2—
Growing Up and Limbering Up
The Kingdom of Hanover
Hermann Kolbe's forefathers lived their lives in the rolling, fertile, partly wooded country around Göttingen, in Hanover's southern exclave. Sandwiched between the duchy of Braunschweig to the north, Electoral Hesse to the south and west, and Prussia to the east, this province had been ceded in the seventeenth century from Braunschweig to the country that subsequently became known as Hanover, after its largest city. In 1714 the country's ruler, Elector Georg Ludwig, also became the king of England, and for more than a century thereafter, Great Britain and Hanover had the same sovereign. During the rest of the eighteenth century, Hanover enjoyed a number of benefits from its close association with Britain. The oligarchic privy councils that effectively ran the country's affairs under an increasingly absentee succession of monarchs were relatively liberal and efficient; for instance, sound agrarian laws protected the peasants and censorship was not practiced. The political links also provided an avenue for direct cultural relations between the two countries. The creation of the University of Göttingen in 1737 is an example of the best work of the Hanoverian regime.[1]
Hanover had an ambivalent relationship with its much larger neighbor Prussia, which was becoming an important power in the eighteenth century. This ambivalence was more increased than lessened by the consanguinity of the rulers of the two countries. During the welter of the Napoleonic wars, Hanover was successively occupied by France
and by Prussia, and then it was made part of a new north German kingdom. The French occupation was distasteful and vexatious. Hanoverians welcomed the restoration of the old order upon Napoleon's defeat in 1814, especially as now the country was raised to the dignity of a kingdom and it acquired additional territory from the Congress of Vienna.
But Hanover, like other German states, suffered from centrifugal and reactionary forces during the years after 1815. Hanover's chief minister after the Congress of Vienna was Count Münster, whose despotic rule from London was much resented by wide segments of the populace. As a ripple effect of the Parisian insurrection of 1830, a riot broke out in Göttingen in January 1831 that led to the fall of Count Münster. Two years later, a new and relatively liberal constitution for the kingdom was promulgated, a document that was written loosely on the English model. The University of Göttingen reflected these troubles. In the early postwar years, its enrollment topped 1500; by 1834 it had plummeted to 860.[2]
Even worse was to come. By Hanoverian law, a woman could not ascend the throne. Accordingly, the fifth son of George III, Ernst August, and not Victoria, became sovereign of Hanover in 1837, thus ever after separating the crowns of Great Britain and Hanover. Ernst August was autocratic and illiberal; one wag reported that he had committed every crime in his life except suicide.[3] One of his first official acts was to suspend Hanover's new constitution. The consequent protest of seven of Göttingen's most eminent professors, including Weber and the Grimm brothers, resulted in their dismissal. Despite expressions of outrage across Germany from citizens of a wide range of political belief, the king had the support of the Hanoverian nobility, and an appeal to the diet of the German Confederation proved fruitless. Open opposition to the regime died, and Ernst August continued his reactionary policies until the uprisings of 1848. "The decision of the Göt-tingen Seven," writes one historian, "propelled the German professoriate into the middle of public life. The Frankfurt Parliament of 1848 would be incomprehensible without the Göttingen protest."[4]
However, the protest and expulsion of the Göttingen Seven can also be overinterpreted. Their refusal to break their oaths by repudiating the constitution of 1833 was more a statement of conscience than a political stand. Most members of the German professoriate in the Vormärz, when exercising any political sentiments at all, stood for moderate constitutional reform; very few had sympathy for extreme democratic or republican views.[5] In this sense, as we will see, Hermann Kolbe became a typical representative of his class.
Forefathers
In German, Kolben means both a club or truncheon and a chemist's flask, and both of these denotations bear ironies in the case of Hermann Kolbe. The surname Kolb or Kolbe is not uncommon in central Germany; it is said that the etymology of the family name is related to the sort of person who is inclined to take up cudgels for appropriate use. Kolbe must have had this implication in mind when he designed his signet emblem in 1851: two brawny arms brandishing clubs are depicted, the one crowning the device apparently poised to strike a crushing blow on his opponent.
Kolbe's grandfather, (Johann) Georg Wilhelm Kolbe (ca. 1760-ca. 1825), was described as a preacher or minister and schoolmaster in the hamlet of Gross-Schneen, eight miles south of Göttingen, at the time of his (eldest?) son's birth—Hermann's father, Carl—in 1790. By 1808 he was schoolmaster in Grone, a settlement just outside the city ramparts. Around 1815 he became property inspector and clerk of courts in Adelebsen, a position he still held in November 1821, after which we know nothing more. It was common at this time and later for ministers to combine pastoral duties with teaching and other civil service.[6] G. W. Kolbe probably had a university education, but there is no Göttingen matriculation record, and it is not known whether he formally qualified for the pastorate.[7]
Carl Friedrich Ludwig Kolbe (18 December 1790-4 October 1870) attended the Göttingen Gymnasium, preparatory to the study of theology at the university, matriculating during the French occupation in April 1808. After completing the three-year course, Kolbe taught Latin and Greek at the monastery school in Ilfeld, near Nordhausen in Saxony. In September 1815 he received a call to lead the Lutheran congregation in Elliehausen, three miles west of Göttingen. It was there that he established his family, marrying (Dorette Caroline) Auguste Hempel (10 August 1800-10 April 1856), less than a month after her sixteenth birthday. The first of their fifteen children was (Adolph Wilhelm) Hermann Kolbe, born 27 September 1818.[8]
Auguste's father, Adolph Friedrich Hempel (1767-1834), was professor of anatomy at Göttingen. Her mother was the former Marie Catherine Louise Grabenstein, the daughter of a physician who was also for a time Bürgermeister of Göttingen. She had died by the time of her grandson Hermann's birth. Professor Hempel, as we will see, had daily contact with his adolescent grandson Hermann during the last three years of his life. Born in Neustrelitz (Mecklenburg), Hempel received his M.D. degree from Göttingen in 1789 and taught there the
rest of his life. The author of a popular anatomy textbook, he was described as a straightforward rationalist in his approach toward his science, consistent with the general intellectual tone at the University of Göttingen.[9] There was still a strong current of Enlightenment rationalism in Göttingen in the nineteenth century; such speculative and metaphysical movements as Naturphilosophie, homeopathy, and phrenology never made headway there.
By all reports, Carl Kolbe was a man of energy, strong opinions, great self-confidence, and forthright manner. The words used by Hermann's biographers to describe his father's character—gerade, unerschrocken, bestimmt, fest in sich geschlossen, ausserordentlich energisch, von festem Willen —could as easily be applied to Hermann as well.[10] His life motto "Tue recht, scheue niemand " ("Do the right thing, fear no one") was carefully inculcated in his children.
The income of Lutheran pastors in nineteenth-century Germany was derived from the agricultural production of local church lands leased to tenant farmers, a sort of prebendal arrangement. The endowments of various church districts differed markedly, and so it was common for pastors to seek more lucrative positions as their careers progressed. As it happens, Elliehausen was a relatively well-endowed position, possessing not only fertile prebendal lands but also agricultural assets at the parsonage itself. In fact, the ground floor of the building provided stalls for cows and horses, part of the second story was used as a granary, and a pigpen directly adjoined the house. The pastors were expected to be farmers in their spare time; indeed, the Elliehausen pastoral farm continued in operation until 1926.
Unfortunately, a serious problem emerged soon after Carl Kolbe's arrival. The church there was barely ten years old, but it had been poorly built, indeed fraudulently, with inferior materials and without a proper foundation, and by the time of Hermann's birth, it was already dangerously and irreparably dilapidated. Failing in his attempt to raise the money needed for a new church, in December 1826 Kolbe accepted a call to the prosperous Kirchspiel of Stöckheim, in the Leine valley twenty miles north of Göttingen. The money for tearing down and rebuilding the Elliehausen church was raised only after Carl's brother Georg was called to be pastor there.[11]
Stöckheim was and still is a small village—there were 410 residents in 1826 and about the same number today—but the church district comprised more than 1800 people; Kolbe now presided over a handsome (and sturdy) sixty-five year old church and an ample parsonage. He needed the room. By the time of his transfer he had four young children, and his family continued to grow rapidly during the next few years. Unfortunately, to Kolbe's great distress and against his vehe-
ment objections, in the late 1830s the tenant farmers working the church lands were able to obtain the money and consistorial approval to acquire their property outright. This development suddenly and dramatically reduced his income. He began to look for yet another congregation.[12]
In September 1840 he accepted a call to the village of Lutterhausen, just outside the town of Hardegsen ten miles south of Stöckheim. Lutterhausen was even smaller than Stöckheim, but the district was prosperous, and it was here that Carl Kolbe remained for the rest of his career and the rest of his life. Weakness of old age forced him to retire in 1869, and he died in 1870. The lovely church and the spacious half-timbered parsonage, which still stand, attest to the comfortable nature of Carl Kolbe's last position. Still, Hermann's biographers—with perhaps some exaggeration—emphasize the modest character of the family's lifestyle, portraying Carl as living the life of a "simple country pastor."[13]
It is possible to say something about Carl Kolbe's religious outlook, for he published a catechetical handbook for the religious and moral instruction of children, at just about the time he needed to begin instructing Hermann and his other children. His book begins with the thesis that "we learn of the existence of God through a rational consideration of the heavens and the earth." Only after thoroughly exploring the rational basis of religious belief does he ask children to understand that "we acquire more detailed knowledge of God through the Bible." These and other passages demonstrate that Pastor Kolbe's religious opinions exhibited the moderate rationalism so characteristic of Lutheran theologians during the German Enlightenment, and especially of the anglophilic Hanoverians. One would seek in vain here for precursors of Hermann Kolbe's future fire-and-brimstone "sermons" to his fellow chemists. Pastor Kolbe's instructions are uniformly modest, gentle, and tolerant, and are informed by a positive and liberal view of God and of mankind.[14]
Determining Hermann Kolbe's religious views is not straightforward. Apparently, Kolbe intended to accede to his father's desire for him to enter the ministry, until at the age of eighteen he discovered chemistry.[15] Conversely, Edward Frankland, a good friend who spent much time with Kolbe from 1845 to 1850, described him much later as an "agnostic."[16] However, if we consider that Frankland was a "born-again" Christian during much of this period (before he began to fall into agnosticism himself), that the term agnostic did not even exist at that time, and that there are many demonstrable inaccuracies in Frank-land's memoirs (including incorrectly labeling others as agnostic),[17] this evidence must be viewed with caution. Frankland said that he and
Kolbe had argued over religion. It could be that Frankland's early impressions were never modified by later data; from his fundamentalist perspective, sincere religious feeling tempered by rationalist moderation may have seemed agnostic.
An autograph memorandum in Kolbe's personnel file at Marburg states his religious confession to be "reformirt." This memo is dated 31 January 1854, which is shortly after his marriage, and it is a reasonable supposition that he converted from Lutheranism after becoming engaged. This conjecture is strengthened by the report that he initially experienced opposition from his prospective father-in-law, but that all tensions were fully resolved shortly before the wedding.[18]
Despite this (probably thoroughly formalist) conversion from the faith of his family, most evidence supports the view that Hermann remained a sincere Christian and that his religious philosophy was similar to that of his father. As professor in Leipzig, he placed the Biblical quotation "God has arranged all things by measure and number and weight" (Wisdom of Solomon 11:20) in large letters above the chart of the chemical elements at the front of his lecture theater. When Kolbe's successor Johannes Wislicenus first saw the placard, he said to his guide, "Das muss verschwinden!" When relating this story, Georg Lockemann compared the two chemists by remarking that Wislicenus, too, was the son of a minister, but a man of very different (i.e., free-thinking) religious convictions—indicating that Lockemann regarded Kolbe as a traditional religionist.[19]
In an 1872 work, Kolbe argued that students of theology should be required to study chemistry and the other sciences in order to combat the atheistic and materialistic image of scientists and of science. This sentiment is reinforced in Kolbe's obituary of Liebig (who, Kolbe asserted, shared his views on theology and religion). The proper attitude, he felt, is not blind orthodoxy that teaches miracles and a literal interpretation of the Bible, but rather a rationalistic and science-based religious conviction that has overtones of natural theology. He wrote
The study and recognition of the wonders of nature and of the laws by which the Creator has revealed himself to mankind in so palpable a fashion leads not, as those stupefiers of mankind would want us to believe, to atheism, but in just the opposite direction: it permits the physical as also the spiritual eye to discern the caring and cherishing hand of the Creator in thousands of features.[20]
Many passages from Kolbe's correspondence with publishers Eduard and Heinrich Vieweg also suggest a moderate rationalist Protestantism, similar to that of his father. In 1853 he recommended for publi-
cation by Vieweg a history of the early Christian church by Eduard Zeller (1814-1908), a prominent member of the Tübingen school of higher criticism and a follower of David Strauss. Zeller had recently been called to Marburg, but had been forced from the theological into the philosophical faculty by persistent charges of atheism. Kolbe explained to Vieweg what the higher criticism meant, said he agreed with Zeller's views, and commented that no one is less inclined toward atheism than Zeller, despite his record of defending conclusions that "make the hair of pious theologians stand on end."[21]
All of this suggests that Kolbe never lost the faith given to him by his father. Nonetheless, considering his upbringing as the son of a pastor and the large number of surviving personal letters that provide a window into his private life, the paucity of evidence for the character of Kolbe's religious convictions is striking. All of his biographers emphasize that chemistry was his whole life, and one can only assume that his science, simultaneously his vocation and avocation, overshadowed even his sincere religious beliefs.
Beyhood
It was in the "rustic simplicity" of Elliehausen and Stöckheim that Hermann Kolbe grew to young manhood. These villages were typical rural communities of the day; the principal occupation was growing hops, rye, oats, fruit, and stock, and the region was devoid of any significant industrialization. Indeed, all of the Germanic lands during the Vormärz were essentially rural (only about twenty percent of Germans in 1815 lived in towns or cities). Widespread industrialization did not begin until the 1840s. Moreover, partly due to the unregenerate regime of Ernst August, Hanover was more backward than most German states. It was not until the middle of the century that the power of the guilds in controlling industrial production was broken, the first railroad lines linking Göttingen and nearby cities such as Kassel and Hanover were constructed, and the kingdom of Hanover joined the Prussian—and increasingly pan-German—Zollverein. Despite their relatively modest financial circumstances and rural surroundings, however, the Kolbe family would have regarded themselves as full-fledged members of the German university-educated elite, the Bildungsbürgertum , with all of the attached neohumanist cultural and elevated bourgeois social implications.[22]
Indeed, the central position of Lutheran theology and the pastorate in the intellectual life of nineteenth-century Germany has often been emphasized; as Fritz Ringer recently put it, ". . . one cannot imagine the development of the German intellectual class in the nineteenth
century without the pastors or their sons." They were devoted to learning and placed the highest value on humanist education. A statistical look at the social origin of German professors indicates this orientation: after sons of professors (16%), sons of ministers (15%) constituted the largest class of German academics at mid-century. The relative modesty of pastoral homes may have increased the social aspirations of ministers' sons, including a certain affinity toward the nobility.[23] Kolbe surely must have felt this pull, especially growing up as he did in the aristocratic Hanoverian kingdom and attending the nobility-dominated university of Göttingen. This affinity could only have been increased by the fact that his maternal grandparents had been two of the leading citizens of Göttingen. His best friend while attending Gymnasium there, as we will see, was the son of a nobleman.
Lockemann wrote that Hermann often fondly reminisced in latter years of his youth as "a precious time of harmless rustic freedom." Ernst yon Meyer remarked that it was not the sort of upbringing that one would expect to nurture seeds of scientific greatness. His first instruction was tendered by his father, followed by a village schoolmaster, and then a capable tutor attached to the Kolbe household, whose duties increased with the growing household. It is said that Hermann derived his energetic, self-confident character from his father and a love of science from his mother. He is depicted as a happy and active child, climbing in fruit trees, pursuing gymnastic exercises in' summer and ice skating in winter, preparing homemade wine from birch sap and syrup of violets, assembling beetle and butterfly collections, and assisting in household chores in the kitchen and cellar. As the eldest child, he took great pleasure in instructing his many brothers and sisters. One source of chemical interest for Hermann was a salt brine works in Sülbeck, within walking distance of Stöckheim, which he often visited; early chemical "experiments" on his mother's stove are also reported.
Kolbe's correspondence and other records have revealed the names of most of his fourteen siblings. Sister Emma died unmarried at the age of twenty-one in 1850, a loss that deeply affected Hermann. Sister "Rutsch," also unmarried, lived with Kolbe's family in Leipzig for many years. Sister Bertha, born in 1824, married a pastor in Ellierode (just two miles from Lutterhausen) named Georg Ost. Their son became Kolbe's godson and namesake; Hermann Ost studied with his uncle, had a successful chemical career at the Hanover Technische Hochschule, and became one of Kolbe's biographers.[24] Brother Carl, about fourteen years younger than Hermann, took his brother's chemistry courses at Marburg in 1851-1852. After their father's death in
1870, Hermann became the acknowledged head of the family, working hard to sort out the questions of probate and inheritance for the ten surviving siblings. Today, only three graves remain in the Lutterhausen churchyard, those of Kolbe's parents and his sister Emma. In 1878 Kolbe provided three new headstones, landscaping, and a sturdy wrought-iron fence enclosing the small plot.[25]
It is clear from later letters that Kolbe had great regard for and pride in his father. Judging by mentions of his trips in letters, his visits home were of respectable frequency, though only one visit from his father to his own home (in Marburg) is recorded. In December 1851 he personally nursed his father back from a serious illness despite the press of duties of his first semester as ordentlicher Professor in Marburg. His father accepted the role of godfather to Kolbe's first male child. In 1861 and again in 1865, Kolbe traveled to Lutterhausen for the celebrations of his father's fiftieth anniversaries of his first teaching post and his first pastoral call, respectively.[26] When he was named a foreign member of the Swedish Royal Academy of Science, Kolbe wrote Eduard Vieweg asking for a copy of the newspaper in which the news was announced so that he could send it to his father.[27] In contrast, it is remarkable that I have found but one reference to his mother in the 850 surviving letters from his pen.
Gymnasium and University
In 1831 Hermann entered the Göttingen Gymnasium, living at first with his maternal grandfather Professor Hempel at Lange Geismarstrasse 230, a short walk from the Gymnasium on Wilhelmsplatz in the heart of the old city. After Hempel's death (1834), he lived in the Gymnasium complex itself in the home of the Gymnasium's director, the philologist G. F. Grotefend, who, curiously, had had Wöhler as a pupil in Frankfurt twenty years earlier. In both of these residences, Kolbe was exposed to cultured academic households. A possibly even stronger influence was a close friendship with a classmate, a member of the Hanoverian nobility named von dem Knesebeck. Knesebeck was acquainted with the young Privatdozent at the University of Göttingen, Robert Bunsen, who informally instructed him in chemistry. Knesebeck and Bunsen both grew up in Göttingen, and one can imagine that their families may have been acquainted. Knesebeck constructed a small laboratory in his father's garden house, where he shared chemical arcana with the young Hermann. Kolbe later recollected that these events in the summer of 1837 induced him to give up his ambition to follow his father into the ministry, as his father wished, and turn to the academic study of chemistry. Unfortunately, his
friendship with his schoolmate was of short duration. One day in class Knesebeck became ill; Hermann took him to his room, only to have his friend die in his arms. An overdose of opium was blamed, but whether intentional (he had a troubled relationship with his stepmother) or accidental was not determined.[28]
Hermann took a second-class Abitur in April 1838. Unfortunately, the Gymnasium's records from this period have not survived; consistent with his overall score, he is said to have been a good but not exceptional student. Ost wrote
His friends from his years at school depict him as a boy who was eager to learn, who thoroughly studied whatever had once attracted his attention. . . . Kolbe did not possess the capability of learning effortlessly; rather, his natural gift consisted in the drive to direct himself resolutely toward fixed goals, and to immerse himself to the depths in whatever subject he attacked.[29]
Kolbe matriculated at the University of Göttingen for summer semester 1838, now intent upon a career as an academic chemist. He took two semesters of physics with J. B. Listing (Weber's successor), three of mineralogy and geology with J. F. L. Hausmann, two of mathematics with Georg Ulrich, and a course in metaphysics from J. F. Herbart. He resided at Burgstrasse 332, across from the Gymnasium. Kolbe's leaving certificate from the university attests that he studied all these subjects with "exceptional diligence," his only black mark being a four-day incarceration in the student jail on account of having insulted an unnamed personage.[30]
Of greatest importance, Kolbe studied chemistry with Friedrich Wöhler. He attended Wöhler's practicum during each of the eight semesters he spent at Göttingen, and during three of these, it was his only academic occupation. We have examined Wöhler's background and early career in chapter 1. Here it is relevant to underline the attractiveness of Wöhler's teaching and his wholehearted—and single-minded—commitment to his science. A fundamentally nonpolitical man, the traumatic episode of Ernst August's revocation of Hanover's constitution and the storm over the Göttingen Seven, which occurred eighteen months after Wöhler's arrival and six months before Kolbe's matriculation, scarcely seemed to touch his consciousness.[31]
As noted in chapter 1, Kolbe was only the third Göttingen student to register for chemistry after Wöhler's arrival there (the first two are known only from their matriculation entries). It was precisely at this time that both Wöhler and Liebig were, beginning to attract comparatively large numbers of students to their lectures and especially their
labs. Most of these were pharmacy and medical students, but the chemistry contingent, initially quite small, increased rapidly during the early 1840s in both universities. This constituted the first sizable contingent of serious chemistry students in history.
Kolbe's progress, however, seems to have been slower than some of his younger compatriots, for it was not until summer semester 1840 that Kolbe was allowed to perform real research. By this time, a real cohort of research students had emerged. Kolbe's compatriots included Carl Voelckel and Georg Schnedermann (Wilhelm Knop arrived in 1841), all of whom were to begin successful, though admittedly modest, academic careers before Kolbe's first professorial call. Kolbe's first research assignment, given to him by Wöhler, was to study a reaction first published by Döbereiner eighteen years earlier, namely, the preparation of formic acid by oxidizing larger organic molecules. Kolbe distilled starch and alcohol with pyrolusite and sulfuric acid and isolated ethyl formate from the reaction mixture. Neither Kolbe nor Wöhler continued this line of investigation. Wöhler published this note under his name alone, giving Kolbe credit within the article.[32]
Kolbe's second assignment was the analysis of fusel oil residue in grain alcohol. Wöhler described his results in a letter to Berzelius—at variance with the only previous analysis of the material—as "striking". Berzelius mentioned the result in his Jahresbericht for 1842, naming Kolbe explicitly. The paper was subsequently published in Liebig's Annalen —Kolbe's first scientific publication. Kolbe related in later years how Wöhler subjected this first manuscript to a stringent linguistic critique, forcing him to eliminate excess verbiage and to describe the factual details in a direct and clear fashion.[33]
About the time this paper was submitted, Kolbe suffered an attack of jaundice, and he spent the winter of 1841-1842 at home in Lutterhausen. By the spring of 1842 he was sufficiently recovered to begin his dissertation work with Wöhler. This work had been well begun but was by no means completed when Wöhler seized upon an opportunity for Kolbe. Robert Bunsen, Wöhler's successor in Kassel, had been called to Marburg in 1839. Wöhler had sent Voelckel to be Bunsen's Assistent late in 1841, but after one year Voelckel accepted a call to the cantonal school in Solothurn, Switzerland. So now Wöhler recommended his promising student Kolbe, not yet Ph.D., as Assistent for Bunsen, a position boasting the not exactly munificent salary of 200 thalers per year.[34]
Kolbe spent three years with Bunsen and derived great profit from his contact with the only slightly older man. Indeed, Bunsen became Kolbe's Doktorvater , Kolbe officially qualifying for the doctorate on 23 October 1843. Referring to the late 1840s, Tyndall reminisced
Bunsen was a man of fine presence, tall, handsome, courteous, and without a trace of affectation or pedantry. He merged himself in his subject: his exposition was lucid, and his language pure; he spoke with the clear Hanoverian accent which is so pleasant to English ears; he was every inch a gentleman. After some experience of my own, I still look back on Bunsen as the nearest approach to my ideal of a university teacher.[35]
The circumstances that Bunsen, like' Kolbe, was a native Göttinger, and that Kolbe's first introduction to chemical operations had come through Knesebeck second-hand from Bunsen, could only have increased Kolbe's regard. In later years, his relationship with Wöhler cooled somewhat (as happened with most of Kolbe's friendships) but never his ties to Bunsen. It may seem anomalous that the kind and gentlemanly Bunsen never lost his affection for his obstreperous student. This circumstance may be partly explained by the fact that in 1844 Kolbe saved Bunsen's life by carrying him unconscious out of a laboratory filled with carbon monoxide.[36]
Heinrich Debus, who studied with Bunsen from 1845 to 1847 and then served as his assistant until 1851, has provided a detailed description of Bunsen's lectures and practicum in Marburg. A set of student lecture notes from 1850 has also survived.[37] Bunsen was clearly one of the century's best lecturers on chemistry, and his numerous and apposite illustrative experiments never failed. He especially loved physical and inorganic chemistry, and he emphasized precise quantitative measurements in all operations. The small amount of theory included in his courses was thoroughly in the spirit of Berzelius' electrochemical dualism. His most popular course was his famous Publikum on electrochemistry, where every seat was always occupied.
In the early 1840s Bunsen taught about five to ten Praktikanten at a time, although there were sixteen by winter semester 1845/46 and occasionally over twenty in the late 1840s. Most were pharmacy or medical students who only worked the standard eight hours per week; normally four to six were advanced workers, mostly chemistry majors, who had permission to work all day every day in the lab. The practicum course started with two or three weeks on the nature of flame and blowpipe analysis. The remainder of the semester was devoted to wet qualitative analysis, for which each student analyzed (at his own pace) progressively more challenging unknowns. For four to six weeks of the analytical portion, Bunsen provided most of the supervision, but as the students gained knowledge and confidence he turned much of the burden over to his assistant. However, he was always present in the laboratory—supervising or doing his own research—to answer any student's question.
Kolbe's duties as Assistent included instruction and supervision of the Praktikanten in the laboratory and assisting Bunsen with preparations for his lecture demonstrations. Kolbe also had time and use of the lab for his own research. Why he never sought to qualify as Privatdozent is not clear; certainly his research was of the requisite quality and quantity for the necessary thesis (the Habilitationsschrift ). A final occupation of his first years in Marburg was the preparation of a German translation of the first volume of Gerritt Mulder's Proeve eener algemeene physiologische scheikunde (Rotterdam, 1844). Wöhler had no doubt recommended him for this purpose to the Braunschweig publisher Eduard Vieweg, whom he had gotten to know through their mutual friend Liebig. The first seventeen letters by Kolbe to Vieweg, of what would become a total of 524 extending over forty years, concern this translation.[38]
Wöhler and Bunsen, Kolbe's two direct mentors, had much in common, and their influence on Kolbe was strong and unmistakable. Both chemists were enormously prolific and, moreover, extraordinarily skilled, inventive, and precise in laboratory operations. Wöhler, thoroughly schooled by the master Berzelius, must have often repeated to his own students his teacher's well-remembered injunction against "geschwind aber schlecht" (speedy but poor) experiments, and he provided his students innumerable models of research following this ideal. Bunsen, a virtuoso if there ever was one, was also famous for the care and accuracy of his work. In the 1830s he worked out methods for the analysis of mixed gases that were far superior to those previously used and that were only slowly spread from his laboratory. In 1841 he developed a much improved carbon-zinc battery that could be used for electrolysis experiments as well as more practical applications. Kolbe was to make immediate and highly productive use of both of these innovations.
Wöhler and Bunsen were also alike in their brilliant teaching abilities, their predilection for experimental investigations, and their habitual avoidance of theory. This is not to suggest that they always ignored the theoretical implications of their studies, nor to deny that many of their papers were theoretically important. Wöhler's work on urea and benzoyl derivatives and Bunsen's work on cacodyl are examples of theoretically rich papers. But, significantly, both scientists left the discipline of organic chemistry just when it began to explode theoretically in the early 1840s—and just when Kolbe arrived on the scene—and neither of them ever returned to the field in the succeeding decades.[39] After 1843 Bunsen excluded organic chemistry ever more effectively from both his teaching and his research. That Kolbe, the student of both of these men, became a theoretically inclined organic
chemist is curious but not truly anomalous. After all, both were still doing organic chemistry when Kolbe was their student, and the strong theoretical orientation of Wöhler's teacher Berzelius and Wöhler's best friend Liebig were clearly of decisive influence on Kolbe's development.
There were also a variety of personal bonds between Wöhler and Bunsen. Since their first meeting in Giessen in 1832, their lives became curiously entwined. Stromeyer was Bunsen's Doktorvater and Wöhler's Doktorgrossvater (through Leopold Gmelin); the decisive scientific influence for both Wöhler and Bunsen, however, was exerted by Berzelius. As Privatdozent in Göttingen, Bunsen succeeded Stromeyer unofficially and temporarily until Wöhler replaced him; Bunsen then took over Wöhler's position in Kassel. Three years later, he succeeded Wöhler's first chemistry professor in Marburg, Wurzer. When Bunsen started his practicum in 1840, he closely imitated that which Wöhler had begun in Göttingen, even to the same eight hours of the week. Finally, Wöhler provided Bunsen with two of his first Assistenten, Voelckel and Kolbe. Their commonalities in personality, temperament, and scientific style resulted in strong bonds of mutual regard.
In conclusion, Kolbe must have felt it extremely natural and comfortable to work in Marburg after his education in Göttingen. Certainly he was given no reason to doubt the basis of the science of chemistry in Berzelian electrochemical dualism. 'This psychological and theoretical commitment was only increased by the research he carried out at both universities.
Doctoral Work
For his Doktorarbeit , Wöhler assigned Kolbe the task of studying the action of chlorine on carbon disulfide. This proved to be a crucial choice because it led Kolbe inexorably toward an examination of the link between organic and inorganic chemistry, a topic that was at the heart of many of the theoretical debates then raging in Europe. Carbon disulfide (modern CS2 ) was a familiar substance at that time, but it was commercially unavailable, so Kolbe had to prepare it in Wöhler's lab directly from the elements. As Wöhler reported to Berzelius in a letter of 26 July 1842, it initially appeared that the products of high-temperature chlorination in a porcelain-filled tube were sulfur dichloride and a new substance that Wöhler formulated as CS+>C l. Berzelius considered this a "very interesting compound" in his preferred "four-volume" (i.e., doubled) formulation of it, CS2 +CC l2 , precisely because of its position on the organic-inorganic interface.[40] (The barred symbols represent Berzelian "double atoms," so that C l = C l2 .)
Within two weeks after writing to Berzelius, Wöhler and Kolbe had determined that no novel compound was formed after all, the second product being merely a mixture of sulfur dichloride and the previously known Kohlensuperchlorid (literally, carbon superchloride, known today as carbon tetrachloride). Still, the reaction was significant as a smooth route to preparing large quantities of the latter material, much superior to Regnault's inefficient chlorination of chloroform. Moreover, Wöhler and Kolbe found that a new compound of carbon, chlorine, and sulfur was in fact formed when the same reaction was carried out at room temperature, though at the time of writing (September 1842) they had not yet determined its formula.[41]
Berzelius wrote that both results were "extremely important" because they represented the formation of organic products from purely inorganic reactants, and he offered advice on how it might still be possible to obtain the product that Wöhler and Kolbe had thought they had isolated in July. He concluded by once again stressing the theoretical importance of these kinds of compounds and exhorting Wöhler to look at a few additional related compounds, including one he and Alexandre Marcet had discovered three decades earlier, the substance now known as trichloromethylsulfonyl chloride (formulated in Berzelian terms as CC l2 +SO2 ).[42]
Wöhler and Kolbe followed Berzelius' prescriptions and found that the compound formed by room-temperature chlorination was indeed the half-chlorinated carbon disulfide. In the paper as it appeared in Liebig's Annalen[ 43] —curiously, published under the single name "Heinrich Kolbe"—the new substance was formulated first as CSCl2 (Wöhler's preference). Kolbe then noted that the formula "probably" should be doubled to CCl4 +CS2 (Berzelius preference).
The Decline of Dualistic Organic Chemistry
Behind Kolbe's two mentors, Wöhler and Bunsen, lay the dominating spirit of Berzelius, whose reign as virtual king of chemical theoreticians was only beginning to be challenged while Kolbe was studying at Göttingen. Wöhler was Berzelius' official (and extraordinarily industrious) representative in Germany. The correspondence of the two chemists over two dozen years occupies nearly 1500 pages in Wallach's massive edition; the warmth of their scientific and personal relationships emerges clearly from virtually every page. Wöhler's own theoretical convictions, such as they were, nearly always came directly from the Jahresberichte and the Lehrbuch of Berzelius.
We have seen how Berzelius offered advice to Wöhler on both the
experimental and interpretational aspects of Kolbe's first extended investigation; Wöhler certainly shared these suggestions with his student. Although no letters between Berzelius and Kolbe have survived, it is known that they had begun to correspond on an occasional basis at least by the summer of 1843.[44] It is clear from both correspondence and his reports in the Jahresberichte that Berzelius became ever more excited about Kolbe's research.[45] For his part, Kolbe revered Berzelius; he kept one highly complimentary letter from the Swede (dated 3 August 1844) for the rest of his life as a "talisman" against the seductiveness of fraudulent hypotheses.[46]
The bedrock of Berzelian chemistry, dating from Berzelius' first important paper (1803), was the conviction that chemical affinity was reducible to polar forces between electrically dissimilar molecular components. The system built up over the years by Berzelius and his school was thus called electrochemical dualism. Applied to inorganic combinations, this system became widely accepted in the 1810s and 1820s.
Applied to the emerging field of organic chemistry in the 1830s, the theory led to the formulation of various organic radicals that were thought to function integrally and electropositively, adding to negative components as metallic elements do in inorganic compounds. Whether electronegative oxygen could enter into an organic hydrocarbon radical was a disputed point right from the beginning of the older radical theory. Berzelius expressed strong doubts about the reasonableness of oxygenated radicals, preferring conceptually clean, theoretically consistent, purely electropositive hydrocarbon radicals. Liebig took the more empirical viewpoint that at least some of the oxygen associated with many organic radicals seemed to appear in entire series of compounds of the radical and so ought to be considered as an integral part of the radical.[47]
Upsetting the coherence of dualistic organic chemistry was the phenomenon of chlorine substitution, wherein highly negative chlorine appeared to replace highly positive hydrogen in organic compounds, without major alteration of the properties of the compound. Since a cardinal thesis of dualistic organic theory was the direct dependence of chemical properties on electrical characteristics of molecular components, this appeared distressingly anomalous. Adumbrated by Liebig's mentor Gay-Lussac, then by Liebig's and Berzelius' archrival Dumas, organic chlorination reactions were first thoroughly explored by Dumas' student Auguste Laurent beginning in 1830. Laurent developed a nonelectrochemical theory of organic reactions based on chlorine substitutions which he initially called the theory of fundamental and derived radicals and later the nucleus theory. According
to this theory, fundamental radicals could be transformed into derived radicals either by substitution within the radical or by addition or elimination of atoms outside the radical. The most important factor for Laurent was not the identity of an atom but its position. The electrochemically opposite substances hydrogen and chlorine could play the same chemical role inside a radical; conversely, a chlorine atom inside or outside a radical would exhibit different chemical properties. Similarly, oxygen could replace hydrogen inside the radical with no great alteration of properties, but oxygen introduced outside the radical would make a neutral substance acidic.
Dumas at first attempted to salvage dualism in the face of Laurent's discoveries. Briefly allied with Liebig at the end of 1837, they published a joint manifesto that advocated a complete analogy between inorganic elemental radicals and organic compound radicals. Attacked by Berzelius as having been corrupted by Laurentian theory, Dumas indignantly refuted the charge. But in August 1838 Dumas discovered chloroacetic acid, and he noted to his surprise that the replacement of three-fourths of the electropositive hydrogen content of acetic acid by highly electronegative chlorine had little real effect on its properties.
Dumas promptly abandoned dualistic organic chemistry, formulating a new theory loosely based on Laurent's work. According to Dumas' "type theory," there are series of organic compounds each of which must be considered to be based on a single "type" formed from the same number of chemical equivalents combined in the same way. Contrary to dualistic organic theory, substitution reactions—even between electrochemical extremes, such as chlorine for hydrogen in chloroacetic acid—cannot alter the chemical type, hence cannot alter the fundamental chemical properties. In a modification of this theory (1840), Dumas conceded that substitution sometimes does alter the fundamental chemical properties without changing the total number or apparent mode of arrangement of the chemical equivalents. In such instances, a new chemical type is created, but without altering the "molecular or mechanical" type. Only addition or elimination of atoms could create a new mechanical type.
Despite his joint declaration with Dumas, Liebig had also been traveling away from the Berzelian orthodoxy. One of his greatest experimental and theoretical masterpieces, the joint work with Wöhler on benzoyl derivatives (1832), had suggested to him that oxygen must be considered as an integral part of certain organic radicals and that the hydrogen atom of benzaldehyde (benzoyl hydride) is replaceable by oxygen, chlorine, or other electrochemically dissimilar substances. Thus the door to apostasy was opened for Liebig.
For Berzelius, all acid-base reactions resulted from electrochemical
addition of a negative anhydrous (usually oxy-) acid to a positive base. Just as potassium sulfate, for example, was regarded as the result of the combination of sulfuric acid (anhydride) and potash:
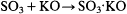
so potassium acetate was regarded as the combination of acetic acid (anhydride) and potash:
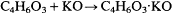
It must be noted that Berzelius did not regard vapor densities of compounds as indicative of molecular size, and so there was no problem with formulating acetate as a "four-volume" molecule. Indeed, only a four-volume (and not a modern two-volume) formula succeeds in representing acetic acid anhydride without fractional oxygen atoms. He also used an atomic weight for potassium that was twice what later chemists adopted, hence he required only half the number of potassium symbols in the formula as compared to what we have become used to. Looking at the issue in the reverse sense, we note that the modern two-volume formula for acetic acid is C2 H4 O2 ; doubling the formula and then subtracting a molecule of water (H2 O) yields the Berzelian formula, which can be depicted as coupled to a (modern) potassium oxide component, K2 O. Although Berzelius was led to these ideas by apparently consistent application of his basic assumptions, and although they were widely accepted for many years, the result of these manipulations was that inorganic and organic chemistry became based on two different fiducial standards: two- and four-volume formulas, respectively. This conflict was to create serious difficulties for Berzelian chemistry during the 1840s and led to its destruction in the 1850s.
Initially, Berzelius preferred to consider the acid, as in the equation cited above, as the trioxide of the acetyl radical, C4 H6 . We will see in the next chapter how he shifted his position after 1838 to view the oxygen content as combined with half the carbon of the molecule, the other half being a hydrocarbon moiety: C2 H6 ·C2 O3 . Since C2 O3 is the (two-volume) formula for oxalic acid (anhydride), all carboxylic acids could subsequently be formulated as homologous hydrocarbons coupled with oxalic acid.
From earlier work by Davy and Dulong and his own benzoyl radical paper, Liebig developed a new theory of acid-base reactions that depended not on dualistic addition of bases to acids but rather on
substitution by bases for a replaceable hydrogen atom of acids. The nonelectrochemical and substitutionist implications of this theory appeared to ally Liebig with Laurent, a point stressed by Berzelius in correspondence with his recalcitrant German friend. In fact, Liebig's organic hydracid theory led directly to a tragic polemic between them, at first waged quietly and privately, then increasingly publicly. The differences separating Berzelius and Liebig were quite real: Liebig had essentially abandoned dualistic precepts in organic chemistry, though he also had no patience for the French type theorists. The kindly Wöhler, who dearly loved both men, was caught in the middle. Unable to understand such passion over matters of theory, Wöhler tried for years without success to effect a reconciliation.
The wear on Liebig's nerves and emotions was also substantial, although not sufficient to overcome his firm (Berzelius would say obstinate) convictions. Worn down to the point of physical and psychical illness both by his arguments with Berzelius and by an increasingly vitriolic series of priority disputes with French substitutionists such as Dumas, Laurent, Malaguti, and Persoz, Liebig ostentatiously declared to Wöhler in early 1840 his abandonment of all chemical theory and his resolution henceforth to devote himself to practical pursuits such as agricultural and physiological chemsitry. Liebig was not alone in feeling an uncomfortable sense of disorientation and dismay at the rapid evolution of theory. J. F. W. Johnston described the science in 1840 as being "unhinged . . . tottering and disjointed." He called for a return to the comfortable Berzelian orthodoxy, condemned the "rage for the new " sweeping the Continent, and labeled Liebig as well as Dumas "chemical chartists."[48]
Liebig's abandonment of theory was not mere rhetoric. After 1839, Liebig abandoned his own hydracid theory and really ceased to participate in the organic-chemical theoretical dialectic. One indication of his disgust was his unauthorized decision in February 1840 (which was during Kolbe's Göttingen years) to publish a whimsical and hilarious French-language lampoon of the type theory, which had been written on a lark by Wöhler.[49] The putative author, S. C. H. Windler (Schwindler = swindler), claimed to have succeeded in gradually replacing all the atoms in copper acetate by chlorine, producing a material composed entirely of chlorine but retaining the properties of copper acetate. The paper portrayed with some justice, but also with malice, the extent to which Dumas, Laurent, and others were inclined to exaggeration. Whether due to the strain of the disputes, or to the Schwindler critique, or to the attainment of a level of professional success that reduced the hunger for acclaim, Dumas also retreated after
1840 from a leading theoretical role. Since many of the remaining leaders of German chemistry, including Wöhler, Bunsen, Gmelin, Rose, Mitscherlich, Will, and Kopp, were not inclined toward theory, and the great Berzelius increasingly appeared to the chemical world to be old-fashioned, something of a vacuum in German organic theory emerged in the 1840s—a void into which Kolbe eagerly stepped.