11—
Breeding Birds
Ned K. Johnson and Carla Cicero
Illustrated by N. John Schmitt and Gene M. Christman
A rich variety of birds nest in the White-Inyo Range, including a number of species not widely distributed elsewhere in California. In this chapter, our chief objective is to introduce the reader to the most frequently seen nesting species and to supplement this list with comments on life history, habitats, and distribution. For reasons of space, we emphasize the montane species and exclude from treatment a host of other, equally interesting kinds of birds that breed in the lowland habitats of the region, for example, Owens Valley, Deep Springs Valley, and Fish Lake Valley. Furthermore, we pass over species known solely in the region as transients, winter visitants, or vagrants. The list of such species is long; both Oasis and Deep Springs Ranch, on the east side of the mountains, are popular birding localities where many unusual distributional records have been gathered in recent years. For an up-to-date summary of the status in the region of all excluded species, we refer the reader to Garrett and Dunn (1981). Johnson and Cicero (1986) have recently summarized records of all species of birds thought to breed in the White-Inyo mountains. Their analysis provided the basis for a quantitative comparison of the boreal avifaunas of the two ranges with those of several nearby mountain systems. Common names, scientific names, and the sequence of species follow the style of the American Ornithologists' Union (1983).
In each of the following species accounts, our goal is to provide an accurate statement on the relative abundance, seasonal status, local distribution, and known elevational range of each form. Descriptions of relative abundance (e.g., "common," "fairly common," "uncommon") are based on the definitions and philosophy offered by Grinnell and Miller (1944). Names of ecologic formations (capitalized) follow Miller (1951). Elevations were determined from specimen labels and field notes, and through direct measurement with an altimeter. Accurate determination of elevational range in this region is especially important because some of the highest known records for certain species are from the White Mountains. The data on lengths and weights are offered to allow gross estimates of body size. These estimates assist in field identification and enable rough comparisons of species. We hasten to note that the information on length is too imprecise to serve any other useful purpose. Figures on lengths and weights are averages of at least five individuals of each sex from study specimens in the Museum of Vertebrate Zoology, University of California, Berkeley (MVZ). Where possible, specimens from Mono County or Inyo County were used for these size data. Measurements in the metric system have been converted to English units; the former were taken directly from specimens and therefore are more accurate.
Each species account contains information on habitat occurrence, foraging and nesting habits, voice and other useful identifying features of behavior, and, occasionally, unusual characteristics of life history or ecology. Much of this information is derived from our own annual field experience in the Great Basin and eastern Sierra Nevada, which for the first author dates more than 30 years and includes many visits to the White-Inyo mountain region. For some species, however, we have drawn details from the literature cited at the end of each account. These citations also serve to introduce the serious student of birds to other sources of information for each species.
For general information on distribution, the notes and collections in MVZ were particularly useful. These were obtained by field parties that visited the White Mountains in 1917 and 1955. The notes of Joseph G. Hall, Ward C. Russell, and Francis S. L. Williamson were especially helpful. In addition, Ward C. Russell (pers. comm. ) provided information on the habitat of Blue Grouse in the White Mountains. We sincerely thank these individuals for the detailed permanent record they left on the status of the avifauna 30 years ago.
We do not emphasize field identification in the accounts because such information is now readily available in several excellent, new pocket guides. Thus, the beginner will want to carry a guide to the identification of North American birds, along with this book, when exploring these fascinating mountains.
Cathartidae (New World Vultures)
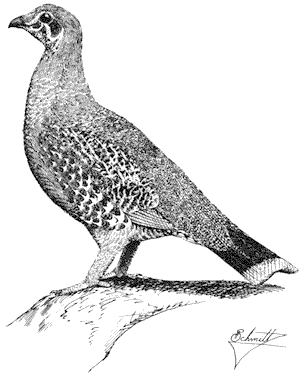
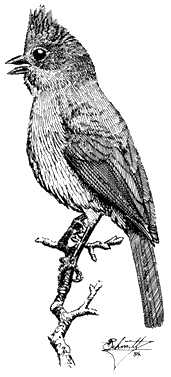
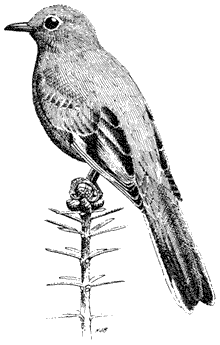
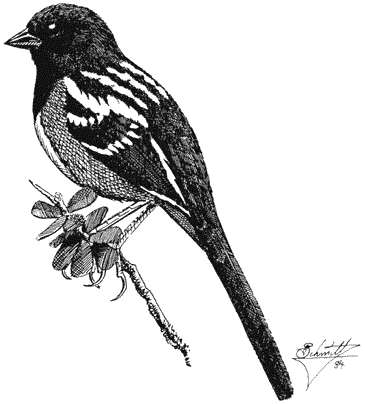
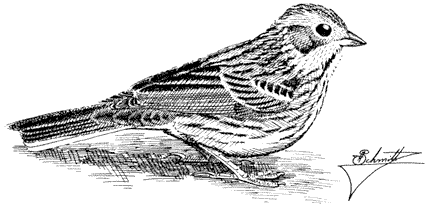
Turkey Vulture,Cathartes aura. (Fig. 11.1) Male length 25 1/2 in (65 cm), female length 27 in (68 cm); male weight 3 1/2 lb (1,559 g), female weight 3 2/3 lb (1,660 g). Uncommon summer resident in the White-Inyo Range; occurs at all elevations.
Turkey Vultures scavenge over open woods, brush, and grassland in varied terrain. Ranches and farming country are also visited. Although most common over valleys and foothills, they occasionally appear at higher elevations. The species is easily recognized by its very small head and broad wings held in a dihedral while soaring. Turkey Vultures forage singly, in pairs, or in loose groups. Odors wafting from carrion are picked up by their keen sense of smell, a rare trait in birds. Because vultures depend largely on thermals for uplift, they are less active on windless days or during stormy weather. This species generally roosts in two separate sites; the main one is used during the night. The second roost, used at dawn and dusk, serves mainly for sunning and preening. These bulky birds prefer to perch in open-branching live or dead trees, on poles, or even on the ground. Such perches enable easy takeoffs and landings. Nest sites are sheltered pockets in cliff faces or among large rocks on steep, brushy slopes. References: D. Davis (1979), Rea (1983).
Accipitridae (Hawks and Eagles)
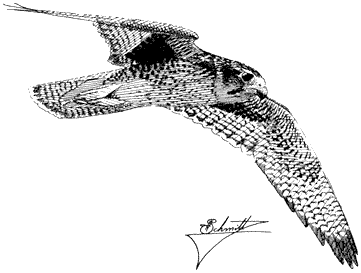
Cooper's Hawk,Accipiter cooperi. (Fig. 11.2) Male length 15 1/2 in (39 cm), female length 17 1/2 in (45 cm); male weight 10 1/3 oz (291 g), female weight 16 1/2 oz (466
Figure 11.1
Turkey Vulture.
Figure 11.2
Cooper's Hawk.
g). Scarce permanent resident in the White Mountains from the valleys to 9,500 ft (2,896 m).
This hawk prefers to nest in aspens, willows, and cottonwoods in canyon bottoms. Foraging takes them over adjacent slopes grown to pines, broken woodland, and brush. A skilled hunter, the Cooper's Hawk maneuvers through openings in foliage, around trees, and over clearings in search of small birds and mammals. Reptiles, amphibians, and large insects are taken infrequently. Like other accipiters, it actively pursues its prey while using vegetation to screen its approach. It may also dash toward prey from a concealed perch in dense foliage. Prey are commonly seized near the ground, in shrubs, or in the canopy of low cover. The hawk usually returns with its kill to feed within 100 ft (30 m) of the nest tree. References: Snyder and Wiley (1976), Reynolds and Meslow (1984).
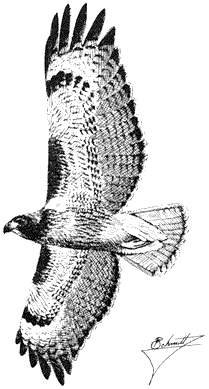
Red-tailed Hawk,Buteo jamaicensis. (Fig. 11.3) Male length 20 1/2 in (52 cm), female length 23 in (58 cm); male weight 2 lb (910 g), female weight 2 2/3 lb (1,223 g). Common permanent resident in the White-Inyo Range, at all elevations up to 10,500 ft (3,200 m).
Figure 11.3
Red-tailed Hawk.
Red-tailed Hawks are one of the most widespread species in the region, occurring throughout a variety of habitats. They hunt for small and medium-sized mammals over broken woodland, brushy slopes, and grassland. Invertebrates, birds, reptiles, and amphibians are taken less commonly. Red-tailed Hawks search for terrestrial prey while soaring high above the ground or while perching quietly in a tree or on a pole. They attack with long dives or sudden plunges. The bulky stick nest is built on a cliff shelf or in a tree and, once abandoned, may serve importantly as a nesting site for large owls. During the breeding season, male Red-tailed Hawks swoop and ascend in exaggerated flight while carrying a snake or other conspicuous prey item in their talons. This courtship display may signify to the female that he is a competent hunter. References: Fitch, Swenson, and Tillotson (1946); Weathers (1983).
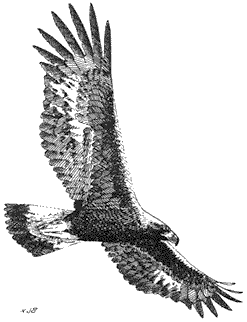
Golden Eagle,Aquila chrysaetos. (Fig. 11.4) Male length 32 in (81 cm), female length 34 in (87 cm); male weight 7 1/4 lb (3,293 g), female weight 8 7/8 lb (4,030 g). Uncommon resident in the White-Inyo Range, from the valley floors to the summits of the highest peaks.
Golden Eagles soar throughout these mountains over all available habitats, including brushland, Pinyon-juniper Woodland, Subalpine Forest, riparian cover in canyon bottoms, and alpine steppes. Open country is preferred for foraging. Mammals are taken primarily, but birds and some snakes may also be eaten. The composition
Figure 11.4
Golden Eagle.
of the diet depends on prey availability, but all animals ranging in size from mice to fawns and songbirds to grouse are fair game. This species is notorious for killing domestic livestock, especially lambs, but such events occur uncommonly. Golden Eagles hunt either from a perch or from the air while near the ground or soaring at great heights. The large stick nest is built on an inaccessible cliff ledge or in tall trees. Early in the breeding season, pairs of eagles conduct elaborate aerial courtship displays that involve mutual dives and other acrobatics. References: Brown and Amadon (1968), Olendorff (1975).
Falconidae (Falcons)
American Kestrel,Falco sparverius. (Fig. 11.5) Male length 10 in (26 cm), female length 10 1/2 in (27 cm); male weight 3 3/8 oz (97 g), female weight 4 1/8 oz (116 g). Uncommon permanent resident in the White-Inyo Range at all elevations.
This species forages in open terrain where vegetation is sparse and low-growing, and where suitable perch sites are available; meadows, unforested slopes, sagebrush flats, and rocky outcrops are frequented. The American Kestrel is a generalized
Figure 11.5
American Kestrel
predator of invertebrates and small vertebrates. Grasshoppers, crickets, and other insects make up the bulk of their diet, but reptiles, mammals, and birds may also be taken. This small falcon typically searches for and attacks its prey from an exposed perch at a moderate height, striking on or near the ground surface. On windy days, however, the birds may hunt by hovering over the ground, especially where perches are sparse or lacking. American Kestrels also "hawk" insects on the wing or, uncommonly, forage directly on the ground for nonflying invertebrates. This species nests commonly in tree cavities, especially in old Northern Flicker holes, and rarely in earth banks or cliff crevices. Being highly maneuverable, American Kestrels harass larger birds of prey and Common Ravens by long stoops and swift ascents. Reference: Balgooyen (1976).
Prairie Falcon,Falco mexicanus. (Fig. 11.6) Male length 15 1/2 in (39 cm), female length 18 in (46 cm); male weight 1 1/8 lb (519 g), female weight 1 7/8 lb (849 g). Scarce resident in the White-Inyo Range at all elevations.
An arid-adapted species, the Prairie Falcon avoids heavily wooded areas. Open rocky canyons and ridgetops grown to Singleleaf Pinyon, sagebrush flats and slopes, alpine scrub, and grassland are preferred. There it searches for small mammals, birds, and invertebrates such as grasshoppers and crickets. Horned Larks are a common food item in open terrain. Prairie Falcons are swift and highly maneuverable in flight, and they deftly take prey from either the air or the ground. They also hunt while perched on a post or pole. Nests are built on rocky ledges or in cliff cavities, commonly far from water but near suitable foraging habitat. The Prairie Falcon can travel long distances daily away from the nest site in search of food. Reference: Cade (1982).
Figure 11.6
Prairie Falcon.
Phasianidae (Partridge, Grouse, and Quail)
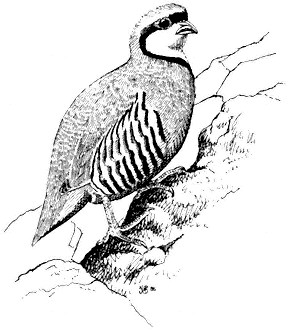
Chukar,Alectoris chukar. (Fig. 11.7) Male length 13 in (33 cm), female length 13 in (33 cm); male weight 21 2/3 oz (614 g), female weight 17 2/3 oz (500 g). Introduced. Common resident from the foothills to at least 10,000 ft (3,050 m) in the Inyo Mountains and 13,400 ft (4,090 m) in the White Mountains. Most numerous between 4,000 and 8,000 ft (1,220 and 2,440 m). Scarce in the northern portion of the region and at very high elevations.
Initially released into California in 1932, this partridge is now widely established in many arid portions of the state. It favors rough and inhospitable canyonsides where scattered clumps of brush and cheatgrass are interspersed with rock outcrops and steep talus slopes. Water from tiny seeps, steadily flowing streams, or tanks must be accessible daily during warm periods and may dictate the local occurrence of birds. Chukars feed primarily on the blades, seeds, and buds of cheatgrass and annuals, although grasshoppers and caterpillars are taken in the spring. Seeds are most important in the summer diet. The birds move continually while feeding, walking up steep boulder faces with agility and skulking across openings. When flushed, the covey explodes into the air and typically heads downhill, alternating rapid wing beats with short glides. Shrill alarm notes accompany the departure of disturbed adults. Foraging adults utter a throaty, resonant chuckling call, which carries for great distances in mountain canyons. Reference: Christensen (1954).
Figure 11.7
Chukar.
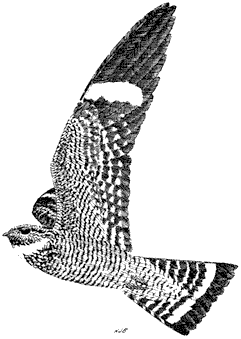
Blue Grouse,Dendragapus obscurus. (Fig. 11.8) Male length 19 in (49 cm), female length 16 in (41 cm); male weight 2 5/8 lb (1,213 g), female weight 1 3/4 lb (803 g). Rare to fairly common, but local, permanent resident in the northern and central White Mountains; recorded between 8,000 and 10,200 ft (2,440 and 3,110 m) elevation. Numbers fluctuate annually. No known records from the Inyo Mountains.
Blue Grouse occur primarily on the east side of the White Mountains, inhabiting upper stream canyons lined with willow and aspen, as well as open Limber Pine forest on adjacent north- and northeast-facing slopes. The diet is well known for populations in the Sierra Nevada and other regions but has been poorly documented in the White Mountains. Spring and summer food in the Sierra Nevada reportedly consists of buds, twigs, catkins, and conifer needles, with fir needles becoming predominant during fall and winter. Because no native firs occur in the White Mountains, it is uncertain what the local grouse eat during those months. However, Limber Pine needles could be an important food. The species supplements its diet with willow leaves in the summer. Blue Grouse leave piles of characteristic droppings on the ground. Each dropping, approximately
Figure 11.8
Blue Grouse.
1 in (2.5 cm) long and 1/4 in (0.6 cm) in diameter, is slightly curved and composed of tightly stacked, coarse plant material that shears neatly into individual cylinders. Males "boom" from trees during the breeding season, amplifying these hoots by expelling air held in vivid orange neck sacs. Reference: Johnsgard (1973).
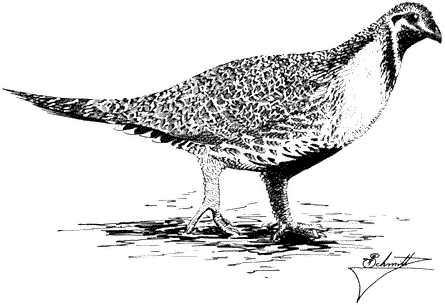
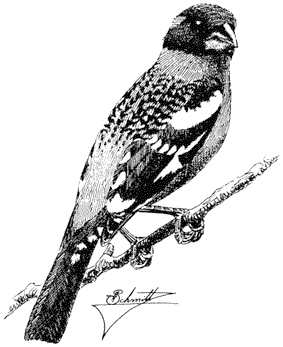
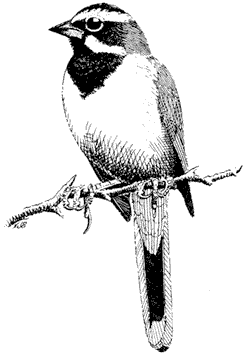
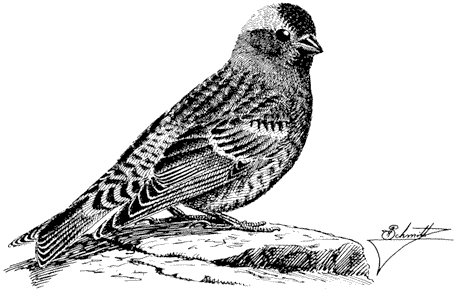
Sage Grouse,Centrocercus urophasianus . (Fig. 11.9) Male length 25 in (63 cm), female length 19 in (48 cm); male weight 4 1/4 lb (1,954 g), female weight 2 3/8 lb (1,085 g). Scarce summer resident in the White Mountains between 8,500 and 12,000 ft (2,590 and 3,660 m) elevation, especially in alpine sagebrush north of White Mountain Peak.
Sage Grouse are strictly tied to the sagebrush association, especially on flat or gently rolling terrain. Sage leaves constitute the main diet of adult birds, although herbaceous legumes and grasses are also eaten; chicks feed primarily on insects. The nest is usually well hidden underneath a sage bush. Prior to breeding, males congregate in large arenas or "leks" subdivided into individual territories. Here they conduct elaborate courtship displays for a group of females. The more dominant males typically occupy central territories in the lek and perform most of the matings. After courtship, the two sexes separate until the fall, when flocks are formed. The range and abundance of the Sage Grouse in California have declined dramatically during this century as a result of the devastation of sagebrush habitat by livestock and agriculture. Reference: Wiley (1973).
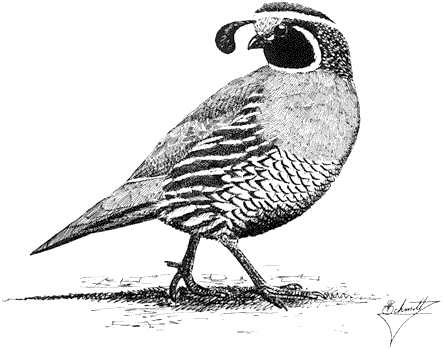
California Quail,Callipepla californica. (Fig. 11.10) Male length 8 1/2 in (22 cm), female length 9 in (23 cm); male weight 5 5/8 oz (159 g), female weight 5 1/2 oz (155 g). Relatively common resident in the White-Inyo Range; occurs between 3,300 and 8,400 ft (1,010 and 2,560 m), although scarce at higher elevations.
This species occurs in localized areas of favorable habitat near streams and springs. Willows and other riparian vegetation, as well as sagebrush and Pinyon-juniper Woodland on adjacent slopes, are frequented. The quail forage mainly in the drier habitats but prefer to nest and roost under the concealing thicket cover. Seeds of Rabbitbrush and sagebrush make up the bulk of their diet in the Great Basin, although in other regions legumes and annuals are a more important seed source. Grass or legume leaves and the fruits, buds, and catkins of brush and juniper are also eaten. In addition, adults take small quantities of animal food, especially during the spring and summer; chicks subsist mainly on such food for the first month. Nests are typically placed on the ground in tall, dense cover of herbaceous vegetation. The California Quail is highly gregarious except during the breeding season. In the fall and winter, coveys forage together in alleyways through brush or alongside roads and other corridors. The social structure of coveys is strictly organized. Reference: Leopold (1977).
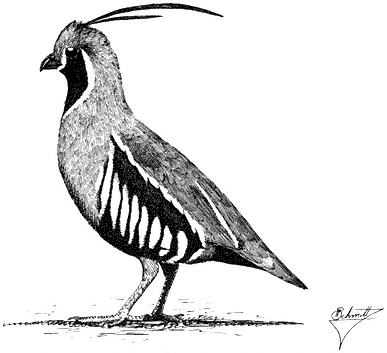
Mountain Quail,Oreortyx pictus. (Fig. 11.11) Male length 10 in (25.5 cm), female length 9 1/2 in (24 cm); male weight 8 1/2 oz (242 g), female weight 7 5/8 oz (218 g). Scarce permanent resident in the White-Inyo Range; current status unclear.
Figure 11.9
Sage Grouse.
Figure 11.10
California Quail.
Figure 11.11
Mountain Quail.
Mountain Quail occur locally in the region on slopes and in canyon bottoms where dense brush is interspersed with open woodland. Sagebrush, Rabbitbrush, and currant within either broken Pinyon-jumper Woodland or Mountain Mahogany are favored. Water is required on a daily basis. Seeds of various perennial plants are taken year-round, with leaves and some fruits, flowers, and insects supplementing the diet. Young chicks subsist primarily on insects. Nests are well concealed under fallen branches, in tall weeds and dense shrubs, or near large, shaded rocks surrounded by thick vegetation. Like other species of quail, Mountain Quail spend the year in coveys except when breeding. Pairs form within coveys while these social groups are intact. In spring, males give a Far-reaching, single inflected whistle that resembles the call of the Northern Pygmy-Owl. Males call frequently in the morning during the early breeding season. This species is highly secretive and prefers to remain hidden in dense brushy cover. In other regions, especially where the birds summer at high elevations, postbreeding coveys are thought to migrate downslope on foot into snow-free, warmer environments. References: Gutiérrez (1980), Miller and Stebbins (1964).
Columbidae (Pigeons and Doves)
Mourning Dove,Zenaida macroura. (Plate 11.1) Male length 11 in (28 cm), female length 10 1/2 in (26.5 cm); male weight 4 1/4 oz (122 g), female weight 4 oz
(113 g). Fairly common summer (permanent?) resident in the White-Inyo Range; occurs up to approximately 10,000 ft (3,050 m).
This species inhabits a variety of habitats, including pinyon woodland, sagebrush, riparian thickets, and meadows. The birds travel long distances daily in search of both food and water. Mourning Doves feed primarily in open areas such as meadows and grassland, where they can find an ample supply of seeds from herbaceous plants. Grain can also constitute an important part of the diet; this is taken mainly in cultivated fields at lower elevations. Tiny pebbles and gravel are also consumed to help grind seeds in the muscular stomach. Nesting habitat requirements are flexible. The preferred nesting substrate consists of a horizontal branch in a tree or shrub, but the flimsy nest may also be built on the ground if woody vegetation is lacking. In addition, Mourning Doves commonly use abandoned nests, either of their own species or of others. Strongly monogamous, Mourning Doves are commonly seen in pairs. This species is easily detected by its characteristic call, a mournful awoo-coo-coo-coo . Reference: Johnsgard (1975).
Strigidae (Typical Owls)
Western Screech-Owl,Otus kennicottii. (Fig. 11.12) Male length 8 1/2 in (22 cm), female length 9 in (22.5 cm); male weight 4 3/4 oz (133 g), female weight 5 1/2 oz (155 g). Uncommon permanent resident in the White-Inyo Range; recorded from 7,200 to 8,200 ft (2,200 to 2,500 m) elevation.
Although this species is locally common in old-growth cottonwoods and tree willows in Owens Valley, only small numbers occur in the White-Inyo mountains. There they prefer the warmer portions of the Singleleaf Pinyon belt. Western Screech-Owls dwell in the lower stretches of canyons in mature trees on gently sloping ground. Natural or woodpecker-excavated cavities in large pinyon trunks are used for roosting and nesting. Foraging takes these owls into more open habitats, but at least some scattered pinyons or junipers seem essential for the presence of this species. Western Screech-Owls feed on a variety of insects, including grasshoppers, crickets, beetles, moths, caterpillars, and spiders. Vertebrates are occasionally eaten, especially during the winter months. The bouncing-ball song of the local subspecies (O. k. inyoensis ) is shorter and composed of fewer notes than the vocalizations of related forms. Like all small owls, the Western Screech-Owl has suffered from fuel-wood cutting in the Singleleaf Pinyon Woodland and from the removal of riparian trees generally. Numbers nearly everywhere have been declining over the past several decades. Reference: Miller and Stebbins (1964).
Great Horned Owl,Bubo virginianus. (Fig. 11.13) Male length 18 in (45.75 cm), female length 19 1/2 in (49.75 cm); male weight 2 lb (914 g), female weight 2 1/2 lb (1,142 g). Fairly common permanent resident in the White-Inyo Range; occurs at all elevations up to approximately 9,500 ft (2,896 m).
The Great Horned Owl is widespread through the region in a variety of habitats. Mammals of small to medium size predominate in their diet, but birds such as quail,
Figure 11.12
Western Screech-Owl.
woodpeckers, and passerines are also taken. To a lesser extent, the species may eat reptiles or amphibians, large insects (e.g., crickets, beetles), and carrion found along highways. Great Horned Owls are most active at night, but they can be seen abroad during the day under overcast conditions. However, most days are spent roosting in large pinyons, in other conifers or aspens, or in cavities in canyon walls. Stick nests abandoned by other raptors, either on cliff shelves or in trees, serve as the primary nesting sites. This species breeds in late winter or early spring. Toward the onset of the nesting season and during the breeding period, both sexes emit deep, resonant hoots. These hoots are deeper pitched in males despite their smaller size relative to females. Great Horned Owls are generally shy and difficult to approach, even at night. References: Burton (1973), Fitch (1947).
Long-eared Owl,Asio otus. (Plate 11.2) Male length 13 in (33.25 cm), female length 13 in (33.5 cm) male weight 8 5/8 oz (245 g), female weight 97/8 oz (279 g). Uncommon permanent resident throughout the White-Inyo region, occurring up to 9,500 ft (2,900 m).
Although in this region the Long-eared Owl favors dense pinyon forest, it also frequents stands of cottonwoods, tree willows, aspens, and Mountain Mahogany. At night, the owls hunt through woodland and along the edges of clearings in search of prey, which consists primarily of small mammals. These owls also feed occasionally on
Figure 11.13
Great Horned Owl.
small birds and, more rarely, on reptiles or amphibians. During the day, the species roosts in dense clumps of Singleleaf Pinyon, Mountain Mahogany, cottonwoods, or aspens. Long-eared Owls are generally solitary except during the winter, when they form loose roosting aggregations in riparian thickets in the foothills and lowlands. Breeding takes place in abandoned nests of other large birds, particularly Red-tailed Hawks, Common Ravens, and, in the lower valleys, Black-billed Magpies. The Long-eared Owl is shy and difficult to attract by means of imitated calls. References: Grinnell and Storer (1924), Marti (1976).
Northern Saw-whet Owl,Aegolius acadicus. (Fig. 11.14) Male length 7 in (17.75 cm), female length 7 1/2 in (18.75 cm); male weight 2 5/8 oz (75 g), female weight 3 1/4 oz (91 g). Uncommon resident in the White Mountains. Winter status is uncertain; at that time, individuals from other regions may augment the local population. Recorded up to approximately 9,000 ft (2,740 m).
This owl occurs in dense riparian thickets and on remote canyon slopes far from water. They forage at the edges of meadows or clearings, particularly among open
Figure 11.14
Northern Saw-whet Owl.
stands of small to medium-sized conifers. There, mice and an occasional bird are taken. Nest sites consist of abandoned woodpecker holes or natural cavities at medium heights in trees. During the day, individuals roost in the dense foliage of small trees, commonly within 10 ft (3.0 m) of the ground. Mated males call infrequently. In contrast, unmated Northern Saw-whet Owls call steadily in the late winter and spring. These calls carry up to 0.5 mi (0.8 km), and thus the owls can easily be detected. They are difficult to locate, however, because the steady whistles quaver in pitch and fade in and out in intensity. Once a Northern Saw-whet Owl is found, either during the day or at night, commonly it can be closely approached. Reference: Earhart and Johnson (1970).
Caprimulgidae (Nightjars)
Common Nighthawk,Chordeiles minor. (Fig. 11.15) Male length 9 in (22.75 cm), female length 9 in (22.75 cm); male weight 2 1/4 oz (63 g), female weight 2 1/2 oz (72 g). Scarce summer resident in the White Mountains; more common in the northern part of Owens Valley to the west. Recorded from 4,000 to 6,750 ft (1,220 to 2,060 m) elevation.
Figure 11.15
Common Nighthawk.
Common Nighthawks breed in the sagebrush-pinyon-covered alluvial fans on both sides of the White Mountains, and in adjacent ranch country with riparian growth in the valleys. Each evening in June and early July, they perform spectacular courtship dives in which the male plunges toward the ground and recovers suddenly with a loud, muffled vvrrrrr sound. The call commonly delivered during high aerial foraging flights is a nasal peent . Females lay their eggs directly on the ground among gravel, sticks, and other plant debris, either in brushland or in large openings in forest. If disturbed while incubating, she flops awkwardly across the ground with fanned wings and tail, luring the intruder away from the nest site. This species feeds exclusively on insects caught on the wing, commonly over water surfaces. References: Armstrong (1965), Caccamise (1974).
Common Poorwill,Phalaenoptilus nuttalli. (Fig. 11.16) Male length 7 1/2 in (18.75 cm), female length 7 in (18 cm); male weight 1 3/4 oz (48 g), female weight 1 3/4 oz (48 g). Fairly common summer resident in the White-Inyo Range; winter status uncertain. Recorded up to 9,500 ft (2,900 m).
Poorwills prefer warm, open terrain with scattered trees or brush where there is an abundance of exposed sand, gravel, and rocks. This habitat is common in both the sagebrush and Pinyon-juniper zones of the region. Mountain Mahogany is also
Figure 11.16
Common Poorwill.
visited, especially when mixed with pinyon. Poorwills commonly rest on gravel road beds, where in the evening their reflected eye shine can be seen in car headlights or with a flashlight. They feed at dusk and dawn, when they take short flights from the ground in search of flying insects such as moths. The whistled poor-will call is followed by a brief dup note, delivered at a somewhat lower pitch and audible only at very close range. This species can become torpid during cold weather; such dormant individuals hide in protected crannies in rocks. References: Jaeger (1948), Marshall (1957).
Apodidae (Swifts)
White-throated Swift,Aeronautes saxatalis. (Plate 11.3) Male length 5 3/4 in (14.5 cm), female length 5 1/2 in (14 cm); male weight 1 1/10 oz (31 g), female weight 1 oz (29 g). Locally common summer resident in the White-Inyo Range, between 6,000 and 10,300 ft (1,830 and 3,140 m).
White-throated Swifts inhabit deep, rocky canyons that offer steep cliff faces for nesting and roosting. Foraging birds fly over a wide variety of habitats where insects are taken on the wing. The daily cruising range of the White-throated Swift exceeds that of most other species of birds in the region. Nests of twigs are cemented together and attached to cliff crevices by means of saliva. Granitic rock is apparently preferred as a nest substrate because it provides for easier nest attachment. White-throated Swifts commonly nest and roost colonially. They forage singly or in groups, darting swiftly at heights from near the treetops to high overhead. A series of excited shrill notes is commonly uttered in flight. During cold snaps, this species becomes dormant and Individuals remain together in rocky crevices until warmer temperatures resume. Reference: Weathers (1983).
Trochilidae (Hummingbirds)
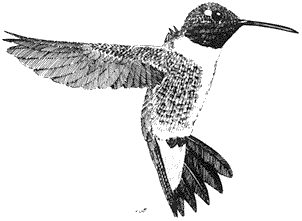
Broad-tailed Hummingbird,Selasphorus platycercus. (Fig. 11.17) Male length 3 1/2 in (9 cm), female length 3 3/4 in (9.5 cm); male weight 1/10 oz (3.8 g), female weight 1/8 oz (4.5 g). Relatively common summer resident locally in the White-Inyo Range; occurs between approximately 6,000 and 9,000 ft (1,829 and 2,743 m) elevation.
This hummingbird inhabits the pinyon-juniper-Mountain Mahogany belt, where it prefers riparian thickets along wet or dry watercourses or near springs. There it feeds primarily on nectar from a variety of plants. Insects and spiders on flowers, near sapsucker borings, or in the air are also taken. The small, cup-shaped nests are typically placed on low horizontal branches of streamside trees. Like most hummingbirds, the Broad-tailed Hummingbird constructs its nest with spider webs, fine leaves, shreds of bark, and cottony material. Prior to breeding, one or several males display with U-shaped dives to a perched female from 20 ft (6 m) or more above the ground. During this display, sharp clicking notes are commonly uttered. In ordinary flight, males produce with specialized wing feathers a characteristic cricketlike trill. Reference: Johnsgard (1983).
Picidae (Woodpeckers)
Red-naped Sapsucker,Sphyrapicus nuchalis. (Plate 11.4) Male length 7 in (18 cm), female length 7 1/2 in (19 cm); male weight 1 1/2 oz (44 g), female weight 1 1/2 oz (43 g). Locally fairly common summer resident in the White Mountains, between approximately 9,000 and 9,600 ft (2,740 and 2,930 m).
Figure 11.17
Broad-tailed Hummingbird.
This woodpecker lives in canyons on the east side of the range, in streamside growth of aspens and willows. Mountain Mahogany and Bristlecone Pine are visited to a lesser extent. The adults drill linear rows of rectangular holes in the trunks and limbs of these trees and dip up the exuding sap with their brushy-tipped tongues. Such borings commonly lure insects, which, in turn, attract other species of birds. The Red-naped Sapsucker usually excavates its nest cavity in a large dying aspen, where decaying wood provides for easy digging. As the nestlings mature, their loud begging cries can be heard up to 100 yd (91 m) from the nest. In response to these cries, the parents arrive independently every few minutes carrying billsfull of large Timber Ants and other insects. Other cavity-nesting species of birds commonly use abandoned sapsucker nests for raising their own young. Consequently, sapsuckers and other woodpeckers play an important role as primary cavity excavators for a significant group of secondary hole-nesting species. For decades this form was combined with the Yellow-bellied Sapsucker (S. varius ) and the Red-breasted Sapsucker (S. ruber ) as a single species. References: Johnson and Johnson (1985), Short (1982).
Hairy Woodpecker,Picoides villosus. (Fig. 11.18) Male length 8 in (20.25 cm), female length 8 1/4 in (21 cm) male weight 2 1/8 oz (60 g), female weight 2 1/5 oz (62 g). Fairly common permanent resident in the White-Inyo Range, between 6,750 and 9,600 ft (2,060 and 2,930 m).
This species is perhaps the most generalized of all North American woodpeckers. In the White-Inyo mountain region, it is at home in such diverse habitats as Pinyon-juniper Woodland, aspen-willow associations, and coniferous forest of Limber and Bristlecone pines. Pinyon is used less commonly toward the south; there the trees must be large and in relatively thick groves on cool slopes or in canyons. Nest cavities are excavated in dead trees of any kind. The species forages mostly in dead or dying trees, where adult and larval insects are obtained by digging into decaying wood. In these vast mountains, Hairy Woodpeckers are sparsely distributed, and usually only one or two pairs may be encountered in a single day. Reference: Short (1971).
Northern Flicker,Colaptes auratus. (Plate 11.1) Male length 11 1/4 in (28.5 cm), female length 11 in (28.25 cm); male weight 5 3/8 oz (151 g), female weight 5 oz (141 g). Relatively common permanent resident in the White-Inyo Range, up to 10,350 ft (3,155 m).
Northern Flickers breed throughout the region in all types of woodland habitats, preferably where there are extensive tracts of good-sized trees near openings. Large, dying pinyons, aspens, or pines are all used for excavating nest cavities. Similar flexibility is shown in foraging behavior. Flickers search for ants, caterpillars, grubs, termites, beetles, and other terrestrial or foliage insects in live and dead tree trunks, leaf litter, bare soil, and ant hills. Because of its unusual terrestrial habits, this woodpecker is likely to be flushed from the ground in meadows, sagebrush flats, or other openings in wooded country. The attractive salmon-red color underneath the wings and tail, clearly seen in flight, is based on red pigments obtained through the diet. Occasional individuals that exhibit yellowish wing or tail feathers have not
Figure 11.18
Hairy Woodpecker.
eaten sufficient pigment-containing food prior to and during feather growth. Reference: Short (1965).
Tyrannidae (Tyrant Flycatchers)
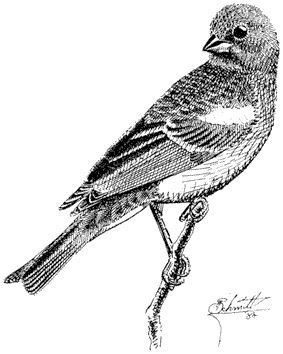
Olive-sided Flycatcher,Contopus borealis. (Fig. 11.19) Male length 6 1/2 in (16.5 cm), female length 6 1/2 in (16.5 cm); male weight 1 1/8 oz (32.6 g), female weight 1 1/8 oz (32.6 g). Fairly common summer resident locally in the White Mountains. Recorded from 8,200 to 10,500 ft (2,500 to 3,200 m).
This large flycatcher breeds only in the higher coniferous forests of Limber Pine, Bristlecone Pine, and Lodgepole Pine. It prefers open stands of mature timber, particularly where high perches and prominent song posts are available. Such perches are in the tops of tall dead and dying conifers. Olive-sided Flycatchers have the highest foraging beat of all North American members of their family, the Tyrannidae.
Figure 11.19
Olive-sided Flycatcher.
They feed mainly on large, flying insects. In contrast, nests are typically built at lower heights, on the outer live branches of Limber or Bristlecone pines. Males may sing continuously, but pairs are usually widely spaced on extensive territories. The easily recognizable song, quick-three-beers , accented on the second note, can be heard from up to 0.5 mi (0.8 km) away. Late migrants passing through the valleys and foothill canyons during late May and early June may also sing occasionally. Reference: Grinnell and Storer (1924).
Western Wood-Pewee,Contopus sordidulus . (Fig. 11.20) Male length 5 3/4 in (14.5 cm), female length 5 1/2 in (14 cm); male weight 1/2 oz (13 g), female weight 1/2 oz (13 g). Common summer resident in the White Mountains, between 7,400 and 9,000 ft (2,260 and 2,740 m) elevation.
This flycatcher is most common in woodland and coniferous forest along streams. Because it prefers to forage from tall trees of open branchwork, dense pinyon and Mountain Mahogany are shunned. Large Quaking Aspen, Bristlecone Pine, and Limber Pine are frequented most commonly. Western Wood-Pewees seek flying insects from moderate heights above the ground; their foraging beat is therefore between that of the Dusky Flycatcher and that of the Olive-sided Flycatcher, with overlap at both
Figure 11.20
Western Wood-Pewee.
the upper and lower levels. Wood-Pewees migrate well into June. Then they may occur in Pinyon-juniper Woodland, willow thickets, and other nonbreeding habitats. The nasal buzz of the Western Wood-Pewee is commonly given throughout the day, even on sunny summer afternoons when few other species of birds are calling. Reference: Marshall (1957).
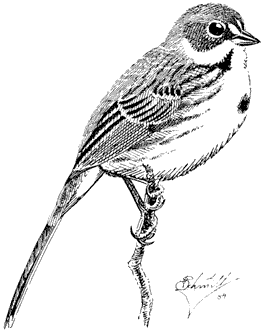
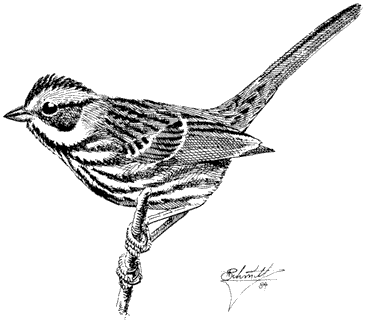
Dusky Flycatcher,Empidonax oberholseri . (Fig. 11.21) Male length 5 1/4 in (13.5 cm), female length 5 in (13 cm); male weight 2/5 oz (11.6 g), female weight 2/5 oz (11.6 g). Common summer resident in the White-Inyo Range, between 8,200 and 10,500 ft (2,500 and 3,200 m).
Although this species avoids Singleleaf Pinyon except occasionally during migration, most other trees as well as willow thickets are used for breeding. In particular, it frequents Mountain Mahogany groves, streamside aspen woodland, and open forests of Bristlecone and Limber pines. Nests are usually built within 5 ft (1.5 m) of the ground in upright twigs of tall, dense shrubs (such as Wild Rose), aspen saplings, and willows. The adults feed near the nest and higher up in the openings between tall trees. The singing and sentry posts are often well above the ground. Dusky Flycatchers feed on miscellaneous flying insects such as small beetles, wasps, flies, and bugs, all of which are snapped from the air. Reference: Johnson (1963).
Gray Flycatcher,Empidonax wrightii . (Fig. 11.22) Male length 5 1/4 in (13.25 cm), female length 5 in (13 cm); male weight 2/5 oz (11.6 g), female weight 2/5 oz
Figure 11.21
Dusky Flycatcher.
(11.6 g). Common summer resident in the White-Inyo Range from 7,200 to 10,500 ft (2,200 to 3,200 m).
Gray Flycatchers are most numerous in the vast tracts of Singleleaf Pinyon growing on either flat or sloping terrain. They also breed sparingly in sunny stands of Limber and Bristlecone pines up to 10,500 ft (3,200 m) elevation in the Inyo Mountains. This is the highest known nesting station for the species. In other regions, such as near the south end of Mono Lake, they breed in stands of tall sagebrush and Bitterbrush. Such growth does not seem to be used extensively in the White-Inyo mountain area. Nesting pairs prefer medium to large Singleleaf Pinyons near openings with relatively sparse brush. The nest straddles a horizontal branch and is commonly placed right near the trunk. Males commonly perch on a bare twig near the nest tree, where they are conspicuous by virtue of their silvery white underparts and tail-dipping mannerism. Typical perch sites for singing or foraging are within 12 ft (3.7 m) of the ground. Insects are caught from bare or nearly bare patches of ground between sagebrush, Bitterbrush, lupine, and small Beavertail Cactus. Like its relative, the Dusky Flycatcher, the Gray Flycatcher catches small insects while in flight. Although they maintain separate territories, the two species commonly occur side by side where their preferred habitats meet in these mountains. Reference: Johnson (1966).
Figure 11.22
Gray Flycatcher.
Say's Phoebe,Sayornis saya. (Plate 11.1) Male length 6 3/4 in (17 cm), female length 6 3/4 in (17 cm); male weight 3/4 oz (21.6 g), female weight 7/10 oz (20.4 g). Sparse summer resident in the White-Inyo region, between 5,900 and 9,500 ft (1,800 and 2,900 m).
Say's Phoebes inhabit the pinyon-juniper-sagebrush zone in the region. Although the species is not numerous, nesting pairs can usually be seen in arid canyon mouths where sunny gravel banks and/or human constructs are present. Nests are placed on protected shelves in cliffs or buildings, and the adults forage nearby. Insect food is snapped from the air or the ground as the birds momentarily leave their low perches on bushtops or rocks, or as they hover. Postbreeding adults and independent young are widespread in shrubland or ranch country, where they perch singly on fenceposts, wires, or bushtops. Reference: Weathers (1983).
Ash-throated Flycatcher,Myiarchus cinerascens. (Fig. 11.23) Male length 7 1/2 in (18.75 cm), female length 7 in (18 cm); male weight 1 oz (28.6 g), female weight 9/10 oz (26.4 g). Uncommon summer resident in the White-Inyo Range, from the valley floors to approximately 7,500 ft (2,290 m).
This flycatcher inhabits Pinyon-juniper Woodland and brush on warm, dry slopes, and open riparian growth with at least scattered trees. Because much of the foraging
Figure 11.23
Ash-throated Flycatcher.
activity occurs within 20 ft (6 m) of the ground, low perching sites are essential. Bushtops or exposed dead twigs in a dead or dying tree are used most typically. The Ash-throated Flycatcher captures large wasps, flies, beetles, and bugs by means of aerial sorties. Nests are built of twigs and lined with grass in a natural or woodpecker-excavated cavity in an old Singleleaf Pinyon or juniper. Both parents are usually conspicuous near the nest site. Pairs are widely spaced through the habitat on large territories and announce their presence with a loud, rolling ka-brick or prit-wherr call (accented on the second syllable). Reference: Miller and Stebbins (1964).
Alaudidae (Larks)
Horned Lark, Eremophila alpestris . (Fig. 11.24) Male length 6 1/4 in (16 cm), female length 6 in (15 cm); male weight 1 oz (29.2 g), female weight 9/10 oz (26.5 g). Locally common summer resident in the White-Inyo Range. Occurs in two separate elevational belts: from the valley floors to 8,000 ft (2,440 m), and from 10,000 ft (3,050 m) to the summit of the White Mountains (14,246 ft or 4,340 m).
The Horned Lark nests over the widest elevational span of any bird species in the region. Because it prefers open and flat or gently sloping terrain, its local distribution is sharply divided into two main parts: lower valleys and flats covered with brush, up to 8,000 ft (2,440 m); and rolling upper sagebrush steppes and alpine fell-fields above timberline, from approximately 10,000 ft (3,050 m) to the highest peaks. This species is most at home in the skimpy grass, herbs, and rocks of windswept wastes,
Figure 11.24
Horned Lark.
where all nesting and foraging activities take place. Grass seeds and insects compose the diet. Breeding males announce their territories by spectacular song flights above their nesting areas. Early in the breeding period, two or more males swiftly chase over bushtops and high overhead. After being aloft for several minutes, they plunge from the sky and perch on the ground, on the tops of rocks, or on prominent shrubs. Postbreeding flocks of coalesced family groups or scattered singles wheel over open country and then land in the rocks and grass, each individual vanishing by virtue of its remarkable concealing coloration. References: Behle (1942), Verbeek (1967).
Hirundinidae (Swallows)
Violet-green Swallow,Tachycineta thalassina. (Plate 11.3) Male length 4 3/4 in (12 cm), female length 4 1/2 in (11.5 cm); male weight 1/2 oz (15.6 g), female weight 1/2 oz (15.5 g). Common summer resident in the White-Inyo mountain region, between 6,750 and 10,300 ft (2,060 and 3,140 m).
The Violet-green Swallow, one of the most conspicuous birds in the mountains, can be seen overhead on virtually any day. It is most common near cliffs or rocky outcrops above 7,500 ft (2,290 m), with numbers declining in the warmer areas below. Several pairs may form loose colonies in the vicinity of such cliff sites. Unlike most other swallows, this species build nests in two distinct kinds of places: either in rock crevices or in abandoned woodpecker cavities in aspens and conifers. The adults
commonly perch on dead limbs of pines or aspens near the nest. This species feeds entirely on small insects caught on the wing. Reference: Grinnell and Storer (1924).
Corvidae (Crows and Jays)
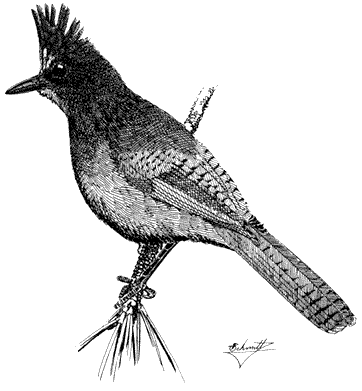
Steller's Jay,Cyanocitta stelleri. (Fig. 11.25) Male length 10 3/4 in (27.5 cm), female length 10 1/2 in (26.5 cm); male weight 3 7/10 oz (105 g), female weight 3 1/2 oz (98 g). Fairly common permanent resident locally in the White-Inyo Range. Recorded between 6,200 and 9,500 ft (1,890 and 2,900 m).
Most records of this jay are from streamside willows and aspens, especially where the latter are mixed in canyon bottoms with large Singleleaf Pinyons or, at higher elevations, with Bristlecone and Limber pines. Diet is exceptionally varied; conifer seeds, fruits, large adult insects, caterpillars, and eggs and young of small birds are all devoured. Steller's Jays are also known to pilfer the seed stores of the Clark's Nutcracker. Nests in the White Mountains have been found in dense willow thickets. The breeding cycle starts in early spring so that stubby-tailed young, attended by
Figure 11.25
Steller's Jay.
parents, can be seen in late May. In other regions, Steller's Jays have been shown to defend group territories, where each pair is dominant to other members of the group on a portion of the total territory. These jays are therefore gregarious year-round. The handsome crest reveals the relative degree of aggressiveness (crest erect) or submissiveness (crest flattened) displayed during interactions with other members of their own species or with potential enemies. Reference: Brown (1964).
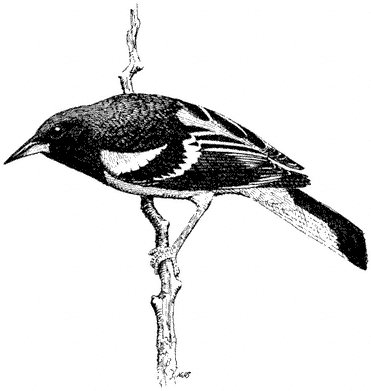
Scrub Jay,Aphelocoma coerulescens. (Fig. 11.26) Male length 11 1/2 in (29.3 cm), female length 10 3/4 in (27.3 cm); male weight 2 4/5 oz (79 g), female weight 2 3/4 oz (77 g). Fairly common permanent resident in the White-Inyo Range, from 6,000 to 9,500 ft (1,830 to 2,900 m).
This species occurs in Pinyon-juniper Woodland on the slopes of the White-Inyo Range. Willows in canyon bottoms and groves of Mountain Mahogany are also visited if the preferred Singleleaf Pinyon is nearby. Brush near pinyon is used sparingly. The Scrub Jay is omnivorous wherever it occurs: seeds, nuts, fruits, large insects, and eggs and nestlings of small birds are all taken when available. Pinyon nuts are a particularly important food in these mountains. Scrub Jays living in pinyon
Figure 11.26
Scrub Jay.
woodlands of southeastern California are grayer and have longer, thinner bills than the oak-chaparral birds west of the Sierra Nevada. Presumably, the drabber coloration in the arid pinyon environment aids in concealment, and the bill shape is adapted for extracting pinyon seeds from cones. Reference: Ritter (1983).
Pinyon Jay,Gymnorhinus cyanocephalus . (Fig. 11.27) Male length 10 1/2 in (26.5 cm), female length 10 in (25 cm); male weight 4 oz (112 g), female weight 3 1/2 oz (97 g). Common permanent resident in the White-Inyo Range; recorded between 7,200 and 10,500 ft (2,195 and 3,200 m) elevation. Annual numbers fluctuate dramatically in any one place in concert with the availability of pinyon seeds.
Pinyon Jays are one of the most characteristic species of the interior Pinyon-juniper Woodland. The loud kraw call, given in chorus from the tops of trees or in flight, usually announces the presence of these conspicuous birds. When foraging, garrulous flocks, commonly numbering into the hundreds, sift through arid woodlands and over adjacent sagebrush country. Pinyon seeds are the major component of their diet and are also important in feeding displays and other courtship rituals. During the fall and early winter, large quantities of the seeds are harvested, transported in the throat, and stored on traditional nesting grounds. Because pinyon seed production is highly sporadic, the foraging range as well as the size of the flock depend on local seed availability. Pinyon Jays also eat some insects, which are taken from open ground by probing or from bark crevices. Pairs nest in loose colonies in large pinyons or junipers in fairly open woodland. Like other jays, the Pinyon Jay is very aggressive and will commonly harass hawks and other threatening birds of prey, especially when the latter are near the nesting area. Reference: Balda and Bateman (1973).
Clark's Nutcracker,Nucifraga columbiana. (Plate 11.2) Male length 11 1/2 in (29.5 cm), female length 11 1/4 in (28.7 cm); male weight 5 oz (139 g), female weight 4 5/8 oz (131 g). Common permanent resident in the White-Inyo Range; recorded from 6,800 to approximately 11,000 ft (2,073 to 3,353 m).
The Clark's Nutcracker is one of the more conspicuous species in the region and is commonly seen or heard overhead in a variety of habitats. The loud, grating kraaa call reveals its presence 0.5 mi (0.8 km) away. According to other studies, this species conducts seasonal altitudinal migrations in the Sierra Nevada, and it may show similar behavior in the White Mountains. Summers are commonly spent in the timber at higher elevations, and the onset of winter initiates a downslope movement into Pinyon-juniper Woodland. Although Clark's Nutcrackers are opportunistic feeders, they subsist mainly on the seeds of various conifers. Whitebark Pine and Jeffrey Pine are the primary trees utilized in the Sierra Nevada, but in the virtual absence of these conifers in the White Mountains, the local populations must eat the seeds of Limber pine and possibly Bristlecone Pine; little work has been done to document this, however. Nutcrackers also commonly visit pinyon in the summer, and when pinyon nuts are plentiful, they may be an important food source. This species is known to store vast seed reserves throughout the year in the Sierra Nevada. A small sac behind the tongue, unique to nutcrackers in the crow family, enables individuals
Figure 11.27
Pinyon Jay.
to carry seeds long distances between sources and storage sites. In addition to conifer seeds, Clark's Nutcrackers also eat insects and spiders, especially during the warmer months. Uncommonly, berries, freshly killed vertebrates (small mammals, nestling birds), and carrion are eaten. Nests are preferably built in fairly small conifers such as junipers. Typically, the birds begin nesting in late winter or early spring, so that by early summer the fully grown young are able to accompany their parents on long feeding flights. References: Tomback (1978a, 1978b).
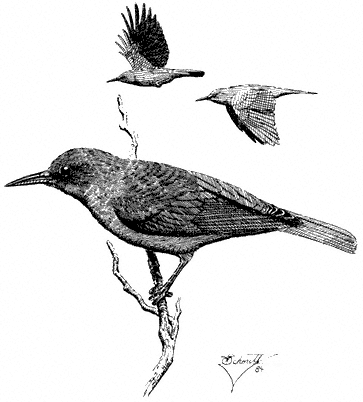
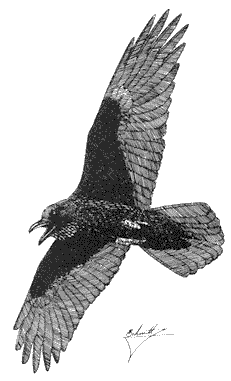
Common Raven,Corvus corax. (Fig. 11.28) Male length 21 3/4 in (55.25 cm), female length 21 1/4 in (54 cm); male weight 2 lb (891 g), female weight 1 7/10 lb (767 g). Relatively common permanent resident at all elevations in the White-Inyo Range.
Although the species is conspicuous, total numbers in the region are probably small. Typically a bird of open country, the species is attracted to carrion of livestock and rabbits, large insects such as grasshoppers, and small mammals. Ravens move long distances daily in search of food. Postbreeding movements bring groups of ravens
Figure 11.28
Common Raven.
to largely barren alpine wastes. Nests are bulky piles of sticks placed on shelves in cliffs; buildings may also be used. The Common Raven is commonly harassed in the air by smaller birds such as American Kestrels and Violet-green Swallows. Our largest perching bird, the Common Raven soars like a hawk near windswept crags and over ridges. Reference: Goodwin (1976).
Paridae (Titmice)
Mountain Chickadee,Parus gambeli. (Plate 11.4) Male length 5 in (12.5 cm), female length 5 in (12.5 cm); male weight 2/5 oz (11.4 g), female weight 3/8 oz (10.7 g). Very common resident in the White-Inyo Range, from 6,750 to 10,500 ft (2,057 to 3,200 m).
This species is most at home in the coniferous forests of Limber Pine, Bristlecone Pine, and Lodgepole Pine occurring above 8,900 ft (2,710 m). However, it also uses lower-elevation habitats of pinyon woodland and streamside thickets of willow-rose-birch, particularly during the nonbreeding season. Nesting activities are concentrated in small cavities in dead conifers or aspen snags. The species forages in the lower foliage layers, where it takes caterpillars and various insects from needle clusters,
twigs, and small branches. When the birds are not breeding, they are gregarious and forage in flocks, either with other chickadees or in mixed groups with nuthatches, creepers, and other small insect-eating species. Mountain Chickadees utter a plaintive series of whistles, teeee-tee-tee , with the first note on a higher pitch and all three or four notes given in a minor key. This vocalization is a common sound in the White Mountains, especially early in the nesting period, and functions in territorial defense. The species also has a variety of buzzy and sputtering calls. References: Dixon (1965), Grinnell and Storer (1924).
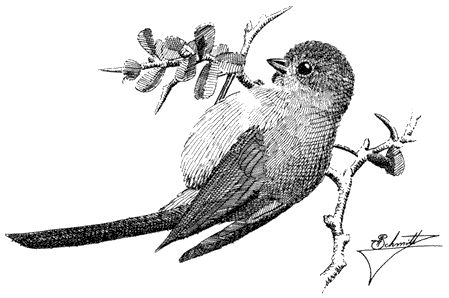
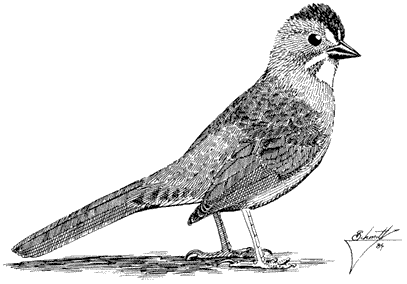
Plain Titmouse,Parus inornatus. (Fig. 11.29) Male length 5 in (13 cm), female length 5 1/4 in (13.25 cm); male weight 5/8 oz (16.3 g), female weight 1/2 oz (15.4 g). Uncommon permanent resident in the White-Inyo Range; occurs from 7,200 to 7,900 ft (2,200 to 2,410 m).
This species is distributed sparsely through the warm, arid portion of mixed Pinyon-juniper Woodland; cool tracts of pure pinyon on northeast slopes are usually avoided. Plain Titmice favor open groves with at least some large, old trees that offer substantial limbs and trunks containing rotted cavities for nest placement. They hop
Figure 11.29
Plain Titmouse.
leisurely on large branches and twigs while searching the foliage for adult insects and caterpillars. Pinyon seeds are pounded open with the stout bill. The species commonly delivers a clear, ringing pee-two, pee-two, pee-two song while foraging. References: Dixon (1949); Johnson, Bryant, and Miller (1948).
Aegithalidae (Long-tailed Tits and Bushtits)
Bushtit,Psaltriparus minimus. (Fig. 11.30) Male length 4 1/3 in (11 cm), female length 4 1/4 in (10.75 cm); male weight 1/5 oz (5.5 g), female weight 1/5 oz (6.1 g). Fairly common resident in the White-Inyo Range, from 6,750 to 8,200 ft (2,060 to 2,500 m).
Despite their small size, Bushtits are conspicuous in the summer as they forage in areas of pinyon-juniper intermixed with sagebrush and Mountain Mahogany. Streamside willow thickets are also inhabited. During the breeding season, in early spring, pairs are generally quiet and fairly shy. By June, however, they join other Bushtits to form tight flocks commonly comprising 20 or more individuals. Such flocks move widely through tree canopies and tracts of tall brush, following lead birds from one foraging site to another while constantly conversing in soft, twittery notes. Small insects and some vegetable matter are eaten as the birds forage among outer leaves and twigs in an acrobatic manner, moving with agility in the fine foliage. The Bushtit nest is an elongated pouch woven from fine plant materials and usually suspended from the end of a pinyon bough. References: Ervin (1977), Marshall (1957).
Figure 11.30
Bushtit.
Sittidae (Nuthatches)
Red-breasted Nuthatch,Sitta canadensis . (Fig. 11.31) Male length 4 1/4 in (10.75 cm), female length 4 in (10.25 cm); male weight 2/5 oz (10.9 g), female weight 3/8 oz (10.3 g). Uncommon summer (permanent?) resident of the White Mountains; recorded from 8,900 to 9,500 ft (2,710 to 2,900 m).
This nuthatch occurs very sparingly and only in Subalpine Forest of Limber and Bristlecone pines. It is the least numerous of the three species of nuthatch that breed in the mountains. Red-breasted Nuthatches feed in the thickly foliaged tops of pines, where they can be extremely difficult to watch. They decoy readily, however, to imitated calls of small owls and will approach to within a few feet if one sits motionless next to a tree trunk. Like other nuthatches, this species excavates its own nest cavity in dead trunks or large limbs by digging vigorously with the bill. References: Anderson (1976), Kilham (1973).
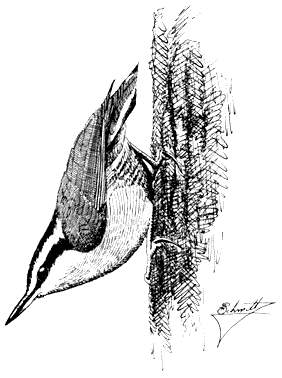
White-breasted Nuthatch,Sitta carolinensis. (Plate 11.2) Male length 5 1/3 in (13.5 in), female length 5 1/4 in (13.25 cm); male weight 5/8 oz (16.6 g), female
Figure 11.31
Red-breasted Nuthatch.
weight 3/5 oz (16.3 g). Relatively common permanent resident in the White-Inyo Range, from 7,200 to 10,500 ft (2,200 to 3,200 m).
This species prefers relatively large Singleleaf Pinyon and Limber pines for foraging but will also use Bristlecone and Lodgepole pines to a lesser extent. An agile climber, the White-breasted Nuthatch ascends, descends, and circles large limbs and trunks in search of insects. Most prey is taken from the bark and from bark fissures. However, they also retrieve insects from deeper inside the wood by woodpeckerlike pounding and digging with their bills. Occasionally, this species drops to the ground to secure fallen items. During the breeding season, pairs of White-breasted nuthatches are widely spaced through the coniferous forest. At other times, they may join other nuthatches and insect-eating species in loose foraging groups. Like the Red-breasted and Pygmy nuthatches, the White-breasted Nuthatch nests in cavities in dead pine or aspen trees. References: Kilham (1972), McEllin (1979).
Pygmy Nuthatch,Sitta pygmaea. (Plate 11.4) Male length 4 in (10.25 cm), female length 4 in (10.25 cm); male weight 4/10 oz (10.1 g), female weight 3/8 oz (10.8 g). Relatively common resident in the White and northern Inyo mountains, between 9,500 and 10,500 ft (2,900 and 3,200 m).
The Pygmy Nuthatch typically inhabits Ponderosa Pines and other Yellow Pine associations throughout most of its range. In the White Mountains, in contrast, where Yellow Pines are virtually absent, it occurs almost exclusively in Bristlecone Pines. Aspen stands may be surveyed infrequently for possible nest sites, but the Bristlecone Pines largely fulfill requirements for food, nesting, and roosting. Pygmy Nuthatches feed on both plant and animal matter. Through the year, the bulk of their diet is composed of pine seeds, but insects predominate during the warmer months. Unlike the Red-breasted and White-breasted nuthatches, these small birds prefer to forage at upper levels in the pines, on the smallest, outermost twigs and needle clusters; trunks and large limbs are usually avoided. This species commonly forages in groups. Occasional individuals dart out after flying insects. The Pygmy Nuthatch uses its bill to dislodge insects from crevices in bark and also to wedge pine seeds into fissures before cracking them open. Some seeds are also stored in such crevices. Nest and roost sites are in tree cavities that they or other species excavate. Occasionally, several birds in addition to the nesting pair may assist in the digging. Some nests may also be shared by three nuthatches instead of two, the third individual typically being an unmated male helper. This species is gregarious when roosting in cavities. This occurs year-round, from before sunset to well after sunrise. Reference: Norris (1958).
Certhiidae (Creepers)
Brown Creeper,Certhia americana. (Fig. 11.32) Male length 4 1/2 in (11.75 cm), female length 4 3/4 in (12 cm); male weight 3/10 oz (8 g), female weight 3/10 oz (7.9 g). Uncommon permanent resident in the White-Inyo Range; recorded from 7,700 to 10,000 ft (2,350 to 3,200 m).
Figure 11.32
Brown Creeper.
Brown Creepers inhabit Subalpine Forest of Limber Pine, Bristlecone Pine, and Lodgepole Pine and woodlands of large Singleleaf Pinyon. They prefer mature timber in fairly dense stands for both foraging and nesting. These birds are excellent climbers, spiraling up tree trunks while probing into bark fissures for insects with their long, decurved bills. Their pale white throats may aid in reflecting light into these dark cracks. After attaining a certain height in the tree, creepers drop to the base of the same or another tree and continue their foraging. Brown Creepers nest in crevices or spaces underneath slabs of loosened tree bark. Although the back is camouflaged and resembles bark, the species can be detected by its constant movement. Reference: C. Davis (1979).
Troglodytidae (Wrens)
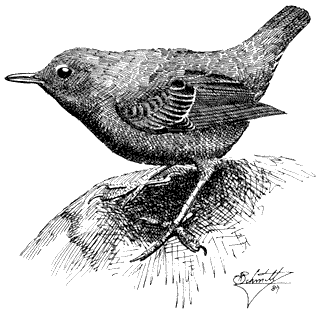
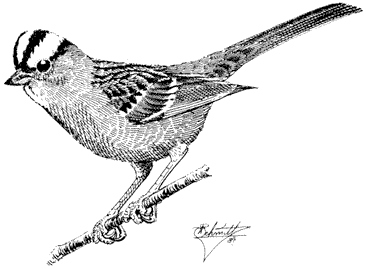
Rock Wren,Salpinctus obsoletus. (Plate 11.3) Male length 5 in (13 cm), female length 5 in (12.75 cm); male weight 1/2 oz (15.1 g), female weight 3/5 oz (16 g). Common resident in the White-Inyo Range, from the base of the ranges to the summit of White Mountain Peak, at 14,246 ft (4,342 m).
Rock Wrens occur through an exceptionally wide elevational range in the region, on generally arid canyon slopes or flats wherever rocky outcrops or surfaces occur. They even persist in areas inhabited by few other species, such as the high-elevation, windswept wastes covered sparsely by low scrub, grass, and rocks. Insects are removed from rock crevices and exposed rock surfaces. This species also uses such crevices for nesting, although it may nest in tunnels in earth banks. Rock Wrens sing from prominent rocks, their buzzes and trills ringing and echoing across canyon walls. References: Kroodsma (1975), Weathers (1983).
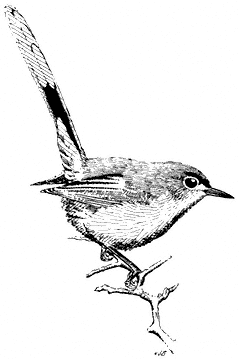
Canyon Wren,Catherpes mexicanus. (Plate 11.3) Male length 5 in (12.5 cm), female length 4 3/4 in (12.25 cm); male weight 2/5 oz (10.8 g), female weight 3/8 oz (9.7 g). Rare to locally relatively common resident in the White-Inyo Range, with records between 5,100 and 7,400 ft (1,555 and 2,256 m).
Canyon Wrens occur as very widely scattered pairs in the region, their local presence dictated by the availability of either steep, rocky canyon walls, with shady overhangs near pools, or boulder piles with deep, shady crevices. The surrounding vegetation can be varied, ranging from brush or woods to open forest. Males announce their presence by a cascading series of clear, mournful whistles that reverberate through the habitat during the nesting period. The adults sneak through shady cracks among the boulders, apparently using their stark white throats to reflect light into the dark recesses, revealing prey of insects and spiders. Nests are also hidden in such crannies. Reference: Grinnell and Storer (1924).
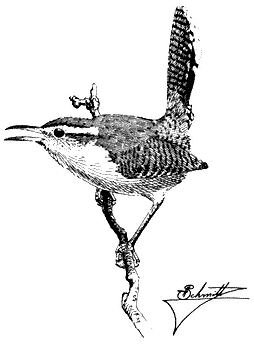
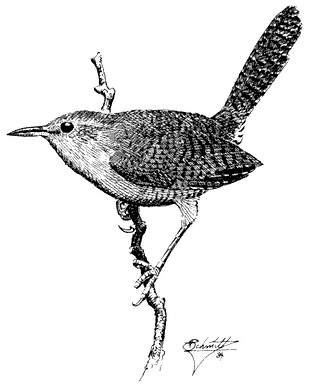
Bewick's Wren,Thryomanes bewickii. (Fig. 11.33) Male length 4 3/4 in (12 cm), female length 4 3/4 in (12 cm); male weight 3/8 oz (9.3 g), female weight 3/8 oz (9.7 g). Common permanent resident in the White-Inyo Range; recorded from 6,750 to 9,500 ft (2,060 to 2,900 m).
This species frequents mountain slopes and canyons grown to Pinyon-juniper Woodland and mixed brush, especially sagebrush. Nearby Mountain Mahogany will occasionally be entered, but it is not preferred. There is some evidence for a partial winter exodus of the population into riparian willows at lower elevations in canyons and valleys. Bewick's Wrens are active near the ground, where they are commonly out of sight, but foraging birds visit the upper and outer foliage of pinyons and junipers. A variety of insects are gleaned from needle surfaces, twigs, and cracks in bark or trunks. The male sings from exposed perches 5 to 15 ft (1.5 to 4.6 m) above the ground. Members of a pair typically forage within several yards of each other. Nests are hidden in or near the ground in natural hollows or crannies in pinyons and junipers as well as in cavities or protected nooks in human-made structures such as cabins and woodpiles. References: Miller and Stebbins (1964), E. Miller (1941).
House Wren,Troglodytes aedon. (Fig. 11.34) Male length 4 1/2 in (11.5 cm), female length 4 2/5 in (11.25 cm); male weight 3/8 oz (9.8 g), female weight 2/5 oz (10.4 g). Common resident in the White Mountains; recorded from 6,750 to 9,950 ft (2,060 to 3,030 m).
Figure 11.33
Bewick's Wren.
Figure 11.34
House Wren.
House Wrens live in riparian settings, where they nest in natural cavities or in abandoned woodpecker holes in aspens. Preferred nest sites are commonly within 5 ft (1.5 m) of the ground. However, in areas of human settlement, they may also nest under the eaves or in cracks in walls of cabins. Foraging similarly takes place at low levels, in brush, willows, or other such growth around fallen trees and rocks. A diversity of insects, especially those of small size, make up their diet. The bubbling song of male House Wrens is a characteristic summer sound of the aspen zone in the range. References: Kendeigh (1941), Marshall (1957).
Cinclidae (Dippers)
American Dipper,Cinclus mexicanus. (Fig. 11.35) Male length 7 in (17.5 cm), female length 6 3/4 in (17 cm); male weight 2 1/8 oz (60 g), female weight 1 3/4 oz (49 g). Small numbers of this species occur year-round in the deep, wet canyons of the White Mountains, up to elevations of approximately 10,000 ft (3,050 m).
The American Dipper occurs only along permanently flowing streams characterized by cool, swift, clear water. Aquatic insect larvae compose the bulk of their diet, but small fish and other invertebrates are occasionally eaten. Dippers get most of their prey directly from the fast-moving waters. They also flycatch and pick insect larvae from streamside rocks. The ability of dippers to forage in mountain streams is truly remarkable. With their strong feet they grasp the rubble on the bottom and wade
Figure 11.35
American Dipper.
underwater in search of food; in deeper, faster water, they typically use their wings while diving and swimming. The American Dipper usually stands on emerged rocks at the water's edge while foraging. Flight between such perches is rapid and low over the water. This species also breeds in the dampness of the stream environment. Preferred nest substrates are overhanging cliff ledges in areas protected from predators and inclement weather but exposed to water spray. Large rocks, tree roots, and undercut banks are commonly used. The mossy covering of the nest is moist and springy. Birds tend to breed in early spring, an adaptation that allows them to take advantage of an ample insect supply before late spring runoff decreases the availability of food. References: Morse (1979), Price and Bock (1983).
Muscicapidae (Muscicapids). Sylviinae (Kinglets and Gnatcatchers)
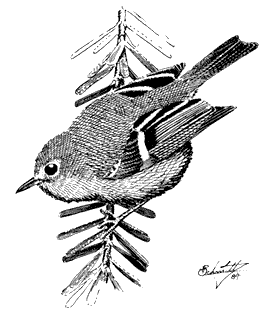
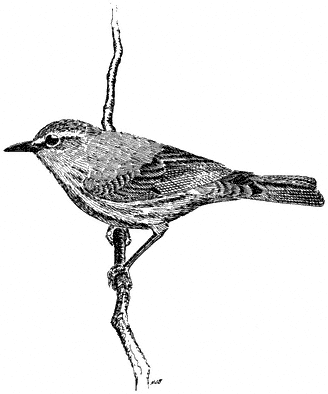
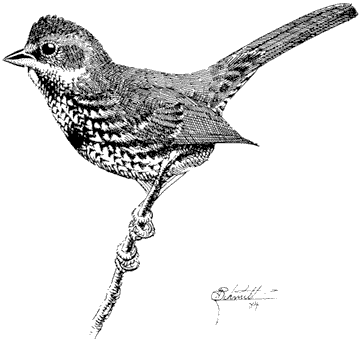
Ruby-crowned Kinglet,Regula calendula . (Fig. 11.36) Male length 4 in (10.25 cm), female length 4 in (10 cm); male weight 1/5 oz (6.2 g), female weight 1/5 oz (6 g). Relatively common summer resident locally in the White-Inyo Range; occurs between approximately 9,500 and 10,600 ft (2,900 and 3,230 m) elevation.
This species is most common in coniferous forest of Bristlecone, Limber, and Lodgepole pines. Aspen groves are visited occasionally. Foraging is concentrated on the terminal needle clusters at low to middle heights in the canopy. Here, this kinglet picks small insects from the foliage while nervously flicking its wings. Perches
Figure 11.36
Ruby-crowned Kinglet.
are changed frequently. Occasional sorties after flying insects and hovering flights near foliage are additional common foraging maneuvers. References: Franzreb (1984), Grinnell and Storer (1924).
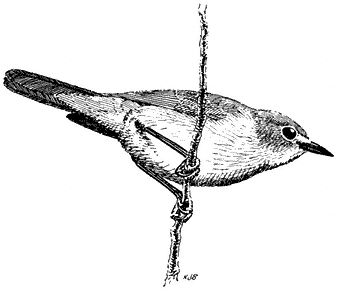
Blue-gray Gnatcatcher,Polioptila caerulea . (Fig. 11.37) Male length 4 1/4 in (10.75 cm), female length 4 in (10.5 cm); male weight 1/5 oz (5.4 g), female weight 1/5 oz (5.7 g). Common summer resident in the White-Inyo Range, from 5,100 to 9,400 ft (1,555 to 2,870 m).
Blue-gray Gnatcatchers frequent arid Oak Woodland and Chaparral throughout most of their breeding range west of the White-Inyo mountain region. However, in these desert mountains, the species switches to a more xeric habitat of Pinyon-juniper Woodland interspersed with sagebrush and, in some areas, Mountain Mahogany. Nearby streamside thickets of willows and tall sagebrush are used less commonly. Gnatcatchers are conspicuous where they occur, uttering a series of high, thin, wheezy notes while actively foraging among the dense foliage of trees and shrubs. Alighting momentarily on a branch, the bird quickly scans the area for food before flying to the next perch. Large insects are usually picked from leaves and twigs, but some may also be taken on the wing. Nests are small, compact cups attached to upright branch
Figure 11.37
Blue-gray Gnatcatcher.
forks in a tree or bush. Although sometimes placed in the open, they are nonetheless well hidden by virtue of their camouflaging outer material. Blue-gray Gnatcatchers respond readily to human squeaks and imitated owl calls. References: Root (1967), Root (1969).
Turdinae (Thrushes)
Mountain Bluebird,Sialia currucoides . (Plate 11.2) Male length 6 3/5 in (16.75 cm), female length 6 1/2 in (16.5 cm); male weight 1 oz (28.7 g), female weight 1 oz (28.8 g). Common summer resident in the White-Inyo Range, from 7,200 to 10,500 ft (2,200 to 3,200 m).
This species lives in exposed places: meadows, sagebrush flats, sparsely vegetated slopes, and open coniferous forest. There they perch on bush tops, prominent rocks, fences, or dead treetops. Insects are taken from either the air, the ground, or the foliage and woody parts of trees and shrubs. The Mountain Bluebird commonly hovers buoyantly before securing its prey. Nests are mostly placed in cavities in dead conifers or aspens. In late summer, postbreeding family groups forage widely over open country. The Mountain Bluebird winters in flocks at lower elevations, either in agricultural regions in valleys, in the desert, or on mountain slopes grown to juniper, the berries of which are often a staple food during that season. References: Power (1966), Power (1980).
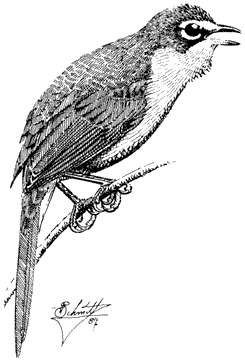
Townsend's Solitaire , Myadestes townsendi . (Fig. 11.38) Male length 7 9/10 in (20 cm), female length 8 1/10 in (20.5 cm); male weight 1 1/8 oz (31.9 g), female weight 1 1/4 oz (35.6 g). Rare summer resident in the White-Inyo Range, between 9,300 and 10,500 ft (2,840 and 3,200 m) elevation. The solitaire also occurs in winter on the lower slopes of these ranges, although these birds are not necessarily from the local summering population.
As a nesting species, solitaires occur only in the best stands of subalpine Limber and Bristlecone Pine forest. Males sing a far-reaching, jumbled series of warbles from spires in the treetops. A more commonly heard vocalization, however, is the brief, haunting whistle that simulates a creaky hinge. Townsend's Solitaires commonly take shady perches near the trunk, below the main foliage canopy of pines. They obtain insects in summer, occasionally by flycatching; winter birds commonly feed on juniper berries. Nests are built in protected crevices in banks, stumps, and exposed tree roots. Reference: Salomonson and Balda (1977).
Hermit Thrush,Catharus guttatus . (Plate 11.4) Male length 6 3/4 in (17.25 cm), female length 6 1/4 in (16 cm); male weight 1 oz (28.5 g), female weight 1 oz (28.8 g). Common summer resident in the White-Inyo Range; recorded from 7,700 to 10,500 ft (2,350 to 3,200 m).
This species lives in the shadiest habitats of the White-Inyo Range: aspen woodland, willow thickets, and coniferous forest of Limber, Bristlecone and Lodgepole pines. Dense groves of Mountain Mahogany, especially on northeast slopes or in
Figure 11.38
Townsend's Solitaire.
ravines, are also suitable; these provide good shade and a ground layer of plant debris in which the thrushes forage. In the morning and again at dusk, males deliver their enchanting, fluty song from prominent perches. The environment occupied by the Hermit Thrush in these mountains seems more open and arid than places where the species occurs in the Sierra Nevada. References: Dilger (1956), Morse (1971).
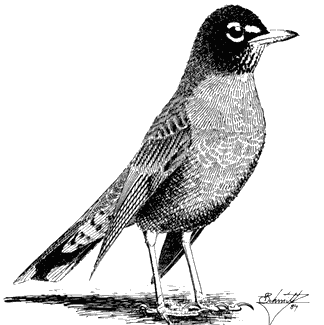
American Robin,Turdus migratorius . (Fig. 11.39) Male length 9 1/8 in (23.25 cm), female length 9 1/4 in (23.5 cm); male weight 2 5/8 oz (74 g), female weight 2 3/4 oz (78 g). Common summer resident in the White Mountains, from 6,750 to 10,500 ft (2,060 to 3,200 m). Scarce in the Inyo Mountains; there is one June record from Waucoba Canyon, at 7,200 ft (2,200 m).
In the White Mountains region, this familiar species breeds in moist canyon bottoms where meadows and dense thickets of willow, rose, and birch prevail. Such habitats provide soft, open ground for foraging, where worms and other ground invertebrates may be taken. Mud for nest construction is also available in these places. The American Robin builds its nest in many kinds of trees situated near wet areas. Typically, the cup-shaped nest is placed on a branch or in a fork at moderate heights. Males begin to sing on their breeding territories in early spring. They sing most vigorously at dawn and during the early morning hours, and their caroling
Figure 11.39
American Robin.
song can often be heard continuously along streamcourses throughout the summer. References: Howell (1942), James and Shugart (1974), Shedd (1982).
Mimidae (Mockingbirds and Thrashers)
Sage Thrasher,Oreoscoptes montanus . (Fig. 11.40) Male length 7 7/8 in (20 cm), female length 7 7/10 in (19.5 cm); male weight 1 2/5 oz (40 g), female weight 1 1/2 oz (44 g). Present in both ranges during the spring and summer months, from approximately 6,000 to 11,000 ft (1,830 to 3,350 m) elevation in the White Mountains.
Although never numerous, Sage Thrashers are most common in the tall, dense sagebrush present on the alluvial fans in the northern White Mountains. Populations at high elevations, where the brush is of short stature, are sparse. In those areas, however, the birds typically seek out the scattered, thicker patches of brush and use the tallest available sages for both song posts and nest sites. This species inhabits no other plant assemblage in the region. Foraging concentrates on the ground between and below the shrubs, but insects in the foliage are also taken; preferred prey items include grasshoppers, cicadas, beetles, and other large species. The Sage Thrasher builds its bulky nest in a well-concealed position in the top of a sage bush. Four or five spotted blue eggs are laid. Males also sing their rich, warbled song from bush
Figure 11.40
Sage Thrasher.
tops and may commonly be heard across the open terrain for at least 0.5 mi (0.8 km). These birds can also be detected by their strong and direct flight just above the sagebrush tops. Reference: Reynolds and Rich (1978).
Vireonidae (Vireos)
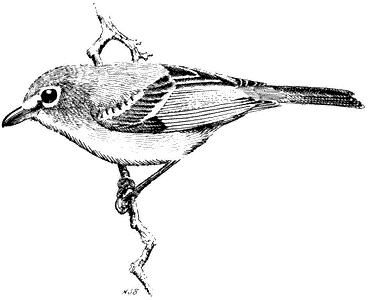
Solitary Vireo,Vireo solitarius . (Fig. 11.41) Male length 5 4/5 in (14.3 cm), female length 5 2/5 in (13.8 cm); male weight 3/5 oz (16.2 g), female weight 3/5 oz (16.8 g). Relatively common summer resident in the White-Inyo Range, between 6,200 and 8,200 ft (1,890 and 2,500 m).
In these mountains, Solitary Vireos inhabit wooded canyon slopes, draws, and flats dominated by large pinyons, junipers, and, to a lesser extent, Mountain Mahogany. Foraging activity ranges from the tree crowns to near the ground. There birds forage for adult and larval insects by moving deliberately through clumps of foliage. The nest is a cup constructed of fine plant fibers, delicate inner bark strips, and grass tops. It is camouflaged externally with grayish or greenish lichens or dried leaves, and it is suspended by its rim from the horizontal fork of a pinyon or juniper branch. Solitary Vireos can be detected from a distance of over 0.25
Figure 11.41
Solitary Vireo.
mi (0.4 km) by their song of rich, inflected slurs or burred phrases, often uttered in a question-and-answer pattern. Occasionally, the male may sing from the nest while covering the eggs in the absence of the female. Pairs of these vireos are generally widely spaced, so that four or five territories at most are encountered in a hike of several miles through favorable habitat. The form V. solitarius plumbeus , which inhabits the White-Inyo Range, is a clean, grayish white bird, in contrast to the yellowish green form, V. solitarius cassinii , that breeds on the west side of the Sierra Nevada. V. s. plumbeus is a relative newcomer to the region, unrecorded there prior to the 1960s. Reference: James (1981).
Warbling Vireo,Vireo gilvus . (Fig. 11.42) Male length 4 3/4 in (12 cm), female length 4 3/4 in (12 cm); male weight 3/8 oz (10.8 g), female weight 2/5 oz (11.8 g). Common summer resident in the White-Inyo Range, between 6,200 and 10,500 ft (1,890 and 3,200 m).
Warbling Vireos dwell in the canopy foliage of riparian aspen, willows, and Water Birch. They take insects from their leafy surroundings by deliberate foraging motions. This species can be difficult to find in the treetops, but its presence is revealed by incessant singing, which commonly continues to midday or even into the afternoon, when other birds are silent. In the early morning, especially in spring soon after their arrival, chases of male vireos in territorial encounters, and males and females in pairing flights, are announced by silvery white flashes through the foliage. The nest is a neatly woven cup suspended from a horizontal fork and placed 8–25 ft (2.4–7.6 m) above the ground in deciduous foliage. Brown-headed Cowbirds commonly
Figure 11.42
Warbling Vireo.
parasitize Warbling Vireo nests in the Sierra Nevada, and possibly also do so in the White Mountains. In favorable habitat, the linear territories of the species occur in uninterrupted succession along mountain streams. References: Grinnell and Storer (1924), Walsberg (1981).
Emberizidae (Emberizids). Parulinae (Wood Warblers)
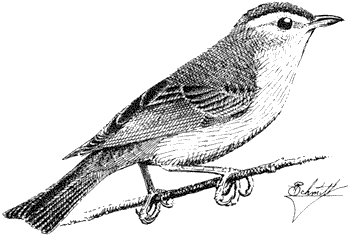
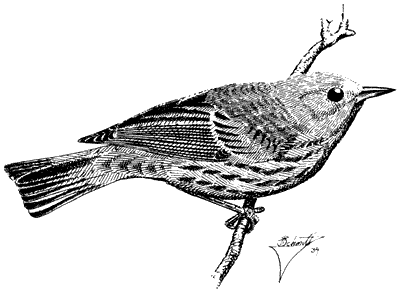
Orange-crowned Warbler,Vermivora celata . (Fig. 11.43) Male length 4 2/5 in (11.25 cm), female length 4 5/8 in (11.75 cm); male weight 2/5 oz (9.2 g), female weight 2/5 oz (9.1 g). Relatively common summer resident in the White Mountains and locally in the Inyo Mountains, between 6,650 and 9,500 ft (2,030 and 2,900 m).
The interior form of this species lives only in moist canyon bottoms, in the cool riparian strip of aspen, Water Birch, and associated thickets of willow and Wild Rose. Such habitat contrasts greatly with the warm Oak-Chaparral growth at low elevations occupied by the form on the west side of the Sierra Nevada. Like most other species in the genus Vermivora , the Orange-crowned Warbler builds its nest in a concealed place on the ground. The adults are well hidden as they search for insects and spiders in the foliage of willow, rose, and aspen saplings. Most challenging of all is to pinpoint the male as he sings from the crowns of aspen, birch, or willow along the stream. His song — a continuous, insectlike trill of two or three parts, each on a slightly different pitch — is usually delivered in long series from a concealed perch. Although exposed briefly while flying between song perches, he quickly vanishes again in the leafy treetops. References: Mengel (1964), Morrison (1981).
Virginia's Warbler,Vermivora virginiae . (Fig. 11.44) Male length 4 1/4 in (11 cm), female length 4 1/4 in (11 cm); male weight 2/5 oz (8.4 g), female weight 1/4 oz (7.5
Figure 11.43
Orange-crowned Warbler.
Figure 11.44
Virginia's Warbler.
g). Relatively common summer resident locally in the White Mountains, between 6,750 and 9,500 ft (2,060 and 2,900 m).
This warbler is most abundant on upper slopes in groves of Mountain Mahogany, but it also occurs sparingly in riparian willows and around clumps of seviceberry in the pinyon zone. The plant growth these birds occupy is often clumped among boulder piles or near rock slides. A secretive species, it perches in the leaves just below the foliage top and can be difficult to find even when it is singing. Nonetheless, a squeak or hissing note will usually bring the bird into view. Then it characteristically flicks its tail while uttering chip notes in alarm. Virginia's Warblers take insect food from twigs and leaves of deciduous brush; thus, their sphere of activity is focused within several feet of the ground. Reference: Johnson (1976).
Yellow Warbler,Dendroica petechia . (Fig. 11.45) Male length 4 1/2 in (11.25 cm), female length 4 1/4 in (10.5 cm); male weight 3/10 oz (9.3 g), female weight 3/10 oz (8.5 g). Uncommon local summer resident in the White Mountains, between 6,750 and 8,200 ft (2,060 and 2,500 m).
This species dwells in the canopy of riparian vegetation, mainly in canyons on the east side of the range. It also breeds at scattered oases in the valleys on each side of the mountains. Yellow Warblers prefer tree willows and cottonwoods but will venture sparingly into aspen at higher elevations. Low willow and alder growth along streams is used for foraging, although much insect food is also taken well up in the foliage of large trees. Nests too are placed in these trees from near the ground to middle heights. Elsewhere this species is heavily parasitized by Brown-headed Cowbirds, and
Figure 11.45
Yellow Warbler.
this activity is suspected to contribute to the uncommonness of the Yellow Warbler in the White Mountains. References: Biermann and Sealy (1982), Griscom and Sprunt (1957).
Yellow-rumped Warbler,Dendroica coronata. (Plate 11.4) Male length 5 in (13 cm), female length 5 in (13 cm); male weight 2/5 oz (12.3 g), female weight 2/5 oz (12.5 g). Common summer resident in the White-Inyo Range, occurring from 8,200 to 10,500 ft (2,499 to 3,200 m).
This species summers in Subalpine Forest of Bristlecone Pine, Limber Pine, or Lodgepole Pine, and in associated riparian trees such as aspen. One of the most generalized foragers of all warblers, it takes insects from the foliage by gleaning and hovering and from the air by flycatching. All levels of the habitat are visited, from the ground to the tops of tall conifers. During the nonbreeding season, berries are often eaten. The Yellow-rumped Warbler also varies its nest sites from near the ground to high in the conifers. Reference: Hubbard (1969).
Black-throated Gray Warbler,Dendroica nigrescens. (Plate 11.2) Male length 4 5/8 in (11.8 cm), female length 4 1/2 in (11.5 cm); male weight 1/4 oz (8.1 g), female weight 3/10 oz (8.9 g). Very common summer resident in the White-Inyo Range, from 6,750 to 9,500 ft (2,060 to 2,900 m).
This warbler's preference for sunny and warm environments is reflected in its increasing abundance from north to south in the woodlands of the White-Inyo mountains. Arid portions of Pinyon-juniper Woodland are favored, with occasional use of Mountain Mahogany. The Black-throated Gray Warbler must occur in huge numbers, as it is one of the most commonly encountered species in the region. Its "see-saw," buzzy song, rising in pitch near the end, is a characteristic sound of the pinyon zone through the spring and summer. Adult Black-throated Grays hunt for insects and spiders in dense terminal needle clusters of pinyon. Nests are built in pinyons or in nearby brush. References: Marshall (1957), Morrison (1982).
MacGillivray's Warbler,Oporornis tolmiei. (Fig. 11.46) Male length 4 4/5 in (12.25 cm), female length 4 3/4 in (12 cm); male weight 3/8 oz (10.8 g), female weight 3/8 oz (10.7 g). Common summer resident in the White Mountains, between 6,750 and 9,500 ft (2,060 and 2,900 m).
This warbler inhabits thick undergrowth along streams, attaining its greatest numbers between 8,200 and 9,000 ft (2,500 and 2,740 m) in willows, alder, Water Birch, nettles, and aspen saplings. Shade and dampness are prominent features of the species' habitat. MacGillivray's Warblers forage for insects and nest near the ground in dense foliage and branchwork; thus, they can be very difficult to observe. When singing, however, males are commonly exposed in bush tops from 5 to 15 ft (1.5 to 4.6 m) above the ground. During migration, this species occurs much more widely and can be found even in very open country near damp spots, as long as there is relatively thick brush nearby with shady hiding places. References: Grinnell and Storer (1924), Hutto (1981).
Figure 11.46
MacGillivray's Warbler.
Figure 11.47
Yellow-breasted Chat.
Yellow-breasted Chat,Icteria virens. (Fig. 11.47) Male length 6 7/8 in (17.5 cm), female length 7 in (17.75 cm); male weight 7/8 oz (24.9 g), female weight 1 oz (27.8 g). Local and rare summer resident in the White Mountains, with records at 6,750 ft (2,060 m), an unusually high elevation for the species.
The Yellow-breasted Chat nests only in the densest riparian thickets in foothill canyons on the east side of the range. Total numbers in the region are probably very small. Lush growth of willows, Wild Rose, nettles, and cottonwood saplings are used for all activities. Because of these habitat preferences, chats are usually very difficult to see in their impenetrable haunts. Their presence is typically revealed by an unusual song, a mixture of harsh and varied rattles interspersed with clear whistles. The singing male is commonly obscured by foliage, but patience will be rewarded by a spectacular song flight occurring between thickets, in which the legs are dangled and wings slowly fluttered. Food consists of insects, spiders, and small fruits. References: Petrides (1938), Thompson (1977).
Thraupinae (Tanagers)
Western Tanager,Piranga ludoviciana. (Plate 11.4) Male length 6 1/4 in (16 cm), female length 6 1/4 in (16 cm); male weight 1 oz (27.5 g), female weight 1 oz (27.8 g). Relatively common summer resident in the White-Inyo Range, from 6,750 to 9,800 ft (2,060 to 2,990 m).
This species shuns open Singleleaf Pinyon as breeding habitat in favor of the larger conifers occurring at higher elevations. Occasionally, however, tall pinyon trees intermixed with Mountain Mahogany, as well as aspen, may be used for nesting. Pinyon Woodland is visited much more extensively when the birds are migrating, which may occur well into June. Western Tanagers forage for wasps, ants, beetles, and other insects in the pine canopies, either by picking the insects from the branches and foliage or by snapping them from the air during short jaunts from their perches. Other foods, particularly fruits, are eaten by postbreeding or migrating individuals. Nests of twigs and fine roots are placed on the outer ends of large limbs, from middle heights to the top of the canopy. Although this species prefers the security afforded by dense conifer foliage, dead limbs or trees are known to be used. Brightly colored males are easily spotted by flashes of yellow as they traverse openings in the forest. The short, melodious, languid warble given by the male, or the short pit-er-ic call uttered by both sexes, also announces the presence of the species. Reference: Grinnell and Storer (1924).
Cardinalinae (Cardinals, Grosbeaks, and Allies)
Black-headed Grosbeak,Pheucticus melanocephalus. (Fig. 11.48) Male length 7 in (18 cm), female length 7 1/4 in (18.25 cm); male weight 1 1/2 oz (41 g), female weight 1 1/2 oz (44 g). Very common summer resident in the White-Inyo Range, between 6,750 and 8,900 ft (2,060 and 2,710 m).
Figure 11.48
Black-headed Grosbeak.
This species is a characteristic mid-elevation bird of the region in diverse woodlands such as pinyon, aspen-willow, and Mountain Mahogany. Between approximately 7,000 and 8,000 ft (2,130 and 2,440 m) elevation, it is very prominent and is almost constantly within sight or sound. The male's rich, warbled song is so loud that it virtually drowns out the voices of most other singing birds in its neighborhood. Male Black-headed Grosbeaks even sing loudly from the nest as they cover the eggs during the absence of the female. Extensive edges and diverse plant mixtures are preferred, these providing the species with foliage insects, buds, and fruits for consumption. References: Weston (1947), Bent (1968).
Lazuli Bunting,Passerina amoena. (Fig. 11.49) Male length 5 in (12.75 cm), female length 4 9/10 in (12.5 cm); male weight 1/2 oz (14.7 g), female weight 1/2 oz (13.6 g). Fairly common summer resident in the White Mountains, recorded between 6,750 and 9,000 ft (2,060 and 2,740 m), but rare above 8,200 ft (2,500 m) except as a postbreeding vagrant.
Lazuli Buntings are restricted in the White Mountains to streamside thickets and are most common in the middle and lower reaches of canyons. Dense tangles of willows, Wild Rose, and Water Birch are chosen for both foraging and nesting, especially where the plant growth is mixed in terms of species composition and stature. The colorful male commonly sings from the tops of tall bushes or small
Figure 11.49
Lazuli Bunting.
trees. Occasionally, however, he ascends to the top of an aspen, pinyon, or other conifer and sings from 30 to 40 ft (9 to 12 m) above the ground. The song is characteristically variable, with phrases and short trills at different pitches and often in couplets. The closely related Indigo Bunting (P. cyanea ), with which the Lazuli Bunting is known to hybridize, has been expanding its range southwestward in recent years, and males have been recorded in the White-Inyo mountains. References: Emlen, Rising, and Thompson (1975); Thompson (1976).
Emberizinae (Towhees, Sparrows, and Allies)
Green-tailed Towhee,Pipilo chlorurus. (Fig. 11.50) Male length 6 3/4 in (17 cm), female length 6 1/2 in (16.75 cm); male weight 1 oz (28.9 g), female weight 7/8 oz (24.9 g). Locally common summer resident in the White-Inyo Range, from 6,200 to 10,000 ft (1,890 to 3,050 m).
Green-tailed Towhees breed in brushland in this region, occurring in greatest numbers in open pinyon with mixed brushland. High-elevation habitats of sagebrush mixed with willows and birch are visited sparingly. A particularly favorable site is sagebrush interspersed with serviceberry, currant, and scattered pinyons in a canyon bottom. Arid flats of pure sagebrush are avoided. The adults are skilled runners, moving while crouched in alleyways between the shrubs. They forage on the ground
Figure 11.50
Green-tailed Towhee.
for insects and fruit in leaf litter. Nests are placed near the ground in bushes. Adult Green-tailed Towhees decoy readily to a squeak and commonly respond with a catlike mew . Males sing from open perches in pinyon tops at approximately 20 ft (6 m) above the ground. Reference: Grinnell and Storer (1924).
Rufous-sided Towhee,Pipilo erythrophthalmus. (Fig. 11.51) Male length 7 3/4 in (20 cm), female length 7 1/2 in (19.5 cm); male weight 1 3/10 oz (37.4 g), female weight 1 3/10 oz (37.6 g). Common resident in the White-Inyo Range, recorded from 6,000 to 9,000 ft (1,830 to 2,740 m).
This species frequents dense brushy habitats along slopes and canyon bottoms in the pinyon-juniper-mahogany belt. Sagebrush and streamside growth of willow-rose-birch are particularly favored. A ground cover of litter is essential to the presence of Rufous-sided Towhees; there the species rakes up seed of herbaceous vegetation and insects by simultaneously scratching with both feet. While foraging, it stays well hidden in the dense cover of brush or riparian thickets. Nests are usually placed on or near the ground at the base of a shrub or in an otherwise concealed place. During the breeding season, males commonly sing from more exposed areas, such as branches of small pinyons, junipers, or Mountain Mahogany. Although this species is difficult to see except when singing, it will readily announce its presence by a buzzy trill, which is subordinated in this region by one or more harsh introductory notes. References: Kroodsma (1971), Davis (1960).
Chipping Sparrow,Spizella passerina. (Fig. 11.52) Male length 5 1/4 in (13 cm), female length 5 in (12.5 cm); male weight 1/2 oz (12.6 g), female weight 2/5 oz (12.1
Figure 11.51
Rufous-sided Towhee.
Figure 11.52
Chipping Sparrow.
g). Very common summer resident in the White-Inyo Range, from 7,200 to 10,400 ft (2,200 to 3,170 m).
This sparrow is very numerous in open woods of pinyon, juniper, and Mountain Mahogany. Territories are defended in areas of scattered trees, open brush, rocks and grass; heavy brush is avoided. Chipping Sparrows search the ground for small seeds and insects but will also take buds in bushes and trees, especially during the spring. The dry, harsh trill of the male is a common summertime song in this area. References: Grinnell and Storer (1924), Reynolds and Knapton (1984).
Brewer's Sparrow,Spizella breweri. (Fig. 11.53) Male length 5 1/4 in (13 cm), female length 5 in (12.5 cm); male weight 3/8 oz (11 g), female weight 3/8 oz (10.4 g). Very common summer resident in the White-Inyo region, from 6,750 to 10,300 ft (2,060 to 3,140 m).
Although the Brewer's Sparrow nests over an impressively wide elevational range in these mountains, only sagebrush is occupied. Extensive stands, such as those on the alluvial fans below the pinyon zone or those on benches and flats at high elevations, are used most commonly. Small numbers also nest in sagebrush growth in local openings of otherwise narrow canyons. This sparrow compensates for its plain plumage by emitting an elaborate song — a series of buzzes and trills given at different pitches, one of the longest vocalizations of any species in the region. Brewer's Sparrows forage
Figure 11.53
Brewer's Sparrow.
on the ground or in bushes for insects and seeds. Nests are well hidden in sagebrush tops. References: Reynolds (1981), Weathers (1983).
Black-chinned Sparrow,Spizella atrogularis. (Fig. 11.54) Male length 5 1/4 in (13 cm), female length 5 1/4 in (13 cm); male weight 3/8 oz (10.6 g), female weight 3/8 oz (10.9 g). Rare and local summer resident in the White Mountains; recorded between 6,750 and 7,000 ft (2,060 and 2,130 m).
The Black-chinned Sparrow occurs sparingly in the White Mountains, at the northern edge of the species' distribution, where it lives in a rather distinctive and characteristic habitat. Although sagebrush is usually prevalent where this sparrow breeds in eastern California, it typically requires a diverse mixture of shrubs in addition to the sage, including Rabbitbrush, Bitterbrush, and Mormon Tea. Furthermore, most records of the species are from brushland sites in varied, sloping terrain, often with jumbled rock outcrops and a scattering of pinyon and/or juniper. Males sing from either the tops of these small trees or from the tops of prominent shrubs and usually fly substantial distances between song perches. Thus, territories
Figure 11.54
Black-chinned Sparrow.
are large, and pairs are widely spaced. The easiest way to detect this trim, handsome sparrow is through its distinctive song — a rapid trill introduced by three clear, sweet notes and increasing in cadence toward the end. Reference: Johnson, Bryant, and Miller (1948).
Vesper Sparrow,Poocetes gramineus. (Fig. 11.55) Male length 6 in (15 cm); female length 5 3/4 in (14.75 cm); male weight 7/8 oz (24.9 g), female weight 4/5 oz (22.7 g). Uncommon summer resident in the White-Inyo Range, from approximately 7,800 to 10,500 ft (2,380 to 3,200 m).
This species occurs in the sagebrush-grassland habitat both below and above the pinyon belt. There only small numbers can be found. Vesper Sparrows are shy birds and commonly remain undetected even when present because of their inconspicuous habits. They forage mostly on the ground, but males usually perch on prominent sage tops when delivering their advertising song. References: Kroodsma (1972), Grinnell and Storer (1924).
Black-throated Sparrow,Amphispiza bilineata. (Fig. 11.56) Male length 5 1/4 in (13 cm), female length 5 in (12.5 cm); male weight 1/2 oz (13.5 g), female weight 1/2 oz (13.2 g). Fairly common summer resident in the White-Inyo Range, occurring from the valley bottoms up to approximately 6,800 ft (2,070 m) elevation.
Black-throated Sparrows occur only in brushland on the alluvial fans on both slopes of the mountains. They generally prefer the warmer, drier areas to the south. Consequently, greatest densities are attained in the southern Shadscale zone, with the species becoming relatively scarce on the cooler sagebrush slopes to the north. The sparrows favor areas that are sparingly vegetated and where an exposed hard rock or gravel surface is evident. Seeds taken from the ground between shrubs constitute the primary food. Nests are placed on the ground in a thick cover of brush. During
Figure 11.55
Vesper Sparrow.
Figure 11.56
Black-throated Sparrow.
the breeding season, these birds can be seen or heard singing from bush tops, and after June, postbreeding family groups filter through the brushland. This strikingly patterned species is often easy to watch and seems tamer than its somewhat plainer relative, the Sage Sparrow. References: Heckenlively (1970), Weathers (1983).
Sage Sparrow,Amphispiza belli. (Fig. 11.57) Male length 5 3/4 in (14.5 cm), female length 5 1/2 in (14 cm); male weight 3/5 oz (16.5 g), female weight 1/2 oz (14.7 g). Very common summer resident in the White-Inyo Range; occurs from the valley floors to approximately 7,500 ft (2,290 m) elevation. Postbreeding birds occur higher, to 8,500 ft (2,590 m), even in early June.
This species occurs continuously along the west-facing slope from the north end of the White Mountains to the southern portion of the Inyos. Curiously, it is rare or lacking on the east side of the mountains south of Trail Canyon. Flats and alluvial fans between 3,500 and 6,500 ft (1,067 and 1,981 m) support the greatest numbers; very high altitudes are avoided. Like its close relative the Black-throated Sparrow, the Sage Sparrow lives only in brushland, and both species commonly occur side by side in the White-Inyo Range. In the north the Sage Sparrow occupies essentially pure sagebrush, while in the south Shadscale (saltbush) is the predominant habitat. Seeds of annual plants provide the primary food source. This sparrow also uses the tops of
Figure 11.57
Sage Sparrow.
bushes for song posts as well as for concealed nest sites. Occasionally, several males can be heard singing from very close range. The tinkling song carries for several hundred yards unless the bird is downwind. Nesting begins in April in the south, so that fully grown juveniles abound in the saltbush scrub by June, when family groups are commonly encountered. Northern populations breed several weeks later. These birds are typically shy and difficult to approach in the open brushland; when pressed, they will fly long distances and may drop to the ground and run among the shadows below well-spaced shrubs. References: Moldenhauer and Wiens (1970), Wiens (1982).
Fox Sparrow,Passerella iliaca. (Fig. 11.58) Male length 6 1/2 in (16.75 cm), female length 6 1/2 in (16.75 cm); male weight 1 oz (29.7 g), female weight 1 oz (28.1 g). Locally common summer resident in the White Mountains, between 6,750 and 9,600 ft (2,060 and 2,930 m) elevation. Known to occur locally in the Inyo Range (Lead Canyon, 9,600 ft [2,930 m]).
Fox Sparrows breed in dense riparian thickets of willows, Water Birch, aspen saplings, and Wild Rose, especially in the deep canyons on the east side of the mountains. This habitat, shared by all Great Basin and Rocky Mountain subspecies, contrasts strikingly with the chaparral used primarily by all forms in the Sierra Nevada. In favorable streamside growth, Fox Sparrow territories are placed in a
Figure 11.58
Fox Sparrow.
continuous linear sequence, and two or more males are commonly heard at once. Song perches, from 5 to 15 ft (1.5 to 4.6 m) high, are typically in bush tops, which offer partial concealment by sprays of foliage. This species forages principally on the ground in leaf litter, which is kicked away by vigorous backward strokes of the legs. Insects exposed in this way are fed to the nestlings. References: Martin (1977), Martin (1979).
Song Sparrow,Melospiza melodia. (Fig. 11.59). Male length 5 3/4 oz (14.75 cm), female length 5 1/2 in (14.25 cm); male weight 3/4 oz (21.7 g), female weight 3/4 oz (20.4 g). Locally common summer resident in the White Mountains with records up to 9,600 ft (2,930 m); more numerous in Owens Valley to the west of the White-Inyo Range.
The highest elevational record for the form M. m. fisherella occurs in Cottonwood Basin in the White Mountains. There, and throughout this generally arid region, the species is restricted to canyon bottoms with thick, low cover near water. Willow and Wild Rose thickets along streams are commonly occupied. Song Sparrows forage near the water's edge or on damp ground below the willows. Males sing from exposed bush tops, usually no more than several feet above the ground. Food consists of insects and seeds taken from the ground. References: Nice (1937); Smith, Yom-Toy, and Moses (1982).
Figure 11.59
Song Sparrow.
Figure 11.60
White-crowned Sparrow.
White-crowned Sparrow,Zonotrichia leucophrys. (Fig. 11.60) Male length 6 1/2 in (16.5 cm), female length 6 in. (15.5 cm); male weight 1 oz (30 g), female weight 9/10 oz (26.1 g). Common summer resident in the White Mountains, between 8,200 and 10,500 ft (2,500 and 3,200 m).
White-crowned Sparrows frequent high-altitude meadows where willow thickets, young or stunted pines, scattered aspen saplings, and wildflowers are clumped on the boggy ground. Because they favor places with running water, the headwaters of mountain streams are a good place to find the species. The adults are sleek and appear clean-cut. During the summer, they forage for insects on the damp ground, never far from cover. Nests are placed either on the ground under willows or other bushes, or a few feet above the ground in willows or small conifers. In the Subalpine Zone of the high Sierra Nevada, the volume of winter and spring snowfall drastically influences both the timing of summer breeding and the amount of space available for nesting. When the snowpack is heavy, fewer birds breed, and these individuals nest two weeks later than during normal years. Populations in the White Mountains presumably respond in a similar fashion. References: Morton (1978); Morton, Horstman, and Osborn (1972).
Dark-eyed (Oregon) Junco,Junco hyemalis. (Fig. 11.61) Male length 5 1/2 in (14.25 cm), female length 5 1/2 in (14 cm); male weight 3/5 oz (17 g), female weight 5/8 oz (18.3 g). Common summer resident in the White-Inyo Range, between 7,400 and 10,500 ft (2,260 and 3,200 m).
Gray-headed Junco,Junco caniceps. (Fig. 11.62) Male length 6 in (15.25 cm), female length 6 in (15 cm); male weight 5/8 oz (18.4 g), female weight 5/8 oz (18.8 g). Rare to relatively common summer resident in the White-Inyo Range, between 7,400 and 10,500 ft (2,260 and 3,200 m).
Because these two forms hybridize in the region, some authorities combine them into one species (Dark-eyed Junco, Junco hyemalis ). However, most individuals encountered in this area appear to be either pure parental types or very close to one species or the other; thus, we prefer to consider them as two full species. Furthermore, studies from several areas where both forms coexist have shown that each kind prefers to pair with a mate of similar appearance, further evidence that two distinct species are involved.
Juncos prefer cool and shady places where grass or forb cover provides a substrate for foraging and nesting. Thus, woodland of large pinyons on north- or east-facing slopes, riparian growth of willows and aspen, shady groves of large Mountain Mahogany, and Subalpine Forest of Bristlecone, Limber, and Lodgepole pines are all used. Openings in these habitats are favored. Most activities are concentrated on or near the ground. There juncos take insects and seeds and commonly conceal their nests under overhanging plants or turf. However, exposed twigs or tree tops at heights ranging from 10 to 50 ft (3 to 15 m) are chosen by males for song posts. The song is a ringing, musical trill. Juncos give their distinctive popping note when an intruder is
Figure 11.61
Dark-eyed (Oregon) Junco
Figure 11.62
Gray-headed Junco.
near the nest, especially after the eggs have hatched. References: Balph (1977), Hadley (1969), A. Miller (1941).
Icterinae (Blackbirds, Orioles, and Allies)
Brewer's Blackbird,Euphagus cyanocephalus. (Plate 11.1) Male length 9 1/4 in (23.5 cm), female length 8 1/4 in (21 cm); male weight 2 5/8 oz (76 g), female weight 2 1/10 oz (59 g). Common summer visitant and local summer resident in the White-Inyo mountain region; recorded up to 9,500 ft (2,900 m) elevation.
This blackbird is a common resident in the vicinity of towns, ranches, and other centers of human activity. It nests in parks, hedgerows, windbreaks of ornamental trees, and riparian growth. In the White Mountains, the Brewer's Blackbird is most likely to be seen as a visitant near meadows and along streams. Wandering individuals may turn up in any kind of habitat and terrain, even at high elevations. References: La Rivers (1944), Williams (1952).
Brown-headed Cowbird,Molothrus ater . (Plate 11.1) Male length 6 3/4 in (17 cm), female length 6 1/4 in (15.75 cm); male weight 1 1/2 oz (41 g), female weight 1 3/10 oz (37 g). Fairly common summer resident in the White-Inyo Range; recorded from the lowland valleys up to 11,600 ft (3,540 m) elevation.
Being nest parasites with no fixed breeding territory, Brown-headed Cowbirds are most commonly seen on the move. The males wander widely when advertising for mates, stopping for several minutes in a treetop or on a power pole to display before departing. This display consists of a gurgling call uttered while the chest is expanded and the tail is spread. A similar liquid note given in flight usually announces the presence of this species. The Brown-headed Cowbird is typically recorded overhead in areas of Pinyon-juniper Woodland, riparian willows, and tall sagebrush. Streamside habitats are especially frequented; thus, the small birds of these environments suffer most from cowbird parasitism. Wandering birds commonly mingle with blackbirds around agricultural areas and pack stations, where they forage on the ground for insects, seeds, grass leaves, and gravel. They feed near piles of manure, prying it forward with their bills to uncover food items. Cowbirds drink water daily in arid regions. References: Scott and Ankney (1983), Verner and Ritter (1983).
Scott's Oriole,Icterus parisorum. (Fig. 11.63) Male length 7 1/2 in (19 cm), female length 7 1/4 in (18.5 cm); male weight 1 3/8 oz (38 g), female weight 1 1/2 oz (41 g). Summer resident in the White-Inyo Range; rare in the north, uncommon in the south.
This species occupies arid woodlands of pinyon, juniper, and, in the northern Mojave Desert, Joshua Tree. Because of their huge territories, the population densities of Scott's Orioles in the region are low. Males announce their positions with a far-reaching song of great beauty — rich, clear whistles reminiscent of the Western Meadowlark. Insect food is taken from the foliage of pinyon or yucca. Nests of these
Figure 11.63
Scott's Oriole.
northern populations are commonly built in pinyons; more southerly populations nest in Joshua trees. References: Marshall (1957), Weathers (1983).
Fringillidae (Finches and Allies)
Rosy Finch,Leucosticte arctoa. (Fig. 11.64) Male length 6 in (15.5 cm), female length 6 in (15 cm); male weight 9/10 oz (26 g), female weight 7/8 oz (25 g). Fairly common summer resident in the White Mountains, from above timberline to the summit, at 14,246 ft (4,340 m) elevation. The species does not nest in the Inyo Mountains, where breeding habitat is lacking.
The Rosy Finch is the only species of bird strictly confined to the Alpine Zone in these mountains during the summer. These birds prefer to feed at the edges of snowfields and cirques, and in alpine rocklands or other areas with much exposed bare ground. Seeds and insects, even those frozen in snow, are taken. Strong fliers, Rosy Finches are highly mobile and forage over wide reaches of the Alpine Zone. Nests are placed in damp cracks below cliffs or in rock piles. In winter, flocks have been encountered in the pinyon-sagebrush zone near old mines. There they roost for the
Figure 11.64
Gray-crowned Rosy Finch.
night in protected niches in the walls of abandoned vertical shafts. References: Grinnell and Storer (1924), Johnson (1965).
Cassin's Finch,Carpodacus cassinii . (Fig. 11.65) Male length 5 3/4 in (14.75 cm), female length 5 3/4 in (14.5 cm); male weight 1 oz (27.3 g), female weight 1 oz (26.6 g). Common summer resident in the White-Inyo Range, between 6,750 and 10,500 ft (2,060 and 3,200 m).
In the White and Inyo mountains, Cassin's Finches have been recorded most commonly in the aspen-willow association and in both Bristlecone and Lodgepole pines. They prefer open forest growth in cool, dry places. Locally, they may nest in cool portions of the pinyon zone, particularly where trees are large and mixed with Mountain Mahogany. This finch takes seeds from the ground and nips buds of aspen and conifers. Females of all ages and males up to approximately 14 months old are similar in appearance, with a streaked gray and brown plumage. Older males contrast strikingly, with their vivid hues of pinkish and rose-red. Studies in other areas have shown that the population size of Cassin's Finches fluctuates annually, presumably in response to the abundance of tree buds. Furthermore, males usually outnumber females. Like most true finches, this species demonstrates no real territorial system. Instead, the male defends the space around his mate wherever she goes rather than the area of the nest site. References: Mewaldt and King (1985), Samson (1976).
House Finch,Carpodacus mexicanus. (Fig. 11.66) Male length 5 1/4 in (13.25 cm), female length 5 in (13 cm); male weight 5/8 oz (18.4 g), female weight 5/8 oz
Figure 11.65
Cassin's Finch.
Figure 11.66
House Finch.
(19 g). Uncommon and local summer resident in the White-Inyo Range; occurs from the lowlands to 9,000 ft (2,740 m) elevation.
Although this species is common around lowland ranches and urban plantings, it is sparse in the mountains proper. Most records are from the pinyon-juniper belt. Springs are especially favored because they can fulfill the species' daily water requirements. Desert cactus fruits, when available, also provide some moisture. House Finches feed on a variety of seeds and small fruits. Nests are commonly placed in steep canyon walls or bluffs. Because they prefer warm and sunny environments, there is minimal overlap in the region between this species and its congener, the Cassin's Finch. References: Thompson (1960), Weathers (1983).
Red Crossbill,Loxia curvirostra. (Fig. 11.67) Male length 53/4 in (14.75 cm), female length 5 3/4 in (14.5 cm); male weight 1 1/8 oz (33 g), female weight 1 1/5 oz (34 g). Irregular permanent resident in the White-Inyo Range; recorded from 6,750 to 10,500 ft (2,060 to 3,200 m). Numbers fluctuate annually; usually the species is either uncommon or relatively common.
This erratic species has been recorded uncommonly from the ranges, where its presence and numbers depend on conifer seed production, which also varies annually.
Figure 11.67
Red Crossbill.
Trees producing requisite seeds include Singleleaf Pinyon, Limber Pine, Bristlecone Pine, and Lodgepole Pine. Lodgepole Pine, an important food source for crossbills in the Sierra Nevada and the Cascade Mountains, is presumably of slight importance in the White-Inyo Range because of its very local occurrence there. This finch is usually first detected overhead by its kip-kip or jip-jip call notes. With good fortune, one may encounter a feeding group in a conifer top. The birds quietly work over terminal cones but then burst away without notice in a flurry of call notes. Breeding may occur in any season if seeds are abundant. In the White Mountains, family groups were recorded in the pinyon zone in early June. By the third week of June, these groups had coalesced into larger flocks. References: Beedy and Granholm (1985), Grinnell and Storer (1924).
References
American Ornithologists' Union. 1983. Check-list of North American birds , 6th ed. Allen Press, Lawrence, Kansas.
Anderson, Stanley H. 1976. Comparative food habits of Oregon Nuthatches. Northwest Science 50:213–221.
Armstrong, Joseph T. 1965. Breeding home range in the nighthawk and other birds; its evolutionary and ecological significance. Ecology 16:619–629.
Balda, Russell P., and Gary C. Bateman. 1973. The breeding biology of the Pinyon Jay. Living Bird 11:5–12.
Balgooyen, Thomas G. 1976. Behavior and ecology of the American Kestrel (Falco sparverius L.) in the Sierra Nevada of California. University of California in Zoology 103:1–83.
Balph, Martha H. 1977. Winter social behavior of Dark-eyed Juncos. Communication, social organization, and ecological implications. Animal Behaviour 25:859–884.
Beedy, Edward C., and Stephen L. Granholm. 1985. Discovering Sierra birds . Yosemite Natural History Association and Sequoia Natural History Association.
Behle, William H. 1942. Distribution and variation of the Horned Larks (Otocoris alpestris ) of western North America. University of California Publications in Zoology 46:205–316.
Bent, Arthur C. 1968. Life histories of North American cardinals, grosbeaks, buntings, towhees, finches, sparrows, and allies . U.S. National Museum Bulletin 237, Smithsonian Institution, Washington, D.C.
Biermann, Gloria C., and Spencer G. Sealy. 1982. Parental feeding of nestling Yellow Warblers in relation to brood size and prey availability: Auk 99:332–341.
Brown, Jerram L. 1964. The integration of agonistic behavior in the Steller's Jay, Cyanocitta stelleri (Gmelin). University of California Publications in Zoology 60:223–328.
Brown, Leslie, and Dean Amadon. 1968. Eagles. hawks, and falcons of the world . 2 vols. McGraw-Hill, New York.
Burton, John A. (ed.). 1973. Owls of the world . E. P. Dutton Inc., New York.
Caccamise, Donald. 1974. Competitive relationships of the Common and Lesser Nighthawks. Condor 76.1–20.
Cade, Thomas J. 1982. Falcons of the world . Cornell University Press, Ithaca, N.Y.
Christensen, Glen C. 1954. The Chukar Partridge in Nevada . Nevada fish and Game Commission Biological Bulletin no. 1, State Printing Office, Carson City, Nevada.
Davis, Cheyleen M. 1979. A nesting study of the Brown Creeper. Living Bird 17:237–263.
Davis, Deborah. 1979. Morning and evening roosts of Turkey Vultures at Malheur Refuge, Oregon. Western Birds 10:125–130.
Davis, John. 1960. Nesting behavior of the Rufous-sided Towhee in coastal California. Condor 62:434–456.
Dilger, William C. 1956. Hostile behavior and reproductive isolating mechanisms in the avian genera Catharus and Hylccichla . Auk 73:313–353.
Dixon, Keith L. 1949. Behavior of the Plato Titmouse. Condor 51:110–136.
Dixon, Keith L. 1965. Dominance-subordination relationships in Mountain Chickadees. Condor 67:291–299.
Earhart, Caroline M., and Ned K. Johnson. 1970. Size dimorphism and food habits of North American owls. Condor 72:251–264.
Emlen, Stephen T., James D. Rising, and William L. Thompson. 1975. A behavioral and morphological study of sympatry in the Indigo and Lazuli Buntings of the Great Plains. Wilson Bulletin 87:145–179.
Ervin, Stephen. 1977. Flock size, composition, and behavior in a population of Bushtits. Bird-Banding 48:97–109.
Fitch, Henry S. 1947. Predation by owls in the Sierran foothills of California. Condor 49:137–151.
Fitch, Henry S., Freeman Swenson, and Daniel F. Tillotson. 1946. Behavior and food habits of the Red-tailed Hawk. Condor 48:205–237.
Franzreb, Kathleen E. 1984. Foraging habits of Ruby-crowned and Golden-crowned Kinglets in an Arizona montane forest. Condor 86:139–145.
Garrett, Kimball, and Jon Dunn. 1981. The birds of southern California . Los Angeles Audubon Society, Los Angeles.
Goodwin, Derek. 1976. Crows of the world . Cornell University Press, Ithaca, N.Y.
Grinnell, Joseph, and Alden H. Miller. 1944. The distribution of the birds of California . Pacific Coast Avifauna no. 27, Cooper Ornithological Club, Berkeley, California.
Grinnell, Joseph, and Tracy I. Storer. 1924. Animal life in the Yosemite . University of California Press, Berkeley.
Griscom, Ludlow, and Alexander Sprunt, Jr. 1957. The warblers of America . Devin-Adair, New York.
Gutiérrez, Ralph J. 1980. Comparative ecology of the Mountain and California Quail in the Carmel Valley, California. Living Bird 18:71–93.
Hadley, N. F. 1969. Breeding biology of the Gray-headed Junco, Junco caniceps (Woodhouse) in the Colorado Front Range. Colorado Field Ornithologist 5:15–21.
Heckenlively, Donald B. 1970. Song in a population of Black-throated Sparrows. Condor 72:24–36.
Howell, J. C. 1942. Notes on the nesting habits of the American Robin. American Midland Naturalist 28:529–603.
Hubbard, John P. 1969. The relationships and evolution of the Dendroica coronata complex. Auk 86:393–432.
Hutto, Richard L. 1981. Seasonal variation in the foraging behavior of some migratory western wood warblers. Auk 93:765–777.
Jaeger, Edmund C. 1948. Does the Poor-will "hibernate"? Condor 50:45–46.
James, Frances C., and Hank H. Shugart, Jr. 1974. The phenology of the nesting season of the American Robin (Turdus migratorius ) in the United States. Condor 76: 159–168.
James, Ross D. 1981. Factors affecting variation in the primary song of North American Solitary Vireos (Aves: Vireonidae). Canadian Journal of Zoology 59:2001–2009.
Johnsgard, Paul A. 1973. Grouse and quails of North America . University of Nebraska Press, Lincoln.
Johnsgard, Paul A. 1975. North American game birds of upland and shoreline . University of Nebraska Press, Lincoln.
Johnsgard, Paul A. 1983. The hummingbirds of North America . Smithsonian Institution Press, Washington, D.C.
Johnson, David H., Monroe D. Bryant, and Alden H. Miller. 1948. Vertebrate animals of the Providence Mountains area of California. University of California Publications in Zoology 48:221–376.
Johnson, Ned K. 1963. Biosystematics of sibling species of flycatchers in the Empidonax hammondii-oberholseri-wrightii complex. University of California Publications in Zoology 66:79–238.
Johnson, Ned K. 1966. Bill size and the question of competition in allopatric and sympatric populations of Dusky and Gray Flycatchers. Systematic Zoology 15:70–87.
Johnson, Ned K. 1976. Breeding distribution of Nashville and Virginia's Warblers. Auk 93:219–230.
Johnson, Ned K., and Carla Cicero. 1986. Richness and distribution of montane avifaunas in the White-Inyo region. California. In C. A. Hall, Jr. and D. J. Young (eds.). Natural history of the White-Inyo Range, eastern California and Western Nevada, and high altitude physiology , pp. 137–159. University of California White Mountain Research Station Symposium, August 23–25, 1985, Bishop, Calif., vol. 1.
Johnson, Ned K., and Carla B. Johnson. 1985. Speciation in sapsuckers (Sphyrapicus ): II. Sympatry, hybridization, and mate preference in S. ruber daggetti and S. nuchalis . Auk 102:1–15.
Johnson, Richard E. 1965. Reproductive activities of Rosy Finches, with special reference to Montana. Auk 82:190–205.
Kendeigh, S. Charles. 1941. Territorial and mating behavior of the House Wren . Illinios Biological Monograph no. 18, University of Illinois Press, Urbana.
Kilham, Lawrence. 1972. Reproductive behavior of White-breasted Nuthatches. II. Courtship Auk 89:115–129.
Kilham, Lawrence. 1973. Reproductive behavior of the Red-breasted Nuthatch. I. Courtship. Auk 90:597–609.
Kroodsma, Donald E. 1971. Song variations and staging behavior in the Rufous-sided Towhee, Pipilo erythrophthalmus oregonus. Condor 73:303–308.
Kroodsma, Donald E. 1972. Variations in songs of Vesper Sparrows in Oregon. Wilson Bulletin 84:173–178.
Kroodsma, Donald E. 1975. Song patterning in the Rock Wren. Condor 77:294–303.
La Rivers, Ira. 1944. Observations on the nesting mortality of the Brewer Blackbird, Euphagus cyanocephalus. American Midland Naturalist 32:417–437.
Leopold, A. Starker. 1977. The California Quail . University of California Press, Berkeley.
Marshall, Joe T., Jr. 1957. Birds of Pine-oak Woodland in southern Arizona and adjacent Mexico . Pacific Coast Avifauna no. 32, Cooper Ornithological Society, Berkeley, California.
Marti, Carl D. 1976. A review of prey selection by the Long-eared Owl. Condor 78:331–336.
Martin, Dennis J. 1977. Songs of the Fox Sparrow. I. Structure of song and its comparison with song in other Embenzidae. Condor 79:209–221.
Martin, Dennis J. 1979. Songs of the Fox Sparrow. II. Intra- and interpopulation variation. Condor 81:173–184.
McEllin, Shaun M. 1979. Nest sites and population demographics of White-breasted and Pygmy Nuthatches in Colorado. Condor 81:348–352.
Mengel, Robert M. 1964. The probable history of species formation in some northern wood warblers (Parulidae). Living Bird 3:9–43.
Mewaldt, L. Richard, and James R. King. 1985. Breeding site faithfulness, reproductive biology, and adult survivorship in an isolated population of Cassin's Finches. Condor 87:494–510.
Miller, Alden H. in 1941. Speciation in the avian genus Junco. University of California Publications in Zoology 44:173–434.
Miller, Alden H. 1951. An analysis of the distribution of the birds of California. University of California Publications in Zoology 50:531–644.
Miller, Alden H., and Robert C. Stebbins. 1964. The lives of desert animals in Joshua Tree National Monument . University of California Press, Berkeley.
Miller, Edwin V. 1941. Behavior of the Bewick Wren. Condor 43:81–99.
Moldenhauer, Ralph R., and John A. Wiens. 1970. The water economy of the Sage Sparrow, Amphispiza belli nevadensis. Condor 72:265–275.
Morrison, Michael L. 1981. The structure of western warbler assemblages: Analysis of foraging behavior and habitat selection in Oregon. Auk 98:578–588.
Morrison, Michael L. 1982. The structure of western warbler assemblages: Ecomorphological analysis of the Black-throated Gray and Hermit Warblers. Auk 99:503–513.
Morse, Douglass H. 1971. Effects of the arrival of a new species upon habitat utilization by two forest thrushes in Maine. Wilson Bulletin 83:57–65.
Morse, Pamela J. 1979. Pairing and courtship in the North American dipper. Bird-Banding 50:62–65.
Morton, Martin L. 1978. Snow conditions and the onset of breeding in the Mountain White-crowned Sparrow. Condor 80:285–289.
Morton, Martin L., Judith L. Horstman, and Janet M. Osborn. 1972. Reproductive cycle and nesting success of the Mountain White-crowned Sparrow (Zonotrichia leucophrys oriantha ) in the central Sierra Nevada. Condor 74:152–163.
Nice, Margaret M. 1937 (reprinted 1964). Studies in the life history of the Song Sparrow . 2 vols. Dover, New York.
Norris, Robert A. 1958. Comparative biosystematics and life history of the nuthatches Sitta pygmaea and Sitta pusilla . University of California Publications in Zoology 56:119–300.
Olendorff, Richard R. 1975. Golden Eagle Country . Knopf, New York.
Petrides, George A. 1938. A life history of the Yellow-breasted Chat. Wilson Bulletin 50: 184–189.
Power, Harry W. 1966. Biology of the Mountain Bluebird in Montana. Condor 68:351–371.
Power, Harry W. 1980. The foraging behavior of Mountain Bluebirds with emphasis on sexual foraging differences . Ornithological Monographs no. 28, American Ornithologists' Union, Washington, D.C.
Price, Frank E., and Carl E. Bock. 1983. Population ecology of the Dipper (Cinclus mexicanus) in the Front Range of Colorado . Studies in Avian Biology no. 7, Allen Press, Lawrence, Kansas.
Rea, Amadeo M. 1983. Once a river . University of Arizona Press, Tucson.
Reynolds, John D., and Richard W. Knapton. 1984. Nest site selection and breeding biology of the Chipping Sparrow. Wilson Bulletin 96:488–493.
Reynolds, Richard T., and E. Charles Meslow. 1984. Partitioning of food and niche characteristics of coexisting Accipiter during breeding. Auk 101:761–779.
Reynolds, Timothy D. 1981. Nesting of the Sage Thrasher, Sage Sparrow, and Brewer's Sparrow in southeastern Idaho. Condor 83:61–64.
Reynolds, Timothy D., and Terrell D. Rich. 1978. Reproductive ecology of the Sage Thrasher (Oreoscoptes montanus ) on the Snake River Plain in south-central Idaho. Auk 95:580–582.
Ritter, Lyman V. 1983. Nesting ecology of Scrub Jays in Chico, California. Western Birds 14:147–158.
Root, Richard B. 1967. The niche exploitation pattern of the Blue-gray Gnatcatcher. Ecological Monographs 37:317–350.
Root, Richard B. 1969. The behavior and reproductive success of the Blue-gray Gnatcatcher. Condor 71:16–31.
Salomonson, Michael G., and Russell P. Balda. 1977. Winter territoriality of Townsend's Solitaires (Myasdestes townsendi ) in a Pinyon-Juniper-Ponderosa Pine ecotone. Condor 79:148–161.
Samson, Fred B. 1976. Territory, breeding density, and fall departure in Cassin's Finch. Auk 93:477–497.
Scott David M., and C. Davison Ankney. 1983. The laying cycle of Brown-headed Cowbirds: Passerine chickens? Auk 100:583–592.
Shedd, Douglas H. 1982. Seasonal variation and function of mobbing and related antipredator behaviors of the American Robin (Turdus migratorius ). Auk 99:342–346.
Short, Lester L. 1965. Hybridazation in the flickers (Colaptes ) of North America. Bulletin of the American Museum of Natural History 129:307–428.
Short, Lester L. 1971. Systematics and behavior of some North American woodpeckers, genus Picoides (Aves). Bulletin of the American Museum of Natural History 145:1–118.
Short, Lester L. 1982. Woodpeckers of the world . Monographs, Delaware Museum of Natural History, Greenville.
Smith James N. M., Yoram Yom-Tov, and Richard Moses. 1982. Polygyny, male parental care, and sex ratio in Song Sparrows: An experimental study. Auk 99:555–564.
Snyder, Noel F. R., and James W. Wiley. 1976. Sexual size dimorphism in hawks and owls of North America . Ornithological Monographs no. 20, American Ornithologists Union, Washington, D.C.
Thompson, Charles F. 1977. Experimental removal and replacement of territorial male Yellow-breasted Chats. Auk 94:107–113.
Thompson, William L. 1960. Agonistic behavior in the House Finch. Part II: Factors in aggressiveness and sociality. Condor 62:378–402.
Thompson, William L. 1976. Vocalizations of the Lazuli Bunting. Condor 78:195–207.
Tomback, Diana F. 1978a. Foraging strategies of Clark's Nutcracker. Living Bird 16:123–161.
Tomback, Diana F. 1978b. Pre-roosting flight of the Clark's Nutcracker. Auk 95:554–562.
Verbeek, Nicolaas A. M. 1967. Breeding biology and ecology of the Horned Lark in alpine tundra. Wilson Bulletin 79:208–218.
Verner, Jared, and Lyman V. Ritter. 1983. Current status of the Brown-headed Cowbird in the Sierra National Forest. Auk 100:355–368.
Walsberg, Glenn E. 1981. Nest-site selection and the radiative environment of the Warbling Vireo. Condor 83:86–88.
Weathers, Wesley W. 1983. Birds of Southern California's Deep Canyon . University of California Press, Berkeley.
Weston, Henry G., Jr. 1947. Breeding behavior of the Black-headed Grosbeak. Condor 49:54–73.
Wiens, John A. 1982. Song pattern variation in the Sage Sparrow (Amphispiza belli ): Dialects or epiphenomena? Auk 99:208–229.
Wiley, R. Haven. 1973. Territoriality and non-random mating in Sage Grouse, Centrocercus urophasianus. Animal Behaviour Monographs 6:87–169.
Williams, Laidlaw. 1952. Breeding behavior of the Brewer Blackbird. Condor 54:3–47.