Early Work on Salicylic and Salylic Acids
Salicylic acid (2-hydroxybenzoic acid), a constituent of a number of plant extracts with noted febrifugal properties, had been studied since the late 1820s, as had the related substance phenol (carbolic acid or hydroxybenzene). Benzoic acid (benzene carboxylic acid) had long been known. For some reason, Kolbe became interested in these compounds shortly after his arrival in Marburg.
Since salicylic acid was known to decarboxylate easily (mild heating converted the substance to phenol, with evolution of carbon dioxide), Kolbe speculated that salicylic acid might be simply an ester of phenol and carbonic acid. Accordingly, it should have been possible to synthesize the acid by esterifying the two components (e.g., reacting sodium phenolate with phosgene, then hydrolyzing the remaining chlorine). Repeated experiments along this line consistently failed, but without shaking Kolbe's assumption.[4]
Kolbe put his first Ph.D. student, Wilhelm Gerland, to work on closely related problems. Gerland succeeded in making a second aminobenzoic acid, "benzaminic acid" (the first such was anthranilic acid). Since anthranilic acid could be oxidized smoothly to salicylic acid, Kolbe suggested publicly that these two compounds had analogous structures, that is, the former must be an amide if the latter (as he still assumed) was an ester. This would explain the isomerism of anthranilic with benzaminic acid, the latter being the true aminobenzoic acid. But Kolbe confessed continued failure in proving this notion by making salicylic acid through esterification.[5] Gerland then found that oxidation of benzaminic produced an isomer of salicylic acid, which he named "Oxybenzoësäure." He suggested that it was this new isomer, not salicylic acid, that was the carbonic acid ester. This seems to have been Gerland's proposal and not Kolbe's.[6]
By 1859, after a hiatus caused partially by his ill health, Kolbe had put two new students to work on related problems. One was Rudolf Schmitt, not yet Ph.D. but already an assistant, who attempted to expand the isomerism of anthranilic/benzaminic acids to analogous aromatic sulfoacids. The other was Eduard Lautemann, then an ad-
vanced Praktikant, who was assigned the study of a number of salicylic acid homologs.[7] This work was going on simultaneously with Kolbe's attempts, with Lautemann's and Carl Ulrich's help, to show that glycolic and lactic acids were monobasic hydroxy derivatives of acetic and propionic acids, respectively, to counter Wurtz' contentions that they were dibasic, or rather (as Wurtz later argued) diatomic and monobasic.
I have suggested in chapter 9 that Kolbe's discomfort with the idea of polybasic organic acids originated in his theoretical commitments to radical theories and the earliest version of the type theory. Kolbe himself stated that his effort to show that salicylic acid was an ester was motivated not so much by an otherwise unexplained isomerism but by his disagreement with those who viewed the compound as dibasic.[8] Piria's demonstration in 1855 of double salts of salicylic acid appeared to provide an irrefutable demonstration of its dibasic character. However, late in 1859 Kolbe and Lautemann finally succeeded in synthesizing sodium salicylate from phenol by simultaneously introducing gaseous carbon dioxide and finely divided sodium metal into hot phenol.[9]
Kolbe initially thought that this synthesis demonstrated his ester hypothesis.[10] However, by April 1860 he abandoned that idea because he realized that his conjectural structure could not account for the known reduced forms of salicylic acid: salicylaldehyde, and salicyl alcohol. Nonetheless, the new synthesis provided proof (he thought) that salicylic acid was indeed monobasic. The substance was, in fact, entirely analogous to glycolic and lactic acids, in that it consisted of benzoic acid in which one hydrogen atom of the phenyl radical was substituted by the radical HO2 . That the hydrogen of this "hydrogen peroxide" radical could be replaced by a metal atom in the double salts of salicylic acid did not mean that the acid was dibasic. He went so far as to assert that only monoacids, such as salicylic acid, have corresponding aldehydes and alcohols and that any assertions to the contrary were "unscientific frivolities" that did not even deserve to be discussed. This remarkable statement contradicted Kolbe's own published views, and he soon had to retract it.[11]
Kolbe's new view of the constitution of salicylic acid seemed to have solved one problem, but it created another: now Kolbe was designating both salicylic and its isomer oxybenzoic acid by the same rational formula and so the cause of the isomerism was mysterious. Fortunately, a recent discovery by Lautemann suggested a resolution. Reduction products of salicylic acid included not only salicylaldehyde but also what appeared to be an isomer of benzoic acid, for which Lautemann and Kolbe suggested the name "salylic acid." Thus, benzoic acid could
be converted to benzaminic and then to oxybenzoic acid, whereas salylic acid was related genetically to both anthranilic and salicylic acids. There were therefore two independent isomeric series. All of this could easily be explained if there were two isomeric parent aromatic hydrocarbons having the equivalent formula C12 H6 , and Kolbe suggested precisely that. The parent radical of the salylic acid series Kolbe suggested should be called "phenyl," which is the radical in phenol; the progenitor of the benzoic acid series could then be called "benzyl," the radical of benzoic acid. Such a hypothesis could also explain the known isomerism of benzyl alcohol versus cresol.[12]
Kolbe admitted that there were problems with this suggestion. No second isomer of benzene was actually known (decarboxylation of salylic acid, for instance, apparently yielded ordinary benzene), and there was no second phenol. Moreover, there should be a distinct salyl aldehyde and salyl alcohol, a sulfosalylic acid, and so on. Nor was there any way to know in what the isomerism of the parent hydrocarbon may consist. However, Kolbe was willing to speculate: benzyl may be
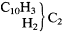
while phenyl could be (C10 H5 )'"C2 . One radical thus has a structure in which one or two additional functional groups may substitute for two hydrogens of methylene, while the other contains a carbon (C2 ) that is bound only to a single triatomic group; he symbolized these isomeric radicals as b(C12 H5 ) and p(C12 H5 ). Kolbe offered no empirical justification for these structural details, nor is one evident to the historian.
Kekulé's former roommate in London, Hugo Müller, verified the existence of salylic acid soon thereafter, or so he thought at the time, and wrote Kekulé about it.[13] Kekulé then published an article that followed up on Kolbe's research, expanding Kolbe's suggestion of two isomeric series to include such compounds as the two known chlorobenzoic acids. He stated that the existence of an isomer of benzoic, i.e., salylic acid, was no longer in doubt, and he thought that there may also be two isomers of benzene.
However, I consider it inappropriate even now to enter into a theoretical discussion, since the necessary factual foundation for this is missing. Even Kolbe, who has to date penetrated the furthest toward true knowledge of the actual arrangements of atoms, was able to discover no other difference in the constitutions of these substances than that, in addition
to the elements that otherwise compose organic compounds, the first contains a soft "b," the other a hard "p."
Kekulé had evidently been dismayed by Kolbe's public charge (presumably directed at Wurtz, but arguably also at Kekulé) of "unscientific frivolities," for he followed this little witticism with a more direct barb:
But what further deters me from discussing this question is the circumstance that I probably could not do that without criticizing Kolbe's theoretical views (which hitherto from one paper to the next have constantly changed), and also his manner of dealing with other chemists in his publications—a critique I wish to avoid, if possible entirely, or at least as long as possible.[14]
Since 1857 Kekulé had been working with benzoic and salicylic acids himself, and he had hinted at a "denser" and "next-simplest" arrangement of the carbon skeleton of benzene in his structure theory paper of 1858. But he was not yet ready to enter into an explicit theoretical discussion, presumably for the reason stated—the still scanty empirical record. It is probable that he had not yet formed any satisfactory hypothesis regarding the isomerisms.[15]
Kolbe's conjecture that his "phenyl" (in contrast to his "benzyl") radical had a carbon with no hydrogen atoms suggested to him that one might be able to hydrogenate this carbon, producing compounds with more hydrogen than aromatic compounds but less than any aliphatic compounds. He had had good results with sodium amalgam as a reducing agent, and so in December 1860 he tried it on phenol, salylic acid, and salicylic acid, and other "phenyl" compounds. In February 1861, he reported excitedly to Liebig that he thought he had succeeded in his goal and that the discovery promised to yield years worth of exciting research. Reduction of salylic acid yielded much aldehyde, but also a certain percentage of a weakly smelling liquid that appeared to be an acid with a larger percentage of hydrogen than the starting material.[16] Liebig was enthralled; this was an "extremely fortunate idea, which opens up a treasure-trove of new discoveries."[17]
Kolbe published a short preliminary communication in the Annalen on this subject and tried to follow it up, but he was unable to isolate enough of the compound to prove his idea of an intermediate link between aromatic and aliphatic compounds. As he wrote to Liebig, there were severe experimental difficulties, including a multiplicity of small amounts of products that were hard to separate. In the meantime, he and his students were more successful (or so they initially thought) at further substantiating his claim of two isomeric series of aromatic com-
pounds. For example, Schmitt applied the idea to benzenesulfonic acids, and Lautemann prepared a variety of new iodo and hydroxy derivatives of salicylic acid and of phenol, all depicted as "phenyl" or "p" compounds.
Unfortunately, both lines of research soon reached dead ends. The chief difficulty was with salylic acid. Kolbe had thought he had more than sufficient evidence to identify this substance as an isomer of benzoic acid. The two solids were different in appearance and their crystal forms were distinct; salylic was only about a third as soluble in water as benzoic, it was more volatile, and it melted at 119ºC rather than 121ºC.[18] The existence of the acid was accepted by nearly everyone, including De La Rue, Müller, and Kekulé (although Kolbe later revealed that Lautemann himself, the actual discoverer, had always expressed doubt regarding the real existence of salylic acid).[19] But as early as the spring of 1861, Cannizzaro argued convincingly that decarboxylation of salylic and benzoic acids both produce a single substance, benzene, and that there is no other isomer of benzene. Later that year, Rudolf Fittig, an assistant of Limpricht and Wöhler at Göttingen, discovered that even tiny amounts of impurities in benzoic acid could dramatically alter its properties, such as crystal structure and solubility; he thought that salylic was simply impure benzoic acid. Two years later, Friedrich Beilstein established that whereas nitrodracylic was a true isomer of nitrobenzoic acid, "dracylic" and benzoic acids were identical. The following year he definitively confirmed Fittig's observations on "salylic acid."[20]
For some time, Kolbe attempted to defend the existence of salylic acid and its putative parent hydrocarbon. Forgetting the reasoning that had led him to abandon the ester hypothesis for the constitution of salicylic acid, he argued in 1863 that there must be four different isomers possessing the composition of salicylic acid. There are two parent hydrocarbons, hence there must be two different ester compounds (salicylic being one), as well as two true (hydr)oxybenzoic acids. After he had failed to crack the intractable aromatic reduction problem, Kolbe gave it to a student, Max Herrmann. In 1864 Herrmann published a paper claiming to have succeeded in adding four atoms of hydrogen to benzoic acid. The next year he thought he had accomplished an analogous transformation with hippuric acid, but an "unfortunate accident" prevented him from precisely characterizing the novel reduction product.[21]
But just about the time this last paper was published, Kolbe became convinced by Beilstein's work: salylic acid did not exist and benzoic acid had no known isomer. With the fall of salylic acid and the increasing evidence against a second isomer of benzene, Kolbe's isomerism
hypothesis appeared to be in trouble. When in 1865 Kolbe published a monograph reprinting his papers and those of his students since 1859, he omitted five aromatic papers, including the one announcing the synthesis of salicylic acid and the preliminary note that proclaimed the existence of intermediate links between aromatic and aliphatic compounds. When salylic acid was mentioned in another reprinted paper, Kolbe attached editorial notes denying its real existence and explaining the source of his error.[22]