CONTRIBUTORS
Sarah G. Allen, Point Reyes Bird Observatory, 4990 Shoreline Highway, Stinson Beach, CA 94970
George A. Antonelis, National Marine Mammal Laboratory, NOAA, National Marine Fisheries Service, 7600 Sand Point Way, Seattle, WA 92115
Tom A. Arnbom, British Antarctic Survey, Natural Environment Research Council, High Cross, Madingley Road, Cambridge CB3 OET, United Kingdom, and Department of Zoology, Stockholm University, Stockholm, Sweden
Tomohiro Asaga, Tokyo University of Fisheries, 4-5-7 Kohnan Minato-ku, Tokyo 108, Japan
Marthan N. Bester, Mammal Research Institute, University of Pretoria, Pretoria 0002, South Africa
Ian L. Boyd, British Antarctic Survey, Natural Environment Research Council, High Cross, Madingley Road, Cambridge CB3 OET, United Kingdom
Michael M. Bryden, Department of Veterinary Anatomy, University of Sydney, NSW 2006, Australia
Harry R. Burton, Australian Antarctic Division, Channel Highway, Kingston, Tasmania 7050, Australia
Michael A. Castellini, Institute of Marine Sciences, University of Alaska, Fairbanks, AK 99775
C. Chambers, Sea Mammal Research Unit, Natural Environment Research Council, High Cross, Madingley Road, Cambridge CB3 OET, United Kingdom
Walter L. Clinton, 5844 NE 75th St., No. A312, Seattle, WA 98115
Daniel P. Costa, Department of Biology and Institute of Marine Sciences, University of California, Santa Cruz, CA 95064
Daniel E. Crocker, Department of Biology, University of California, Santa Cruz, CA 95064
Robert L. DeLong, National Marine Mammal Laboratory, NOAA, National Marine Fisheries Service, 7600 Sand Point Way, Seattle, WA 92115
Charles J. Deutsch, Department of Biology, University of California, Santa Cruz, CA 95064
Michael A. Fedak, Sea Mammal Research Unit, Natural Environment Research Council, High Cross, Madingley Road, Cambridge CB3 OET, United Kingdom
Clifford H. Fiscus, National Marine Mammal Laboratory, Alaska Fisheries Science Center, National Marine Fisheries Service, 7600 Sand Point Way, Seattle, WA 92115
J. Harwood, Sea Mammal Research Unit, Natural Environment Research Council, High Cross, Madingley Road, Cambridge CB3 OET, United Kingdom
Roger D. Hill, Wildlife Computers, Woodinville, WA 98072
Mark A. Hindell, Department of Zoology, University of Tasmania, P.O. Box 252c, Hobart, Tasmania 7001, Australia
Harriet R. Huber, National Marine Mammal Laboratory, NOAA, National Marine Fisheries Service, 7600 Sand Point Way, Seattle, WA 92115
Ronald J. Jameson, U.S. Fish and Wildlife Service, National Ecology Research Center, Piedras Blancas Research Station, P.O. Box 70, San Simeon, CA 93452
Vicky Lee Kirby, Department of Biology, University of California, Santa Cruz, CA 96064
Richard M. Laws, St. Edmund's College, University of Cambridge, Cambridge CB3 OBN, United Kingdom
Burney J. Le Boeuf, Department of Biology and Institute of Marine Sciences, University of California, Santa Cruz, CA 96064
Mark S. Lowry, Southwest Fisheries Science Center, National Marine Fisheries Service, La Jolla, CA 92038
T. S. McCann, British Antarctic Survey, Natural Environment Research Council, High Cross, Madingley Road, Cambridge CB3 OET, United Kingdom
B. J. McConnell, Sea Mammal Research Unit, Natural Environment Research Council, High Cross, Madingley Road, Cambridge CB3 OET, United Kingdom
Patricia Morris, Department of Biology, University of California, Santa Cruz, CA 95064
Yasuhiko Naito, National Institute of Polar Research, 9-10, Kaga l-Chome, Itabashi-ku, Tokyo 173, Japan
Nadav Nur, Point Reyes Bird Observatory, 4990 Shoreline Highway, Stinson Beach, CA 94970
C. Leo Ortiz, Department of Biology, University of California, Santa Cruz, CA 96064
Joanne Reiter, Institute of Marine Sciences, University of California, Santa Cruz, CA 96064
Haruo Sakurai, National Institute of Polar Research, 9-10, Kaga l-Chome, Itabashi-ku, Tokyo 173, Japan
David J. Slip, Australian Antarctic Division, Channel Highway, Kingston, Tasmania 7050, Australia
Brent S. Stewart, Hubbs-Sea World Research Institute, 1700 South Shores Road, San Diego, CA 92109
William J. Sydeman, Point Reyes Bird Observatory, 4990 Shoreline Highway, Stinson Beach, CA 94970
Philip H. Thorson, Department of Biology, University of California, Santa Cruz, CA 95064, and Hubbs-Sea World Research Institute, 1700 South Shores Road, San Diego, CA 92109
Ian S. Wilkinson, Sea Fisheries Research Institute, Private Bag X2, Rogge Bay 8012, South Africa
Pamela K. Yochem, Hubbs-Sea World Research Institute, 1700 South Shores Road, San Diego, CA 92109
One—
Elephant Seals:
An Introduction to the Genus
Burney J. Le Boeuf and Richard M. Laws
ABSTRACT. The two species of elephant seals, M. angustirostris and M. leonina , inhabit different parts of the world, in opposite hemispheres and contrasting climatic zones, they breed at different times of the year, and they differ in size and elaboration of male secondary sexual characteristics. The northern species is lacking in genetic variation, apparently the result of an extreme population bottleneck caused by sealing in the last century; the population has increased rapidly in this century. The southern species has decreased in overall number over the last half century. There are three (or four) stocks, with little genetic interchange; the major stock appears to be stable, others have decreased, and one population is increasing. Despite these differences and thousands of years of separation without intermingling, the two species are remarkably similar in morphology, behavior, and life history traits serving reproduction and the diving pattern.
The aim of this chapter is to provide information on the origins, nomenclature, and key features in the ecology and life history of elephant seals that will serve as background for the specialized chapters that follow. Research on both species has been both intensive and extensive. The more accessible northern species has been particularly closely studied at Año Nuevo Island, where large cohorts of known-age marked animals provide longitudinal data, and intensive year-round studies of behavior have been made. Research on the southern species has been concentrated on the breeding season because the remote and rigorous environment in which it lives has made year-round studies more difficult. Nevertheless, a recent review remarked that it "is one of the most exhaustively and widely studied pinnipeds" (Ling and Bryden 1992).
While the northern species' breeding range is comparatively limited, with extensive interchange between colonies, the southern elephant seal has a number of separate populations, with limited interbreeding, over a cir-
cumpolar range and in a broad range of latitudes (Laws, this volume). Its population ecology is more varied.
Origins
Elephant seals are the largest in size of the 34 extant species of pinnipeds (King 1983). There are two species in the genus Mirounga , the northern elephant seal, M. angustirostris , and the southern elephant seal, M. leonina . The genus is in the family Phocidae, the true seals, as distinct from the other two families in the suborder Pinnipedia: Otariidae, the fur seals and sea lions, and Odobenidae, the walrus.
Northern elephant seals are distributed along the west coast of North America from mid-Baja California, Mexico, to the eastern Aleutian Islands in Alaska (Stewart et al., this volume). The distribution of southern elephant seals is circumpolar on island and mainland sites, from the Antarctic continent to Patagonia, but concentrated on subantarctic islands (Laws, this volume).
The origins of elephant seals, like all pinnipeds, are obscure, but there is general agreement that phocids originated in the Tethyan-Mediterranean part of what is now Asia during the middle Miocene, about 15 to 20 million years ago (King 1964; Hendey 1972; Hendey and Repenning 1972; Repenning 1980). J. L. Davies (1958), drawing heavily on J. E. King (1956), argues that Antarctic seals derived from ancestral monachinoid seals that spread southward to the Caribbean at the time of seawater cooling in the Miocene and thus took advantage of the absence of the Central American isthmian barrier and entered the East Pacific cold-water route to the south. C. A. Repenning (1980) also thinks that phocid seals first invaded the south during the Miocene, approximately 10 million years ago. The earliest fossil records of seals in Argentina and South Africa date from this time.
J. L. Davies (1958) and Q. B. Hendey (1972) argue that the elephant seal genus developed in the Antarctic and the present species, M. leonina , colonized most of the anti-Boreal zone. They reason that elephant seals reinvaded the Northern Hemisphere by retracing the ancestral route along the west coast of South America during a Pleistocene glacial age, a time when passage to the Caribbean was closed. When the rewarming of the seas occurred, the group in the lower California region, which we now know as M. angustirostris , was cut off from the main elephant seal population to the south. According to Hendey (1972:108), "Mirounga angustirostris can thus be regarded as a relict species, surviving in isolation far from the origins of the genus."
K. T. Briggs and G. V. Morejohn (1976) present a different interpretation. They argue that the presence of fossil elephant seals in California and relative primitiveness of the northern species (more complicated teeth and reduced sexual dimorphism) are inconsistent with a putative southern
origin. They propose that the genus originated in the subtropics from unspecialized ancestors of modern monk seals. Ancestors of Mirounga entered the Pacific by way of the Central American Seaway. Uplift of the Tertiary Central American Seaway and poleward retreat of elephant seal populations, due to climatic conditions, led to geographic isolation, speciation, and the present distribution of the two species. According to this interpretation, the northern species is the older of the two. The southern species either evolved directly from the northern congener or they shared a common ancestor.
The poor fossil record does not permit an adequate test of these competing hypotheses. It is also difficult to say how long the two species have been separated. Perhaps the separation was as recent as the last major glaciation, which ended about 5 to 10 thousand years ago, or possibly it began as long ago as the early Pleistocene. Elephant seals were in California 100 to 130 thousand years ago as revealed by fossils found in the San Diego formation (Miller 1971), about the time of the beginning of the last glaciation. Other fossil material from California may be from 1 to 4 million years old, but whether the material is definitely Mirounga is in question (C. A. Repenning, pers. comm.). That the two species are considered distinct and differ in many ways argues for a separation long enough to have permitted the evolution of different structures and behaviors. The distance separating the breeding ranges of the two species today is more than 8,000 km, and there is no evidence of intermingling.
Nomenclature
Whether the ancestors of elephant seals headed north after entering the Pacific or headed south to the Antarctic, or took both directions, remains moot, but it is clear that the name for the genus has a southern origin. Mirounga is from miouroung , an old Australian Aboriginal name for elephant seals. A more fitting name, Macrorhinus , was proposed by Cuvier in 1824, but the name had already been given to an insect, so Mirounga , suggested by J. E. Gray (1827), prevailed.
The species names have even more frivolous origins (King 1964; Le Boeuf 1989). T. Gill (1866) proposed angustirostris , or narrow nose, for northern elephant seals on the coasts of western North America, to differentiate them from leonina in the Antarctic. He was impressed by the "peculiarly narrowed and pronounced snout" of the only northern specimen he had for examination, a female skull. This is ironic because the most outstanding feature of the animal is the elaborate nasal appendage of the male, not the subtle anatomical character of the female skull. Linnaeus's name for the southern species, leonina , was based on Lord Anson's erroneous description (Linnaeus 1758); the southern species bears no resemblance to a lion or a sea lion.
Even among the common names, there are errors and inconsistencies that have come into common usage. Calling adult elephant seals "bulls" and "cows" and the newborns "pups" is the legacy of sealers. Seals breed on "rookeries," a term first used to describe the breeding grounds of gregarious birds, and polygynous male seals defend "harems" of females.
Appearances
Elephant seals are extremely sexually dimorphic, fully adult males being up to ten times larger than adult females (figs. 1.1 and 1.2). Males also have distinctive secondary sexual characteristics besides size which females lack—an enlarged proboscis and thick skin on the sides and underside of the neck, ruddy and more rugose in the northern species. The short pelage of adults varies from gray to brown, except for immediately following a drastic annual molt when the pelage takes on a gray or silver hue. The elephant seals share with the monk seals, Monachus sp., a drastic molt process unlike that of any other mammal, during which the hairs are shed attached by their roots to large sheets of molted epidermis (Ling and Bryden 1992; Worthy et al. 1992). Pups have a woolly black natal pelage that is molted at 4 to 6 weeks of age and replaced by a silver-gray coat (Laws 1956a ). The new hair pushes the old coat upward and out. The new hair is short and flat but grows to a length of about 12 to 14 mm during the two weeks after the old coat has been shed. (The duration and location of molting are discussed below.) The fusiform body has been modified by selection to reduce drag in the water; the male penis and testicles and female mammary glands are internalized when not in use.
Similarities and Differences between the Two Species
The close relationship between the two species is at once obvious to the casual observer from the similarity in size, appearance, and movements. Behavior of the two species is, on the whole, remarkably similar on land and at sea. For example, the social organization of males competing for access to females during the breeding season is similar. We describe these similarities in more detail in later sections.
Laws (1956a ) described the behavior of a small population of southern elephant seals in the South Orkney Islands, breeding on sea ice in spring and present under fast ice, in which they kept open breathing holes, in early winter. Similar behavior occurs in the South Shetland Islands, and elephant seals have been seen hauled out on pack ice (R. M. Laws, unpubl. observ.). This is in extreme contrast to some subtropical breeding locations of the northern species, for example, desert islands on the west

Fig. 1.1
A northern elephant seal bull bellows a threat vocalization to a competitor.
An adult female is in the foreground, and others are in the background.
Photograph by Franz Lanting.

Fig. 1.2
Two southern elephant seal bulls fighting at Península Valdez, Argentina.
Photograph by Burney J. Le Boeuf.
coast of Baja California, Mexico. The dive depths and dive durations during transit and foraging are remarkably similar despite occurring in disparate parts of the ocean (Le Boeuf et al. 1988; Le Boeuf, this volume; Stewart and DeLong 1991; Stewart and DeLong, this volume; Hindell 1990; Fedak et al., this volume).
Differences between the species have not been subjected to special study; we describe the most obvious. The southern male is longer and heavier than its northern counterpart. Southern bulls in harems weigh 1,500 to 3,000 kg, with maximum weights reaching 3,700 kg (Ling and Bryden 1981). The largest northern males weigh 2,300 kg (Deutsch, Haley, and Le Boeuf 1990). Females of the two species do not appear to differ significantly in mass. Southern females range widely in mass from 350 to 800 kg shortly after giving birth, with most of them in the range 400 to 600 kg (Fedak et al., this volume). Northern females have a postpartum mass ranging from 360 to 710 kg (Deutsch et al., this volume). The mean mass of northern pups, however, may exceed that of southern pups at weaning, 131 kg versus 121 kg (Le Boeuf, Condit, and Reiter 1989; Deutsch et al., this volume; Fedak et al., this volume), perhaps owing to a difference in suckling duration (Le Boeuf, Whiting, and Gantt 1972; Laws 1953b ; McCann 1980). However, at South Georgia, mean male weaning mass in four years ranged from 118.7 to 137.2 kg (SCAR 1991), and more southerly populations of M. leonina have higher weaning weights; for example, R. M. Laws (1953b ) found weaning weights of about 200 kg at Signy Island in 1948–1949, and H. Burton (pers. comm.) reports high weaning weights at King George Island.
Laws (1953b ) and R. Carrick, S. E. Csordas, and S. E. Ingham (1962) report southern bulls measuring 6.2 m, and others refer to males of 7.62 to 9.14 m; the early records, however, probably included hind flippers and are not comparable (see Scheffer 1958). C. M. Scammon (1874) reports a northern male that measured 6.71 m, but the longest northern bull measured in recent times was 5.03 m long (B. Le Boeuf, unpubl. data). The average lengths of males seem to be less disparate across species; Laws (1960) estimated 4.72 m for southern males, and mean estimates for northern males range from 4.33 m (Deutsch et al., this volume) to 4.48 m (Clinton, this volume).
Some morphological differences between the species beg explanation. For example, the southern elephant seal can bend its body backward into a Ushape over a much greater angle than the northern elephant seal. Perhaps because of this difference, southern males also seem to be able to rear up higher during fighting than northern males. Paradoxically, despite greater sexual dimorphism in skull characteristics in southern elephant seals, the fleshy exterior of the northern species is more sexually dimorphic. The proboscis of the northern male is larger and the integumentary neck and
chest shield are more highly developed than these features in the southern male (Murphy 1914; Laws 1953b ).
Developmental differences in early life deserve further study. Southern pups are weaned at 22 to 23 days of age (Laws 1953b ; McCann 1980; Campagna, Lewis, and Baldi 1993); northern pups are weaned at 24 to 28 days, nursing duration increasing with the age of the mother (Reiter, Panken, and Le Boeuf 1981). Some southern elephant seal pups begin molting while still suckling; a small percentage molt in utero (Laws 1953b , 1956a ; Carrick et al. 1962; Le Boeuf and Petrinovich 1974). For pups at Signy Island, Laws (1953b ) reports that the molt lasts about 24 days and is completed by 30 to 38 days of age. The molt in northern elephant seal pups is equally long but does not begin until after weaning, at about 28 days of age; the process begins slightly later in males than in females (Reiter, Stinson, and Le Boeuf 1978).
In the southern elephant seal, weaned pups fast for an average of 37 days, and the duration of the postweaning fast increases linearly with increasing weight at weaning. Pups continue to fast until they reach a lower weight threshold of about 70% of weaning weight (Wilkinson and Bester 1990). The duration of the fast in the Northern Hemisphere varies with the date of weaning. Pups weaned early fast for a mean of 73.5 ± 7.6 days; pups weaned late in the season fast for a mean of 55.6 ± 13.2 days (Reiter, Stinson, and Le Boeuf 1978). As in the south, the duration of the fast is positively correlated with weaning mass, and at departure from the rookery, pups have lost an average of 25 to 30% of their weaning weight. Some pups of both species remain near the rookery for up to 2 to 2 1/2 months before going to sea for the first time (Reiter, Stinson, and Le Boeuf 1978; Condy 1979).
The threat vocalizations of males are quite distinct, evidently a divergence that has come about with geographic separation (Le Boeuf and Petrinovich 1974). The threat call of northern males is composed of 3 to 20 discrete expulsive, low-frequency bursts that are emitted at a relatively constant rate, with some individuals adding a longer embellishment to the beginning or end of the call. The mean duration of the call is 6.8 ± 2.5 seconds. In contrast, the threat vocalizations of southern males are more than twice as long (mean = 19.1 ± 8.3 seconds) and are composed of long roars that vary in pitch and loudness, the result of a sound being produced during inspiration as well as during expiration.
The near-annihilation of the northern elephant seal population by sealers in the last century, compared to the less destructive sealing efforts in the larger and more widely distributed elephant seal populations in the Southern Hemisphere, has apparently resulted in differences in genetic variation. The extreme population bottleneck experienced by the northern species—a reduction to less than 100 individuals breeding on only one
island in the late 1880s—is interpreted as being responsible for lack of allozyme diversity and reduced DNA sequence diversity in two mtDNA regions, relative to southern elephant seals (Bonnell and Selander 1974; Hoelzel et al. 1993).
Terrestrial Habitat
The traditional rookeries and haul-out sites of elephant seals are islands or remote continental shores. Preferred breeding and resting sites are usually located on gradually sloping, sandy beaches or sand spits. Given similar accessibility, elephant seals settle first on beaches with a fine sandy substrate, next on pebbles, and as a last resort on boulders or rocky shores. A sandy substrate is ideal for the caterpillarlike movements of these large animals. Moreover, in both species, flipping damp sand, loose dirt, or small damp pebbles on their backs aids in temperature regulation on warm days (Laws 1956a ; White and Odell 1971; Heath and Schusterman 1975).
The Annual Cycle on Land
The annual cycle of the two species is similar except that the time scale is shifted (fig. 1.3). M. leonina breeds in the austral spring (early September to mid-November), while M. angustirostris breeds in the northern winter (December to February). Temporal intervals between reproductive events are similar (Carrick et al. 1962; Le Boeuf and Petrinovich 1974). There are slight shifts in peak events, such as the maximum number of females during the breeding season, among rookeries distributed widely across latitudes. This is especially well documented in the southern elephant seal (Laws 1956a ; Condy 1979; McCann 1985; Hindell and Burton 1988). As one proceeds from northern rookeries such as Peninsula Valdez, Argentina, to southern ones like the islands of King George and South Georgia (across 22 degrees of latitude or about 1, 736 km), the peak number of females is delayed by 22 days, from October 3 to October 25 (McCann 1985; Campagna, Lewis, and Baldi 1993). Variation in the onset of the season in the northern species, from one rookery to the next, has not been reported, but differences, if they exist, are thought to be slight.
The annual cycle of northern elephant seals at Año Nuevo, California, seems to be typical of that of other colonies of seals in the Northern Hemisphere (with the possible exception of numbers during the female and juvenile molt; see fig. 1.3a) but differs in some respects from southern colonies, not only in the shift in seasons but in the timing of haul-out in different age and sex groups. The annual cycle is described below for Año Nuevo and for Macquarie Island.
Northern Species
It is useful to divide the annual cycle into four terrestrial phases: breeding season, female and juvenile molt, male molt, and juvenile haul-out. The relative numbers present on the rookery during each phase is shown in figure 1.3a.
Breeding Season:
December to Mid-March
The breeding season at Año Nuevo begins in early December with the arrival of the adult males. Usually, the older bulls arrive first, and all serious competitors are on the rookery by the end of December. Concurrent with the arrival of adult males, there is a rapid decline in the number of juveniles, 1 to 4 years old, that previously predominated on the rookery. Pregnant females begin arriving in mid-December, reach a peak during the period from January 26 to February 2, and then their numbers decline until all of them have returned to sea by the end of the first week in March (fig. 1.4). Younger males begin leaving the rookery in late February, but the larger bulls remain on the rookery until the end of March, long after the last female has departed.
The pupping period is from about the third week in December to the end of the first week in February. Copulations occur from the first week in January through the first week in March, with February 14 being the peak day of copulation frequency.
Female and Juvenile Molt:
Mid-March through May
As the last females wean their pups and return to sea, the first females that gave birth early in December begin returning from sea in mid-March to molt, a process that takes about one month to complete. This influx of adult females continues for about two months. The adult females are joined by juveniles, 1 to 4 years old, of both sexes. The highest number of animals present on the rookery are seen in late April. Present in the spring but declining in number are adult males, all of whom return to sea by the end of March, and the newly weaned pups, 80% of whom leave the rookery by the end of April. Rather suddenly, in early to late May, there is a rapid decline in total numbers on the rookery.
Male Molt:
June through August
The lowest number of animals are observed on the rookery in June, July, and August (fig. 1.3a), when breeding-age males molt. There is a tendency for the younger pubertal males, 5 to 6 years old, to arrive in early summer, and they are followed by the older males in late summer (fig. 9.1, this volume). A few young of the year and 1 ½-year-olds are observed early in this period, but they make up less than 5% of the total number of seals in residence. Juvenile numbers begin to increase in August, and in some years, they outnumber molting males by the end of the month.
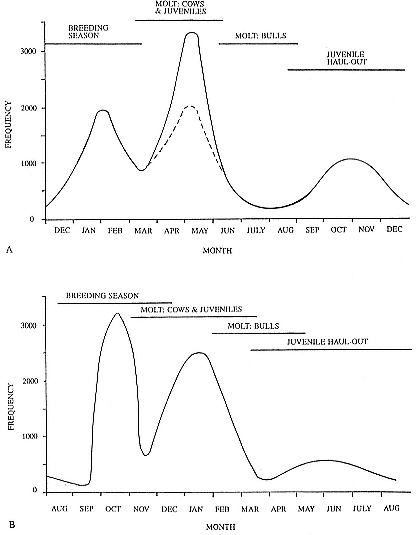
Fig. 1.3
A schematic representation of the annual cycle of northern and southern
elephant seals showing the relative number of animals present throughout the
year. The times of breeding and other events during the annual cycle are
indicated by horizontal bars. (A) The relative number of northern elephant
seals on a rookery such as Año Nuevo Island during the late 1970s (solid line).
The dashed line indicates that for some rookeries (e.g., San Nicolas and San
Miguel), the number of animals during the molt of cows and juveniles is
nearly the same as the number observed during the breeding season
(Le Boeuf and Bonnell 1980). (B) The relative number of southern elephant
seals present on a typical rookery during the annual cycle (based on
data from Laws 1956a and Hindell and Burton 1988).
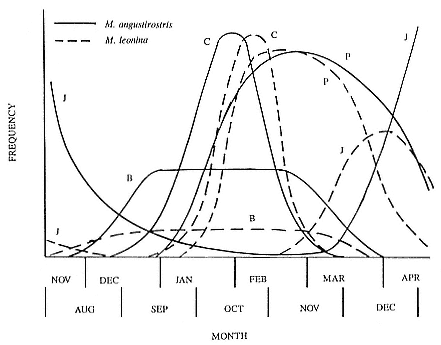
Fig. 1.4
A schematic diagram showing the increase and decrease in categories of elephant
seals present during the breeding season on Año Nuevo Island (solid line;
adapted from Le Boeuf 1972) and a typical rookery of the southern elephant seal
(dashed line; adapted from Laws 1956a and Hindell and Burton 1988).
Juvenile Haul-Out:
September through November
By the first week in September, all but a few males have gone to sea to feed and fatten up for the approaching breeding season. Young of the year and juveniles up to four years of age increase steadily in number to a peak in October. At Año Nuevo, many of these juveniles are immigrants from southern rookeries. Some yearlings exhibit a pathological skin and pelage called "scabby molt," but as a rule, normal molting does not occur during this period. Pubertal, subadult males begin to arrive on the rookery during the middle of November, a time when juveniles are decreasing in number.
The schematic representation of the total number of elephant seals present on Año Nuevo throughout the year (fig. 1.3a) shows the relative number of animals present in each phase of the annual cycle. The temporal patterning of peaks and troughs in total animals, as well as the relative size of the peaks to each other, has varied little at this rookery during the last two decades. Elephant seals are in residence throughout the year, but their
numbers fluctuate greatly because animals of both sexes and different age groups are moving in and out predictably.
The pattern in figure 1.3a resembles that observed at other northern elephant seal colonies, such as San Nicolas, San Miguel, and Santa Barbara islands (Odell 1974; Bonnell et al. 1979), during the same period that Año Nuevo was monitored (Le Boeuf 1981). The difference is that the female-juvenile molt numbers are equal to or only slightly higher than breeding season numbers. This is because animals from southern California rookeries may haul out to molt in a different place from where they breed, showing up to be counted on northern California rookeries like Año Nuevo. (Similarly, some South Georgia elephant seals haul out to molt at Signy Island, Elephant Island, and South Shetland Islands; Macquarie Island animals haul out on the Antarctic continent. Also, bulls haul out to molt in limited numbers at Año Nuevo compared with colonies of the southern species.)
Southern Species
Information for the southern species is drawn from R. M. Laws (1956a ), R. Carrick et al. (1962), and M. A. Hindell and H. R. Burton (1988) (fig. 1.3b).
Breeding Season:
August to Mid-December
At Macquarie Island, the adult males arrive in early August, and all serious competitors are present by mid-September (fig. 1.4). Small numbers of juveniles (females of 1 to 4 years; males of 1 to 6 years) haul out in winter but decline to near zero by the end of August. Pregnant females begin to arrive in mid-September, reaching a peak about October 16, subsequently declining so that all have returned to sea by the end of November. Adult bulls remain until mid-December. The pupping period is from late September to the end of October. Copulations occur from mid-October to mid-November, with peak frequency in early November.
Female and Juvenile Molt:
January and February
Mature females begin returning from sea about the beginning of January, to molt for about one month, reaching peak numbers about the end of January; all have returned to sea by the end of February. The juvenile haul-out begins much earlier, in mid-November, reaches a peak about mid-December, and ends in late January.
Male Molt:
November to April
Subadult males (6 to 8 years old) molt from early November to early March, with a peak at the end of December. Adult males haul out for molt from early February until May, with a peak in mid-March. The lowest numbers are observed on the rookeries in winter, July to August, representing a haul-out of adult males and juveniles.
Juvenile Haul-Out:
Mid-March to the End of August
By the end of August, males have completed their molt and gone to sea to feed and lay down blubber for the breeding season. At Macquarie Island, there is a winter haul-out of juveniles from April to August, with peak numbers in early June less than half of the peak molting haul-out. At Macquarie, juveniles are present ashore for a greater proportion of the year than any other population component. At Signy Island and South Georgia, there is no significant winter haul-out.
At Signy Island, because the breeding population is small, the highest numbers are ashore molting in the last week of January, representing immigrants from South Georgia. But at Macquarie Island and South Georgia, the largest numbers are present in the breeding season.
Thus, the general haul-out cycles for northern and southern elephant seals are broadly similar in the breeding season, but there are significant differences in detail throughout the rest of the year. The overall breeding season is longer in the southern species, mainly due to earlier haul-out of the bulls, but the pupping season is more synchronous with a more pronounced peak in numbers of cows; weaned pups depart to sea earlier (fig. 1.4). The female and juvenile molt is slightly shorter and later at Macquarie Island than at Año Nuevo. The period when males are ashore for the molt is twice as long at Macquarie as at Año Nuevo, starting earlier and ending earlier at Macquarie; also, few adult males molt at Año Nuevo (i.e., only the males that breed there). The juvenile haul-out is similar in the two locations, although slightly longer and earlier at Macquarie. At South Georgia and Signy Island, south of the Antarctic Convergence, it would be expected to be earlier than at Año Nuevo or Macquarie owing to lower air temperatures (and pack ice around Signy Island) from April onward. The limited data confirm this (Laws 1956a ).
Some colony and species differences in the shape of the annual cycle of total animals are to be expected given that there are differences in molting locations, in the time that food is abundant, and in the distance of prey from the rookery. By and large, the presence of elephant seals on traditional rookeries and hauling grounds is predictable by age and sex.
Time at Sea
It is useful to consider the time at sea from the individual point of view. This varies with age and sex (fig. 13.1, this volume). In northern elephant seals, pups make their first contact with the water within two weeks of weaning at about six weeks of age, learning to swim and dive in shallow water near the rookery, until they depart on their first trip to sea at 3 ½ months of age (Reiter, Stinson, and Le Boeuf 1978; Thorson and Le Boeuf, this volume). After 4 to 5 months at sea, they return to the rookery for
about one month in the fall, then return to sea for another 4 to 5 months. Both sexes take two trips to sea per year until they undergo puberty. When they come of reproductive age, the fall haul-out is skipped, and the seal remains at sea for 11 months of the year. Thereafter, the adult pattern is adopted. Females make a postlactation trip to sea lasting a mean of 72.6 ± 5.0 days, followed by a one-month molt on land, followed by another 8-month trip to sea over the course of pregnancy. Thus, adult females are at sea for 10 months of the year (Le Boeuf et al. 1988; Le Boeuf, this volume). Adult males also make two trips to sea per year but spend less total time at sea, 8 months, than females (Le Boeuf, this volume; Stewart and DeLong, this volume). Southern elephant seals appear to hold to a similar schedule at sea (Laws 1956a ; Hindell and Burton 1988).
Research on the migratory paths and location of foraging areas of both species is in progress (Hindell, Slip, and Burton 1991; DeLong, Stewart, and Hill 1992; Bester and Pansegrouw 1992; McConnell, Chambers, and Fedak 1992; Le Boeuf et al. 1993) and is treated in several chapters in this volume (chaps. 5, 13, 16, and 20).
Reproduction
Social Organization and Mating Behavior
Bulls are belligerent when they arrive on the rookery at the start of the breeding season, threatening and fighting with each other in areas where females will settle to give birth. When pregnant females begin arriving, they seek each other out for safety from the sexually aggressive males and gather in groups, or harems. The result of the fierce, bloody encounters between males is a dominance hierarchy at each breeding area (Le Boeuf and Peterson 1969; Le Boeuf 1974; McCann 1981). This social structure effectively reduces access to the grouped females to a few of the highest-ranking males in the area. One male, the alpha or beachmaster, dominates all other males and keeps them away from females with the species-specific threat vocalization delivered with the head and neck elevated, braced up with the foreflippers. The second-ranked male keeps all other males away from females, too, but defers to the top-ranked male, and so it goes with decreasing rank. Social rank is directly associated with access to females; the alpha bull situates himself in the middle of the group of females. Although small harems of 100 females or less may be ruled by one bull, as the female group increases in number, it becomes too time- and energy-consuming for the alpha male to keep all other males out. Other males, next in rank, take up peripheral positions in the harem. In small colonies, the harems are smaller and discrete, each controlled by a single dominant bull, with subordinate bulls positioned around but outside the harem. In very large colonies, like
some beaches at South Georgia, there may be a nearly continuous mass of breeding females stretching for a kilometer or more, without individual harems. Alpha males and subordinate bulls are then positioned at intervals among the females (Laws 1956a ).
Mating Success of Males
The ultimate result of this power structure is that few males mate, and male mating success is directly related to social rank in the dominance hierarchy. At Año Nuevo, as few as 5 out of 180 males were responsible for up to 92% of the copulations observed with up to 470 females during a breeding season; one male held the alpha position in a large harem and dominated mating for four consecutive years, inseminating an estimated 200 females (Le Boeuf 1974). In the southern elephant seal, M. N. Bester and I. S. Wilkinson (this volume) recorded that "dominant bulls controlling the harems achieved over 98% of all matings."
A study of lifetime reproductive success at Año Nuevo confirms that there is great variance in male reproductive success (Le Boeuf and Reiter 1988). In a sample of 91 male pups, only 19 reached breeding age. Three males were extremely successful, inseminating an estimated 121, 97, and 63 females, respectively; 5 other males apparently inseminated 69 females, and the remaining 11 surviving males (as well as the 72 nonsurvivors) failed to mate. The males most successful at mating achieved high ranks in the dominance hierarchies associated with harems.
Although males undergo puberty at about 5 years of age in the northern species and 4 to 5 years of age in the southern species, they do not achieve high rank until they are older, at least eight years of age, and larger (Laws 1956a ; McCann 1985; Clinton, this volume; Deutsch et al., this volume). Prime breeding years for northern males are at age 9 to 12, males are in decline at age 13, and 14 years is the maximum life span (Le Boeuf and Reiter 1988). The majority of breeding bulls in the southern species are also 9 to 12 years old, but the maximum life span is 20 years (Laws 1953a ; McCann 1985). Despite the predominance of adult males in mating and male-male competition, four other categories of subadult males (SAMs) are present during the breeding season: SAM1 = 4 years old, SAM2 = 5 years old, SAM3 = 6 years old, and SAM4 = 7 years old (Le Boeuf 1974; Cox and Le Boeuf 1977; Clinton, this volume).
The Reproductive Cycle of Females
There is strong circumstantial evidence that most females of both species mate for the first time at sea (Laws 1956a , 1956b , this volume; B. J. Le Boeuf, unpubl. observ.). At Año Nuevo, only a few identifiable (marked) virgin females mate on land each year, late in the breeding season; the vast majority must mate at sea because they are not observed elsewhere on land,
either at this time or later in the season. Studies on the southern species demonstrated that in parous females, after copulations and fertilization, development is suspended and the blastocyst remains free in the uterus for about 4½; months; implantation occurs at the end of the summer molt, and active embryonic growth occupies about 7½; months (Laws 1956b ).
Within 6 days after a pregnant northern elephant seal arrives on the rookery during the breeding season, she gives birth to a single pup (this period in the southern elephant seal is 5 days). She keeps it near her and nurses it daily for 27 days (23 days in the southern elephant seal), all the while remaining in the harem and fasting from food and water. During the last 3 to 5 days of nursing, she copulates (about 4 days in the southern elephant seal). Four weeks after giving birth, 34 days after arriving on the rookery (28 days in the southern elephant seal), and on her last day of estrus, the female weans her pup by returning to sea (Le Boeuf, Whiting, and Gantt 1972; Laws 1953b , 1956a , 1956b ).
Age at Primiparity and Natality
In expanding colonies in northern California, females give birth for the first time at age 3 to 6, with 4 years of age being the mean age at primiparity (Reiter, Panken, and Le Boeuf 1981; Huber 1987; Le Boeuf and Reiter 1988). High local density of breeding females is correlated with deferred maturity (Huber et al. 1991). Most females produce a pup annually until they die at a maximum age of about 20 years; however, skipping a year has been observed in some rookeries, especially following first breeding (Huber 1987; Huber et al. 1991).
At South Georgia, M. leonina females also give birth for the first time at age 3 to 6, the majority at age 4. At Macquarie Island, there was deferment of the range and mean by one year (McCann 1980; Carrick et al. 1962). The reason for the difference is not clear, although body growth rates are lower at Macquarie Island. M. A. Hindell and G. J. Little (1988) report two known-age females observed suckling pups on Macquarie Island at age 23.
Female Reproductive Success
Female weaning success increases with age up to at least age 8 (Reiter, Panken, and Le Boeuf 1981; Le Boeuf and Reiter 1988; Huber 1987; Huber et al. 1991; Sydeman et al. 1991; Deutsch et al., this volume). Reproductive experience may have a positive (Reiter, Panken, and Le Boeuf 1981) or a negative effect on subsequent weaning success (Sydeman et al. 1991; Sydeman and Nur, this volume). An important factor in weaning success is female mass, which increases with age (Deutsch, this volume). At Año Nuevo, older, larger females dominate younger, smaller females, displacing them or their pups and preempting areas in the harem that are less prone to nursing disruptions caused by peripheral males or high water (Christenson and Le Boeuf 1978).
Preweaning Pup Mortality
The major cause of pup mortality on the rookery and lack of weaning success is the trauma-starvation syndrome that begins with mother-pup separation and ends with starvation or lethal injury inflicted by adults (Le Boeuf and Briggs 1977). Trauma results from crushing by breeding bulls, or from orphaned pups being bitten while attempting to suckle alien females. Three classes of variables affect motherpup separation and increase pup mortality either directly or indirectly: female aggression; the number; distribution, and density of females; and winter storms that flood the harems at peak season.
An important variable affecting the maximum pup mortality rate is the size of the breeding unit in relation to the topography of the breeding site. At Año Nuevo, if mothers and their pups can move inland to higher ground when surf and high water threatens, such as occurs on large island beaches or mainland breeding sites, the annual pup mortality rate is usually less than 10% of pups born. If, however, there is no fallback position, due to wave cut platforms or high cliffs, the pup mortality rate may rise to 75 to 100% (Le Boeuf and Condit 1983; Stewart and Yochem 1991). On northern elephant seal island colonies, where the pup mortality rate has been monitored closely, the annual rate is usually in the range of 10 to 40% (Le Boeuf and Reiter 1991; Huber, Beckham, and Nisbet 1991). In the southern species, it has been recorded at 2 to 6% on average (Laws 1953b ; Condy 1978). It is higher in unfavorable conditions. Thus, at South Georgia, in some situations on land at the beginning of the season, pups melted deep holes in the snow, which prevented them from sucking and resulted in mortality as high as 30%. Mortality (80%) was associated with ice-breeding colonies at Signy Island due to breakup of the fast ice in storms (Laws 1953b ).
Nursing and Adoption
Most females nurse their own pups exclusively until weaning; however, some females that lose their own pups adopt orphans and raise them as they would their own. In a study by M. L. Riedman and B. J. Le Boeuf (1982) at Año Nuevo, 5% of the orphans reunited with their mothers, 27% were adopted or frequently cared for by foster mothers, and 68% remained orphaned and died. Since young, inexperienced females most often lost their pups, they were the ones most likely to foster an orphan. Usually, they adopted pups that were the same age as those they had lost. Adoption of a single pup was most common, but pupless females also attempted to steal suckling pups, adopted a weaned pup, adopted two pups, or indiscriminately nursed any orphan that approached. Nursing between unrelated cows and pups has been observed in southern elephant seals, but it is unusual. Orphaned pups that attempt to steal milk are usually unsuccessful (McCann 1982).
At Año Nuevo, a few "superweaners" may be produced when pups are
suckled by two "mothers" or are adopted by a pupless female after being weaned by their own mother (Reiter, Stinson, and Le Boeuf 1978; Le Boeuf 1981). These weanlings may attain twice the mass of normal weanlings. In addition, some weanlings attempt to steal milk from nursing females by stealth or perseverance; the majority of these milk thieves are males.
Philopatry and Site Fidelity
Like most other pinnipeds and many terrestrial mammals, most female elephant seals give birth on the rookery where they were born in roughly the same site from year to year. Seventy-one percent of the females born at Año Nuevo during the 1970s returned to give birth there for the first time (Reiter, Panken, and Le Boeuf 1981). The rest moved to adjacent rookeries as their birth site became crowded (Le Boeuf, Ainley, and Lewis 1974). Similarly, 70% of females monitored returned to give birth at the original site the following year. Movement to a new site was associated with failing to wean a pup or high density of breeding females. Similarly, D. G. Nicholls (1970) reported that at Macquarie Island, 77% of branded cows up to 11 years old were found breeding within 4 km of their birth site, and Hindell and Little (1988) reported two 23-year-old cows breeding within 1 km of their birth site.
Fasting:
A Key Life History Trait
Fasting on land is an integral part of the life history strategy of elephant seals (Bartholomew 1970). Consider the breeding bull. Clumping of females into groups enhances the potential for polygyny (Emlen and Oring 1977). An alpha bull that can keep others away from a group of females will mate with many females and sire many offspring, provided that he remains near the females night and day keeping competitors away. If he has to make periodic trips to sea to feed, other males will mate in his absence. Clearly, the mating success of a male that dominates his competitors will depend on the length of his uninterrupted tenure on the rookery during the mating season. Breeding males fast for over 100 days while fighting for social rank and competing for mates (Le Boeuf 1974; Wilkinson and Bester 1990; Deutsch et al., this volume; Clinton, this volume).
Females fast while nursing, which provides numerous advantages. By not having to feed while nursing, as sea lions and fur seals do, an elephant seal transfers a great deal of milk energy to her pup in a short time (Costa et al. 1986; Fedak et al., this volume). This frees the mother to prepare for future offspring, to forage on her own without encumbrance.
Fasting is imposed on weaned pups by the departure of the mother. They fast while learning to swim and dive before embarking on a trip to sea to find food (Reiter, Stinson, and Le Boeuf 1978; Thorson and Le Boeuf, this volume).
To reap the benefits of fasting, elephant seals must be able to accumulate sufficient energy during their long trips to sea, they must have an enormous capacity to store fat, and they must economize this energy store while on land. Evidently, they have no problem satisfying the first two requirements (Laws 1953b ; Le Boeuf et al. 1988; Le Boeuf, this volume; Fedak et al., this volume). We emphasize some behaviors that minimize energy expenditure on land.
When elephant seals are not fighting, mating, nursing, or learning water skills, as in the case of newly weaned pups, they sleep. Sleep does not follow a diel pattern. It occurs at all hours but especially during the heat of the day. Among breeding males and nursing females, it is clearly opportunistic. Moreover, elephant seals undergo long apneas during sleep lasting up to 25 minutes (Blackwell and Le Boeuf 1993; Castellini, this volume). This aperiodic pattern of breathing is one of several ways to economize water and save energy (see also Huntley, Costa, and Rubin 1984). Since a major avenue of water loss is through respiration, reducing the number of exhalations by breath holding during sleep reduces water loss. This means a reduction in lipid metabolism, the sole source of water for a fasting seal (Ortiz, Costa, and Le Boeuf 1978).
Besides serving as an energy reserve and a source of water during long fasts, the fat stores, or blubber, provide insulation against the cold both on land and at sea. Conversely, overheating as a result of active movement is minimized in seals by a countercurrent system in the uninsulated flippers.
Foraging
Elephant seals exhibit long, deep, and nearly continuous diving at sea (Le Boeuf et al. 1986, 1988, 1989; Hindell, Slip, and Burton 1991; Boyd and Arnbom 1991; Stewart and DeLong 1991). Foraging occurs during these journeys that take them long distances from their rookeries. Prey, foraging locations, and the unusual diving pattern of both species are subjects of much current interest and intensive study (e.g., Hindell, Burton, and Slip 1991; DeLong, Stewart, and Hill 1992; McConnell et al. 1992; Bester and Pansegrouw 1992); these topics are treated in detail in several chapters in this volume (chaps. 5, 11, 13, 14, 15, 16, and 20).
Predation
The major predators on elephant seals are white sharks, Carcharodon carcharias , and killer whales, Orca orcinus (Laws 1953b ; Ainley et al. 1981; Le Boeuf, Riedman, and Keyes 1982). Shark attacks on northern elephant seals have been observed throughout the breeding range. White sharks kill elephant seals near the surface, victimizing both sexes and all age groups, including
the largest bulls. At the Farallon Islands, near the mouth of San Francisco Bay, white shark predation is restricted to juveniles during the fall haul-out and is localized in the zone of shallow water (< 11 m) within 0.4 km of the island (Klimley et al. 1992). At Año Nuevo, peak attacks on elephant seals occur in December and January, and breeding age males are most frequently victimized (Le Boeuf, Riedman, and Keyes 1982). The contribution of white shark predation to total mortality at sea is unclear, but the diving behavior of elephant seals in the high-risk zone, moving to and from the Año Nuevo rookery over the continental shelf, suggests selection for predator avoidance (Le Boeuf and Crocker 1993). Predation by killer whales has been documented less frequently than predation by sharks (Laws 1953b ; M. Pierson, pers. observ.). Leopard seals, Hydrurga leptonyx , will occasionally take a southern elephant seal but cannot be classed as an important predator (Laws 1953b ).
The cookiecutter shark of the genus Isistius bites out circular chunks of skin and blubber the size of a tennis ball from northern elephant seals in the southern part of their range, but since the craterlike injuries are not lethal, this is more a matter of parasitism than predation (Le Boeuf, McCosker, and Hewitt 1987).
Historically, man has been the major predator on both species of elephant seals, killing them for their oil. The history of sealing in the Southern Hemisphere is reviewed by Laws (this volume) and W. N. Bonner (1982); the decline in numbers and the virtual annihilation of northern elephant seals due to sealing is reviewed by G. A. Bartholomew and C. L. Hubbs (1960) and B. C. Busch (1985) and addressed in chapter 2 of this volume.
References
Ainley, D. G., C. S. Strong, H. P. Huber, T. J. Lewis, and S. H. Morrell. 1981. Predation by sharks on pinnipeds at the Farallon Islands. Fishery Bulletin 78: 941–945.
Bartholomew, G. A. 1970. A model for the evolution of pinniped polygyny. Evolution 24: 546–559.
Bartholomew, G. A., and C. L. Hubbs. 1960. Population growth and seasonal movements of the northern elephant seal, Mirounga angustirostris. Mammalia 24: 313–324.
Bester, M. N., and H. M. Pansegrouw. 1992. Ranging behaviour of southern elephant seal cows from Marion Island. South African Journal of Science 88: 574–575.
Blackwell, S. B., and B. J. Le Boeuf. 1993. Developmental aspects of sleep apnoea in northern elephant seals. Journal of Zoology, London 231: 437–447.
Bonnell, M. L., B. J. Le Boeuf, M. O. Pierson, D. H. Dettman, and G. D. Farrens. 1979. Summary Report 1975-1978, Marine Mammal and Seabird Surveys of the Southern
California Bight Area. III. Pinnipeds , Bureau of Land Management, Department of the Interior, Contract AAa550-CT7-36.
Bonnell, M. L., and R. K. Selander. 1974. Elephant seals: Genetic consequences of near extinction. Science 184: 908–909.
Bonner, W. N. 1982. Seals and Man: A Study of Interactions . Seattle: University of Washington Press.
Boyd, I., and T. Arnbom. 1991. Diving behaviour in relation to water temperature in the southern elephant seal: Foraging implications. Polar Biology 11: 259–266.
Briggs, K. T., and G. V. Morejohn. 1976. Dentition, cranial morphology and evolution in elephant seals. Mammalia 40: 199–222.
Busch, B. C. 1985. The War Against the Seals . Montreal: McGill-Queen's University Press.
Campagna, C., M. Lewis, and R. Baldi. 1993. Breeding biology of southern elephant seals in Patagonia. Marine Mammal Science 9: 34–47.
Carrick, R., S. E. Csordas, and S. E. Ingham. 1962. Studies on the southern elephant seal, Mirounga leonina (L.). IV. Breeding and development. CSIRO Wildlife Research 7: 161–197.
Carrick, R., S. E. Csordas, S. E. Ingham, and K. Keith. 1962. Studies on the southern elephant seal, Mirounga leonina (L.). III. The annual cycle in relation to age and sex. CSIRO Wildlife Research 7: 119–160.
Christenson, T. E., and B. J. Le Boeuf. 1978. Aggression in the female northern elephant seal, Mirounga angustirostris. Behaviour 64: 158–172.
Condy, P. R. 1978. The distribution and abundance of southern elephant seals, Mirounga leonina (Linn.), at Marion Island. South African Journal of Antarctic Research , 8: 42–48.
———. 1979. Annual cycle of the southern elephant seal Mirounga leonina (Linn.) at Marion Island. South African Journal of Zoology 14: 95–102.
Costa, D. P., B. J. Le Boeuf, A. C. Huntley, and C. L. Ortiz. 1986. The energetics of lactation in the northern elephant seal. Journal of Zoology, London 209: 21–33.
Cox, C. R., and B. J. Le Boeuf. 1977. Female incitation of male competition: A mechanism in sexual selection. American Naturalist 111: 317–335.
Davies, J. L. 1958. The Pinnipedia: An essay in zoogeography. Geographical Review 48: 474–493.
DeLong, R. L., B. S. Stewart, and R. D. Hill. 1992. Documenting migrations of northern elephant seals using day length. Marine Mammal Science 8: 155–159.
Deutsch, C. J., M. P. Haley, and B. J. Le Boeuf. 1990. Reproductive effort of male northern elephant seals: Estimates from mass loss. Canadian Journal of Zoology 68: 2580–2593.
Emlen, S. T., and L. W. Oring. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197: 215–223.
Gill, T. 1866. On a new species of the genus Macrorhinus. Proceedings of the Chicago Academy of Science 1: 33–34.
Gray, J. E. 1827. Mammalia . Vol. 5 of The Animal Kingdom Arranged in Conformity with Its Organization , by the Baron (G.) Cuvier, with additional descriptions by Edward Griffith and others. 16 vols. London: George B. Whittaker.
Heath, M. A., and R. J. Schusterman. 1975. "Displacement" sand flipping in the northern elephant seal (Mirounga angustirostris ). Behavioral Biology 14: 379–385.
Hendey, Q. B. 1972. The evolution and dispersal of the Monachinae (Mammalia: Pinnipedia). Annals of the South African Museum 59: 99–113.
Hendey, Q. B., and C. A. Repenning. 1972. A Pliocene phocid from South Africa. Annals of the South African Museum 59: 71–98.
Hindell, M. A. 1990. Population Dynamics and Diving Behaviour of a Declining Population of Southern Elephant Seals. Ph.D. dissertation, University of Queensland, Australia.
Hindell, M. A., and H. R. Burton. 1988. Seasonal haul-out patterns of the southern elephant seal (Mirounga leonina L.) at Macquarie Island. Journal of Mammalogy 698: 81–88.
Hindell, M. A., H. R. Burton, and D. J. Slip. 1991. The foraging areas of southern elephant seals, Mirounga leonina , inferred from water temperature data. Australian Journal of Marine and Freshwater Research 42: 115–128.
Hindell, M. A., and G. J. Little. 1988. Longevity, fertility and philopatry of two female southern elephant seals (Mirounga leonina ) at Macquarie Island. Marine Mammal Science 4: 168–171.
Hindell, M. A., D. J. Slip, and H. R. Burton. 1991. The diving behaviour of adult male and female southern elephant seals, Mirounga leonina (Pinnipedia: Phocidae). Australian Journal of Zoology 39: 595–619.
Hoelzel, A. R., J. Halley, C. Campagna, T. Arnbom, B. J. Le Boeuf, S. J. O'Brien, K. Ralls, and G. A. Dover. 1993. Elephant seal genetic variation and the use of simulation models to investigate historical population bottlenecks. Journal of Heredity 84: 443–449.
Huber, H. R. 1987. Natality and weaning success in relation to age of first reproduction in northern elephant seals. Canadian Journal of Zoology 65: 1311–1316.
Huber, H. R., C. Beckham, and J. Nisbet. 1991. Effects of the 1982–83 El Niño on northern elephant seals on the South Farallon Islands, California. In Pinnipeds and El Niño: Responses to Environmental Stress , ed. F. Trillmich and K. Ono, 219–233. Berlin: Springer Verlag.
Huber, H. R., A. C. Rovetta, L. A. Fry, and S. Johnston. 1991. Age-specific natality of northern elephant seals at the South Farallon Islands, California. Journal of Mammalogy 72: 525–534.
Huntley, A. C., D. P. Costa, and R. D. Rubin. 1984. The contribution of nasal countercurrent heat exchange to water balance in the northern elephant seal, Mirounga angustirostris. Journal of Experimental Biology 113: 447–454.
King, J. E. 1956. The monk seal genus Monachus. Bulletin of the British Museum of Natural History (Zool.) 3: 203–256.
———. 1964. Seals of the World . London: British Museum of Natural History.
———. 1983. Seals of the World . 2d ed. Ithaca: Cornell University Press.
Klimley, A. P., S. D. Anderson, P. Pyle, and R. P. Henderson. 1992. Spatiotemporal patterns of white shark (Carcharodon carcharias ) predation at the South Farallon Islands, California. Copeia 3: 680–690.
Laws, R. M. 1953a . A new method of age determination for mammals with special reference to the elephant seal, Mirounga leonina Linn. Falkland Islands Dependencies Survey, Scientific Reports 3: 1–11.
———. 1953b . The elephant seal (Mirounga leonina Linn.). I. Growth and age. Falkland Islands Dependencies Survey, Scientific Reports 8: 1–62.
———. 1956a . The elephant seal (Mirounga leonina Linn.). II. General, social and reproductive behavior. Falkland Islands Dependencies Survey, Scientific Reports 13: 1–88.
———. 1956b . The elephant seal (Mirounga leonina Linn.). III. The physiology of reproduction. Falkland Islands Dependencies Survey, Scientific Reports 15: 1–66.
———. 1960. The southern elephant seal (Mirounga leonina Linn.) at South Georgia. Norsk Hvalfangst-Tidende 49: 466–476, 520–542.
Le Boeuf, B. J. 1972. Sexual behavior in the northern elephant seal, Mirounga angustirostris. Behaviour 41: 1–26.
———. 1974. Male-male competition and reproductive success in elephant seals. American Zoologist 14: 163–176.
———. 1981. Elephant seals. In The Natural History of Año Nuevo , ed. B. J. Le Boeuf and S. Kaza, 327–374. Pacific Grove, Calif.: Boxwood Press.
———. 1989. The wars of the noses. BBC Wildlife 7: 144–149.
Le Boeuf, B. J., D. G. Ainley, and T. J. Lewis. 1974. Elephant seals on the Farallons: Population structure of an incipient breeding colony. Journal of Mammalogy 55: 370–385.
Le Boeuf, B. J., and M. L. Bonnell. 1980. Pinnipeds of the California Islands: Abundance and distribution. In The California Islands: Proceedings of a Multidisciplinary Symposium , ed. D. M. Power, 475–493. Santa Barbara, Calif.: Santa Barbara Museum of Natural History.
Le Boeuf, B. J., and K. T. Briggs. 1977. The cost of living in a seal harem. Mammalia 41: 167–195.
Le Boeuf, B. J., and R. C. Condit. 1983. The high cost of living on the beach. Pacific Discovery 12–14.
Le Boeuf, B. J., R. C. Condit, and J. Reiter. 1989. Parental investment and the secondary sex ratio in northern elephant seals. Behavioral Ecology and Sociobiology 25: 109–117.
Le Boeuf, B. J., D. P. Costa, A. C. Huntley, and S. D. Feldkamp. 1988. Continuous, deep diving in female northern elephant seals, Mirounga angustirostris. Canadian Journal of Zoology 66: 446–458.
Le Boeuf, B. J., D. P. Costa, A. C. Huntley, G. L. Kooyman, and R. W. Davis. 1986. Pattern and depth of dives in northern elephant seals, Mirounga angustirostris. Journal of Zoology, London 208: 1–7.
Le Boeuf, B. J., and D. E. Crocker. 1993. Diving behavior of elephant seals: Implications for predator avoidance. Paper presented at a symposium on the biology of the white shark, held at the Bodega Marine Laboratory, University of California, Davis, March 4–7.
Le Boeuf, B. J., D. E. Crocker, S. B. Blackwell, P. A. Morris, and P. H. Thorson. 1993. Sex differences in diving and foraging behavior of northern elephant seals. In Marine Mammals: Advances in Behavioural and Population Biology , ed. I. Boyd, 149–178. Symposia of the Zoological Society of London no. 66. London: Oxford University Press.
Le Boeuf, B. J., J. E. McCosker, and J. Hewitt. 1987. Crater wounds on northern elephant seals: The cookiecutter shark strikes again. Fishery Bulletin 85: 387–392.
Le Boeuf, B. J., Y. Naito, A. C. Huntley, and T. Asaga. 1989. Prolonged, continuous, deep diving by northern elephant seals. Canadian Journal of Zoology 67: 2514–2519.
Le Boeuf, B. J., and R. S. Peterson. 1969. Social status and mating activity in elephant seals. Science 163: 91–93.
Le Boeuf, B. J., and L. F. Petrinovich. 1974. Elephant seals: Interspecific comparisons of vocal and reproductive behavior. Mammalia (Paris) 38: 16–32.
Le Boeuf, B. J., and J. Reiter. 1988. Lifetime reproductive success in northern elephant seals. In Reproductive Success , ed. T. H. Clutton-Brock, 344-362. Chicago: University of Chicago Press.
———. 1991. Biological effects associated with El Niño Southern Oscillation, 1982–83, on northern elephant seals breeding at Año Nuevo, California. In Pinnipeds and El Niño: Responses to Environmental Stress , ed. F. Trillmich and K. Ono, 206–218. Berlin: Springer Verlag.
Le Boeuf, B. J., M. L. Riedman, and R. S. Keyes. 1982. White shark predation on pinnipeds in California coastal waters. Fishery Bulletin 80: 891–895.
Le Boeuf, B. J., R. J. Whiting, and R. F. Gantt. 1972. Perinatal behavior of northern elephant seal females and their young. Behaviour 43: 121–156.
Ling, J. K., and M. M. Bryden. 1981. Southern elephant seal—Mirounga leonina . In Handbook of Marine Mammals. 2. Seals , ed. S. H. Ridgway and R. J. Harrison, 297–327. London: Academic Press.
———. 1992. Mirounga leonina. Mammalian Species 391: 1–8.
Linnaeus, C. 1758. Systema naturae. Regnum animale . Vol. 1., pt. 1. 10th ed. rev. Stockholm: Laurentii Salvii.
McCann, T. S. 1980. Population structure and social organization of southern elephant seals, Mirounga leonina (L.). Biological Journal of the Linnaean Society 14: 133–150.
———. 1981. Aggression and sexual activity of male southern elephant seals, Mirounga leonina. Journal of Zoology 195: 295–310.
———. 1982. Aggressive and maternal activities of female southern elephant seals (Mirounga leonina). Animal Behaviour 30: 268–276.
———. 1985. Size, status and demography of southern elephant seal (Mirounga leonina ) populations. In Sea Mammals of South Latitudes: Proceedings of a Symposium of the 52d ANZAAS Congress in Sydney—May 1982 , ed. J. K. Ling and M. M. Bryden, 1–17. Northfield: South Australian Museum.
McConnell, B. J., C. Chambers, and M. A. Fedak. 1992. Foraging ecology of southern elephant seals in relation to bathymetry and productivity of the Southern Ocean. Antarctic Science 4: 393–398.
Miller, W. 1971. Pleistocene vertebrates of the Los Angeles Basin and vicinity (exclusive of Rancho La Brea). Bulletin of the Los Angeles County Museum of Natural History 10: 35.
Murphy, R. C. 1914. Notes on the sea elephant, Mirounga leonina (Linn.). Bulletin of the American Museum of Natural History 33: 63–79.
Nicholls, D. G. 1970. Dispersal and dispersion in relation to birth site of the southern elephant seal, Mirounga leonina (L.), of Macquarie Island. Mammalia 34: 598–616.
Odell, D. K., 1974. Seasonal occurrence of the northern elephant seal, Mirounga angustirostris , on San Nicolas Island, California. Journal of Mammalogy 55: 81–95.
Ortiz, C. L., D. P. Costa, and B. J. Le Boeuf. 1978. Water and energy flux in elephant seal pups fasting under natural conditions. Physiological Zoology 51: 166–178.
Reiter, J., K. J. Panken, and B. J. Le Boeuf. 1981. Female competition and reproductive success in northern elephant seals. Animal Behaviour 29: 670–687.
Reiter, J., N. L. Stinson, and B. J. Le Boeuf. 1978. Northern elephant seal development: The transition from weaning to nutritional independence. Behavioral Ecology and Sociobiology 3: 337–367.
Repenning, C. A. 1980. Warm water life in cold ocean currents. Oceans 13: 18–24.
Riedman, M. L., and B. J. Le Boeuf. 1982. Mother-pup separation and adoption in northern elephant seals. Behavioral Ecology and Sociobiology 11: 203–215.
Scammon, C. M. 1874. The Marine Mammals of the North-Western Coast of North America, Described and Illustrated, Together with an Account of the American Whale-Fishery . San Francisco: J. H. Carmany and Co.
SCAR. 1991. Report of the workshop on southern elephant seals, Monterey, California, May 22–23, 1991.
Scheffer, V. 1958. Seals, Sea Lions and Walruses . Stanford: Stanford University Press.
Stewart, B. S., and R. L. DeLong. 1991. Diving patterns of northern elephant seal bulls. Marine Mammal Science 7: 369–384.
Stewart, B. S., and P. K. Yochem. 1991. Northern elephant seals on the southern California Channel Islands and El Niño. In Pinnipeds and El Niño: Responses to Environmental Stress , ed. F. Trillmich and K. Ono, 234-243. Berlin: Springer Verlag.
Sydeman, W. J., H. R. Huber, S. D. Emslie, C. A. Ribic, and N. Nur. 1991. Age-specific weaning success of northern elephant seals in relation to previous breeding experience. Ecology 72: 2204–2217.
White, F. N., and D. K. Odell. 1971. Thermoregulatory behavior of the northern elephant seal, Mirounga angustirostris. Journal of Mammalogy 52: 758–774.
Wilkinson, I. S., and M. N. Bester. 1990. Duration of post-weaning fast and local dispersion in the southern elephant seal, Mirounga leonina , at Marion Island. Journal of Zoology, London 222: 591–600.
Worthy, G. A. J., P. A. Morris, D. P. Costa, and B. J. Le Boeuf. 1992. Moult energetics of the northern elephant seal (Mirounga angustirostris). Journal of Zoology, London 227: 257–265.