5.2—
Morphology
5.2.1—
Morphology in Situ
Mitochondria in living cells are highly pleomorphic, as shown by phase contrast microcinematography by Hongladarom et al., (1965). Pleomorphism is reflected
also in thin section electron microscopy, in which mitochondria appear as roughly circular profiles as well as highly elongated or irregular cross sections (Fig. 5.1a). The circular sections may represent transverse or oblique sections through an otherwise elongated organelle. The diameter of the elongated mitochondrion appears to be about 0.4 to 0.5 µm, while the length may be several micrometers long. Although rods or apparent spheres are the most common profiles seen, sections derived from branched or cup shaped organelles have also been discerned (Bagshaw et al., 1964). The recent analysis of serial sections of yeast cells by Hoffman and Avers (1973), which showed that yeast contains a single, giant, branched mitochondrion, suggests that the irregular cross sections of mitochondria of other cells might also be sections of a single branched organelle.
The mitochondrion consists of a double membrane system with an inner convoluted membrane enclosing the matrix, and surrounded by a smooth outer membrane (Fig. 5.1a, 5.1b). High resolution electron micrographs of material
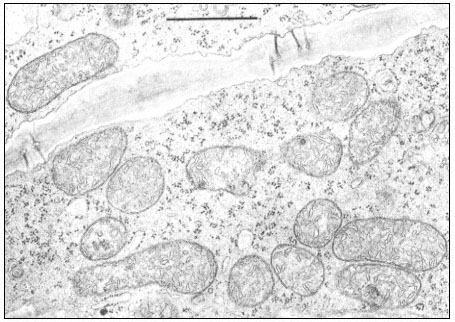
Figure 5.1a
Mitochondria in phloem parenchyma cells of a maize leaf. Magnification bar = 1 m m.
(Micrograph courtesy of O. E. Bradfute and Diane C. Robertson.)
fixed with glutaraldehyde and post-fixed with osmium tetroxide show the tripartite nature of both the inner and outer membranes. Each membrane has a thickness of approximately 9 nm (Baker et al., 1968).
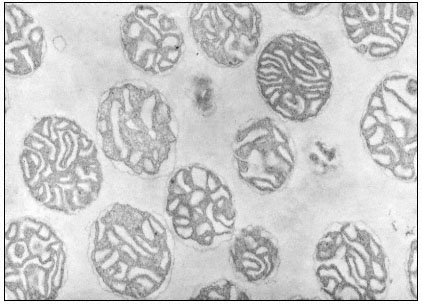
Figure 5.1b
Isolated mitochondria from mung bean hypocotyls. Mitochdria have been
suspended in 0.3 M mannitol prior to fixation. Magnification × 26,000.
(Micrograph courtesy of W. D. Bonner, Jr.)
5.2.2—
Morphology of Isolated Mitochondria
Electron micrographs of isolated mitochomdria show circular cross sections, presumably reflecting a spherical shape when released from their cellular environment. The electron micrographs of the intact isolated mitochondrion show clearly the two membrane systems, as well as the tripartite organization of each membrane. The fine structure of isolated mitochondria is highly dependent upon the osmolarity of the suspending medium (Baker et al., 1968). When mitochondria are suspended in 0.3 to 0.4 M sucrose or mannitol, the matrix appears contracted and electron dense (Fig. 5.1b), but when suspended in 0.2 M sucrose, the matrix appears more expnaded and less electron dense, and resembles that of mitochondria seen in sity . The dense matrix of mitochondria suspended in 0.3 to 0.4 M sucrose or mannitol is due to the hypertonicity of the suspending medium. Since the inner membrane is generally regarded as the osmotic barrier, the dense nature of the matrix reflects a water loss, which is reversible when the organelles are suspended in 0.2 M sucrose.
Negatively-stained water-lysed mitochondria show that the inner membranes have the characteristic stalked particles similar to those reported for mammalian mitochondrial membranes (Fernandez-Moran, 1962; Parsons et al., 1965). The particles have a headpiece with a diameter of 10 nm, attached to a stalk 3.5 to 4.5 nm wide and 4.5 nm long (Fig. 5.2). These resemble the particles identified with ATPase function in heart mitochondria (Racker et al., 1969).
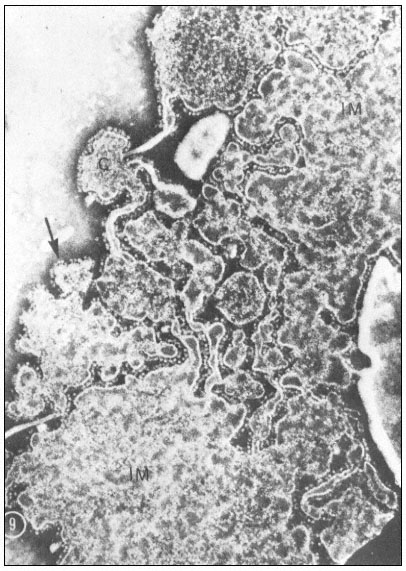
Figure 5.2
Portion of a surface spread and negatively stained summer squash
mitochondrion. The large areas of membrane (IM) are presumed to be
part of the inner membrane forming the shell of the mitochondrion.
The cristae (C) appear as smaller pieces of membrane of rounded shape
connected together by narrower (possibly tubular) pieces. The membranes
are coated with projecting knob-like subunits which are best seen lying in
the plane of the object at the edge of the pieces of membrane (arrow). The
dimension of the head of the subunit is 10 nm and the stem is 3.5–4.0 nm wide
and 4.5 nm long. (Parsons et al., 1965). (Reproduced by permission of the
National Research Council of Canada from the Canadian
Journal of Botany, Volume 43, 1965. pp. 647–55.)