Sequelae of Chronic Valvular Disease
Chronic valvular disease involves permanent deformity of a cardiac valve or its neighboring structure. The defect may be congenital (present since birth) or acquired during life in a variety of ways.
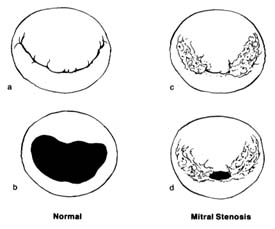
Figure 31. The mitral valve as seen from the left atrium. (a) Normal mitral valve, closed position,
showing two leaflets, one large and one small, covering the orifice completely. (b) Normal mitral
valve, open position. (c) Mitral stenosis, closed position, showing the valve with its peripheral
portion joined together at the commissures; the central opening still can close completely in
systole. (d) Mitral stenosis, open position; during diastole only a small central opening
permits blood flow, causing serious obstruction.
Stenosis of a valve, unless congenital, is always the result of a slow process, taking years or decades to reach the point of compromising heart function. Incompetence of a valve can develop abruptly or gradually: it may be caused by a disease of the valve itself, of the ring to which it is attached, or of the auxiliary structures supporting closure of the valve. The two left-sided valves—the mitral and aortic valves—are exposed to pressure several times higher than that exerted on the right-sided valves. The wear and tear on the valves as well as the fact that most cardiac diseases predominantly affect the left ventricle make these two valves more vulnerable. Furthermore, strain on the left ventricle from overload in valvular disease has more serious implications than does strain on the right ventricle.
Stenosis of the mitral valve (fig. 31), when significant, produces resistance to the blood flow during diastole from the left atrium to
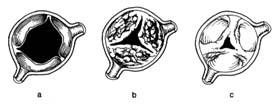
Figure 32. The aortic valve in its open position. (a) Normal valve.
(b) Aortic stenosis caused by calcium deposition producing stiffening
of the leaflets; the mobility of the valve is limited even though the
commissures are free. (c) Aortic stenosis caused by joining together of
the peripheral parts of its commissures, similar to mitral stenosis.
the left ventricle. To overcome the resistance and squeeze blood through the narrowed orifice, pressure in the left atrium has to rise (typically to 25 mm Hg instead of the normal 10 mm Hg), whereas the diastolic pressure in the left ventricle, normally identical with that in the atrium, remains unchanged. Mitral stenosis thus produces a pressure gradient across the valve, the magnitude of which depends on the severity of stenosis. High pressure in the left atrium makes the pressure rise in the pulmonary blood vessels, leading to pulmonary hypertension, which in turn overloads the right ventricle and may cause its failure. This mechanical dam at the mitral orifice has an effect on circulation through the lungs similar to that of left ventricular failure (see chap. 5).
Stenosis of the aortic valve (fig. 32) causes resistance of the ejection of blood into the aorta during ventricular systole, which has to be overcome by increased pressure in the left ventricle. Normally during systolic ejection pressures in the left ventricle and the aorta are identical. Aortic stenosis causes a pressure gradient across the aortic valve. For example, in severe aortic stenosis the pressure needed to eject blood into the aorta (which itself has a pressure of 120 mm Hg) may be as high as 240 mm Hg (a gradient of 120 mm Hg), doubling the systolic workload of the left ventricle and leading to its hypertrophy.
In mitral regurgitation the left ventricle, while ejecting blood into the aorta, also pumps some blood back into the left atrium. If the volume of backflow is appreciable—sometimes equal to the
forward flow into the aorta—the left ventricular workload is significantly increased.
In aortic regurgitation the incompetent aortic valve cannot keep blood from being sucked back into the left ventricle during diastole. In severe cases the amount of blood ejected by the left ventricle may be double or triple the normal cardiac output so as to compensate for the backflow, resulting in volume overload and hypertrophy.
In general, then, the effect of valvular disease on the heart is an increase in workload. In contrast to the cardiac consequences of coronary-artery disease, where the heart muscle is damaged and weakened, increased workload produces compensatory hypertrophy of the muscle of the affected ventricle. Thus the cardiac pump becomes stronger than normal, permitting the affected person to lead a normal life for many years. Disability develops late, when hypertrophy of the myocardium can no longer cope with the high workload. Valvular disease is the ideal target for surgical removal of the cause of overload. Not only can surgery correct the disabling symptoms and manifestations of heart failure in such cases, but it may lead to regression of cardiac hypertrophy as well.
The notion of dilating a stenotic mitral valve was expressed as early as 1900, though the means for doing so were not then available. In 1948 surgical technique reached the point where mitral valvotomy could be successfully performed. Throughout the 1950s stenotic valves were dilated by placing fingers or instruments into the beating heart. Mitral stenosis was the most frequent target for so-called closed-heart surgery. Closed mitral valvotomy produced great successes—even today the operation is performed on selected younger patients. (Operations on stenotic aortic and pulmonary valves were less successful and have been abandoned in favor of open-heart repair.)
But during closed operations on a beating heart no actual repair of incompetent valves was possible. The next breakthroughs in valvular surgery came with the introduction of the pump-oxygenator, which made open-heart surgery possible, and then the development of functioning prosthetic valves.
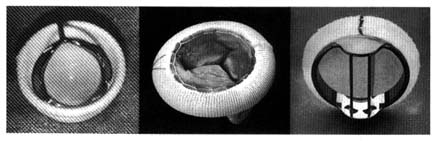
The first successful prosthesis consisted of a plastic ball within a metal cage (fig. 33, left). With the heart stopped, its functions temporarily assumed by the pump-oxygenator, the damaged valve
Figure 33. Examples of prosthetic cardiac valves. Left: Prototype mechanical ball
valve introduced in the early 1960s. Center: Biological valve taken from a pig's
left ventricle mounted on a specially designed ring.
Right: A mechanical tilting-disk valve.
was removed and the prosthetic valve sutured into the aortic orifice. Once the heart was restarted, the ball was propelled to the top of the cage by left ventricular contraction, allowing the blood to enter the aorta by passing around it. Ventricular relaxation pulled the ball back into the base, sealing the orifice. A similar ball valve was used to replace faulty mitral valves, with the cage inside the ventricle: ventricular relaxation pulled in the ball and opened the valve, permitting blood to flow from the atrium to the ventricle. Systole forced the ball back to its base, closing the mitral orifice.
Prototype ball valves worked well, with some patients surviving for more than 20 years. Yet the many technical problems and complications spurred the development of new devices. A new approach was introduced a decade after the ball valves, namely the use of biological valves, consisting of human or other animal tissue. The most successful and widely used biological valve is the aortic valve of a pig (porcine heterograft), mounted into a special frame that can be sutured into the mitral or aortic orifice (fig. 33, center). This three-leaflet valve is an ideal substitute for the aortic valve, but it also works well in the mitral position. Modifications of ball valves led to improvements that reduced some of the untoward consequences of the earlier designs. Other prosthetic valves now widely used are fitted with hinged discs to control bloodflow (fig. 33, right).
The major disadvantage of prosthetic valves is that as foreign bodies they are a prime site for the formation of blood clots. These clots may interfere with valvular function, but more often they
represent a potential source of emboli, which can cause stroke or other complications. Hence patients with prosthetic valves must remain on anticoagulant therapy throughout their lives. Biological valves greatly reduce the risk of thrombus formation; here anticoagulant treatment is administered only in selected cases. The disadvantage of biological valves, however, is their low durability, for the valve may stiffen and even calcify. The average duration of their normal function is seven to ten years, after which replacement is usually necessary. In children and young adults the process of biological-valve deterioration is greatly accelerated, so that their use in this age group is avoided.
Valvular surgery represents one of the most important advances in the treatment of heart disease, yet there are still many unsolved problems. None of the presently available replacement valves can function as efficiently as the normal natal valve. In addition to clot formation, a number of other complications may develop after successful surgery. And the risk of surgery is not negligible: surgical mortality is estimated at 5–10 percent and may be even greater in high-risk patients. Consequently, deciding the optimal time for valvular surgery is often difficult. Patients in heart failure or seriously disabled by a valvular disease are prime candidates for surgery. Those whose symptoms are still mild enough to allow a reasonably active life may best manage with nonsurgical medical treatment. Early operations in asymptomatic patients aimed at preventing future problems are inadvisable except in special circumstances. In considering candidates for valvular surgery, the physician usually takes into account the patient's occupation and life-style as well as the prognoses with and without surgery.