New Forms of Conception
Since the birth of the world's first IVF baby in 1978, the basics of the procedure have become familiar to the public, though medical details may remain vague. At the time of ovulation, doctors remove at least one mature egg (also called an ovum or oocyte) from a woman's ovary. This process involves passing a long needle through the vaginal or abdominal wall, puncturing fluid-filled ovarian follicles, sucking out (aspirating) the follicular fluid, and examining this fluid with a microscope to see whether it does, in fact, harbor an egg. Sperm, gathered through masturbation, meet egg in a specially prepared laboratory dish, where they bathe in nutrients intended to promote conception.[1] If a sperm does fertilize an egg and cell division proceeds to a stage and quality the embryologist considers adequate, the embryo is removed from the dish and placed into a catheter, which the doctor injects through the vagina and cervix into the woman's uterus, where, it is hoped, the embryo, released from its catheter, will burrow into the uterine lining and grow normally.
In a medical specialty too often characterized by exaggerated claims, IVF did mark revolutionary changes in fertility treatments and human reproduction itself. Most profound was moving egg, sperm, and conception from the body to a laboratory; sexual intercourse was no longer required. A second major change evolved after the earliest attempts at in vitro fertilization, as in vitro programs attempted to increase the likelihood of conception and successful pregnancy by removing more than one egg from the ovary and transferring more than one embryo per menstrual cycle. Normally, a single egg matures each month, bursting through the ovary's wall in the process of ovulation. To force maturation of several eggs in one cycle, the woman takes a combination of hormones, in most
cases by daily injections that she or her partner can give, in a regimen known as controlled ovarian hyperstimulation—also known as "superovulating" the woman.
Initially, IVF was intended for the relatively few women with blocked or absent fallopian tubes whose naturally ovulated egg cannot otherwise reach the uterus. Although the chances of a successful IVF pregnancy were always small for these women (hovering between 10 and 15 percent in the more experienced programs during earlier years, up to 15–20 percent by the mid-1990s), they were better than nothing. If the woman did become pregnant, the IVF procedure—bypassing the problematic fallopian tubes—was responsible. Birth of a healthy baby could rightfully be called an IVF "success." Once physicians and the public have access to a procedure like IVF, however, its use extends beyond the original target population. Doctors offer the new treatment for a broader range of conditions—wanting to "do something" when no better medical alternative exists—without systematically evaluating the outcomes. The number of patients and types of diagnoses indicating "need" for a treatment grow independently of biological rationale or scientific data. Doctors were soon trying IVF on women with conditions other than intractable tubal problems, prescribing this technique for patients with endometriosis, ovulation disorders, and "unexplained infertility." IVF even became a treatment aimed at male fertility problems that may lower a couple's chance of conceiving. By the 1990s, the American Society for Reproductive Medicine (ASRM) deemed IVF an appropriate treatment for anyone who had failed in attempts with conventional therapies (i.e., fertility drugs, surgery, inseminations). "The primary medical indication" for IVF, according to the society's rather broad definition-by-default, was "failure of conventional therapy to provide a pregnancy for the infertile couple."[2] One fertility specialist, chagrined but not surprised at the growth of IVF, put it succinctly: "If all I have is a hammer, the whole world looks like a nail."
The original in vitro fertilization process, with transfer of embryos to the uterus, has engendered variations that have, in turn, expanded in use (see Figure 1). For example, as with IVF, gamete intrafallopian transfer (GIFT) entails retrieving eggs from the ovary and combining them with sperm. In GIFT, however, the doctor places eggs and sperm (the female and male gametes) through a small incision near the woman's navel into the fallopian tube for fertilization, rather than into a laboratory dish. If conception occurs, the fertilized egg must then travel to the uterus in the usual, unassisted manner. GIFT is possible only if a woman has at least one open fallopian tube; the hope is that a more natural fertilization, within the tube, will allow for more successful embryo growth and
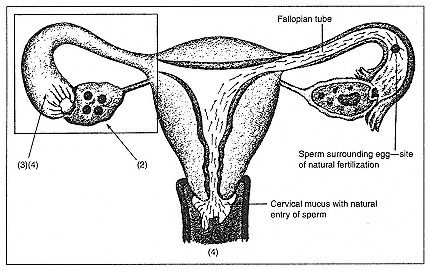
Figure 1
The assisted reproductive technologies. In this illustration of the female
reproductive tract, the natural process of fertilization is shown to the right,
with sperm entering at the cervix and proceeding through the uterus to meet a
released egg in the fallopian tube. On the left, the sites of ART interventions
are indicated, corresponding to the steps listed.
Step 1: (not shown) Ovarian hyperstimulation to mature several eggs.
Step 2: A needle puncture (usually ultrasound-guided, through the vaginal wall)
to aspirate eggs from mature follicles (usually 4–20 per cycle).
Step 3: Each egg mixed with prepared sperm for fertilization—done in individual
laboratory dishes for 1–5 days for IVF, TET, ZIFT; for GIFT, eggs retrieved by
laparoscopy are returned to fallopian tubes immediately, along with prepared sperm.
Step 4: Return of embryos vaginally to uterus (IVF) or during laparoscopy to
fallopian tube (TET, ZIFT). For intrauterine insemination with controlled ovarian
hyperstimulation (IUI/COHS) women undergo Step 1, followed by the injection of
the hormone human chorionic gonadotropin (hCG) to trigger ovulation; prepared
sperm are then placed through the cervix into the uterus. For intracytoplasmic
sperm injection (ICSI, a micromanipulation technique) a single prepared sperm is
injected directly into each mature egg (as adjunct to Step 3); if fertilization is
achieved, resulting embryo is then transferred to uterus and/or fallopian tubes.
(Illustration based on C. Harkness. 1992. The infertility book: A comprehensive
and emotional guide . Berkeley, Calif.: Celestial Arts.)
implantation. Less commonly used variations transfer eggs fertilized in vitro into a woman's fallopian tube. The specific timing of this transfer procedure aims at catching the most opportune embryonic stage for entering and implanting within the uterus. Zygote intrafallopian transfer (ZIFT) uses one or more fertilized but undivided eggs, and tubal embryo transfer (TET) uses fertilized
eggs that have undergone cell division. Additional spin-offs of the in vitro procedure include cryopreservation, the freezing and storing of unused embryos, which can be thawed and transferred during a later attempt at pregnancy; micromanipulation techniques, which assist the actual fertilization process by helping sperm enter an egg (see Chapter 4); the use of "donor eggs," usually from younger women, for transfer after fertilization into women nearing or beyond menopause (see Chapter 5); and combining donor sperm and/or eggs in various types of surrogate gestational relationships, during which one woman carries a pregnancy for another.