6.5.1—
The Glycollate Pathway
The pathway of glycollate metabolism in leaves has been elucidated by infiltrating excised leaves or leaf discs with 14 C-labelled glycollate or other intermediates of the pathway. Glycollate is converted rapidly to glyoxyllate which may be oxidized non-enzymatically to carbon dioxide and formic acid, in the presence of hydrogen peroxide. In this reaction the carbon dioxide is derived from the carboxyl carbon of glycollate and the formate from the methyl carbon (Tolbert & Burris, 1950). However the presence of catalase, which breaks down peroxide, is thought to preclude such a total degradation of glyoxyllate in the peroxisome and the supply of 14 C-labelled glycollate or glycoxyllate to leaf tissue in the light has been found to give rise initially to labelled glycine and serine and subsequently to labelled glyceric acid hexoses and sucrose (Tolbert, 1963; see Fig. 6.7).
Infiltration of [2-14 C] glycollate into leaves in the light was found to give [2-14 C] glycine but serine was found to be labelled in the 2 and 3 carbons (Tolbert & Cohan, 1953; see Fig. 6.7). From this evidence it was concluded that two molecules of glycine give rise to one molecule of serine with a loss of one molecule of carbon dioxide; the 1 and 2 carbons of serine are derived from carbons 1 and 2 of glycine respectively, while the 3 carbon of serine is derived from the 2 carbon of glycine and carbon dioxide arises from carbon 1 of glycine. In wheat leaves in light [3-14 C] serine was converted to [3-14 C] glycerate
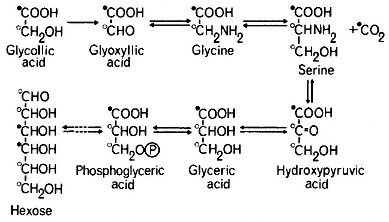
Figure 6.7
The glycollate pathway. The labelling pattern of intermediates of the
pathway ane hexose is indicated for when they are derived from
[1-'4 C]-glycollate (

and this was incorporated into hexose presumably by the reactions of the Embden-Meyerhof-Parras pathway (Rabson et al., 1962). This pathway of hexose formation from glycollate has been confirmed by the finding that [2-14 C] glycollate gives rise to glycose labelled in the 1, 2, 5 and 6 carbons and [3-14 C] serine to glucose labelled in the 1 and 6 carbons (Jiminez et al., 1.962).
The glucollate pathway is therefore gluconeogenic in light. The conversion of glyceric acid derived from glycollate, to glucose and sucrose is inhibited by DCMU an inhibitor of Photosystem II (Miflin et al., 1966). In the dark, supplied [14 C]-glycollate is converted into TCA cycle acids rather than sugars which may be due to an oxidation of pyruvate derived from glyceric acid, or may result from a direct conversion of glyoxyllate to malate.
Glycollate produced during photosynthesis in the presence of 14 CO2 is usually found to be uniformly labelled i.e. both carbons have the same specific activity. This distribution of radioactivity would be expected if the glycollate was derived from carbons 1 and 2 of ribulose-1,5-bisphosphate. Consequently all the compounds arising from glycollate are uniformly labelled. Serine, in particular, has been found to be uniformly labelled while phosphoglyceric acid was predominately carboxyl labelled, indicating that serine was produced from glycollate rather than directly from phosphoglyceric acid formed in CO2 -fixation (Rabson et al., 1962). The uniformly labelled glyceric acid formed in this pathway in turn produces uniformly labelled hexoses instead of the 3,4-14 C-hexoses resulting from incorporation of 14 C from the photosynthetic carbon cycle.
The operation of the glycollate pathway in leaves has been shown by tracer experiments but it has been confirmed by the detection of enzymes in leaves which are necessary for some reactions of the pathway. The initial reaction, the oxidation of glycollate to glyoxyllate is catalysed by glycollate oxidase, an enzyme first isolated by Zelitch and Ochoa (1953). It has FMN as the prosthetic group and utilizes molecular oxygen as the electron acceptor. The enzyme is competitively inhibited by bisulphite addition compounds of aldehydes, a -hydroxy-sulphonates, which have the general structure R-CHOH-SO3 H and
are therefore structural analogues of glycollate. The most commonly used a -hydroxysulphonate is a -hydroxypyridylmethane sulphonate (HPMS) and treatment of leaf discs or infiltration of excised leaves with this compound results in the accumulation of glycollic acid while having no effect upon the rate of CO2 -fixation. Experiments of this type have shown that 40 to 70: of the carbon fixed in photosynthesis will accumulate as glycollate in tissues treated with HPMS and these results have been interpreted as indicating that a large fraction of the carbon fixed in photosynthesis is metabolized by this pathway.
Transaminases are present in leaves which catalyse two steps of the pathway: a glutamate-glyoxyllate transaminase catalysing the formation of glycine is widespread in plant tissues, and a serine-pyruvate aminotransferase catalysing the formation of serine to hydroxypyruvate is found in leaves. The conversion of glycine to serine is catalysed by serine hydroxymethyltransferase which has been found in pea and wheat leaves (Cossins & Sinha, 1966). This reaction is inhibited by isonicotinyl hydrazide, and treatment of leaf tissue with this compound during photosynthesis in 14 CO2 causes an accumulation of [14 C] glycine and [14 C] glycollate and a decrease in the incorporation of 14 C into glucose (Miflin et al., 1966). Use of this inhibitor thus provides additional evidence for the operation of the pathway.
Two types of glyoxyllate reductase have been found to occur in leaves. One, an NADP-linked enzyme, is thought to be located in the chloroplast, while a second NAD-linked enzyme is present in the cytoplasm and is referred to as hydroxypyruvate reductase since the enzyme isolated from some sources has a higher activity to hydroxypyruvate than to glyoxyllate. The enzyme appears to catalyse the reduction of hydroxypyruvate to glycerate in the glycollate pathway.