Five—
Population Ecology of Southern Elephant Seals at Marion Island
Marthan N. Bester and Ian S. Wilkinson
ABSTRACT. Research has highlighted the continued decline in the southern elephant seal population at Marion Island, and life table data implicate recently matured cows as the most vulnerable part of the population. Observation of onshore behavior suggests that the factors producing the elevated mortality rates of these cows operate at sea. Current research is concentrated on the investigation of at-sea behavior of this component of the population.
Declines in the southern elephant seal populations in the southern Indian Ocean, including the population at Marion Island, have been evident since the 1970s (Condy 1978; van Aarde 1980; Skinner and van Aarde 1983; Burton 1985). Notwithstanding a number of hypotheses that have been offered to explain the observed declines (ibid.), the driving forces behind them are, as yet, poorly understood.
Comprehensive tagging studies commenced at Marion Island in 1983 to permit the first assessment of life history parameters such as age-specific survival, age-specific fecundity, and age at maturity for females in the Marion Island population based on data collected at Marion Island. Previous comments on population characteristics for this population (Condy 1977) were based largely on statistics drawn from the populations at Macquarie Island (Carrick and Ingham 1962) and South Georgia (Laws 1960). Such population parameters cannot tell us what the cause of the decline is, but they can highlight weak links within the population at this site, which allows the focusing of future research efforts.
This chapter describes the present status of the Marion Island population and analyzes the population based on parameters derived from the 1983 cohort. In addition, we present the first data on movements of cows from Marion Island during the pelagic phase of their life cycle.
Methods
Counts of pups were conducted in a main study area (MSA) representing ± 10% of the coastline at Marion Island (46°54¢ S, 37°45¢ E) during the breeding seasons from 1974 to 1989 following the methods of P. R. Condy (1978). In 1976 and 1986–1989, the number of births for the whole of Marion Island was determined from counts made at all the breeding beaches at the end of October (Wilkinson and Bester, in press). The number of pups born in the MSA expressed as a percentage (range: 24–31%) of the total island pup production was calculated for 1976 and the years 1986–1989. Given that the proportion of births occurring in the MSA did not change significantly in these years (Wilkinson 1991), the pup population in the MSA is assumed to be representative of the entire population as an index of population size. Rates of population change were calculated using the exponential equation (Caughley 1977)
Nt = No ert
for the periods 1974–1989 and 1983–1989. The years 1983–1989 coincide with the period for which data on population parameters are available. From 1983 onward, weaned pups were tagged and beaches around the whole of Marion Island searched at 7- to 10-day intervals to resight these individuals (Bester 1989; Wilkinson 1991). Age-specific survival rates incorporating tag losses were calculated using Jolly-Seber mark-recapture analysis (see Wilkinson 1991). Rates of loss for individual tags ranged from 0.5% in the first year to 4.5% in the fifth year, which yielded combined loss rates (two tags) of 0.0 to 0.6%. Intercohort differences in first-year survival were calculated according to I. S. Wilkinson (1991). Calculation of age at first reproduction followed the method of A. E. York (1983), and fecundity followed the method of Wilkinson (1991). Sex ratio at birth was taken to be the same as the ratio of male:female weanlings that were tagged between 1983 and 1989 (Wilkinson 1991). The net reproductive rate (Ro ), calculated using the equation
Ro = S lx mx
where S lx mx = the sum of the product of the age-specific survival and fecundity values of females of a cohort (Caughley 1977), of the population at Marion Island and other components of the life table are described in Wilkinson (1991). In calculating the life table, empirical data were used up to age 5, after which the mortality rate was assumed to be constant (Harwood and Prime 1978) until a year or two before death (McCann 1985). Fecundity rates were assumed to remain constant after the age of full recruitment (age 6 in this population) to the breeding population until death, with no reproductive senescence (Hindell and Little 1988). All cows were
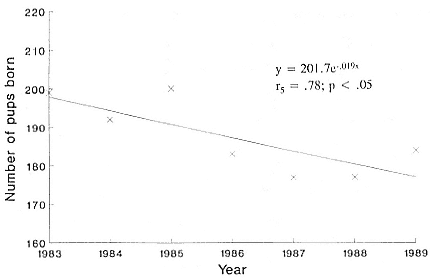
Fig. 5.1
Annual pup production for southern elephant seals within the MSA, Marion
Island, for the years 1983 to 1989. The equation refers to the line fitted
through the data points using least squares regression analysis (Zar 1984).
assumed to produce only one pup (Laws 1956), at a sex ratio found in the present study, and female longevity was 23 years (Hindell and Little 1988).
Data on individual movement of postbreeding cows (n = 3, aged 4, 7, and 7 years) at sea were collected with microprocessor-controlled Time-Depth Recorders (Wildlife Computers, Woodinville, Wash.) using the geolocation option, which included measurement of surface water temperature. The recorders were deployed on cows that were immobilized chemically using a remote injection method (Bester 1988b ) and following procedures detailed in M. N. Bester and H. M. Pansegrouw (1992). The Geolocation analysis software package of Wildlife Computers was used to calculate the daily longitude and latitude from light-level data, the theory and precision of which are discussed by R. D. Hill (1991).
Results and Discussion
Trends in Population Size and Population Parameters
The Marion Island population declined an average of 4.8% per annum between 1974 and 1989; it had slowed to 1.9% per annum between 1983 and 1989 (fig. 5.1). The total island pup production for 1989 was 585 individuals. As immigration and emigration are virtually nonexistent (Burton
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1985; Bester 1989; Gales, Adams, and Burton 1989), the observed decline must be a consequence of an imbalance between births and deaths within the population.
Cows of the 1983 cohort produced pups for the first time at age 3. An estimated 26.2% of cows had produced pups at age 3, 56.5% at age 4, 76.3% at age 5 and 100% of those cows known to still be alive at age 6. The sex ratio of 3,856 pups tagged between 1983 and 1989 was 1.04:1 (male:female), which does not differ significantly from unity (c 2 (1) = 1.82, p >.05). The mean age of these females at first pupping was 4.41 years.
Survival of females was 61.8% to age 1, 45% to age 2, 35.2% to age 3, 25.6% to age 4, and 21.1% to age 5 (table 5.1). The mortality rate
decreased up to age 3, then increased in the fourth year, and further decreased from age 4 to 5 (table 5.1). Intercohort comparison of first-year survival showed no differences for the years 1983 to 1988.
An indication of the viability of a population is shown by its net reproductive rate (Ro ). The Ro represents the number of daughters born to a female during her lifetime, or the rate of increase in the population with each passing generation (Caughley 1977). Therefore, to maintain itself, a population must have an Ro of 1. In the case of the population at Marion Island, the Ro is 0.661, resulting in a loss of 34% of the population with each passing generation. As Ro is a composite of both survival and fecundity, it can be affected by age-specific survival, pregnancy rate, and sex ratio of offspring.
The mean age at first pupping and hence recruitment to the adult component of the population is similar for Marion Island and the stable South Georgia population (McCann 1985; McCann and Rothery 1988) and occurs a year earlier than at Macquarie Island (Hindell 1991). The apparent 100% pupping rates of females age 6 and older are higher than the 85% reported at South Georgia (McCann 1985), and only reports for hooded seals (98%; Øritsland 1964, in Riedman 1990) and northern elephant seals (97.8%; Le Boeuf and Reiter 1988) are similar in magnitude.
Observations of sexual behavior at a single beach at Marion Island over three consecutive summers showed that dominant bulls controlling the harems in these years achieved over 98% of all matings and that the success of these matings was not affected by their timing in the season, or by prior (during the same season) levels of sexual activity of the bull (Wilkinson 1991). In view of the high pupping rates observed and the observed effectiveness of the few bulls that did breed, the "paucity of males" hypothesis (Skinner and van Aarde 1983) can be dismissed as a possible factor in the decline.
Manipulation of Life Table Data
The proportion of female pups at Marion Island (48.9%) is higher than the figure of 47% quoted for South Georgia, which resulted in an Ro of greater than 1 (McCann 1985). The proportion of female pups born would need to increase to 75% to allow the Ro to reach a value of 1 if pupping rates and age-specific survival remained constant (table 5.2).
When considering the age at primiparity, maturity would have to be advanced by two years, with breeding beginning at age 1 (26.2% pupping) and recruitment to the adult population complete by age 4 (100% pupping), to realize an Ro of 1. The present mean age at maturity (4.41) is already lower than previously reported at this site (Condy 1977) and similar to the stable South Georgia population (McCann 1985). Given that the cows are reproducing at an age that is already early for this species and
| ||||||||||||||||||
| ||||||||||||||||||||||
that they are producing offspring at the maximum rate, it would seem that age-specific survival is the key to the decline. Higher survival rates would improve age-specific survival/fecundity values and the resultant Ro value.
Assuming all other parameters are held constant, first-year survival would have to increase to 95% to produce a stable population (table 5.3). The present observed survival to age 1 is already the highest ever reported for this species, and given the major improvement that is required to stabilize the population, it seems unlikely that this is the vulnerable age group. Studies on the reproductive success of cows at Marion Island also showed low preweaning mortality rates (Wilkinson 1991).
The annual adult survival rate of females (82.4%) is too low to maintain
| ||||||||||||||||||||||
the population and needs to be increased to 90% to stabilize the population (table 5.4). The assumption made that survival rate of adults remains constant from age 5 until a year or two before death at age 23 (Hindell and Little 1988) contrasts with the view of T. S. McCann (1985) and R. M. Laws (1960) who assumed survival to remain constant from maturity to age 10 and then decline annually until death at age 20. However, the assumption of constant adult survival agrees with a study on gray seals, Halichoerus grypus , by J. Harwood and J. H. Prime (1978) and provides the highest Ro that is possible. If survival declined after age 10, the Ro would be lower still.
This manipulation of life table data shows the relative importance of the first year and adult components of the population. A 1% change in annual adult survival results in an approximately 5% change in Ro (table 5.4) but only a 1% change in Ro when first-year survival is changed by a similar amount (table 5.3). As mentioned, survival to age 1 is higher than ever reported in this species, and if it is then assumed that adult annual survival rate is not abnormally low, then it may be juvenile survival rates that are the problem, resulting in lowered recruitment to the adult age class.
If the figures for first-year survival and adult annual survival (from age 5 onward) described above are assumed correct and maintained, while replacing juvenile survival (l2 – l4 ; see table 5.1) with the values calculated for South Georgia (McCann 1985), the Ro value exceeds one (table 5.5).
Mortality Rates of Recently Matured Cows
The data for the 1983 cohort show that there is an increase in the mortality of 3-year-old females. This increase in mortality comes at a crucial time for the population. Although this age group is not the most reproductively
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
valuable (evidenced by the lx mx values in table 5.1), a high mortality rate at this age will, combined with high juvenile mortality, reduce the level of recruitment to the adult population and thus affect the survival/fecundity schedule, lowering Ro . The sharp increase in mortality among 3-year-old females comes at a time when animals at Marion Island are maturing sexually and are exposed to greater physiological stress levels resulting from gestation and lactation.
Gestation and the postpartum lactation period impose increased ener-
getic demands on the female, and these costs are relatively higher among young females (Reiter and Le Boeuf 1991) that are still in a more rapid phase of growth and development than their older counterparts (Laws 1953; Reiter, Panken, and Le Boeuf 1981). During the lactation period the cows may also lose up to 43% of their initial prepartum mass (Costa et al., 1986). Cows at Marion Island that were observed leaving the breeding beaches at the end of lactation were noticeably emaciated (Wilkinson 1991), implying a severe drain on their body reserves. In contrast, some cows at South Georgia were observed to leave the beaches with large blubber stores intact (McCann, Fedak, and Harwood 1989), possibly indicating that the nutritional status of the cows at the two sites differs.
Given current knowledge of the terrestrial component of the life cycle at Marion Island, it would appear that factors operating during the pelagic phase of the annual cycle, of subadult and adult cows in particular, hold the key to the decline process.
Cow Movements at Sea
The three cows that were tracked ranged over entirely different areas (fig. 5.2). None were followed for the total time at sea (67, 69, and 78 days, respectively), but two cows appeared to be returning to the island when recordings ceased. The cows spent a large proportion of their time in reasonably well-defined areas at, or near, the limit of their feeding range (between 1,100 and 1,400 km) (Bester and Pansegrouw 1992). The most circumscribed foraging area (that of the 4-year-old cow) lay south of the Antarctic Polar Front at 54°–57°S latitude and 25°–29°E longitude in cold surface water (minimum temperature of –1.7°C). The two other cows moved north to widely separated (by 15°–20° longitude) areas between approximately 40°–45°S latitude (fig. 5.2). The northwest-bound cow encountered warmer surface water (maximum temperature of 14.1°C) consistent with the mean position of the Subtropical Convergence at 41°40¢ S latitude which is recognizable at the sea surface by a mean decrease in temperature from 17.9°C to 10.6°C (Lutjeharms, Walters, and Allanson 1985). The northeast-moving cow appeared to remain in water between 5.3°C to 7.3°C at the Subantarctic Front (central surface temperature 7°C; Lutjeharms 1985). The cows therefore ranged widely without overlap in their feeding areas, the choice of which might have been influenced by biological enhancement at oceanic frontal systems (see Lutjeharms, Walters, and Allanson 1985). It is likely, however, that the small sample size has identified only a small part of the actual feeding range of elephant seal cows from Marion Island during the postbreeding period (Bester and Pansegrouw 1992), since three of five postbreeding cows from Macquarie Island foraged in deep oceanic waters off the Antarctic coast (Hindell, Burton, and Slip 1991).
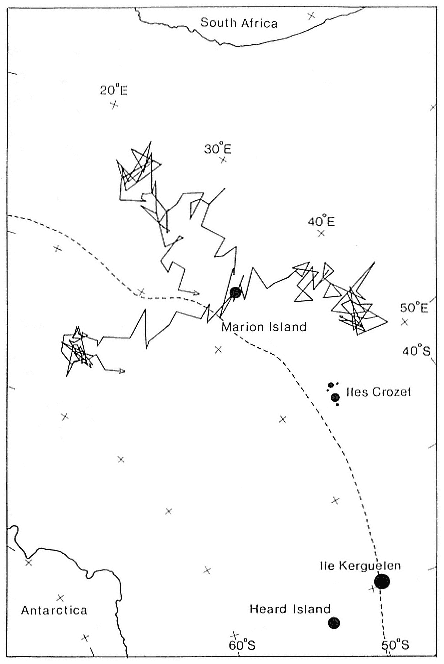
Fig. 5.2
At-sea movements of 3 southern elephant seal cows from Marion Island,
during their postbreeding pelagic period. The mean position of the
Antarctic Polar Front is shown (dashed line).
References
Bester, M. N. 1988a . Marking and monitoring studies of the Kerguelen stock of southern elephant seals, Mirounga leonina , and their bearing on biological research in the Vestfold Hills. Hydrobiologia 165: 269–277.
———. 1988b . Chemical restraint of Antarctic fur seals and southern elephant seals. South African Journal of Wildlife Research 18: 57–60.
———. 1989. Movements of southern elephant seals and subantarctic fur seals in relation to Marion Island. Marine Mammal Science 5: 257–265.
Bester, M. N., and H. M. Pansegrouw. 1992. Ranging behaviour of southern elephant seal cows from Marion Island. South African Journal of Science 88: 574–577.
Burton, H. R. 1985. Tagging studies of male southern elephant seals (Mirounga leonina L.) in the Vestfold Hills area, Antarctica, and some aspects of their behaviour. In Sea Mammals of South Latitudes: Proceedings of a Symposium of the 52d ANZAAS Congress in Sydney—May 1982 , ed. L. K. Ling and M. M. Bryden, 19–30. Northfield: South Australian Museum.
Carrick, R., and S. E. Ingham. 1962. Studies of the southern elephant seal Mirounga leonina (L.). V. Population dynamics and utilization. CSIRO Wildlife Research 7: 198–206.
Caughley, G. 1977. Analysis of Vertebrate Populations . New York: John Wiley and Sons.
Condy, P. R. 1977. The ecology of the southern elephant seal, Mirounga leonina (Linnaeus 1758), at Marion Island. D.Sc. dissertation, University of Pretoria, South Africa.
———. 1978. The distribution and abundance of southern elephant seals Mirounga leonina (Linn.) on the Prince Edward Islands. South African Journal of Antarctic Research 8: 42–48.
Costa, D. P., B. J. Le Boeuf, A. C. Huntley, and C. L. Ortiz. 1986. The energetics of lactation in the northern elephant seal, Mirounga angustirostris. Journal of Zoology, London 209: 21–33.
Gales, N. J., M. Adams, and H. R. Burton. 1989. Genetic relatedness of two populations of the southern elephant seal, M. leonina. Marine Mammal Science 5: 57–67.
Harwood, J., and J. H. Prime. 1978. Some factors affecting the size of British grey seal populations. Journal of Applied Ecology 15: 401–411.
Hill, R. D. 1991. Geolocation by Light-Level Readings . Woodinville, Wash.: Wildlife Computers Geolocation Instruction Manual.
Hindell, M. A. 1991. Some life-history parameters of a declining population of southern elephant seals, Mirounga leonina. Journal of Animal Ecology 60: 119–134.
Hindell, M. A., and H. R. Burton. 1987. Past and present status of the southern elephant seal (Mirounga leonina ) at Macquarie Island. Journal of Zoology, London 213: 365–380.
Hindell, M. A., H. R. Burton, and D. J. Slip. 1991. Foraging areas of southern elephant seals, Mirounga leonina , as inferred from water temperature data. Australian Journal of Marine and Freshwater Research 42: 115–128.
Hindell, M. A., and G. J. Little. 1988. Longevity, fertility and philopatry of two
female southern elephant seals (Mirounga leonina ) at Macquarie Island. Marine Mammal Science 4: 168–171.
Krebs, C. J. 1985. Ecology: The Experimental Analysis of Distribution and Abundance . New York: Harper and Row.
Laws, R. M. 1953. The elephant seal (Mirounga leonina Linn.). I. Growth and age. Falkland Islands Dependencies Survey, Scientific Reports 8: 1–62.
———. 1956. The elephant seal (Mirounga leonina Linn.). II. General, social and reproductive behaviour. Falkland Islands Dependencies Survey, Scientific Reports 13: 1–88.
———. 1960. The southern elephant seal (Mirounga leonina Linn.) at South Georgia. Norsk Hvalfangst-Tidende 49: 466–476, 520–542.
Le Boeuf, B. J., and J. Reiter. 1988. Lifetime reproductive success in northern elephant seals. In Reproductive Success , ed. T. H. Clutton-Brock, 344–362. Chicago: University of Chicago Press.
Lutjeharms, J. R. E. 1985. Location of frontal systems between Africa and Antarctica. Deep-Sea Research 32: 1499–1509.
Lutjeharms, J. R. E., N. M. Walters, and B. R. Allanson. 1985. Oceanic frontal systems and biological enhancement. In Antarctic Nutrient Cycles and Food Webs , ed. W. R. Siegfried, R. M. Laws, and P. R. Condy, 11–31. Berlin: Springer Verlag.
McCann, T. S. 1985. Size, status and demography of southern elephant seal Mirounga leonina populations. In Sea Mammals of South Latitudes: Proceedings of a Symposium of the 52d ANZAAS Congress in Sydney—May 1982 , ed. L. K. Ling and M. M. Bryden, 1–17. Northfield: South Australian Museum.
McCann, T. S., M. A. Fedak, and J. Harwood. 1989. Parental investment in southern elephant seals, Mirounga leonina. Behavioral Ecology and Sociobiology 25: 81–87.
McCann, T. S., and P. Rothery. 1988. Population size and status of the southern elephant seal (Mirounga leonina ) at South Georgia, 1951–1985. Polar Biology 8: 305–309.
Reiter, J., and B. J. Le Boeuf. 1991. Life history consequences of variation in age at primiparity in northern elephant seals. Behavioral Ecology and Sociobiology 28: 153–160.
Reiter, J., K. J. Panken, and B. J. Le Boeuf. 1981. Female competition and reproductive success in northern elephant seals. Animal Behavior 29: 670–687.
Riedman, M. L. 1990. The Pinnipeds: Seals, Sea Lions, and Walruses . Berkeley and Los Angeles: University of California Press.
Skinner, J. D., and R. J. van Aarde. 1983. Observations on the trend of the breeding population of southern elephant seals, Mirounga leonina , at Marion Island. Journal of Applied Ecology 20: 707–712.
Taylor, R. H., and G. A. Taylor. 1989. Reassessment of the status of southern elephant seals (Mirounga leonina ) in New Zealand. New Zealand Journal of Marine and Freshwater Research 23: 201–213.
van Arade, R. J. 1980. Fluctuations in the population of southern elephant seals Mirounga leonina at Kerguelen Island. South African Journal of Zoology 15: 99–106.
Wilkinson, I. S. 1991. Factors affecting reproductive success of southern elephant seals, Mirounga leonina , at Marion Island. Ph.D. dissertation, University of Pretoria, South Africa.
Wilkinson, I. S., and M. N. Bester. In press. Population parameters of the southern elephant seal population at Marion Island. Antarctic Science .
York, A. E. 1983. Average age at first reproduction of the northern fur seal (Callorhinus ursinus). Canadian Journal of Fisheries and Aquatic Science 40: 121–127.
Zar, J. H. 1984. Biostatistical Analysis . 2d ed. Englewood Cliffs, N.J.: Prentice-Hall.