10—
The Theory of Chemical Structure and the Structure of Chemical Theory
We have now moved through more than half of our narrative, and this is an appropriate point to pause and consider in more detail four important themes that we have hitherto barely adumbrated: the development of synthetic methods in organic chemistry; philosophical questions connected with chemical theory; craft skills and tacit knowledge; and the problem of formula notation and interpretation.
Organic Synthesis
Histories of organic synthesis traditionally used to begin with Wöhler's preparation of artificial urea in 1828, which is supposed to have sent the enigmatic "vital force" into well-deserved oblivion. The situation was in fact extraordinarily complex, and a brief summary of this episode and its latter day interpretations is appropriate here.[1]
We now know that there were as many varieties of "vitalism" as there were of "materialism" and of "mechanism" in the eighteenth and early nineteenth centuries; that there is no simple—nor even any sophisticated—correlation between vitalistic and metaphysical, teleological, or theological habits of thought; that there were examples of the artificial preparation of "organic" materials from inorganic ones before 1828; that "total" and "direct" syntheses were only performed long after that date; that many chemists—including many "vitalists"—expressed confidence in the future possibility of unlimited organic syntheses during the first three decades of the nineteenth century; and that Wöhler's accomplishment in no way refuted vitalism at a stroke,
nor could it have done so even in principle. The semantic problem alone is daunting, for it would require an army of interpreters to match the manifold senses of the word "synthesis" against the protean character of "vitalism," not to mention the necessity of a careful winnowing and analysis of historical events to judge which could be considered the critical tests. In conclusion, a full answer to the question "in what year and by what event was vitalism overthrown" is not possible and not worth the effort if it were.
All that said, with respect to the insignificance claim for Wöhler's urea, there is a danger in proving too much. Wöhler obviously thought that he had done something rather dramatic in his excited letter of 22 February 1828 to his mentor Berzelius, stressing that he could make urea without a kidney, or even a living creature. Berzelius' reply two weeks later is just as enthusiastic and just as focused on the issue of organic synthesis; Wöhler had produced a "jewel" for his "laurel-wreath" that would "immortalize" his name. Wöhler clearly noted in his letter that his accomplishment would fail to convert a committed skeptic, and his published article did not assert that vitalism had been refuted. Moreover, both Wöhler and Berzelius recognized that the reaction was very relevant to a separate issue, the emergent study of isomerism, for Wöhler had simultaneously discovered that urea and ammonium cyanate had identical compositions. However, these qualifications do not negate the fact that both men regarded the new reaction to be important for an evaluation of vitalist beliefs. Wöhler's pride in his feat was still in evidence thirty-five years later, when we find him urging his combative friend Liebig to respond to Berthelot's attempted usurpation of the entire field of organic synthesis, but modestly adding that Liebig should do this without explicit mention of the 1828 paper.[2]
The "myth" of Wöhler's overthrow of vitalism in 1828 (and myth it surely was) was not created, as has been argued, in the spate of celebratory articles published during the centennial year, nor even in Hofmann's 1882 obituary of Wöhler. It was created in the immediate aftermath of the event itself. As early as 1843, Hermann Kopp, writing as a historian, urged that this was the deed that destroyed vitalist belief, and ignored the reaction's relevance for isomerism; Kopp's portrayal became an important source for later writers.[3] The fact that most commentators on this "epochal" discovery during the first few years after the event emphasized its impact on the theory of isomerism may simply indicate an implicit recognition of the slippery character of the vitalist debate and simultaneous enthusiasm over a current live topic—not an assertion of its irrelevance for the demarcation between organic and inorganic chemistry.[4] Relevant it was; a refutation of vitalism it was not. However, by the early 1850s, if not before, the myth of
a definitive refutation had become ensconced in the German textbook literature.[5]
Even Wöhler and his most partisan mythologists recognized that urea had not represented a total or direct synthesis from the elements themselves, that this was a very simple organic substance whose physiological significance could easily be marginalized, and that the event represented at best a first hesitant step toward a distant goal. For the next quarter century, chemists were ambivalent, at times predicting a glorious future of artificial organic compounds creating better living through chemistry, at other times racked with doubt about the possibility of extending Wöhler's work to ever more complex substances.[6]
Additional steps were eventually made, and here Wöhler's student Kolbe was in the forefront. We noted in chapter 3 that in 1845 Kolbe became the first to publish a total synthesis of an organic compound, namely, acetic acid from inorganic carbon and water, also using sulfur and chlorine. In this paper, Kolbe used the term synthesis perhaps for the first time in a chemical context and boldly predicted the artificial preparation of sugar, starch, and other organic products. A few years later, he and Frankland published what I have termed the first two important general synthetic manipulations (carboxylation through nitrile formation and the Kolbe electrolysis reaction).
From that time on, the synthetic repertoire of organic chemists quickly grew: Frankland's organometallic routes, Strecker's preparation of alanine and lactic acid from aldehyde, Hofmann's various eponymous amine reactions, Gerhardt's acid anhydrides, Williamson's ether synthesis, Kekulé's sulfurations, the Wurtz reaction and his glycol work, Wanklyn's preparation of propionic from carbonic acid, Berthelot's total syntheses of acetylene and other simple organics, the first commercial synthetic dyestuffs mauve and fuchsine, and so on. All of these examples are taken from the years 1847-1859; in the following decade the growing stream of organic-chemical novelties became a torrent. As late as 1850, Strecker thought it necessary to italicize his claim to have prepared artificial lactic acid; by 1858 Kolbe was confidently predicting that artificial indigo, alizarin, and quinine would soon make their appearance.[7] By the latter date—about the time of the formulation of structure theory—expressions of caution and doubt disappeared from organic textbooks. In sum, what undid chemical vitalism was not a single discovery, nor any small handful of them, but rather a gradually increasing sense of the grand possibilities of organic-chemical manipulations, closely connected with fast-growing empirical success.
Kolbe remained in the vanguard of synthetic organic chemistry during his burst of research in the years 1858-1865—in fact, he was
arguably the leading personality. One of the most important of his contributions was a method for the preparation of salicylic acid from phenol, carbonic acid, and sodium metal, carried out in collaboration with his student Lautemann (this subject is explored in chap. 12). His role in the prediction and extended study of branched-chain secondary and tertiary alcohols and acids, and his participation in the further study of polyfunctional alcohols and acids, were discussed in chapter 9. Another instance began with Kolbe's published speculation that taurine, a component of a bovine bile acid discovered a generation earlier by Leopold Gmelin, was nothing other than aminoethylsulfonic acid. Since he regarded Magnus' isethionic acid (prepared from sulfuric acid and alcohol) as the analogous hydroxy compound, a reaction route was evident, and in 1862 he reported a synthesis of taurine from isethionic acid.[8] He also succeeded in reversing Strecker's synthesis of lactic acid, by converting the latter to alanine.[9] In both cases, he made important theoretical arguments, interrelating the constitutions of alanine and glycine with lactic, propionic, and acetic acids.
Jacob Volhard's project in Kolbe's lab in the eventful summer of 1862 was a study of sarcosine, long known as a hydrolysate of creatine (found in meat extract). Volhard proposed that sarcosine was N-methylglycine, and he proved the idea by preparing the compound from methylamine and Kekulé's monochloroacetic acid.[10] With another junior colleague, Rudolf Schmitt, Kolbe carried out a modification of his salicylic acid synthesis by reacting carbonic acid with metallic potassium in the presence of water and isolating formic acid from the reaction mixture.[11] In the same issue of the Annalen in which this paper appeared, Kolbe and Schmitt published a second article that signaled the isolation of a red dyestuff from the oxidation of commercial phenol by oxalic and sulfuric acids. The product, "rosolic acid," was subsequently shown to be identical to a compound first prepared by F. F. Runge many years earlier.[12]
These events occurred just at that heady time when a new science-based industry was burgeoning: the coal tar dye trade. There are indications that Kolbe was quite interested in principle in taking economic advantage of this development however he could, but for whatever reason, he failed to follow up on his and Schmitt's discovery. A few years later, several scientists and industrialists (including Heinrich Caro, James Wanklyn, Carl Graebe, Carl Schorlemmer, R. S. Dale, and J. F. Persoz) investigated the compound further, and this work led to the commercial dyes known as corallin and aurine. Between 1876 and 1880, the cousins Emil and Otto Fischer elucidated the complex structures of these and related dyes. They are triphenylmethane derivatives, just like fuchsine (magenta)—the second commercially suc-
cessful color and the foundational product for several of the largest chemical companies today. Kolbe had failed to patent his method of preparing rosolic acid and his priority complaints were ignored. As for the Fischers' work, despite its structuralist basis Kolbe was "astonished" to find that it was "truly excellent." "It must only be translated into chemistry," he added.[13]
Such generosity of sentiment did not characterize Kolbe's reaction to Adolf Baeyer's structural and synthetic work on indigo. Kolbe himself had long been interested in indigo, both scientifically and for its potential pecuniary rewards;[14] he thought Baeyer's structural suggestions were unscientific absurdities. It must have bitterly rankled that Baeyer became the first to achieve a successful laboratory synthesis; mercifully, he did not live to see its lucrative commercial application.
After his transfer to Leipzig, Kolbe continued the sort of synthetic work that we have exemplified for the Marburg period; on the whole, however, his personal research activity declined. He did achieve fabulous financial success with one project, however—an improved version of his salicylic acid synthesis of 1859. This subject is covered in chapter 12.
Structure Theory and the Philosophy of Chemistry
During the eighteenth and much of the nineteenth centuries, the science of chemistry was more thoroughly imbued with the methodology and general culture of natural history than with that of the physical or mathematical sciences. Chemists were usually depicted—and depicted themselves—as naturalists exploring the earth's great garden of chemical species, in just the same way that the true naturalists were widening our horizons on biological species. This was the reason why systems of chemical classification were regarded as so central to the science, why Linnaeus' search for a natural botanical system was emulated by chemists, why genetic and parental relationships among chemical compounds were emphasized, and why biological metaphors were so often employed in chemistry.[15] It also partially explains the antitheoretical attitudes of many nineteenth-century chemists.
But this naturalist's scientific self-image was also mixed with some of the naturalist's scientific self-doubt. Many chemists felt the need for a general theory to guide their investigations, on the model of the exact sciences, but this goal proved to be elusive. Lavoisier, as much a physicist as a chemist, sought to create a general theory centered on oxygen and embracing physics and physiology as well as chemistry. Dalton's efforts to extend chemical atomism to physics were largely unsuccess-
ful. His atomic theory became the closest thing to a generalized theory for the science, but most chemists innocently maintained the fallacy that this theory was coterminous with the law of definite proportions. Berzelius attempted to develop an electrochemical theory that would span the science, but he ran into increasing difficulties. Advocates of the radical and type theories of the 1830s and 1840s likewise tried to create general theories that could serve as guides to chemical investigations. Both theories came to be seen as insufficiently flexible and general until the advent of structure theory, which combined some of the best features of each.
As they began to search for a general theory analogous to that of the physicists, chemists also increasingly adopted the hypothetico-deductive methodology that had already begun to penetrate the exact sciences.[16] For a time, the inductivist commitments of most chemists kept hypothetico-deductivism suppressed, at least on the rhetorical level, but by mid-century it was breaking through into the science. As early as 1836 Dumas opined
Theories have always been regarded as things quite different from truth; for this reason theories have long been accorded an importance proportional to the service that they render. . . . In chemistry, our theories are crutches; to show that they are good, they must be used to walk. . . . A theory established by twenty facts must explain thirty, and lead to the discovery of ten more; but nearly always it is modified or succumbs to ten new facts added to all that went before.[17]
Dumas may have acquired his taste for deductivism from Ampère, who was a particularly adept user of the method,[18] or from the physical chemist C. L. Berthollet. It appears that he communicated the same predilection to his protégé Wurtz.
In England, John Herschel's popular Preliminary Discourse on the Study of Natural Philosophy offered fervent praise of deductivism (despite the Baconian overlay); the book is known to have strongly influenced Michael Faraday. Both Herschel and Faraday were, of course, chemists as well as physicists. Herschel's book may also have been influential for Alexander Williamson; not only Williamson's hypothetico-deductivism, but also some of his language (the search for verae causae and for experimenta crucis ), may well have derived from Herschel.[19]
As far as Germany is concerned, throughout the 1820s and 1830s Liebig was inclined toward hypothetico-deductivism, as was Berzelius, who was so influential for the direction of German chemical theory. Given the prevailing inductivist atmosphere in chemistry, however, both men were rhetorically cautious about their methodology and were
also no doubt to a degree self-deluded. In 1862, after the method of hypothesis had become more generally accepted, Liebig composed a devastating critique of Baconian induction.[20] German physics was beginning to become perceptibly hypothetico-deductivist as early as the 1830s, with the work of such men as Gauss, Weber, and Neumann.[21] Kolbe, a student of the inductivists Wöhler and Bunsen, was an instinctive hypothetico-deductivist from the time of his earliest scientific work. His first extended paper contains repeated and explicit references to the method of hypothesis, at a time (1845) when this was uncommon in chemistry.[22] Liebig or Berzelius are the most probable sources for this predilection.
It is clear from all of this that the beginnings of a trend toward development of a strongly generalized and hypothetico-deductivist pattern of theorization modeled on the exact sciences could be discerned in chemistry at the time of Kolbe's entry into the field around 1840. These trends, however, had not yet entered the lifeblood of chemistry, certainly much less so than for physics and astronomy. The model of natural history was still dominant for chemists. In this methodological sense, it was the theory of structure that made the difference and created a new era for the field. Structure theory was a general theory that could be applied to inorganic as well as organic chemistry. In a stronger sense, its success also demonstrated the success of the underlying and more general atomic theory; after the 1860s, few chemists ever again seriously questioned the theory or equated it with the law of definite proportions.[23]
Moreover, structure theory was well suited for application of hypothetico-deductive method. Once its principles were understood and accepted, the theory generated hypotheses almost effortlessly, each of which could suggest one or more experimental tests. One of the qualities that made the theory so powerful for the method of hypothesis was the gradually acquired ability of organic chemists to synthesize new artificial compounds. As their synthetic repertoire rapidly enlarged during the middle decades of the century, chemists were increasingly able to ask and answer their scientific questions experimentally.
Perhaps the earliest legitimate example of this theoretical technique is Williamson's ether synthesis of 1850. Ether had first been prepared from alcohol in the sixteenth century, which illustrates the point that synthesis per se was nothing new to the nineteenth century. However, Williamson's innovation created a way to produce novel "tailored" ethers at will. In doing this, his intent was not the naturalist's ambition to add as many new chemical species as possible to the world's store of knowledge, but rather to provide a specific test, a crucial experiment,
for two theories that could thereby be made to dictate two different experimental outcomes. Whether or not retrospective logical analysis can sustain Williamson's claim to have provided an irrefutable test, empirical history supersedes logical necessity, for as we have seen, his argument was soon universally regarded as compelling.
The polemic between Kolbe and Wurtz during the years 1857-1861 on the structure of lactic acid provides innumerable additional examples of this sort of approach. Kolbe and Wurtz not only explored new oxidations, reductions, and halogenations, they also created larger molecules by esterifications and ether syntheses, and each of these reactions represented an attempt to provide evidence for a particular point of view. Kolbe's predictions of the synthesis of new diacids and new secondary and tertiary alcohols, which soon led to their preparation, are particularly striking examples. Finally, it needs to be stressed that synthesis now could be placed beside analysis—indeed, by the 1860s it had already usurped the pride of place—in structural investigations. We have seen several examples of this trend in the previous section.
Craft Skills and Tacit Knowledge in Organic Chemistry
All of this suggests that a certain convergence of chemical toward physical hypothetico-deductive methodology took place around mid-century, attributable in substantial part to the emergence of the theory of structure. There is more to the methodological question than that, however. Physics and chemistry were distinguished by a host of different qualities, values, exemplars, habits of thought, details of practice, and so on, and the result of all of these particulars was that the two sciences maintained quite distinct cultures. Especially on the European continent, physics gradually became less concrete, more abstract, and more firmly based on an axiomatized mathematical foundation. The influence of technology was evident in increasingly complex instrumentation and apparatus, and the culture of precision measurements combined with rigorous error analysis imbued the practice of physics ever more strongly.[24]
None of these qualities characterized chemistry by and large, particularly not the organic field that became so dominant in the middle and later decades of the century. Today as a hundred years ago, most organic chemists have little need for higher mathematics. As far as precision measurements are concerned, they need to know how to weigh out stoichiometric quantities, measure densities, record melting and boiling points, and determine the correct atomic ratios in combustion
analyses,[25] but these modest calculational and metrical demands cannot compare with those of the physicists. There was technology in the chemical laboratory, as in the physical laboratory, but it consisted mostly of simple materials in simple combinations and could not compare with the complex optical and electrical apparatus being used by the physicists.
What the chemists had, and needed in the fullest measure, were craft and observational skills. Chemistry has sometimes been compared to cookery, and the simile is apt in many respects. Substantial manual dexterity and technical know-how—for glass-blowing, devising and assembling apparatus, sealing connections, cutting rubber and cork, heating, cooling, pouring, mixing, grinding, and on and on—were imperative qualities. There was a standard repertoire of procedures, such as distillation, filtration, solvent extraction, titration, and recrystallization, that quickly became well-practiced habits for the novice. Precise observation was vital—color, viscosity, clarity, smell, taste, texture, crystal size and shape, and so on. An excellent memory was a virtual necessity to deal with the thousands of compounds regularly encountered.
There was much truth, therefore, in the perception that organic chemists were like naturalists exploring exotic ecosystems, only in the chemists' case, the object of study had often just been created for the first time by the investigator himself. When the discipline of physical chemistry was organized in the 1880s, the leading promoters advertised their field as the "chemistry of the future" and battled for social and institutional support with the well-entrenched organikers over the next two or three decades. Organic chemists resented having their lovely science depicted as a mere compounding of novelties, and their more laboratory-oriented values were even shared by a few of the physical chemists themselves. "Of course a man must specialize," W. D. Bancroft said. "He must be a chemist rather than a physicist." About the same time, he confessed privately, "I abominate exact measurements myself." But as a physical chemist, Bancroft was in a distinct minority.[26]
It must be emphasized that the organic chemist's laboratory repertoire was not a set of simile mechanical operations that all learned and performed equally. Cognitive activity, both conscious and unconscious, must accompany all craft operations. The ultimate quality and success of a reaction or of a recipe, even of a single operation in the process, is closely tied to what is happening in the chemist's or the cook's mind. Consequently, both the manual and the intellectual sides of the craft had to be mastered. This was a good reason for Kolbe's (and many others') emphasis on practical laboratory work as the centerpiece of
chemical education. Precepts, ideas, and data could be learned in the classroom, but the craft skills and tacit knowledge of the professional could only be assimilated side by side with the master and his assistants.
There was a real art to be learned, and this is not simply a trope. Everyone with experience in the laboratory, including the greatest masters, knows of struggles with reactions that just will not go, products that emerge in gummy resins and refuse to give distinct crystals, yields that evaporate to a pittance, and product mixtures that resist every attempt at separation. The virtuosi, however, know how to overcome and circumvent problems and are often able somehow to coax success out of failure. The other side of the coin is the rapture of success, when the dazzling crystals of product suddenly and mysteriously blossom in the recrystallization flask. The ineffable "master's touch"—that of a Wöhler, a Bunsen, or a Kolbe—is the most difficult quality to teach and may well be impossible to impart.
There was an even deeper cognitive side to the chemist's craft. The epistemological technique of transdiction —inferring invisible submicroscopic details from macroscopic observables—was habitual with chemists long before physicists developed a similar art. Ever since chemical atomism arose in the early years of the nineteenth century, chemists were comfortable in routinely inferring the atomistic compositions of molecules from macroscopic gravimetric measurements. There was, of course, an epistemological debate, but most chemists refused to worry excessively about fine philosophical distinctions, and the radical phenomenalism that Gerhardt defended for a time never really became popular.[27] As the century progressed, transdiction became ever more elaborate, and inferences to composition were supplemented by inferences to molecular structure. When the chemist adds methyl iodide to an etherial solution of potassium ethoxide in a Williamson synthesis, for example, her mind's eye is focused on the activity at the molecular level, watching for signs of smooth assimilation of the two reactants, and hoping that she has sufficiently dried the solvent. Examples of such mental habits can be extracted from the host of reactions discussed in this book.
In general, reactions are assessed by analysis of the product—the payoff of an experiment—which also involves transdictive procedures and assumptions. The object of the game here is separating the components of a reaction mixture and purifying the substance on which interest is focused. Procedures for accomplishing these goals were developed, mostly empirically and many of them during the eighteenth century, and assessment criteria were well standardized. Every chemist knows, and knew in the period with which we are concerned, that con-
stant sharp boiling and melting points are a primary indicator of purity and that admixture of impurities tends to lower and spread out these transition points. Every chemist knows, and knew, that attractive, well-formed, uniform crystals are another positive indicator. If such signs are not observed, then the chemist must try again—another distillation, sublimation, recrystallization, or repetition or modification of the reaction itself.
Elemental analysis is the final step, but only after product purity is attained. If a product matches the crystal appearance and sharp melting point of a known substance, and its percentage of carbon, hydrogen, and oxygen comes within the experimental error of that calculated for the relevant formula, then a match is achieved and the collegial community will probably be satisfied. If the purity indicators are good and the chemist has reason to expect a novel product, then the percentage composition of the observed product is again compared to the predicted formula, using accepted values for atomic weights. Such an identification was usually accompanied by at least a brief examination of chemical and physical properties and characteristic reactions, as well as preparation and characterization of simple derivatives such as salts of acidic or basic compounds, and so on.
Often the point of the organic chemist's investigation was achieved by establishing the very existence of a new compound—such as Williamson's asymmetric ethers. For other research, it was necessary to create and enumerate isomers corresponding to a single formula in order to test an idea. In still other cases, synthetic or analytical reactions needed to be explored to establish or refute conjectures, such as those concerning structural details of a molecule or genetic relationships among compounds. Synthesis was often a goal per se, especially of natural products. Finally, in some cases it was the particular properties of a new substance that provided the point of interest.
Although there certainly were distinctions in the details of laboratory practice in different universities and different countries, the generic chemical procedures, standards, and goals just outlined were quite uniform throughout the world during the period with which we are concerned. Thus, in principle, consensus was attainable regarding the general course of individual reactions and the existence of novel organic compounds. However, there were many potential points of challenge throughout the series of operations just described. Disputes over the "facts" were certainly not uncommon, and we have seen a number of them here, but what is striking is how infrequently experiments by members of the profession were successfully challenged. The story of Kolbe's chimerical salylic acid, which is related in chapter 12, is notable in its relative singularity.
I have gone through this material, at risk of trying the patience of chemically sophisticated readers, because I believe it is helpful for other readers to understand something of the mental equipment, operational repertoire, and epistemological habits of thought to properly appreciate and evaluate the historical action in this narrative. One theme of this book is pedagogy; chemistry students start out at the layman's level and have to acquire all of that equipment during their apprenticeship. We began in the first chapter with the guild model for the German university, and regarding chemistry as both a cognitive and a manual craft fits well in that model. It also needs to be stressed once more that, although chemistry had much in common with physics as a natural or exact science, it had a culture that was quite distinct in many ways, and acquisition of this culture was also part of the educational process.
Exploring Atomic Ecologies:
Erlenmeyer and Kolbe
Ultimately, what made the theory of structure so powerful was an aspect that brought it closer to the physicists' scientific culture, namely, its ability to be axiomatized. In its simplest form, it could be reduced to a series of simple algorithmic or combinatorial rules, whereby possible molecular structures could be schematically derived by following straightforward valence assignments. The theory in this simple, powerful form is roughly the same as what Kekulé and Wurtz referred to in the late 1850s as the theory of "atomicity of the elements." It had roots in and similarities to both radical and type theories, but it was cleaner and more mechanical and was manipulable by simple algorithms rather than complex chemical intuition.
For example, most radical theorists regarded radicals as irreducible molecular moieties that were to be considered as wholes. A simple valence bond between two atoms was foreign, even antithetical, to the theory. Similarly, the type theory suggested more than mere genetic relationships among series of compounds: it asserted familial chemical similarities among members of the same type. This was one of the central claims of the theory that Kolbe (and others) began to contest in the 1840s and early 1850s. It was also what Wurtz was referring to when he wrote in 1861, "In a word, the idea of types is an artifice, a pure convention. In my opinion it must be subordinated to a more fundamental notion, that of the atomicity of the elements ."[28]
Although Kekulé and Couper laid the foundation for the theory of atomicity of elements in 1857-1858, even advocates of this theory did not immediately eliminate old type/radical thought patterns from their
minds and begin thinking in the simple structural combinatorics that would soon become second nature to organic chemists. There was a certain period of transition for the field, during which the language and thoughts of leading theorists were somewhere in between the two stages. By the time Hofmann was composing his Introduction to Modern Chemistry in 1865, this transition period was about over, and Hofmann could correctly refer there to the "revolution" that had taken place in the science.[29]
By the late 1860s, then, many if not most leading theoreticians had become full structuralists and thereafter thought largely in terms of those simple algorithmic patterns. However, this does not mean that organic chemistry had become a simple arithmetic problem, as Kolbe often charged. The schematic simplicity of the theory had to be compared and constantly checked and modified against a sometimes bewildering array of chemical peculiarities on the actual atomic—molecular level. The empirical contingency of atoms and their idiosyncratic chemical interrelationships contrasted starkly with the schematic simplicity of the theory. In effect, chemists had to emulate the naturalists once more, in exploring and determining what amounted to seemingly arbitrary (or at least then unmotivated) "ecological" relationships among the atoms in a molecule. In such a situation, it was vital that chemists maintain open attitudes. The theory was an indispensable guide in a general sense, but empirical flexibility was the most pragmatic course for questions of detail.
Nothing could have predicted, for instance, that "double bonds" would become the best way to view the relationship between adjacent carbon atoms in olefins, as opposed to maintaining free carbon affinities or two affinities satisfying each other, or even reducing the actual valence. Nothing could have predicted the eventually established circumstance that the four valences of carbon are all chemically equivalent. It was not at all clear why certain simple structures perfectly consistent with the theory, such as OCH2 and C(OH)4 , resisted all attempts at realization. Kolbe asserted as early as 1850 that the carbonyl carbon of acetic acid was bound both to oxygen and to alcohol functions. In 1859, Kekulé made the same statement in structural terms and argued that this atomic arrangement defined organic acid behavior in the general case. But, again, exactly why this was true remained mysterious.
Chapter 9 examined the work of some of the leading organic chemists of the 1860s in exploring some of these contingent "chemical ecologies" and pondered the effects such activity had in reshaping the character of the science. This section and the next continue this line of investigation, concentrating on the thoughts and writings of two
of the most avid converts to structural ideas: Emil Erlenmeyer and Aleksandr Mikhailovich Butlerov. Both men had interesting relationships with Hermann Kolbe.
A former apothecary and a student of Liebig and Will, Erlenmeyer first entered our story in chapter 7.[30] Closely associated with Kekulé as a fellow Privatdozent in Heidelberg in 1856-1858, Erlenmeyer was one of the most inventive of the early structural chemists. In 1859, Kekulé trusted Erlenmeyer so implicitly that he asked him to perform all copyediting and proofreading for the first sheets of his textbook, then being printed, and he did not even want the final version to be sent back to him before printing. Predictably, this procedure created friction between the two men.[31]
In 1858, Kekulé in collaboration with three other Heidelberg colleagues decided to found a new critical journal devoted principally to book reviews of the scientific literature and entitled Kritische Zeitschrift für Chemie, Physik und Mathematik . Kekulé's intent, as he wrote to Liebig, was to create a "dam" against the flood of "Schmierliteratur" then afflicting science.[32] However, neither Liebig nor any other first-rank chemist lent his name publicly to the enterprise.
Kekulé had departed for Ghent long before even the first volume of this journal was complete, and Erlenmeyer took over the chief editor's role. From this time on, the journal restricted its scope to chemistry alone and began to publish reprinted (and occasionally original) articles. Beginning with volume three in 1860, Erlenmeyer took sole command of editorial production. The dramatic increase in the pace of chemical research and in the number of chemists, Erlenmeyer noted in a preface to that volume, meant that researchers were now in constant danger of unintentionally trespassing on each other's work. All the more vital was it that an organ be established that provided for rapid publication of preliminary results and small projects. Consequently, the Zeitschrift was henceforth to appear twice monthly in numbers of two sheets (thirty-two pages) each.[33] What Erlenmeyer did not say publicly, but in private letters to colleagues in an attempt to boost circulation, was that he and others were also growing increasingly impatient with the slow publication schedule of Liebig's Annalen . His new model for the Zeitschrift was the Comptes rendus in Paris.[34]
Kolbe began to send Erlenmeyer offprints of his Annalen articles for reprinting. In 1862 they began a friendly scientific correspondence, and by the end of the next year this had developed into a real friendship, including regular visits. Kolbe perceived enough commonality in their views that he began to think of Erlenmeyer as a compatriot.[35] "I do not doubt," he wrote, "that my and your views will in time come into
their own and win general approval. Without this certainty the isolation in which I now find myself with my views would have become unendurable to me."[36]
According to Erlenmeyer's later opinion, both he and Kekulé remained advocates of type theory (that is, they were not full structuralists) when Kekulé was in Heidelberg, even after he had enthusiastically adopted the point of view advocated in Kekulé's 1857-1858 papers. His conversion from this "one-sided" viewpoint to true structuralism came only late in 1861. The problem with the earlier viewpoint, Erlenmeyer said, was that its advocates always strove to identify a single de-terminative type, or atom defining the type, that could be used unambiguously to classify and characterize the molecule. He thought the correct viewpoint was to regard all pieces of a molecule, all of its radicals and atoms, to be ontologically equal, so that a molecule might be considered to be schematically dissectable in any possible fashion.[37]
Examination of volumes four and five (1861 and 1862) of Erlenmeyer's Zeitschrift supports this autobiographical contention. In a paper published in April 1861, Erlenmeyer stated that he considered Kolbe as much a typist as Williamson, Gerhardt, and Wurtz, and he argued, in a Gerhardtian vein, that a significant accomplishment of the type theory was to have shown that one cannot prove absolute molecular constitutions. However, the goal of the paper was to argue a point that Kolbe would have considered constitutional, namely, to demonstrate that all alcohols and acids are monobasic.[38] Erlenmeyer's thesis was pure Kolbean theory, and it would seem that at this time he would have described himself as a follower simultaneously of Gerhardt, Kekulé, and Kolbe, creating a useful syncretic viewpoint from these various influences.
However, early the following year Erlenmeyer began to publish a series of remarkable theoretical ideas that shows significant evolution from the previous year, ideas that are quite in the spirit of full structuralism. In a fervent defense of chemical theory in general and the newer theories in particular, Erlenmeyer provided one of the best early statements of the basic assumptions of the theory of atomicity of elements. The article contains the first suggestion (if not positive assertion) of the existence of carbon-carbon double bonds in olefins. He also proposed that the various affinity units (valences) of an atom have differing strengths, for on this assumption such otherwise anomalous compounds as carbonic oxide and nitrous gas could be assimilated into the theory of atomicity, since in such compounds not all affinities of some atoms are engaged with those of other atoms.[39] Soon thereafter, Erlenmeyer developed the idea, apparently derived from Wurtz and Kekulé, that n-valent atoms are accretions of n-monovalent subatoms,
the subatoms differing in their absolute and relative affinities toward subatoms of various other atoms. This was a potentially powerful theory, but it proved to be empirically inaccessible and so was abandoned before the end of the decade.[40]
In April 1862 Erlenmeyer published another critical article, this time on the lactic acid controversy. He rhetorically asked "die Herren Typiker" (among whom he obviously no longer counted himself) why they believed their type formulas so literally when doing so generated many misleading predictions. The proper approach, he said, was to consider a chemical formula as a "positional diagram" (Situationsplan ) for its constituent atoms, which specifies the "topographic positions" (topographische Lage ) of all of its components. So as not to be misunderstood, he added,
No one could imagine that with these formulas I intend to indicate the actual positions of the components. But I will remark that I very much intend thereby to express a topographic analogy of the derivative with the mother substance and to show that the mere reaction formulas of the typists no longer suffice, but rather we need to use relative constitutional formulas.[41]
Erlenmeyer's "relative constitutional formulas" look to modern eyes, and no doubt to many of his contemporaries as well, like structural formulas. Indeed, he became the earliest German advocate of the graphic formulas pioneered by Couper and Crum Brown, which proliferated in his articles especially after 1865.
At the end of 1863, Erlenmeyer published a defense of Kolbe's views. Kolbe was being misunderstood by nearly everyone, Erlenmeyer argued; he was a typist in the full sense and had lost credit for some of his discoveries only because of the unusual form of his theories, while the content was quite conventional. Indeed, Kolbe's types were in some respects superior to other versions, Erlenmeyer asserted; they were more consistent, more genetically and synthetically derived, and more exclusively focused on carbon. Kolbe's biggest real differences with the other typists were his insistence on the goal of establishing absolute constitutional formulas and his use of equivalents for carbon and oxygen. There was a certain irony in each of these points of conflict, Erlenmeyer continued. For one, the typists' "reaction" formulas, despite the conventional rhetoric of their users, do ordinarily specify constitutions in Kolbe's sense, and more often than not they correspond essentially with Kolbe's formulations. Moreover, Kolbe may well be right in viewing carbon as C2 (C = 6) rather than
C (C = 12). (Here Erlenmeyer was making reference to his own subatomic speculations.)[42]
Kolbe was very grateful. After thanking Erlenmeyer, he added,
By undertaking to prepare the way for a better understanding of my views on the composition of organic compounds, against which there is widespread prejudice, you are taking on a somewhat dangerous task, since a large number of chemists want to know absolutely nothing about them, and will resent you for demanding that they think seriously about chemical questions. . . . Even though our views differ in points of detail, I am pleased that they are nearly the same regarding carbon.[43]
Erlenmeyer was fast earning a reputation, and not altogether a favorable one, as a prolific but increasingly acerbic and incautious critic; even his formerly close relationship with Kekulé went on the rocks. It was partially for this reason that his journal was not flourishing. By the fall of 1864, there were only 150 subscribers (and more than half of them were Russians). He decided to give up his editorship and gave the journal over to three Göttinger Dozenten (Beilstein, Fittig, and Hübner).[44] In its place, he did what many of his theoretically inclined colleagues were doing and began writing a textbook. In 1868 Erlenmeyer's future was finally secured (he was then a forty-three year old Privatdozent) by a call to the Munich Technische Hochschule.
Erlenmeyer maintained a close relationship with Kolbe until the latter's increasingly strong attacks on structure theory finally drew his fire. In June 1871, he wrote Kolbe saying that he was composing a public lecture to be given in honor of King Ludwig's twenty-fifth birthday. Without giving away details, he wanted, among other things, to persuade Kolbe "that your formulas are nothing more than my formulas, that at most a difference between them arises when we have a different view of the constitution itself."[45]
The lecture was printed in the course of the summer, equipped with a ponderous 3500-word footnote dealing with Kolbe,[46] and Erlenmeyer sent a copy of it to his friend. Kolbe was one of the few who were still contesting structure theory, Erlenmeyer noted, but what Kolbe did not realize is that operationally, his formulas were nearly identical to those of the structuralists. He was a type theorist in the strict sense, the sort that he (Erlenmeyer) and Kekulé had been before both began to operate as full structuralists about 1861. According to Erlenmeyer, the only substantial difference between Kolbe and the structural chemists was that Kolbe denied the existence of not only direct connections between carbon atoms (chain theory) but also the very concept of valence bonds.
Erlenmeyer argued that Kolbe's position was not logical or consistent. Kolbe averred that his theory was based upon atomicity, that carbon atoms were (almost always) tetratomic, and that methyl, for example, was monatomic because one of the carbon atomicity units was unsatisfied. Kolbe had angrily rejected the conclusion that it was precisely that fourth valence of carbon that formed the point of attack or attachment for the methyl radical. It was the methyl radical as a whole , he said, that substituted for the hydrogen of water to form methyl alcohol, of methane to form ethane, of formic acid to form acetic acid, and so on. However, Erlenmeyer protested, it was Kolbe himself who had begun as early as 1850 to speak of the "point of attack" of chemical action, in this case referring to the carbonyl carbon atom of acetic acid.
Erlenmeyer also chided Kolbe for the one-sidedness that characterized all genuine type theories. Kolbe's search for the dominating "Grundradikal" or "Hauptkohlenstoffatom" resulted in an over-emphasis on one radical or atom to the detriment of every other one in the molecule. It was downright silly to search for a single carbon atom in an alcohol that confers "family character" (Gattungscharakter) on the compound; it is rather functional group analysis (emphasis on hydroxyl groups, in this case) that has chemical significance. Kolbe had used various typographical techniques, including bolding, boxes, and size differentials, to distinguish among the carbon atoms in organic compounds. Were the alcohol of the eighteen-carbon stearic acid to be prepared, Erlenmeyer concluded, and were Kolbe to use sizing to distinguish all of the carbons, one would need a microscope to see the eighteenth.[47] Erlenmeyer summarized his position in the following way:
Kolbe has remained a typist while most other chemists have abandoned the type viewpoint. He considers chemical compounds not according to their entire composition and constitution and all properties determined from these factors, but only as alcohols, as aldehydes, as carbonic acids, as sulfonic acids, as amines, etc. etc.[48]
That this conservatism had operational hazards Erlenmeyer was also able to show. Kolbe had predicted as a consequence of his point of view that the two chlorine atoms of the unknown compound 1,3-dichloropropane would be found to be chemically distinct, whereas chain theory (structure theory) would predict them to be chemically identical. Erlenmeyer succeeded in testing this prediction, at least for the bromine analog: he had the compound synthesized and reported
that both bromine atoms hydrolyzed simultaneously to form the dialcohol.[49]
Erlenmeyer could hardly have devised a more stinging rebuke than to say that Kolbe was a one-sided type theorist and needed to think more seriously and more chemically about compounds and reactions, for this was precisely Kolbe's longstanding complaint against the type theorists and the structuralists. Kolbe's fury can only be imagined. In September he composed yet another general attack on structure theory for his new Journal für praktische Chemie . To Vieweg he commented, "In this piece I become personally unpleasant only once, namely against Erlenmeyer, a man who has not a trace of logic in his head. He challenged me and forced me to this. Liebig may not like it, but I cannot help that."[50] In that event, however, Kolbe decided to suppress his comment.[51] But he did not hesitate to disparage Erlenmeyer ever after in his private correspondence. "Once a pharmacist, always a pharmacist," he grumbled to Volhard.[52]
Butleroy, Kekulé, and Kolbe
The accession of Czar Alexander II to the Russian throne in 1855 soon led to a quickening interest in modern science, and his government embarked on an urgent program to encourage foreign education of eligible candidates, especially at German and Swiss universities.[53] One of the earliest of these scholars was Aleksandr Mikhailovich Butlerov,[54] who was already full professor at Kazan (although he had not yet done any significant chemical research) when he arrived in western Europe in 1857 on a government-sponsored komandirovka (educational tour). Butlerov was influenced by two visits with Kekulé in Heidelberg, but he spent his longest period (five months) in Wurtz' laboratory in Paris. This was just when Couper was there as well, formulating his ideas on what members of Wurtz' research group were already beginning to call the "structure" of chemical compounds. Butlerov participated in this program as well, presenting a lecture to the new Société Chimique (on 17 February 1858) that outlined a carbon type theory similar to that of Odling, Kekulé, Frankland, and Kolbe.[55] Unfortunately, this lecture was never published. By the end of May, Butlerov was back in Kazan, but the year that he had spent in Germany and France determined the course of his research for the rest of his life.
Three years later Butlerov was back in the West. In September 1861 he presented to the Speyer meeting of the Gesellschaft Deutscher Naturforscher und Ärzte a paper "On the Chemical Structure of Com-
pounds" that Soviet historians have always considered the locus classicus of structure theory.[56] The real purpose of the paper was to try to move the chemical community from a halfway position, mixing older type and radical theories with the new theory of atomicity of elements, to the position of full structuralism. This was just the sort of transition to which Erlenmeyer later referred, and the timing matches, as well. It is therefore likely that Erlenmeyer's and Butlerov's conversions were connected, but it is not easy to determine who played the leading role for the other. Erlenmeyer wrote Butlerov eight months after the Speyer meeting to lodge a mild priority protest. "I think I can assume with certainty," he stated, "that the ideas [in Butlerov's Speyer paper] were, in large part at least, influenced by our conversations." These ideas included the constancy of tetratomicity of carbon, double bonds in unsaturated hydrocarbons, and the variable strength of the four valences of carbon, all of which, he asserted, he had been earlier to emphasize than Butlerov.[57]
It is in any case clear that Erlenmeyer and Butlerov agreed closely with each other from 1861 on. Erlenmeyer printed Butlerov's Speyer speech in full; it was otherwise unpublished, perhaps otherwise unpublishable due to its purely theoretical content. In part because Heidelberg was proving to be increasingly popular with Russian chemistry students, Erlenmeyer began to teach ever larger numbers of Russians; at times this national contingent comprised more than a third of his students. Similarly, his Zeitschrift was the most common publication outlet for Russian chemists in the early 1860s.[58]
In articles published in the Zeitschrift over the next few years, Butlerov instantiated his theoretical manifesto by means of innumerable examples from the science of organic chemistry, most of them having to do with the structural elucidation of isomers and many incorporating novel syntheses emanating from his laboratory.[59] In so doing, he established himself in the forefront of the fast-moving field of structural organic chemistry.
Butlerov was also concerned to integrate what seemed to be a fractured and fractious field, by attempting a substantive and semantic analysis of the language and theories of some of the leading chemists of the day. The leading personalities among the theoretically active chemists were Kekulé and Kolbe. Despite their strong apparent differences, Butlerov wrote, their "basic principles are nearly, or perhaps entirely identical," the conflicts arising only because of their incomplete or inconsistent application of those principles. Kolbe was inconsistent, Butlerov felt, because he sometimes assumed divalent or even trivalent carbon and because he used equivalents rather than atomic weights. Kekulé was inconsistent because of his assumption of "molecular com-
pounds" for certain structures, which violate atomicity rules, and because he sometimes failed to propose a single unique structure for every compound.[60]
Semantically, Butlerov also tried to shed light rather than heat. If one really examines what each author means by his denotations, whether he uses the term "reaction formulas," "constitutions," "relative constitutions," or "topographic position" is of little consequence. Kekulé, Kolbe, Erlenmeyer, Wurtz, Heintz, and other advocates of the new theory, he claimed, were talking about essentially the same things and interpreting their formulas largely the same way. This was why Butlerov thought a relatively new and unused expression should be introduced, namely, chemical structure . Butlerov wrote
In Kolbe, as in Kekulé, one sees the same tendency to judge the chemical connections of the individual atoms forming a complex molecule, or the points of attack of chemical affinity, which is saying the same thing. Kolbe defines constitution as the manner of this connection; I call this chemical structure . . . Be that as it may, most of Kekulé's as well as Kolbe's formulas are clearly based on the principle of chemical structure. I might add that ever since the importance and role of atomicity was recognized this principle has formed the real point of departure for almost all theoretical considerations.[61]
During the 1860s, Kolbe agreed with Butlerov's identification of "constitution" with "structure," for he commented in a letter to Frankland that the two terms had identical denotations.[62] After 1870, however, Kolbe began to draw increasingly sharp distinctions between them.
Butlerov had high respect for both Kolbe and Kekulé as leaders of the structuralist school. The influx of Russian chemists, mostly from Kazan, into Kolbe's lab during the years 1862-1867 can only have been due to Butlerov's recommendations to his students. Butlerov also knew of the importance of Kekulé's work for the rise of structure theory; he only resented Kekulé's attitude, especially his imperialistic manner of promoting his own work.
Kekulé had been reading all of this literature as it came out and was induced to add some clarifying comments. It makes little difference, he said, if one chooses to speak of "chemical structure," "constitutions," or "topographic positions"; the ideas were those on atomicity of elements that he had been the first to publish in 1857-1858.[63] He took strong exception to Erlenmeyer's and Butlerov's opinion that he had remained tainted by residues of type-theoretical thinking until 1861. Recent historical assessments suggest that Kekulé's claim to exclusive right to valence and structure theory is at the very least simplistic (as he later implicitly conceded), but that his avowal that he had been a
full structuralist since 1857-1858 is consistent with what is known of his views in the late 1850s.[64]
It was during these years that Kekulé's relationship with Kolbe went from uneven to very bad. Despite his early close association with Kolbe's enemies such as Gerhardt and Williamson, Kekulé's view of Kolbe's work was not unremittingly negative. During the Kolbe-Wurtz polemic over lactic acid, Kekulé expressed to Erlenmeyer the wish to "negotiate" with Kolbe and to accustom his friend Wurtz to "better manners." In a letter to Lothar Meyer, he referred to Wöhler's and Kolbe's "lovely " syntheses (his emphasis), and a few years later publicly praised Kolbe's "sagacity" in successfully predicting the existence of secondary and tertiary alcohols.[65]
But Kolbe never had any use for Kekulé. He considered Kekulé a "foolish chatterer," a self-promoting and ostentatious pretender whose thin reputation would very soon collapse.[66] In 1861 and 1862 their work came uncomfortably close together, when both made tentative attempts to explain certain of the isomeric relationships of aromatic monoacids and unsaturated diacids. In one of his papers, Kekulé refused to comment on Kolbe's views on the first of these subjects, since the whole matter was still uncertain; moreover, he said, Kolbe's views changed so often that he had become a moving target, difficult to hit.[67] Kekulé had a second opportunity to upbraid Kolbe when he publicly identified two errors in one of Kolbe's papers. Kolbe conceded "a slip of the pen" for one of them, but tried to excuse the other by an unsatisfactory semantic ploy that did not satisfy the editor of the Annalen , Kolbe's good friend Hermann Kopp, who added an explanatory footnote.[68]
A third occasion caused Kolbe the most embarrassment. Shortly after arriving in Marburg, Kolbe had one of his students electrolyze succinic acid, expecting from theory to obtain ethylene. Kolbe was surprised to find his student reporting both eudiometric and gravimetric combustion analyses of the product as indicating methyl ether. He was never able to explain this result; moreover, he lost the data given to him by the student and even forgot the student's name. But he never forgot this curious result. In the fall of 1859, in the midst of that wondrous burst of activity that did so much to burnish his reputation, he appended a description of the experiment, including a statement only on the eudiometry and no analytical data, to a series of short notices published in the Annalen .[69]
In 1864 Kekulé repeated Kolbe's experiment and stated that Kolbe had misidentified the product: it was ethylene, after all. He then added insult to injury by expressing surprise that such a "skilled chemist" as Kolbe could confuse two such different compounds and pointing out
that eudiometric combustion analysis is never sufficient to establish organic formulas.[70] Kekulé's reproof was printed in Erlenmeyer's journal, and Kolbe wrote Erlenmeyer soon thereafter:
Kekulé is a cheeky insolent wretch to purport to teach me that eudiometric analysis alone does not suffice to determine the composition of a gas. But I will have a hard time answering him. . . . I can well imagine that Kekulé is very unhappy that his type theory no longer satisfies even him or gives him nourishment, and I can understand that such a petty man would loose his anger on someone who is traveling a better road with his chemical views. But regarding the succinic acid electrolysis he may be right.[71]
He was right—again—and Kolbe was forced to admit it publicly. Herr Kekulé "ought to have proceeded just a bit more modestly," but in the matter at hand he was "completely correct." Indeed, methyl ether as a product of succinic acid electrolysis had always seemed inconsistent with Kolbe's own theories. "By his demonstration of the error and by clearing the matter up Herr Kekulé has freed me from this embarrassment, and I am therefore doubly grateful to him."[72] Kolbe had done the right thing by graciously conceding to a man he despised. What is curious is how he had gotten himself in this position in the first place. He had published a highly anomalous observation that was eight years old, without data, without even remembering the name of the student who had performed the work, and without even attempting to repeat the relatively straightforward experiment. It was a careless and thoughtless act, and Kolbe was justly embarrassed by it. But years later, he would find repeated occasion to repay Kekulé for his insolence in pointing out Kolbe's mistake to all the world.
The Problem of Formulas and Their Interpretation
In a little-known passage, Albert Ladenburg, a student of Bunsen, Carius, Kekulé, and Wurtz, painted an intriguing picture of the Frankfurt Naturforscherversammlung of 1868. Butlerov had come to Germany for a third time, but Kekulé and several other Germans had felt aggrieved by Butlerov's critiques and were no longer very friendly. On a walk to a restaurant for lunch, Kekulé and Butlerov got into an angry argument, so when they arrived, some of the younger members of the group made sure to sit between the disputants. Later at one of the sessions, Kekulé and Erlenmeyer contested long and earnestly over questions of formulation. Ladenburg sat there silently, thinking that they were disputing unessential matters and had little disagreement in
substance. He said that he was able to persuade both men of this in private conversation afterward.[73]
Such disputes were common in the 1850s and 1860s. Kekulé had personally contributed to some of the confusion, for in 1857-1858, he had formulated a substantially new theory (atomicity of the elements, or structure theory) from the socially safe haven of type theory, using type-theoretical language and formulas. This conventional (Gerhardtian) garb later led some colleagues, notably Erlenmeyer and Butlerov, to view Kekulé incorrectly as having come only halfway at first.[74]
In 1864 Kekulé attempted to straighten out what he regarded as misinterpretations of his formulas. Rational formulas are ordinarily written as reaction formulas, that is, incompletely resolved formulas designed to illustrate specific reactions, functions, or familial relationships. This is normally the most useful and appropriate way to write formulas, Kekulé argued, since excessive or unnecessary detail can degrade clarity of presentation. However, there are certain situations when one wishes to specify the bonding relationships of every atom in the molecule, and this is possible if the compound has been sufficiently investigated. One can write such a completely resolved formula using either type-theoretical or graphical notation (the latter being, for Kekulé, his curious sausage-shaped structures); graphical formulas are clearer but also more cumbersome. According to Kekulé, when one writes a completely resolved formula, whether in terms of types or sausages,
compound radicals completely vanish. . . . Thus, one goes back to the elements themselves which compose the compound. But these are the very considerations that led to the view that carbon is a tetratomic element, and that carbon atoms have the property of bonding to themselves.[75]
It would appear that Kekulé was trying here not only to defend himself from Butlerov's critiques but also to explain Wurtz' position with regard to Kolbe, for Kolbe had scorned Wurtz' view that multiple rational formulas are possible for one and the same compound. Wurtz found himself making a similar defense against his Russian friend Butlerov. After Butlerov's "Erklärungsweisen" appeared, Wurtz published it in his Répertoire de chimie pure and sent him a letter complimenting him on the work:
I find expressed there ideas which I share myself; among others, that the idea of atomicity of elements is based on Gerhardt's types and gives them their true sense. . . . But I would regret it if you were to renounce type notation before being able to replace it with a more advantageous
one. Are you not struck by the simplicity of type formulas? . . . When bodies of complex composition are involved, these [structural] formulas are necessarily complicated, and in such cases indiscriminate use could hurt the clarity of the exposition. . . . Aside from these small reservations I am with you . . .[76]
Butlerov responded,
I am very happy to know that you share my thoughts, and I must add at the same time that what I said about the signification of Gerhardt's types is of course your idea, an idea that you have long been expressing in your publications. I believe that those typical formulas that suffice for the majority of relations of bodies do nothing more than express their chemical structure, or at least the most salient part of that structure.[77]
Butlerov was writing a new textbook of organic chemistry just at this time, the first fascicle appearing in the course of the summer of 1864. Since (as Beilstein put it in a letter to Baeyer) the book was based on German theories, namely on a "Kolbe-Kekulé foundation," Butlerov sought and eventually found a publisher for a German edition.[78] Here he repeated what he had said in his 1863 article, acknowledging that Kekulé was the author of structure theory but averring that his partial allegiance to type theory had led him to a number of inconsistencies. It was these inconsistencies and gaps that he had tried to call attention to and remedy in 1861. Still, Kekulé "recognized the principle of chemical structure more completely and applied it more generally" than Kolbe, who nonetheless deserved major credit (in Butlerov's opinion) for developing the theory. However, it had been Erlenmeyer, Butlerov said, who had been the most consistent and definite in recent years.[79]
This was not Beilstein's view. He wrote Butlerov about this time,
I also spoke during this vacation with Erlenmeyer about this point [atomicity of elements]. I told him that his papers often caused me a great deal of trouble, because each time I had to think myself into his theories, which cost a lot of time. The explanation for this is simple. These days there is no single orthodoxy or type theory, each chemist has his own beliefs & acts accordingly. Everyone is therefore used to thinking only in his own fashion & finds it difficult, i.e., is unaccustomed, to think in another fashion. For instance, Kolbe believes his formulas to be the simplest possible ones & once told Erlenmeyer that he has never been able to properly understand Gerhardt's theory.[80]
Erlenmeyer, for one, professed to have no trouble understanding other chemists' formulas. He wrote Butlerov,
I think we are the only two chemists in the world who understand each other and everyone else. Besides us, I know of no one else who understands us, and none who understands everyone else, as we do. Most typists understand Kolbe not at all, Kolbe understands the typists not at all, Kekulé doesn't understand himself, Kolbe, or us. Wurtz is in the same position. Heintz understands Wislicenus and Wislicenus understands Heintz, but no one else understands anyone else. Heintz thinks he understands you, but he too misunderstands you. Who's left?[81]
Erlenmeyer's viewpoint seems to have been fairly common among his peers. Each thought he understood the various formula styles quite well, thank you; it was only the others who were guilty of misunderstanding. Beilstein's comment cited above complains not about the impossibility but merely the labor of formula translation; Ladenburg averred that he succeeded in serving as interpreter between Erlenmeyer and Kekulé in 1868 and that their disagreement had been insubstantial.
There always were, and still are, pitfalls in understanding caused solely by a lack of complete comprehension of the notation, formula, or language used. Despite Erlenmeyer's sarcasm, however, most of the misunderstandings in organic chemistry of the 1860s were relatively minor or short lived, such as those described between Kekulé, Erlenmeyer, Butlerov, and Wurtz. There is little basis for the thesis that the primary cause of chemical controversy at this time was due to competing formula styles. Priority for structure theory was a lively subject, to be sure, in part due to slippery semantics. But when the discourse concerned specific reactions and specific substances, competitors generally managed to understand each other pretty well. Moreover, by the end of the 1860s, graphical structural notation had largely supplanted the diverse type notations, and there was thereafter little cause for confusion among the majority of organic chemists.
The one significant exception to this generalization was Kolbe, who sometimes found it difficult to follow the details of structure-theoretical arguments and whose own idiosyncratic formulas were sometimes misinterpreted, especially during the 1850s when his notation kept shifting. This problem is explored in greater detail in the first section of chapter 13. But even here the difficulties can be exaggerated. There were some substantive differences between Kolbe and the structuralists. These distinctions were understood by both Kolbe and his rivals, and it was usually on these that they rightly focused. This was particularly the case after he finally adopted atomic weights in 1868.

Kolbe's birthplace and home from 1818 to 1826, the Elliehäuser
parsonage. Photograph taken in June 1992 by the author.

Lutheran church in Stöckheim, Kolbe's home from 1826
to 1840. Photograph taken in June 1992 by the author.

Lutheran church in Lutterhausen, Kolbe's parents' home from
1840 to 1870. Photograph taken in June 1992 by the author.
Hermann Kolbe, ca. 1860, pen-and-ink drawing by F. Justi (1880), from a photograph.
Courtesy of the Erbengemeinschaft Justi, Bildarchiv Foto Marburg.

Deutsches Haus, Marburg—Kolbe's Institute, 1851-1865.
Photograph taken in June 1992 by the author.

Chemical Institute of the University of Leipzig, ca. 1908. From the
Festschrift zur Feier des 500 jährigen Bestehens der
Universität Leipzig (Leipzig: Hirzel, 1909, page 72).
Kolbe's lecture theater, Leipzig, 1872.
Courtesy of the Deutsches Museum, Munich.

Kolbe's teaching laboratory, Leipzig, 1872.
Courtesy of the Deutsches Museum, Munich.

Villa Adolpha, Dresden, 1874: site of first production of
salicylic acid. Courtesy of the Deutsches Museum, Munich.

Hermann Kolbe, ca. 1880. Photograph
from the author's collection.

Justus Liebig. Courtesy of The Edgar Fahs Smith
Collection, Special Collections Department, Van Pelt-
Dietrich Library Center, University of Pennsylvania.

Friedrich Wöhler. Courtesy of The Edgar Fahs Smith
Collection, Special Collections Department, Van Pelt-
Dietrich Library Center, University of Pennsylvania.
Robert Bunsen. Courtesy of The Edgar Fahs Smith
Collection, Special Collections Department, Van Pelt-
Dietrich Library Center, University of Pennsylvania.

Edward Frankland. Courtesy of The Edgar Fahs Smith
Collection, Special Collections Department, Van Pelt-
Dietrich Library Center, University of Pennsylvania.
August Kekulé. Courtesy of The Edgar Fahs Smith
Collection, Special Collections Department, Van Pelt-
Dietrich Library Center, University of Pennsylvania.
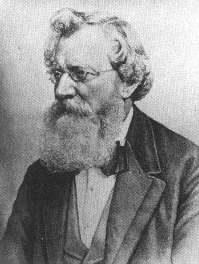
August Wilhelm Hofmann. Courtesy of The Edgar Fahs Smith
Collection, Special Collections Department, Van Pelt-
Dietrich Library Center, University of Pennsylvania.

Adolphe Wurtz. Courtesy of The Edgar Fahs Smith
Collection, Special Collections Department, Van Pelt-
Dietrich Library Center, University of Pennsylvania.

Charles Gerhardt. Courtesy of The Edgar Fahs Smith
Collection, Special Collections Department, Van Pelt-
Dietrich Library Center, University of Pennsylvania.