B. Modern Chemical Definitions[1]
acetic acid = essence of vinegar: CH3 CO2 H
acetoacetic ester: CH3 COCH2 CO2 CH2 CH3 , produced by self-condensation of ethyl acetate, CH3 CO2 CH2 CH3
acetone: CH3 COCH3 , the simplest ketone
acetyl: CH3 CO- (the oxygen is double bonded to carbon, which has an untilled valence)
acetylene :
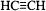
acid chloride: compound of the form RCOCl
alanine: CH3 CH(NH2 )CO2 H, aminopropionic acid
alcohol: loosely, a compound with a hydroxyl (-OH) group
aldehyde: any compound whose carbon chain ends in -CH=O
aldehyde (loosely) = acetaldehyde: CH3 CHO
aliphatic compound: non-benzenoid organic compound
[1] Organic chemists will note that some degree of generality and rigor has been sacrificed here for the sake of clarity. Terminology sometimes differed in the nineteenth century; such distinctions are explained in the text.
alkyl: saturated hydrocarbon radical, often symbolized generically as "R"
amide: compound of the form RCONH2 (this particular example is a primary amide)
amine: compound of the form NR3 , where the R's are hydrogen or hydrocarbon groups
amino acid: compound with both amine and carboxylic acid groups
amyl: a five-carbon saturated hydrocarbon radical
amylene: a five-carbon olefin
aniline: C6 H5 NH2 , aminobenzene
anthranilic acid:ortho -aminobenzoic acid
aromatic compound: organic compound derived from benzene
-ate: suffix indicating ester or salt of an acid
benzaldehyde: C6 H5 CHO, benzoyl hydride
benzene: C6 H6
benzoic acid: C6 H5 CO2 H, benzenecarboxylic acid
benzonitrile: C6 H5 CN
benzoyl: C6 H5 CO-, phenyl carbonyl
benzyl alcohol: C6 H5 CH2 OH
butane: a four-carbon saturated hydrocarbon
butyl: a four-carbon saturated hydrocarbon radical
butylene: a four-carbon olefin
butyric acid: a four-carbon carboxylic acid
caproic acid: a six-carbon carboxylic acid
carbohydrate: a compound exhibiting a multiple of the formula CH2 O
carbonyl: functional group consisting of a carbon atom double bonded to oxygen
carboxyl: -COOH, confers acid character on a molecule
carboxylic acid: loosely, an organic acid, or a compound with a carboxyl group
chloroform: CHCl3
condensation: amalgamation of two molecules into one, often with loss of H2 O
cresol: methyl phenol, MeC6 H4 OH
cyanogen: NC-CN
cyclohexamethine: any proposed structure for benzene exhibiting a cyclical array of six trivalent CH groups
cyclohexatriene: Kekulé's hexagonal benzene formula, possessing three carbon-carbon double bonds and three carbon-carbon single bonds; an example of a cyclohexamethine theory
dibasic acid: acid having two replaceable hydrogen atoms; a double acid
dimer: compound formed of two identical parts, a "doubled" molecule
dithionic acid = hyposulfuric acid: H2 S2 O6
ester: compound of the form RCO2 R, resulting from condensation of an organic acid and an alcohol
ethane: CH3 CH3
ether: compound of the form R-O-R, where the R's are hydrocarbon radicals
ether (loosely) = diethyl ether: CH3 CH2 OCH2 CH3 or EtOEt
ethyl: CH3 CH2 -, often symbolized Et
ethyl alcohol = (loosely) alcohol: CH3 CH2 OH or EtOH
ethylamine: EtNH2
ethylene: CH2 =CH2 , the simplest olefin or alkene
ethylidene: CH3 CH= (a radical, or incomplete molecule)
fatty acid: large organic acids found as components of fats
formic acid: HCO2 H
functional group: any site on an organic molecule that has a multiple bond or a heteroatom
glyceride: ester of fatty acid(s) plus glycerin; triglycerides are fats
glycerin: CH2 OHCHOHCH2 OH, a trialcohol
glycine = glycocoll: aminoacetic acid
glycol: compound with two hydroxyl groups, a dialcohol
glycol (loosely) = ethylene glycol: CH2 OHCH2 OH
glycolic acid: CH2 OHCO2 H
glyoxal: OHCCHO
halide: chloride, bromide, iodide, or fluoride
halogen: chlorine, bromine, iodine, or fluorine
heteroatom: in an organic molecule, any atom other than hydrogen or carbon
homolog: a member of a homologous series
homologous series: series of organic compounds that differ only by successive CH2 (methylene) groups, each methylene representing a link in the carbon chain
hydracid: compound whose acidity is unconnected with its oxygen content
hydride: compound of the form X-H, where X may be almost any atom or radical
hydrocarbon: a compound consisting only of hydrogen and carbon
hydrolysis: reaction with water, often splitting the reactant molecule
hydroxyl: -OH, confers alcohol character
iso-: prefix indicating either (1) a new isomer of a familiar compound hitherto thought to have no isomers; or (2) a branched-chain hydrocarbon
isoamyl: Me2 CHCH2 CH2 -
isobutyl: Me2 CHCH2 -
isobutyric acid: Me2 CHCO2 H
isomers: compounds with the identical formula but different structures, that is, compounds that have the same sorts and numbers of atoms but arranged differently
isopropyl alcohol: CH3 CH(OH)CH3
ketone: compound of the form RCOR, that is, carbonyl flanked by two alkyl radicals
lactic acid: CH3 CH(OH)CO2 H
leucic acid: a six-carbon hydroxy acid
leucine: a six-carbon amino acid
malic acid: a four-carbon hydroxy diacid; hydroxysuccinic acid
malonic acid: HO2 CCH2 CO2 H, a three-carbon diacid
methane: CH4 , called "marsh gas" in the nineteenth century
methine: CH, a trivalent radical
methyl: CH3 -, often symbolized Me
methylamine: MeNH2
methylene: CH2 , a divalent radical
methylsulfonyl chloride: CH3 SO2 C1
methylsulfonic acid: CH3 SO3 H
moiety: organic-chemical term indicating a portion of a molecule
nitrile = cyanide: compound with the group -CN
normal: possessing a straight (unbranched) carbon chain, symbolized n -
octane: an eight-carbon saturated hydrocarbon
olefin: a hydrocarbon with a carbon-carbon double bond; alkene
ortho/meta/para: referring to aromatic isomers distinguished by virtue of two substituents occupying different positions on the benzene hexagon-on adjacent carbons (1,2), separated by one carbon (1,3), or on opposing carbons (1,4), respectively
oxalic acid: HO2 CCO2 H
oxyacid: compound whose acidity is related to its oxygen content
paraffin: a hydrocarbon with no multiple bonds; alkane
pentane: a five-carbon saturated hydrocarbon
phenol = carbolic acid: phenyl hydroxide or hydroxybenzene, C6 H5 OH
phenyl: C6 H5 -
phosgene = carbonyl chloride: COCl2
polymer: compound formed of many identical parts
primary alcohol or amine: compound of the form RCH2 OH or RNH2
propane: CH3 CH2 CH3
propionic acid: CH3 CH2 CO2 H
propyl =n-propyl = normal propyl: CH3 CH2 CH2 -
propylene: CH3 CH=CH2
salicylic acid:ortho -hydroxybenzoic acid
saturated: organic compound possessing no carbon-carbon multiple bonds
secondary alcohol or amine: compound of the form R2 CHOH or R2 NH
succinic acid: a four-carbon saturated dicarboxylic acid
sulfonic acid: a compound of the form RSO3 H
tartaric acid: a four-carbon dihydroxy diacid; dihydroxysuccinic acid
tertiary alcohol or amine: a compound of the form R3 COH or R3 N
tertiary butyl alcohol =t-butyl alcohol: Me3 COH
toluene: methylbenzene
unsaturated: possessing carbon-carbon multiple bonds (can absorb hydrogen gas)
valence: number of bonds a given kind of atom can form to other atoms
valeric acid: a five-carbon carboxylic acid