MARINE BACTERIA AND THEIR ROLE IN THE BIOLOGICAL AND CHEMICAL CYCLES IN THE SEA
Because of the specific role of bacteria in the nutritional cycle of the sea, it is fitting at this point to consider in some detail the general biology and activities of these microorganisms.
Although the existence of marine bacteria has long been recognized and was early verified even in depths of over 1000 m in the Gulf of Naples (Russell, 1892), yet it is only in recent years that the study of bacteria in the metabolism of the sea has gotten well under way. For a synoptic review of works especially relevant to the development of the subject the student is referred to Benecke (1933).
Structure and Reproduction
Bacteria are unicellular organisms although some of them form chains or groups of cells. Morphologically there are the following three forms:
In their structure and activities they form a rather well-defined group of organisms, although some possess animal characteristics, especially in chemical composition, while others approach more nearly the plants, particularly the blue-green algae, with which they share the characteristic of having scattered nuclear material. Some authors consider them as occupying an intermediate position between the plants and animals (Jordan, 1931). A distinct plant characteristic is the ability of some to synthesize such complex substances as amino acids with only ammonia as a source of nitrogen. It is important to note that none possess chlorophyll, nor are their cell walls composed of cellulose. For convenience, however, they are all placed with the plants; but owing to the absence of chlorophyll in their structure they must be regarded as fungi, and since their common method of multiplication is by simple fission, they are named fission fungi or Schizomycetes.
Structurally, the marine bacteria are represented mostly by motile rods and various types of vibrios or comma-shaped forms, and there are fewer spore-formers in the sea than on land (Waksman, 1934). About 70 per cent of marine bacteria are colored as opposed to 15 per cent of the terrestrial forms.
Characteristically, bacteria are the smallest of all organisms, some measuring only 0.0005 mm in diameter. This extreme minuteness has a profound bearing on the activities of bacteria and also introduces very special problems and difficulties in the technique of collecting and in the estimation of actual numbers and mass.
Characteristic also of bacteria is their rapid rate of reproduction. This is accomplished by vegetative cell division. It may again be emphasized here that in nearly all instances where swarms of organisms—bacteria, diatoms, dinoflagellates—occur in such large numbers that the water is discolored by the accumulation of their bodies, the method of reproduction has been one of binary fission. Assuming an uninterrupted physiological state and optimum growing conditions, the accumulation of individuals can be extremely rapid since the increase is by geometric progression. The rate of division of bacteria may be as frequent as once every twenty or thirty minutes. Hence, biologically, there is a tremendous potentiality to build up an excessively large mass. However, large masses do not accumulate because of natural checks which hold the tendency to mass production within bounds. Among such checks may be mentioned availability of food supply, formation of toxic metabolic products, physical-chemical conditions of the water, nature of substratum, and consumption by other organisms. An understanding of these checks or controlling factors is one of the ends sought in the study of marine bacteriology, for such an understanding provides an explanation
Associated with bacterial reproduction is the faculty of spore formation, whereby the individual bacterium of certain species is enabled to withstand adverse conditions while remaining in a state of quiescence for long periods of time. How significant this faculty is in the marine environment, where physical and chemical conditions are relatively uniform, at least with respect to the open water, is not clear. Viable bacteria have been taken from strata of marine mud where they must have remained buried for thousands of years (p. 920). However, the functions of these buried forms as effective transformers in the water or immediate bottom have apparently ceased, and the likelihood of their revival in the sea bottom is remote. Those not too deeply buried may serve to some extent as food for burrowing detritus-feeding animals.
Bacterial Modes of Life
For our immediate study of the relationship of bacteria to other organisms and to the chemical cycles of the sea, it is necessary to take into consideration the vital implications of their activities as a part of the dynamic energy cycle within the marine population of which they are a part. The investigations of marine bacteria are therefore concerned chiefly with their physiological processes as applied to the sea and its chemical and biological problems. Their indispensable function in the economy of the sea is primarily one concerned with transformation of organized substances and not with accumulation or storage of organic matter.
In order to understand better the activities of bacteria, it is necessary to distinguish between certain of the different modes of life. These are concerned especially with the source of nutrition and the oxygen supply.
Autotrophic Bacteria. These resemble green plants in their ability to build carbohydrates and proteins out of the simple substances carbon dioxide and inorganic salts. Some of these, known as photosynthetic, possess coloring material, or bacteriochlorin, and use radiant energy in building up protoplasm, while others, known as chemosynthetic, derive their energy from the oxidation of various inorganic compounds such as H2S, S, or NH4.
The amount of organic material synthesized in the sea by bacteria is small when compared to that produced by the chlorophyll-bearing plants. It is not fully known to what extent the chemosynthetic forms contribute to organic material on the sea bottom where depths are so great that there is insufficient light for photosynthesis, but the concentration of benthic animals suggests correlation mainly with the pelagic and benthic algae as the source of primary food and not with the bacteria.
However, autotrophic purple sulphur bacteria have been on several occasions reported as being so abundant in isolated inshore situations as to impart a red tint to the water, to the surface of algae, or to the bottom (Benecke, 1933).
Heterotrophic Bacteria. These obtain their energy by the oxidation of organic compounds. Hence they live as saprophytes or parasites. Most bacteria of the sea are of this type.
We have learned that in the cycle of organic material in the sea it falls to the phytoplankton, in particular, but also to other algae to synthesize organic substance from such inorganic raw materials as carbon dioxide, nitrates, phosphates, and others. But the store of certain of these materials may be exhausted in the euphotic layer and become bound as part of the substance of organisms. However, over a long period of time organisms die at the same rate as they are born, and thus a continuous return of the raw material is possible if a transforming agency is provided. Such an agency exists in the heterotrophic bacteria, whose enormous task is to perpetuate this phase of the cycle through mineralization of organic matter. Animals take part in the general cycle, but owing to the fact that as a group they are neither producers nor transformers (in the sense that plants and bacteria are), a reduced cycle could go on without them; indeed, considerable quantities of plants must at times by-pass the animals and be directly reduced to inorganic state by bacteria (fig. 248, p. 926). The interposition of animals no doubt functions to smooth out the cycle so that bacterial activity goes on at a more even rate throughout the year. There appears to be no evidence that the number or kind of bacteria show any marked seasonal cycles (Lloyd, 1930, ZoBell, 1938). During seasons of plant production, rapidly growing animals incorporate large quantities of plant substance into their own structure and, owing to the longer life cycles of many animals, much of this material may become more gradually available to bacteria at periods of minimum plant production. Illustrative of this is Lohmann's study of the cycle of plankton organisms over the whole year at Kiel. According to his observations the plants for the year averaged 56 per cent of the volume of the total pxsankton However, it was found that from December to February plants formed scarcely a third of the total plankton. At Plymouth the winter production of plants is set at about one fourth of the summer rate. These rates apply only to calculations based on given diatom populations during twenty-four hour periods of production during these seasons and may vary with other species and conditions (Harvey et al, 1935).
Utilization of Dissolved Organic Matter. In discussing the chemistry of the sea, it was pointed out (p. 248) that an appreciable quantity of organic matter is present in solution in sea water. The concentrations per unit volume of water are very much smaller than those
It was believed by Pütter (1907) that many marine animals are able to absorb dissolved organic matter through their gills and integuments and may thus obtain a portion of their food through utilization of the organic matter in solution in the sea. There is little evidence that other forms than bacteria make any considerable use of this supply of nutriment (Krogh, 1934b, Bond, 1933).
According to some authorities the low concentration and uniform distribution of dissolved organic matter in the sea may result from the action of heterotrophic bacteria. Sea water may be looked upon as a dilute culture medium in which the upper limit of concentration of dissolved organic substance is a threshold value maintained by the bacteria at a level below 10 mg/l. In apparent contradiction it must be mentioned that this may appear too dilute for bacterial growth, since for some non-marine bacteria 10 mg/l of different carbon compounds is the minimum concentration in which successful growth occurs (Stephenson, 1939). This may also be true for marine bacteria suspended free in the water. But when the bacteria and other organic material are adsorbed to solid surfaces a greater efficiency in utilization of dilute organic matter is possible, and growth occurs although concentrations may be much lower than 10 mg/l.
Experimentally it has been shown by ZoBell and Anderson (1936a) that in normal filtered sea water bacteria may flourish but do so best when grown in receptacles providing the greatest solid surface area per unit volume of sea water. Increased surfaces were obtained by introducing sterile beads, siliceous sand, and so forth. Increasing the ratio of solid surface to water leads to increased bacterial activity only when the nutrient medium is of low concentration such as that prevailing in the sea. It is explained that the increased surfaces offer places for attachment of greater numbers of periphytic bacteria (that is, those which grow attached to solid surfaces). Clean glass microscope slides submerged in the sea also quickly show a concentration of bacteria following adsorption of organic food and provision of solid surface.
The efficiency of the activities of periphytic bacteria is believed to be enhanced owing to adsorption of organic matter to solid surfaces and to the reduction of diffusion at the immediate surfaces of bodies and within the interstices at the tangent of the bacterial cell and the solid surfaces. Thus these micro-volumes serve as concentrating foci for bacterial
In nature the solid surfaces are provided by all types of particulate matter, animate or inanimate, on the bottom, in the plankton, and on the nekton.
Oxygen Relations. It is convenient to classify the bacteria further on the basis of their different oxygen requirements or tolerances. There are obligate aerobes which use free oxygen, obligate anaerobes which function in the total absence of free oxygen, facultative forms which may live in either type of environment, and microaerophiles which require a reduction of free oxygen but not to the point of anaerobic conditions. Most marine bacteria are said to be of this type.
Bacterial Chemical Transformations. To facilitate study of the cycle of substances in the sea, it is necessary further to classify the marine bacteria into several convenient general groups, namely nitrifying, denitrifying, nitrogen fixing, sulphur, and iron bacteria, and so on, based upon their metabolic activities in the transformation of these substances. To describe these groups is to describe the role of bacteria in the chemical and biological cycles of the sea.
The Nitrogen Cycle
The main features of the nitrogen cycle must be considered because of the outstanding importance of nitrogen to all forms of life and because of the great general interest it holds in illustrating the successive ecological implications with respect to bacteria.
It has been pointed out (chapter VI) that nitrogen exists in the sea in combination with other elements, for example, in ammonia (NH3), and as oxides of nitrogen in nitrite ion (NO2), and nitrate ion (NO3). Nitrogen enters into the composition of all living things. It is one of the building blocks (nutrients) used by plants in forming the complex protein molecules of their bodies from which animals must derive their nitrogen. However, not all forms of nitrogen can be used by plants, hence the complex nitrogenous compounds found in both plants and animals must, upon death of the organisms, be decomposed, along with their products of excretion, to chemically simpler compounds utilizable by the plants. This decomposition, we know, is accomplished mainly by the activity of proteolytic bacteria.
The process of decomposition involves a series of steps in which specifically different bacteria are concerned. The early stages of the transformations are not fully known, but amino acids result and the nitrogen compounds—ammonia, nitrites and nitrates—are a part of this process. It is these inorganic compounds and also, to some extent, the amino acids that can be used directly by the plants for their supply of nitrogen.
The main store of nitrogen in the sea has been considered elsewhere (pp. 181 and 242). We need to be concerned here only with the main features of the chemical circulation of this element as activated by organisms including the plants, animals, and bacteria living within the sea. We may begin with the large and complex protein molecule of plant or animal tissue which is broken down into more simple products containing nitrogen. The cycle is composed of six transitions known as ammonification, nitrification, nitrogen assimilation, denitrification, and nitrogen fixation.
Ammonification. It is well known that ammonia is a product associated with decay of organic material. There are a number of stages and different products involved in this simplification of the molecule, among which products are the amino acids, considered present in the sea only by inference (Cooper, 1937). The bacterial process of deaminization results in splitting off the NH2 group in the amino acids, and there are many types of bacteria in the sea which are endowed with the ability to carry on this process. In weak solutions the ammonia may be intercepted at this point and the nitrogen assimilated directly by diatoms, as is known to occur also with higher plants. ZoBell (1935) and Cooper (1937) review the literature, in which there is much evidence that ammonia nitrogen constitutes one of the important sources of nitrogen utilized by unicellular algae. Ammonia is present usually only in small quantities and its distribution and concentration appear to indicate the place and intensity of organic decomposition (Redfield and Keys, 1938).
Nitrification. It was formerly suggested by some investigators that the sea water must derive its nitrates through drainage from land or through electrical discharges and photochemical processes. The nitrifying bacteria occurring near shore were thought to have washed in from land and not to live normally in the sea. In more recent years investigations have concluded from bacteriological and chemical studies that this type of bacteria is also beyond doubt truly marine. Carey (1938) summarizes briefly the development of our knowledge of these microorganisms in the sea.
The complete process of nitrification includes the formation of nitrates from ammonia and nitrites (NH3 → NO2 → NO3). The organisms responsible for converting the ammonia to nitrite are called Nitrosomonas and Nitrosococcus. They may live in the absence of organic material, obtaining their carbon through assimilation of carbon dioxide and their energy to carry on life processes through the oxidation of ammonia to nitrites. Investigations by Carey indicate that this takes place mainly in two regions, on or near the bottom in coastal areas and in the water in association with plankton at moderate depths, though some nitrites may also be formed at mid-depths. Oxidation of ammonia to nitrite may also occur photochemically (p. 256).
Following the conversion to nitrites, another group of bacteria oxidizes the nitrites to nitrates. The source of carbon for the nitrateformers is apparently also the carbon dioxide, and the energy is derived through oxidation of nitrities.
Nitrogen Assimilation. The process of nitrogen assimilation is primarily a function of the plants, the phytoplankton and benthic algae, but bacteria also assimilate nitrogen. Nitrogenous nutrients such as amino acids, ammonia, nitrates, or nitrites are the available sources of the nitrogen used by the plants in building up the amino-nitrogen of the protoplasm. It is not clear which of the last three mineralized compounds are preferred by phytoplankton as a source of nitrogen, but field observations provide evidence that they may be used simultaneously. During periods of low plant production the process of nitrification results in the storage of nitrogen as nitrates, and subsequent outbursts of diatoms then draw heavily upon this supply. After assimilation these plants may die and be dissociated directly by bacteria, or what is perhaps the most usual occurrence, they may be eaten by animals which require organic nitrogen from the plants directly or indirectly. In any event, a return to the inorganic nitrogen compounds is eventually effected by the bacteria, although animals preying directly upon each other may keep the organic nitrogen within their own cycle for some time. However, due to constant losses, this cycle cannot be self-perpetuating, and there must therefore be a large group of herbivorous animals to serve as food for the carnivorous forms.
Nitrate Reduction and Denitrification. The nitrogen cycle in the sea is more involved than is indicated by the above discussion. Gran (1901) and later Baur (1902) showed that denitrifying bacteria also exist in the sea. These denitrifiers and nitrate-reducers produce the effect just opposite to nitrification. Accordingly, nitrates are reduced to nitrites by splitting off a part of the oxygen, and other bacteria, the true denitrifiers, may carry the process even further with complete reduction of the nitrites and evolution of free nitrogen (NO3 → NO2 → N2). This last step, which is apparently not of great importance in the sea, constitutes a loss to the cycle in the sea unless the elementary nitrogen can be reclaimed by nitrogen-fixing bacteria (see below). The relatively uniform distribution of dissolved nitrogen in the sea also suggests that true denitrifiers and nitrogen fixers are not important in the general economy of the sea (Hamm and Thompson, 1941).
It has been held that the process of reduction of nitrates and nitrites by bacteria is characteristic of an environment lacking or poor in oxygen. Under these anaerobic conditions and in the presence of organic matter the bacteria find their source of needed oxygen in the molecules of these compounds. Rather little is known about the extent of denitrification in the sea, but since most sea waters have at least a small supply of
Nitrogen Fixation. In the terrestrial environment, the fixation of free nitrogen by bacteria is an important factor. To what extent similar nitrogen fixation occurs in the sea through the activity of bacteria is not well known. However, nitrogen fixers are reported from coastal areas, some in symbiosis with algae, and the extent to which they function in fixing free nitrogen depends upon environmental conditions involving the amount of available nitrogen compounds at their disposal, since nitrogen fixation is not an obligatory process (Benecke, 1933).
Phosphorus, Carbon, and Sulphur Cycles
Though they are allotted relatively less space for discussion, it is not intended to minimize the importance of bacterial activity in the transformation of these elements.
Phosphorus is another of the important plant nutrients which has a biologically activated cycle involving alternation of organic and inorganic phases. Whereas carbon dioxide is always present in sufficient quantity for the needs of marine plants, the phosphorus, like the nitrogen, may be depleted to the extent of interfering with the fullest growth. In laboratory experiments phosphate is apparently quickly regenerated by bacteria and other agencies following the death of plants and animals. In studies on stored sea water, Renn (1937) and Waksman et al (1938) found that phosphates are assimilated by bacteria in the growth of their cell substance but that bacterial competition with diatoms for this element is not serious under these conditions, since upon death and autolysis of the bacteria the phosphates are returned in a few days in mineralized form. When a supply of decomposing diatoms is at hand, the phosphates are regenerated from these more rapidly than they are consumed by the bacteria. Under these experimental conditions, approximately two thirds of the total phosphorus present in the diatoms was liberated within 132 hours through bacterial activities. Cooper (1935) found a more rapid regeneration of phosphates from animal plankton than from diatoms.
It must be emphasized, however, that these quick regenerations of phosphates in laboratory experiments are at variance with findings relative to the rate of regeneration in the sea, where renewal in the water is much slower, requiring three to four months (p. 260).
The complex carbon compounds built up by marine plants and animals are decomposed through bacterial action with the formation of carbon dioxide. This formation supplements the carbon dioxide that is produced through respiration by all other organisms and helps maintain the
Sulphur is one of the essential constituents of living matter, and its compounds, like those of other elements of protoplasm, are acted upon by bacteria. Transformations wrought by bacteria in the chemical compounds of sulphur may have far-reaching effects on the associated plant and animal populations and also upon chemical and geological phenomena. First, it may be mentioned that plants utilize a small quantity of sulphur in their metabolism; the compound used, namely sulphate, is produced by chemical or biological oxidation. Second, in the decomposition of organic compounds containing sulphur, hydrogen sulphide is produced as a disintegration product which, when present in large quantities, is inimical to plant and animal life. The odor of hydrogen sulphide is frequently noticeable at low tide in the organically rich muds of protected bays and in muds brought up from deeper water.
This hydrogen sulphide may be formed by splitting off the H2S group of the protein molecule or through a process of reduction of the proteins by heterotrophic bacteria. The inorganic compounds of sulphur, such as sulphates and sulphites, may also be reduced to hydrogen sulphide by heterotrophic bacteria under anaerobic conditions in the presence of organic material. Hence it is important to note that through intramolecular respiration oxidation of organic matter can continue even though all free oxygen has been removed. In areas with little or no circulation of water near the bottom and with an accumulation of organic detritus these heterotrophs as well as true sulphur bacteria abound, but the hydrogen sulphide evolved inhibits any other forms of life. In the Black Sea hydrogen sulphide is found from 180 m to the bottom; and from a depth of 300 m to 1500 m it increases from 1.48 cm3/l to 6.17 cm3/l (fig. 237, p. 872, Nikitin, 1931). Other classical examples are found in the oyster pools of threshold fjords (p. 802).
Not all bacteria that are involved in the sulphur cycle produce hydrogen sulphide. The metabolic requirements of the true sulphur bacteria produce the opposite effect through the process of oxidation. During the assimilation of carbon dioxide by autotrophic sulphur bacteria in the presence of free oxygen, the hydrogen sulphide is oxidized according to the following chemical equation:
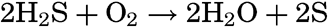
In some types of bacteria, including many purple bacteria, the sulphur is deposited as reserve material within the bacterial cell. The nitrogen needed is obtained from ammonium salts.
Bacteria and Bottom Deposits
Only brief mention can be made of the activities of bacteria in the processes of sedimentation on the ocean bottom. Further consideration
The activities of the various types of bacteria that occur in great abundance in the bottom sediments are believed to have a significant role in determining the character of the bottom deposits and the diagenesis of rock strata. Some of the results of chemical transformations wrought by these microorganisms are:
Formation of humus, a very stable organic end product of decomposition with a characteristic carbon-nitrogen ratio varying from 8:1 to 12:1 (Jensen, 1914).
Calcium precipitation in the presence of calcium salts and high pH.
It is believed by some investigators—Drew (1914), Bavendamm (1932), and others reviewed by Benecke—that bacteria in the sea are important agents in the precipitation of calcium carbonate in marine sediments and may therefore be of special geological importance. Many bacteria are known experimentally to produce an alkaline reaction in the presence of calcium and organic material, yielding ammonia. Such organisms may encrust themselves with areolas of calcium carbonate. The extent to which this type of precipitation can occur is still a moot question. Lipman (1929) was of the opinion that in the sea there can be no calcium precipitation because sea water does not contain sufficient concentration of calcium, but the general opinion seems to be that under certain natural conditions bacterial precipitation of calcium can and does occur in the sea, especially in bays in the tropics where there is an abundance of organic material.
Iron and manganese may be precipitated by bacteria that form sheaths of compounds of these metals (Harder, 1919). For example, they obtain energy from the oxidation of the soluble ferrous bicarbonate using the carbon dioxide liberated and precipitating ferric hydroxide.
Distribution of Bacteria in the Sea
The greatest numbers of marine bacteria have been found in the coastal waters where the greatest abundance of plant and animal life is also produced. In vertical distribution we find two main centers, on the bottom a few millimeters below the mud-water interface, and in the pelagic zone attached to the floating plants and animals and other particulate matter (fig. 247). Reference to table 100, from ZoBell and Anderson (1936b), will illustrate typical vertical distribution off the California coast. It should be noted that even though one center of distribution is in the pelagic zone, associated with the plankton, the existence of bacteria is apparently not truly planktonic but sessile upon organisms, and must therefore also closely coincide vertically and horizontally with the maximum distribution of these organisms.
It has been indicated by Carey and Waksman (1934) and others that bacteria are also present in relatively small numbers in the water even to a depth of 5000 m, and to that extent must be considered planktonic. But since the favorite habitat is one of attachment, it is not possible at present to know how normal the true planktonic existence is. Many individuals may be there through accident and be able to survive and multiply only to the degree that they are capable of utilizing the dilute organic matter that is in solution in the water. The advantages of a periphytic habit were discussed elsewhere (p. 912). A planktonic distribution must, however, be of great significance in the dispersal of these microorganisms.
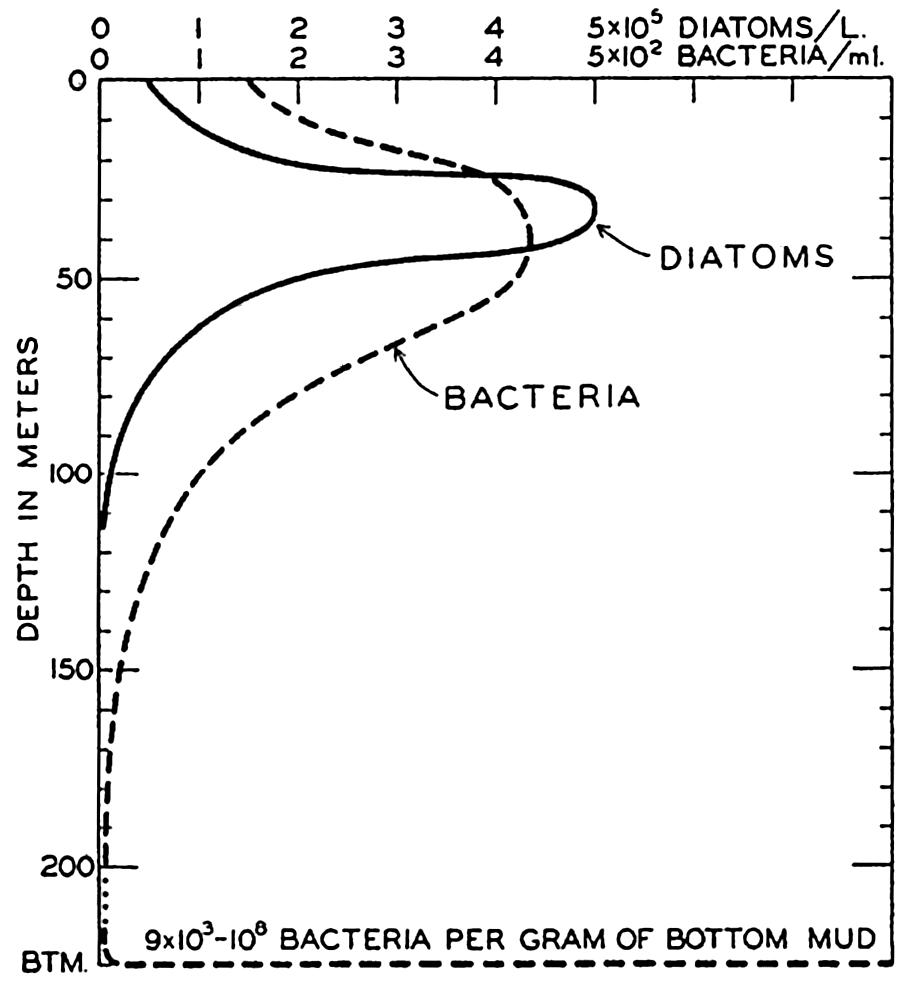
The vertical distribution of bacteria in the sea.
The greatest density by far of bacterial populations is found on the bottom, where as many as 420,000,000 cells per gram of wet mud may occur (ZoBell and Anderson, 1936b). Such dense populations are possible mainly because in a thin layer representing an interface between mud and water there is concentrated a large portion of the organic detritus, dead bodies of plants and animals, which is constantly sinking and forming an abundant rich food supply for bacteria as well as for bottom-dwelling animals which compete with them. Most bacteria occur in the first few millimeters of bottom ooze and there is a gradual diminution of numbers with the increasing thickness of the bottom deposits. Viable
In fig. 247 is shown the vertical distribution of bacteria and its relation to the diatom population. It will be noted that the large bacterial population of the pelagic region coincides roughly with the vertical range of floating plants and animals. This apparently results from the periphytic habit of many bacteria. The plankton organisms—diatoms, copepods, and so forth—living most abundantly in the euphotic zone, serve as surfaces for attachment and offer a favorable environment for multiplication (p. 912). The presence of a large periphytic bacterial population in the plankton results in prompt bacterial decomposition of large quantities of dead organisms before they have sunk to great depths; thus a large portion of mineralized plant nutrients is regenerated within or only a little below the euphotic zone.
| Sample | Depth (m) | Number of bacteria | ||
|---|---|---|---|---|
| Water | 1 | 147 | 344 | 261 |
| Water | 10 | 238 | 400 | 360 |
| Water | 20 | 292 | 528 | 395 |
| Water | 50 | 86 | 620 | 208 |
| Water | 100 | 14 | 17 | 53 |
| Water | 200 | 3 | 2 | 0 |
| Water | 500 | 2 | 0 | 0 |
| Sediment | Bottom | 2,170,000 | 768,000 | 16,200,000 |
| E. W. Scripps Station No. | 32A1 | 32B2 | 33A3 | |
| Water depth | 704 m | 610 m | 1287 m | |