5—
Trees
Deborah L. Elliott-Fisk and Ann M. Peterson
A tree is a woody plant 6 ft (2 m) or more in height with a single main stem (trunk). However, some tree species may take on a shrub growth form under environmental stress. We typically envision trees composing a forest, which has a relatively closed canopy such that little direct sunlight reaches the forest floor. In the White-Inyo Range, true forests are rare, as low amounts of precipitation prohibit forest development. Instead, there are open woodlands of scattered trees, with the ground (vegetation) cover also discontinuous. For this reason, it is correct to refer to tree communities in the range as woodlands; riparian and aspen forests are the only exception to this rule.
Traveling to higher elevations in the range (e.g., by driving up Westgard Pass and the White Mountain Road), one passes through a series of vegetation zones, where plant communities and their associated species each occupy a particular elevational belt. Riparian woodlands and forests are seen along streams at lower elevations (Fig. 5.1), and Pinyon or Pinyon-juniper Woodland inhabit most sites between 6,500 and 9,500 ft (2,000 and 2,900 m). Above this, instead of the montane forest/woodland of Red and White Fir (Abies magnifice and A. concolor ), various pines, Western Juniper (Juniperus occidentalis ), and Mountain Hemlock (Tsuga mertensiana ) so characteristic of the nearby Sierra Nevada, is a shrubland dominated by Great Basin Sagebrush. This treeless zone extends up to the subalpine Bristlecone-Limber Pine Woodland, and locally to the Alpine Tundra Zone, depending on the type of rock the plants are growing on.
The absence of a montane forest/woodland is a consequence of the range's floristic history and its setting in the rain shadow of the Sierra Nevada. Beginning in mid-Tertiary time (approximately 25 million years ago), western North America experienced a cooling and drying trend, which forced more mesic species coastward. As mountain building activity further restricted the movement of moisture-laden air masses from the Pacific Ocean inland, much of the Great Basin woodland disappeared. However, some mesic species were able to persist in high-elevation habitats where soils or microclimates were favorable (Fig. 5.2). Thus, most tree stands in the White-Inyo Range are relicts of the more extensive woodlands and possibly forests that once inhabited the area (Elliott-Fisk, 1986).
A number of different types of evidence support this hypothesis. Several tree species that form extensive forests in the Sierra Nevada and Rocky Mountains exist only as patches (relict stands) of trees on special substrates in the White-Inyo Range (Fig. 5.3). The soils that develop on these substrates possess either a higher water-holding capacity or more favorable nutrient status, or both. Thus, Ponderosa Pine (Pinus ponderosa ), Jeffrey Pine (Pinus jeffreyi ), Lodgepole Pine (Pinus murrayana ), Western Juniper

Figure 5.1
Riparian forest along Silver Canyon at an elevation of 6,790 ft (2,070 m). This community
is dominated by Water Birch, Arroyo Willow, and Narrowleaf Willow. Sagebrush Scrub
covers the upper slopes.

Figure 5.2
Ponderosa Pine on north-facing colluvial slope in Lone Tree Creek canyon at 7,000 ft (2,
35 m). Trees are anchored on rocky outcrops along this steep, talus-covered slope. Growth
rings show that the trees have been injured many times by rockfalls.

Figure 5.3
Isolated stand of Lodgepole Pine, with a few Limber Pine on south-facing slopes, at Cabin
Creek, elevation 10,000 ft (3,050 m). These trees are on a deposit of early Quaternary
alluvium and outwash, where a well-developed soil with a high water-holding capacity has
formed. Sagebrush Scrub is present on the adjacent, drier colluvial slopes.
(Juniperus occidentalis ), Quaking Aspen (Populus tremuloides ), Narrowleaf Cottonwood (Populus angustifolia ), and Water Birch (Betula occidentalis ) still have small populations existing in the range.
Although the Bristlecone-Limber Pine Woodland occurs on several different substrates (including Reed Dolomite, sandstone of the Campito Formation, and Barcroft Granodiorite), it too is commonly edaphically and/or competitively restricted to particular lithologies. Wright and Mooney (1965) have documented the dominance of Bristlecone Pine (Pinus longaeva ) on dolomite (Fig. 5.4); such trees are able to tolerate the poor nutrient and water status of these soils. In contrast, Limber Pine (Pinus flexilis ) is more abundant on granitic soils. Although these two species are not as restricted as those listed in the preceding paragraph, it is very likely that they too were more widespread in the past and have been edaphically restricted in range due to low precipitation.
The irregular distribution of riparian communities is not as well understood, although it is probably related to changes in surface and subsurface hydrology. Upper

Figure 5.4
In the southern half of the White Mountains, Bristlecone Pine Woodlands are best
developed on the light-colored Reed Dolomite, with Sagebrush Scrub inhabiting the darker
Campito sandstone. The stand shown here occurs along White Mountain Road between
Campito and Sheep mountains in the Ancient Bristlecone Pine Area, elevation approximately
10,650 ft (3,250 m).
valleys commonly possess a deep mantle of weathered, coarse debris, usually of glacial origin. Water percolates rapidly into this regolith, eventually forced to the surface down valley as the groundwater table rises. Most commonly, riparian trees are present just below the point where water first appears at the surface in the stream channel, but this is not always the case. Ranching practices may have played a role in the eradication of some of these communities (Peterson and Elliott-Fisk, 1988).
The marginal nature of the environment for tree existence is also attested to by the presence of hybrids and genetic dwarfs. Although Quaking Aspens occur as large trees in moist settings in the White Mountains, they also occur as (genetic) dwarfs at scattered high-elevation (treeline) sites (Fig. 5.5). Dwarf Aspens also occur at other alpine treeline locations in the mountain ranges of western North America. The intermediate appearance of some individuals in the Yellow-Jeffrey Pine stand at Jeffrey Mine Canyon suggests that Pinus ponderosa × P. jeffreyi hybrids may be present here. Juniper hybrids (Juniperus occidentalis × J. osteosperma ) are also present in the White-Inyo Range (Vasek, 1966). Because hybrids more commonly occur where stressful climatic conditions shorten the flowering season, possibly resulting in overlapping pollen shed and fertilization for two species whose flowering periods may nor normally be time-synchronous, it is not unusual that hybrids occur in the White-Inyo Range.

Figure 5.5
At the alpine rockfall along McAfee Creek, elevation 10,800 ft (3,290 m), an extensive
stand of Dwarf Aspens occurs. These individuals range from 3 to 6 ft (1 to 2 m) in height.
Photo taken in early July, before the trees had fully leafed out. In the valley immediately
below, nondwarfed aspens are widespread on both the valley walls and along the creek itself.
Although many of the tree species of the White-Inyo Range have interesting ecological adaptations and distributional patterns (Lloyd and Mitchell, 1973), it is the Bristlecone Pine (Fig. 5.6) that has generated the most scientific interest. This species is the longest-lived plant documented, with some individuals in the White Mountains reaching ages of 4,500 years or more. It is interesting to note that the oldest trees are not the largest, healthiest-appearing individuals, but instead are somewhat shortened trees with spike (dead) tops and incomplete bark (strip growth). These old trees occur on marginal, dry sites, and their growth is very slow, as evidenced by narrow tree-ring widths. Because the wood of the Bristlecone Pine is very dense and resinous, it is resistant to decay, with fallen or even upright dead individuals persisting on the landscape for thousands of years.
The presence of both long-lived individuals and dead tree remnants has allowed dendrochronologists, such as E. Schulman, C. W. Ferguson, and V. C. LaMarche, Jr., of the Laboratory of Tree-Ring Research at the University of Arizona, to construct long tree-ring series. As tree growth is primarily a function of climate, these chronologies can be used to reconstruct climate changes. C. W. Ferguson has constructed a Bristlecone Pine chronology that spans approximately the last 8,700 years. This chronology and others have many scientific applications; for example, the rate of production of

Figure 5.6
Bristlecone Pines are the oldest living individual organisms known, with some attaining
ages over 4,500 years. The individuals shown here in the Patriarch Grove, elevation
11,200 ft (3,415 m), exhibit partial die-back and strip-bark growth, with exposed wood
weathering into beautiful patterns and colors.
radiocarbon (isotope C14 ) in the atmosphere has been determined by its extraction from Bristlecone Pine wood (tree-ring) samples, which has allowed scientists to calibrate radiocarbon dates for this period. LaMarche (1973) has determined that the elevation of the upper Bristlecone Pine limit (treeline) has decreased in postglacial time, due to a general cooling. Dead trees above the current alpine treeline may be seen on Sheep Mountain above Patriarch Grove.
The remainder of this chapter lists and describes the tree species that occur in the White-Inyo Range.
Pinaceae (Pine Family)
Pinyon Pine,Pinus monophyllaTorr. & Frém . (Fig. 5.7a ) 10–33 ft (3–10 m) in height; locally multiple-stemmed above base; bark gray to dark brown (as tree ages), with narrow, flat ridges and thin scales; one needle per fascicle, rigid, incurved, sharp, gray-green, 1–1.6 in (2.5–4 cm) long; male catkins 0.2 in (5–6 mm) long; female cones initially sticky, bright green, concentrated on upper branches, 2–4 in (5–10 cm) in length, broadly spherical to globose, opening widely after seeds ripen,
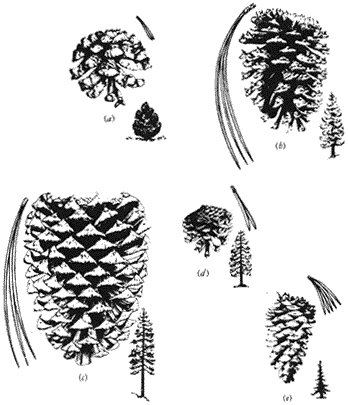
Figure 5.7
(a) Pinyon Pine. (b) Ponderosa Pine. (c) Jeffrey Pine. (d) Lodgepole Pine. (e) Limber Pine.
turning shiny russet brown, scales thickening at outer edge; large seeds to 0.8 in (20 mm) in length with short wings, dispersed in autumn.
Distribution. Common in Pinyon-juniper Woodland on all aspects and topography from 5,900 to 9,500 ft (1,800 to 2,900 m) throughout range; well-developed stands may be seen along the Westgard Pass Road in the Cedar Flat area and on the White Mountain Road on the way to Sierra Vista.
Pinyon Pines are the dominant trees in all of the lower woodlands of the Great Basin, Mojave Desert, and Colorado Plateau. The Two-needled Pinyon (Pinus edulis Engelm.) occasionally hybridizes with the Single-needled Pinyon (P. monophylla ) where their ranges overlap. Single-needled Pinyon Pine has a wide range of environmental tolerance and can persist on severe sites, where individuals may live to be at least several hundred years old, displaying erratic growth patterns. It may occur in association with various juniper and pine species in the White-Inyo Range, including Bristlecone Pine on granitic substrates.
The seeds (commonly referred to as nuts) of the Pinyon Pine tree have been not only a valuable food resource for native American populations but are collected by various birds and rodents. In fact, animals are a valuable dispersal agent for the tree's seeds. For an excellent treatment of the tree's ecology and use, see Lanner and Lanner (1981).
Ponderosa (Yellow) Pine,Pinus ponderosaDougl. ex P. & C. Lawson. (Fig. 5.7b ) 30–80 ft (10–25 m) in height; typically straight trunk; crown occasionally conical to spreading with spike tops on older trees; bark thick (up to 4 in [10 cm] on oldest trees), yellow-brown to pink with broad plates and shallow furrows, inner surface yellow, resinous odor; young green branchlets, turning brown with age; three needles per fascicle, yellow-green, 4.5–7 in (12–18 cm) long, tufted, rows of stomates largely indistinguishable; male catkins 1 in (2–3 cm) long, clustered; female cones terminal to subterminal, typically 2.8–5 in (7–13 cm) in length, outturned prickles on needle scales, young cones green, yellow-brown to russett brown with age, oval; seeds about 0.2 in (5–6 mm) in length with long, narrow wings.
Distribution. Rare, occurring in isolated relict patches on west slope of the White Mountains from 6,800 to 7,550 ft (2,085 to 2,300 m) in Lone Tree Creek and Jeffrey Mine Canyon; best developed on north-facing slopes.
Although Ponderosa Pine is perhaps the most common and widespread conifer in the western United States, its distribution in the White-Inyo Range is very restricted, most likely as a consequence of aridity, because the range is situated in the rain shadow of the Sierra Nevada. The isolated stands on the west slope of the White Mountains could represent relicts from a montane forest that might have existed in the range prior to major uplift of the Sierra Nevada.
Trees in the Ponderosa Pine stands occur on steep slopes and are widely spaced, attesting to the severe competition for water. Single-needled Pinyon Pine and Jeffrey Pine, other xerophytic conifers, occur as associates.
Jeffrey Pine,Pinus jeffreyiGrev. & Balf. (Fig. 5.7c ) 30–80 ft (10–25 m) in height; thick, straight trunk; crown spreading but symmetrical, branches widely spaced, thickening with age; bark thick, reddish brown (darker than Ponderosa Pine), deep furrows with typically narrow plates, inner surface pink to brown, vanilla odor; three needles per fascicle, blue-green, 4.5–7 in (12–18 cm) long, visible rows of stomates; male catkins 1 in (2–3 cm) long; female cones subterminal, 4.5–6 in (12–15 cm) in length, inturned prickles on needle scales, young cones purple, turning russet brown when ripe, long oval; seeds 3–4.5 in (8–12 cm) long, with wings 1 in (2–3 cm) in length.
Distribution. Rare, known only from Jeffrey Mine Canyon, although individuals may also be present in nearby canyons along the western slope of the White Mountains; elevational range 6,775–7,710 ft (2,065–2,350 m); best developed on perched alluvial fan deposits.
Jeffrey Pine is commonly the dominant and largest tree in the lower montane forest of the eastern Sierra Nevada. The Jeffrey Pine community of the western White Mountains is probably a relict of a former montane forest in the range (as may be the case for Ponderosa Pine). The stand at the apex of the Jeffrey Mine Canyon alluvial fan is composed of individuals of all ages, with the oldest perhaps 600 to 800 years old. Trees are widely spaced (forming an open woodland), as seen elsewhere in more arid parts of their range.
A few Pinus ponderosa × P. jeffreyi hybrids appear to be present here, although confirmation is not yet complete. The two species do hybridize elsewhere in California (Haller, 1962).
Lodgepole Pine,Pinus murrayanaGrev. & Balf. (Fig. 5.7d ) 50–80 ft (15–25 m) in height; trunk straight, widening at base; open crown with heavy branching, particularly with age (and the concurrent development of a spiked top); bark relatively thin with small, thin scales, light gray-brown; two needles per fascicle, bright green, 1.2–2 in (3–5 cm) long, densely clothing branches; male catkins to 0.3 in (8 mm) in length; female cones clustered, commonly subterminal, ovoid, semiserotinous, 1.2–2 in (3–5 cm) long, slender (semi-deciduous) prickles, purple-green when young, turning clay-brown when ripe; seeds to 0.2 in (4 mm) in length with long wings.
Distribution. Although Lodgepole Pine has been reported from several sites in the White Mountains (Lloyd and Mitchell, 1973), it has been collected (verified) only from Cabin Creek and adjacent Chiatovich Flats and the Middle Creek cirque. The well-developed stand along Cabin Creek occurs at about 10,000 ft (3,050 m) on early Quaternary alluvial and outwash deposits derived from metavolcanic material. Scattered, rare individuals may occur elsewhere on both the western and eastern slopes of the White Mountains.
Lodgepole Pine has a wide range throughout the mountains of western North America, occurring even near the Arctic treeline in the Yukon Territory. The taxon has now, however, been divided into separate species (which were originally all classified as Pinus contorta Dougl.), with Pinus murrayana largely restricted to the Pacific coastal region.
Although Lodgepole Pine has a wide tolerance range, commonly occurring from the upper to lower montane treelines and in wet as well as dry sites, its existence in the White Mountains is very marginal. As with Ponderosa and Jeffrey pines, Lodgepole Pine is probably relictual in nature. Soil moisture retention is greater on these older alluvial and glacially derived soils — a possible explanation for the restriction of the main population to the Cabin Creek site. The trees here are some of the most massive in California and may be up to 1,000 years in age.
Limber Pine,Pinus flexilisJames. (Fig. 5.7e ) 30–50 (rarely 65) ft (10–15 [rarely 20] m) in height; trunk straight to contorted with age and increasing environmental severity, wide at base; symmetrical to broadening crown with age; young trees with silvery gray, smooth bark, turning into broken plates with thin scales with age; five
needles per fascicle, dark green, stiff and erect, with visible rows of stomates, 1–2.8 in (2.5–7 cm) long, in dense tufts at ends of branches; male catkins red, up to 0.4 in (1 cm) long; female cones subcylindric, 4–7 in (10–18 cm) long, without prickles, scales thickened and incurved, green to yellow-brown when ripe; seeds dark brown, about 0.4 in (1 cm) long with narrow wings.
Distribution. Most commonly present as a codominant with Pinus longaeva , forming a subalpine woodland between 10,000 and 11,500 ft (3,050 and 3,500 m), although individuals extend down to 6,850 ft (2,085 m) in isolated canyons (such as Lone Tree Creek) and thus can occur in association with most of the other tree species in the range. Several vigorous stands occur along White Mountain Road between the Schulman and Patriarch groves.
Limber Pine is perhaps the most widely distributed tree in the White-Inyo Range. It grows on all substrates and is especially important in forming the subalpine woodland on granitic soils, although Pinus longaeva commonly forms the alpine treeline proper. Although the species prefers mesic sites, it occurs in xeric habitats, where it reaches its maximum age. Though not as long-lived as Pinus longaeva , the species displays basically the same growth pattern (as it too is a five-needled pine) and so is similar dendrochronologically.
The relatively large seeds of Limber Pine are a food resource for birds and small mammals; they may have also been used by native Americans inhabiting high summer camps in the White Mountains.
Bristlecone Pine,Pinus longaevaD. K. Bailey. Typically 15–50 ft (5–15 m) in height; trunk commonly thick and contorted or split, but straight when young; very stressed trees may have large, spreading crowns almost shrubby in appearance; old trees commonly spike-topped, with strip-bark growth; relatively thin, reddish to dark brown bark with flat, irregular ridges; five needles per fascicle, slender, 1–1.5 in (2.5–3.5 cm) long, with characteristic bristle-like appearance on branches; male catkins about 0.4 in (1 cm) long, red-purple; female cones ovoid, dark purple to brown when ripe, 2.8–3.5 in (7–9 cm) long, slender, incurved prickles; seeds about 0.3 in (8 mm) long, with wings up to twice the length of seeds, light brown.
Distribution. Common in the subalpine from 10,000 to 11,650 ft (3,050 to 3,550 m), occasionally extending down to 8,500 ft (2,600 m), forming a mixed Pinyon Pine-Bristlecone Pine Woodland. Best developed and dominant on dolomite and limestone, especially along the southern half of the White Mountain summit surface. Occurs on outcrops of Reed Dolomite along White Mountain Road in and between Schulman and Patriarch groves.
The White Mountains are better known for their Bristlecone Pines than for any other natural feature. The science of dendrochronology (tree-ring dating) has advanced through work on the White Mountains' Bristlecone Pines (Ferguson, 1968, 1970). Trees over 4,500 years old have been dated, with their growth-ring sequences matched to those of dead individuals whose existence overlapped in time. To date, a yearly chronology has been developed to 6,700 B.C. , giving a detailed record of postglacial
climatic change and radiocarbon fluctuation in the atmosphere (Ferguson and Graybill, 1983). The Bristlecone Pine tree-ring chronology is an extremely important dating tool for natural scientists (Suess, 1970).
As one walks through the Bristlecone Pine Woodlands, many fallen, dead individuals are seen (which is not unusual for any forest/woodland where fire frequency is low). Perhaps it is more surprising to see these same types of specimens outside of the distribution of the living trees. Scientists have used dead Bristlecone and Limber pines to infer shifts in the upper and lower treelines (LaMarche, 1973). This is another valuable tool for the reconstruction of climatic (environmental) change.
Cupressaceae (Cypress Family)
Utah Juniper,Juniperus osteosperma (Torr.) Little. (Fig. 5.8a ). Small tree, usually 6–20 ft (2–6 m) in height, although larger individuals occur; usually one stem at base, dividing to four to eight stems above 3 ft (1 m); gray-brown bark, weathering ash white; leaves mostly in threes (ternate), with "hidden" glands (imbedded in mesophyll with overlying layers obscuring their presence), 0.1 in (2–3 mm) long; male cones 0.1–0.2 in (3.5–4.5 mm) long; female cones ("berries") 0.3–0.4 in (7–9 mm) long, red to reddish brown, with sweet, dry pulp; seeds 0.04 in (1 mm) long usually one per cone with four cotyledons per embryo; usually monoecious.
Distribution. An important codominant of the Pinyon-juniper Woodland (here and in the Great Basin as a whole), Utah Juniper ranges from 6,850 to 10,335 ft (2,085 to 3,150 m) and is occasionally associated with Limber and Bristlecone pines. Well developed on alluvium, as at junction of Westgard Pass and White Mountain roads (Cedar Flats).
Juniper trees of short stature and shrublike appearance are commonly seen in lower montane woodlands in western North America. Although the species vary geographi-
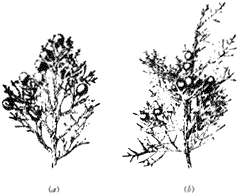
Figure 5.8
(a) Utah Juniper. (b) Western Juniper.
cally (with J. scopulorum Sarg. in the Rocky Mountains, J. monosperma (Engelm.) Sarg. throughout the southern Rocky Mountains and the Southwest, J. california (California Juniper) common along the California desert margins, etc.), the physiognomy of the vegetation is similar, with the understory as open in appearance as the tree canopy.
Although Utah Juniper appears to prefer deep alluvial soils in the White-Inyo Range, it occurs on rocky volcanic outcrops, on steep slopes covered by colluvial deposits, and on most other substrates. Changes in its geographical range over the last several thousand years have been documented using plant macrofossils from woodrat middens (Elliot-Fisk, 1986; Jennings, 1988).
Western (Sierra) Juniper,Juniperus occidentalisHook ssp.australis Vasek. (Fig. 5.8b ) 15–50 ft (5–15 m) in height, locally contorted or depressed (stunted); one well-defined trunk; reddish brown to brown, shreddy bark; spreading branches; leaves mostly ternate (in threes), gray-green, each with a conspicuous gland that secretes a whitish, sticky substance, 0.1 in (3 mm) long; male cones ca. 0.15 in (3.5–4 mm) long; female cones ("berries") ca. 0.3 in (7–8 mm) long, blue-black at maturity, with resinous pulp; seeds ca. 0.2 in (5–6 mm) long, one or, more commonly, two per cone, with two to three cotyledons per embryo; primarily dioecious.
Distribution. Rare in the White-Inyo Range, with individuals reported between 9,050 and 10,570 ft (2,760 and 3,220 m) at the upper Pinyon-juniper and lower Bristlecone-Limber pines woodland transition in the central eastern canyons of the White Mountains (east Cottonwood and Crooked Creek areas) and the upper eastern slopes of the Inyo Mountains (Seephole Trail Springs and New York Butte).
Western Juniper is a spectacular tree commonly seen on rocky outcrops in the montane forest of the Sierra Nevada, especially on granitic substrates. Although its geographical range in the White-Inyo Range is not well documented, a large stand of several hundred trees exists in the eastern Cottonwood Creek drainage on glacially sculpted and jointed quartz monzonite outcrops (Elliott-Fisk, 1986; Elliott-Fisk and Ryerson, 1988; Jennings et al., 1988). One problem hindering its identification is its possible hybridization with Utah Juniper. Trees of apparently hybrid origin occur at the head of San Lucas Canyon in the Inyo Mountains and at the northern end of the White Mountains (Vasek, 1966).
Betulaceae (Birch Family)
Water Birch,Betula occidentalis Hook. (Fig. 5.9) 10–30 ft (3–9 m) in height; bark glossy, reddish brown, smooth, not separating into thin layers; twigs rough; leaves round-ovate, 0.8–1.6 in (2–4 cm) long, rounded at the base, acute at the apex, margins sharply serrate (uncommonly doubly serrate) except near the base, glabrous, dull green above, glandular, dotted, light green beneath; petioles 0.2–0.4 in (5–10 mm) long; monoecious, catkins 0.8–1.6 in (2–4 cm) long.
Distribution. Forming scattered stands, some thick, in riparian areas and springs throughout the range from 6,500 to 8,500 ft (1,675 to 2,600 m) on the east side of

Figure 5.9
Water Birch.
the range along South Fork Perry Aiken Creek (7,610–8,530 ft [2,320– 2,600 m]) and Wyman Canyon (7,400 ft [2,255 m] and below) and on the west side of the range along Silver Canyon (6,800 ft [2,075 m]).
Water Birch is a common tree species throughout the White Mountains and occurs predominantly in the Pinyon-juniper Woodland zone (6,500–9,500 ft [1,980–2,895 m]). This tree forms thick stands in riparian areas and is intermixed with willows, aspen, and cottonwood. Water Birch stands are best developed on the east side of the range (Peterson and Elliott-Fisk, 1988).
Water Birch is a common species in the Great Basin-Rocky Mountain vegetation complex. According to Lloyd and Mitchell (1973), this species once had a wider range and has become restricted to higher elevations and riparian areas.
Salicaceae (Willow Family)
Black Cottonwood,Populus trichocarpaT. & G. (Fig. 5.10a ) 40–100 ft (12–30 m) in height; trunk 1–3 ft (0.3–1 m) in diameter; grayish, smooth bark that is furrowed with age; branches spread to form a broad, open crown; leaves ovate, 1.5–3 in (4–8 cm) long (less commonly longer), truncate or cordate at the base, acute at the apex, margins finely serrate, shiny dark green above, paler and glaucous beneath; petioles rounded, 0.6–1.6 in (1.5–4 cm) long; dioecious, male and female catkins rare, 1.5–3 in (4–8 cm) long.
Distribution. Sporadic in the range, occurring mostly near streams, springs, or meadows from 4,900 to 8,850 ft (1,500 to 2,700 m). This species forms part of the riparian community in Wyman Canyon (7,610 ft [2,320 m]), Queen Canyon (7,200–8,300 ft [2,195–2,530 m]), and at Toll House Springs (5,970 ft [1,820 m]).
Black Cottonwood is the largest of the American poplars. It commonly occurs as part of the riparian community in both the desert scrub and pinyon-juniper vegetation zones in the White Mountains. Black Cottonwood is able to capture, use, and store great quantities of water from streams, springs, and the groundwater table. It is commonly intermixed with various willows (Salix spp.). Black Cottonwood is very shade-intolerant, and an individual must occupy a dominant position to thrive.
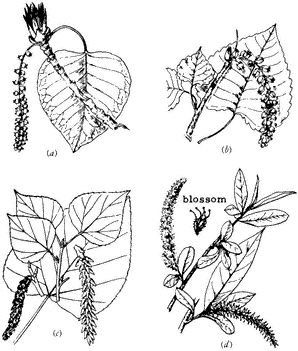
Figure 5.10
(a) Black Cottonwood. (b) Fremont Cottonwood.
(c) Quaking Aspen. (d) Willow.
Male and female flowers are present on separate trees. Female flowers form hanging clusters that are covered with cottony hairs. When the seeds are released, they are scattered by the wind and form small, white drifts in sheltered places, hence the name cottonwood .
Fremont Cottonwood,Populus fremontii Wats. (Fig. 5.10b ) 40–100 ft (12–30 m) in height; bark whitish gray, roughly cracked; branches spreading, forming a broad, open crown; twigs stout; leaves deltoid, 1–3 in (3–8 cm) long, 1.5–3.5 in (4–9 cm) wide, truncate to cordate at the base, sharply pointed at the apex, margins crenate, yellowish green and glossy above, paler beneath; petioles flattened, 1.5–3 in (4–8 cm) long; dioecious, catkins dense, 2–4 in (5–10 cm) long, female catkins loosely flowered; seeds with long white hairs.
Distribution. Spotty along creeks and streams from 5,580 to 7,875 ft (1,700 to 2,400 m), forming part of the riparian community along Wyman Canyon (6,990 ft [2,130 m]), Silver Canyon (6,800 ft [2,075 m]), and Montgomery Canyon (7,790–8,010 ft [2,375–2,440 m]).
Fremont Cottonwood was named for John C. Fremont. He discovered this large arid-land cottonwood in 1844 on an expedition to Nevada and California. Typically a
southwestern species, it occurs in a few scattered locations in the White Mountains. Fremont Cottonwood occurs in the riparian community at Wyman and Indian canyons, on the east side of the White Mountains, and at Silver, Lone Tree, and Montgomery canyons, on the west side of the range (Peterson and Elliott-Fisk, 1988).
Fremont Cottonwood has been widely planted as an ornamental tree in the West. Whether this species is native or was introduced to the White Mountains is unclear. The distribution of Fremont Cottonwood is very patchy, and the number of individuals at each location is small.
Narrowleaf Cottonwood,Populus angustifoliaJames. 30–60 ft (10–20 m) in height; grayish bark; narrow crown; slender twigs; leaves mostly lanceolate, 1.5–3 in (4–8 cm) long, 0.4–1.2 in (1–3 cm) wide, rounded at the base, somewhat acuminate at the apex, margins coarsely serrate, light green and glabrous; dioecious.
Distribution. Rare; moist habitats; known only from two sites in the range — at Wyman Canyon (7,020–8,200 ft [2,140–2,500 m]) and Queen Canyon (8,300 ft [2,530 m]).
Narrowleaf Cottonwood is common in much of the Rocky Mountain region. Isolated sites in California define the western edge of this species' distribution.
Quaking Aspen,Populus tremuloides Michx. (Fig. 5.10c ) 10–50 ft (3–20 m) in height; straight trunk; smooth, greenish-white bark (darkening at base with age); slender twigs that commonly droop; leaves round-ovate, 1–2 in (2.5–5 cm) long and about as wide, broadly rounded to cordate at the base, sharply pointed at the apex, margins crenate or serrulate to almost entire, glabrous and green above, paler beneath; petioles flattened, 1–2.5 in (2.5–6 cm) in length; flowers rare, dioecious, male catkins 1.5–2.5 in (4–6 cm) long, female catkins 2–4 in (5–10 cm) long; tiny seeds (2 million/pound), brown with white hairs.
Distribution. Occurs predominantly in the Subalpine Zone from 9,025 to 10,825 ft (2,750 to 3,300 m) in rocky areas and moist locations along streams or at the edges of meadows. Major stands occur on the east side of the range on the South Fork McAfee Creek (10,825 ft [3,300 m] and below), Crooked Creek (9,850 ft [3,000 m]), and Indian Creek (8,850 ft [2,700 m]).
Quaking Aspen forests are one of the most productive communities in the White Mountains. Major stands are located on the eastern slopes of the range. This species does not inhabit a distinct zone, as in some parts of the Great Basin region; instead it has a patchy distribution. It is possible that Quaking Aspen was previously more extensive in the range and that these are relict stands (Peterson and Elliott-Fisk, 1988).
Even though an aspen stand can produce millions of seeds annually, reproduction — or, more properly, stand growth and maintenance — is predominantly vegetative. New trees arise from a common root system; groves of these trees are referred to as clones. All of the trees that make up an individual clone are genetically identical.
Quaking Aspen is intolerant of both shade and competition from conifers. Therefore, this taxon does not occur intermixed with coniferous woodlands in the White-Inyo Range, but only in disjunct stands. Quaking Aspen is best developed on moist soils derived from colluvium or glacial till.
Dwarf Quaking Aspen trees occur in two localized exposed sites at the head of Silver Canyon (10,400 ft [3,170 m]) and on the slopes surrounding the South Fork McAfee Creek (10,800 ft [3,292 m]). These trees are considered to be genetic dwarfs. According to Strain (1964), Dwarf Aspen exist in a more severe environment than nondwarfs. Dwarf Aspen take on a recognizably different growth form and typically have short trunks with many low branches, giving the tree a shrublike appearance. The leaves of Dwarf Aspen are somewhat larger than those of nondwarfs.
Willows,SalixL. spp. (Fig. 5. 10d ) May attain height of 10–20 ft (3–6 m); bark light in color, on older trees fissured and darker; commonly multistemmed above base; twigs slender, yellow to reddish; leaves long, simple, mostly narrow, pinnately veined, and alternately arranged; petioles short; dioecious, catkins distinct, yellow to green; seeds tiny (2–3 million/pound) with small tufts of hair.
Distribution. Common in riparian areas throughout the range, 4,500–10,000 ft (1,375–3,050 m). Salix exigua rarely occurs in nonriparian sites.
Willows line creeks and streams throughout the White Mountains. Eight species are known to exist in the range. The most common include Salix exigua, S. lasiolepis, and S. lutea , which occur from 4,500–10,000 ft (1,370–3,050 m). The other species have a more sporadic distribution and include S. geyeriana, S. laevigata, S. lasiandra, S. pseudocordata , and S. orestera (Peterson, 1986; Peterson and Elliott-Fisk, 1988).
Willows commonly have multiple, branching trunks and take on either a tree or a shrub form. Of the 175 species native to North America, only 30 attain tree size. Willows are commonly associated with Quaking Aspen, Water Birch, and cottonwoods in the White-Inyo Range and locally form an impenetrable wall of vegetation.
Although willows are an easily recognizable element of the riparian community, the identification of individual species can be a difficult task. Male and female flowers occur on separate plants, and both are needed to make a correct identification.
Willows are intolerant of shade and dry soils. Germination rates are low, but many seeds are produced by each plant. The seeds have fine, cottonlike hairs, which aid in their dispersal by wind. Because this species is riparian, seeds are also transported by water.
Aceraceae (Maple Family)
Mountain Maple,Acer glabrumTorr. var.diffusum(Greene) Smiley. (Fig. 5.11) Shrub to small tree 6–20 ft (2–6 m) in height; twigs whitish gray; leaves simple, palmately lobed; leaf blades 0.6–1 in (1.5–2 cm) long, 0.5–1.1 in (1.2–2.8 cm) wide, with few blunt teeth on lobes; peduncle plus pedicel 0.4–0.8 in (1–2 cm) long; fruit a samara, three to six pairs, 0.8–1.2 in (2–3 cm) long.

Figure 5.11
Mountain Maple.
Distribution. Rare in White-Inyo Range; on rocky slopes between 8,000 and 9,000 ft (2,440 and 2,750 m). Collected from Birch Creek (8,400 ft [2,560 m]), Wyman Creek (8,600 ft [2,620 m]), and Cottonwood Creek (8,700 ft [2,650 m]) drainages.
Mountain Maple prefers moist, shaded habitats near streams and springs or under a canopy. It is the smallest of the Great Basin maples. This variety also grows in the Panamint Mountains and along the eastern Sierra Nevada. It is a favorite deer browse (Lanner, 1984).
Box Elder,Acer negundoL. ssp.californicum(Torr. & Gray) Wesmael. Tree 20–65 ft (6–20 m) in height with broad, rounded crown; twigs slender, pubescent, greenish; leaves pinnately trifoliate, three to five lobes, ovate, 2–4.8 in (5–12 cm) long, coarsely serrate, densely pubescent beneath; flowers unisexual, greenish; pedicels filiform; four to five stamens; samara straw-colored when mature, finely pubescent, 1–1.2 in long.
Distribution. Rare in the White-Inyo Range. Lowland and riparian tree, often introduced. Present at old Roachville town site along Cottonwood Creek at 5,800 ft (1,770 m).
Box Elder occurs throughout North America, but its distribution is patchy, especially in eastern California and the Great Basin. This tree has been widely planted for shade in semi-arid regions. The sap of this tree has been collected as a source of syrup (Lanner, 1984).
Simarubaceae (Quassia Family)
Tree of Heaven,Ailanthus altissima (Mill.) Swingle. Spreading tree up to 50 ft (15 m) in height; bark thin, gray, rough; branchlets stout, hairy; leaves odd-pinnately compound, 12–24 in (30–60 cm) long, 11–25-foliate; leaflets lanceolate to oblong, 2.8–6 in (7–15 cm) long, with 2–4 teeth near base; fruit a samara, 1.2–2 in (3–5 cm) long, reddish.
Distribution. Introduced at Toll House Springs along Westgard Pass Road at 5,970 ft (1,820 m).
The Tree of Heaven, a native of southeastern Asia, has become naturalized in California as the result of extensive planting by Chinese miners in the mid- and late 1800s. The tree is moderately drought-resistant and rapid-growing, quickly producing shade in areas that native trees cannot readily colonize. The male flowers are unpleasant-smelling, and suckers may spread the species to sites where it is not wanted.
Ulmaceae (Elm Family)
Smooth-leaved Elm,Ulmus carpinifolia Gleditsch. Tree to 65 ft (20 m) in height; branchlets juicy, terete, subglabrous; leaves simply or doubly serrate, with axillary tufts beneath, 2– 3 in (5–8 cm) long; flowers in fascicles (cymes), appearing before the leaves; fruit an elliptic samara, cuneate at the base.
Distribution. Introduced at Toll House Springs along Westgard Pass Road at 5,970 ft (1,820 m).
The Smooth-leaved Elm, a native of Europe, was introduced to North America after it was discovered to be highly resistant to Dutch Elm disease. This species has been widely planted across the United States, becoming naturalized (as an escapee) in some areas.
Key to Trees
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
Elliott-Fisk, D. L. 1986 Relict tree populations in the White Mountains. In C. A. Hall, Jr. and Young (eds.) Natural history of the White-Inyo Range, eastern California and western Nevada, and high altitude Physiology . University of California, White Mountain Research Station Symposium, August 23–25, 1985, Vol. 1, pp. 64–67. University of California, Los Angeles.
Elliott-Fisk, D. L., and A. D. Ryerson. 1988. The dendroecological potential of east-central California. In C. A. Hall, Jr. and V. Doyle-Jones (eds.) Plant biology of eastern California . Natural
History of the White-Inyo Range, symposium vol. 2, pp. 212–222. University of California, Los Angeles.
Ferguson, C. W. 1968. Bristlecone Pine: Science and esthetics. Science 159(3817):839–846.
Ferguson, C. W. 1970. Dendrochronology of Bristlecone Pine, Pinus aristata : Establishment of a 7, 484-year chronology in the White Mountains of eastern central California, U.S.A. In I. V. Olsson (ed.). Radiocarbon variations and absolute chronology , Nobel symposium 12, pp. 237–259. John Wiley & Sons, New York.
Ferguson, C. W., and D. A. Graybill. 1983. Dendrochronology of Bristlecone Pine: A progress report. Radiocarbon 25:287–288.
Haller, J. R. 1962. Variation and hybridization in Ponderosa and Jeffrey pines. University of California Publications in Botany 34: 123–166.
Jennings, S. 1988. Late Quaternary vegetation change in the White Mountain region. In C. A. Hall, Jr. and V. Doyle-Jones (eds.). Plant biology of eastern California . Natural History of the White-Inyo Range, symposium vol. 2, pp. 139–147. University of California, Los Angeles.
Jennings, S., D. L. Elliott-Fisk, J. Watkins, and M. Winter. 1988. Dynamics of Juniperus populations in the White Mountains, CA-NV. Association of American Geographers Program and Abstracts , Phoenix, p. 88. Washington, D.C.
LaMarche, V. C., Jr. 1973. Holocene climatic variations referred from treeline fluctuations in the White Mountains, California. Quaternary Research 3:632–660.
Lanner, R. M. 1984. Trees of the Great Basin: A natural history . University of Nevada Press, Reno.
Lanner, R. M., and H. Lanner. 1981. The Piñon Pine, a natural and cultural history . University of Nevada Press, Reno.
Lloyd, R. M., and R. S. Mitchell. 1973. A flora of the White Mountains, California and Nevada . University of California Press, Berkeley.
Peterson, A. M. 1986. The distribution and ecology of deciduous trees in the White Mountains, California-Nevada. M.A. thesis, University of California, Davis.
Peterson, A. M., and D. L. Elliott-Fisk. 1988. The distribution and ecology of deciduous trees in the White Mountains. In C. A. Hall, Jr., and V. Doyle-Jones (eds.). Plant biology of eastern California . Natural History of the White-Inyo Range, symposium vol. 2, pp. 59–68. University of California, Los Angeles.
Strain, B. 1964. Physiological and morphological variability of local Quaking Aspen clones. Ph.D. dissertation, University of California, Los Angeles.
Suess, H. E. 1970. Bristlecone Pine calibration of the radiocarbon time scale, 5200 B.C. to the present. In I. U. Olsson, (ed.). Radiocarbon variations and absolute chronology , Nobel symposium 12, pp. 303–311. John Wiley & Sons, New York.
Vasek, F. 1966. The distribution and taxonomy of three western junipers. Brrittonia 18:350–372.
Wright, R. D., and H. A. Mooney. 1965. Substrate-oriented distribution of Bristlecone Pine in the White Mountains of California. American Midland Naturalist 73:257–284.