Chapter 10—
Protein Synthesis in the Cytoplasm
10.1—
Introduction
Since gene expression occurs almost exclusively via the synthesis of proteins, an understanding of the mechanism of this process is important. Although living organisms make several thousand different proteins with a variety of functions, present evidence suggests that they are all made, by the same basic mechanism, on polysomes. These structures consist of a template RNA molecule, messenger-RNA (mRNA), with several associated ribosomes, and protein synthesis is a complex, energy requiring, multi-enzymic process. A template mechanism of synthesis is dictated by the fact that the protein product has a specificity which depends upon the precise sequence of the constituent amino acid residues in the polypeptide chain. This automatically rules out the normal enzymic method by which most other cell constituents are made, since each enzyme of a set specific for the synthesis of one protein would, in turn, require a further set for its synthesis, ad infinitum. However, since some small polypeptides are synthesized by a non-template mechanism, it is possible that some proteins with highly repetitive sequences of amino acid residues, may be synthesized similarly.
Because all organisms probably synthesize proteins by a similar mechanism, the tendency has been to use an organism which is particularly amenable for basic studies. This organism is the prokaryote, Escherichia coli, and higher plant material has not been much used because of associated technical difficulties. However, recognition of the importance of the deposition of proteins in plants as our major source of protein food, is leading to an increased spate of work in the desire to understand, and possibly manipulate, this important process in the major food plants.
Proteins are synthesized on free and membrane-bound polysomes in the cytosol or in various sub-cellular organelles, e.g. mitochondria, chloroplasts. Only the biochemistry of protein synthesis by cytoplasmic ribosomes will be reviewed in this chapter; organelle protein synthesis is dealt with in chapter 11. Different aspects of the control of protein synthesis are covered in chapters 13 and 15, but it should be re-iterated here that the level of protein in the cell depends on a dynamic balance between synthesis and degradation.
10.2—
Polysomes
10.2.1—
Structure of Ribosomes
Ribosomes are classified as eukaryotic or prokaryotic in type, on the basis of: (a) sensitivity to various antibiotics; (b) functional interchangeability of soluble factors and ribosomes from different sources; and (c) structure and sedimentation characteristics, e.g. 70s (prokaryotic) or 80s (eukaryotic).
Bacterial ribosomes, plant chloroplast and mitochondrial ribosomes are classified as prokaryotic, even though they may not always exhibit all of the above characteristics, e.g. plant mitochondrial ribosomes have sedimentation values of about 80s.
Plant and animal cytoplasmic ribosomes are of the eukaryotic type and have sedimentation values of about 80s. This class of ribosomes are heterogeneous in size however, with most of the differences accounted for by differences in the large subunit, the small subunit having been conserved during evolution (Cammarano et al., 1972a,b & c).
Measurements of the size of plant cytoplasmic ribosomes in the electron microscope vary, but they are approximately 25 × 20 nm. Miller et al., (1966) have described them as being acorn-shaped, and several workers have described a cleft in the small subunit. In the rat liver ribosome model of Nonomura et al., (1971) there is a tunnel between the two subunits, directly under the cleft in the small subunit, which is thought to accommodate the mRNA. Ribosomes require Mg2+ for structural integrity and dissociate at low Mg2+ concentrations into a large and a small subunit with sedimentation values of about 60s and 40s respectively (Ajtkhozhin et al., 1972). There is good evidence that mRNA attaches to the small subunit before the addition of the large subunit completes the ribosome structure, and the different roles of the two subunits are a feature of all the models of ribosome structure and function (see Noll et al., 1973). The 80s ribosome of plants corresponds to a molecular weight of 3.9 × 106 daltons, with molecular weights for the large subunit of 2.4 × 106 daltons and the small subunits, 1.5 × 106 daltons; the small subunit is of the same molecular weight as its mammalian counterpart, the large subunit is smaller. The large subunit of plant ribosomes contains one molecule of 25s RNA, hydrogenbonded to 1 molecule of 5.8s RNA (Payne & Dyer, 1972), and 1 molecule of 5s RNA; the small subunit contains 1 molecule of 18s RNA. The molecular weight of the 25s RNA is 1.3 × 106 daltons, and that of the 18s is 0.7 × 106 daltons (Loening, 1968). The large subunit contains 46% protein, and the small 54%.
The large subunit of pea seedling ribosomes contains 44–45 proteins and the small subunit 32–40 proteins, of which most are basic and of molecular weights between 20 × 103 and 30 × 103 daltons. Some proteins may be represented by more than one copy per ribosome. Proteins extracted from cytoplasmic
and chloroplast ribosomes of the same species, show little similarity in two-dimensional electrophoresis or by immunological comparison and are significantly less similar than are the cytoplasmic ribosomes of different species, e.g. beans and wheat (Gualerzi et al., 1974).
Before a complete understanding of the mechanism of protein synthesis is elucidated, it will be necessary to know the spatial relationships and three-dimensional structures of the proteins and the RNAs of the ribosome, as well as those of the associated molecules which also play a part in protein synthesis. Information on the structure of the proteins and the RNA molecules of the E. coli ribosome, together with reconstitution experiments, is now well advanced (Traub & Nomura, 1968; Nashimoto et al., 1971; Wittmann, 1973; Anderson et al., 1974; Nierhaus & Dohme, 1974).
10.2.2—
Free and Membrane-Bound Polysomes
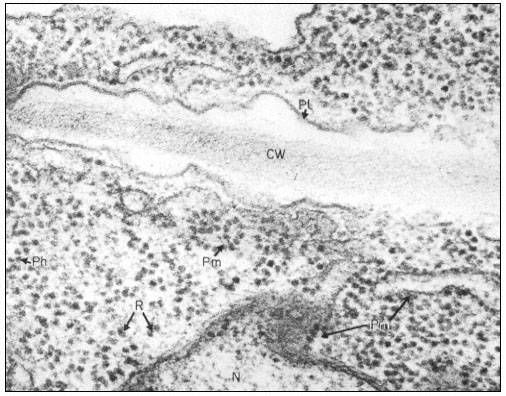
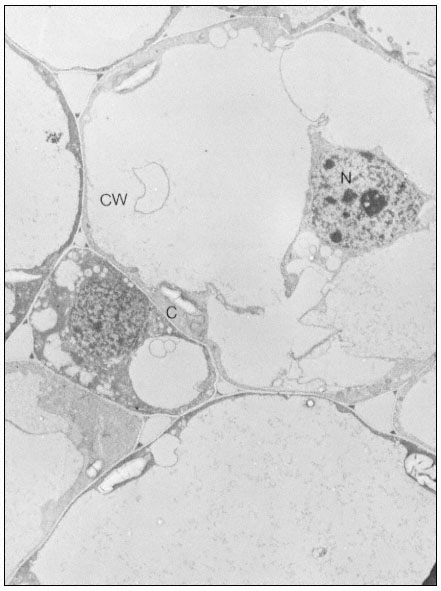
Ribosomes associate with mRNA to form polysomes, as can be seen in the electron micrograph (Fig. 10.1); the size of the polysome varies according to the length of the mRNA and the number of attached ribosomes. Polysomes are found either free in the cytoplasm or attached to the surface of membranes of the endoplasmic reticulum (ER) and the nucleus. In animal embryonic cells, most of the polysomes are free and engaged in protein synthesis for internal use, whereas in those differentiated animal cells from which large amounts of protein are exported, most of the polysomes are found attached to the ER. Thus, the generalization arose that membrane-bound ribosomes synthesize protein for export and free ribosomes for intracellular use. It soon became clear however, that membrane-bound ribosomes occur in some tissues which do not export protein, and that in cells where all the protein synthesized is for internal use, different classes of protein are synthesized on the two types of ribosomes. Less information is available for plants. In the developing broadbean seed the highly vacuolate, relatively membrane-free cells of the cotyledon are transformed just prior to the onset of storage protein synthesis, into cells whose cytoplasm is vesicular and in which ER with attached ribosomes is very prominent. This new protein synthesis machinery is assembled at a precise time in the course of seed development for the production, in large amounts, of the storage proteins of the seed (Bailey et al., 1970; see Fig. 10.2). Later, during dehydration of the seed, ribosomes become detached and the population of free ribosomes thereby increased. A similar series of events has been recorded for the developing seeds in many other plants.
The way in which ribosomes attach to membranes is not clear; the ribosomes themselves, the membranes and the protein being synthesized, have all been suggested as being involved in the binding. There is evidence from animals that the large subunit is in close contact with the membranes, and Baglioni et al. (1971) have postulated that the large subunit binds directly to the membrane, probably at a specific binding site (Sunshine et al., 1971), and that a protein on
Figure 10.1
Electron micrograph of a thin section of parts of two adjacent cells in a
shoot apex of pea showing ribosomes and polyribosomes; CW = Cell Wall, Pl
= Plasmalemma, N = Nucleus, Pm = Membrane bound polysomes, R = Ribosome
Ph = Polysome helix. By courtesy of A. D. Greenwood, Department of Botany
and Plant Technology, Imperial College of Science and Technology, London.
the membranes is responsible for the attachment (James et al., 1969). Alternatively, it has been suggested that one of the proteins of the large subunit is responsible, whereas other observations suggest that the binding may be dependent on the nascent polypeptide chain. It now seems likelly that, in vivo, membrane-bound ribosomes synthesize a different class of protein to free ribosomes, whereas in vitro, proteins of both classes are synthesized by both types of ribosomes, suggesting the control is not a function of the ribosome itself.
10.2.3—
Isolation and Purification
Ribosomes and polysomes are normally isolated from plants and purified by sedimentation through sucrose cushions as originally described by Wettstein
Figure 10.2
Electron micrographs of developing seeds of Vicia faba.
(a) 25 days after fertilization. Ribosomes free in the cytoplasm. Very little ER present.
et al. (1963). In a typical procedure, the material is homogenized in 0.25 M sucrose containing 200 mM -Tris-HCl, pH 8.5 at 2ºC, 500 mM -KCl and 15 mM -MgC12 , with a Willems Polytron for 3 seconds at a speed setting of 8. The homo-
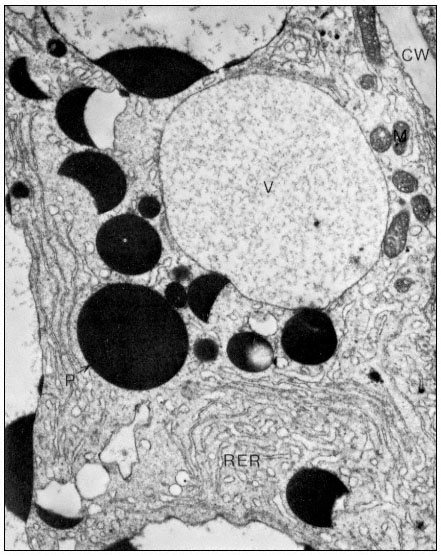
Figure10.2
(b) 55 days after fertilization, showing most of the polysomes bound to ER and
storage protein being laid down. C = Cytoplasm, P = Protein body, CW = Cell Wall,
RER = Endoplasm reticulum with polysomes attached, N = Nucleus, V = Vacuole.
From Boulter et al. (Qual. Plant. XXIII, 239–50, 1973).
genate is filtered through Miracloth and Triton X-100 is added to a final concentration of 2% (v/v). The filtrate is centrifuged at 104 × g for 10 min at 2°C and the ribosomes and polysomes are recovered from the supernatant by layering over a 3 ml cushion of 0.1 M sucrose in 50 mM -Tris-HCI, pH 8.5, 50 mM -KCl
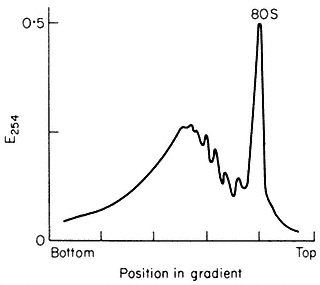
and 10 mM -MgCl2 , followed by centrifugation for at least 44 x 107 g-min, as described by Leaver & Dyer (1974) (Fig. 10.3). The inclusion of the detergent
Figure 10.3
Sucrose density profiles of ribosomes and polysomes from Pisum
sativum. Ribosomes recovered after 6 h. centrifugation through
1 M -sucrose cushion. E254 = absorbance at 254 nm
(From Leaver & Dyer Biochem. J. 144, 165–7, 1974.)
Triton X-100 is necessary since plant materials contain a proportion of polysomes which are membrane-bound and the addition of Triton X-100 solubilizes the endoplasmic reticulum, so releasing them. The method gives a preparation of both polysomes and ribosomes and the latter can be removed by centrifugation techniques or, alternatively, the whole preparation can be converted to ribosomes by exposure of the plants to nitrogen gas for at least 1 hour prior to extraction. Since different plants and even different tissues from the same plant contain different amounts of membrane-bound to free polysomes and different free polysomes to free monoribosome ratios, isolation conditions may need to be varied for optimum results with different experimental materials. The situation is further complicated by the fact that plant mitochondrial ribosomes, although not functionally of the 80s type, sediment at 80s (Leaver & Harmy, 1973), and can contaminate cytoplasmic preparations to varying extents, depending on the experimental material. Damage to ribosomes, both structural and by the removal of associated proteins, can occur during isolation and preparation. The extent of this damage can be assessed, to some extent, by extracting the RNA and fractionating it by polyacrylamide gel electrophoresis (see Leaver & Key, 1970).
10.3—
The Biochemical Mechanism of Protein Synthesis
Protein synthesis, i.e. the assembly of polypeptide chains from their amino acid constituents, occurs on the ribosomes. The information specifying the amino acid sequence of the polypeptide chain is contained in mRNA molecules, which
in the case of eukaryotes are complexed with specific proteins in vivo. Messenger-like RNA-protein particles called 'informosomes' have been isolated from the cytoplasm and from polysomes of germinating wheat embryos (Ajtkhozhin et al., 1973; Ajtkhozhin & Akhanov, 1974). So far, however, the RNA of these particles has not been definitely established as mRNA. When an mRNA molecule associates with ribosomes, the information it contains is translated; the number of ribosomes involved depends largely upon the size of the polypeptide chain being synthesized, since generally, ribosomes are evenly spaced along mRNA molecules.
The translation process can be arbitrarily split into initiation, elongation, and termination and release. However, prior to the initiation of the process on the ribosome, the twenty different protein amino acids must be activated and attached to specific tRNA molecules. Also, after release of the polypeptide chain from the ribosome, a series of post-translational changes may occur.
10.3.1—
Amino Acid Activation and Aminoacyl-tRNA Synthesis
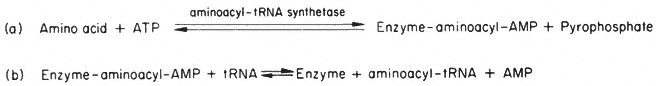
This energy-requiring process is accomplished in the cytosol by enzymes called aminoacyl-tRNA synthetases or ligases. It occurs as a two-stage reaction, both stages being catalysed by the same aminoacyl-tRNA synthetase enzyme (Fig. 10.4). The product of the reaction, aminoacyl-tRNA molecules, contain in the
Figure 10.4
The reactions of aminoacyl-tRNA synthesis. (In the cell a pyrophosphatase
acts on the pyrophosphate formed to make reaction (a) irreversible in practice).
aminoacyl-tRNA bond, sufficient energy for the subsequent formation of peptide bonds to occur spontaneously and, in addition, the anticodon triplet of bases of the tRNA ensures that the amino acid will be located at a particular residue position in the amino acid sequence of the protein product. This reaction, therefore, controls the proportion of free to aminoacylated-tRNAs in the cytoplasm, and theoretically therefore, could affect the rate and/or type of protein being synthesized.
10.3.1.1—
tRNA
The code words (codons) of the genetic code were first established for E. coil. This work showed that there were 61 codons shared between twenty protein amino acids, i.e. there is more than one codon for some amino acids and these can attach to more than one tRNA species. Chemically different tRNA species which can be acylated by the same amino acid are called isoacceptor tRNAs.
However, it was soon realized that not every codon has a corresponding tRNA with a specific anticodon and that some tRNAs recognize more than one codon. Crick's 'wobble hypothesis' (1966) to account for this, proposed alternative base-pairing to occur between the base in the third position of the mRNA codon and the corresponding base in the tRNA anticodon. The proposed rules are set out in Fig. 10.5. Thus, inosine in the 5' position in the anticodon of an
Figure 10.5
The basis of the 'wobble' hypothesis. (X =
Any nucleotide; I = Inosine; G = Guanine;
U = Uridine; A = Adenine; C = Cytosine;)
(From Boulter et al. (Biol. Rev. 47, 113–75, 1972.))
aminoacyl-tRNA molecule allows this base to pair with either U, C or A in the third 3' position of a messenger codon. Similarly, with G or U in the 5' position in the anticodon, two codons may be recognized by a single aminoacyl-tRNA, whereas with C or A only a single codon is recognized. Since alternative base-pairing is only possible with the base in the third position of the codon, codons for the same amino acid which differ in either of the first two base positions, base-pair with different tRNAs. The only example of 'wobble' in the first base of a codon occurs with tRNAiMet , which recognizes both the initiator AUG or GUG codons (see later). Even so, separation of tRNAs by counter current-distribution, MAK columns, BD-cellulose columns and reversed phase chromatography has shown that there are more isoacceptor tRNAs than can be accounted for by the degeneracy of the genetic code. Similarly, isoacceptor tRNAs for amino acids exist in plants; for example, soyabean seedlings have at least six different tRNALeu species (Anderson & Cherry, 1969). Although few experiments have been carried out, it has been found in every case investigated that plants use the same codon in amino acid assignments as those of E. coli. However, plants may not use all of the possible codons for a particular amino acid, since some isoacceptor tRNA species may be absent, or because of the 'wobble' effect; e.g. Caskey et al., (1968) have shown that the isoleucyl tRNA from guinea pig liver recognizes AUU, AUC and AUA codons, whereas the corresponding isoleucyl-tRNA from E. coli only recognizes two of these, AUU and AUC. This response to different codons in the two organisms is explained if the liver tRNA had 5' inosine in the anticodon, while E. coli tRNA had guanine.
The whole question of the number and the activity of tRNAs in different tissues of plants is complicated by the fact that, (a) it is not certain whether the methods for separating tRNAs are completely effective; (b) it is known that during isolation and purification, partial modification of tRNA molecules can occur, which may affect their charging ability with amino acids or their ability to transfer amino acids to proteins, or both (see Sueoka & Kano-Sueoka, 1970); (c) the relative importance of the three protein synthesizing systems, cytoplasmic, chloroplast and mitochondrial, each with its associated tRNAs and synthetases may differ in different tissues. Nevertheless, different complements of isoacceptor tRNAs may occur in different tissues and changes in pattern have been demonstrated during development (Bick et al., 1970; Littauer & Inouye, 1973), and it has been suggested that isoacceptor tRNAs are involved in the control of protein synthesis. Garel et al., (1973) have shown that the complement of tRNAs synthesized in the silk gland is related to the mRNAs being translated and they have suggested defining this phenomenon as 'modulated tRNA biosynthesis'.
10.3.1.2—
Aminoacyl-tRNA Synthetases
Aminoacyl-tRNA synthetases have been isolated and purified from a variety of plants (see Boulter 1970; Boulter et al., 1972; Zalik & Jones, 1973), and although a considerable amount of work has been done on their properties, several problems remain. For example, the question of the detailed substrate specificity of different aminoacyl-tRNA synthetases is still under investigation. Since activation is a two-step process, both amino acid and tRNA specificity have to be considered. The synthetase is normally absolutely specific towards the tRNA; few mismatches have been reported in homologous systems (Arca et al., 1967, 1968). However, a synthetase specific for one amino acid can, in some instances, activate to a lesser extent another amino acid. Even so, synthetases are remarkably amino acid specific. A second amino acid attached to a tRNA molecule normally specific for another amino acid, will be located in the polypeptide chain according to the tRNA anticodon, i.e. where the first amino acid would have been placed. With regard to amino acids not normally found in the cell, specificity is not always so pronounced, e.g. azetidine-2-carboxylic acid is activated and transferred to tRNAPro by the proline enzyme of mung bean (Peterson & Fowden, 1965), whereas Polygonatum multiflorum, which contains a high level of this amino acid in nature, contains a prolyl-tRNA synthetase which will not activate it. In Mimosa and Leucaena, where mimosine, an analogue of phenylalanine, occurs naturally and where it is non-toxic, the phenylalanyl-tRNA synthetase discriminates against mimosine. In several other species where mimosine is toxic, it is activated by the phenylalanine-tRNA synthetase but the enzyme does not attach it to tRNAPhe (Smith & Fowden, 1968).
It is not clear if there is one or more than one synthetase whenever there are
a number of isoacceptor tRNAs. In soybean seedlings where there are at least three different leucyl aminoacyl-tRNA synthetases (Kanabus & Cherry, 1971) which have different specificities towards the six leucine isoacceptor tRNAs, it would appear that there is more than one cytoplasmic leucyl-tRNA synthetase. However, in some instances, a single aminoacyl-tRNA synthetase can recognize different isoacceptor tRNAs. When both cytoplasmic and chloroplast tRNA species are present, there are probably at least two sets of aminoacyl-tRNA synthetases. In light-grown Euglena for example (Reger et al., 1970; Krauspe & Parthier, 1973), there are two aminoacyl-tRNA synthetases for each of phenylalanine and leucine, whereas dark-grown Euglena, which is without chloroplasts, contains only one; there are various reports of chloroplast aminoacyl-tRNA synthetases which only acylate chloroplast tRNAs and not their cytoplasmic counterparts and vice versa. However, in heterologous systems such as chloroplast tRNA plus cytoplasmic activating enzymes, specificity may range from none to complete (Burkard et al., 1970; Boulter et al., 1972), although the biological significance of these findings is not clear.
There is evidence that, as well as the tRNAs, the complement of aminoacyltRNA synthetases may change during the life cycle of plants (Bick & Strehler, 1971).
10.3.2—
Translation of mRNA
10.3.2.1—
Initiation of the Polypeptide Chain
Initiation of protein synthesis on plant cytoplasmic ribosomes starts with the assembly of a mRNA•40s subunit complex which requires ATP for its formation and this step is followed by the addition of methionyl-tRNAi to form a mRNA•40s subunit•methionyl-tRNAi complex. The process requires GTP and at least two initiation factors (Weeks et al., 1972; Marcus et al., 1973). Initiation is completed by the addition of the 60s subunit and the 80s ribosome so formed is then able to accept the next aminoacyl-tRNA, thus starting the elongation process. The tRNAi Met is a special tRNA charged with unformylated methionine, which can enter the so-called 'P' site of the ribosome complex and base-pair with the initiator codon, AUG or GUG in the mRNA. Although mRNAs are translated from the 5'® 3' end, evidence from sequence studies of bacteriophage messages shows that the initiator triplet is not at the 5' terminus, but is set a considerable number of nucleotides in. For example, the first initiator codon of bacteriophage R17 RNA is preceded by 91 nucleotides which are not translated (Adams & Cory, 1970). In the case of polycistronic messengers, one or more initiator triplets will occur intramolecularly.
Evidence that plants have a special type of initiator tRNA, tRNAi Met , comes from the wheatgerm system (Leis & Keller, 1970; Marcus et al., 1970a; Tarrago et al., 1970; Ghosh et al., 1971), and from Vicia faba (Yarwood et al., 1971a),
where it has been shown that two major and one minor tRNAMet species are present. The minor and one of the major tRNAMet species function as chain initiators as shown by AUG-dependent binding (Tarrago et al., 1970; Yarwood et al., 1971a), and by N-terminal analyses of either the product of tobacco mosaic viral RNA-directed (Marcus et al., 1970a) or poly-AUG, poly-GU and endogenous messenger-directed incorporation (Yarwood et al., 1971a). Neither of the major tRNAMet species from either wheat or beans is formylated, in contrast to the initiator tRNAiMet of prokaryotes, and it is presumed that these are the cytoplasmic tRNAMet s for initiator and internal methionine residues. The minor, formylatable tRNAMet , is presumed to be the initiator of chloroplast protein synthesis (see chapter 11)
The hydrolysis of ATP precedes the formation of the first peptide bond and may be required for recycling of the methionyl-tRNAi binding factor. The requirement for ATP has also been demonstrated in the rabbit globin synthesizing system (Schreier & Staehelin, 1973), but not in those of prokaryotes. The order in which the initiator tRNA and mRNA bind to the small subunit in mammalian and prokaryote systems is in dispute; the order suggested above for wheatgerm by Marcus and his coworkers, therefore, whilst agreeing with some workers on those systems disagrees in particular with the suggestion of Legon et al., (1973) and Schreier and Staehelin (1973) that the initiator binds prior to the mRNA in rabbit globin synthesis.
Knowledge of the two plant initiator factors involved in binding methionyl-tRNAi and mRNA is much less complete than for the similar factors in bacterial and mammalian systems; details of the latter are, therefore, relevant. In bacteria there are three factors, IF-1, IF-2 and IF-3 involved in the initiation process. Factor IF-1 has a molecular weight of 9,400 daltons and is the most basic of the three proteins. Factor IF-2 has been shown to consist of two sub-components, both active, with molecular weights of 9 x 103 –100 × 103 daltons and 80 × 103 daltons although the latter is probably derived from the former by proteolysis during purification. Factor IF-3 is a protein or group of proteins with molecular weight(s) of 21.5 × 103 –23.5 × 103 daltons (Grunberg-Manago et al., 1973). The three factors attach to the small subunit; IF-2 binds GTP and formyl-methionyl-RNA, while IF-3 binds mRNA. Factor IF-I increases the affinity of factors IF-2 and IF-3 for the 30s subunit, and is also necessary for the release of IF-2 from the ribosome.
The order of the attachment of the different factors to the small subunit and the order of the binding of mRNA and acylated initiator tRNA, is still a matter of debate. Some results suggest that IF-1 is attached before IF-2 and IF-3, whilst others indicate that it can only be attached after. Similarly, it is possible that an unstable intermediary complex consisting of formyl-methionyl-tRNAi •30s subunit•initiation factors is formed, i.e. that mRNA is bound after initiator-tRNA, or that a 30s subunit•mRNA•IF-3 complex is formed, which is then stabilized by the addition of formyl-methionyl-tRNAi and IF- I and IF-2. The next step in the process, the addition of the large subunit, does not require GTP, but
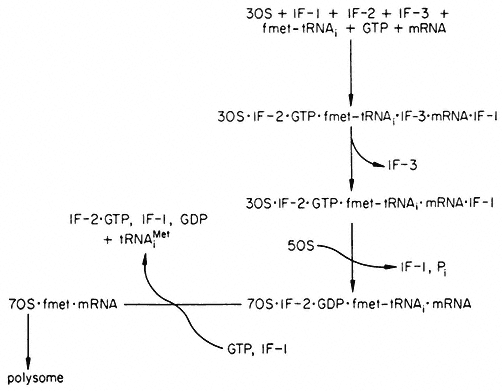
GTP hydrolysis is required to release IF-2 from the 70s ribosome complex. The role of GTP hydrolysis in initiation is still not fully understood, but it is not only required for the release of IF-2. Factor IF-3 is not only required for the binding of natural mRNAs, but also functions later either to dissociate the subunits after chain termination, or to keep dissociated subunits apart until another cycle of initiation is instituted. This activity of the IF-3 factor is referred to as its DF (ribosome dissociation factor) activity. None of the factors occurs on the polysomes and they recycle in protein synthesis, as shown in Fig. 10.6.
Figure 10.6
Recycling scheme for initiation factors in prokaryotes. The exact order
in which the factors interact with the 30s subunit is still not clear, nor is
whether fmet-tRNAi attaches before or after mRNA. IF-2•GTP•fmet-
tRNAi may exist independent of the 30s subunit. (fmet-tRNAi =acylated
formylmethionyl-tRNAi ; tRNAiMet =deacylated formylmethionyl-tRNAi ).
(Modified from Haselkorn & Rothman-Denes (1973).)
Several proteins which form an integral part of the small subunit have also been shown to be involved in initiation. Small subunit proteins, s1, s11, s13, s19, participate in IF-2 binding and s1, s11, s12, s13, s14, in IF-3 binding; s12 is responsible for recognition of mRNA (Haselkorn & Rothman-Denes, 1973; Anderson et al., 1974). Shine and Dalgarno (1974) have proposed a model of how the large subunit.initiation factor complex may recognize and attach mRNA. It would appear that the sequence (5') GGAGGU (3') is present in the same relative position with respect to the first translatable AUG triplet in all prokaryotic messengers so far analyzed. Furthermore, the 3' proximal end of 16s RNA has the sequence GAUCACCUCCU UA (OH), so that the underlined nucleotides could potentially base-pair with the GGAGGU sequence in the mRNA.
Further complexity of the initiation process is indicated by the work of
Schreier and Staehelin (1973), who have characterized five initiation factors (IF-E1 to IF-E5 ) for mammalian protein synthesis. Their results with rabbit globin mRNA are consistent with the formation initially of a methionyl-tRNA1 •IF-E2 •GTP complex, which is bound independently of mRNA to the 40s ribosome subunit by IF-E3 . Subsequent to this, mRNA and the 60s subunit are joined by the cooperative action of IF-E4 , ATP, IF-E1 and IF-E5 . The binding of natural mRNA requires IF-E4 and ATP. IF-E5 promotes the joining of the 40s complex with the 60s subunit and IF-E1 inhibits complex formation in the absence of mRNA binding. However, it has been suggested that the apparent need for additional factors results from deproteinization of ribosomal subunits and mRNAs, i.e. the requirement may be for structural proteins rather than initiation factors. The probable relationship between the different factors 1s given in Table 10.1. The little information available suggests that eukaryotic factors are not exchangeable with their prokaryotic counterparts.
| ||||||||||||||||||||||||||||||||||||||||
Factor EIF-3, contrary to its prokaryotic counterpart, consists of a number of different sized polypeptide chains; the question as to whether there are different EIF-3s or whether selectivity is controlled by additional mRNA specific factors, is still unresolved. A similar uncertainty to that described for the prokaryotic system surrounds the question as to whether or not methionyl-tRNAi binds to the 40s subunit prior to mRNA.
The initiation process is extremely complex and still not fully understood. A variety of proteins are involved, some being ribosomal structural proteins and others proteins which recycle during the process. These proteins interact, changing the conformation of the ribosome and thereby allowing the different steps of initiation to proceed in a sequential and orderly manner. Different mRNAs probably differ in their rate of attachment to the ribosome and/or in their efficiency in other stages of the initiation process, so affecting the frequency and rate of translation. With proteins which contain prosthetic groups their
absence may inhibit initiation, since it has been shown that lack of haemin in globin synthesis, results in the formation of an inhibitor of the binding of methionyl-tRNAi to 40s subunits (Adamson et al., 1972; Gross & Rabinovitz, 1973; Legon et al., 1973). Furthermore, a variety of mRNA specific factors have been proposed called 'interference' or 'i-factors', which affect the specificity of the initiation factor IF-3 (Groner et al., 1972). Thus, Strycharz et al. (1973) have identified a supernatant factor in Krebs II ascites cells, which is specifically required for the initiation of the translation of encephalomyocorditis viral RNA. However, Lodish (1974), from a kinetic analysis of protein synthesis, has proposed a model which precludes the necessity for mRNA specific factors. He points out that these have often been observed in systems which are less active than the corresponding in vivo system, and that the three eukaryotic systems available which translate exogenous mRNAs efficiently in vitro, do so with a variety of mRNAs.
10.3.2.2—
Elongation of the Polypeptide Chain
The elongation process starts once the 80s plant ribosome containing mRNA and methionyl-tRNAi , has been assembled and takes place by the following repeated cycle of events, each cycle being separated into:
(a) codon-directed binding of aminoacyl-tRNA;
(b) peptide bond formation; and
(c) translocation.
The ribosome has two binding sites; a 'P' site for the binding of methionyl-tRNAi and an 'A' site where all other aminoacyl-tRNAs bind. After the initiation complex has been formed, the next aminoacyl-tRNA, i.e. the one carrying the anticodon to the next codon of the mRNA, binds at the 'A' site. Once the aminoacyl-tRNA has been bound in the 'A' site, a peptide bond is formed by a peptidyl-transferase enzyme, which is part of the large subunit, such that the 'A' site now carries the aminoacyl-tRNA joined to methionine by a peptide bond. The 'P' site now carries the deacylated tRNAiMet , which is subsequently removed leaving an open 'P' site, and the peptidyl-tRNA moves from the 'A' to the 'P' site, so moving the message relative to the ribosome. The 'A' site is now empty and is ready for the next aminoacyl-tRNA to enter according to the next codon in the message. This cycle of reactions requires K+ , Mg2+ , GTP and various elongation factors, proteins, which are found in the soluble fraction of cell homogenates.
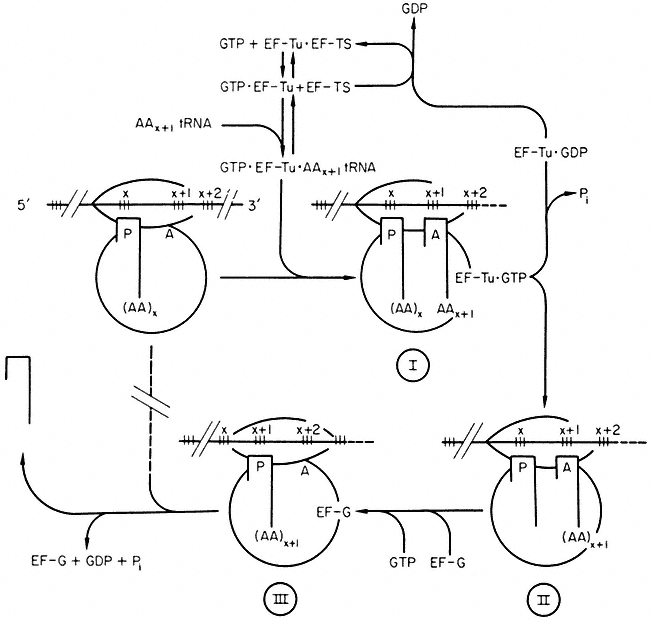
Our knowledge of polypeptide chain elongation is most complete with E. coli, where three elongation factors are known, EF-Tu, EF-Ts and EF-G (Fig. 10.7). Factor EF-Tu binds stepwise with GTP and aminoacyl-tRNA and this ternary complex transfers the aminoacyl-tRNA to the 'A' site of the ribosome, releasing an EF-Tu•GDP complex and inorganic phosphate. Factor EF-Ts displaces GDP to form the complex EF-Ts•EF-Tu, from which GTP displaces EF-Ts to regenerate EF-Tu•GTP. The third elongation factor, EF-G,
and free GTP, are responsible for the translocation of the peptidyl tRNA from the A' to the 'P' site with the prior removal of the deacylated tRNA from the 'P' site; GTP is hydrolyzed during the process. The elongation factors, like the initiation factors, recycle during protein synthesis (Fig, 10.7). The protein chain grows from the N-terminal end, and probably starts to fold into its three-dimensional conformation whilst the process of elongation is proceeding.
Figure 10.7
Polypeptide chain elongation in prokaryotes. Ribosomes cover more
than two codons on mRNA. Reaction I ® II is catalysed by peptidyl transferase.
( ![]() = tRNA, (AA)x = peptide with N-terminal amino acid x. AAx+1 = amino acid x+ 1.)
= tRNA, (AA)x = peptide with N-terminal amino acid x. AAx+1 = amino acid x+ 1.)
(Modified from Haselkorn & Rothman-Denes (1973).)
Fewer details of the elongation factors are available in eukaryotic systems. However, two elongation factors, designated as the binding enzyme EF-1 and the translocase EF-2 (Hardesty et al., 1963; Gasior & Moldave, 1965) have been purified from ungerminated wheat embryos (Golinska & Legocki 1973; Twardowski & Legocki, 1973; Legocki, 1973; Allende et al., 1973). The mechanism of binding of aminoacyl-tRNA to wheat ribosomes is on the whole similar to
that in E. coli, the main difference being the absence in the eukaryotic system of a factor with the properties of EF-Ts, i.e. EF-1 = EF-Tu and EF-2 = EF-G.
Elongation factor EF-I, which requires the presence of Mg2+ at low concentration, K+ and GTP, is responsible for the binding of aminoacyl-tRNA to wheat ribosomes. It contains three active forms differing in molecular weight. The larger EF-1 forms dissociate to a single species of molecular weight 6 × 104 daltons during gel electrophoresis in the presence of SDS. The three forms observed may represent a monomer, trimer and tetramer of a single protein unit, of which the trimer seems to be the most stable form. Some experimental evidence supports the view that the light species is the active form of the EF-1 enzyme. For example, in calf brain and wheat embryo, it has been shown that the interaction of EF-1 with GTP and aminoacyl-tRNA yields a ternary complex which contains the light form of the enzyme, i.e. molecular weight 5 × 104 to 6 × 104 daltons (Moon et al., 1972; Legocki, 1973). It is suggested that the amino group of the esterified amino acid plays an important role in aminoacyl-tRNA binding (Jerez et al., 1969) and this differs from the bacterial system where EF-Tu can react with the deaminated product of phenylalanyl-tRNA, phenyl-lactyl-tRNA (Fahnestock et al., 1972).
The second elongation factor, EF-2, is involved in translocation of the peptidyl-tRNA from the 'A' site to the 'P' site during elongation. It is a protein of molecular weight 7 × 104 daltons and requires thiol compounds for activity (Twardowski & Legocki, 1973).
Ribosomes and elongation factors from the cytoplasm of eukaryotic organisms are not functionally interchangeable with their E. coli counterparts (Krisko et al., 1969). Furthermore, Perani et al., (1971) have shown absolute ribosome specificity, either for 70s or 80s ribosomes, of two sets of elongation factors, T and G (EF-1 and EF-2), isolated from yeast, and for factor EF-2 isolated from the alga, Prototheca zopfii. It is generally accepted that elongation factors within each ribosomal type (70s or 80s) are exchangeable between eukaryotes (Ciferri, 1972).
10.3.2.3—
Termination and Release of the Polypeptide Chain
No reliable information is available on chain termination in plants, most of the work having been done with E. coli. However, as there are some differences between the prokaryote and mammalian eukaryote systems, these will be mentioned briefly. In E. coli the elongation cycle is repeated until certain termination codons on the mRNA come into the 'A' site; these codons are UAG, UAA and UGA. At least three release factors, proteins, which recognize the terminator codons and bring about the release of the completed polypeptide chain from the tRNA, have been identified (Caskey et al., 1969; Capecchi & Klein, 1969; Milman et al., 1969). Originally designated R-1, R-2 and S these are now called RF-1, RF-2 and RF-3. RF-1 recognizes UGG or UAG, RF-2
recognizes UAA or UGA, and RF-3 affects the rate of release of the polypeptide chain. In mammalian systems, only a single release factor which responds to all three codons has been identified, and furthermore the prokaryotic termination requirement for GTP appears to be absent (Haselkorn & Rothman-Denes, 1973).
In addition, several other termination factors have been proposed from different organisms (see Haselkorn & Rothman-Denes, 1973). Although little is known about chain termination in plants, the presumed universality of the code suggests that similar terminator triplets and proteins are involved in plants also.
Originally, it was thought that on chain termination ribosomes dissociated into subunits and that the free ribosomes found in the cell were inactive in further protein synthesis. Noll et al. (1973) have presented evidence that at least some of the free ribosomes found in the cell interact with a factor and partially dissociate, so that the small subunit with bound IF-1, IF-2 and IF-3, can initiate protein synthesis.
In conclusion, it may be said that small differences exist between the mechanism of protein synthesis in the pro- and eukaryotic systems, e.g. the initiator tRNA, the involvement of GTP in different steps, as well as in the possible number of elongation factors. In view of the technical difficulties involved, work on the detailed mechanisms of eukaryotic systems could be considered hardly worthwhile. However, it is possible that translation level controls are much more important in eukaryotes than they are in prokaryotes. This possibility, together with the importance of plant proteins as a source of food, are justification enough for an attempt to characterize the process in detail in plants and, thereby develop suitable assay systems.
10.3.2.4—
Cell-Free Systems
In order to gain detailed knowledge of the mechanism of protein synthesis in plants, it is necessary to develop in vitro assay systems. Such systems must satisfy the following criteria:
(a) be dependent on the exogenous component(s) to be tested;
(b) be efficient, i.e. of comparable activity to in vivo rates, in order to allow quantitative analysis;
(c) the product, whose synthesis is monitored, must be clearly defined.
Two basic types of cell-free systems are used: fractionated systems, in which enzymes and components are isolated, purified and then reconstituted into an assay system, and unfractionated systems, in which the cells are broken open and cell-debris, nuclei and mitochondria removed by centrifugation leaving the ribosomes and the various components for protein synthesis in the supernatant, which is then used as the assay system. If all of the components of the assay are isolated from one organism, the system is said to be homologous as opposed to heterologous when they are not.
In addition to the fractionated E. coli prokaryotic system, there are four
fractionated eukaryotic systems which satisfy the above criteria. These are the rabbit reticulocyte (Lockard & Lingrel, 1969; Gilbert & Anderson, 1970), the mammalian liver (Prichard et al., 1971), the Krebs II ascites cell (Mathews & Korner, 1970), and the only plant system, that of wheatgerm (Allende & Bravo, 1966; Allende, 1970; Leis & Keller, 1970; Marcus et al., 1970a,b; Tarrago et al., 1970; Ghosh et al., 1971; Legocki & Marcus, 1970; Klein et al., 1972; Lundquist et al., 1972). Other fractionated cell-free systems from plants, such as those from developing legume seeds (Gumilevskaya et al., 1971; Payne et al., 1971a,b; Yarwood et al., 1971a,b; Beevers & Poulson, 1972; Wells & Beevers, 1973), are not well characterized and assays often lack quantitative accuracy. This is because in isolating the constituent enzymes and components, damage is caused in breaking the cell wall by hydrodynamic sheer, by cell vacuoles breaking and releasing acids, phenolics, tannins and other substances, and by activation of proteases and nucleases (Payne & Boulter, 1974). If the preparations used contain several components and structural damage to ribosomes has occurred, interpretation of the results may be qualitatively ambiguous, since enzyme preparations may contain proteins needed for the reconstitution of protein-leached ribosomes.
Unfractionated cell-free systems cannot be made from tissues which have a high nuclease or protease activity, and until better methods for the inhibition of degradative enzymes become available, most unfractionated systems from plants will be of limited value. Recently, Davies et al. (1972) have used media of high ionic strength and high pH with some success, and Gray and Kekwick (1973) have developed a system from pea seedlings using 0.2 mM vanadyl sulphate as an inhibitor. This system has been shown to synthesize the small subunit of ribulose bisphosphate carboxylase (Fraction 1 protein), since the tryptic peptides of the in vitro product were similar to those of the naturally occurring protein. The identity of the product was also proved by immunoprecipitation. The requirement of product identification is essential in complete cell-free assays (see also chapter 11, where the synthesis of the large subunit of Fraction I protein by the chloroplast cell-free system is described).
The wheatgerm system is by far the most successful unfractionated cell-free system from plants. Its preparation is as follows (Marcus et al., 1974):
Dry wheat embryos are ground thoroughly with a small amount of sand in a precooled mortar in a total volume of 3.3 ml of 90 mM KC1, 2 mM CaCl2 , 1 mM Mg (Ac)2 , 6 mM KHCO3 . The embryos are initially ground in 1.0 ml with 0.5 and 1.8 ml increments added subsequently. The slurry is then centrifuged for 10 minutes at 23,500 × g and the supernatant is removed with a Pasteur pipette, taking care to leave behind as much as possible of the upper lipid layer. Just prior to use, 0.5–3.0 ml are dialyzed against 500 ml of 1 mM Tris–acetate, pH 7.6, 50 mM KCl, 2 mM Mg(Ac)2 , 4 mM 2-mercapto-ethanol for 1.75 hours; this preparation is termed S23.
The wheatgerm system is attractive in spite of needing some additions, (e.g. tRNA), since it shows great promise as a 'translation' system for various mRNAs. This is an important development as the isolation of mRNAs from plants is now feasible using binding to oligo-dT columns (since a proportion of eukaryote messengers contain a poly-A sequence), by gradient centrifugation and by immunoprecipitation of polysomes (Haselkorn & Rothman-Denes, 1973; see also chapter 9).
Four RNAs of brome mosaic virus (BMW) induce amino acid incorporation into proteins when used as messengers in the wheatgerm system. RNA4 is translated with an efficiency comparable to that of bacteriophage RNA in E. coli extracts. The product is homogeneous and indistinguishable from the coat protein of BMV (Shih & Kaesberg, 1973). Satellite tobacco necrosis virus RNA has also been translated in this system and the product shown to be coat protein (Klein et al., 1972). Several natural eukaryotic messengers have also been translated in the wheatgerm system, e.g. rabbit globin mRNA (Efron & Marcus, 1973) and leghaemoglobin RNA (Verma et al., 1974). The latter is the only naturally occurring plant messenger to be isolated so far. It is a poly A-containing 9s–12s mRNA which was isolated from soybean root nodule polysomes. When used to programme the wheatgerm system by Verma et al., (1974), the product was identified serologically as mainly leghaemoglobin S (LbS ) with a little leghaemoglobin F (LbF ); there are two types of leghaemoglobin found in soybean nodules and the reason for the preferential synthesis of the LbSin vitro, is not understood. Factors other than those already present in the wheatgerm system were not required, and there was no need for the addition of heme.
There are several unfractionated cell-free systems from animals (Haselkorn & Rothman-Denes, 1973). Of particular interest is that of the Xenopus oocyte since intact eggs are used to translate exogenous messages (Gurdon et al., 1971). Natural plant messages have not been used but there is evidence that tobacco mosaic virus RNA can be translated (Knowland, 1973). Coat protein is not synthesized, however, and the main product is a polypeptide with molecular weight 14 × 104 daltons. Although no function has been assigned to this protein, it is known that plant cells infected with TMV also make a protein of the same molecular weight (J. Gurdon, personal communication).
The use of heterologous systems assumes the interchangeability of the various enzymes and components between different systems and as mentioned previously, it is generally accepted that this is not usually possible between prokaryotic and eukaryotic organisms. Generally, attempts to demonstrate interchangeability between different eukaryotic systems have been positive, but several workers have suggested that this is because discrimination can only be seen under optimum conditions. The general conclusion is that tissue and organism specificity may occur but only in exceptional cases (Mathews, 1973).
10.3.2.5—
Inhibitors
Several antibiotics and other inhibitors block various steps in protein synthesis, and are, therefore, extremely useful in establishing the mechanism of the process (Pestka, 1974). Table 10.2 lists the more common inhibitors and their sites of action; many of these have been used with plants. It is important to realize
| ||||||||||||
that the concentration of the inhibitor is often critical for the production of a specific effect; it is essential to establish the conditions for the correct use of an inhibitor with each tissue and to maintain effective controls. It has been suggested by Glazer and Sartorelli (1972) that in rat liver, membrane-bound 80s ribosomes are more susceptible to a range of inhibitors than are the 80s ribosomes free in the cytosol.
10.3.3—
Post-Translational Changes
Changes which take place after the synthesis of the polypeptide chain are called post-translational. The most common of these are concerned with the many proteins which consist of more than one polypeptide chain. Since there is no evidence that unpartnered subunits occur in any quantity, machinery must exist in the cell to ensure that approximately correct numbers of each constituent polypeptide chain are synthesized and correctly assembled, although little is known about the mechanisms. Some mRNAs may be polycistronic but in other
cases mRNAs for each polypeptide chain are translated independently. Self-assembly by interaction at specific sites on different polypeptide chains may occur.
Several examples are known where some subunits of a protein are made in the cytoplasm and others on the chloroplast or mitochondria. For example, ribulose bisphosphate carboxylase is a large molecule (also known as Fraction I protein) consisting of two types of subunit; the small subunits are synthesized on 80s cytoplasmic ribosomes, whilst the large subunits are synthesized on 70s chloroplast ribosomes and assembly of the two types to form the complete molecule occurs in the chloroplast (see chapter 11).
Post-translational changes also occur by the addition of prosthetic groups or by modification of the polypeptide chain. Such modifications may be the formation of disulphide linkages, the removal of N-terminal portions, the removal of intramolecular segments, the derivation of amino acids and the addition of carbohydrate or lipid units to form glycoproteins and lipoproteins respectively. These changes are all brought about by specific enzymes and probably after the polypeptide chain has left the ribosome.
The formation of disulphide linkages is often part of the assembly of multichain proteins, e.g. legumin in Vicia faba (Wright & Boulter, 1974), although the enzymes involved have not been demonstrated from plants. Few plant proteins have methionine at their N-terminus when isolated, although this amino acid must be presumed to be the N-terminus when synthesized, and, therefore, removal of one or more residues at the N-terminal region must have occurred subsequently. Enzymes for this purpose have been demonstrated in bacteria but not in plants, and it is possible that the process in plants is non-specific and mediated by leucine aminopeptidase, a ubiquitous plant enzyme which removes amino acids from the N-terminus of proteins and may continue to do so until the conformation of the protein inhibits its activity. The removal of intramolecular segments of plant proteins similar to the situation with proinsulin in animals, has not been demonstrated, but there is no reason to suppose that it does not occur. Modification of individual amino acids is quite common in plant proteins; examples are, S -N-trimethyllysine in some cytochromes c (Ramshaw et al., 1974), and hydroxyproline in extensin, a protein of cell walls (Lamport, 1965). Where investigated, the protein when synthesized contained the parent amino acid, the substituted amino acid arising secondarily, probably after the protein had left the ribosome. Some residue positions of a particular amino acid may be substituted and others not, though the specificity of the changes is not understood.
Synthesis of the protein moiety of glycoproteins takes place on the membrane-bound ribosomes of the ER, but the sugar groups are added sequentially at different sites in the cell. Those closely linked to the polypeptide chain such as glucosamine and mannose, are added immediately after release from the ribosome or in the ER itself, other more terminal groups may be added later, some in the Golgi apparatus (Whaley et al., 1972).
10.4—
Protein Synthesis in vivo
The rate of protein synthesis in vivo can be measured by determining the amount of protein in a plant, organ or tissue at two different times; this will give the net rate since proteins 'turnover' and the amount of protein found would represent the balance between synthesis and degradation (Huffaker & Petersen, 1974).
Various equations describing the relationship between the synthesis and degradation of protein have been proposed but there are many technical and interpretive difficulties in this type of experiment. The turnover rate for an individual protein is not the same as the value obtained for the total protein and although the latter may be of interest agriculturally, most biochemical and physiological studies require information on individual proteins. This information has only been obtained so far with a few plant proteins, although most are thought to turnover (Huffaker & Petersen, 1974; see also chapter 12). Possible exceptions are storage proteins which are sequestered from the metabolically active cytoplasm and stored in membrane-bound protein bodies until subsequently required at a more active phase of the life-cycle.
A variety of studies using acrylamide gel electrophoresis has shown that the protein complement of organs and tissues changes during the life-cycle of a plant, and also in response to environmental conditions. Organ-specific proteins have been demonstrated serologically and often one or a few proteins may predominate in an organ, e.g. ribulose bisphosphate carboxylase, which often accounts for 50% of the protein of leaves. The changing complement of proteins during development is due to changes in the type or rate of protein synthesis and/or degradation. For example, Beevers and Poulson (1972) followed changes in the protein content of pea cotyledons during the period 9–33 days after flowering. Initially, protein content increased gradually, then rapidly between 21–27 days to decline once more as the seed dehydrated and matured. Incorporation experiments with 14 C-leucine indicated that albumin proteins were synthesized early in cotyledon development whilst globulin synthesis predominated with increasing maturity. During the period of rapid protein synthesis, greater amounts and a higher percentage of polysomes to monosomes were extracted than at other stages in the development of the seed. However, unlike in bacteria, where induced enzyme synthesis is widespread and the product is not 'turned over' but diluted out by a rapid rate of cell division, plants have a much slower rate of cell division and protein degradation appears to be a common method for regulating the amount, and hence the activity of proteins.
The relative amounts of different seed proteins synthesized may vary considerably in different varieties and breeding lines of a crop; for example, in maize where the major storage proteins of the seeds are prolamins and glutelins normal maize contains a relatively high proportion of prolamin, whereas in the mutant Opaque 2, the proportion of prolamin is greatly reduced. This is of particular significance since prolamin is very deficient in the essential amino
acid lysine, so that seed meals obtained from Opaque 2 have a higher content of lysine and are consequently more nutritious than those from normal maize.
Protein synthesis is a concerted process involving the biochemical machinery of the cell as a whole, since it consists of a series of co-ordinated reactions, the constituents of which may be synthesized in the nucleus (mRNA, tRNA), the mitochondria (ATP), the cytosol (amino acids and aminoacyl tRNAs), the ribosomes (enzymes). Although changes in the rate of protein synthesis occur, very little is known about the mechanisms involved. Theoretically, control could be exerted at any one of the many constituent reactions, this being the pacemaker reaction for protein synthesis in that particular cell/tissue. However, the control mechanisms must allow the overall process to adjust in response to genetic, physiological and environmental stimuli, so that the rate and/or the type of proteins being synthesized can change.
Further Reading
Allende J.E. (1970) Protein biosynthesis in plant systems. Techniques in Protein Biosynthesis, vol. 2 (eds. P.N. Campbell & J.R. Sargeant), pp. 55–100. Academic Press, London and New York.
Anderson W.F., Bosch L., Gros F., Grunberg-Manago M., Ochoa S., Rich A. & Staehelin Th. (1974) Initiation of protein synthesis in prokaryotic and eukaryotic systems. FEBS Letters48, 1–6.
Boulter D. (1970) Protein synthesis in plants Ann. Rev. Pl. Physiol.21, 91–114.
Boulter D., Ellis R.J. & Yarwood A. (1972) Biochemistry of protein synthesis in plants. Biol. Rev.47, 113–75.
Haselkorn R. & Rothman-Denes L.B. (1973) Protein synthesis. Ann. Rev. Biochem.42, 397–438.
Kurland C.G. (1972) Structure and function of the bacterial ribosomes. Ann. Rev. Biochem.41, 377–408.
Mans R.J. (1967) Protein synthesis in higher plants. Ann. Rev. Pl. Physiol.18, 127–46.
Stewart P.R. & Letham D.S. (Eds.) (1973) The Ribonucleic Acids. Springer-Verlag.
Wittman H.G. (1973) Structure and function of bacterial ribosomes. In: Regulation of Transcription and Translation in Eukaryotes, (eds. E.K.F. Bautz, P. Karlson & H. Kersten), pp. 211–212. 24 Colloquium der Gesellschaft für Biologische Chemie. Springer-Verlag Berlin, Heidelberg, New York.
Zalik S. & Jones B.L. (1973) Protein biosynthesis. Ann. Rev. Pl. Physiol.24, 47–68.