6—
Confronting the Reform Movement
The "quiet revolution" of the 1850s transformed the theoretical foundations of chemistry and provided the basis for its explosive growth in intellectual and technological applications during the second half of the century. It was a complicated affair that had many interconnecting aspects.
1. First was the decline of electrochemical dualism . By the mid-1840s, virtually everyone had lost faith in at least the strong program of dualism, and many French chemists opposed it ardently. It had been weakened by innumerable examples of substitution reactions in organic chemistry, accumulating since the 1820s, few of which appeared to agree with dualistic principles. The gradual loss of what had been such an important and productive principle was distressing and disorienting to many. The general retreat from pursuit of theory by such leading figures as Liebig and Dumas was related to this perceived theoretical chaos.
2. A direct and obvious consequence was the rise of a unitary view of molecules . If molecules are not dissectible into paired, electrically opposed components (classical radicals) as demanded by dualism, some drew the conclusion that they must instead be holistic structures whose internal details cannot be determined by chemical reactions at all. This view, implied by some of Dumas' rhetoric in his type theory papers of 1838-1840 and pursued in more than rhetorical fashion by other Frenchmen,
such as Gaudin, Baudrimont, and Gerhardt,[1] appeared from the German perspective to be the overriding French orthodoxy of the 1840s and 1850s.
3. This unitarian viewpoint, however, was simultaneously opposed, also in France, by new (substitutionist) arrangement theorists . Dumas, who during the heyday of the old radical theory had practiced the art of constructing "rational formulas" with the best of the German Berzelians, did not really abandon the quest for molecular constitutions when he embraced type theory, as his defense of "mechanical types" and his use of other structuralist metaphors show.[2] The very notion of a "type" and the centrality of substitution suggested that the arrangement of the atoms within a molecule must be just as important as their identities. The structuralist program was thus an obvious concomitant of type theory. These ideas were intimately related to Laurent's nucleus theory; Laurent frequently remonstrated with Gerhardt regarding the latter's apparently absolute rejection of arrangement theories.[3] Those pursuing typist arrangement theories were not so different in principle, then, from those Germans attempting to rescue the rational formulas of dualism by means of an increasingly obsolescent copula theory.
4. The decline of copulas was directly connected to the emergence of a new type theory . The natural outgrowth of all of the previous trends (except the radical unitarian doctrine), this theory developed suddenly in 1849-1850. Wurtz' synthesis of primary amines, Hofmann's synthesis of secondary and tertiary amines, and Frankland's work on zinc alkyls, all published in 1849, were each viewed by their authors essentially as substitutions of organic radicals for the hydrogen atoms of inorganic hydrides (in the cases just mentioned, ammonia and the hypothetical metal hydrides). Paul Thenard's work of 1847 on the methylation of phosphine (PH3 ) was now interpreted by the new type theorists in similar terms, though Thenard had not initially done so himself.[4] The following year (1850), Alexander Williamson provided a fourth new type and the most important one of all, the water type. In this work, Williamson not only ensured the success of the new type theory but also effectively promoted two central reforms championed earlier by Laurent and Gerhardt.
5. One of these reforms was the establishment of consistent molecular magnitudes . Organic chemists had never regarded vapor densities as consistently determinative of molecular size,
partly because to do so would have created a number of apparent anomalies. To maintain elements of dualistic theory, it had proven necessary to use "four-volume" organic formulas double the size of chemically comparable inorganic ones, and most chemists subscribed to these formulations. For largely schematic and esthetic reasons, hence ones that few of their contemporaries found compelling, Gerhardt from 1842 and Laurent (with greater consistency and cogency) from 1845 had advocated taking all formulas, organic as well as inorganic, at the same number of gaseous volumes, preferably two.
6. A largely separate but equally critical issue was atomic weight reform . By 1850 this came down essentially to the issue of whether or not to double the atomic weights of carbon, oxygen, and sulfur, that is, to halve the usual number of such atoms in organic formulas. The smaller weights, implying, for example, a formula for water of HO (or H2 O2 ) and a formula for the ethyl radical of C4 H5 , had become nearly universally accepted.
Williamson's Asymmetric Synthesis Argument
Williamson's work proved to be the pivot on which turned the ultimate breakthrough of the movement as a whole, because an effective experimental argument that he devised in 1850 and that saw various applications in the next five years essentially settled the molecular magnitude issue, provided compelling grounds for adopting the newer type theory and the atomic weight reform, and further weakened the vestiges of dualism enshrined in the copula theory. During his student years in the 1840s in Heidelberg, Giessen, and Paris, Williamson (1824-1904) was a student of Gmelin and Liebig, and he became the first major advocate of Laurent's and Gerhardt's chemistry.[5] In 1850, newly installed as professor of analytical chemistry at University College London, he decided to try to settle the important issue of relative molecular magnitudes of alcohol (in modern terms, CH3 CH2 OH or Et-OH) and ether (CH3 CH2 OCH2 CH3 or Et-O-Et).[6]
From the 1820s through the 1840s, Liebig and most other chemists had assumed that alcohol was simply the hydrate of ether, since acid dehydration of alcohol yields ether—this despite the fact that the vapor density of ether is nearly twice that of alcohol.
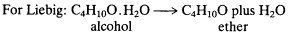
Laurent and Gerhardt, however, argued from 1846 on that alcohol is a monoethyl and ether is a diethyl water derivative. Reacting Frank-land's reagent ethyl iodide together with a substance first prepared by Liebig, potassium ethoxide, Williamson succeeded in substituting ethyl for the replaceable hydrogen of alcohol, resulting in a novel ether synthesis that was elegant, smooth, low-temperature, and high-yield. In Williamson's terms,
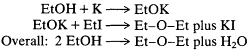
This reaction could still be explained by the older theory if one assumed that the potassium ethoxide and ethyl iodide were each separately transformed, yielding two ether molecules rather than one. But an additional advantage of the Williamson reaction was its flexibility in producing new "tailored" ethers, and an ingenious modification by Williamson cut off any retreat. By reacting methyl iodide with potassium ethoxide, the two theories would now predict two distinct results. The Gerhardt-Laurent (Williamson's) theory required the production of a single, homogeneous product, an asymmetric ethyl methyl ether. Liebig's theory would require a mixture of products to result from the reaction, the symmetrical (normal) ethyl ether, derived from the ethoxide, and a new symmetrical methyl ether, derived from the iodide.
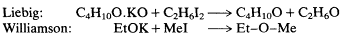
The consequent production of a single ethyl methyl ether settled the issue, at least for Williamson. As Gerhardt and Laurent had been urging, the ether molecule must be essentially twice the size of the alcohol molecule, not simply a dehydrated version of it; more generally, there is no pre-formed water in alcohols or acids, nor pre-formed oxides in salts. The Gerhardt-Laurent molecular magnitudes reform appeared to be established at a stroke, and dualistic formulations were further weakened, if not destroyed. Many chemists agreed with Williamson, as we shall see in the next section.
The atomic weight reform required another kind of argument. The focus of the ether molecule was the oxygen, which Williamson saw no reason to subdivide. A single oxygen atom, O = 16, could then serve as the material link or bond between two ethyl radicals in ether, an ethyl and a hydrogen in alcohol, or two hydrogens in water. The argument
was cogent, but not as compelling as that for the structure of ether; it involved an appeal both to Ockham's razor and to structuralist instincts.[7]
The best evidence for the strong impact of Williamson's asymmetric synthesis argument is its widespread deployment in the five years after 1850. In 1851 Williamson used it again himself, in conjunction with a messy ketone reaction, to advocate Gerhardt's and Laurent's formula for acetone—and solving one of their troubling anomalies in the process. As we saw in chapter 4, in the spring of 1852 Gerhardt synthesized organic acid anhydrides by a reaction route analogous to Williamson's (and already predicted by Williamson the year before). He also applied Williamson's logic as well, by producing asymmetrical anhydrides (such as acetic-benzoic anhydride) that could be explained only by the new chemistry. Gerhardt thought this accomplishment was "terribly revolutionary," the best paper of his life. Indeed, it convinced a wide variety of skeptics both that monobasic organic acids contain no water even in their "hydrated" form and that the water molecule must have two hydrogen atoms. This was the event that propelled Gerhardt from his pariah status to career success—if unfortunately only briefly, as he died in 1856.
The next chapter will show how in 1854 William Odling and August Kekulé, then living in England, independently applied versions of Williamson's argument. Also in 1854 Williamson himself used it once more. He succeeded in chlorinating sulfuric acid in two separate stages, producing in the first reaction a substance now known as chlorosulfonic acid, HO.SO2 .Cl. If one were to reason on the basis of the conventional formula for sulfuric acid, HO.SO3 , the halfway chlorination ought to have produced at best a mixture of HO.SO3 and Cl.SO3 , and not a homogeneous asymmetric product. It was clear to Williamson from this reaction that there is no preformed water even in dibasic inorganic acids. Again, the reaction had indicated the inconsistent molecular magnitudes of the hitherto prevailing views: the conventional sulfuric acid formula HO.SO3 , like the HO formula for water, needed to be doubled to compare consistently with the usual organic formulas (in more unitary, nondualistic terms, H2 S2 O8 ), and then the larger Gerhardtian atomic weights had to be applied to oxygen and sulfur (yielding H2 SO4 ). Viewed in this light, his argument was that, in sulfuric acid, the sulfuryl radical SO2 is the material link between HO and Cl in chlorosulfonic acid and between two HOs in sulfuric acid, just as oxygen is the linking element in ether and in water.[8]
Assumptions of relative molecular sizes and atomic weights were also crucial in interpreting the new radicals created by Kolbe and Frankland during the years from 1847 to 1850. The radicals formed in
these reactions are now thought to combine together as they are formed, to create dimers:
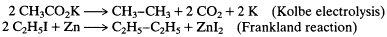
Nothing in their reactions of formation, however, indicates this dimerization, and Kolbe and Frankland wrote versions of the simpler equations as follows:
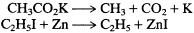
These implied production of the actual monomeric radicals. Although Gerhardt and Laurent immediately argued for the dimer formulas of the new substances, it is understandable that Kolbe and Frankland, and initially most other chemists, were not persuaded of the truth of a more complex and less obvious reaction route—especially because the central argument that the Frenchmen used was their mysterious and unmotivated even-number rule.[9]
But by March 1850, newly converted from copula to type formulations, Hofmann became convinced that Gerhardt and Laurent were right.[10] He attempted to settle the issue by an experiment designed to decarboxylate valeric acid pyrolytically, but the results of the experiment were messy and unhelpful. Still, he appended a thorough brief for the thesis that the "radicals" were dimeric molecules. They had none of the properties that one would expect from true radicals, such as extreme reactivity, or additive reactions with oxygen or halogens. That they had proven so difficult to extract from their compounds suggested all the more strongly that they ought, if they were true monomeric radicals, to have properties similar to the highly active "radicals" (i.e., metals) extracted by Davy from soda and potash. On the contrary, the new hydrocarbons had all of the indifferent paraffinic properties of the predicted homologues of marsh gas.
Another anomaly was that the differences between the boiling points of three adjacent homologous "radicals" were found in each case to be 47ºC. This was about twice the fairly consistent boiling point differences between nearly all adjacent homologs, as found by Hermann Kopp. The anomaly would vanish if the formulas for the "radicals" were doubled and intermediate unknown hydrocarbons were interpolated. Again, whereas amylene (C10 H10 ) and amyl hydride (C10 H12 ) boil at 39ºC and 30ºC, respectively, "amyl" (Frankland's and Kolbe's C10 H11 ) boils at 155ºC, which did not seem reasonable to
Hofmann; a doubled formula would again restore consistency in the place of anomaly. Finally, the "radicals" all had doubled vapor densities compared to similar olefins and hydrides.
In short, both physical and chemical properties suggested that these molecules were twice the size of what Frankland and Kolbe thought. Hofmann conceded that this meant supposing an in situ and in statu nascenti dimerization reaction, for which there was no direct evidence. He pointed out, however, that in situ polymerization or condensation reactions were already known and accepted, such as the formation of acetone from acetates and mesitylene from acetone.
In December 1850 Benjamin C. Brodie, Jr., adduced additional arguments in favor of the dimer formulas.[11] At the end of his paper, he suggested a crucial experiment modeled on Williamson's recently published asymmetric synthesis argument. Frankland had reacted ethyl iodide with zinc to produce what he thought was ethyl and what Brodie and Hofmann were interpreting as ethyl-ethyl. If one instead used mixtures such as ethyl iodide and amyl iodide for the reaction, one might be able to produce asymmetric radicals, in this instance ethyl-amyl. The formation of such an asymmetric radical as a product would demonstrate that dimerization was occurring, since according to Frank-land's conception one would expect to produce only mixtures of symmetrical radicals, ethyl and amyl. But Brodie reported that neither he nor Hofmann had had success with the reaction.
That success was achieved by Wurtz in 1855.[12] In place of Frank-land's, Brodie's, and Hofmann's zinc (Frankland, presumably following an earlier suggestion of Liebig's, had also tried potassium), Wurtz got the reaction to work using sodium, which he said combined the advantages of acting less energetically and being cheaper than potassium. Following the Williamsonian strategy indicated by Brodie and Hofmann,[13] Wurtz produced the "mixed" radicals ethyl-butyl, ethyl-amyl, buty-amyl, butyl-caproyl, and methyl-caproyl. The argument from this evidence for the larger dimeric formulas for the Kolbe-Frankland "radicals," averred Wurtz, was "of the same order and just as conclusive" as Williamson's for the larger ether formula. One of Hofmann's predicted interpolated compounds was also a product of this research, namely, butyl-amyl (in Wurtz' four-volume formulas, C8 H9 .C10 H11 ), which was half-way between "butyl" (Kolbe's "valyl," in Wurtz' formulation C8 H9 .C8 H9 ) and "amyl" (C10 H11 .C10 H11 ). The boiling point, Wurtz emphasized, just matched Hofmann's prediction. Wurtz even succeeded in producing similar asymmetric radicals from Kolbe electrolyses.
Dimerization introduced a new complexity into the explanation of the reactions, but Wurtz, again following Brodie and Hofmann, cited
other accepted instances of this kind of reaction. He was even able to make reference to a project he had carried out under Liebig in 1842 involving the reaction of hydrochloric acid and copper hydride with release of hydrogen gas, which supported Dumas' 1828 speculation on the "dimerization" of hydrogen atoms to form hydrogen molecules.[14] Wurtz also pointed out that this was one of the theses defended by Laurent when, in 1846, he clarified Gerhardt's ideas on atoms and molecules. He mentioned that it had also been proposed and defended more than forty years earlier by Ampère.
The "Wurtz reaction" is still taught in organic chemistry classes today, but it is a messy reaction producing problematical product mixtures, and it is no longer synthetically useful. This is all the more reason for admiring Wurtz' achievement in unraveling the complex chemistry and properly analyzing the liquid and gaseous products, then drawing the important theoretical conclusions—even if the latter were not original to him. He concluded the paper with an advocacy brief for the new chemistry. He used the new atomic weights in this section, "for greater simplicity,"[15] but in general continued to employ the older conventional equivalents for another four years.
It is interesting that all of the protagonists in the reform movement sketched here expressed themselves after the new type theory emerged in terms of compatibility and complementarity rather than conflict. In 1850, Frankland, Hofmann, Wurtz, and Williamson all implied independently that their work on organometallics, complex amines, primary amines, and ether, respectively, suggested a consolidation of radical and substitution theories.[16] The same year Gerhardt used similar language in attempting to reconcile with Liebig, and Liebig returned the rhetoric two years later.[17] Also in 1852, Frankland stated unequivocally that his and others' recent research promised "to assist in effecting a fusion of the two theories which have so long divided the opinions of chemists, and which have too hastily been considered irreconcilable."[18]
Finally, in 1855 Wurtz put forth an extended argument having to do with "condensed" or multiple types. He pointed out that although the old radical theory used a binary conception of constitution with addition mechanisms, the new chemistry used a similar binary constitutional orientation with substitution mechanisms. Thus, "far from being contradictory, they complement one another."[19] These visions of continuity and complementarity were clear-sighted and not simply an instance of soft-pedaling revolution. The newer type theory was a natural and logical outgrowth of the development of the science to 1849; it incorporated much of the older theories, while at the same time justifying many of the new ideas of Laurent and Gerhardt.
Attack and Counterattack:
Kolbe Versus Williamson
As we saw at the end of chapter 5, Kolbe was taken by surprise by Gerhardt's and Williamson's papers of the early 1850s and he recognized their importance. Kolbe was far from alone in seeing the compelling character of the new research, and he became increasingly alarmed by the rapid gains that Gerhardt seemed to be making. In France, establishment figures such as Fremy, Regnault, and especially Dumas and Thenard ceased quarreling with Gerhardt and began to support his academic career. Not only did Gerhardt's students such as Chancel and Chiozza adopt elements of his system but also Wurtz, Cahours, Pelouze, Malaguti, and Quesneville. A few Russians and Americans, as well as such Britishers as Odling, Brodie, Henry Roscoe, and Williamson, became converts.
Of even greater concern to Kolbe, there were obvious signs that Gerhardt's views were gaining currency among younger and mid-career German chemists as well. Leopold Gmelin had adopted Laurent's classification scheme in 1848, and Liebig had had high praise for Gerhardt's acid anhydride research. Worse, Kolbe's good friends Hofmann and (German-educated) Frankland had clearly been moving toward the "typist" camp since 1849. Hofmann privately opined at the time of the acid anhydride work that this sort of argument "removes the last support for the idea that [alcohols and acids] contain water." Later he characterized the argument as "irresistibly convincing."[20] A new generation of German chemists—including Kekulé, Karl Weltzien, Emil Erlenmeyer, Heinrich Limpricht, Ludwig Carius, Lothar Meyer, Adolf Baeyer, and Leopold von Pebal—were declaring themselves as converts during the early to mid-1850s.
Kolbe could take little comfort that the éminences gris of German chemistry had not signed on, for he well knew that they were uninterested in the theoretical dialectic in general. "What opinion Liebig now has on the subject [of Gerhardt's work] I do not know," he wrote Vieweg. "I suspect none at all, like Wöhler, since neither one seems to have much interest for such matters."[21] The same was true for Bunsen, as Kolbe well recognized.[22] One member of the "classical" generation who did convert to Gerhardt's system, if not to his notation, was Otto Erdmann, Gerhardt's first chemistry teacher and founder of the Journal för praktische Chemie .[23] In sum, Gerhardt's views were making headway in the early 1850s in major chemical centers such as Heidelberg and Göttingen and were establishing outposts all over the Germanic lands.
In addition, virtually the entire chemical faculty at Giessen became
Gerhardtians. Heinrich Will, Liebig's successor, is discussed shortly. Adolf Strecker had been successively student, assistant, and Privatdozent under Liebig before his call to Christiania (Oslo) in 1851 and had written Kolbe's favorite short organic chemistry textbook. Strecker had always been favorably inclined toward Gerhardt's and Laurent's research, and by 1854 he was clearly a convert; at the Karlsruhe Congress he was quite vocal in his defense of the new chemistry.[24] He was a talented chemist, much respected in his day. Finally, it took little reading between the lines of the annual Jahresbericht der Chemie to perceive that its editor Hermann Kopp, Ordinarius for theoretical chemistry at Giessen after Liebig's departure, had by about 1854 become attracted by the French ideas. The sober and cautious Kopp, seven years older than Williamson, later recalled the latter's work as providing "brilliant confirmation" of the new chemistry, "demonstrating beyond question" the Gerhardtian constitutions and quickly garnering willing converts.[25] The defections of Strecker and Kopp were particularly bad blows for Kolbe. They were both intimate friends of his (Duzfreunde ), and Kolbe had always admired their careful conservative approaches.
Kolbe worried about how to respond to the new research, especially because he was writing the critical theoretical sections of his textbook in 1853, just as the tide appeared to be turning against him. During the spring, summer, and early fall of that year, Kolbe worked furiously to devise counterarguments to the Williamson-Gerhardt theory. When he thought he had the desired disproofs, he incorporated them into his first textbook installment, which appeared in June 1854. But he was so concerned at the signs of revolution in the air that he decided to hurry his critiques into print as articles at the earliest possible moment.
The first of these was an attempt to disprove Williamson's theory that acetic acid consists of Williamson's "othyl" radical C2 H3 O united to oxygen, the latter atom also linked to a hydrogen atom.[26] Erroneously translating his own conventional equivalents into Williamson's atomic weights by doubling the number of his hydrogen atoms (rather than using the correct transformation algorithm of halving the numbers of his carbon and oxygen atoms), Kolbe concluded that Williamson's acetic acid formula implied the presence of two methyl groups. If that formula were true, then one of the methyls could in principle be replaced by another radical, ethyl for instance. But experiment failed to achieve this end, so Williamson's theory was at least weakened, if not directly falsified.
This line of thought demonstrates both that Kolbe fully grasped the import and compelling nature of the asymmetric synthesis argument in particular—he was after all trying to turn Williamson's own powerful
gun upon him—but also that he had only a superficial understanding of the new chemistry in more general terms. Two years earlier, upon publication of the German translation of Williamson's third ether paper, Liebig had even provided his readers with two versions of the correct transformation algorithm, but Kolbe must have misunderstood it.[27] Having rejected the atomic weight reform years earlier as a passing French fancy and supremely confident of the truth of the older system, he had simply never successfully worked through the details of the arguments.
Kolbe's attempted refutation was actually performed and written by an English student of Kolbe's, Francis Wrightson, who is otherwise little known; Kolbe later affirmed that the reasoning was his. The research was complete by about June 1853 and was published in the August issue of the Philosophical Magazine . The following month a reply by Williamson appeared. Without explaining the precise character of the fallacy committed by Wrightson, Williamson contented himself with demonstrating that "the result which he failed in obtaining is incompatible with the othyle theory of which he conceived it a consequence, and the result he obtained is decidedly confirmatory of the theory which he expected it to upset."[28] Wrightson's confused and ungracious rebuttal shows that he had not understood Williamson's reply[29] —nor apparently did his teacher, who repeated the same arguments the following year.
In the July and August 1853 issues of the Annalen der Chemie , a two-part German translation of Gerhardt's detailed memoir on acid anhydrides appeared, and in September a German translation of his and Chiozza's work on amides was published. It was to these articles in particular that Kolbe must have been referring when he wrote Vieweg of the "research of the very greatest scientific importance . . . which threatens to overturn" the entire existing theoretical foundation of chemistry. By the time he wrote this letter (on 16 October 1853), he thought he had hit upon "the strongest proofs against Gerhardt's theory" without having had to enter the laboratory.[30] But that fall, Wrightson having left Marburg, Kolbe put another English student, Frederick Guthrie, to work on additional organic electrolyses to refute Williamson. By late fall he had written a detailed critique, which he read to the Marburg Naturforschende Gesellschaft and sent to Liebig for the Annalen and to Hofmann for the Journal of the Chemical Society .[31]
Kolbe brought a number of points to bear against Williamson (and secondarily against Gerhardt). Williamson had taken no notice, it seemed, of his proof that methyl exists in acetic acid and its derivatives. Synthetic methods of the sort Williamson had used are untrust-
worthy indicators of constitutions, Kolbe felt, less certain at least than analyses. In addition, Wrightson's evidence, he thought, had not been effectively countered in Williamson's rebuttal. As for the new asymmetric compounds, nothing prevented one from assuming that the new methyl ethyl ether, for instance, was a combination of ethyl oxide (ethyl ether) and methyl oxide (methyl ether), i.e., C4 H5 O.C2 H3 O. To the possible objection that such electrochemically similar molecules would have no reason to cohere, Kolbe responded that many other similar instances were known, such as compounds of chlorine and iodine. Another point of difference between the two theories was that Kolbe's acetic anhydride formula posited three methyl hydrogens, whereas Gerhardt's and Williamson's had six. Were the latter correct, acetic anhydride with an odd number of chlorine atoms would be possible; conversely, such compounds would be excluded by Kolbe's formula because they could not be represented without use of non-integral atomic coefficients. But no such compounds were known to exist.
Finally, Kolbe regarded the electrolysis of acetic acid as irrefutably falsifying Williamson's theory. According to Williamson, the constitution of acetic acid was analogous to water, with the othyl radical taking the place of one of water's hydrogen atoms. Accordingly, electrolysis of the acid ought to proceed analogously to that of water, with othyl and potassium (or at least their decomposition products) appearing at the negative electrode and oxygen emerging at the positive pole. In fact, nothing but hydrogen is found on the negative side, with methyl and carbonic acid appearing at the positive. Especially for this reason, Kolbe concluded that Williamson's theory was "easily refuted."
Kolbe's paper was read at a meeting of the Chemical Society on 20 February 1854; there was an element of drama as Williamson had prepared a reply to be read immediately following. Henry Watts wrote to H. E. Roscoe at Heidelberg shortly before the event, very much looking forward to the "jolly row" in store.[32] Williamson wrote the same correspondent
Of course I will answer [Kolbe's paper], though from what I have seen of its contents there seems little chance of my converting him to more rational views on the subject, as he does not enter into or understand the point of view opposed to his view.[33]
Williamson's reply was a polemical masterpiece, in which carefully reasoned refutations alternate with less substantive forms of verbal pyrotechnics.[34] In response to Kolbe's methodological critique of synthesis as a valid means of determining constitutions, Williamson
attacked Kolbe's favorite approach, analysis, at the same time blasting his use of undefined and empirically illfounded symbolic distinctions:
It would be just as reasonable to describe an oak-tree as composed of the blocks and chips and shavings to which it may be reduced by the hatchet, as by Dr. Kolbe's formula to describe acetic acid as containing the products which may be obtained from it by destructive influences. A Kolbe botanist would say that half the chips are united with some of the blocks by the force parenthesis ; the other half joined to this group in a different way, described by a buckle ; shavings stuck on to these in a third manner, comma ; and finally, a compound of shavings and blocks united together by a fourth force, juxtaposition , is joined on to the main body by a fifth force, full stop . The general use of unmeaning signs has become so habitual to Dr. Kolbe, that whenever anything has to be explained, he performs the task to his own satisfaction by inventing a sign for its unknown cause. . . . Signs and words are doubtless indispensable means for the expression of facts or thoughts; but Dr. Kolbe uses them instead of facts, and as a substitute for ideas.[35]
Williamson averred that in admitting the existence of the new compound ethyl methyl ether, however he may wish to formulate it, Kolbe had thereby conceded the essential point at issue, the doubled size of ethers relative to alcohols. (This is an example of rhetorical sleight of hand, as Williamson must have been aware that Kolbe intended his argument about the combination of ethyl and methyl "oxides" to apply only to the asymmetric compounds, not to ordinary ether itself.) He reaffirmed his response to Wrightson, expressing satisfaction at being permitted now to direct his rebuttal to the real source of that fallacious reasoning. Finally, he pointed out that the crucial experiment that Kolbe had conceded would win the day for the Williamson-Gerhardt theory—production of an acetic acid anhydride with an odd number of chlorine atoms—was easily produced from the reaction of trichloroacetyl chloride with sodium acetate.
Williamson's mastery of both the rhetorical and substantive forms of scientific discourse is highlighted by comparing his reply to Kolbe with Gerhardt's response.[36] Published in the same issue of the Annalen immediately preceding Williamson's, Gerhardt could only sputter with indignation that Kolbe had attacked "Williamson's" theory, when the ideas that the "overly sensitive" Kolbe opposed so vehemently were his (Gerhardt's) alone. The matter was serious because, by appearing in Liebig's journal, the German public might infer that Kolbe's views were shared by the master (here Liebig added a footnote cautioning that "This should not be assumed"[37] ). Gerhardt's outburst once more indicates his tragic flaws: arrogance, bad grace, and lack of tact. His
priority claim was not unreasonable, but here it served only to alienate his friends. And he did not bother to attempt to refute Kolbe's arguments rationally.
While all these events were taking up Kolbe's attention, his friend Edward Frankland was also directing a friendly but devastating critique at the copula theory. Frankland's most famous memoir, which contains a limited statement of the concept of valence, was published in the Philosophical Transactions in 1852, but in German not until March 1853, when presumably Kolbe first read it.[38] In this work, Frankland argued not only that the reactions of organometallic compounds showed that metal atoms have a maximum combining capacity with other atoms or radicals. He also suggested that the only proper way to envision the reactions is to depict organic radicals as replacing inorganic atoms such as oxygen, rather than simply adding to the metal without altering its chemistry, as in the copula theory.
For instance, according to the copula theory, it should have been easy to oxidize or halogenate the highly electropositive zinc methyl, but all such attempts failed. The only way further combination predicted by the theory could take place was if the organic copulas became detached from the metal atom. In this way, Frankland demonstrated that metal atoms were very much chemically altered by their "copulation" and that their combining capacity was indeed reduced—moreover, precisely by the number of copulas. This applied even to the prototype of copulated radicals, cacodylic acid, in which arsenic was connected to three oxygen atoms and could not be further oxidized to the five-oxygen analog of uncopulated arsenic acid. In Frank-land's new type-theoretical interpretation, this was because two methyl groups had taken the place of two oxygens. In short, the postulates and predictions of the copula theory had been systematically contradicted at every turn. Only an application of type theory could save the phenomena.
Accommodation and Defiance
Little wonder, then, that a note of panic appeared in Kolbe's October 1853 letter to Vieweg. Having adjusted his theories and devised what seemed to him to be decisive refutations, Kolbe wrote the critique discussed above, and incorporated it into the first installment of his textbook, which appeared in June 1854. In his introduction, he commented that a number of empirically shallow theories, proposed mostly by French chemists, threatened to destroy the true basis of organic chemistry, the electrochemical radical theory. It was therefore necessary to begin his text by treating these ideas in detail.
The task facing organic chemists today, Kolbe averred, is the elucidation of chemical constitutions by discerning the "rational compositions" of molecules, that is, the manner in which their proximate components are combined. Kolbe had nothing but contempt for those empiricists whose love of experimentation (Experimentirkunst ) was greater than that for true scientific research and who only sought to produce new substances as fast as possible without investigating their constitutions. He stated that we will "never" be able to gain a "clear view" of the spatial arrangements of the atoms in a molecule; but we are now and will be in the future able to discern the "functions" that atoms and proximate components perform with respect to each other.[39]
The only sure guide to rational constitutions, Kolbe continued, is Berzelius' electrochemical radical theory, founded on the conviction that organic chemical theory must be based on analogies to inorganic chemistry. There are problems with organic electrochemical theory, Kolbe conceded. Above all, we often cannot isolate the components of organic molecules, and even when we can, the determination of their electrochemical properties is problematic. But we should maintain a sense of perspective and not abandon an old, trusted, and useful theory at the first sign of difficulty. Have confidence in the flexibility of a good theory, he counseled, and trust to the future to work through the anomalies.[40]
Consciously or not, Kolbe illustrated this flexibility by the adjustments that he continued to make in his own version of the theory. In the 1840s he had viewed oxalic acid, HO.C2 O3 , as the progenitor of all organic acids. Beginning in 1848 he had preferred to view acids in a more general fashion, as deriving from an ultimate resolved radical C2 to which one could add methyl to form the more proximate acetic acid radical
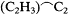
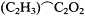
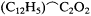
Thus, whereas in 1850 he had conceded to type theory the thesis that chlorine and other atoms may substitute in organic radicals with-
out necessarily causing major alterations of properties, he was now expanding this concession to include all-important oxygen. Moreover, he now viewed hydrogen substitution as the schematic means of constructing all organic acids and acid derivatives from formyl. Other systems were analogous: amines were copulated nitrogens produced by substitution of hydrogens by hydrocarbon radicals, and so on. The fact that halogen substitution does not much alter chemical properties of hydrocarbon radicals proves that "the grouping of their proximate components remains the same" and that their "types" are unaltered. This is true in some cases for inorganic substituting groups as well, such as amido, nitro, and sulfuryl groups.[42] Dumas could not have argued with greater force or clarity for the assumptions of type theory than Kolbe did here.
Despite these concessions, however, Kolbe remained resolutely faithful to dualistic organic chemistry. Although halogen substitution for hydrogen cannot be explained by means of electrochemical precepts, that does not mean that the precepts are wrong. Chlorine, perhaps even hydrogen, might undergo an alteration of electrochemical properties when it enters organic molecules. After all, elemental phosphorus has several chemically different states, and nascent hydrogen is very different from the stable gaseous element. Thus, he believed it would be "frivolous" to abandon dualism because of the single anomaly of substitution.[43]
Kolbe also retained the copula theory as the last major element of dualism left in organic theory. Copulas, he continued to affirm, have little influence on properties of the prototype acid. For example, formic, acetic, and propionic acids are quite similar despite the presence of hydrogen, methyl, and ethyl as copulas, respectively. Accepting Frankland's demolition of the copula theory would require conceding that atoms have fixed combining capacities, irrespective of the electrochemical properties of the adducts—a challenge directed to the very heart of dualism.
But Frankland's evidence was hard to deny. Although stressing that he did not agree with Frankland's conclusion, Kolbe admitted that there did seem to be a general law at work. One might be able to account for it, he thought, by a qualitative electrochemical argument relating to the varying affinities for oxygen of different elements. Since positive elements such as potassium and calcium combine with oxygen in only a small number of proportions, whereas negative elements such as halogens, sulfur, and nitrogen combine in a much larger number, perhaps the addition of alkyl groups to, say, nitrogen makes the nitrogen less negative, hence less capable of combining with additional oxygen. Thus, the total number of atoms or radicals combined with
nitrogen remains roughly the same. Even so, he noted that his tentative suggestion "in no way explains" the extreme regularity or exactness of the relationship revealed by Frankland.[44]
Although often sounding here much like a "typist" himself, in a historical section he poured scorn over the "games with formulas" that
. . . for many years the best chemists of France have been playing, with unbelievable self-deception. Rarely indeed has a new theory been introduced into science with such ostentation and such great confidence in its infallibility, as Dumas' type theory. Seldom has unscientific behavior and the desire immediately to derive general laws of nature from isolated facts been more severely punished as in this case. Of the numerous laws with which the type and substitution theories wanted to enrich science, one after another had to be retracted, and finally nothing more remained than the naked fact that in many organic compounds hydrogen can be substituted by chlorine without essentially altering the molecular grouping of the atoms or in many cases the characteristic properties of the compound.[45]
Kolbe noted the "remarkable fact" that it was almost exclusively German scientists who had developed the radical theory, and he ventured the opinion that the French had opposed the theory "because it had not developed on French soil." Laurent's theory of fundamental and derived nuclei, which some had considered a sort of radical theory, was nothing of the kind to Kolbe; it was a "painting of the imagination," reminiscent of Naturphilosophie. An example of Laurent's penchant for "paper-and-pencil laws of nature" was his notion that oxygen substituting within the nucleus does not alter the characteristic neutrality of organic nuclei (to form aldehydes, ketones, ethers, etc.), whereas if one, two, or three oxygen atoms are added to the exterior of the nucleus they create mono-, di-, or tribasic acids, respectively.[46]
Some, he noted, had criticized the radical theory for hypothesizing fictitious entities, just as he was criticizing Laurent's nuclei. These cavils no longer had any force since he and Frankland had isolated a number of hydrocarbon radicals. Others had suggested that methyl, ethyl, etc. were not real (monomeric) radicals but rather dimeric molecules. To the extent that this argument was based on the application of Laurent's even-number rule, it was just another example to Kolbe of a fictitious French law. Hofmann's claim that one ought to have expected extreme chemical lability from hydrocarbon radicals is also flawed; after all, the radicals are homologous with hydrogen, which is stable and fairly unreactive except regarding oxidation. Finally, the evidence from boiling points could also be countered. Kopp's laws were known to have many exceptions, such as comparing H-OH with
CH3 -OH, and it could well be that the new radicals represented further exceptions. (This despite the fact that in 1847 Kolbe himself had used boiling points to argue for his interpretation of the constitutions of the fatty acids.) In Kolbe's view, the case for monomer versus dimer formulas was simply unproved, and in such instances one must prefer the simpler hypothesis.[47]
At the end of the general section of his textbook, Kolbe printed a truncated version of his critique of the Williamson-Gerhardt ether theory. He chose to omit the Wrightson argument and the methodological critique of synthesis as providing access to constitutions, although this was probably not in reaction to Williamson's reply, which he had presumably not yet seen. However, the week after his critique and Williamson's rebuttal were read in London, Guthrie's experiments in Marburg cast further light on the matter, and Kolbe just had time to incorporate the consequent changes into his manuscript. If, as Williamson had argued, potassium ethoxide has a constitution analogous to water, then in electrolysis the constituents occupying the places of the hydrogen—potassium and ethyl—should share the latter's electrochemical behavior and emerge at the negative electrode. As Guthrie found that only hydrogen appeared there, the theory appeared to be weakened.[48]
Kolbe, however, put the matter more strongly. To Vieweg he declared Guthrie's evidence a "fundamental proof of the untenability of [Williamson's] hypotheses," and in his text he stated that this "fundamental experiment . . . completely refutes" the French-English theory.[49] Words such as "proof" and "refutation" leave no space for doubt or debate. It is more than a little ironic to see such strong wording coming from Kolbe, who here and elsewhere openly conceded the anomalous character of many electrolyses and the unpredictability of the electrochemical properties of organic compounds and their components. Furthermore, Heinrich Will was soon to note that Kolbe failed to see that his own formulas were fully as susceptible to his own critique as Williamson's. But even without such internal contradictions, the investment of perfect certainty in a purely analogical argument suggests methodological problems.
The Battle Lost
There is considerable evidence here of defensiveness, along with often emotional and even shrill counterattacks. Despite Kolbe's brief sense of triumph with Guthrie's electrolyses, there continued to be good reason for his discomfort. Four months after the premiere of Kolbe's textbook and a month after the appearance in German of Wil-
liamson's and Gerhardt's rebuttals, Heinrich Will published a devastating critique of Kolbe's views. Will (1812-1890) had been a Liebig student in the late 1830s, subsequently becoming Privatdozent and first assistant in Giessen. When Liebig opened a branch laboratory for beginners in 1843, Will was placed in charge. As a consequence, he taught large numbers of students, many of whom achieved prominence in the next decade. Many of those who are counted (and counted themselves) as Liebig students actually were taught mostly by Will, including Williamson, Hofmann, Strecker, Kekulé, and Erlenmeyer.
Although Will had a strongly empirical approach, it is interesting to note that the topic of his disputation for Habilitation (1844) was the thesis that no compounds exist that have an odd number of equivalents of carbon—a cardinal thesis of Gerhardt's chemistry.[50] A fellow student and confidant of Kekulé's during the years 1849-1851 reported that Will's lectures opened many windows.
Later our discussions, both in private and in the larger circle of our friends, moved increasingly into the purely theoretical realm. At that time in our circle the perception was already stirring, partly still unconsciously, that the strict radical theory was not the sole all-redeeming dogma of chemistry. The seed of this perception, I believe, was to be found in Will's lectures on organic chemistry.[51]
Will succeeded Liebig as ordentlicher Professor when Liebig transferred to Munich in 1852. By 1853, according to his student Volhard, Will was giving a "clear, convincing, indeed enthusiastic portrayal" of Gerhardt's system in his lectures, to the point that Volhard and his comrades came to swear on type theory and honor Gerhardt as the reformer of organic chemistry.[52] The next year Will published his commentary "On the Theory of the Constitution of Organic Compounds" in the Annalen .[53]
Will argued that Gerhardt's atomic weight reform must be adopted simply because it was becoming ever clearer that formulas written in conventional equivalents always have even numbers of carbon and oxygen atoms. There was thus no empirical justification for retaining C2 in preference to C (i.e., C = 6 in preference to C = 12). Furthermore, the asymmetric synthesis argument as applied by Williamson and Gerhardt, and the new bases of Wurtz and Hofmann, had placed the molecular magnitude issue (the doubled sizes of ethers and acid anhydrides with respect to alcohols and acids) beyond any question.
Unlike Williamson, Will understood that Kolbe's depiction of ethyl methyl ether as a compound of ethyl oxide with methyl oxide was not intended to apply to ordinary ether. But if the asymmetric Williamson reaction links these two compounds together so firmly, then it must
also link ethyl oxide with itself in the symmetrical reaction, which is the equivalent of Williamson's ether formula. The latter makes much more chemical sense, Will argued, for a dimerized ethyl oxide has no analog in either organic or inorganic chemistry.
As for Kolbe's crucial electrolysis argument, Will suggested that it would have some force if directed against the existence of such oxygenated radicals as Williamson's othyl or ethoxide. But since Kolbe had now conceded the existence of oxygenated radicals, his and Williamson's formulas were equivalent. Translating Williamson's formula for acetic acid into Kolbe's notation, the two were
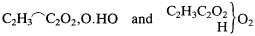
These formulas depict the same atoms combined in the same way. The only significant difference between them is that Kolbe suggested a chemical distinction between the two extra-radical oxygen atoms, a distinction, Will noted, that has no empirical basis. But even conceding this distinction, any electrochemical argument that applies to one of these formulas must perforce apply to the other. If Kolbe had disproved Williamson's formula, he had simultaneously disproved his own.
The issue here as elsewhere, Will concluded, was that inorganic chemistry could no longer remain the analogical guide to organic chemistry. The reform of organic chemistry, begun by Gerhardt, provided the entree into the chemistry of the future, and it could no longer be in doubt that his reform would win the day. Will, the model of the mid-career establishment German chemist and the newly appointed successor to Liebig as ordentlicher Professor at Giessen, was declaring himself for the young (French and English) Turks.
Much later, in a biography of Will, Hofmann wrote of this period of rapid gains for the new views: "With some, this transition happened silently, they slipped as it were right into the new theory; in fact there were some who the night before had been implacable opponents of Gerhardt and Laurent, and awoke the next morning completely converted." In his biography of Wurtz, Hofmann chose similar language to describe the period: "The time had arrived in which, one after another, the most ardent opponents—often overnight, and without providing any reasons whatever for their conversions—made their pronouncements."[54] Hofmann later affirmed that a "revolution" had taken place during the 1850s—and he was not the only one to apply this word.[55] The evidence presented here suggests that this transition came earlier, faster, and more completely in Germany than has hith-
erto been thought. Most active and theoretically oriented German chemists had converted several years before the Karlsruhe Congress of 1860, the event usually described as the turning point.
Wurtz's discovery in 1855 of the asymmetrical or "mixed" radicals was the final blow for Kolbe. Kolbe may have been able to satisfy himself that ethyl oxide and methyl oxide are sufficiently distinct electrochemically to provide a basis for combination, but it was difficult to imagine such a distinction between, say, the eight-carbon butyl and the ten-carbon amyl, especially considering the absence of any heteroatoms and the extreme stability of their combination. Butyl-amyl's exact fulfillment of Hofmann's boiling point prediction—based on Kopp's laws—was another problem for Kolbe. It was fortunate that the imminent publication of the fascicle of volume six of the Handwörterbuch der reinen und angewandten Chemie that contained the beginning of letter "R" gave Kolbe the impetus to write a summary article on "radicals," further modifying his views. He wrote the article in late December 1855, immediately after a summary of Wurtz' paper appeared in Liebig's Annalen .[56]
The piece contains a strong affirmation of the complete substitutability of the hydrogen of organic radicals by virtually any element, even electrochemical opposites, sometimes with minimal alteration of properties. How this undeniable truth can be reconciled with electrochemistry "remains for the moment still an open question," but by no means falsities that theory. He portrayed the issue of monomeric versus dimeric radicals likewise as open, and rehearsed all his previous rebuttals to Gerhardt, Laurent, Hofmann, and Brodie.
However, Wurtz' new research was "without a doubt . . . of much greater significance for this question" than the earlier boiling point argument. The vapor density and boiling point of the mixed radical butyl-amyl, for instance, fit right between butyl and amyl, and it just matched Hofmann's prediction, which strongly suggested that the symmetrical radicals were butyl-butyl and amyl-amyl. But after fairly summarizing this evidence, Kolbe still demurred: "It remains for the moment undecided and questionable whether these facts have sufficient probative force" to compel a doubling of the radical formulas. After proclaiming Wurtz' evidence highly important, he did not even attempt a reasoned refutation, but rather simply judged the issue still open.
Frankland later stated that through correspondence during the year 1856 he was able to persuade his friend Kolbe to retract the critique of the law of maximum combining capacities that had appeared in his textbook in June 1854.[57] Unfortunately, none of these letters have been found. It would seem, however, that at least the beginning of the correspondence on this subject may have been earlier than Frankland in-
dicated, for this article reveals that Kolbe's conversion dates from the end of 1855. Significantly, Kolbe now not only accepted Frankland's law for metals, nitrogen, phosphorus, antimony, and arsenic but expanded it to include carbon as well. If one takes as one's type carbonic acid, formulated as 2HO.C2 O4 , and assumes that it has a maximum combining capacity of four, then replacement of one oxygen atom by methyl results in acetic acid, HO.(C2 H3 )C2 ,O3 . (In Berzelian fashion, the half-molecules of water HO were considered external and not essential to the composition of the acid. One needed simply to omit an HO group in moving from a dibasic to a monobasic acid.) A second methyl in the place of oxygen would yield the neutral substance acetone, (C2 H3 )2 C2 C2 (omitting the second half-molecule of water).[58]
Kolbe's "carbonic acid theory," as it evolved into its final formulation by 1860, is described in detail in chapter 8. Here it suffices to note that it was essentially a "newer type theory" very similar to, and clearly influenced by, those of Williamson, Gerhardt, Hofmann, Frankland, and Wurtz. Kolbe later conceded that he had become a type theorist (but always insisted that his types were sharply distinct from those of Gerhardt and his followers). He also eventually conceded that he had been wrong when in 1854 he attacked Williamson's interpretation of his ether synthesis.[59]