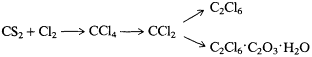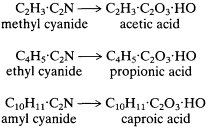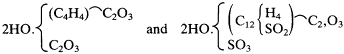3—
A Journeyman Chemist
The Copula Theory
Kolbe must have been an avid observer of the disputes raging in organic chemistry during his student years in Göttingen. Laurent's nucleus theory, Dumas's type theory, Liebig's hydracid theory, Berzelius's defenses of dualism, the angry self-justificatory letters by the last three of these chemists published almost biweekly for a time in the Comptes rendus , and the Schwindler parody, not to mention the lively private correspondence that Wöhler conducted with his feuding intimates Berzelius and Liebig, all occurred while Kolbe was studying with Wöhler.
This was also the time when Berzelius was fashioning the theory of copulated or conjugated compounds, his chief line of defense in response to the arguments of the French substitutionists.[1] Since 1837 Berzelius had been noting the discovery of a number of organic derivatives of inorganic acids and bases (especially alkyl sulfates and sulfonic acids, and ammines) that retained typical properties of the original acid or base, especially an unchanged saturation capacity. In 1839 Gerhardt coined the term copulé to refer to such combinations, the organic adduct being the copule . Berzelius then expanded this concept to explain the near identity in properties of acetic and chloroacetic acid. In the latter substance, the "sesquichloride of carbon," or C2 Cl6 —the copula—was combined with "oxalic acid," or C2 O3 ·H2 O, without affecting the fundamental (acidic) properties of the latter moiety. When Dumas' assistant Louis Melsens found a method to reduce
the chlorinated derivative back to the starting material, Berzelius responded that acetic acid itself must be a "copulated" compound:
![]()
Berzelius then developed a general theory of copulated or conjugated compounds. He supposed conjugation to be a nonelectrochemical form of chemical combination, which was why the saturation capacity of the acid remained unaltered. The hydrogen of the organic copula could be substituted by chlorine or other atoms, Berzelius conceded, but since this substitution occurred only in the passive, chemically unimportant copula, it was not surprising that the fundamental chemical properties of the compound did not change. For every substance substituted by chlorine, Berzelius now hypothesized a copula structure, always placing the chlorine in the copula. The theory provided a way for Berzelius to concede the increasingly unavoidable fact of chlorine substitution while maintaining the basis of dualistic organic chemistry. But what seemed to Berzelius to be a flexible modification showing the strength of dualism, seemed instead in the eyes of his opponents to be an unacceptably ad hoc retreat for a dying theory. In his posthumous chemical manifesto, the embittered and dying Laurent blasted the theory:
A word let fall from the pen of Gerhardt, was thus transformed into a luminous idea for dualism. From this time everything was copulated. Acetic, formic, butyric, margaric, &c., acids,—alcohols, ethers, amides, anilides, all became copulated bodies. So that to make acetanilide, for example, they no longer employed acetic acid and aniline, but they re-copulated a copulated oxalic acid with a copulated ammonia. I am inventing nothing—altering nothing. Is it my fault if, while writing history, I appear to be composing a romance? What then is a copula? A copula is an imaginary body, the presence of which disguises all the chemical properties of the compounds with which it is united. . . . The dishonesty is flagrant.[2]
But the copula theory proved to have a scientifically fruitful life. Just before Kolbe's arrival in Marburg, Bunsen provided a compelling argument in favor of Berzelius' theory by isolating the cacodyl radical and a large number of its derivatives, all of which also fit into the emerging copula theory. The acidity of oxygenated arsenic ("arsenious acid," formulated as AsO) did not seem at all affected by the addition of methyl radicals (producing "cacodyl oxide," formulated as
C2 H6 AsO). Cacodyl oxide could be reduced to cacodyl, the first unquestionably organic radical ever isolated and the first organometallic compound ever synthesized. Berzelius was thrilled at this new support for his ideas. "This is a triumphal chariot, which will overrun and smash [Dumas'] flimsy theoretical barricades," he exulted to Wöhler.[3]
The foundations of the copula theory would have been severely weakened, however, had Gerhardt's and Laurent's arguments been accepted, namely, that all compounds must be related to the same number of gaseous volumes (two), that hydrated acids are not oxides combined with water but rather are radicals with replaceable hydrogen, and that certain acids are polybasic. For instance, in response to Berzelius' suggestion that acetic acid consists of oxalic acid, C2 O3 ·H2 O, paired with the conjugate radical methyl, C2 H6 , Gerhardt and Laurent argued that the true formulas for the two components are half of those indicated, that there is no oxalic in acetic acid, that the true oxalic acid is dibasic rather than monobasic, and that it cannot be separated into an oxide and a molecule of water. They had similar problems with cacodyl oxide and other copula formulas.
Despite these objections, in its initial historical context Berzelius' copula theory was a successful theory that was both empirically supported and heuristically fruitful. Indeed, at the time of its formal introduction in 1839-1841, Gerhardt had not yet even fully formulated the arguments just rehearsed. Moreover, one could imagine a variety of potential compromise positions between the Berzelians and the French reformers if the two sides had been willing to look at the matter from their opponent's point of view. Despite the tendency of modern chemists to view Gerhardt's and Laurent's theories as correct, it might be said that there was as much truth to the copula formulas as there was to the French nucleus and type formulas.
Berzelius was excited by the subject of Kolbe's first research, for this was the same sort of work that had just yielded the wonderful fruit of cacodyl derivatives. This point was not lost on Kolbe, who from autumn 1842 had become a student of the chemist whose cacodyl series was still appearing in Liebig's Annalen . Kolbe's first discovery in Marburg was that moist rather than dry chlorine reacting with carbon disulfide resulted in an oxygenated and rearranged product, namely, one predicted earlier by Berzelius now known as trichloromethylsulfonyl chloride. Alkaline hydrolysis produced the corresponding sulfonic acid:
![]()
This last substance Kolbe named Chlorkohlenunterschwefelsäure , or dithionic acid (i.e., hyposulfuric acid anhydride) copulated with Kohlensuperchlorür (i.e., carbon "protochloride," or fully chlorinated ethane).[4]
It will be useful for what follows to understand this compound and its formulation from both a modern and a Berzelian-Kolbean perspective. The first point to note is that dualists always worked schematically with the anhydrides of acids, whether or not they had ever been isolated. For dithionic acid (Unterschwefelsäure ), the schematic transformation from modern (hydrous) to Berzelian anhydride composition is H2 S2 O6 - H2 O = S2 O5 . Second, vapor densities were not regarded as a consistent indication of molecular size, so that formulas could be halved or doubled as required by theory. Most organic formulas were doubled with respect to inorganic formulas—that is, four-volume organic formulas were usually standard. Thus, whenever dithionic acid, including its associated water molecule (S2 O5 +H2 O), occurs in a dualistic organic formula, it is equivalent to the modern sulfonic acid moiety: -SO3 H. Finally, the copula—carbon protochloride—could be considered either a two-volume representation of the fully chlorinated ethane discovered by Faraday (= modern C2 Cl6 ) or a four-volume representation of the fully chlorinated methyl radical (= modern -CCl3 ). The first was initially preferred by Berzelius since the copula could then be represented as a known isolated compound. After methyl was also discovered as a (seemingly) stable isolated substance, however, Kolbe preferred the second interpretation. Hence, what is seen by modern chemistry as the combination of two monovalent moieties, sulfonic acid and methyl, was viewed by copula theorists as the nonelectrochemical "coupling" of two stable, theoretically isolable molecules, dithionic acid and carbon protochloride.
In this same brief notice of 1844, Kolbe revealed that the carbon superchloride (CCl4 ) formed by chlorination of carbon disulfide could be partially transformed by passage through a hot reduction tube into another of Faraday's chlorocarbons, CCl2 , today known as tetrachloroethylene. The latter compound could be further chlorinated, or chlorinated and oxidized to chloroacetic acid, depending on conditions and how many molecules were thought to take part in the reactions. In Kolbe's terms (using unbarred atoms), these reactions are
In 1845 Kolbe published his first extended paper, summarizing his work with these compounds over the previous three years, revealing a number of new derivatives, and interpreting all of them as Berzelian copulas.[5] He showed how one could reduce the trichloro sulfonic acid derivative in a stepwise fashion to dichloromethyl-, monochloromethyl-, and fully reduced methylsulfonic acid using either chemically or electrically produced nascent hydrogen. This represented his first application of Bunsen's powerful new battery, and he sagely predicted that it would doubtless prove an important instrument for the future study of chemical constitutions. The paper was fairly bursting with new compounds, new reactions, and new methods—a bravura performance.
But the paper is at least as notable for its theoretical content as for its experimental novelties. Kolbe argued that his new derivatives of dithionic acid formed a perfect analogy to the chlorinated acetic acids of the French chemists, but that the analogy only made sense when formulated as copula compounds and not as "types." The sulfur acids could be chlorinated without significantly affecting their properties only because the methyl that actually undergoes substitution is the passive copula, in precisely the same way that the hydrocarbon copula of acetic acid can be chlorinated without much affecting the "oxalic acid" to which it was coupled. In an ironic twist of language, he argued that the copulated dithionic acids could stand as "prototypes" for all copulas.[6] Furthermore, by starting with a chlorocarbon and ending with the synthesis of chloroacetic acid, Kolbe thought he had proven the preexistence of the former in the latter, confirming Berzelius' prediction.
This new research, Kolbe averred, thus transformed copulas from "the realm of mere hypothesis to a high degree of probability." Of course, the work also further broadened the field of chlorine substitution and could, like A. W. Hofmann's 1843 papers on chlorinated anilines, be viewed as supporting the "newer substitution theory" of the French chemists. This would be a hasty conclusion, he cautioned. Indeed, one could hardly imagine two more different chemical "types" than chloroform and carbon disulfide, yet Regnault and Kolbe had independently shown that each reacts with chlorine to yield an identical product, carbon superchloride. Contrary to Dumas, chemical types were not conserved. Hofmann's research only showed that aniline, too, was a copulated compound.[7]
Kolbe also followed up on Berzelius' comment to Wöhler about Kolbe's compounds occupying the border region between organic and inorganic compounds. As we have seen, one of the new reactions
Kolbe discovered was the transformation of what is now called tetra-chloroethylene into chloroacetic (i.e., trichloroacetic) acid. Because he had prepared the former substance (like all of his chlorinated carbon compounds) from carbon disulfide and ultimately from inorganic carbon and sulfur, and because the product of the reaction was reducible by electrolytic hydrogen to acetic acid, here was a "nearly direct" synthesis of vinegar from the inorganic elements. These and the other reactions Kolbe had discovered showed a "continuous chain" from inorganic to organic compounds, so much so that any distinct boundary between them had disappeared. Kolbe waxed eloquent: as one can now synthesize vinegar, it may prove possible in the future to reduce acetic acid to alcohol, then to synthesize sugars and starches, in which case an immense new field for our endeavors will open up.[8]
This was the first significant organic synthesis since Wöhler's classic urea paper of 1828,[9] and the first total synthesis in history. The previous paragraph shows that Kolbe was well aware of its importance. It is also said that Kolbe was the first here to use the word Synthese in its modern chemical sense. In many respects, his accomplishment was even more dramatic than Wöhler's, since Wöhler had begun with starting materials derived from living creatures, whereas Kolbe had started with carbon and sulfur, whose ties with life were distant and indirect at best. To be sure, Kolbe's acetic acid synthesis did not destroy vitalism any more than his mentor's urea had, but it was an important benchmark on the route to modern synthetic organic methods.
This, Kolbe's first major paper, established patterns he would follow for the rest of his life. Above all, the article reveals his intense interest in chemical theory and, in particular, in theories concerning the inner constitutions of the molecules of organic compounds (especially organic acids). Kolbe's starting point was Berzelian radical theories. Under attack by the French substitutionists, Berzelius had devised a modification of his theory, copulas, that could countenance the distasteful new phenomenon. However, unlike Berzelius, Kolbe embraced chlorine substitution with enthusiasm and never exhibited any discomfort with the notion of electropositive radicals containing electronegative elements. Like Liebig's, his dualism was moderated by the undeniable fact of chlorine substitution. But in all essential respects, he was from first to last a committed, indeed zealous, Berzelian. For Kolbe, radicals were not mere schematic or conventional entities, but real, preexisting parts of molecules. The distinguishing characteristic of his entire career was the discernment and (wherever possible) physical isolation of these radicals.
As might be expected, Berzelius himself was delighted and highly impressed by Kolbe's work; Kolbe treasured the Swede's letter of con-
gratulations of 3 August 1844 to the end of his life. Berzelius described Kolbe's experiments and interpretations in glowing terms in his Jahresberichte and praised the significance of the work in letters to both Wöhler and Bunsen. He averted publicly that Kolbe had provided "as complete a proof as is possible in chemistry" for the existence of copulas.[10] The following year (1845) he met Kolbe, for the first and only time, during his last visit to Germany.
Berzelius even used Kolbe's disproof of Dumas' types as the final decisive argument in a paper entitled "Views Regarding Organic Composition," which he submitted in February 1846 to the Swedish Academy of Sciences and subsequently published in Poggendorff's Annalen der Physik —the last important article of his life. Berzelius thought that Kolbe's work provided "positive proof" for the existence of copulas; it "completes the refutation of the substitution theories and the imagination-game of chemical types." He regarded as quite novel and striking the stepwise replacement of chlorine by hydrogen and vice versa, with no significant change in the properties of the compound. In short, it was the best work he had seen since Bunsen's cacodyl series.[11]
One can imagine the effect such praise from the venerated master must have had on the young Kolbe. Indeed, much of the substance and rhetoric of Berzelius' last article can be discerned in often little-altered form in many of Kolbe's own later diatribes. Berzelius here repeated (no fewer than three times, and once in italics) that the only sure guide to theories of organic compounds is the dualistic theory of inorganic chemistry. He also rebuked the French chemists, especially Dumas, Laurent, and Gerhardt, for letting their imaginations dictate "Phantasiebilder" that have little or no empirical support or connection to the existing theoretical structure of the science. Determining constitutions of organic compounds is both the most important and the most difficult of all goals in chemistry, he averred; all the more vital that it be done with caution, care, and circumspection.
Assistant to Playfair
In 1845 an opportunity arose for a temporary foreign position for Kolbe. The British government had just established a Museum of Economic Geology near St. James' Park in London and had hired Lyon Playfair, a former student of Liebig, as the museum's organic chemist. One important assignment given Playfair was the analysis of mixtures of naturally occurring hydrocarbons, required in connection with a Parliamentary Commission on Explosions in Coal Mines. The acknowledged master of such analytical methods was Bunsen, to whom Play-fair turned for advice. Bunsen thought to send Kolbe as assistant to
Playfair and persuaded Kolbe to accept the position. This was to be Kolbe's only extended trip of his life outside the European continent.
Kolbe entered into his duties in London in October 1845, the same month that another assistant, Edward Frankland, arrived at the museum and the same month that August Wilhelm Hofmann arrived from Bonn as Professor of Chemistry at the new Royal College of Chemistry. Hofmann later related that he met Kolbe soon after their arrival at a meeting of the Chemical Society. He had been given a large official residence rent-free, and he invited Kolbe to live with him there. They became intimate friends.[12]
Frankland was seven years younger than Kolbe and not very knowledgeable in chemistry, especially regarding the latest experimental methods then current in Germany, having come directly from an unhappy pharmaceutical apprenticeship. When Hofmann married in August 1846, Kolbe established lodgings on Belvedere Road, near Frankland's residence on Doris Street. Frankland later recollected
At this time Kolbe could speak only a few words of English, but we arranged to give each other lessons in German & English and we met, for this purpose on two evenings in the week at his lodgings. . . . He made rapid progress and was soon able to speak with facility. It was not long after this intercourse became established between us, before he began to explain to me his great interest in organic chemistry.[13]
Frankland reported that Kolbe had a "supreme contempt" for inorganic analysis, of the type that Frankland had been hired to perform, as it was of "little or no theoretical interest."[14] He was soon infected by his friend's enthusiasm for experimental and theoretical organic chemistry. Kolbe instructed him both in Bunsen's "then but little known but beautiful & delicate processes of gas-analysis" and in Berzelian theory.[15] In addition to Hofmann and Frankland, Kolbe became acquainted with most of the London chemical community, and it is said he made a particularly strong impression on Thomas Graham and Michael Faraday.[16]
Frankland described the "profound impression upon all of us" made from the first by Kolbe's exemplary care and skill in laboratory operations. "He never grudged any amount of trouble in fitting up apparatus or performing an operation, if a greater amount of accuracy could thereby be secured." After Kolbe sent a sample apparatus for explosion eudiometry, Wöhler replied with thanks, in awe of Kolbe's glass-blowing skill. If all else failed, his former teacher opined, Kolbe could easily make a living as a skilled artisan.[17]
Kolbe's official duties were to analyze mixtures of gases gathered
from coal mines in the hope of providing for better means of preventing explosions. He reported on this work both to Bunsen and officially to Playfair for the British government.[18] Frankland related that there was insufficient room at the museum's laboratory for these analyses, so they were all performed in Kolbe's lodgings. Playfair, as it happened, was not often present in London due to other governmental duties such as service on a commission on the potato blight and a lectureship at the military college at Addiscombe.[19] Perhaps due to this circumstance, Kolbe had considerable free time on his hands, and by December 1845 we find him using Playfair's lab for his own research purposes.[20] Toward the end of his stay in England, in the spring of 1847, he published two important papers, the second of which was a joint project with Frankland.
Kolbe knew that he of all European chemists was in a unique position to exploit a certain new field of research. He had mastered improved and as yet unpublished gas-analytical methods, as well as the mode of construction of a novel and very powerful carbon-zinc battery, both of which he had learned from Bunsen, and unlike his master, he was entranced by the theoretical goal of investigating the constitutions of organic molecules. With these tools, Kolbe set out to accomplish what decades earlier had repeatedly frustrated Berzelius himself: the electrolysis of organic acids. One of his laboratory notebooks preserved in the Deutsches Museum in Munich records experiments in this direction from 1 October 1846 to February 1847.[21] With solutions of potassium acetate, butyrate, and valerate, Kolbe made the electrolysis work, but he obtained a daunting number of solid, liquid, and gaseous products. After electrolyzing potassium valerate, for instance, he isolated, in addition to potash, hydrogen, and carbonic acid, a new saturated hydrocarbon possessing the formula C8 H9 , a new olefin C8 H8 , and an ethereal oil apparently of the formula C8 H9 O+C8 H9 C2 O3 . In his first paper on this subject Kolbe conceded that these were still preliminary results, but he stressed that they supported the copula formula for valeric acid, which would be C8 H9 ·C2 O3 ·HO.[22] Although he was not able to bring this work to a fully satisfactory conclusion while in England, this was the origin of the still useful "Kolbe electrolysis" reaction.
Frankland joined Kolbe in an attack from a different direction on the question of the constitution of organic acids. It was known that upon hydrolysis, cyanogen and benzonitrile yield oxalic acid and benzoic acid, respectively. The latter reaction, developed in 1844 by Fehling, suggested the correctness of the copula formulas for benzonitrile and benzoic acid:
![]()
Kolbe and Frankland determined to generalize this reaction for the simple aliphatic acids and succeeded admirably:
In the view of the authors, this work supported the existence of copulas and, in Frankland's later assessment, established "for the first time the internal molecular structure of these acids . . . ."[23]
But in the published paper, Kolbe and Frankland expressed their views with diffidence. Well cognizant of their youth (Frankland was only twenty-one) and lack of established positions, they wisely chose to soft-pedal their novelties. Investigations of the molecular constitutions of compounds, they wrote,
. . . are always attended with more or less danger, and those who, leaving the safer road of experiment, plunge into the depths of hypothesis, and build up theories apparently ingenious, though often untenable, frequently stumble and fall amongst a host of contradictions. It is a common error, as experience teaches, into which young chemists are very apt to fall, that, persuaded of the infallibility of their own views, and blind to well-founded objections, they endeavor to convince by quick and ready argument rather than by solid reasoning, and consequently they either offend others or feel themselves offended when contradicted.
Hence, they felt a "certain degree of timidity" in presenting these views, against those "generally received." They professed no intention of giving a "decided preference" to the ideas here defended or of forcing their opinion on others. They did, however, aver that the views were worthy of consideration.[24] One can imagine Kolbe writing these lines, worrying about being put in the. same category as the French chemists and bearing Berzelius' published cautionary remarks of the previous year in mind.
In fact, Kolbe and Frankland were aware that one could explain these reactions without recourse to copulas, or even to any constitutional hypothesis, by simply using empirical formulas, as Liebig had
begun to do since 1840. However, they argued that their explanation, using what we would now refer to as more resolved structural formulas, had manifold advantages. It was simpler and more consistent than empirical formulas, and it revealed direct analogies to other reactions of nitriles and cyanides otherwise hidden. Furthermore, one could then propose a single homologous series of hydrocarbon radicals as the constitutional basis not just for nitriles and acids but also for alochols and hydrides, not to mention Kolbe's own alkyl hyposulfuric acids. They even speculated on the reaction mechanism of the oxidation of ethyl alcohol to acetic acid. This was, therefore, a bold publication for two professionally unestablished academic chemists. No one else at that time was publishing this sort of experimentally grounded theoretical work in organic chemistry.
It would be hard to overemphasize the significance of the Kolbe and the Kolbe-Frankland papers. Read to the Chemical Society on the same day (19 April 1847), they represent inverse synthetic methods: a carbon atom-increasing carboxylation reaction (through the corresponding nitrile) and, apparently at least, a carbon atom-decreasing decarboxylation reaction. These reactions were the first general synthetic routes between hydrocarbons and organic acids and represent the two first great general synthetic methods ever published. Together with Kolbe's acetic acid synthesis, they were the earliest planned reactions where the carbon content of an organic molecule was deliberately altered. They were also the first synthetic reactions whose purpose was to elucidate "constitutions," or what we now refer to as chemical structures. The few pre-1847 reactions that might be considered "synthetic" were fortuitous transformations whose constitutional import was only dimly or not at all appreciated.
Back to Marburg
In the second week of May 1847, Kolbe returned to his assistantship in Marburg in the company of his new English friend. Frankland had had two job offers, one of which he declined and the other he postponed in order to study with Bunsen during that prospective summer semester. He later said that this decision "had much to do with shaping all my future life," and he always had felt grateful to Kolbe for persuading him to make it.[25] In his memoirs, Frankland described the trip, his first abroad, in captivating detail—including such important incidents as Kolbe's disgust with Frankland's negative reaction to his first taste of real German Rhine wine. Having arrived in Marburg, they rented separate rooms in the Hotel Europäischer Hof, on Elisabethstrasse across from the Chemical Institute (still today Marburg's largest
hotel). The next day Kolbe introduced his friend to Bunsen, and they both began their work.[26]
In his memoirs, Frankland described the many social functions to which he was invited that summer. At the first of these he met his future wife, Sophie Fick, the sister of professor of anatomy Adolf Fick. It is amusing to read Frankland's detailed judgments of the pulchritude (or lack thereof) of a number of Marburger Mädchen , each carefully identified by name. Another time he accompanied Kolbe home to Lutterhausen for the wedding of one of Kolbe's sisters. This, his first introduction to German domestic life, was very interesting and agreeable to him; he spent time in the garden of the house with Kolbe's many younger siblings, the youngest of whom helped Frank-land with his German.[27]
But Kolbe and Frankland must have worked efficiently that summer, for in three months they published a second paper on the nitrile hydrolysis reaction and completed another project as well. They expanded the series of acids synthesized from nitriles to a total of eight (formic, acetic, propionic, butyric, valeric, caproic, benzoic, and cuminic) and made a stronger argument for the correctness of the copula formulations than they had made in London. A further support would be the reverse process, the reduction of an acid to the corresponding nitrile. They noted that Hofmann had long been attempting this reaction and had informed them by letter that he had recently succeeded—as had Dumas. They also applied Kopp's boiling point regularities to support their formulas.[28]
Kolbe's and Frankland's second joint project in the summer of 1847, actually begun already in London, involved treating ethyl cyanide with potassium in an effort to free the ethyl radical when the potassium united with the cyanide. To the delight of Kolbe and Frankland, the reaction seemed to proceed well, but to their disappointment, the product as analyzed seemed to be methyl rather than ethyl:
![]()
This makes an anomalous chemical equation, and they were unable to sort satisfactorily through what turned out to be a rather messy reaction. As Frankland later determined, the product was actually ethane (dimeric methyl); the extra hydrogen must have come from water or alcohol contamination. In the 1847 context, however, the isolation of "methyl" was a very notable result, even if the details of the reaction were still cloudy.[29] Their paper was written by Kolbe and was presented in both their names in Frankland's English translation to the
Chemical Society on 7 February 1848; it was, Frankland noted, "warmly applauded" there.[30]
In early August 1847, before the end of the summer semester, Frankland was prevailed upon by his new employer at Queenwood College (Hampshire) to return to England. He spent fourteen months in this position, another fifteen months back in Germany (acquiring a Marburg Ph.D. with Bunsen followed by a semester with Liebig in Giessen), and fourteen more months at Putney College. Frankland then received a call in March 1851 to Owens College, the predecessor of the University of Manchester. During this peripatetic period, Frank-land single-mindedly and quite successfully stalked the hydrocarbon radicals. Frankland later reminisced: "The isolating of the alcohol radicals was, at this time, the dream of many chemists, whilst others doubted or even denied their existence. I was also smitten with the fever and determined to try my hand at the solution of the problem."[31] Frankland apparently allowed himself some dramatic license for this statement, for in his first paper of the series, described below, he wrote (I believe accurately) that after cacodyl, no chemist seemed to be even trying to isolate the hydrocarbon radicals except Kolbe and himself.[32] No doubt this was because almost no one besides Frankland and Kolbe believed any longer in the older conception of radicals as real, preexistent parts of molecules.
Bunsen had prepared cacodyl from the reaction of cacodyl chloride with zinc. In the spring of 1848 Frankland tried heating potassium with ethyl iodide (rather than ethyl cyanide as in his first attempt), hoping to free the ethyl. He only succeeded in isolating "ethylic hydride," C4 H5 ·H, which appeared isomeric with his and Kolbe's "methyl." He decided he might have better luck if he tried a less active metal, and so he selected Bunsen's reagent, zinc. The experiment, performed early the following year in Marburg, was successful, and it led to the publication of a milestone paper entitled "On the Isolation of the Organic Radicals." Frankland had not only isolated ethyl, but he had also synthesized a new organometallic substance, zinc ethyl, which he formulated C4 H5 Zn. The reaction of methyl and amyl iodide with zinc produced analogous substances, including the free (apparently stable and isolated) radicals methyl and amyl. The isolation of such radicals "excludes every doubt of their actual existence, and furnishes a complete and satisfactory proof of the correctness of the theory [of the ethyl radical in alcohol and ether] propounded by Kane, Berzelius and Liebig fifteen years ago."[33]
Frankland's proof, of course, had a flaw: his (and Kolbe's) methyl, ethyl, and so on could not be distinguished from their dimers ethane,
butane, and so on (as we now regard them) without applying vapor density data to achieve consistent molecular magnitudes. Nonetheless, the evidence for isolated radicals appeared to be compelling. While Frankland was working in his laboratory in December 1849, Liebig reported to Hofmann: ". . . Frankland has isolated methyl, ethyl, and this week amyl. So what was desired as the foundation for the [radical] theory is now from this side finally here."[34] One of Liebig's reasons for abandoning chemical theory in 1840 had been the uncertain and shifting status of organic radicals, but after 1849, Liebig never again doubted their reality. This return to an earlier article of faith did not, however, induce him to return to the strife of theoretical chemistry.
Frankland and Kolbe had now isolated what they considered to be four different radicals by three distinct methods, all in less than three years. Unfortunately, Berzelius, who would have been overjoyed by these events, had died in August 1848.
Vieweg Verlag and Braunschweig
Within days of Frankland's departure from Marburg in August 1847 to take up his post at Queenwood College, an opportunity arose for Kolbe as a professional writer and editor at Vieweg Verlag in Braun-schweig. Friedrich Vieweg (1761-1835) had founded this publishing house in Berlin in 1786. Distressed by Prussian censorship, in 1799 he accepted the invitation of Duke Karl Wilhelm Ferdinand to move his firm to the capital of the Duchy of Braunschweig; he built an imposing neoclassical edifice on the Burgplatz to house his firm. From its founding, the house prospered from the publication of theological and literary works. Friedrich's eldest son Eduard Vieweg (1796-1869), who became a partner in the firm in 1825 and full owner upon his father's death, changed the orientation of the press toward scientific and technological subjects, chemistry in particular. Before his entry into the firm, Eduard spent three years traveling in France and Great Britain. In Paris he met the young Justus Liebig, who was then studying with Gay-Lussac, and he formed a very close lifetime friendship with him; most of Liebig's books were published by Vieweg.[35]
In 1832, Liebig and J. C. Poggendorff (soon joined by Wöhler) hatched a plan to publish a chemical dictionary-style handbook, the Handwörterbuch der reinen und angewandten Chemie , and persuaded Eduard Vieweg to publish it. This was to be a detailed summary of the state of the art in chemical science, eventually issued in twelve large volumes and much imitated in other countries. Subsequent editions of this monumental project continued to appear until 1930. In the fall of 1832, Liebig set to work with great energy on some of the 400 planned
"A" articles, many of which were highly important ones: Äther, Äthyl, Aldehyd, Alkohol, Analyse , and so on. The editors hoped to have the first volume on the market by 1834. But Liebig had more projects planned than time, and Poggendorff was dilatory; the first fascicle did not appear until late 1836 and the first complete volume not until 1842. By 1847 only two volumes had come out, covering the alphabet from A to E, and Liebig was quickly losing interest in the completion of the project. In a letter to Vieweg on 28 April 1847, he suggested that younger talents be recruited and named nine possibilities, one of whom was Kolbe. A memo by Vieweg on this letter indicates that it was his intention to ask Buff, Will, Knapp, Varrentrapp, Erdmann, and Marschner to contribute.[36]
It was at Wöhler's urging that Kolbe was asked to become the chief editor of the project. Kolbe had just completed a translation for Vieweg of Mulder's textbook of physiological chemistry, so Vieweg had some indication of his writing skills. On 6 August 1847, Vieweg wrote Kolbe and invited him to come to Braunschweig for this assignment. Kolbe accepted immediately, indicating his intention to arrive in late September.[37]
Kolbe's Braunschweig years were happy and productive ones. He formed an extremely close relationship with Eduard Vieweg, though Vieweg was his elder by twenty-one years—a relationship that remained untroubled to Vieweg's death in 1869. He also became very friendly with Vieweg's son Heinrich (1826-1890) and with Franz Varrentrapp (1815-1877), a pharmacist who had received his doctorate with Liebig, then settled in Braunschweig. Varrentrapp taught at the Anatomisch-Chirurgisches Lehranstalt and performed research for the Braunschweig Gewerbeverein, a state-supported research institution for the promotion of trades and industries. He also wrote for Vieweg on the side. When he received a call to the University of Aachen in 1868, Vieweg persuaded him to stay in Braunschweig by making him a partner in the firm. The next year Eduard Vieweg died, and the firm was directed for the following eight years by Heinrich Vieweg and Varrentrapp.[38]
While on holiday from the University of Marburg in early June 1849, Frankland visited Kolbe for three days and spent "a very pleasant evening with Kolbe at Varrentrapp's home." Frankland reported that through the Varrentrapp family Kolbe met a woman named Franziska yon Spilker and became engaged. Frankland met her on this trip and commented that "fortunately for Kolbe this engagement was soon afterwards broken off," though by which party we are not told.[39]
The four years Kolbe spent in Braunschweig were a time of great
unrest throughout the continent of Europe, and Kolbe was not unmoved or unaffected by political forces. The duchy of Braunschweig had close historical, political, and family ties to the electorate of Hanover, and in the eighteenth century, Braunschweig like its neighbor had enjoyed a progressive regime under the duke that brought Friedrich Vieweg from Berlin. The medical school and trade society for which Varrentrapp worked were two examples of typically Enlightenment institutions founded in Braunschweig. A third was the Collegium Carolinum—eventually renamed the Technische Hochschule—founded in Braunschweig in 1745 on English and Dutch models and intended to educate the middle class for practical services to state and society.[40]
But the religious tolerance and civil freedoms introduced in the eighteenth century were disturbed as a result of the French wars. In 1830 the mad, reactionary, and heartily despised Duke Karl was driven from the land, and the new ruler, Karl's younger brother Wilhelm (d. 1884), granted the state its first constitution. He reinstituted the previous reforms, along with farsighted economic provisions such as entry into the Prussian customs union and introduction of one of the earliest state railroad lines in Germany. Until the founding of the German empire, Braunschweig remained a model, very much in the minority, of a progressive small German polity.
Eduard Vieweg participated fully in the political events of his day, founding at various times three influential newspapers. The longest lived was the Deutsche Reichs-Zeitung , begun shortly after the March 1848 revolution, which agitated for the joining of the Germanic lands into a unified empire. Vieweg was by no means always well inclined toward or in agreement with the Prussian regime, but he saw in Prussia the nucleus from which a united Germany could and must grow; Austria must in all events be excluded. Whether it was from daily contact with Vieweg or from his own earlier convictions, Kolbe's views were substantially the same. Kolbe joined a Vaterländisches Verein (Patriots' Society) in Braunschweig, though this likely was different from the similarly named clubs of marked republican and even radical orientation that were proliferating in the Germanic lands during the 1840s. Frankland accompanied Kolbe to a meeting of this society (1 June 1849) and reported that the members were "all unanimous in accepting the Prussian Constitution."[41]
It would appear that Vieweg's and Kolbe's political sentiments were similar to those of the so-called Casino faction of the Frankfurt Parliament, usually described as center right liberals. This group, consisting mostly of professors and industrialists, were Kleindeutsche who sought
a unified Germany under Prussian and not Austrian leadership, who wanted constitutional guarantees but also protection against the centrifugal forces represented by republicans, democrats, socialists, and anarchists. In August 1848, at the peak of the power and optimism (such as it was) of the Frankfurt Parliament, Kolbe wrote to Frankland in the only English language words to survive from his pen: "As to politics, I pay a great confidence to our present government in Frankfurt. I hope, there will be in future but one Germany and only one king, as you have but one queen."[42]
Ost reported that it was Kolbe who safely and in secret conducted the Hessian classical historian and journalist Adam Pfaff (1820-1886) from Braunschweig to Hamburg. Pfaff had made himself persona non grata in Hesse-Kassel by his activity as editor of the liberal Neue Hessische Zeitung . When the paper was suppressed during the reaction in 1850, he had fled to Braunschweig, where he was hired by Vieweg to be editor of Vieweg's Deutsche Reichs-Zeitung . His escape to Hamburg, and subsequently to Brussels, was necessitated by continued pursuit by the Hessian regime.[43] This adventure underlines Kolbe's (moderate) liberalism during this period and the influence of his friend and employer Eduard Vieweg.
Chemical Editor
At Vieweg Verlag, Kolbe set to work energetically at his editorial tasks. One of his earliest decisions was notational. There was at that time disagreement on certain important points, and there had been several recent international shifts in formula conventions. Above all, should one take H = 1, H = 2, or H = 2, in other words, should one notate benzoyl chloride, for example, as C14 H10 O2 Cl2 (Berzelius' notation until 1826 and Liebig's until 1844), C14 H5 O2C l (Berzelius' and Wöhler's notation since 1826), or C14 H5 O2 Cl (Liebig's notation since 1844)? To Wöhler, Kolbe expressed a preference for H = 2. Wöhler agreed but remarked that Kolbe had used H = 2 in his dissertation, and he noted that there are problems of consistency in the Handwör-terbuch as a whole since the first volumes, following Liebig's preference of the 1830s and early 1840s, had used H = 1. Wöhler suggested Kolbe check with Liebig before settling the point. Kolbe did this, and Liebig agreed with the proposal to use barred letters to remove any possible ambiguity.[44] Kolbe's pattern in his own articles at this time was to use barred letters for German publications and the unbarred (H = 2) letters in England, as had become accepted there. The English system was soon thereafter generally adopted in Europe. This system
is notationally equivalent to taking H = 1, C = 6, O = 8, Cl = 35, and so on, which are the conventional equivalents that became so universally popular during the late 1840s and 1850s.[45]
Kolbe discussed these and other notational issues in articles written for the Handwörterbuch sometime in the middle of 1848. He explained his preference for the barred symbols, while sharply rebuking the French for maintaining Dumas' hybrid system of H = 1, C = 6, and O = 16 with four-volume organic formulas. This was, he thought, an "irresponsible" system that only increased the existing confusion, and was defended only to attempt to maintain an "imagined law" based on "vague hypotheses."[46] This, his first strong public derogation of the French, may have been partially motivated by feelings aroused by the violence of the February revolution in Paris and the consequent upheavals in Germany of the "March days." Kolbe's moderate liberalism did not extend to toleration of insurrection or anarchy.
In this article Kolbe also discussed how the notation of chemical formulas
. . . attains great importance by offering us a means of representing with greater sharpness and precision the different conceptions concerning the chemical constitution of a compound, solely by the various ways of grouping a few symbols, thereby simultaneously expressing a summary of ideas that can be reproduced so briefly in no other manner.[47]
So, for example, the constitution of acetic acid might be represented by the various formulas C+HO, HO.C4 H3 ,O3 , or ![]() . Whereas the first is little more than an empirical formula, the second accounts for the acidic and some of the electrochemical properties of the substance. The last, however, also accounts for the evidence supporting a copula formula: hydrogen of the methyl copula can be substituted by halogen without breaking up the structure of the radical, or the methyl copula can even be transferred to another radical entirely, as, for example, in the transformation of acetyl derivatives into methyl cyanide or cacodyl compounds.
. Whereas the first is little more than an empirical formula, the second accounts for the acidic and some of the electrochemical properties of the substance. The last, however, also accounts for the evidence supporting a copula formula: hydrogen of the methyl copula can be substituted by halogen without breaking up the structure of the radical, or the methyl copula can even be transferred to another radical entirely, as, for example, in the transformation of acetyl derivatives into methyl cyanide or cacodyl compounds.
Two months later, Kolbe provided even more detail on his ideas of the constitution of acetic acid. The radical of acetic acid is methyl copulated to "oxatyl," C2 , the resulting hydrocarbon radical to be called "acetyl," ![]() . Oxatyl was "the exclusive point of attack for the affinities of those elements," such as oxygen, that formed the various compounds of acetyl. For instance, the first stage of oxidation of acetyl was aldehyde
. Oxatyl was "the exclusive point of attack for the affinities of those elements," such as oxygen, that formed the various compounds of acetyl. For instance, the first stage of oxidation of acetyl was aldehyde ![]() , the third stage being acetic acid
, the third stage being acetic acid ![]() . In other words, reagents add only to oxatyl, and conversely, oxatyl undergoes only addition reactions. Substitution
. In other words, reagents add only to oxatyl, and conversely, oxatyl undergoes only addition reactions. Substitution
reactions (i.e., those that occur without major alteration of chemical properties) could take place only in the copula: for example, ![]() . Organic radicals could now be subsumed into two distinct groups: hydrocarbon radicals and conjugate radicals such as those of the acetyl class, which could then form "secondary" conjugate radicals (those that have undergone substitutions).[48]
. Organic radicals could now be subsumed into two distinct groups: hydrocarbon radicals and conjugate radicals such as those of the acetyl class, which could then form "secondary" conjugate radicals (those that have undergone substitutions).[48]
In this fashion, Kolbe had schematically resolved the acetic acid molecule one step further than ever before by distinguishing and focusing on what has become known as the carbonyl carbon atom. He had also created a theory of greater generality and wider application: radicals fully analogous to oxatyl could be formed from such elements as arsenic and sulfur, forming the constitutional basis for cacodyl and sulfonyl compounds. In this article, Kolbe thus altered his interpretation of the organic acids from formulas based on copulated oxalic acid to a more generalized concept using copulated oxide hydrates of carbon and other atoms—a progressive and fruitful shift.[49]
The buckle symbol, ![]() , Kolbe noted, had been proposed to him by Friedrich Otto (1809-1870), a professor of technical and pharmaceutical chemistry at the Collegium Carolinum, as a convenient way to indicate the special form of chemical bonding in copulated compounds. As for periods and commas, Kolbe defined them as equivalent in denotation; from this time forward, however, he tended to use the period for indicating the attachment of "basic water" to the remainder of an organic formula and the comma for separating oxygen from hydrocarbon moieties—though he sometimes omitted it. Parentheses presumably further emphasized the integral character of certain radicals. The option of dispensing with all of these punctuation marks and relying on simple juxtaposition of grouped letters, as he did, for instance, with C and H in hydrocarbon radicals, apparently did not sufficiently satisfy Kolbe's electrochemical instincts.
, Kolbe noted, had been proposed to him by Friedrich Otto (1809-1870), a professor of technical and pharmaceutical chemistry at the Collegium Carolinum, as a convenient way to indicate the special form of chemical bonding in copulated compounds. As for periods and commas, Kolbe defined them as equivalent in denotation; from this time forward, however, he tended to use the period for indicating the attachment of "basic water" to the remainder of an organic formula and the comma for separating oxygen from hydrocarbon moieties—though he sometimes omitted it. Parentheses presumably further emphasized the integral character of certain radicals. The option of dispensing with all of these punctuation marks and relying on simple juxtaposition of grouped letters, as he did, for instance, with C and H in hydrocarbon radicals, apparently did not sufficiently satisfy Kolbe's electrochemical instincts.
In these articles, which contain his most extended descriptions of the notational conventions that he followed with minor variation for the rest of his life, Kolbe made few of these details explicit. What does emerge clearly, though, is his conviction that carefully constructed formulas are extraordinarily useful semiotic and heuristic devices for the development and communication of theoretical ideas. In fact, to a degree perhaps unmatched by any other chemist of his day, Kolbe's notation cannot be separated from his theories. This is true because every formula that Kolbe wrote was a deliberate theoretical statement, namely, an assertion regarding the constitution of the molecule being discussed.
The Handwörterbuch project, barely alive for so many years, took off immediately under Kolbe's leadership. He solicited scores of arti-
cles from competent authorities and rode herd on them until they delivered; he then edited their contributions. He wrote a number of significant articles himself, among them Formeln, chemische, Formyl, Gepaarte Verbindungen, Kakodyl , and Kohlenwasserstoffe , and he revised articles on Acetyl, Aethyldithionsäure , and Benzoësäure . By 1851, the third and fourth volumes were complete through letter L, as well as a supplement volume. More progress was made in four years under Kolbe's leadership than had been made in the previous fifteen years. Liebig, Wöhler, and Poggendorff remained the "Herausgeber, " with Kolbe as "Redakteur. "
Kolbe also accepted another literary assignment early in his tenure in Braunschweig, which would lead to an enormous literary opportunity—and burden—occupying him for the rest of his life. Someone, probably either Friedrich Otto or Eduard Vieweg himself, conceived the idea that it would be a valuable (or profitable) undertaking to publish a German version of Thomas Graham's popular English textbook of chemistry. The first German edition (1840-1843) was a simple translation by Otto. For the second edition, conceived as a substantial rewrite of the original, Otto wrote the first volume, on general, physical, and theoretical chemistry (1845-1847), and Kolbe was asked if he would edit the organic portion of the text. On Christmas day 1847 we find Bunsen replying to Kolbe's request for advice on this point; Bunsen urged him to accept Vieweg's offer, but cautioned him not to overcommit on too many projects—sage counsel![50] We can only conclude that he must have worked at least occasionally on this project during the next three and a half years, since by the time of his arrival in Marburg, he had a manuscript of at least a portion of it, which he put to good use in preparing his lectures. But the manuscript went through several incarnations before it actually began to appear in fascicles in 1854.[51] By then, Kolbe had long been referring to it as "meine Organische. " It was no longer a translation, nor even a translated revision of Graham's work, but a detailed advanced organic chemistry text written by Kolbe ab ovo —although still published with a second title page giving the Graham-Otto imprimatur.
Frankland mentions, and we are told by biographers, that Kolbe had occasion to work in Varrentrapp's laboratory during his years in Braunschweig. No evidence for this is apparent from Kolbe's publications or letters, and fifteen years later he explicitly stated to Frankland that he had had no opportunity for laboratory work in Braunschweig.[52] He published only one article containing original experimental work during this period, and that work appears to have been done when Kolbe returned to Bunsen's lab in Marburg for six weeks or so in the summer recess of 1848 and again for "a week or so" in the summer re-
cess of 1849, as Frankland reported. He returned for a third time in the summer of 1850, spending a month in Marburg, Giessen, Frankfurt, and Heidelberg.[53] Frankland related: "He was then in his prime, full of enthusiasm for organic chemistry and earnestly hoping for a professorship, which would afford him the much desired opportunity for work."[54]
The work done by Kolbe on the first of these occasions was particularly fruitful. On the first day of August 1848, Kolbe wrote Frankland from Marburg, hoping that Frankland would arrive before he had to return to Braunschweig. He was working on the electrolysis of malonic and acetic acids. Six days later, he reported to Vieweg, "My chemical work, for which Bunsen's laboratory offers in fullest measure all the necessary equipment, has already achieved the geatest success, so that within a short time I will have reached the goal I had set for myself. Bunsen himself shows the greatest interest in my experiments. . . ."[55] A paper summarizing the results of this work was sent early the following year to Liebig for the Annalen and to London for the Journal of the Chemical Society .[56] Liebig thought the work "magnificent" and agreed with Kolbe's conclusions. "It has been a long time," he wrote, "since I have read an article that has excited me as much as yours has. The thoughts are as lovely as the methods, and the development is masterly." He had dabbled along similar lines himself, he said, but without this kind of success, and was happy to leave the field entirely to Kolbe.[57]
In this paper, Kolbe succeeded in sorting out many of the difficult details of the electrolysis reaction he had been working on since London. The hydrocarbon radical from decarboxylated potassium valerate he named "valyl"; he guessed that it must be the radical of the still unknown butyl alcohol. Even more important, acetic acid, barely mentioned in the London paper, now gave clean results: hydrogen, carbon dioxide, and methyl were all among the products, and the methyl gas proved chemically identical to that which he and Frankland had prepared from ethyl cyanide. (Kolbe's "radicals" are now considered to be their respective dimers, octane and ethane.) Whereas in 1844 he had fully synthesized acetic acid, this most fundamental organic substance, he had now successfully analyzed it into its component parts. They were the radicals predicted by his Berzelian theory.
This success gave Kolbe the impetus to write a long critical review of recent research on organic radicals, together with the various theories that were then contending to explain those results. Although he was at work on this review as early as March 1850, it was not until that fall that he completed and submitted the article, both to the Annalen and to the Journal of the Chemical Society . Hofmann was
Foreign Secretary to the Chemical Society, and in a reply to Kolbe, he offered either to translate Kolbe's article or to have it translated under his direction.[58]
Kolbe began by summarizing the disputes of the 1830s and 1840s between the (principally German) Berzelians and the French substitutionists, centering on the constitution of acetic acid and its derivatives. It was now beyond question that substitution does take place without altering the fundamental chemical properties of the substance and that organic radicals are by no means inviolable, as Berzelius had initially wanted to maintain. But Kolbe insisted that this by no means destroyed, or even substantially weakened, the electrochemical theory. One need only hypothesize further structure in one's radicals to provide a fully adequate theoretical explanation for all the new reactions.
For acetic acid, this meant that it was no longer sufficient to suppose a single integral "acetyl" radical, C4 H3 , combined with three equivalents of oxygen to form the anhydrous acid, as Liebig had been doing for the past decade. Kolbe's new formula was ![]() , in which the oxatyl group C2 "presents the exclusive point of attack for the powers of affinity of oxygen, chlorine, &c.," as described in his Handwörterbuch article of 1848. This in turn suggested a new mechanism for the reaction in which alcohol is oxidized to aidehyde:
, in which the oxatyl group C2 "presents the exclusive point of attack for the powers of affinity of oxygen, chlorine, &c.," as described in his Handwörterbuch article of 1848. This in turn suggested a new mechanism for the reaction in which alcohol is oxidized to aidehyde:
![]()
Kolbe thought that the oxygen must induce the ethyl radical to split into methyl plus C2 H2 , whereupon the two hydrogen equivalents in the latter moiety are captured by the extra oxygen, while the existing oxygen in the compound remains. Oxidation of aldehyde proceeds then by the direct addition of two more oxygens to the C2 . Substitution can complicate the notation of conjugate radicals, such as sulfobenzoic acid:[59]
![]()
In addition to the conjugate radicals found in copulated compounds, Kolbe named his second class of hydrocarbon radicals, possessing different chemical properties, the "ether" or "alcohol" radicals, which provided an idea of the constitutions of the homologous ethers and alcohols and their derivatives. A third class of radicals, new to this article, was the "homologizing hydrocarbon radicals," Cn Hn , as in succinic or adipic acids:
![]()
For the first time, we see here Kolbe admitting the possibility of dibasic organic acids, and he conceded that it might be necessary to reformulate succinic and sulfobenzoic acids as
where the numbers of atoms are simply doubled and rearranged in the first formula and another sulfate group is added in the second.[60] A curious aspect to this particular manipulation is that the second formula does not maintain the correct atomic ratios.
It is unclear precisely how Kolbe assigned some of his structures, in particular how he knew which compound was in the ether and which in the conjugate radical series. For example, according to Kolbe's theory, methane and ethane are actually methyl hydride and ethyl hydride, whose radicals are in the ether series. The chemically similar chlorinated derivatives perchloroethylene and perchloroethane are, however, conjugate radicals:[61]
![]()
To be sure, Kolbe had shown six years earlier that one could transform perchloroethylene into chloroacetic acid, justifying the first formula. He had also, however, shown how to convert methyl hydride to methyl chloride to methyl cyanide to acetic acid, which ought to have suggested that the methyl in methyl hydride is also a conjugate radical.
Another weakness of Kolbe's position, and one that he recognized, was the uncertain status of electrochemical-dualist precepts in his theory. Although his organic electrolyses appeared to demonstrate anew the general validity of electrochemical ideas, his specific predictions of the outcome of the experiments not infrequently failed verification.[62] He conceded that in many organic substances, it even seems impossible to determine which elements are positive and which are negative, such as carbon and nitrogen in cyanogen or carbon and hydrogen in hydrocarbons. Perhaps hydrogen's electrochemical properties are different in organic versus inorganic compounds—after all, hydrogen certainly has different properties in the normal gaseous versus the nascent state. So might chlorine have a less negative character in organic compounds than in its natural condition. These, he admitted, must be seen as conjectures regarding yet unsolved difficulties.[63]
In any case, Kolbe had developed here a detailed electrochemical radical theory that he was proud to compare with the type theories of the French. His view was that Dumas, Laurent, and Gerhardt had rushed to discard Berzelius' theoretical edifice on the basis of a single new phenomenon—substitution. This was overly hasty, to say the least, since all one needed to do was discard the now untenable belief in the immutability of radicals in order to resurrect the time-tested Berzelian theory. He concluded
It would be ridiculous to allow a single fact, difficult of explanation, to induce us to throw aside at once a theory which has served us for so long a period as a trustworthy guide in the difficult field of organic chemistry, and has preserved us most securely from the errors of a code of laws like that which has been laid down by Laurent and Gerhardt—unless we had some better theory to substitute for it. . . . [C]hemistry is indeed something better than a mere arithmetic problem, into which Laurent and Gerhardt endeavor to convert it.[64]
In many respects, this was an impressive performance. The article is detailed, well documented, and (aside from some nationalistic aspersions in the conclusion) a fair-minded summary of much recent research in organic chemistry. And in one respect at least, it was revolutionary. Kolbe here adumbrated for the first time a concept that would prove central in the future development of the science, what modern organic chemists refer to as functional groups , and he began the process of locating functionality on specific parts of the "constitutions" or structures of molecules. It was Berzelius who first suggested that acetic acid could be considered as schematically dissectible into hydrocarbon and oxycarbon moieties; it was Kolbe who generalized that notion and drew out its implications. Kolbe showed that it is at the "oxatyl" carbon (in modern vocabulary, the carbonyl carbon) where the chemical functionality of the molecule is concentrated. This thesis was amply supported by dozens of reactions of acetyl derivatives and other methyl compounds. He attempted the same thing with all of the scores of compounds discussed in his paper.
But it was precisely in this respect where the greatest weakness of the paper lies. Kolbe aggressively followed the same pattern of identifying functionality and its location within the molecule that proved so successful for acetic acid, even for those much more numerous cases where little empirical data existed from which to conclude such details. His paper is full of formulas suggesting specific details of constitution and implying predictions of chemical behavior that were unsupported or unexamined in 1850. Along the same lines, he continued the prolif-
eration of notational symbols that were poorly defined or even undefined. Berzelius had used periods or commas, while others among his contemporaries used parentheses and brackets, but Kolbe's pen was exceedingly restless with regard to the density of such symbols, and he added single and double buckle symbols to identify conjugate and homologizing hydrocarbon radicals. This often produced quite complex formulas (some examples of which have been cited) to which might be attached various empirical interpretations or predictions. For psychological and structural reasons, he could not write a formula without deliberately suggesting these interpretations and predictions.
For instance, the implication of his formulas for dibasic sulfobenzoic or succinic acids (as cited earlier) suggests that the two acid functions of each molecule are not chemically the same. It was eventually determined that in one case, this suggestion was correct, while in the other case it was not. More damaging and more to the point, in neither case did he have any evidence on which to judge the issue. Kolbe's readers had the right to expect the verbal and symbolic distinctions between alcohol and conjugate radicals to match consistent differences between the chemical behavior of those radicals, such as their replaceability with halogen atoms, but he simply failed to pursue this evidence. As a final example, the formulas for the chloroethylenes and chloroethanes cited above imply chemical nonequivalence of both the carbon atoms and the chlorine atoms within these molecules, but there was at that time no evidence for either equivalence or nonequivalence.
The Defection of Hofmann and Frankland
Some additional problems in this paper concern Kolbe's orientation toward Hofmann's and Frankland's research on substitution reactions. As already noted, Kolbe and Hofmann were exact contemporaries, arrived in London the same month, and became very close friends during the next two years. Hofmann later reported the results of some joint work that they performed in this period.[65] At this time, Hofmann and Kolbe were of very similar minds regarding constitutional theories. Hofmann's work on aniline appeared fully to support Berzelius' copula theory, the aniline derivatives all being formulated as ammonias with the structurally unspecified hydrocarbon C12 H4 as a copula. Consistent with the theory, all the substituted anilines retained the characteristic basic properties of ordinary aniline, analogous to free ammonia. In June 1848, two months before Berzelius' death, Hofmann published a paper extolling Berzelius and his theory and adducing all
the recent powerful experimental support for copulas, including Kolbe's work on acids and his own on bases. Still, he plaintively noted, "vainly have I hoped" to split aniline directly into NH3 and C12 H4 .[66]
But the following year he found compelling reasons to abandon the copula theory, turning to embrace a thoroughgoing substitution theory and Liebig's amidogen theory (NH2 plus a radical). Hofmann's conversion appears to have turned on one notable failed analogy between ammonia and aniline: whereas ammonia and benzoic acid can react together losing two water molecules (four "water equivalents") to form benzonitrile, there is no corresponding reaction between aniline and benzoic acid. The only way theoretically to rationalize this fact was to consider aniline not as a compound containing proximate ammonia but rather as one containing proximate amidogen (NH2 ) in its structure; then there would be insufficient hydrogen in aniline to yield two water molecules unless some additional hydrogen were abstracted from the hydrocarbon moiety. Thus, aniline is not NH3 ,C12 H4 , but rather NH2 ,C12 H5 .[67]
As trivial an adjustment as this may seem, the implications were large. Copula theorists insisted wherever possible on using isolable compounds as their proximate components and explaining their combination as nonelectrochemical addition. This joining of proximate components, they averted, had nothing to do with substitution of hydrogen. By contrast, Hofmann's new constitution for aniline did indeed suggest substitution of Laurent's phenyl radical for a hydrogen atom of ammonia, and it denied the preexistence of ammonia in aniline. In short, Hofmann was saying that aniline could not be a copulated ammonia; it was instead a substituted ammonia.
In this paper, Hofmann placed all the weight motivating his conversion on the nitrile-forming reaction just discussed. Nonetheless, he did mention Wurtz's discovery earlier that year of methylamine, ethylamine, and amylamine (what Gerhardt called primary amines), which appeared to be ammonia with one hydrogen substituted by organic radicals. This new development was surely a motivating factor as well. At the very end of the paper, he mentioned one more new kind of reaction that, he thought, put the matter beyond all question: his own discovery, just made, of secondary and tertiary amines, representing further substitutions of the second or third hydrogens of ammonia or of the second hydrogen of aniline, by various hydrocarbon radicals.
This paper was read to the Chemical Society on 5 November 1849. By 26 December, Hofmann had submitted to the Royal Society a detailed memoir on the subject just broached, detailing one of the most classic pieces of organic chemical research in the nineteenth century. Using alkyl iodides as reagents, Hofmann described the preparation,
properties, analysis, and theoretical classification of dozens of new substituted ammonias, including mono-, di-, and tri-methyl, ethyl, and amyl amines and anilines.[68] In a letter to Kolbe written while the paper was in press he described the work and related how much he had enjoyed it: "This investigation was a great deal of fun for me, for in 6 weeks the whole matter was settled. These reactions are so precise, that not a single experiment failed. The number of bases has now become virtually unlimited."[69]
In a letter that has apparently not survived, Kolbe must have communicated to Hofmann in 1850 an outline of his ideas for the review article on organic radicals, for in the letter just cited (undated, but ca. March 1850 by context), Hofmann reacted to some of Kolbe's ideas. Kolbe must have been attempting to maintain a strict interpretation of copulas, for Hofmann, now a true substitutionist, responded:
I cannot yet accommodate myself to your point of view, in particular it seems more complicated than mine, and moreover it does not explain why ethyl can be inserted twice into aniline and three times into ammonia. This is a fact which I, as you can see, have established by these experiments. But I will suspend judgment until I have heard all your reasons.[70]
Kolbe's other good friend from his London years was simultaneously undergoing a similar conceptual evolution. It was Frankland who in 1848 had introduced the use of alkyl iodides as reagents, and he used them with phenomenal success in the preparation of new organometallic compounds. These reactions appeared to proceed by substitution mechanisms. The radicals seemed to substitute indifferently for hydrogen, not only in the (mostly hypothetical) metal hydrides but also in Paul Thenard's alkyl phosphines and in Hofmann's and Wurtz' nitrogen bases.
At the same meeting of. the Chemical Society where Hofmann first mentioned his preparation of secondary and tertiary amines from alkyl iodides and signaled his conversion from copulas to substitution, Frankland's first paper on organometallic compounds, in which he drew similar implications, was read in absentia. An alkyl iodide, Frankland wrote, was analogous to hydriodic acid or any other hydracid, and the alkyl radical can substitute for hydrogen in a variety of organic and inorganic substances. He drew a specific analogy to Wurtz', Hofmann's, and Thenard's compounds, all the result of substitution reactions. Frankland was quite serious about this matter: the same comment was repeated in a paper read to the Chemical Society on 18 February 1850.[71]
Thus, in addition to his strong statements and recently revealed evi-
dence in favor of the original radical theory of 1832-1834, from the beginning of his second German period (October 1848 to January 1850) Frankland was also paying close attention to the newest research on substitution and fitting his results into that theoretical program as well. In a classic paper written shortly after his return to England, Frank-land was explicit. One could depict metal oxides as "true molecular types" for the organometallic compounds, he stated, citing Laurent and Dumas as authorities for the concept. For the fourth time in three years, he emphasized the analogy between his new compounds and those of the type theorists: "It is obvious that the establishment of this view of the constitution of the organometallic bodies will remove them from the class of organic radicals, and place them in the most intimate relation with ammonia and the bases of Wurtz, Hofmann, and Paul Thenard." Frankland was clearly offering the olive branch to the French type theorists: "The formation and examination of the organometallic bodies promise to assist in effecting a fusion of the two theories which have so long divided the opinions of chemists, and which have too hastily been considered irreconcilable."[72]
This apparent defection from the copula theory (and implicitly at least toward the French chemists) of his two loyal friends must have given Kolbe pause, and may account for the long delay between draft and publication of his 1850 article. There is evidence in Kolbe's paper of a serious effort toward accommodation of the uncomfortable new research, but also of the construction of a seawall against the incoming tide of typist ideas. Hofmann and Wurtz were "certainly right," he said, regarding the amidogen constitution of aniline and primary amines. Furthermore, Hofmann's work did demonstrate to a certain extent the truth of the type theorists' dictum that substitution of electrochemically foreign elements (such as chlorine or bromine in aniline) does not fundamentally alter the substance's properties. But that same research also showed that the extreme interpretation of type theory is wrong: a steady decrease of basicity occurs as aniline becomes more halogenated, until finally it becomes virtually neutral in character. Hofmann's and Frankland's research also placed beyond any doubt the actual existence of organic radicals as components of compounds, a point that some French chemists such as Dumas and Gerhardt had at times denied.[73]
Kolbe then asked whether it was the "radical hydrogen" of ammonia (i.e., that hydrogen atom whose removal creates amidogen) which is substituted by methyl or ethyl to create methylamine or ethylamine, and he answered his query in the affirmative.[74] Thus, Kolbe assumed the chemical nonequivalence of the three hydrogens of ammonia, simply from the circumstance that in some compounds nitrogen has
only two hydrogens associated with it. The fact that Hofmann had shown how to substitute all three hydrogens in succession by similar and smooth reactions did not seem to weaken this assumption.
This is an example that once more illustrates Kolbe's habit of assuming theoretical details that are unmotivated, or even subtly contradicted, by empirical information; others have been cited earlier. His mind was so intensely and habitually oriented toward chemical theory in general, and molecular constitutions in particular, that such assumptions seem to have been an integral part of his mentality. This nearly obsessive concern would continue in the future to be both his greatest strength and his greatest weakness as a scientist.