Chapter Two
The Normal Heart:
Function
Cardiac Impulses and Their Conduction
The heart has the unique property of rhythmicity. To maintain life, it must contract rhythmically; the average rate of contraction of the heart for an adult at rest is seventy times a minute. Proper function of the circulatory system also requires a well-coordinated contraction of the various parts of the heart. The origin of the cardiac impulses and their conduction are the functions of specialized cells distributed throughout the heart, consisting of the pacemaker (the initiator of cardiac action) and the conducting system (the distributor of the impulses through the heart). These cells are grouped together into three types of structures: two larger accumulations of cells, or nodes; nervelike conduits, or bundles , with branches; and the terminal portion of the branches, constituting a fine network on the inner surface of the ventricles.
The uppermost node, the sinoatrial node (S-A node ), is located at the junction of the superior vena cava and the right atrium. The lower node, the atrioventricular node (A-V node ), is located in the lower septal wall of the right atrium. From the lower portion of the A-V node emerges the bundle of His , entering the junction between the two atria and the two ventricles, where it divides into two principal branches: the left-bundle branch and the right-bundle branch, which run down either side of the ventricular septum. The branches subdivide into smaller and smaller branches, eventually forming the fine network, or Purkinje network , located
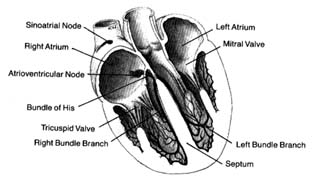
Figure 9. The heart with its front wall removed, shown to
indicate the principal parts of the conduction system.
in the endocardial layer of the ventricles, where it comes in contact with muscle cells to be stimulated. A diagram of the conducting system is shown in figure 9.
Specialized cells of the S-A node generate electrical potential. When that potential reaches a certain level, it is discharged, activating the conducting system throug which an electrical impulse travels, producing contraction of the cardiac muscle. The discharge of electrical potential in specialized cells is called depolarization . Immediately after depolarization the cell begins rebuilding electrical potential (repolarization ) for the next impulse. This process is analogous to discharging and recharging a battery. All specialized cells have the capacity of generating an electrical impulse as well as carrying it rapidly throughout the conducting system. However, impulse formation in cells other than those of the S-A node is suppressed by the function of the S-A node, which as the primary pacemaker has the fastest rate of discharge. Its impulse directly stimulates the atria to contract, then speeds through the atrium to the A-V node, where it slows considerably. When the impulse reaches the junction between the A-V node and the bundle of His, it passes quickly through the remainder of the conducting system down to the Purkinje system, through which it stimulates the ventricles to contract. Slow conduction through the A-V node is essential for appropriate coordination of atrial and ventricular contractions, which should be separated by an interval of about 0.16 seconds to facilitate flow of blood from the atria to the ventricles.
Since all cells within the conducting system are capable of impulse formation, they serve as standby pacemakers activated only when the primary pacemaker fails to discharge. The lower portion of the A-V node, at its junction with the bundle of His, is the secondary pacemaker . Its rate of discharge, 50 times a minute, is slower than that of the primary pacemaker. Lower divisions of the conducting system, including the Purkinje network, represent the third line of defense against failure of the impulse to reach the ventricles. The discharge rate of this tertiary pacemaker is 30 to 40 times a minute. It is most frequently activated when the connection between the A-V node and the Purkinje system is interrupted, in which case the atria may contract at a fast rate, obeying the primary pacemaker, while the ventricles contract more slowly, activated by the tertiary pacemaker (see chap. 6).
The heart muscle, like other muscle tissue, has the ability to contract (that is, reduce its length), thereby exerting a considerable force. Since the heart muscle is globular in shape and envelopes a cavity filled with blood, its contraction expels most of the contents of the cavity. Muscle cells, accepting the stimulus from the conducting system, also discharge electrical potential while contracting (being depolarized) and are recharged (repolarized) during relaxation. However, these cells under normal conditions are not capable of impulse formation.
The primary pacemaker, the S-A node, is under the control of the autonomic nervous system (the part of the nervous system unresponsive to a person's will) through fibers connecting it with both divisions of the autonomic nervous system: sympathetic nerve fibers can quicken impulse formation; parasympathetic nerve fibers can slow it. This nervous control permits necessary adjustments in cardiac function, such as accelerating the heart rate during exercise and slowing it afterward. The secondary pacemaker is less efficiently regulated by the autonomic nervous system. The tertiary pacemaker has no significant connections with that system: its rate of discharge remains the same under all conditions.
Ejection of Blood
The pumping action of the heart results primarily from ventricular contraction. The atria act more as collecting reservoirs, and their
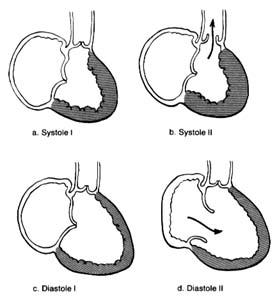
Figure 10. A ventricle during two stages of systole and two stages of diastole.
contraction accounts for only a small part of the blood entering the ventricles from the atria; most of it is sucked in by the ventricles. Since the state of contraction or relaxation of the ventricles determines the overall volume of the heart, it is customary to divide the cardiac cycle into two periods: the period of ventricular contraction, or systole; and the period of ventricular relaxation, or diastole .
During systole the beginning of ventricular contraction and the resulting first rise in the pressure inside the two ventricular chambers causes the two atrioventricular valves to close (fig. 10a ). The sudden tensing of the atrioventricular valves produces a loud noise—the first heart sound. Now the pressure can effectively build within the ventricles as they contract, until it exceeds the pressure in the aorta and the pulmonary artery, at which point the two semilunar valves are forced open and blood begins to flow into the two arterial trunks (fig. 10b ). Blood is pumped with considerable force and velocity during the first half of the ejection, and then gradually slows down. At the moment ventricular contraction ends and the period of relaxation begins (diastole), pressure in the cavities starts to fall, causing the semilunar valves to be sucked into
closed position (fig. 10c ). Closure of the semilunar valves produces the second heart sound. Relaxation of the ventricular muscle now produces a rapid fall in pressure in the two ventricular cavities, and the moment ventricular pressures fall below atrial pressures the two atrioventricular valves open widely, permitting the ventricle to fill with blood from the atria (fig. 10d ). As stated, relaxation enlarges the ventricular cavities and sucks in atrial blood; this occurs mostly during the first third of the diastole; during the middle third relatively little pressure change and flow occur. This is the period of rest, diastasis . The final third of the diastole involves the contraction of the atria, at which time the small remainder of blood (20 percent of the total volume or less) enters the ventricle from the atria. From the above description two points are clear: (1) During both systole and diastole there are short periods of time during which flow of blood ceases; these occur between the time one set of valves closes and the other opens, as shown in figures 10a and 10c . These two periods are referred to as isometric contraction and relaxation of the cardiac muscle; they are important in permitting efficient and rapid rise and fall in pressure. (2) Flow through the two sets of orifices does not occur with uniform volume and velocity; maximum flow occurs during the earliest part of ventricular ejection and ventricular filling.
Blood Pressure and Blood Flow
During systole the semilunar valves are wide open, and the pressure within the cavities of the two ventricles and the arterial trunks on the respective sides is identical. The highest level of pressure within the ventricle and the arterial system on the corresponding side is called systolic pressure . The systolic pressure within the left ventricle and the aorta is about five times higher than the corresponding pressure within the right ventricle and the pulmonary artery. The onset of diastole and the closure of the semilunar valves signal the separation of pressure between the ventricles and the arterial trunks; pressures in the ventricular cavities drop sharply to levels close to zero; pressures in the aorta and pulmonary artery level off to a point slightly lower than that of semilunar-valve closure. The lowest pressure in the ventricues and the lowest pressure levels in the arterial trunks are termed diastolic pressure . Since
during most of diastole pressures in the two ventricles and their respective atria are identical, ventricular diastolic pressure and atrial pressure are usually the same. Normal average pressures in the adult are as follows:
|
The heart, being a pressure pump, functions properly if it can maintain an adequate flow of blood and adequate pressure. Both blood flow and blood pressure have to be regulated in response to needs of the body. The quantity of blood ejected into each circulatory system—the volume of blood flow—is the cardiac output . Cardiac output can be expressed in two ways: either as the quantity of blood ejected into the arterial system with each ventricular contraction (stroke volume ), expressed in cubic centimeters per heartbeat; or the total quantity of blood ejected into each arterial system within a minute (minute volume ), expressed in liters per minute. It is customary to use the general term "cardiac output" to indicate minute volume and to specify stroke volume as such. The maintenance and regulation of cardiac output is one of the most intricate functions of the circulatory system; it will be discussed later in this chapter in connection with the physiology of exercise.
Blood pressure commonly refers to pressures in the systemic arterial circulation. The arterial blood pressure can be maintained at its level of 100 mm Hg or above only because the blood is enclosed within a system of vessels so regulated that the same amount of blood
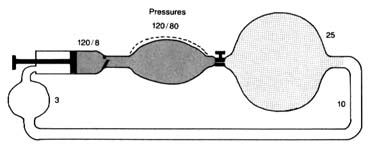
Figure 11. A circulatory model resembling the human circulation.
is pumped into it and discharged from it simultaneously. Thus if a stroke volume of 70 cc of blood is ejected into the aorta with each beat, an equal amount leaves the arterial system through the "exit"—the arterioles—into the capillary system. The arterial system is protected at one end by the aortic valves and at the other end by the sum total of the arterioles. A simplified diagram of such a system is provided in figure 11, in which a pump (at left) ejects fluid into an elastic container that has at its end a stopcock; that container is connected to a much larger container from which a system of tubes returns the fluid into the pump. The pump represents the left side of the heart; the first "closed" container, the high-pressure arterial system; the stepcock, the arterioles; the larger, "open" container, the capillary and venous reservoir of blood; the returning pipes, the larger veins; and the container at the lower left, the right atrium. The arterioles are normally in a semiconstricted state and are regulated by impulses reaching them from the central nervous system via the nerves. These impulses are capable of either constricting the arterioles further or relaxing them, thereby regulating outflow from the first reservoir and hence the arterial pressure. In the arterial system resistance to blood flow is very high; in the pulmonary arterial system where arterioles are wide open, resistance and pressures are much lower than on the systemic side. The capillary and venous systems in both circuits have no narrow area offering any resistance to flow and hence their pressure is close to zero.
This relationship between pressure, flow, and resistance is usually presented in the form of an equation derivative of Poiseuille's law and analogous to Ohm's law in electricity: pressure equals flow (cardiac output) times resistance, or P = F × R . It follows that
pressure can be maintained at a constant level only if resistance falls each time cardiac output increases. This actually takes place because of a barostat, a mechanism analogous to the thermostat that regulates pressure rather than temperature. The human barostat consists of pressure-sensitive points within the walls of some arteries. If the volume of blood ejected into the aorta increases, raising the cardiac output, the aorta becomes distended. Pressure-sensitive receptors react to the distention of arterial walls by sending signals through the nervous system to relax the arterioles just enough to let the excess blood out of the arterial system and to maintain constant arterial pressure.
The systemic circulation consists of many circuits connected in parallel (see fig. 1, p. 2). Each of these smaller circuits has its own resistance at the arteriolar level. The general equation mentioned above applies to each of these circuits as well as to their sum total; consequently, arteriolar resistance in each region determines blood flow independently within the systemic flow and pressure available. Thus, if in a given organ the arterioles were to constrict, its blood supply would be reduced, since blood would flow more easily through alternate circuits; if the arterioles relaxed in a given circuit, blood flow would increase proportionally. This mechanism, mediated less through the central nervous system than through local reflexes, controls blood flow through individual organs and parts of the body. Such a regulating mechanism (regional flow control ) provides for increased blood flow in areas where it is most needed. Thus during exercise the working muscles receive a more abundant blood supply, and after meals the digestive tract is provided with increased blood flow—all without disrupting the general balance of the total flow or altering the arterial pressure in the systemic circulation.
The blood pressure is the same in all arteries up to the beginning of the arterioles. There the pressure falls abruptly from a systolic level of 120 mm Hg to 25 mm Hg in the capillaries, a level just sufficient to drive the blood into the venous system through the narrow capillary channels. On the venous side blood flows slowly toward the heart. The veins have virtually no driving power, and the blood flow is aided only by the venous valves and by the massaging action of the various muscles of the body.
The aorta and its principal branches are, as mentioned, elastic
vessels. This property plays an important part in causing the blood to flow through the body evenly rather than intermittently. During systole all the elastic arteries are expanded by the addition of blood ejected from the left ventricle; the elastic vessels then revert to their original size as the excess blood drains into the capillaries. If blood were ejected into a system of totally rigid pipes, then forward flow of blood would occur only during systole and would stop during diastole. Elastic tissue in arterial walls makes for a reservoir of variable capacity that acts as a pressure and flow equalizer. This principle is often used in perfume and cologne atomizers: simple dispensers with one bulb spray only when the bulb is squeezed; dispensers with a double bulb are capable of spraying continuously because the second bulb, which is not squeezed, distends with air when the first bulb is squeezed and acts as a pressure and flow equalizer.
The sudden dilation of the aorta by the blood ejected from the left ventricle is transmitted along the arterial system as a wave of elastic displacement of the arterial wall, usually referred to as the arterial pulse . The pulse is an index of the heart rate but also reflects the quantity of blood ejected into the aorta, the mode of its injection, the elasticity of the larger arteries, and the condition of the closed arterial system. It obviously provides important and readily obtainable information concerning the circulatory apparatus. Study of the arterial pulse has been a basis of diagnosing disease for many centuries.
The circulation through the pulmonary circuit is much simpler than the systemic circulation. It is confined to a single organ, the lungs; therefore there is little need for the regulation of blood flow (see fig. 1). Its pressure is low because, under normal circumstances, pulmonary arterioles are wide open and offer little resistance to flow, and nervous regulation of pulmonary arterioles is practically nonexistent. Consequently, the fall in pressure from the arteriolar side to the capillary side of the pulmonary circulation (pressure gradient ) is very low, less than 10 mm Hg.
The final destination of the circulatory system is the capillary network in both circuits. In it the exchange between blood and tissue, and between blood and air, takes place. The thin walls of the capillaries are a semipermeable membrane, a partition through which certain chemical substances can pass back and forth: gases,
water, and simple chemical substances, such as sodium, potassium, and glucose can leave or enter the blood as needed, but blood cells and large molecules (for example, proteins) remain in the blood. The capillaries thus act as an intermediary in two vital body functions: (1) blood-gas exchange, the acquisition of oxygen in the lungs and its delivery to the tissues coupled with the taking of carbon dioxide from the tissues and its excretion through the lungs (by means of a chemical reaction between the gases and hemoglobin, the red dye contained in the red blood cells); and (2) the physical process of regulating the water content in the tissues and blood and permitting an exchange of electrolytes (such as sodium and potassium) between tissue fluid and the blood. Water and chemical substances dissolved in the blood plasma can enter and exit as needed, driven by two forces: the osmotic pressure on the two sides of the capillary walls and the hydrostatic pressure within them. Each substance dissolved in the blood on one side of the capillary wall, and in the tissue fluid on its other side, reaches equal concentration in the two media. The pressure in the capillaries, about 25 mm Hg, maintains a fluid balance within the vascular system, as this pressure is identical with the osmotic pressure exerted by plasma proteins and electrolytes. If the hydrostatic pressure increases, or the osmotic pressure falls, then fluid leaves the vascular system, increasing the fluid content of tissues—a situation that may lead to edema (see chap. 5). A fall in hydrostatic pressure or increase in osmotic pressure draws fluid into the vascular system, raising the volume of circulating blood. These factors play an important role in maintaining blood volume at an optimal level. Water and solutes are quickly and efficiently exchanged in the capillary system.
Exercise
The circulatory system supplies oxygen and other vital substances to tissues of the body. Obviously, the demands on this supply line increase sharply during exercise. Human energy is customarily expressed in terms of oxygen consumption; oxygen consumption is to the body what gasoline consumption is to the automobile. Oxygen consumption is at its minimum during complete rest (resting oxygen consumption ) and measures in an average adult between
200 and 250 cc of oxygen per minute. This level (1 MET ) covers the essential metabolic needs of the body (basal metabolism ). The level of oxygen consumption rises steeply with activity since the oxygen cost of human effort is quite high. For example, walking at a moderate pace increases oxygen consumption to about three times its basal level. Strenuous exercise, such as climbing stairs briskly or running, may increase the basal demands for oxygen by as much as five to eight times. Maximum possible effort for a healthy individual occurs at the cost of 10 to 15 times the basal oxygen consumption; for a trained athlete it may reach 20 times the resting level. This high need for oxygen is one of the principal limiting factors of human exercise. The limitation depends on the possible top performance of the two principal systems involved in the process: respiration, supplying enough oxygen to the air spaces of the lungs; and circulation, delivering enough oxygen to the working tissues.
The burden of delivering 10 to 20 times the basal amount of oxygen to the tissues is considerable, and it is supportable only because of work-saving adaptive mechanisms. These mechanisms apply primarily to the circulatory apparatus, since the respiration is usually capable of increasing its work in proportion to high demands. The circulatory system is so designed that delivery of oxygen can increase much more than the work of the heart and circulation can. This is made possible through three adaptive mechanisms. First, in periods of high demand the circulatory system makes full use of the available oxygen supply. Normally during rest only a small part of the available oxygen is consumed by the tissues. Blood returning to the right side of the heart is, at rest, still 75 percent saturated with oxygen, indicating that only one-fourth of the available supply has been utilized. Better utilization of oxygen is an effective work-saving device for the heart. For example, if a certain form of exercise demands four times the resting amount of oxygen, the tissue can easily draw twice as much oxygen as during rest (reducing the oxygen saturation of the returning venous blood from 75 percent to 50 percent), in which case the volume of blood circulating through the tissues (cardiac output) has only to double instead of quadruple to meet the full demand. Second, blood can be redistributed (by way of regional flow regulation, as discussed earlier) so that the working muscles or organs get a greater share of the flow than do less important regions. Thus during exercise the digestive tract, the kidneys,
the brain, the skin, and other nonparticipating organs are perfused with less blood than during rest in order to supply the heart and the working muscles with more oxygen. Third, working muscles can perform temporarily with a smaller supply of oxygen than needed to meet actual energy demands. This "oxygen debt" is repaid immediately after exercise ceases. This mechanism is particularly important for short-term, high-intensity forms of exercise.
These adaptive mechanisms are essential, since the heart has a rather limited capacity for increasing its work. It is estimated that in a healthy individual, cardiac output can only increase to four or at most five times its resting level (from a normal of 5 liters per minute to 20 or 25 liters per minute). Thus, as a general rule, at peak cardiac performance the potential oxygen supply to the tissues rises fourfold; the tissues can extract up to four times as much oxygen from the blood during exercise than at rest; therefore top muscular performance is about 16 times the resting level when expressed in terms of oxygen consumption.
The following analogy may help illustrate the adaptive circulatory process during exercise. Let us imagine a large industrial plant with ample supplies of raw material but no storage facilities for its principal fuel, coal, which has to be brought in. The normal daily manufacturing activities of the plant require a quantity of coal equivalent to five carloads; however, since the shortest train consists of 20 cars, only one-quarter of each car is unloaded, thereby supplying the needs. At times the plant is called on to increase temporarily the manufacture of one of its principal products. The increased fuel demands are met in part by bringing in longer trains, and in part by unloading more coal from each car. The plant can also, to conserve fuel, slow down or eliminate the manufacture of some less essential product. If one assumes that the rail-loading facilities at the other end of the communication line limit coal delivery to 80 carloads a day, the maximum manufacturing capabilities of the plant would amount to 16 times its ordinary level, if all 80 carloads were brought in and all the coal in them unloaded.
Physiologists and clinicians often find it necessary to calculate the work of the heart, at rest and during exercise, in terms of work delivered (external work ) rather than the actual energy utilized by the heart. External work is expressed by the following formula: work equals blood pressure times cardiac output, or
W = P × F . The increase in work during exercise is directly related to the increase in cardiac output, since blood pressure shows little change, its level being well regulated by the previously described barostatic mechanism.
In spite of the efficient work-saving devices for the heart and the circulation operating in the healthy individual, exercise imposes a heavy strain on the circulatory system. Obviously, under conditions of less than perfect health, certain functions of the circulation begin to lag, and the efficiency of the circulatory adjustment may suffer. Exercise, which may reveal faulty circulatory function long before it becomes evident under resting conditions, thus provides one of the fundamental tests of the circulatory apparatus in the study of cardiac disease.