient light penetration to support attached plants—that is, the eulittoral zone—constitutes about 2 per cent of the sea floor.
Anyone frequenting the seashore is familiar with the covering of brown rockweed, Fucus, the green sea lettuce, Ulva, and a number of other low-growing plants that carpet the rocks in the intertidal zone. These or yet other relatively low-growing or encrusting plants may extend to varying depth below low tide if a suitable substratum for attachment is
The large kelps, such as Nereocystis, Pelagophycus, and Macrocystis, are found typically on rocky reefs some distance from the intertidal zone. Growth may occur on shoal reefs or rocks miles from shore, but the destructive mechanical effect of breaking waves and swells usually prevents any growth of these long-stiped forms in the immediate vicinity of exposed shores or rocks. Hence, the large kelps characteristically form in bands or patches some distance from shore where there is active circulation of water and yet where the danger of abrasion is reduced.
It was pointed out that only a small per cent of the sea floor may be considered to have sufficient light to support attached plants. Although this area may have enough light, it is vastly reduced as a suitable area for attachment of larger plants because of the great coastal stretches of mud, sand, shingle, or other unfavorable features. Therefore the bulk of the material produced by the attached marine plants is relatively small and can support only a small portion of the animal life actually present throughout the vast marine habitat; nevertheless, in more restricted areas along the coasts, attached plants—for example, the eel grasses—may be the chief producers. As a result of this restricted production by the benthic, or attached, plants, the primary food production becomes mainly a function of the unattached floating plants, notably, the diatoms and dinoflagellates, which, though microscopic in size, occur in vast, incalculable numbers.
Accordingly, our study of plant production must be concerned mainly with these floating forms. The means and adjustments by which this extensive community of floating plants—that is, the phytoplankton—is maintained and is related to other forms of life will be dealt with in subsequent chapters. First, however, in order to have a more complete understanding of the whole biological “setup” of the sea, it will be necessary as a point of departure to make a brief review of the various groups that are important to the economy of the sea as a whole.
The entire plant kingdom is divided into four primary divisions: the Thallophyta, Bryophyta, Pteredophyta, and Spermatophyta. Only the first and last of these are represented in the sea.
These primary divisions are each again divided and subdivided into many smaller secondary divisions which are indispensable to the specialist in marine botany, but for our purpose it will suffice to mention these smaller divisions only when they are important to marine economy, or when they have obtained rather widespread inclusion in more or less general literature dealing with the sea.
Only an abridged classification can be given. For a more complete treatment of the systematics the reader can refer to numerous good texts on botany or to publications dealing specifically with the group in which he is interested. A few of these publications are included in the bibliography, and yet others can be traced from those works included.
Thallophyta
Nearly all of the marine plants fall into this botanical division, which is made up of primitive plants in which the body shows little or no differentiation of vegetative organs—that is, no true root, stem, or leaf. Important among these thallus plants are the marine algae and the marine fungi, especially the bacteria. Since bacteria constitute the subject of a more specialized study of the sea, they will be dealt with under a special heading in chapter XVIII.
Most algae are beautifully colored, and sometimes also iridescent. The pigments of the chromatophores intercept solar energy, which is used in the synthesis of organic compounds. The type of pigment or pigment combination occurring in the algae as color manifestations has led to the names commonly used for the classes:
Blue-green algae (Myxophyceae)
Green algae (Chlorophyceae)
Brown algae (Phaeophyceae)
Red algae (Rhodophyceae)
Yellow-green algae (a heterogeneous group variously classified by different authors)
In general, the colors are characteristic of the classes, but other characteristics associated with cell structure and life history are more fundamental in distinguishing the five groups. Each group has a considerable variation in general morphology, some features of which will be pointed out in a review of the classes. The first four, with the exception of some blue-greens, are attached plants, while the yellow-greens are characteristically floating, or planktonic, forms.
Blue-Green Algae (Myxophyceae)
This class contains only small, poorly organized plants, some consisting of only a single cell, while others are multicellular. The blue color of these plants is due to a water-soluble accessory pigment, phycocyanin. In certain inland waters, it has been reported that upon the

Characteristic types of multicellular marine algae. a, Trichodesmium; b, Fucus; c, Alaria; d, Ulva; e, Ectocarpus; f, Sargassum; g, Rhodymenia; h, Polysiphonia; i, Cytosiphon; j, Lithothamnion.
[Full Size]
Methods of Reproduction. Reproduction in this group is by asexual fission. This, the most simple method of propagation, consists of single individuals dividing to form two of lesser size, which in turn again divide after growth. In instances involving blue-green algae that form chains of cells, the chains divide into smaller sections known as hormogonia. Fission of the cells in the hormogonia again increases the length of the filaments.
Distribution. The Myxophyceae are of less general importance in the oceans than are the following algal groups. They are widely distributed in fresh and brackish water. In the sea they are most often found in the warmer waters, where they may cause the phenomenon of sliming. A brackish-water form, Nodularia spumigena, native to calm fjords of the north, may at times cause extensive sliming of the waters. In the Gulf of Bothnia, sliming due to this or similar forms may assume considerable proportions.
Green Algae (Chlorophyceae)
As the name indicates, the algae of this class are green in color. The pigments of the chloroplasts include the two types of chlorophyll, a and b, and the various carotinoids. The yellow and orange of the latter pigments are masked by the abundance of the green chlorophyll. In contrast to the chitinous cell wall of the blue-greens, these plants produce walls that are largely cellulose—a carbohydrate as opposed to the nitrogenous product, chitin. Some green algae of the sea—for example, Halimeda of the Siphonales—become incrusted with calcium carbonate, and thus may contribute materially in some places to the formation of lime deposits in warmer seas. The joints of the plant remain uncalcified, and thus allow flexibility in the moving water.
There is great diversity in the morphological features of this class. Common forms are filamentous with septa (Urospora) or without septa (Codium), tubular (Enteromorpha), and sheet-like (Ulva, or sea lettuce) (fig. 68d).
Methods of Reproduction. Common methods of reproduction may be illustrated by the habit of the cosmopolitan Ulva. In sexual reproduction the contents of any of the ordinary cells of the flat two-layered plant may form biciliated bodies called gametes which, upon escaping into the water, unite in pairs and by cellular division grow to form the new plant, known as the sporophyte, but usually passing first through a filamentous stage. Reproduction may also be asexual, in which case any of the common cells of the sporophyte plant may form microscopic quadriciliate zoospores (spores are simple reproductive cells which differ from seeds mainly in that they do not contain any ready-made embryo plant). These zoospores, upon being discharged, grow directly into gametophytes, the plants that produce the gametes.
During the period of reproduction, large swarms of gametes and zoospores may be released, leaving the parent plant colorless and forming a green “bloom” on the waters of quiet bays. For many filter-feeding animals, the floating microscopic reproductive products of these and other algae form a source of food that must not be overlooked in a study of food of littoral animals. In bays, also, these swimming stages of algae, as well as algal slime, contribute to primary film formation that leads to an eventual fouling growth on ships and other submerged structures.
Distribution of Green Algae in the Sea. The green algae are found mainly in the upper littoral zone, especially in the lower half of the tidal zone, and in the immediate subtidal region down to a depth of 10m or more, and therefore in a relatively well-lighted habitat. It is with the green algae that the fresh-water algae are most closely related.
In geographic distribution, green algae are found most abundantly in the warmer seas. Algologists have remarked on the relative scarcity and dwarfed development of the Chlorophyceae in the Arctic Sea.
Brown Algae (Phaeophyceae)
Brown algae belong almost entirely to the sea, only a very few occurring in fresh water. Here are included the conspicuous brown seaweeds, many of which grow to notably large size. The pigments of this class include green chlorophyll, which is masked by the yellow and brown pigments, xanthophyll, carotin, and fucoxanthin.
Plants of this class of algae form the conspicuous offshore growths popularly known as “kelp beds.” They are the giants among the seaweeds, and form the marine forests among whose waving stipes and fronds myriads of neritic fish obtain their food and seek shelter from their aquatic enemies. These, also, are the kelps commonly harvested in many places for the commercial products they yield.
The brown algae possess a great range of size and structure. There are minute, delicate, filamentous branching plants (Ectocarpus, fig. 68e); coarse, hollow, sausage-like chains a foot or more in length (Scytosiphon, fig. 68i); short-stalked forms with broad thalli (Laminaria, Costaria, and Alaria, fig. 68c, some of which become nearly 2 m broad); many branched forms (Fucus, Egregia); and long-stalked giants of the Pacific with long leathery fronds (Macrocystis, Nereocystis, Pelagophycus).
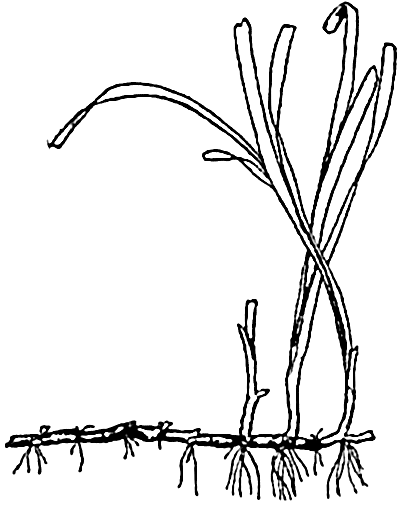
In structure the brown algae are the most advanced of all thallophytes. If we refer only to the more superficial details, Nereocystis
Nereocystis may attain a length of 35 m or more. The plant is anchored to a hard substratum by means of a profusely branched structure known as the holdfast, but there are no true roots. From the holdfast extends the long cylindrical stipe, which is hollow through most of its length and ends distally in a large hollow bulb. This bulb, like the stipe, is filled with gas, giving buoyancy to the plant. Ribbon-like fronds or laminae issue from the distal end of the bulb.
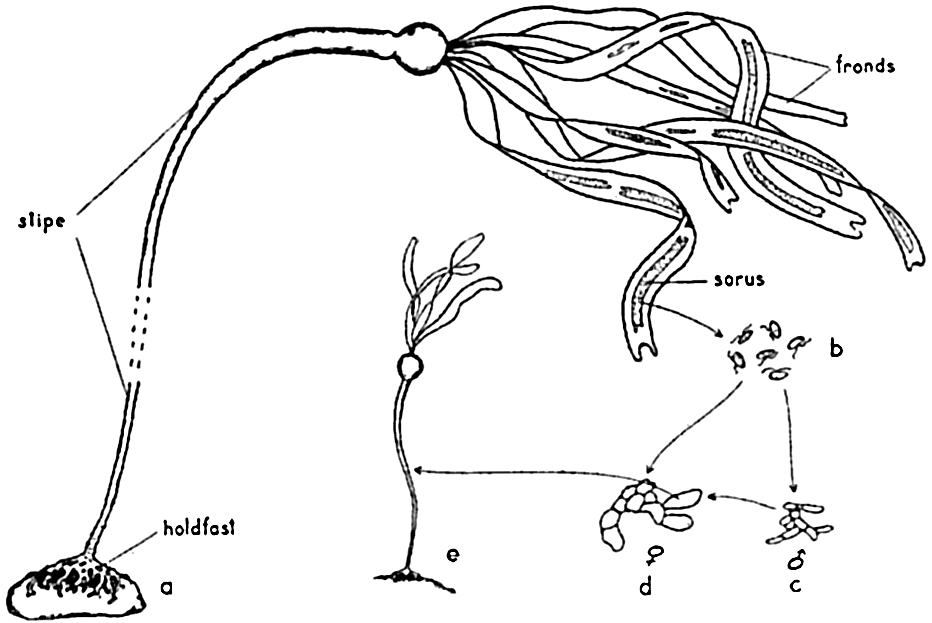
The gross structure and life cycle of Nereocystis. a, sporophyte plant; b, swimming zoospores; c, male and d, female gametophyte plants; e, young sporophyte.
[Full Size]
The hollow bulb and stipe maintain the upper portion of the plant near the surface, exposing the fronds to favorable light conditions. In common with other large algae, the parts are tough, flexible, and slippery in order to withstand with least resistance the effect of frequently violent storm waves and strong currents.
Methods of Reproduction. The life cycle of the brown algae includes various types of alternation of generations. Commonly, in the Laminariales, which includes the large kelps, there is an alternation of generations that may be illustrated by the cycle shown in Nereocystis (fig. 69). Here the large, conspicuous sporophyte plant produces a series of sori, or “fruiting areas,” appearing as dark brown patches running longitudinally along the whole length of the fronds. Beginning at the distal end of the frond, these patches are detached at maturity, leaving a broad gap (3 to 10 cm) in the frond. From the mature sori, innumerable ciliated zoospores escape and, upon reaching a suitable substratum, grow to small filamentous plants, the inconspicuous gametophyte stage. Thus the alternation of generations in this form is
In the brown algal group, Fucales, to which Fucus and Sargassum (fig. 68f) belong, the main plant is a sporophyte, but, within the thousands of tiny cup-like conceptacles forming the bladders, gametes are formed like spores. These unite after being discharged free in the water. Thus the alternation of generations is evident only cytologically. In connection with the “spawning” of Fucus, it is interesting to note that it is rhythmic with the tide, taking place after a period of exposure at low tide.
Distribution. The brown algae reach their maximum development in cooler waters, and are therefore typical of the rocky coast of higher latitudes. Sargassum and others of the Fucales are characteristic, however, of tropical or subtropical regions. Tilden (1935) is of the opinion that the Laminariales arose in the North Pacific, while the Fucales had their origin in the South Pacific. Several species or varieties of Sargassum, or “gulfweed,” are found in large quantities in the Sargasso Sea, whence they have drifted and multiplied after being torn loose from coastal areas. They are kept afloat by air bladders and grow vegetatively, propagating by fragmentation, but apparently do not form fruiting bodies. The drifting masses form a characteristic environment with associations including other algal and animal forms of littoral type.
The vertical distribution of brown algae shows many low-growing forms, especially the Fucales, in the rocky intertidal zone. Near the lowest tide level the medium-sized forms with leathery fronds and short limber stipes begin to prevail, and they increase markedly in the next 15 to 20 m of depth, finally diminishing and disappearing below the eulittoral zone.
Intermingled with these short-stiped algae are the giant long-stiped kelps that usually grow most abundantly some distance from the shore and extend to depths of 30 m or more. Macrocystis, one of the giant kelps of the Pacific, is said to reach to the surface from a depth of 80 m off the coast of Chile (Hesse, Allee, and Schmidt, 1937), but in the North Pacific it has its most abundant growth in water of about 15 m. Kelps of this genus are said to be absent from strictly tropical waters and, as a
Mention should also be made of epiphytic forms such as the filamentous Ectocarpus (fig. 68e), which prefers to be attached to other algae growing at various depths.
Red Algae (Rhodophyceae)
Nearly all of the red algae are marine. From the standpoint of color, they are the most striking of all the marine algae, some of them being also highly iridescent. Many of the delicate forms are among the most beautiful macroscopic objects of the sea. The order Gelidiaceae ranks first in importance commercially since certain of its members form the main source of agar.
The pigments of the chromatophores include the usual chlorophylls together with xanthophyll, carotin, and, in addition, the red phycoerythrin and sometimes phycocyanin. The plants may appear red, purple, violet, or, to some degree, brown or green. The deeper-growing species are the more purely red, a fact which is perhaps associated with their ability to synthesize more efficiently in the subdued light of greater depths than are the shallow-water types (Gail, 1922).
Though usually small in size, the red algae show a diversity of form much greater than the brown, and they are also more numerous. All are multicellular, the simplest being filamentous branching forms like Polysiphonia (fig. 68h), which, together with other filamentous algae, are commonly called “sea moss.” The larger flat types may be illustrated by Rhodymenia (fig. 68g), in which the broad frond may attain a considerable length. However, the maximum length of the larger red algae is only about 1 to 2 m.
Methods of Reproduction. The life cycle of some species is very complicated and cannot be amply discussed here. The reader is referred to the works of Kylin and other texts for a more complete treatment. In the higher types there is a regular morphological alternation of generations in which the sporophyte and gametophyte may superficially appear similar. Polysiphonia is commonly used to illustrate the life cycle of red algae. Here three types of plants are produced—namely, a male and a female gametophyte and an asexual tetrasporic plant. The last arises from the carpospores, which occur on the female plant. The carpospores are the products of union of male and female gametes. Upon germination, the tetraspores of the asexual plant give rise, in turn, to the sexual plants.
One of the most remarkable features of reproduction in red algae however, is the complete absence of any ciliated or flagellated swimming spores or gametes. This feature is a notable departure from the rule
Distribution. The Rhodophyceae are widely distributed geographically, but are most abundant in temperate seas. Their vertical distribution indicates that they prefer to grow in subdued light. A few species may be found in the intertidal zone, but the most luxuriant growth is subtidal. They may occur in abundance in depths less favorable to most of the green and the brown algae, and in the Mediterranean they have been reported from depths of 130 m. Thus, from shallow to deep water the general vertical distribution of the algal groups discussed is successively the green, the brown, and the red, with a wide degree of overlapping.
It should be mentioned here that certain red algae (the Nullipores) play an important role in calcium carbonate precipitation in the sea. They have contributed, and still do contribute, greatly to geological formations. Among these are, especially, the coralline algae, of which Lithothamnion (fig. 68i) is a typical example. They are distributed from lat. 73°5′ S to 79°56′ N (Tilden, 1935) and can be observed as copious encrustations on rocks and shells in the littoral zone of every exposed shore.
Yellow-Green Algae
There is considerable disagreement as to the proper grouping and status of divisions within this heterogeneous assemblage of organisms, some of which, as indicated below, are animal in nature. As a matter of convenience in discussing the more important marine members, we shall here employ only names of more or less familiar usage in biology and oceanography. Many of the members included are classified as animals in zoological texts, but in consideration of their holophytic nature (faculties of photosynthesis) it is most convenient for oceanographic studies to include them a priori among the producers. For more detailed treatment of the systematics of the various divisions, the reader is referred to Fritsch (1935) and the relevant works included in the discussions under the separate groups.
In contrast to the algae previously discussed, the members of this assemblage of plants and plantlike animals are primarily floating forms and will be taken up in the order of their importance in the economy of the sea.
Diatoms. The plants here included are all microscopic in size, the larger species viewed individually appearing only as tiny points. Some earlier authors of marine botany included them with the brown algae. A comprehensive treatment of the group is given by Hustedt (1930). In structure they are unicellular, but individuals may form chains or groups of various types. Examples of types representing the common genera are
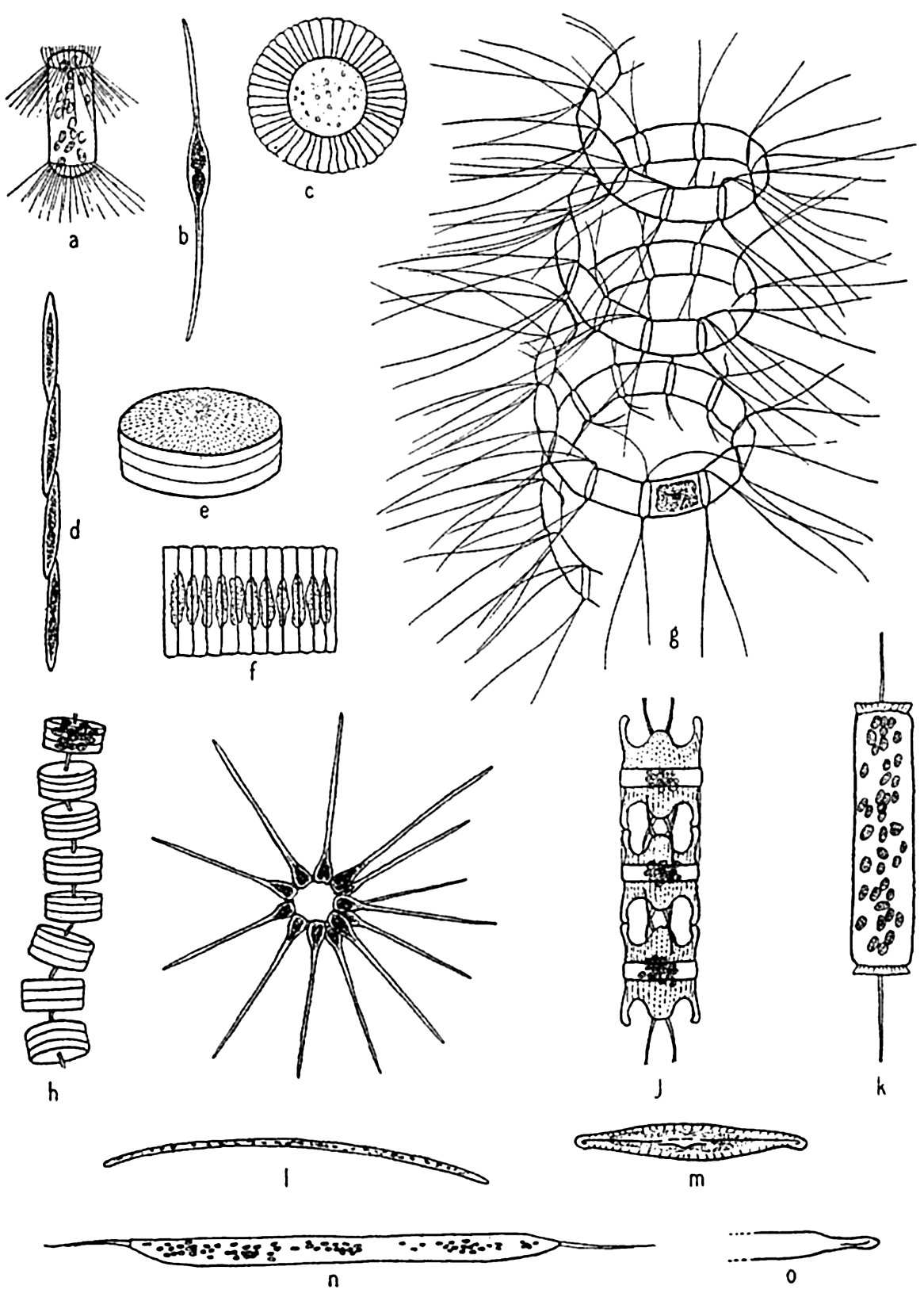
Characteristic types of diatoms. a, Corethron; b, Nitzschia closterium; c, Planktoniella; e, Coscinodiscus; f, Fragilaria; g, Chaetoceros; h, Thalassiosira; i, Asterionella; j, Biddulphia; k, Ditylum, l, Thalassiothrix; m, Navicula; n, o, Rhizosolenia semispina, summer and winter forms.
[Full Size]
Since these plants as a group may be considered the most important in the economy of the sea, it is imperative that we treat them in considerable
The shell structure of diatoms (fig. 71) may be likened to a box with a telescoping lid, because it consists of two nearly equal halves fitted one over the other. The pieces corresponding to the top and bottom of the box are known as the valves, and these are each joined by connecting bands that overlap and together form the girdle. The larger half of the shell is known as the epitheca, and the smaller half, which fits into it, as the hypotheca. The protoplasm lies wholly within the shell, but for exchange of metabolic products it is exposed by a slit (raphae) in the valve of some types and by small pores in others.

The gross structure of a simple diatom (Coscinodiscus). a, valvular view; b, girdle-view section of cell wall.
[Full Size]
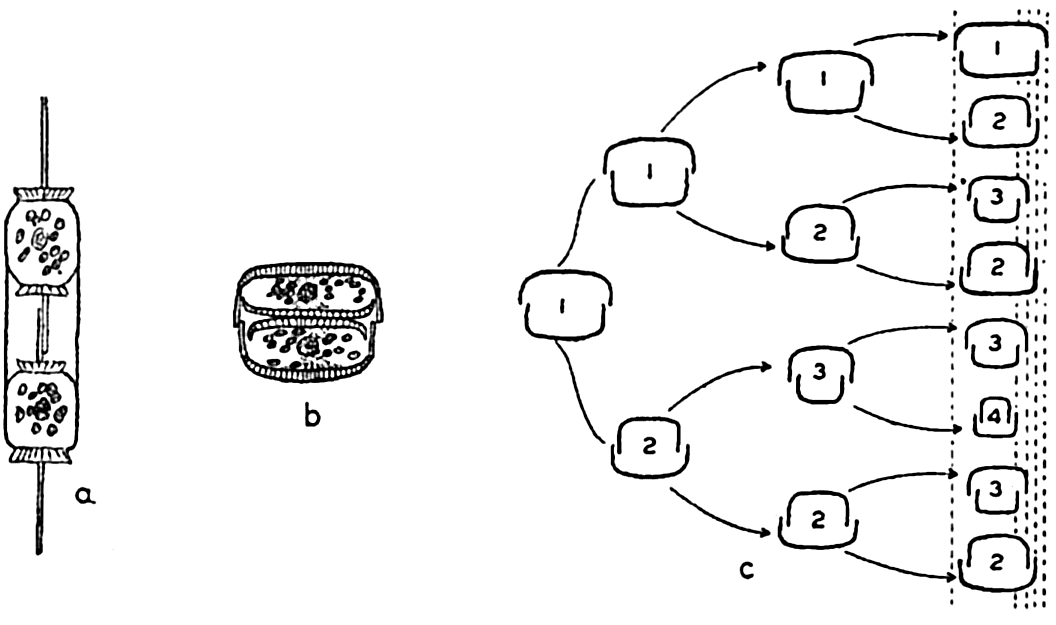
Reproduction in diatoms. a,b, cell division; c, diminution of size resulting from cell division in three generations.
[Full Size]
Diatoms may possess only one or many chromatophores, which may vary in color from yellow to olive-green or brown. Authorities are in poor agreement as to the nature of the pigments present, but there is some indication that the common pigments are masked by the accessory brown pigment diatomin, which may be identical with fucoxanthin of the brown algae. An important product of assimilation is an oil that is frequently visible as droplets within the diatom.
Methods of Reproduction. The most common method of propagation among the diatoms is by simple cell division (fig. 72a). This method has a far-reaching effect on the population in two distinct ways. First, it is conducive to a rapid production of enormous numbers when
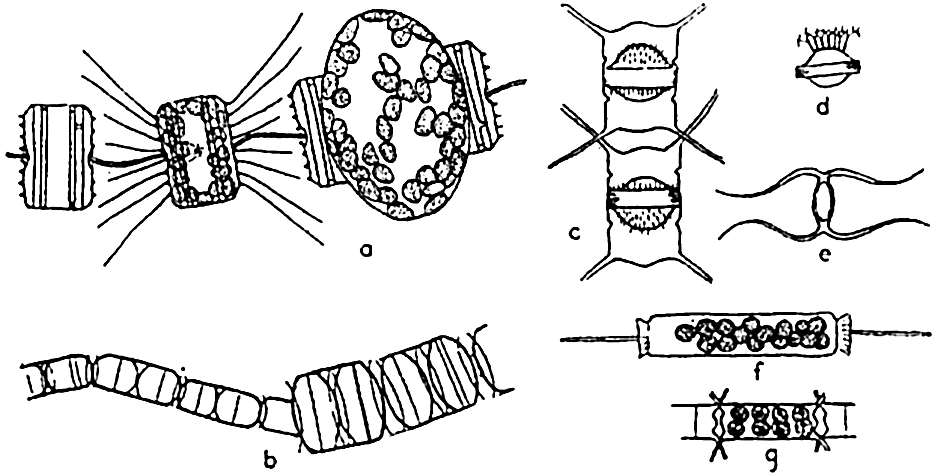
Reproduction in diatoms. a, auxospore formation in Thalassiosira aestivalis (after Gran and Angst); b, increase in cell size following auxospore formation in Melosira nummuloides (after Fritsch); c, resting spores in mother cells, Chaetoceros vanhurckii; d, resting spore of Chaetoceros diatema; e, resting spore of Chaetoceros radicans; f, microspores in Ditylum; g, microspores in Chaetoceros didymus (after Gran and Angst).
[Full Size]
Diatoms may also produce what are known as microspores (fig. 73b). These were early observed by Murray, Gran, and others. They consist of small protoplasmic spheres occupying the shell, and may escape as biciliated spores. The significance of these bodies is not fully known.
Resting spores of characteristic structure (fig. 73c) are also formed in most pelagic neritic species, especially of the centric types, by the cell contents becoming condensed and surrounded by a heavy, siliceous wall. They may be produced at the initial appearance of unfavorable living conditions, and may drift for some time within the old frustule or sink to the bottom to survive the unfavorable seasons of inadequate nutrients, cold, or of varying salinity so characteristic of many coastal areas. Gran (1912) has reported them from Arctic collections in which they were enclosed in ice.
Winter and summer forms of oceanic diatom species have been reported. These are cases of marked dimorphism in which the coarse winter forms have been looked upon as a means of survival from one favorable season to another. However, the dimorphism may be only an adjustment to changes of viscosity inherent with seasonal temperature changes.
Many diatoms grow normally on the bottom in the littoral zone, where they may or may not be attached by stalks or glide freely over the bottom. These benthic forms produce the heavily shelled types with most exquisite designs. Diatoms may also grow in profusion on other plants and animals. The littoral genus Licmophora frequently occurs on pelagic copepods, and the massed growth of Cocconeis ceticola flourishing on the skin of whales that have spent considerable time in the cold antarctic waters has, by its yellow color, given rise to the name “sulphur-bottom” for the blue whale.
Dinoflagellata. These are frequently spoken of collectively as the dinoflagellates (fig. 74). Space will not permit the amount of discussion that this diverse group of organisms requires for adequate treatment (see Kofoid and Swezy, 1921, Kofoid and Skogsberg, 1928, Fritsch, 1935). It is a group concerning which it is not easy to make generalizations without the danger of introducing errors. The members are of great importance in the economy of the sea. A large number are holophytic and rank second to the diatoms as producers in the marine plankton. They are therefore best studied with the phytoplankton. Others are holozoic or animal-like in nutritional requirements, ingesting particulate food and possessing other characteristics that place them clearly with the animals. Some are saprophytic, living upon dead organic matter. All are important as food to filter- and detritus-feeding animals.
Typically, the dinoflagellates are unicellular, some being armored with plates of cellulose, others unarmored or naked. All possess two flagella for locomotion, an important feature in the holophytic forms, for
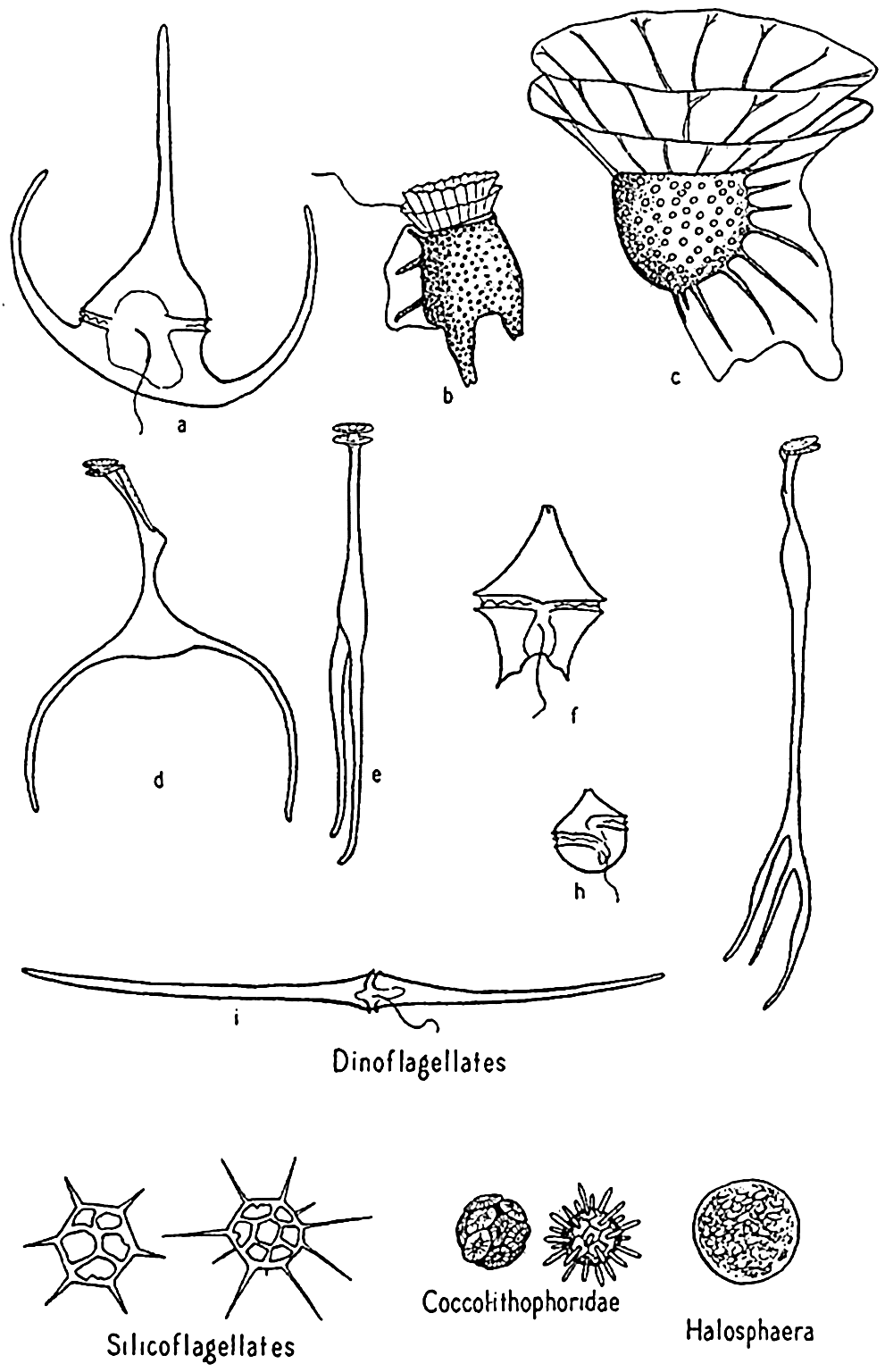
Dinoflagellates and other phytoplankton organisms. a, Ceratium tripos; b, Dinophysis; c, Ornithocercus; d,e, Triposolenia, front and side views; f, Peridinium; g, Amphisolenia; h, Goniaulax; i, Ceratium fusus.
[Full Size]
Methods of Reproduction. Among the dinoflagellates, reproduction is accomplished mainly by processes of cell division, which in some instances result in a chain of individuals clinging loosely together. Temporary structural variations may normally occur in individual cells at opposite ends of the chain. The progressive size reduction that is
Dinoflagellates are found in all seas, but the greatest development of species is met with in the warmer waters, where a number of very bizarre forms are to be found. Owing to the destructibility of their cellulose plates by bacteria and other agencies, they are not preserved in bottom deposits. Important genera are Ceratium, Peridinium, Dinophysis, Gonyaulax.
Phaeocystis. Phaeocystis is a brown, flagellated plant, neritic in habit, that forms colonies in gelatinous, lobed globules visible to the naked eye. The large numbers produced may at times render the surface water quite brown and become a serious cause of clogging in silk plankton nets. Reproduction is accomplished by formation of flagellated spores that escape from the colonies.
Coccolithophoridae. Among the smallest (5 to 20 microns) of autotropic organisms of the sea are the biflagellated (some marine forms are not flagellated) forms of this group (fig. 74). Usually they are not caught by the ordinary net, through the meshes of which they readily escape, and when caught special care must be taken that their calcareous protecting armor is not dissolved by the preservative, leaving only an indefinable mass. The soft parts are shielded by tiny calcified circular plates or shields of various design and projections called coccoliths, or rhabdoliths. These shields had been found in enormous numbers in marine bottom deposits before the organisms of which they are a part were discovered by the Challenger and identified from their living habitat in the plankton, where they were found entangled in protoplasmic strands of pelagic protozoa or in the stomachs of salps and pteropods. Typically, the coccolithophoridae belong to the open sea, but they may occasionally reproduce in large numbers in coastal waters; at one time, according to Gran (1912) numbers of 5 to 6 million per liter gave the waters of Oslo Fjord a milky appearance. Some also occur in fresh water.
Though minute in size, they are of great importance as food to filter-feeding organisms, and also as contributors to calcareous bottom sediments. They occur in geological formations dating from the Cambrian period. Common genera among these organisms are Coccolithus, Pontasphaera, and Rhabdosphaera.
Halosphaera. Halosphaera is a unicellular, microscopic plant of the order Heterococcales (fig. 74). Earlier authors have included it with the green algae. It occurs at times in vast numbers in the plankton, floating mostly near the surface. Halosphaera virides occurs over the whole Atlantic and is abundant both in the warmer waters of the Gulf Stream system and in high southerly latitudes, where the Discovery investigations in the Antarctic found it second in importance to the diatoms. Meringosphaera of this order also occurs in marine plankton.
According to Gran, Halosphaera is practically the only open-sea form in which the predominantly green color of land plants is to be found. Notwithstanding the vast numbers that are often found, it does not reproduce by the quick method of simple binary fission, as in diatoms, but, after having grown for some time to its maximum size, the cell contents are transformed into a large number of zoospores. These swimming spores escape and through some unknown method are transformed back into tiny globular forms that gradually increase to normal size by successively shedding their weakly silicified investing membranes. Resting spores may also be produced.
Silicoflagellates. These flagellate organisms (fig. 74) deserve mention only briefly, since they do not usually occur in sufficiently large numbers to enter materially into the economy of the sea. However, they are such persistent members of plankton communities from nearly all colder seas that their starlike, open, siliceous shells attract a good deal of interest. Many occur in bottom sediments, and their development is shown in fossil marine deposits. That they contribute at least in a small way to the food of animals is shown by their frequent occurrence in food vacuoles of tintinnids.
The Higher Plants in the Sea
The two intermediate phyla of the plant kingdom—namely, the mosses (Bryophyta) and the ferns (Pteridophyta)—are wanting in the sea. However, the highest of plants, the Spermatophyta, are represented by about thirty species of Angiosperms, or flowering plants. These belong to three genera of the Hydrocharitaceae and six genera of the Potamogetonaceae (Arber, 1920). They have not originated in the sea, but have invaded and colonized it by way of fresh water. Their closest affinities are with widespread fresh-water angiosperms belonging to the same families.
Of outstanding importance among the marine angiosperms is the eel grass, Zostera (fig. 75). Botanically, the plant is not a grass despite the long, slender, and flexible grasslike leaves, which are thus adapted to withstand the force of moving water. Unlike the benthic algae, Zostera and its relatives possess true roots that are attached to an underground stem, or rhizome, forming an anchor in the soft substratum. There are fertile and sterile plants, and, since the plants grow submerged, mostly in depths of 4 or 5 m but also to a depth of 14 m (Petersen, 1918), the flowers are pollinated under water through the agency of currents.