Phosphorus, Carbon, and Sulphur Cycles
Though they are allotted relatively less space for discussion, it is not intended to minimize the importance of bacterial activity in the transformation of these elements.
Phosphorus is another of the important plant nutrients which has a biologically activated cycle involving alternation of organic and inorganic phases. Whereas carbon dioxide is always present in sufficient quantity for the needs of marine plants, the phosphorus, like the nitrogen, may be depleted to the extent of interfering with the fullest growth. In laboratory experiments phosphate is apparently quickly regenerated by bacteria and other agencies following the death of plants and animals. In studies on stored sea water, Renn (1937) and Waksman et al (1938) found that phosphates are assimilated by bacteria in the growth of their cell substance but that bacterial competition with diatoms for this element is not serious under these conditions, since upon death and autolysis of the bacteria the phosphates are returned in a few days in mineralized form. When a supply of decomposing diatoms is at hand, the phosphates are regenerated from these more rapidly than they are consumed by the bacteria. Under these experimental conditions, approximately two thirds of the total phosphorus present in the diatoms was liberated within 132 hours through bacterial activities. Cooper (1935) found a more rapid regeneration of phosphates from animal plankton than from diatoms.
It must be emphasized, however, that these quick regenerations of phosphates in laboratory experiments are at variance with findings relative to the rate of regeneration in the sea, where renewal in the water is much slower, requiring three to four months (p. 260).
The complex carbon compounds built up by marine plants and animals are decomposed through bacterial action with the formation of carbon dioxide. This formation supplements the carbon dioxide that is produced through respiration by all other organisms and helps maintain the
Sulphur is one of the essential constituents of living matter, and its compounds, like those of other elements of protoplasm, are acted upon by bacteria. Transformations wrought by bacteria in the chemical compounds of sulphur may have far-reaching effects on the associated plant and animal populations and also upon chemical and geological phenomena. First, it may be mentioned that plants utilize a small quantity of sulphur in their metabolism; the compound used, namely sulphate, is produced by chemical or biological oxidation. Second, in the decomposition of organic compounds containing sulphur, hydrogen sulphide is produced as a disintegration product which, when present in large quantities, is inimical to plant and animal life. The odor of hydrogen sulphide is frequently noticeable at low tide in the organically rich muds of protected bays and in muds brought up from deeper water.
This hydrogen sulphide may be formed by splitting off the H2S group of the protein molecule or through a process of reduction of the proteins by heterotrophic bacteria. The inorganic compounds of sulphur, such as sulphates and sulphites, may also be reduced to hydrogen sulphide by heterotrophic bacteria under anaerobic conditions in the presence of organic material. Hence it is important to note that through intramolecular respiration oxidation of organic matter can continue even though all free oxygen has been removed. In areas with little or no circulation of water near the bottom and with an accumulation of organic detritus these heterotrophs as well as true sulphur bacteria abound, but the hydrogen sulphide evolved inhibits any other forms of life. In the Black Sea hydrogen sulphide is found from 180 m to the bottom; and from a depth of 300 m to 1500 m it increases from 1.48 cm3/l to 6.17 cm3/l (fig. 237, p. 872, Nikitin, 1931). Other classical examples are found in the oyster pools of threshold fjords (p. 802).
Not all bacteria that are involved in the sulphur cycle produce hydrogen sulphide. The metabolic requirements of the true sulphur bacteria produce the opposite effect through the process of oxidation. During the assimilation of carbon dioxide by autotrophic sulphur bacteria in the presence of free oxygen, the hydrogen sulphide is oxidized according to the following chemical equation:
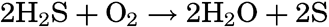
In some types of bacteria, including many purple bacteria, the sulphur is deposited as reserve material within the bacterial cell. The nitrogen needed is obtained from ammonium salts.