MORPHOLOGY
GENERAL CHARACTERISTICS Treeshrews resemble those first, small mammals that skulk in hiding from slavering dinosaurs in the corners of dramatic scenes depicting the Cretaceous. In overall shape they closely mirror some of those ancient mammals: they are of similar size, limb,
The general treeshrew morphology is fine tuned to the individual ecological characteristics of each species in ways that are standard among mammals. Arboreal species have shorter, broader hind feet than do terrestrial ones; the more insectivorous terrestrial species have elongated muzzles, and those that dig have long claws. Pentail treeshrews are particularly adapted for arboreal life: their bauplan resembles that of small marsupials rather than squirrels. Their hands and feet are short, broad, and remarkably strong, with sharp curved claws and digits that can be spread at least 180 degrees across the surface of a branch. On thin supports the hallux is placed above the branch at a wide angle to the other digits, which grip the side of the support (Le Gros Clark 1926; Gould 1978). Their long tail swings in counterbalance when they travel on slender branches (Gould 1978; pers. obs.). Members of the genus Tupaia also have structural adaptations for climbing, including first toes that move independently to clasp the top of a branch and ankle joints that can rotate somewhat and allow the treeshrews to descend trees headfirst (Jenkins1974), as dosquirrels. Ptilocercuslowii likewise runs down tree trunks headfirst (pers. obs.), and its anatomy shows that it rotates its ankles more

Fig. 2.1. The Jurassic pantothere Crusafontia, reconstructed from an almost perfect skeleton (above) (from Benton 1991). A large treeshrew, Tupaia tana (below).
Pentails seem to prefer to travel connected pathways, without much jumping (pers. obs.), but they seem to be able to jump. On the ground they are apparently quite awkward, and move with “a series of short quadripedal hops” (Gould 1978). Tupaia species move with gaits similar to those of most small mammals: at slow speeds they walk, and at a faster pace they gallop or half-bound (Jenkins 1974). They are arboreal leapers and jump to cross small gaps. Even species that forage on the ground are skilled climbers and jumpers.
TEETH The teeth of treeshrews have been the subject of several studies; below is a summary of some of the published conclusions, principally those of P. M. Butler (1980). Treeshrews have modified tribosphenic molars, of the dilambodont type in Tupaiinae (roughly triangular teeth with three high, sharp main cusps separated by basins). This primitive tooth type arose in the Cretaceous period and is also currently found in didelphid marsupials, but it is not as primitive as the teeth of tenrecs or Solenodon. Other insect-eating mammals, such as bats and Insectivora, also retain or have evolved convergent versions of tribosphenic molars. The form of the teeth is more primitive in Ptilocercus than in Tupaia(Butler 1980). Teeth are evolutionarily plastic, and their form follows function. As in other living taxa, the teeth of treeshrews have a mixture of derived and primitive features.
[1] The concept of primitiveness in features of organisms is relative: of two designs or “states” that a character can have, the most primitive is the one closest to the design that the common ancestor of the two organisms that carry them had. In evolutionary time, the most primitive evolved prior to more advanced (derived) models, and advanced characters were derived through evolution from primitive ones. The scenario is trivially simple, and all of modern evolutionary systematics stands on it; but operationally it can be difficult or impossible to know what design an ancestor had. We are fortunate that mammalian teeth are the best and most often preserved fossil mammal bits, so that for teeth (and little else, I may add) we have an excellent record of much of mammalian history and we can say with some confidence how basic patterns of dentition evolved.
Without it being a law, evolution often seems to favor an increase in a lineage's efficiency in certain tasks, at the expense of others; that is, a change to more “specialized” and less “generalized.” A theme in this book will be why I think treeshrews are, or are not, primitive or generalized mammals.
The long face and muzzle of Tupaia species overlies long jaws. The fourth premolar and three molars of treeshrews occlude tightly and form the chewing apparatus. The front portion of the mandible and maxillary forward of the fourth premolar is stretched out such that the anterior premolars and canines are separated by wide gaps (fig. 2.2). The upper
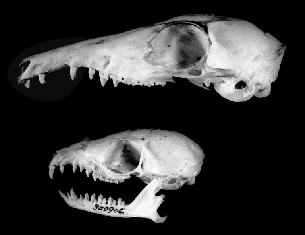
Fig. 2.2 Skulls of treeshrews: large treeshrew,Tupaia tana(above);lesser treeshrew,Tupaia minor(below).
In general, tribosphenic teeth have a shearing and puncturing/crushing occlusal function, well suited to reducing the brittle exoskeletons of arthropods, which can be chopped and crushed into particles with a simple vertical chewing motion. But Tupaia also has some transverse jaw motion and a broadening of the molars compared to those of early mammals (Butler 1980). These features together may increase the variety of foods that Tupaia can efficiently handle (Fish and Mendel 1982). Within the Tupaiidae there is considerable species to species variation in dentition, with the development of stabbing incisors greatest in Ptilocercus.
DIGESTIVE TRACT The parts of a mammalian digestive tract vary in size and structure according to the functional specialization of each part, but generally the more time-consuming and complex the digestion process, the larger and more complex the compartment in which it takes place. The most specialized of all mammalian diets include the structural molecules of the vegetative parts of plants (cellulose). Digestion of these requires an array of microorganisms, a large storage chamber to house them, secretion of a culture medium to keep them viable, digestion time to allow them to work, and area to absorb the products. At the other extreme is a diet of simple carbohydrates (sugars) and proteins (animal tissue), which merely needs a small chamber where enzymes break down ingested tissues into small molecules that can be directly and quickly absorbed.
The digestive tracts of treeshrews are of the simplest gross structure (Hill 1958; see table 2.1 below): the stomach is simple, and the small intestine is narrow and of standard length for a mammal. The caecum is absent in T. tana and narrow, smooth, and rudimentary to short in the other species, with that of T. gracilis the longest. The large intestine is hardly differentiated from the small (it is difficult to identify in T. tana), and it is extremely narrow, smooth, and short.
Among other mammals, only bats as an order possess no caecum, and their whole intestinal tract resembles in its simplicity that of treeshrews, as does that of many insectivorous marsupials. Insectivora likewise have similar simple, tubelike guts. In contrast, the gut of an elephant shrew (Macrocelides), which eats some plant matter, has a very large caecum (Mitchell 1916). Mitchell (1905, 1916), with a remarkably modern systematic view, compared the intestinal morphology of an impressive number of mammalian as well as avian taxa: “[The primitive gut] possesses a caecum, or possibly a pair of caeca, homologous with the paired caeca
| Species (N) | HBL | TGL | Colon | Cecum | TGL/HBL | Colon/TGL | Reference |
|---|---|---|---|---|---|---|---|
| Treeshrews | |||||||
| Tupaia tana (5) | 210 | 908 | 50 | 0 | 4.3 | 0.055 | Emmons 1991 |
| T. montana (1) | 175 | 820 | 30 | 8 | 4.7 | 0.037 | Emmons 1991 |
| T. gracilis (1) | 133 | 280 | 35 | 11 | 2.1 | 0.125 | Emmons 1991 |
| T. minor (2) | 124 | 435 | 42 | 7 | 3.5 | 0.97 | Emmons 1991 |
| Fruit Bats | |||||||
| Pteropus vampyrus | 286 | 1,280 | 0 | 0 | 4.5 | Richardson, Stuebing, and Normah 1987 | |
| Cynopterus brachyotis | 101 | 656 | 0 | 0 | 4.5 | Richardson, Stuebing, and Normah 1987 | |
| Artibeus jamaicencis (2) | 81 | 357 | 0 | 0 | 4.4 | Emmons 1991 | |
| Carollia perspicillata | 58 | 200 | 0 | 0 | 3.4 | Klite 1965 | |
| Insectivorous Bats | |||||||
| Molossus molossus | 78 | 123 | 0 | 0 | 1.6 | Klite 1965 | |
| Pteronotus parnellii | 71 | 186 | 0 | 0 | 2.6 | Klite 1965 | |
| Squirrel Monkey (omnivore) | |||||||
| Saimiri sciureus | 270 | 1,307 | 159 | 39 | 4.8 | 0.12 | Fooden 1964 |
The teeth and digestive tracts of treeshrews are thus similar to those of many other insectivorous mammals and possess a number of both primitive and more derived features.