5.7—
Energy Linked Reactions of Mitochondria
5.7.1—
Oxidative Phosphorylation
5.7.1.1—
Coupling Sites
Oxidative phosphorylation, the process whereby the reaction
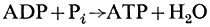
is coupled to the oxidation of reduced electron carriers of the respiratory chain occurs in plant mitochondria in the same manner as does the reaction in animal mitochondria. The sites of phosphorylation are identical (see Baltscheffsky & Baltscheffsky, 1974). These are the sites I in the NADH: ubiquinone reductase segment, II in the cytochrome b: cytochrome c reductase segment and III in the cytochrome oxidase segment of the respiratory chain. It has not been possible to identify precisely those components of the respiratory chain which are the energy transducers in each of the three sites, although these have been approximated from the change in redox potentials (D E0 ') between adjacent carriers (Lehninger, 1965), by application of the crossover theorem (Chance & Williams, 1955), and from shifts in the midpoint potential of certain electron carriers upon the addition of ATP to uncoupled anaerobic suspensions of mitochondria (Wilson & Dutton, 1970a,b; Lindsay & Wilson, 1972; Chance et al., 1970; Ohnishi, 1973; Devault, 1971). The D E0 ' indicates the thermodynamic feasibility of coupling between two adjacent components. Thus, coupling sites were thought to be located between endogenous NAD and the flavoprotein of NADH: ubiquinone reductase; between cytochrome b and cytochrome c of the cytochrome b :cytochrome c reductase; and between cytochrome a and oxygen of cytochrome oxidase. The application of the crossover theorem by and large confirms the general location based on thermodynamic grounds. According to the crossover theorem, electron transport through the coupling site is the rate limiting step in tightly coupled mitochondria. The carrier on the substrate side of the coupling site should become reduced, while the carrier on the oxygen side would become oxidized. Upon the addition of the phosphate acceptor, ADP,
there would be observed a rapid transient oxidation of the carrier on the sub-strate side, and a reduction of the carrier on the oxygen side of the coupling site, concomitant with the release of controlled respiration in the state 4-state 3 transition (see Table 5.4).
| ||||||||||||||||||||||||||||||||||||||||||
Wilson and Dutton (1970a) reported that the midpoint potential (Em ) of cytochrome a3 becomes more negative upon energization of an anaerobic suspension of rat liver mitochondria by ATP. Similarly they report that the Em of one of the b cytochromes (amax 564 nm) increases upon energization by ATP (Wilson & Dutton, 1970b; Chance et al., 1970). These shifts of the midpoint potentials were attributed to changes in ligand interaction energy of the heme iron upon energization, and that the iron atoms of these cytochromes were directly involved in energy coupling.
Such a change in the midpoint potential was postulated by Wang (1970). In his model, the phosphoimidazole group becomes a much weaker coordinating ligand for the Fe(II) than imidazole, and hence should lower the midpoint reduction potential of the corresponding electron carrier. Similar experiments in the iron-sulphur centres of the site I region of the respiratory chain showed that of the five iron sulphur centres identified, ATP affected the reduction potential of only one of these centres, designated as centre I (Ohnishi, 1973). Addition of ATP caused a partial oxidation of centre I when the reduction potential was poised at a value where the iron was almost completely reduced. These observations suggested that the reduction potential of centre I was dependent upon the phosphate potential, and that the addition of ATP caused a lowering of the midpoint potential of the centre I iron sulphur protein. The significance of the changes in the midpoint potential was questioned, at least for the site II region, since none of the b -cytochromes showed an ATP-induced potential shift when investigated in plant mitochondria (Dutton & Storey, 1971; Lambowitz et al., 1974). One must conclude that there is a fundamental difference in the function of the b -cytochromes in plant and animal mitochondria or that the changes in the midpoint potential do not reflect the formation of an energy transducing species. Lambowitz et al., took the latter view and attributed
the midpoint potential changes to a reverse electron flow with cytochrome b –566 and cytochrome a3 equilibrating with the redox mediators by way of cytochrome c, while the iron sulphur centre of site I equilibrated through the nicotinamide adenine nucleotide pool.
5.7.1.2—
ADP:O Ratios
The general location of the three coupling sites means that for tightly coupled mitochondria, predictable ratios of the moles of ADP phosphorylated to the gram-atoms of oxygen consumed (ADP/O or P/O) may be obtained for each substrate. Thus succinate should give a ratio of 2.0, NAD-linked substrates a ratio of 3.0 and a -ketoglutarate a ratio of 4.0 (one phosphorylation via succinyl-CoA, three phosphorylations via the respiratory chain NADH). These ratios are largely confirmed in plant mitochondria. Early attempts gave results much below the predicted ratios, but with refinements in the procedure for the isolation of plant mitochondria, the inclusion of required cofactors, and the exclusion of competing reactions, predicted ratios have been approached, as shown in Table 5.5. Exogenous NADH does not interact with respiratory chain NAD,
| ||||||||||||||||||||||||||||||||
but with flavoproteins of the external dehydrogenase. These ultimately enter the respiratory chain at the level of cytochrome b and hence electrons go through two coupling sites only (Douce et al., 1973).
5.7.1.3—
Energy Coupling in Cyanide Resistant Respiration
Mitochondria of Symplocarpus are fully capable of energy conservation in the uninhibited state. Hackett and Haas (1958) found P/O ratios greater than 3 for a -ketoglutarate oxidation by skunk cabbage mitochondria. In the presence of cyanide, the energy coupling sites associated with the cytochromes are by-passed, and energy coupling involves only the NADH:ubiquinone reductase portion of the respiratory chain. Storey and Bahr (1969b) obtained ADP/O ratios of 1.3 with succinate as substrate in skunk cabbage mitochondria in the absence
of cynide; the ratio was zero in the presence of cyanide. With malate as the substrate, the ADP/O ratios were 1.9 and 0.7 in the absence and presence of 0.3 mM KCN respectively, showing that coupling sites II and III were effectively by-passed while coupling site I, which could be activated by malate but not by succinate, was still functioning in the presence of cyanide.
Wilson (1970a), however, has proposed that energy coupling occurs in the alternate pathway as well. He found that cyanide treated mitochondria of A. maculatum and Acer pseudoplatanus produced ATP, with an ATP/O ratio of 0.5 at high O2 concentration (greater than 100 µM ) with succinate as the substrate. Submitochondrial particles of A. maculatum which lacked malate dehydrogenase activity oxidized succinate with P/O ratios of 0.2 to 0.6 (Wilson 1970b). Since malate dehydrogenase-depleted particles were still capable of phosphorylation, the possibility that the phosphorylation was due to the oxidation of a product of succinate oxidation, i.e., malate, could be eliminated, therefore suggesting that a phosphorylation site exists in the alternate pathway which functions only at high C2 concentration. Cyanide inhibited mitochondria from spadices of A. maculatum and from mung bean hypocotyls still retain an energy related function through the cytochromes. Bonner and Bebdall (1968) showed that a substrate linked reverse electron flow is active in spadix mitochondria. Cytochrome c and cytochrome oxidase of cyanide treated mitochondria were reduced by ascorbate plus, N,N,N',N' -tetramethyl-p -phenylenediamine (TMPD), an electron donor to cytochrome c. Subsequent addition of succinate caused a reoxidation of cytochrome c and cytochrome a with a partial reduction of cytochrome b. The reoxidation of cytochromes c and a was prevented by uncoupling concentrations of carbonylcyanide-phenyl-hydrazone or 2,4-dinitrophenol. Wilson and Moore (1973) also showed that cyanide inhibited mung bean mitochondria could carry out oxidation of ascorbate plus TMPD. The oxidation was thought to involve a reverse electron flow through coupling site II and energized by ATP or the high energy intermediate.
5.7.1.4—
Mechanism of Coupling
The mechanism of energy coupling remains an intractable problem in spite of the impressive array of workers in this area. Three outstanding hypotheses are under active consideration: (a) the chemical intermediate hypothesis; (b) the chemiosmotic hypothesis; and (c) the conformation coupling hypothesis.
The operation of the chemical intermediate hypothesis may be summarized as follows:
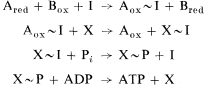
where A and B are adjacent electron carries at the coupling site, X and I are unknown couplers common to all coupling sites (see Greville, 1969). The scheme postulates the existence of both non-phosphorylated and phosphorylated intermediates. A non-phosphorylated intermediate accounts for the action of uncouplers, which is independent of the presence of phosphate. The nonphosphorylated intermediate can also be coupled to work functions such as ion transport, which is sensitive to uncouplers, but not to the phosphorylation inhibitor, oligomycin. The phosphorylated intermediate transfers a phosphoryl group to ADP. Hill and Boyer (1967) showed that the bridge oxygen between the b and g phosphorus of ATP is furnished by ADP. Hence, the mechanism of phosphorylation involves an activation of phosphate.
The chemiosmotic hypothesis in its simplest form as first proposed by Mitchell (1961) involves a vectorial metabolism in which the elements of water are transported to opposite sides of the mitochondrial membrane (Fig. 5.5).
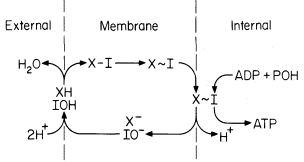
Figure 5.5
Representation of the Mitchell chemiosmotic
scheme for oxidative phosphorylation.
(Redrawn from Mitchell, 1966.)
Thus oxido-reduction reactions create a pH gradient as well as a potential difference (outside positive) which tends to drive H+ back across the membrane into the inner compartment. This force is the proton-motive force, and it is this flow of protons through the coupling site (a reversible ATPase) which drives ATP synthesis. The phosphorylation reaction is represented as follows:
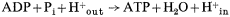
It can then be shown that in the absence of a transmembrane electrical potential, a pH differential of 3.5 units is required to poise the ratio of ATP/ADP = 1 while a potential difference of 210 mV would be required in the absence of pH gradients (Mitchell, 1966). The translocation of protons and equivalent OH– is effected by ionizable groups which are designated XH and IOH, corresponding to components of an ATPase. The proposed intermediate X-I of the ATPase must have a sufficiently low hydrolysis constant at the high electrochemical
potential of H+ in the outer phase to come to reverse equilibrium with water according to the reaction
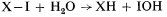
On the other hand, the hydrolysis constant of the intermediate X ~ I must be in the order of 105M , and the intermediate X ~ I must be in equilibrium with the ATP/(ADP + Pi ) couple in the inner phase, so that
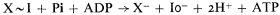
The system vibrates between states in which the intermediate X – I is alternately accessible to the outer and inner phases, being X – I when in contact with the outer phase and X – I when in contact with the inner phase. The transition of X – I to X ~ I is due not to the pumping of energy into X–I, but to a lowering of the ground state energy for X – I hydrolysis by some 10,000 calories per mole on translocation through an anisotropic membrane.
While experimental verification of the chemiosmotic mechanism has been realized only in chloroplasts by acid-base trasitions (Jagendorf & Uribe, 1966; see also chapter 4), similar experiments have not been successful in mitochondria. Cockrell et al., (1967) have shown ATP synthesis after establishing a K+ gradient by valinomycin-treated mitochondria. However, Glynn (1967) argued that ATP synthesis via a K+ gradient could be explained equally well on the basis of a membrane potential rather than cation transport down a chemical gradient through an ATPase.
In their general aspects, the chemical intermediate hypothesis and the chemiosmotic hypothesis differ primarily in the nature of the initial driving force for coupled phosphorylation. The two hypotheses converge at the level of the ATPase in that both call for an unknown intermediate, X ~ I (Greville, 1969).
The conformational coupling hypothesis of Boyer (1967) differs in that the ATPase has a high affinity for Pi and ADP. Tightly bound ATP is formed via a nucleophilic attack by ADP on orthophosphate in a SN2 reaction with the displacement of water (Korman & McLick, 1970). According to the model, there exists an energy requirement for the release of bound ATP from the complex, by changing the complex from one having a high affinity for ATP to one having low affinity (Boyer et al., 1973). The advantages of this hypothesis are that it explains most of the exchange reactions observed in coupled phosphorylation, and the coupling of ATP to energy yielding reactions of mitochondria.
5.7.2—
Reverse Electron Flow
Electron flow and energy transduction through the coupling sites of the respiratory chain are reversible processes. These are shown by the reduction of endogenous NAD and cytochrome b when substrates of higher reduction
potentials are oxidized as well as the ATP induced oxidation of reduced cytochrome a + a3 when the terminal oxidase is inhibited by sulphide. The properties of energy linked NAD reduction by plant mitochondria are similar to those reported in animal mitochondria (Chance, 1961; Chance & Hollunger, 1963). These include the requirement for succinate oxidation and for ATP (Storey, 1971b). Although ATP is required, the reduction of NAD is not sensitive to oligomycin. Hence NAD is reduced by reverse electron flow through the coupling site but without the participation of the ATPase as such. Uncouplers either inhibit the reduction when added before succinate, or reverse the reduction when added after a steady state reduction is attained. This can be interpreted as an effect upon the coupling site in preventing reverse electron flow, as well as a general release of controlled respiration so that the endogenous NADH is now oxidized rapidly through the coupling site. It is not possible to distinguish between the two alternatives.
Reverse electron flow through coupling sites II and III has been demonstrated by Storey (1972) and Lambowitz et al., (1974). When ATP is added to sulphideinhibited or to anaerobic suspensions of mung bean mitochondria, the b -cytochromes become reduced while cytochrome c and cytochrome a + a3 become oxidized. The effect is reversed by uncouplers or by phenazine methosulphate (PMS) which mediates electron flow between cytochromes b and c. The reverse electron flow through coupling site II involves an ATPase (Lambowitz et al., 1974). Hydrolysis of ATP is observed in an anaerobic suspension of mung bean mitochondria supplemented with ascorbate plus TMPD and ATP. Addition of PMS caused an increase in the rate of ATP hydrolysis which is to be expected if PMS formed a shunt of electrons from reduced cytochrome b to cytochrome c.
5.7.3—
Ion Transport
Mitochondria contain two compartments, one of which is readily accessible to low molecular weight solutes such as sucrose or mannitol, and a second in-accessible to sucrose or mannitol. The former is identified with the inter-membrane space, and the latter, the mitochondrial matrix. The volume enclosed by the inner membrane behaves as an osmometer (Lorimer & Miller, 1969; Overman et al., 1970). Selective permeability to solutes and active transport are properties of the inner mitochondrial membrane. The movement of solutes across the inner membrane may be determined by assay for net increases in intramitochondrial contents. If the movement results in the net increase or decrease in the osmolarity of the sucrose-inaccessible volume, such solute movements will be reflected in volume changes of the inner compartment which can be monitored by changes in the light scattering properties of a mitochondrial suspension. Thus mitochondrial swelling is accompanied by a decrease in light scattering, while a contraction is reflected by an increase in light scattering.
5.7.3.1—
Monovalent Cations
Permeability of the inner membrane of plant mitochondria to potassium and chloride ions is restricted. Slow, passive permeability to K+ and C1– may be observed on transfer of mitochondria to isosmotic KCl, but when mitochondria are energized by the addition of substrate (NADH), rapid loss of both K+ and Cl– occurs, accompanied by mitochondrial contraction (Wilson et al., 1969; Kirk & Hanson, 1973). In contrast to the energized extrusion of K+ and C1– , mitochondria undergo an NADH induced swelling' in potassium acetate solution, and K+ and acetate ions are taken up. This has been interpreted as an active transport of acetate, while K+ penetrates along an electrochemical potential and chloride is not transported. The NADH-induced swelling in potassium acetate is inhibited by uncouplers of oxidative phosphorylation, by ADP plus Pi , or by respiratory chain inhibitors (Wilson et al., 1969; Lee & Wilson, 1972), but is restored when oligomycin is present with ADP plus Pi . An intermediate of oxidative phosphorylation possibly mediates active transport of acetate, but not of chloride. This is strengthened by the observation that mitochondria respire with expected respiratory control and ADP/O ratio when suspended in isotonic KCl, but lose respiratory control in potassium acetate.
Ionophorous antibiotics, valinomycin and gramicidin D, increase the permeability of the mitochondrial membrane to potassium ion as shown by the increased rate of passive swelling in potassium chloride solutions (Kirk & Hanson, 1973; Miller et al., 1970a). Valinomycin also increases the rate of NADH-induced swelling in potassium acetate, as well as in chloride, phosphate, sulphate and nitrate salts of potassium (Kirk & Hanson, 1973; Wilson et al., 1972) although the swelling due to acetate and phosphate reverses on exhaustion of NADH. This has been interpreted to mean that the swelling is due to the enhanced permeability of the inner membrane to potassium, as well as an active transport of acetate or phosphate. In the case of the latter anions, swelling is thought to be due to an enhanced permeability toward K+ while the anions diffuse along an electric potential. On cessation of NADH-supported respiration, acetate and phosphate leak out according to their chemical potential, followed by potassium ions, while no leakage of chloride, nitrate or sulphate occurrs as they are distributed along their chemical potential (Wilson et al., 1972). The picture which emerges is that the movement of potassium ion is controlled by the movement of anions, but K+ flux can be modified by ionophores such as valinomycin or gramicidin D. Valinomycin may stimulate K+ uptake via a H+ exchange as found by Rossi et al., (1967) in liver mitochondria, but such K+ uptake due to H+ exchange is not accompanied by swelling of the mitochondria.
5.7.3.2—
Divalent Cations
Ca2+ does not cause marked stimulation of respiration in plant mitochondria as it does in animal mitochondria, except with exogenous NADH as substrate
(Miller et al., 1970b; Miller & Koeppe, 1971). A slight release from controlled respiration is detected with malate plus pyruvate or with succinate as substrates. This is associated with the accumulation of calcium and inorganic phosphate by mitochondria (Miller et al., 1970b). Extensive Ca2+ uptake by plant mitochondria occurs only in the presence of inorganic phosphate (Hodges & Hanson, 1965; Elzam & Hodges, 1968; Earnshaw et al., 1973; Chen & Lehninger, 1973). The phosphate dependent Ca2+ transport is energy dependent and may be supported by substrate oxidation or by ATP (Elzam & Hodges, 1968). The energy dependence seems to be at the level of an intermediate of oxidative phosphorylation. Substrate-supported Ca2+ transport is sensitive to uncouplers and respiratory inhibitors (Chen & Lehninger, 1973) as well as to ADP (Elzam & Hodges, 1968). The ATP supported Ca2+ transport is inhibited by oligomycin. Similar observations have been reported for Sr2+ transport by mitochondria (Johnson & Wilson, 1972). Other anions, e.g. acetate, arsenate, sulphate, chloride or nitrate promote neither Ca2+ nor Sr2+ uptake (Hodges & Hanson, 1965; Johnson & Wilson, 1972) although all will produce a metabolically independent swelling, while arsenate and acetate produce an active swelling as well, indicating that mitochondrial membranes are permeable to these anions, and will actively transport arsenate or acetate, as well as phosphate (Hanson & Miller, 1967; Johnson & Wilson, 1972; Lee & Wilson, 1972). Uptake of Ca2+ and inorganic phosphate results in the deposition of electron dense material in mitochondria which is dependent upon the concentrations of both Ca2+ and phosphate, and the time of incubation (Peverly et al., 1974). The composition of the deposits has not been ascertained, but is believed to be a form of calcium phosphate. The deposition of the phosphate salt of divalent alkaline earth metal ions may account for the contraction of mitochondria induced by Ca2+ . Since the volume changes of mitochondria are measured by light scattering changes, the formation of crystals within the mitochondria may increase the ight scattering properties of the suspension, and be interpreted as a contraction.
5.7.3.3—
Anion Transport
The importance of anion transporters in the movement of solutes across the mitochondrial inner membrane has been studied extensively in mammalian mitochondria (Chappell & Haarhoff, 1967; Harris, 1969). In these studies, the role of the phosphate transporter and the malate transporter is emphasized. Similar investigations were carried out by Phillips and Williams (1973b) and by Wiskich (1974). The presence of anion transporters was demonstrated by the spontaneous swelling of mitochondria in solutions of the ammonium salts of phosphate or malate. Ammonium salts were used because the mitochondrial membrane is readily permeable to ammonium ion. Mitochondrial swelling under these conditions is indicative of an osmotic adjustment due to the net influx of solutes into the mitochondrial matrix (Overman et al., 1970; Wilson et al., 1973). The swelling in ammonium phosphate was inhibited by
N -ethylmaleimide which inhibits the phosphate-hydroxyl antiporter while the swelling in ammonium malate as well as the malate supported respiration was inhibited by 2-butylmalonate, 2-phenylsuccinate, benzylmalonate or p -iodo-benzylmalonate, all inhibitors of the dicarboxylate carrier (Phillips & Williams, 1973a,b). The anion transport system is interpreted as follows: (a) a phosphatehydroxyl antiporter which transports phosphate in exchange for hydroxyl ion; (b) a malate-phosphate antiporter which transports malate in exchange for phosphate; (c) a tricarboxylate-malate antiporter which transports tricarboxylate anions in exchange for malate; (d) other dicarboxylate anions enter by exchange with malate. It is the prevailing view that anions are actively transported, and that cations follow the anion transport along an electric gradient (Hanson & Miller, 1967). The essential role of anion transport in determining cation movement, however, is modified by the presence of cation ionophores.