III—
Foundations of the Rad Lab
1—
The Place
The View from Above
The angel above the usual academic heaven to whom Lawrence first appealed was Frederick G. Cottrell, who had grown rich combating the powers of darkness. In 1906, while an assistant professor of chemistry at Berkeley, Cottrell had invented an electrostatic precipitator that removed noxious particles from the smoke of a local Dupont plant. The trick worked for other industrial polluters too; and in 1914 Cottrell endowed a not-for-profit business, the Research Corporation, to "serve the growing number of men in academic positions who evolve useful and patentable inventions from time to time in connection with their work and [who] without looking personally for any financial reward, would gladly see these further developed for the public good." Its charter required its board of directors, which included T. Coleman Dupont, Elihu Thomson, and other powerful men of American industry, to seek out and support research projects that might lead to profitable patents. Cottrell expected that proceeds from working or leasing the patents would repay the Corporation's investment in the research behind them and replenish its capital fund.[1]
The conservatism of the Corporation's board and the indifferent return on its patents during the war kept it from fulfilling its
charter until the year of the crash, when it gave the Smithsonian Institution a grant to study the biological effects of radiation. Then, under an aggressive president, Howard A. Poillon, who wished to cut a figure in the world of scientific giving, and with the advice of Cottrell, who was retained as a consultant, the Corporation collected what it could from its precipitators and shopped for patentable material at research universities. By the end of 1932 it had given $120,000 in aid of research to universities and other public institutions.[2] At the time the Corporation began to stir, the universities were awakening to the predicament into which the new relations between science and industry, or research and commercialization, had placed them. Who if anyone should protect the patentable results flowing from academic laboratories? Should not the universities whose facilities produced the results? And ought not educational institutions to prosecute their rights with what vigor they could manage during the downturn in the economy, to offset losses in endowment income and state allocations?[3] But then, would it not be compromising, and even immoral, to obtain royalties from inventions made at tax-exempt institutions supported by public monies or private gifts? The farmer whose taxes underwrote agricultural research at a state university would not be pleased to pay for it again through royalties on the patents it furnished. Would not the farmer—and everyone else—decide to leave a university so conducted to make its own way? "Why should gifts intended for the general welfare play the rôle of capitalizing a business?"[4]
These questions were aired frequently during the early 1930s, in the pages of professional journals, in a report by the Committee on Patents of the AAAS, in a Symposium on Patents at the Patent Office, and at the NRC.[5] A minority preached that universities should have nothing to do with patents—the proximity to commer-
cialization would demoralize the professoriate, threaten impartiality and openness, promote jealousy, ruin academic standards, debase learning, and exterminate the race of Faradays and Maxwells who did science for the love of it and made discoveries that transformed the world.[6] Another minority held that "there is nothing laudatory in the fact that [Faraday and Maxwell] and their colleagues failed to visualize the vast network of power utilities and communication systems which have evolved from their investigations" and urged scientists to pursue whatever financial gain their discoveries might bring them; "'Truth for truth's sake' is a delusion of so-called savants."[7] The majority favored some sort of protection. The AAAS's Committee on Patents, on which Cottrell served, urged patenting by or on behalf of universities, and not primarily for profit. That would be counterproductive: "A scientist who is impelled only by a motive of profit is far less likely [than others] to make any important contribution to knowledge." The main interests of both the inventive professor and his university should be to control the products resulting from his research: to oversee their quality, price, and advertising; to protect the public; and to return enough to pay for the oversight and, perhaps, to support further research.[8]
The AAAS's Patent Committee drew attention to two practices that enabled universities to obtain some measure of control and return without entering into business directly. One set up a special board within the institution, as Caltech, MIT, and the universities of Illinois and Minnesota had done. The other left the administration in the hands of a special corporation, of which the cynosure was the Wisconsin Alumni Research Foundation (WARF), established in 1925 with a Wisconsin professor's patent on vitamin D as its capital. WARF put its profits back into research at the university; and by 1937 it had given about $700,000 and an endowment that yielded over $125,000 a year. This success inspired other hard-pressed universities. By 1936 Cincinnati, Columbia, Cornell, Iowa State, Lehigh, Penn State,
Purdue, Rutgers, and Utah had similar organizations.[9] California was exceptional among the great land grant colleges in not having an equivalent of WARF.
The year that WARF began, the president of the University of California charged the Board of Research to formulate a patent policy. An overstrict system resulted: any member of the University who perfected a patentable invention was obliged to bring it to the attention of the president, who would appoint a special board to advise him what to do with it. Neither the institution nor its faculty gained much from this procedure. Six years later, in 1931, when WARF was assisting research at Wisconsin at $1,000 a day, Leonard Loeb, then chairman of the Patent Committee of the Board of Research, decided that more might be done in California. He approached Cottrell. Now Cottrell had originally wished to give his patents to the University and had set up the Research Corporation only when it became clear that no public body acceptable to him had the mandate or enterprise to accept them.[10] It now appeared that the relationship earlier proposed could be reversed and the Research Corporation set up as the holding company for the University. Discussions between Cottrell and the Board of Research in the late spring of 1931 seemed promising to both parties. Armin Leuschner, the board's head, tried the alliance with a successful application to the Research Corporation for $5,000 for general support of work in the natural sciences at Berkeley.[11]
These pourparlers had the endorsement of president Sproul, who met with Cottrell and inclined to put promising material "at the disposal of the Research Corporation or [its partner] the Chemical Foundation for consideration as to whether or not it desires to secure patents." Sproul believed in a symbiosis of capitalistic and academic industry. "This is the age of science and
democracy," he said in his inaugural address in 1930, "an age of strain and steel, electricity, chemistry, and science." "The work of the laboratory capitalized in the factory and by industry has built up a great civilization." Sproul had graduated from Berkeley in engineering in 1913 and returned with two years' experience to begin the climb from the Comptroller's Office to the presidency. He declared that no one belonged on the faculty who did not do research; for research, especially scientific and engineering research, research that might eventuate in patents, was the pump of progress. "Endlessly going over old lessons is a narcotic to progress."[12] He would have liked, though he could not have afforded, a faculty of Lawrences. To him, the aggressive policies of the Research Corporation fit the circumstances of the University perfectly: the strain of the age, the obligation to research, the cut in the budget. He rescinded the patent policy of 1926, in order, he said, to give faculty members full freedom of action. But he recommended that the action go to the Research Corporation.[13]
And the Corporation was aggressive. To work its major asset, it set up a worldwide cartel so restrictive and unfair in its practices that the U.S. government felt obliged to force the American branch to change its ways. The Corporation fought in the courts as vigorously as General Electric, pushed development of the inventions entrusted to it, and hunted out promising professors. It was more combative than WARF, which did not always behave gently either (the Corporation restricted the number of licenses of its primary patents and eventually also had to alter its ways). But WARF's ties to the university moderated its commercialism.[14] The Research Corporation had fewer inhibitions. Lawrence was to adopt its methods as well to profit from its support.
Cottrell became Lawrence's agent as well as his angel. The first job was to secure a big old Poulsen magnet, one of a pair of derelicts belonging to Federal Telegraph. At the prompting of its
former employee and Lawrence's colleague in the School of Engineering, Leonard Fuller, Federal seemed willing to give one to the University. But the magnet required much reworking to answer Lawrence's purpose. Not then believing that the cyclotron had sufficient promise to justify a very large investment by the Research Corporation, Cottrell recommended that Lawrence stop in Saint Louis in the spring of 1931 on his way to a meeting of the NAS; the Radiological Research Institute there might give something toward refurbishing the magnet. The president of the institute, E.C. Ernst, had an interest, but no principal, to invest in Lawrence's projects. Cottrell next suggested the Chemical Foundation, set up by the government in 1920 to administer 5,000 German chemical patents seized during World War I. The Foundation was intended to protect American chemical industry while it developed domestic strength on German innovations, and in the shade of a high tariff wall, it earned royalties that amounted to nearly $9 million by 1932. Its charter obliged it to spend its income "for the advancement of chemical and allied science and industry," a mission very liberally interpreted, or stretched, by its chief officers Francis Garvan and William Buffum. Its adventure of greatest interest to us was the licensing of two patents it held on high-voltage x-ray tubes. When GE brought a suit for infringement against the license, the Foundation and the Radiological Research Institute countersued, with the consequence that both suits were dismissed and the field opened to further development.[15]
This connection may have been important when, on Cottrell's urging, Poillon went to see the executives of the Chemical Foundation some time in July 1931 to work out cooperative support for Lawrence's project. The connection: the quashing of GE's patents on x-ray tubes had saved Federal Telegraph over a million dollars. "[Buffum] is going to approach Federal and see how appreciative they are and suggest that they not only donate the magnet, but condition it." Then, Poillon continued to Cottrell, Lawrence would need but $7,000, which the Research Corporation
and the much richer Chemical Foundation could manage.[16] Federal did not care to square its obligation in this way. Poillon decided to contribute $5,000 and Buffum $2,500 of the $12,000 that Lawrence deemed necessary to rebuild, transport, and set up the magnet. Sproul put up the difference.[17]
From the standpoint of the Research Corporation and the Chemical Foundation, the most promising of the applications of magnets that Lawrence had in mind in the early summer of 1931 was the acceleration not of positive ions but of electrons, in the manner tried with no conspicuous success by Walton and by Tuve and Breit. Lawrence explained in a long memorandum, written in June, how he would improve on Walton's arrangement by multiplying magnets to hold the accelerating electrons on their paths. Lawrence's electrons would have energy high enough to penetrate the marketplace. "[They] carr[y] us straight into the field of high voltage x ray tubes and as such may prove medically and economically highly important," Cottrell wrote Poillon after studying Lawrence's memorandum. He also reminded the head of the Research Corporation that the head of the Chemical Foundation had "very definite ideas on the need for the development of x ray facilities." Following his policy and his penchant, Cottrell urged Lawrence to patent his scheme and to keep quiet about it until he had.[18]
After the pledge by the Research Corporation and the Chemical Foundation of the money toward the cyclotron—and after Lawrence had been told to patent it—Cottrell returned to the business of the early spring, "closer and more effective teamwork in general on patent matters" between the University and the Corporation. The goal might be secured in stages. "The Research
Corporation [should] study the patents and University of California negotiations with the purpose of seeing if it could not more effectively handle exploitation in eastern territory at least as an entering wedge, and if all worked well the Research Corporation could eventually take over the whole works."[19] The regents' secretary, R.M. Underhill, the University's comptroller, R.C. Nichols, and Sproul agreed to this procedure; and Lawrence immediately assigned his patent rights to the Corporation.[20]
Lawrence himself was an important property to the leadership of the small philanthropies that had pledged to support him. Cottrell outlined a grand future: "I believe Lawrence is a mighty promising young man for us to keep in touch with and develop in connection with our major plans. . . . Lawrence's work seems to me a very good peg on which to hang definitely a concrete proposal of cooperation between not only Chem. Found. & R.C. but also with Max Mason & Rockefeller Foundation , and thus bring to a head the tentative contacts already started. This particular work of Lawrence's is right in Max Mason's own field and will keenly interest him technically I am sure, which is an added advantage for the present purpose. Even if you [Poillon] and Buffum feel that you want to cover the complete needs of this particular line of research so as to feel freer with regard to the patent matters developing out of it, I still think it would be well for Lawrence to have a visit with Max Mason and for you all to talk over the larger plan." If the Rockefeller Foundation would play, the Carnegie Corporation and the California State Legislature might join the game. An empire might be built on precipitators. Lawrence was to make the enterprising Research Corporation his headquarters during his many trips to New York to raise money for his Laboratory.[21]
There was a snag, however. Professors did not have the spirit of cooperative enterprise of the industrial research laboratory. Cottrell to Poillon: "I find there is greater unwillingness on the part of people to tell each other their innermost ideas and note
that it is extremely prevalent among scientific men, especially when it comes to do with their colleagues." Nor did they immediately see the point of patents. Poillon to Cottrell: "If he [Lawrence] is one of the men that we are going to make awards to from time to time, it seems that we should develop his protective instincts."[22]
The View from the Ground
When the Research Corporation came into his life, Lawrence shared the inhibitions of the academic scientist against securing a personal financial interest in his discoveries or inventions. This inhibition arose at the end of the nineteenth century, when applications of electricity made physicists newly useful and threatened the identification of professors with high culture and disinterested speculation. Patents and professors should not mix. "Working as he does with public funds, directing as he does the minds and hands of students, it is, to say the least, scarcely honest [for a professor] to go with the results of such work to the Patent Office." Thus a British periodical for applied science editorialized in 1884. As for the Germans, "It is well known throughout the world," they said, "that the physical laboratories of Germany have no windows looking towards the patent office."[23] In the United States, Popular Science Monthly castigated people who would degrade science to a "low, money-making level," and memorialists praised defunct physicists who had resisted the blandishments of industry. That was their morality and their opportunity: "Nature turns a forbidding face to those who pay her court with the hope of gain, and is responsive only to those suitors whose love for her is pure and undefiled."[24]
These inhibitions—"a gesture of repugnance toward money-making as a practice inconsistent with intellectual integrity"—remained strong in the 1930s despite the increasing integration of academic science and industrial development. Hale built it into
his quest for endowment for academic science; donors need have no worry, he said, that their gifts would enrich their recipients; "the men of science . . . may be counted upon to devote their efforts to the advancement of knowledge without thought of personal gain." The unconventional Szilard's constant attendance on the Patent Office caused his friends to warn him of "an opposition to you [from the British physicists] on account of taking patents." "It is not customary [he allowed] to take out patents on scientific discoveries." In defending Steenbeck's claim to the invention of the betatron, the German industrial physicist Carl Ramsauer apologized for the "unusual" character of the documentation, "a prior patent application rather than a [scientific] publication."[25] But there was the difficulty that anyone might seek to patent a process openly described in the scientific literature after adding some small improvement to it; scientific ethos and self-protection did not run in parallel. A way out of the difficulty was to raise patenting to a social responsibility, according to the following formula. "In spite of the fact that I think that university people should not be interested in patents," Urey wrote his collaborator in the elecrolytic separation of deuterium, "for the protection of pure science . . . it would be wise to patent this process."[26] That was the way Lawrence came to think under the tutelage of the Research Corporation.
The University gave him no guidance. The cognizant body, the Board of Research, demonstrated its level of leadership and vigor of oversight in its opinion about what it understood to be a patent on a process for making radium from sodium. Leuschner to Lawrence: "We do not feel ourselves quite competent to judge the ethical side of the question. Your and Mr Poillon's own knowledge concerning the practice of pure physicists in this regard I think should be sufficient to guide you in whatever action you wish to take." And Leuschner to Poillon: "We have faith in the Research Corporation because it is a non-profit corporation and
supports research where its funds, according to its own judgment, are best applied."[27] By the time the ersatz radium came up, Lawrence was ready to put himself in Poillon's hands. He had put off patenting the cyclotron as unbefitting despite the Corporation's urging. Then he learned from John Slater, the new head of MIT's physics department, that an engineer at Raytheon had hit on the idea of a "proton merry-go-round" independently of all its other inventors. Raytheon called in a former student of Slater's, Eugene Feenberg, to calculate the machine's practicality; and, on receiving assurance of its promise, applied for a patent. "It would never occur to me," so the physicist's ethos spoke through Slater, "to patent such a thing." "It never occurred to me to patent the work we are doing either," Lawrence replied, "and I am doing so only at the urgent request of the Research Corporation and the Chemical Foundation, who apparently have a better perspective of practical things than we have."[28]
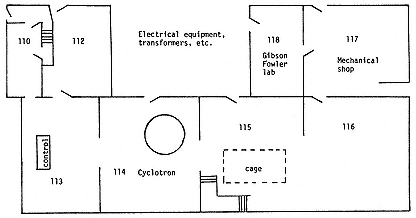
The work and immediate practical problems then fully occupied his time. His magnet, valued at $25,000, weighed over eighty tons. Where to put it? While Federal deliberated over making the gift, Lawrence toured the campus in search of a firm floor. Since he proposed to work on an engineering scale, he faced the challenge of winning a foothold in the preserves of the engineers. Civil Engineers politely declined to house him; Mechanical Engineers robotically refused him the ideal space they underutilized in the Mining Building; and he turned to Cottrell not only for the money to move and refurbish the magnet, but also for somewhere to put it.[29] Sproul supplied the firm floor. On August 26, 1931, also the day that Molly announced their engagement at the New Haven Lawn Club, Lawrence was assigned the Civil Engineering Test Laboratory, "a large frame structure with several substantial concrete piers in the rooms" (plate 3.1), for his experiments. We take this far-seeing decision of Sproul's as the foundation act of the Radiation Laboratory.[30]
Sproul's act freed Lawrence's operations from control and even supervision by the Department of Physics, although the Laboratory remained an integral part of the Department until 1936 and a satellite until 1939. From the beginning the Laboratory had a research budget exceeding the Department's, which remained just under $12,000 from 1930/31 to 1932/33, and fell to $8,000 in the worst Depression year, 1933/34, while the Laboratory's expenditures continually increased.[31] Lawrence spent his money without overscrupulous accounting and, when he required more, raised it from outside the University or by dealing directly with Sproul. Although Lawrence's rapid rise and independent base inspired jealousy in some of his fellow seekers after truth, in general his relations with his colleagues in the Physics Department were cordial, if not close. No senior member of the Department besides Lawrence steadily worked in the Laboratory during the 1930s.
Lawrence did not find it easy to consolidate his domain. The usual administrative burden of establishing a new institution in old surroundings was increased in his case by the weight of the magnet. "It is one hell of a job getting things moving," he wrote Cooksey in December, in the technical language of administrators. "I guess the new magnet is too damn heavy."[32] He got it moved first to the Pelton Waterwheel Company in San Francisco to rebuild the poles (plate 3.2).[33] He saw to the renovation of the new laboratory and to the eviction of most of its tenants. The mapping division of the Forest Service and French phonetics remained to soak up radiation (fig. 3.1).[34] And he laid industry under contribution. Federal Telegraph gave 650 gallons of transformer oil (value $227.50); American Smelting and Refining Company lent lead for shielding; and Federal supplied old, gassy, reject 20-kW oscillator tubes.[35]
Fig. 3.1
Layout of the ground floor of the old Radiation Laboratory. Room 118
belonged to Chemistry. Seaborg, J1, 1 , 12.
Lawrence became very adept at scrounging. He always needed power tubes, power transformers, and just plain power. In 1932 he wanted tubes for an x-ray machine. Federal sold them at $330 a piece, discounted. Lawrence offered $225 and the thought that if the Laboratory made a successful high-voltage x-ray plant with them, Federal's fortune would be made too. He tried the same ploy with the Deforest Radio Company: "The engineering development of our method will lead to a considerable oscillator tube business. In view of this you may feel disposed to furnish us tubes for our experimental development at considerable discount." Deforest allowed 10 percent; Federal agreed to accept $225, and charge the rest to charity; Lawrence stayed with Federal.[36]
Transformers provided an unlikely subject of comedy. GE loaned three 25-kW, and PG&E three 75-kW transformers, total value $2,000, in the fall of 1931, for three months or so. That was not to know Sproul or Poillon, who badgered the companies into extending the loans and then selling at a giveaway discount.[37]
On power, however, PG&E would not budge. In reply to Leuschner's pleas, the Company's president observed that PG&E was the second largest taxpayer in the state and saw no reason to abate its charges to an institution it already supported beyond its desires. "It seems to me the cost of experiments coming in the category of 'pure science' ought to come out of the funds of the University." For years Sproul had to top off Lawrence's power bill from his emergency fund.[38]
With gifts in kind and discounts Lawrence stayed within his first-year budget, that for 1931/32, even though he decided to rebuild the magnet more extensively than he had expected. Federal's gift was asymmetric; to provide a sufficiently uniform field for the cyclotron, however, it needed symmetrical poles on either side of the gap in which the vacuum tank would sit (plate 3.3). The Laboratory accordingly procured the answering pole from the remaining derelict. By November 1931, with almost everything bought or ordered, given and discounted, Lawrence had $600 left from the $7,500 from the Research Corporation and the Chemical Foundation. That, he thought, would get him through the rest of the academic year 1931/32, on the big cyclotron project at least.[39] But he had other things in mind as well.
2—
X Rays the Berkeley Way
Sloan's Tube
While Lawrence was moving into his new domain, an old friend, Joseph Boyce from Chicago, came to see what sort of physics went on in California. He reported his findings to Cockcroft. The work of C.C. Lauritsen and C.D. Anderson at Caltech, he said, was something in its way; "but the place on the Coast where things are really going on is Berkeley." Lawrence had in mind or hand no fewer than six different machines for throwing atomic projectiles. "On paper this sounds like a wild damn fool program,
but Lawrence is a very able director, has many graduate students, adequate financial backing, and in his work so far with protons and mercury ions has achieved sufficient success to justify great confidence in his future." Boyce itemized Lawrence's armamentarium: the second and third cyclotrons, the mercury-ion linac, a larger linac for protons, a Van de Graaff, and what Boyce misidentified as a Tesla coil. It is with this last apparatus, which Ralph Fowler of Cambridge thought the most interesting new apparatus at Berkeley, that we are now concerned.[40]
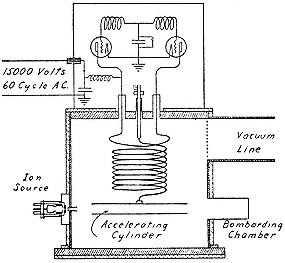
After the 30-stage mercury linac opened fire in the fall of 1931, Lawrence put Sloan to work on a resonant transformer as an alternative to the cyclotron for producing fast protons. The general idea of this apparatus appears from figure 3.2, which represents Sloan's final design. The secondary coil supports the water-cooled secondary tube. When the tube goes strongly negative, protons rush into it from the grounded ion source; they emerge half a cycle later, when the now positive tube drives them into the bombarding chamber. The protons in effect fall twice through the high potential of the secondary (800 kV in this design). When the device was designed, Livingston had not yet reached 1 MeV; Sloan's scheme promised to do so and, what was extremely important, to give a beam far more intense than could be expected from the cyclotron.[41]
Two features of the design in figure 3.2 distinguish it sharply from the Tesla coil used by Tuve's group. First, the secondary is part of a single oscillating circuit rather than a separately tuned one. The advantage of the design is efficiency, bought at the expense of high-power radio engineering, in which Sloan had the advice of Fuller. The second distinctive feature is that the heavy copper secondary coil is in effect an antenna placed inside the evacuated acceleration chamber and supported at a voltage node from the copper roof of the internal tank wall: the all-metallic connection obviated the need for insulation, the failure of which
Fig. 3.2
Sloan's resonance transformer for doubling the
energy of positive ions. Sloan, PR, 47 (1935), 67.
haunted the usual methods of producing and holding high voltages.[42]
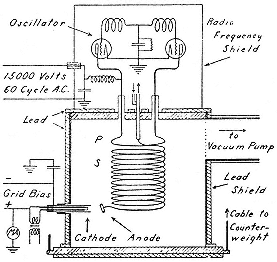
In November, having planned a tube that incorporated the entire oscillating system in the vacuum to eliminate corona discharge, Sloan turned to something that would make a better Christmas present for Lawrence and Cottrell. This was "a helluva x-ray outfit," with an intense beam at 100 kV, which, in Lawrence's opinion, could easily be hardened tenfold. "I feel quite sure [he wrote Cottrell in December] that ere long we will be producing million volt x rays."[43] Lawrence was right in fact but wrong in timing. It took two years of hard work to develop the generator of figure 3.3 into a plant that could be operated continuously at 800 kV.
Sloan's invention opened a new set of technical challenges and financial opportunities for the Laboratory. It promised to give the
Fig. 3.3
Sloan's x-ray generator. The high-energy x rays
arise at the anode attached to the secondary when
it stops electrons from the cathode. Sloan,
PR, 47 (1935), 65.
same sort of beam that Lauritsen obtained from his huge installation, but at a fraction of the cost. It gave a beam with over three times the energy of the output of the largest x-ray plants in use in hospitals with about the same demands on space and power (about 200 kW). And it marked the beginnings of interdisciplinary work at the Laboratory. To proceed, however, it was necessary to raise the amounts necessary to harden Sloan's hundred-thousand volt rays to Lawrence's canonical million.
Lawrence turned to the Research Corporation with the tactics it had taught him. "I know that the General Electric Company would be only too glad to get behind the project because it has immediate commercial possibility, but, of course, I hope this can be avoided." He thought $500 or $1,000 would bring the matter well forward.[44] Sloan's personal needs were covered by his fellowship, which GE renewed. Lawrence longed to proceed; Sloan had no desire to study for his exams; the work stalled for want of a
$400 pump and a little something for contingencies. Vacation was coming, freeing Sloan from his already minimal studies; perhaps, at the end of the summer, he would have a commercially viable machine. And perhaps not. If not, Lawrence held out, Sloan might be able to generate million-volt x rays another way, by bombarding light elements with the intense ion beam from the x-ray machine when adapted to accelerate protons. "This is a possibility that may turn out to be much more important than the production of x rays by electrons. . . . The medical applications of these latter considerations are certainly of considerable importance." Poillon returned a check for $500.[45]
During July 1932 Sloan succeeded in producing plenty of hard x rays without the intervention of protons, x rays powerful enough to penetrate a centimeter of lead or half an inch of steel. Birge, now department chairman, esteemed Sloan's tube "the most important of the discoveries of the radiation laboratory," not excluding the cyclotron; he recognized it as "mainly a commercial proposition;" and he worried that it would result in a "patent war or something with G.E."[46] The University announced Sloan's success to the national press and a mixed pilgrimage of humanitarians and promoters trekked to Berkeley. GE's San Francisco representative came right over and declared that the tube had "very great commercial value, not only for medical work but for the examination of steel welds." The home office quickly confirmed his interest.[47] Meanwhile the chief engineer of the Kelley-Koett Company, a major constructor of x-ray apparatus in the Midwest, put in an appearance. He was followed by a representative of Westinghouse, then entering the x-ray business in
search of a market for its power tubes, by the officer in charge of x-ray work in army hospitals, and by the roentgenologist at the University hospital in San Francisco, Robert Stone, who crossed the Bay, saw Sloan's rays burn through steel, and desired to use them on his patients.[48]
Sloan, J.J. Livingood (a postdoc from Princeton "very anxious to get into [the] nuclear racket," for whose services Lawrence paid nothing), and others improved the apparatus of figure 3.3 until it gave out rays of perhaps 700 kV. Then Lawrence, like the ingénue in The Importance of Being Earnest , outdid his wooers; he advised Poillon to rush commercialization by engineering the tubes in the Research Corporation's laboratories even while Sloan and his unpaid helpers continued development at Berkeley. "Not only do I feel that the method is superior for the production of radiations above a half million volts, but also I am inclined to think that very inexpensive outfits can be manufactured for the production of radiation in the region of 200 or 300 kilovolts. I am told that there is a very big market for such deep therapy outfits."[49]
Hospitalization
Two major research hospitals decided to try what Sloan's tube could do. The first commission came from Francis Carter Wood, the director of the Institute of Cancer Research of Columbia University, who had encouraged Lauritsen's work and knew of Berkeley's alternative through Buffum. The Chemical Foundation dispatched its physicist, Frank M. Exner, to help Sloan build an improved machine, for which Wood's Institute would pay $5,725 from a fund given by Charles Crocker, the son of one of California's railroad barons. How improved? Lawrence told Exner he wanted to push to a million volts; Exner told Wood, and Wood objected. His institute had long experience treating cancer with the gamma rays of radium, at 2 MeV, and they had not "done any miracles." Lawrence thought about it from the "physical point of view" (and also as an entrepreneur with a balky
client) and agreed. "There is not much point in x rays above a half a million volts for therapy purposes."[50] But the Sloan tube was to be developed for the art, as well as for medicine and patents, and also to support life in the Laboratory. Livingston, having graduated, needed a job to sustain him for his essential work on the cyclotron. Lawrence looked everywhere and found nothing. The obvious solution to all difficulties would be to employ Livingston part-time on x-ray money. The goal would stay a million volts.
Everything fell into place with the commissioning of a millionvolt plant by the University's Medical School. The initiative came from Stone and the money—some $12,000 for plant and installation—from William H. Crocker, brother to Columbia's patron and a regent of the University. Livingston became to Stone's machine what Exner was to Wood's. Therefore he was expected—so flimsy were the professional qualifications of radiologists then—to run it as well as to make it. His relevant training consisted of two weeks with Lauritsen to learn technique. He then set dosages for the human guinea pigs at the University hospital.[51]
The estimated costs of the twin Crocker machines included no profit, overhead, or salaries. Their 20-kW Federal power oscillators cost $300 each, after a 20 percent discount, and each machine needed four, or, in the new design, six. Economy was necessary. Sloan salvaged a ton of lead plates from old storage batteries, melted them down, and remolded them into shielding blocks. Safety demanded an additional 6,000 pounds of lead, which Lawrence begged as an additional loan from the American Smelting and Refining Company. Efficiency required an instrument maker, whose salary had to be found. To begin development of the hospital machines, Lawrence needed $4,000 immediately, or so he told the Chemical Foundation. His reckoning omitted the salaries of Sloan and Livingood.[52]
By December 1932 the prototype hospital machine was working beautifully. It could perform briefly at over a million volts, and at 750 kV gave out 14 milliamps so steadily as to melt its water-cooled copper target. It far exceeded their fondest hopes, Lawrence told Wood, being much superior to Coolidge's cascaded tubes or Lauritsen's set of gas-pump cylinders, and, moreover, compact and inexpensive, like the first cyclotron. It was a light that could not be hidden, the equivalent of half the world's supply of radium. "Though we have most of the tube plastered with lead (7,000 pounds in all), we see x rays almost everywhere in the lab with a fluoroscope." "There is no question now [December 1932] but that we have the most powerful x ray tube in the world and that our outfit is incomparably superior to any other, and promises to revolutionize x ray technology. I say this unqualifiedly."[53]
This last disclosure was directed to Poillon, who was trying to patent Sloan's invention when he received it. Poillon had not looked upon the work at Berkeley with unmixed satisfaction. It was the old problem with academics: they innovated, jerry-rigged, experimented, always improving and refining, indifferent to the business side of things. "I have told Sloan and Lawrence [Poillon complained to his patent lawyer] that we wanted this device brought to a certain state of perfection at Berkeley, where it could be engineered for production. They seem to be very anxious to get somebody else to do that secondary step. . . . This of course does not suit our book and I have warned them about directing the attention of others to it lest the others start the development and the patent situation becomes very cloudy." Poillon tried to silence the publicity that the commissioning of the hospital plants had generated.[54]
Poillon's impatience did not deflect Lawrence. Having done the physics, if not the engineering, of million-volt x rays, the Laboratory proposed to replace the heart of the machine, the expensive commercial oscillator, with cheaper devices of its own manufacture. That required patience from Stone and Exner and
money from Poillon and Buffum. Lawrence asked for $500 to $1,000. Poillon, hard-pressed and unenthusiastic, returned $700. "Money as you know is extremely scarce."[55] Fuller took over the direction of the project and assigned an engineering student to help design a practical 200-kW oscillator. A satisfactory design emerged, in which the oscillator shared the vacuum of the discharge tube; Lawrence stopped development of instrumentation for the cyclotron and threw his disposable resources into making and improving the new tube.[56] By June 1933, having spent $550 of the $700, Fuller, Sloan, and company had an oscillator more powerful than Federal's; by September they had licked the remaining vacuum problems; by December Stone's machine was ready for installation, and Sloan, exhausted, was ready for the hospital.[57]
Livingston and Sloan set up the San Francisco machine (plate 3.4). It performed beautifully, at a lower cost for power than expected. Crocker's $12,000 bought a building ($5,000), the machine ($4,000), Livingston's salary ($1,000), and accessories ($2,000). Running at 800 kV and 10 milliamps, it outdid all other x radiators, including Lauritsen's.[58] The business was, in fact, a great success. It showed how a subsidized university, with clever men, out-of-work or underpaid postdocs, and, if it wished, no overhead charge, could outdo industry. As Lawrence pointed out, GE had recently built an x-ray unit for a Chicago hospital that cost $65,000, faltered at 600 kV, and took much longer to install than Sloan's machine. GE's standard tubes cost $25,000, Kelley-Koett's 600 kV tubes $29,000. The Crocker brothers got a great bargain.[59]
Exner began to set up the Columbia machine after his return to New York in late spring of 1933. He eventually had as his helper Wesley Coates, who had completed his doctorate under Lawrence in 1933 with work on the soft x rays he found in targets struck by fast heavy ions from the old mercury linac. Coates went to Columbia to build a machine on Sloan's principles for the acceleration of protons. He then became the physicist at the Crocker Research Laboratory of Columbia Presbyterian Medical Center, where the Sloan x-ray machine had been placed. One day in 1937 the machine declined to hold its rated voltage. Coates peered in to diagnose the trouble, brushed a high-potential line, and fell a martyr to high energy.[60]
The Laboratory continued its efforts to improve Sloan's tubes well into 1935. Wood decided that experimentation with rays comparable in penetrating power with radium's gammas might be valuable after all and made available ample funds for building oscillators large enough to drive the tank at the highest voltage it could take. He paid salaries to Sloan and the instrument maker, E.W. Lehmann, and various shop costs, amounting to upward of $1,700.[61] During this work, Dr. Walter Alvarez of the Mayo Clinic came to town. Lawrence took him to the University's Medical School. "He was exceedingly enthusiastic about it," Lawrence wrote Poillon, "and wants to start negotiations." Lawrence urged the Research Corporation to greater activity: "There is absolutely no question but that you should push the commercial development as rapidly as possible."[62] As symbol and agent of this alliance, Lawrence's student Harry White went to work for the Research Corporation after learning to run the Sloan machine in San Francisco in order to promote it "and possibly . . . other by-products of the Radiation Laboratory work in the future."[63] Several pests then attacked Sloan's x-ray plants. For one,
Berkeley's homemade oscillator appeared to infringe RCA patents; the existing plants switched to commercial tubes, which would have driven the cost of future installations to around $25,000.[64] For another, Sloan suffered a back injury that made him a semi-invalid from 1935 to 1937 and unable to carry development further.[65] And another of the Research Corporation's investments, the Van de Graaff generator, showed itself capable of producing million-volt x rays more economically than the Sloan tube.[66]
As Birge had said, the Laboratory's development of high-voltage x radiators was largely a commercial venture. It was not the sort of thing that won prizes in physics. After crediting the Research Corporation and the Chemical Foundation with the initiative for developing Sloan's ideas into an effective generator of x rays, Lawrence wrote in his report to them for 1932: "From the point of view of physicists the most interesting aspect of the Sloan apparatus is its tremendous effectiveness in producing intense beams of two [!] million volt protons."[67] In his correspondence with physicists, Frédéric Joliot, for example, Lawrence emphasized not the x rays but the protons; and even when, in justifiable pride, he mentioned the fearsome power of Sloan's first machine, he specified the primary purpose of the second as the creation of milliamps of million-volt protons.[68] It was the first task Sloan took up after recovering from overwork. By February 1934 he had observed his protons, but not in the numbers desired. It took well into the year before this, the "primary purpose" of his work, was realized.[69]
3—
The Radio and the Cyclotron
Going on the Air
The distinctive feature of the Radiation Laboratory's approach to particle accelerators was reliance on radio technology. Many of the Laboratory's earliest workers, including Lawrence, had been radio hams in their youth; they continued the sport on a grand scale by a ham link between the Laboratory and one of its earliest satellites at the University of Michigan.[70] The heart of Livingston's first cyclotron was a pair of off-the-shelf Radiotrons, air-cooled, rated at 75 watts each and arranged in a standard radio circuit, with the single dee in place of an antenna. The analogy is not idle. The cyclotron could interfere with commercial broadcasting or police communications; the Laboratory listened to itself on the radio so as to correct spillage into others' air waves and "prevent investigations by the Radio Commission which may result in our having to put in elaborate and expensive protection devices." It is said that Lawrence used to tune his home radio to the cyclotron's operating frequency to monitor its, and his students', performance.[71] The second cyclotron required a 20-kW water-cooled oscillator in another standard radio circuit; the third cyclotron used two 20-kW tubes, which also drove Sloan's x-ray machine.[72] As we know, their cost provoked the Laboratory to make their own and to enter still more deeply into the art of radio engineering. This move so far from ordinary physics was a surprise even to people familiar with Lawrence's methods.[73] The Laboratory was so filled with radio waves that its members could light a standard electric bulb merely by touching it to any metallic surface in the building.[74] Many cyclotron laboratories were to eke out their resources by cannibalizing old radio parts.[75]
The maximum energy given particles by a cyclotron depends not on the power but on the frequency of its oscillator. From the fundamental equation 2.1,
![]()
where R is the radial distance to the collecting cup. Livingston achieved million-volt protons with the second cyclotron with the cup at 11.5 cm and the oscillator at 20 MHz. This last number represented close to the practical limit on frequency: to have gone higher would have required, first, a more intense magnet (since the frequency is proportional to the field strength) and, second, a tube capable of changing polarity over twenty million times a second and delivering around twenty thousand watts of power. The first requirement would have pushed the art of magnet design, the second that of power oscillators, to or beyond the edge of available technology. The only immediate way to increase the energy of the protons by an order of magnitude was to increase R three-fold . Hence the great value to the Berkeley cyclotroneers of Federal's derelict magnet, which could be rebuilt to give a field of appropriate intensity between pole pieces 27.5 inches (70 cm) in diameter.
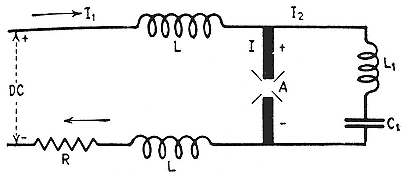
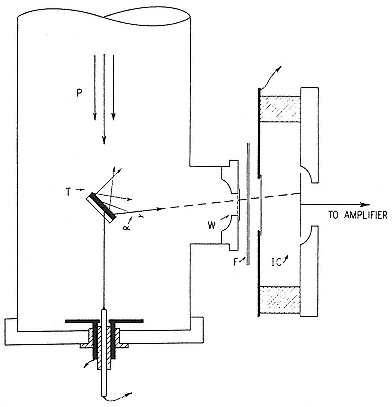
The Federal magnet, like the oscillator tubes, was a product of radio technology, the Poulsen generator, which had at its heart a periodic arc between electrodes in hydrogen. When the arc struck, it carried the oscillatory discharge from a large condenser, which fed an antenna; when the discharge current diminished sensibly, the arc went out and a battery recharged the condenser, which, when full, relit the arc (fig. 3.4). The magnet assisted the extinction of the arc: it made the ions carrying the current run in a curved path from one electrode to another; they therefore could not create by collisions any fresh ions along the straight line between the electrodes; and consequently not enough carriers were available there to continue or restart the arc when the potential across the gap at A (fig. 3.4) no longer sufficed to ionize the gas
Fig. 3.4
Schematic diagram of the electrical arrangement in the Poulsen arc. I1 , the
charging current; I , the rf current through the resonant circuit when the arc
strikes. The choke coils L keep the rf current from the battery circuit.
Heilbron, Museoscienza, 22 (1983), 16.
near the electrodes. In a word, the field insured that the arc went out without flickering (plate 3.5). The objective was to produce an undamped signal of almost constant frequency rather than the broadband output characteristic of the spark transmitters of early wireless.[76]
We are familiar with Federal's growth under navy contracts, and with the culmination of their relations in the commissioning of four 1,000-kW generators, two for each end of a radio link between the United States and its expeditionary forces in France. Their magnets could deliver 18,000 gauss.[77] The war ended before the huge antenna towers—second only in height to the Eiffel tower—could be completed in France. The navy withdrew, leaving France with half a radio station and Federal with four 80-ton magnets. In 1919 the French government decided to proceed with the Lafayette station, as they called it in memory of the American alliance, in order to communicate with its empire in Southeast Asia. Its signal received in San Francisco was four to eight times stronger than the signals from other major European transmitters. That did not recommend completion of the American end of the
link to the navy, however, which now favored development of vacuum-tube oscillators. That left Federal with two war-surplus magnets. Everyone switched to tubes except the Dutch, who constructed a 3,600-kW Poulsen generator to drive an antenna stretched between two hills in Java, which gave the Dutch East Indies a voice audible in Amsterdam.[78]
Lawrence's big magnet, being a piece of high technology, required professional engineering help in its metamorphosis into a tool of science. Fuller advised about renovating the pole pieces and about windings and power supplies; and he procured the assistance of an employee at Federal, Gilbert W. Cattell, who inquired into all sorts of details: the best sort of paper insulator for the windings, of oil for cooling, of cables for connecting, and so on.[79] Cattell wound and insulated the coils at Federal before the magnet went to Pelton for machining (plate 3.6). Toward the beginning of February 1932, the foreman at Pelton's machine shop, Henry Nelson, carted its handiwork across the Bay and erected it in the Radiation Laboratory. Pelton did an excellent job, the pole faces parallel to four-thousandths of an inch, the field homogeneous up to 18 kG.[80] Lawrence hoped to have the cyclotron itself in operation at the end of February and shortly thereafter to pass "the next milestone," protons with energies above 3 MeV.[81] In March he worked "night and day" with Livingston and a graduate student, James Brady, on the machine. "I have neglected everything else—even my fiancée has suffered."[82] Molly's suffering did not make the machine go. In April they thought that they had cured the leaks that the huge magnetic forces kept springing in the cyclotron tank. Still no results. Lawrence went East in May, to Molly and marriage. Livingston
labored on; the chairman of the Physics Department, Elmer Hall, alarmed at his appearance, counselled a long rest.[83]
Vacation cured what slavery had not. By mid September Livingston had overcome his various difficulties and produced hydrogen-molecule ions at 1.6 MeV. "When he gets up in the 3,000,000 volt range [Lawrence wrote Cottrell] he intends to stop and bombard various elements with them before going to higher energies." A week later he had reached 3.6 MeV at about one µA, always with ![]() , since the lighter protons would have required an oscillator with impractically high frequency. Lawrence and Livingston thought they saw their way clear to 5 or 6 MeV, but they decided to "hesitate now awhile on the road to higher voltages [to] do some experiments."[84]
, since the lighter protons would have required an oscillator with impractically high frequency. Lawrence and Livingston thought they saw their way clear to 5 or 6 MeV, but they decided to "hesitate now awhile on the road to higher voltages [to] do some experiments."[84]
Stopping for physics was perhaps a pleasure. It was certainly a financial necessity. Lawrence had started planning for a larger apparatus long before Livingston had got a beam. He did not plan to carry off the Poulsen arc from Java, but to enlarge the pole pieces and vacuum chamber of the new cyclotron from 27.5 to 37.5 inches. He floated this bubble in April 1932. Poillon indicated that he would incline toward granting the $1,100 needed when asked for it. But Lawrence had overreached. Poillon had the same month pledged $2,500 to the Laboratory to enable it to make much needed detectors, a cloud chamber with cinema camera ($1,500) and a magnet to analyze particle beams ($1,000). Somehow Lawrence thought that the Research Corporation and the Chemical Foundation would provide another $800 for instruments and supplies; but when he asked for it in August, he was told that the University should pay for such things. Lawrence already had spent almost the entire allocation for expenses and power for AY 1932/33. It was only August! He would have to close down the Laboratory, he said, unless his hard-fisted backers allowed him to pay for his electricity from the $1,000 granted for
the analyzing magnet. As an inducement to Poillon, Lawrence dropped—only temporarily—the unfunded proposal to enlarge the pole pieces and vacuum chamber. Poillon allowed the redirection of funds.[85]
Broadcasting Upon the Waters
The creation of the 27-inch cyclotron called for an unusual blend of faith, energy, and entrepreneurism. How unusual the combination was may be gathered from the reluctance of other physicists to follow Lawrence's lead. The nuclear physicists in and around the Cavendish at first considered the cyclotron to give too small a current to be useful.[86] They next depreciated it as "ticklish to adjust," a view common among English physicists as late as 1935, although Cockcroft had seen for himself, in 1933, that "all the trick lies in correcting for inhomogeneities of field around the gap by inserting 'shims' or pieces of sheet iron." He had witnessed the operation; the trickster was Livingston, "who does most of the work."[87] A closer observer saw the same thing. "Lawrence does no actual experimental work anymore," Birge wrote another member of the Department. "It keeps him busy just bossing all the men working with him!"[88]
The first Englishman to see the 27-inch run, Ralph Fowler, came to Berkeley in January 1933, when the machine was producing 2.5 MeV hydrogen molecule ions. Neither the machine nor its products interested Fowler. "Probably only trivial stuff," he wrote Rutherford. Six months later Cockcroft did not see that the cyclotron had opened up any important areas of investigation not accessible to the Cockcroft-Walton accelerator. "We can get in long before California in this field [he wrote his co-inventor] and there are a lot of points to be cleared up [about nuclear
processes]."[89] There is no doubt that the 27-inch was a temperamental machine and that the maintenance of its vacuum occupied and irritated many members of the laboratory. But where visitors saw unreliability, and nonvisitors doubted that cyclotrons worked at all, the natives appreciated difficulties overcome and augured a rich harvest when they turned their machine to physics.[90]
Ever zealous and generous in his cause, Lawrence told Cockcroft that he could easily reproduce the Berkeley machine with a magnet that could be had for the asking. He referred to the Lafayette arcs, then about to be junked in favor of vacuum-tube oscillators and to decommissioned 500-kW arcs procurable from navy surplus. "[They] could be obtained for a song and transport," Cockcroft wrote Rutherford, "if Oliphant [Marcus Oliphant, a prominent nuclear physicist at the Cavendish] shows any enthusiasm in this direction." Oliphant did not show enthusiasm, preferring to go to a million volts in the old, direct, dependable, one-step way. Not until 1936 did the Cavendish decide to create a cyclotron, which it did from scratch, following the plans of Berkeley's then newly designed 37-inch machine. The delay put it two generations of accelerators behind Berkeley at the end of World War II.[91]
While the Cavendish was losing its opportunity, Frédéric Joliot, son-in-law and heir apparent to Mme Curie, inquired of Lawrence what it might cost to build a cyclotron. The expense of the large magnet worried him. Lawrence replied with news about the Lafayette monsters and the necessary modifications of the pole pieces.[92] Joliot applied to the engineer at the station and received all the consideration he could have wished. One of the magnets was being dismounted. "It is only a matter of hauling it to the Ecole Normale Supérieure." The refurbishing could be done in the station's shop, which could also supply 20-kW water-cooled
oscillator tubes. The director of the French wireless service authorized the visit.[93] Then Joliot dropped the initiative, because, he later said, he could not get permission to rebuild the magnet. But it is probable that the size of the task, the demand for expertise in radio engineering, and the prospect of dragging an 80-ton magnet through the narrow streets of the Latin Quarter combined to defeat his interest.[94] Like his Cambridge colleagues, Joliot waited until 1936 to begin constructing a cyclotron, and he then required help from Berkeley to correct the many mistakes in its design.
No European center made use of Poulsen arc magnets except the Centre anticancéreux in Marseilles, which made a cyclotron from a small unit decommissioned from the telegraph service in Lyon.[95] The Dutch were the last to consider the option, in 1940, when they asked Lawrence for advice on cannibalizing their Batavian transmitter, which could attain 20 kG.[96] The war put an end to the plan. Cyclotroneering qualities were more easily found in the New World than in the Old. In 1932 New York University inquired into the fate of the 500-kW arcs at Annapolis and reserved one at its decommissioning in June 1934. In 1933 Stanford obtained the mate to Lawrence's magnet from Federal on the understanding that it would be released to the first institution that succeeded in raising the money needed for conversion. In 1934 Cornell tried to obtain one of the decommissioned Annapolis magnets. Columbia, too, was interested; the navy had but one magnet to give, the one that NYU had reserved but had since relinquished; Columbia, which had been frustrated by the navy's sale as scrap of another Poulsen magnet it had coveted, won the prize.[97]
The last cyclotrons built around a Poulsen arc came into existence in 1951. The circumstances were unusual. The Japanese had begun to make cyclotrons as early as the British and the French. At the end of World War II, they had three. The U.S. Army, newly afraid of nuclear physics, threw them all into the ocean. Lacking resources to buy or build a substitute, physicists at the Nishina laboratory in Tokyo scrounged for parts to reconstruct what they had lost. "Fortunately we have a magnet which was originally used for a Poulsen arc generator."[98]
4—
Imported Physics
The year 1932 began at the Radiation Laboratory with the 11-inch cyclotron at 1.2 MeV, the 27-inch cyclotron little more than a magnet, and the Sloan x-ray tube a gleam in its inventor's eye. The staff of cyclotroneers had tripled. Besides Livingston there were now James Brady, who had recently completed his doctoral thesis on Lawrence's discontinued topic, the photoelectric effect in alkali vapor, and remained on, on the payroll of his new employer, Washington University of Saint Louis, to learn something about his professor's new lines of work; Milton White, a Berkeley graduate of 1931, who earned his Ph.D. in 1935; and Malcolm Henderson, from Yale, who already had a Ph.D. and, like Livingood, a private income, which allowed him to contribute his services in an unhurried way to the Laboratory. White worked on the 11-inch cyclotron; Brady, at first, on the 27-inch. When the big machine began to operate, Henderson replaced Brady, who then, with White, had the effrontery to check whether Lawrence and Livingston had, in fact, obtained high-speed resonant protons from the smaller machine. They did not find confirmation easy. By the summer of 1932, however, they had measured the penetrating power of the very meager beam and found it to answer expectations for protons of a million volts or so. This work did not enjoy the glory of publication because, according to Brady, he and
White thought it advisable not to give the impression that they had doubted their professor's claim.[99]
In their hurry to build accelerators, Lawrence and his associates did not find the time to provide themselves with the detectors needed to register the effects of any disintegrations they might provoke. That was only reasonable: the accelerators did give a beam that could be improved; there lay a proven road to progress. Lawrence's group therefore lagged behind Tuve's, which had developed excellent detectors—Geiger counters, linear amplifiers, a novel cloud chamber—during 1931. Not that Tuve's people preferred detection to acceleration. The dilatoriness of their overlords at the Carnegie Institution and their indifference to the possible market value of their x rays (the Carnegie did not pursue patents) had reduced them to small jobs while they awaited permission to rip out their Tesla coil and install their 1-meter Van de Graaff.[100] When the age of disintegration began, therefore, their combination of detectors and high-current accelerator made theirs the preeminent laboratory for nuclear physics in the country. Or so Johnson, a good friend of both Lawrence's and Tuve's, judged the situation early in 1932. "It looks as if he [Lawrence] has some nice work under way but as far as the general technique for working in the field is concerned I cannot help but feel that you are way ahead."[101]
Lawrence's old friends from Yale, Donald Cooksey and Franz Kurie, came to Berkeley in the summer of 1932 to help shorten the lead. They had been asked before—Kurie for the summer of 1931, Cooksey for the spring semester 1932—but had not been able to accept.[102] Both of them were accomplished performers on the sorts of instruments the Laboratory needed most. Kurie's instrument was the cloud chamber. Cooksey was a one-man band, curator of precision instruments in Yale's Sloan Laboratory, an
excellent machinist, and a connoisseur of fine design. They set up with the help of an improbable character, a retired officer of the Italian Navy, Telesio Lucci, who volunteered his services to the Laboratory. The reinforcements from Yale left the Laboratory with the rudiments—but only the rudiments—of the instrumentation of nuclear physics. Both later returned, Cooksey for a lifetime.[103]
Disintegration at Last
Lawrence's boys—he called all his staff, including his senior Cooksey, "boy," there being no women among them—pursued instrumentation with such urgency during the summer of 1932 because of the many great novelties in nuclear physics discovered that spring. In February, Cockcroft and Walton sent to the Royal Society of London a paper describing the acceleration of hydrogen ions down an eight-foot tube connected with their voltage multiplier. They managed to get around 700 kV across the tube and a current of perhaps 10 µA down it; and they mentioned preliminary experiments on fast protons entering a chamber separated from the evacuated tube by a thin mica window. The experiments were not very interesting. Cockcroft and Walton measured the range, or distance of penetration, of the protons through various gases. Their technique for detecting protons was identical to that perfected by Rutherford in the counting of alpha particles: they looked for scintillations where protons struck a fluorescent screen placed in the experimental chamber (plate 3.7). They promised more measurements of the same character and certain improvements in the apparatus by which they hoped to coax it to its design potential of 800 kV.[104]
Here we see again the compulsion of the instrument builder: to perfect the apparatus and to make it the subject of study. For two months after completing their apparatus, Cockcroft and Walton did not search for disintegration products. It is said that they did
not look immediately for material products of disintegration because they expected that much or all of the energy would be carried off by gamma rays, in keeping with the widespread preconception that (as will appear) had protected Joliot and Curie from discovering the neutron. Rutherford became impatient; Cockcroft and Walton should stop playing with their protons and look for disintegration products in the old way, with a fluorescent screen.
On April 14, 1932, they placed a thin film of lithium in the experimental chamber to receive protons and saw flashes of light on a screen that caught particles liberated from the target. A few elementary precautions ruled out the possibility that scattered protons caused the flashes. Two days later they knew the cause: a lithium nucleus that captures a proton can disintegrate into two alpha particles.[105] They had reached their goal: they had split the atom, by a reaction almost the inverse of the process discovered by Rutherford a dozen years before. He had then used alpha particles from natural sources to knock protons from nitrogen nuclei; they had used protons from an artificial source to make alpha particles from lithium. "When one learns that protons and lithium nuclei simply combine into alpha particles," Bohr wrote, on hearing the news from Rutherford, "one feels that it could not have been different although nobody has ventured to think so."[106]
Cockcroft and Walton subjected various targets to beams of various energies and tracked the disintegration particles with a fluorescent screen, a cloud chamber, and an advanced electronic detector developed at the Cavendish. They found that about half their beam was protons and the rest hydrogen-molecule ions; that the threshold for disintegration of lithium was astonishingly low, 125 kV; that the number of disintegrations increased rapidly with the energy of the incident protons, there being one for a billion at 250 kV, and ten per billion at 500 kV; and that the range of the emergent alpha particles, 8.4 cm of air, was consistent with the conservation of energy and momentum, the equivalence of mass and energy, and the reaction Li7 (p,a )a .
Boron and fluorine also gave off alpha particles under proton bombardment and other elements, even silver and uranium, seemed to yield a few. As for agreement with Gamow's theory, "which was largely responsible for stimulating the present investigation," it was not at all good. The theory gave as probability for the entry of a proton into a lithium nucleus one in six at 600 kV and three in a hundred at 300 kV; measurement suggested one in a million in the first case and five in a hundred million in the second.[107] But with elements heavier than iron, theory was much more stingy than fact: there should have been nothing from silver or uranium. In Tuve's opinion, it was this disintegration of heavier elements—"by far the most striking feature . . . , a feature which is distinctly disconcerting from the standpoint of all present-day theoretical ideas of the nucleus"—that had the first claim on the attention of physicists. Cockcroft also felt the difficulty. He appealed to Gamow, who could only suppose that the silver and uranium had contained some light impurity that gave the evidence of disintegration. Cockcroft did not like to lose a discovery or yield to theory so easily. "I always believed it possible for a really good theoretical physicist to explain any experimental result and now you fail me in the first test."[108]
The Berkeley group knew about Gamow's theory by the time Livingston got the 11-inch cyclotron going. He mentioned it in his thesis as the stimulant to efforts to reach high potentials and Lawrence understood that Livingston had given him what he needed to crack nuclei. "These protons have enough energy for nuclear disintegration experiments and it will not be long probably before we are disrupting atoms. But even more exciting are the possibilities of the large magnet, which at this very moment is being installed in our new radiation laboratory."[109] The moment was January 1932, four months before Cockcroft and Walton got their first counts.
The main reason that the Berkeley group did not succeed in splitting atoms before Cambridge is that they did not try. (As
much might be said about Lauritsen, then busy irradiating cancer victims, and Tuve, who had not yet set up his Van de Graaff.) Another reason, as Lawrence freely admitted, was that neither he nor Livingston "kn[e]w very much about nuclear theory."[110] They had not even a wrong idea about what to look for. The news that lithium yielded to particles with the piddling energies attainable at Cambridge was disappointing. "When Cockcroft and Walton . . . disintegrated lithium with only a few hundred thousand volts, the thought naturally suggested itself that perhaps our efforts to obtain very high voltages were hardly worth while." But soon enough, Lawrence wrote Poillon, in a curious mixture of apology and triumph, Berkeley had results more interesting than Cambridge's.[111]
Lawrence learned while preparing for his honeymoon that he had been anticipated by Cockcroft and Walton. He wired Brady, who turned the beam of the 11-inch cyclotron onto a crystal of LiF procured from the Chemistry Department.[112] He found only a few hints of disintegration: he had too weak a current and too insensitive a detector. As late as mid August, the Berkeley group, although enriched by Cooksey and Kurie, had nothing decisive. "Unfortunately [Lawrence wrote Cockcroft] our beam of protons is not nearly as intense as yours although of higher voltage."[113] Just before the group disintegrated—Brady to Saint Louis and Cooksey and Kurie to New Haven—they got counts with a beam of 700 kV protons originating in a filament source constructed by Cooksey and detected with his Geiger counter. In September a new group—Lawrence, Livingston, White, and Henderson—took over the experiments, increased the intensity of the proton beam, and got alpha particles in numbers that agreed, more or less, with the results of Cockcroft and Walton.[114] Thus Berkeley's labor-intensive effort, extending over six months, confirmed the discov-
ery made by a pair of Cambridge physicists in two days. The delay was not a consequence of too many cooks at the broth, but rather of the inexperience of the Berkeley group with detectors and of the difficulty of operating the cyclotron with a steady and useful current. Cockcroft and Walton forced the Laboratory to make its accelerators into instruments of research.
The Deuteron and the Neutron
The combined performance of several generations of chemists on the balance had resulted by World War I in a detailed knowledge of the relative weights of atoms. In particular they had fixed the weight of a hydrogen atom as 1.0077 on a scale defined by taking the weight of oxygen to be 16. The recognition of the existence of isotopes around 1914 opened the possibility that hydrogen or oxygen or both might be mixtures of atoms of different weights. The point was examined by Francis Aston, whose improved mass spectrograph of 1927 disclosed that the weight of a hydrogen atom was 1.00778 (O = 16).
That appeared to be quite satisfactory until Berkeley chemists challenged Aston's assumption that oxygen has but one isotope. Soon evidence from band spectra confirmed the challenge and allowed estimates of the relative abundance of O16 and O18 in ordinary oxygen. When the heavier isotope is taken into account, the weight of ordinary oxygen exceeds that of O16 . Aston's ratio between the weights of ordinary hydrogen and ordinary oxygen, 1.00778:16.00000, could be saved by supposing hydrogen too to be complex. Berkeley's specialist on physical constants, Birge, computed the consequences of the supposition based on the relative abundances of oxygen isotopes (O16 :O18 ::630:1); and he found that everything came into order if about one hydrogen atom in 4,500 has a weight twice that of the more plentiful type.[115]
A Ph.D. from Lewis's school of thermodynamics and chemistry at Berkeley, Harold C. Urey, had a system of classifying isotopes that required the existence of deuterium—to give heavy hydrogen
the name he gave it after a struggle with his colleagues and professors of Greek that lasted longer than his search for the new element.[116] Encouraged by Birge's numbers, he undertook to separate the graver from the lighter hydrogen atoms. Thermodynamic calculations suggested that evaporating liquid hydrogen around its critical point would enrich the liquid in heavy atoms at the cost of the vapor; a still set up at the low-temperature facilities of the National Bureau of Standards by F.G. Brickwedde effected a sufficient separation to allow detection of very faint spectral lines characteristic of deuterium from a fraction of the slightly enriched liquid. The detection made a stir and brought Urey a Nobel prize. "A great scoop for America," said Aston, who, like Birge, had graciously missed the discovery. Urey and Brickwedde gave a sample of the liquid to Aston's rival, K.T. Bainbridge, who worked to more than Cavendish accuracy. Bainbridge reported a mass for deuterium of 2.0126, where O16 = 16.[117]
While Urey was evaporating heavy hydrogen, his former professor Lewis was trying to separate the isotopes of oxygen. It was tedious and unrewarding work. The separation of hydrogen isotopes, with their pronounced difference in mass, appeared easier and, what was better, "much more interesting and important." Following up an observation by E.W. Washburn, chief of the Chemical Division of the National Bureau of Standards, that the water in old electrolytic cells was richer in deuterium than tap water, and guided by his feeling for the thermodynamics at work, Lewis effected a far better separation than did Urey or Washburn and estimated relative abundance at 1:6,500 as against his former student's modest 1:30,000.[118]
Some numbers speak louder than words. In January 1933 Urey's best sample of heavy water contained only 1.2 percent deuterium oxide, about four times the concentration Washburn had managed. They had nothing better in April, when Lewis had 30 percent. "I am very curious to know how G.N. Lewis succeeded . . . with so little effort," Urey wrote his collaborator. "I suppose that he used some electrolytic method and succeeded in hitting the correct conditions exactly."[119] Urey had had no luck with electrolysis. It was uncanny the way everything that Lewis tried succeeded, although he had no surer explicit knowledge than Urey of the electrochemistry at work. On February 28, 1933, the University of California News Service announced Lewis's discovery, which the national press advertised under the whimsical headline, "Record weight of water achieved by California chemist."[120]
The news brought requests from all over for samples to use in place of ordinary hydrogen in the favorite experiments of the petitioners. One could scarcely fail to find something publishable about the behavior of a brand-new substance with a familiar chemistry but unknown physical properties. Lewis gave deuterium freely, almost as fast as he could make it.[121] Several important experiments, as well as many trivial ones, sprang from his generosity. The earliest request came from Bainbridge. Then Otto Stern telegraphed from Hamburg for ammunition for his newly perfected beam machine for measuring magnetic moments. The sample arrived at the end of July. "It was really fabulously nice of you to have answered my cry of need for isotopic water so promptly," Stern wrote, especially since their own attempts to make heavy water had failed.[122] Then there was water for electrolytic studies, for the Stark effect, for spectroscopy, for overvoltage, and for Lewis's own investigations of the physical and chemical
properties of deuterium.[123]
The experiments to conjure with came from the mating of the deuteron and the accelerator. As Fowler watched a 50 percent solution of heavy water brew in Lewis's still, the Radiation Laboratory was planning "to repeat Cockcroft and Walton on Li and B with H particles [deuterons]—also to make He4 by shooting H2 at OH2 H2 ice. All nice and wild of course but exciting." With characteristic openness and generosity, Lewis sent Rutherford "some heavy water to play with," although the Cavendish physicists, with Lauritsen and Tuve, were the world's only competitors of the Berkeley bombardeers.[124] Their competition proved most fruitful.
The deuteron immediately attracted sustained theoretical interest. It was to nuclear physics what the hydrogen atom was to the old quantum theory: the simplest system available for calculation and experiment. According to the systematics of isotopes from which Urey had drawn evidence for the existence of deuterium, the deuteron should have consisted of two protons and an electron. Just before he announced his discovery, however, another intervention from Cambridge simplified the picture. The long-sought neutron, which Rutherford had believed in and which his students had looked for for a decade and more, had at last put in an appearance.[125]
The course of its discovery contains several points of general interest. To go back no further, in 1930 two physicists in Berlin, Walther Bothe and Hans Becker, discovered that beryllium gives off very penetrating rays, more penetrating even than the hardest gamma rays from radium, when hit by alpha particles from
polonium.[126] They supposed that this "beryllium radiation" consisted of gamma rays, for which, indeed, they had been looking. The discovery interested Irène Curie, who worked in her mother's Institut du radium in Paris, where there was more polonium, and less physics, than in Berlin or Cambridge. At the end of 1931 Curie had found beryllium rays so penetrating that, were they gamma rays, as she assumed, their energy would have been between 15 and 20 MeV, three times that of the alpha particles that brought them forth.
Meanwhile a student of Chadwick's at the Cavendish had found that the beryllium rays emitted in the direction of the incident alpha radiation were far more plentiful than those emitted in the opposite direction, an asymmetry suggestive of the collision of particles. As this asymmetry came to light in Cambridge, Curie and Joliot found that when they placed a screen of wax in the path of the beryllium radiation, a detector behind the screen registered protons with energies of around 4.5 MeV. That caused them to increase their estimate of the power of the radiation: were it electromagnetic and obedient to the equations of the Compton effect, it would require about 50 MeV to liberate protons of 4.5 MeV. The discrepancy of a factor of three in their estimates of the energy of the radiation did not encourage belief in their interpretation of its nature.[127]
In February 1932, a month after this second set of results from Curie's institute had come to hand, Chadwick declared the discovery of the neutron. A colleague, Norman Feather, had scavenged enough polonium from old radon tubes (mainly from the Kelly Hospital in Baltimore) to make a source nearly as powerful as the Parisian one. Excellent electronic detectors stood ready. With all this it was not difficult to show that the beryllium radiation knocked protons from other materials than wax and that everything made sense if it consisted of neutral particles of mass close to the proton's. As Fowler summed up for the jury of
theorists: "It seems to be absolutely correct and the case is good enough to hang anyone on, but perhaps not yet scientifically certain."[128]
By the time the news reached Berlin, Bothe and Becker had found that beryllium does emit gamma rays under alpha bombardment, but of a much more modest energy, some 5.1 MeV, than Curie and Joliot required. (In fact the energy is more modest still, since the gamma ray comes from the decay of an excited state of C12 , some 4.4 MeV above the ground state.) Thus in a year and a half the unknown beryllium radiation, which physicists first tried to absorb in a way that did the least damage to received ideas—hence problematic gamma rays rather than an entirely new particle—was unravelled to yield an important discovery and a warning about the complexity of nuclear reactions.
Because of its complexity, the Bothe-Becker radiation was not adapted to a clean determination of the neutron's mass. Chadwick therefore had recourse to the transformations of boron and lithium, which he supposed to occur as follows: B11 (a ,n)N14 and Li7 (a ,n)B10 . Chadwick set the velocity with which the neutrons leave the interaction equal to the maximum velocity of the protons they knocked out of the paraffin screen. With this assumption and the known energy of alpha particles from polonium, conservation of momentum gives the energy of the recoil atoms and conservation of relativistic energy the mass of the neutron. For the latter calculation the isotopic masses of the participating atoms must be known accurately. Here the then recent—and subsequently invalidated—work of Aston was essential. From the first transformation, of boron, mn = 1.0066 (O = 16) with a probable error of 0.001. From the second, of lithium, for which the neutron's velocity had not been measured precisely, mn = 1.0072. Hence the sum of the masses of neutron and proton (Aston's value) appeared to be 1.0072 + 1.0078 (mp ) - 0.0005 (me ) = 2.0139, or some 0.0013 mass units greater than Bainbridge's value for the mass of the deuteron. On the natural supposition
that d = p+n, the deuteron would have the meager binding energy of just over 1 MeV; should it consist of two protons and an electron, it would be more securely bound, by 0.0025 mass units or 2.5 MeV.[129]
Although, as soon appeared, Chadwick's estimate was based on a mistaken reaction and inaccurate isotopic masses, its consequence, that the deuteron possesses a positive binding energy, however meager, seemed an essential postulate. How else could the deuteron and nuclear physics hold together? Whatever one's views about the nature of the neutron—whether it is simple and singular, or a tight union of a proton and an electron—its weight could not be much less than the proton's if they were to join together in a stable deuterium nucleus.[130] Against the Cavendish's heavy neutron and its secure deuteron, Lawrence and his Laboratory placed a light neutron and an unstable nuclear physics.
Scurrying for Position
The discoveries of the neutron and deuteron and the demonstration of nuclear disintegration gave the few laboratories in the world able to do "high-energy physics" great scope for maneuver. They chose according to the opportunities afforded by their machines and circumstances, and by the temperaments of their directors. The Cavendish decided to work accurately and slowly, with very light elements and very modest energies. Carnegie's Department of Terrestrial Magnetism (DTM) worked from Cavendish energies up to a million volts or so and also concentrated on lighter elements; they sought to make their beams as homogeneous and their targets as clean as possible to provide a firm basis for further work. The Kellogg Laboratory at Caltech worked with a similar energy range, but not so energetically as the
DTM. The Radiation Laboratory at Berkeley played its long suit, went to higher energies and heavier targets as quickly as it could, experimented sloppily, and opened and discovered vast new territory. Outclassed for exact measurement by high-tension machines with straight tubes and relatively strong currents, the cyclotron could compete only by shooting faster projectiles than the opposing artillery could field.
Following the detection of disintegration at Berkeley at the end of the summer of 1932, Lawrence's group, which then consisted of himself, Livingston, and White, thought that their minuscule beam of protons, a mµA (a billionth of an ampere), liberated more alpha particles from lithium than Cockcroft and Walton had reported.[131] This cornucopia, sanctioned by Oppenheimer's calculations from Gamow's theory, turned out to be a mistake, an error of reckoning, as Cockcroft observed, by a factor of sixty. With proper arithmetic, the Berkeley results came into rough agreement with Cambridge's. Meanwhile Henderson had taken over the 11-inch cyclotron and Cooksey's Geiger counter. He drove the measurements up to the energy limit, with protons of 1.23 MeV, and made a discovery: the probability of interaction of a proton with a lithium nucleus does not increase with energy of bombardment above 400 keV.[132]
Early in the new year, White came back on the machine, put boron in place of lithium, got a surprisingly high yield, and was anticipated by Cockcroft and Walton.[133] Then Livingston joined the game, with Lawrence. They took on aluminum, which, they reported, gave off alpha particles and, like lithium and boron, had an energy threshold above which the probability of proton absorption became constant. But they had run too fast and taken analogy, rather than Cockcroft and Walton, as their guide. Aluminum's "alpha particles" turned out to be soft x rays. "We are finding it a dickens of a job to make sure whether radiations are protons or alpha particles or gamma rays or the Lord knows
what," Lawrence had written Boyce a week before sending his misidentification of the aluminum x rays into print.[134] The error probably came to light when Livingston started "disintegrating 'to beat hell'" with the 27-inch cyclotron. In January 1933, having figured out how to shim the magnet, he knocked apart lithium and carried Henderson's curve up to 1.5 MeV.[135] But from aluminum the most conspicuous products were soft x rays.
For a month or so in February and March 1933, Lawrence believed that the initial era of machine design at the Laboratory had ended. "Therefore for some time in the future [we] shall devote most attention to the experimental study of the nucleus rather than the development of experimental technique." He was not looking forward to it. "We have decided not to be in any hurry about it and have settled down to patient, painstaking experimental study."[136] They were released from this uncongenial line of work by Lewis's method of collecting heavy water.
Tuve followed up the earliest of the discoveries, that of the neutron, as soon as he heard about it. He tried to assemble a polonium source equivalent to Chadwick's, but found it hard going. Feather had left few old radon tubes on the East Coast and was asking for more. "I have the greatest respect and warm regard for Cambridge physicists, and feel like a small boy speaking up in church when I deflect anything toward myself that might have helped them," Tuve wrote the radiologist at Kelly Hospital, insisting, however, that charity begin at home. His eagerness to confirm and extend Chadwick's discovery contrasts with Lawrence's initial reaction, that the neutron "has no particular effect on our experiment excepting to emphasize that there is a fascinating world of phenomena that will be accessible when we will have developed our method for producing swiftly moving protons."[137]
Tuve's group assembled enough polonium to develop a technique for detecting neutrons with their linear amplifier and improved cloud chamber. When the Van de Graaff came on line, Tuve searched for "artificial neutrons" using protons and alpha particles as projectiles, but found very little even at the maximum potential, some 750 kV, that he could reach in the spring of 1933. Nevertheless, he expected that even at moderate energies a Van de Graaff generator would be a very spectacular neutron source. On Gamow's theory, each µA of alpha particles on beryllium would give only 6 percent of Chadwick's yield (from 5.2-MeV polonium rays) at 800 kV; but twice that yield at 1,200 kV, 15 times it at 1,600 kV, over 50 times it at 2,000 kV.[138] Lauritsen's group checked the prediction as far as they could go; at 950 kV and 30 µA , their tube put out neutrons as copiously as the largest polonium source in use anywhere. Then they tried the same experiment with heavy water from Lewis. This time the artificial output exceeded the natural a hundredfold.[139] Simultaneously Lawrence was obtaining large yields of neutrons from deuterons shot at beryllium by the cyclotron.
When the 1-meter Van de Graaff started working, Tuve repeated Cockcroft and Walton's experiments using the target and detection scheme indicated in figure 3.5. He and his group confirmed the presence and the ranges of the alpha particles reported in the (p,a ) reactions investigated at Cambridge.[140] They then began a series of painstaking bombardments of light elements by protons that led them to a fine discovery. These measurements determined the "excitation function," the yield of a nuclear reaction as a function of the energy of the incident particles. As far as they could go, they found all Berkeley's thresholds wrong.[141] That was not their fine discovery. Their strong, homogeneous beam made possible detection of narrow reaches of energy at which
Fig. 3.5
The method of provoking and detecting nuclear reactions at the Carnegie
Institution. P is the incident proton beam; W, a window closing the
vacuum space; F, an absorber; IC, an ionization chamber. Tuve,
JFI, 216 (1933), 32.
impacting protons are particularly effective in exciting or disintegrating their targets. They found this "resonant excitation" in a characteristic way, by trying to clear up discrepancies between their excitation functions for carbon and Lauritsen's.[142] En route, they provided strong evidence that the apparent disintegrations of elements of medium atomic weight reported by Cockcroft and
Walton—the disintegrations that Tuve had regarded as eminently subversive of theory—originated, as Gamow had suggested, in contamination of the targets.[143] Their view proved correct.
Berkeley experimenters contributed little to this line of work or to the precise determination of the energies involved in nuclear reactions. These energies, measured primarily at the Cavendish and at Caltech, concerned hydrogen, helium, lithium, beryllium, and boron. The measurements established the basis of a system of isotopic masses more accurate than Aston's. One outcome of this system, which constantly underwent revision during the 1930s, was Hans Bethe's theory of the source of the sun's heat. None of this painstaking work will engage us further.[144] It was not the sort of thing that Lawrence's boys did.[145] As Bethe said of another sort of demanding measurement of importance in nuclear physics, the dependence of the range of a fast particle on its energy, "it is not likely to be solved in the ordinary course of research because it is too complex for a graduate student and would involve too much time for a more advanced scientist."[146]
Accuracy is not everything, even in science. In the first two years of its existence, the Rad Lab built a machine that outdid all others in the acceleration of charged particles. It made the most powerful commercial x-ray tube in the world. It developed a linear accelerator. It received national attention. The dilution of effort and the demands of institution building resulted in work that was scarcely fastidious. But they did not cost Lawrence what he valued most: victory in the race for high energy.