3—
Assisted Reproductive Technology:
A Modern Fact of Life
"Whatever we went through was worth it," Sharon affirms now unequivocally, gazing at her daughter, a happy, healthy-looking baby who does all the things babies do. What this doting first-time mother and her husband went through included fertility drugs, which made Sharon feel sick and depressed, then two variations of in vitro fertilization. That was after Jeff's surgery to reverse a vasectomy, the legacy of a previous marriage when he thought he would never want more children. Although Sharon had no reason to suspect she would have problems conceiving, she was in her late thirties, past the age when women are most fertile. And although Jeff's fertility test showed sperm swimming energetically—evidence that his vasectomy reversal was successful—there was the risk of what the doctor called "male factor problems." Faced with these potential difficulties, Sharon decided after only three months of trying to conceive that she would begin fertility treatment. For six months she took clomiphene citrate. Their life revolved obsessively around the moment of her ovulation: Would it be today or tomorrow? Should they have intercourse before going to work or later that night? Had they missed the best time, had they lost the month? Could Jeff go on a business trip next month?
When Sharon's doctor advised a more aggressive approach, they plunged ahead. First, Sharon took stronger fertility drugs so that her ovaries would produce many eggs, which the doctor then extracted for in vitro fertilization (IVF). To maximize the likelihood of pregnancy during these cycles, her doctor performed two differing embryo transfer procedures at the same time. He inserted two embryos through her vagina into her uterus; and, through a small abdominal incision, he placed one embryo at the entry of each fallopian tube.
All the while Sharon and Jeff continued their intense schedule of sexual intercourse.
Sharon's doctor, a fertility specialist, keeps a tally and tells patients his success rates for various treatments. Asked which manner of conception her doctor would list as successful in her case, Sharon jokes, "Both." In every joke, of course, is a grain of truth. For the burgeoning category of fertility treatments known as "assisted (or advanced) reproductive technologies"—shortened perhaps too suggestively to the term ART—that grain can be large indeed. Reports on the success of these interventions have been inadequate and, in some instances, deceptive. As Sharon and Jeff's experience illustrates, moreover, physicians' tallies often ignore another explanation for successful pregnancies. Perhaps their low-tech, at-home process of sexual intercourse actually resulted in the pregnancy. Now, with Rebecca in tow, they do not really care which process did the trick. But for other couples—and for the specialty of fertility medicine—the question is crucial.
Today's fertility patients decide about their own treatments based on numbers from cases such as Sharon's. No patients—even the majority who do not initially undergo assisted reproduction—are untouched by these technologies, which have become pivotal for fertility medicine today and for the field's future directions. Fertility specialists now recommend some form of in vitro fertilization to nearly any woman who does not become pregnant with less invasive treatments. While helpful for some patients, these reproductive technologies highlight dangers in the ongoing expansion of fertility interventions. Only rarely do these dangers erupt into public view as egregious ethical violations.
The less extreme but far more common problems, however, are woven of the same cloth. This chapter traces the growth of assisted reproduction and of its controversies, not only as information important in itself, but as background for what follows in subsequent chapters. Understanding the array of fertility treatments now available to women and men requires an understanding of ART. Understanding ART, in turn, requires taking a broad view that encompasses doctors who are doing too much to too many patients, politics that make for strange reproductive bedfellows, government that abrogates its responsibilities, and technology that in the words of one prominent fertility specialist "is running away from us."
New Forms of Conception
Since the birth of the world's first IVF baby in 1978, the basics of the procedure have become familiar to the public, though medical details may remain vague. At the time of ovulation, doctors remove at least one mature egg (also called an ovum or oocyte) from a woman's ovary. This process involves passing a long needle through the vaginal or abdominal wall, puncturing fluid-filled ovarian follicles, sucking out (aspirating) the follicular fluid, and examining this fluid with a microscope to see whether it does, in fact, harbor an egg. Sperm, gathered through masturbation, meet egg in a specially prepared laboratory dish, where they bathe in nutrients intended to promote conception.[1] If a sperm does fertilize an egg and cell division proceeds to a stage and quality the embryologist considers adequate, the embryo is removed from the dish and placed into a catheter, which the doctor injects through the vagina and cervix into the woman's uterus, where, it is hoped, the embryo, released from its catheter, will burrow into the uterine lining and grow normally.
In a medical specialty too often characterized by exaggerated claims, IVF did mark revolutionary changes in fertility treatments and human reproduction itself. Most profound was moving egg, sperm, and conception from the body to a laboratory; sexual intercourse was no longer required. A second major change evolved after the earliest attempts at in vitro fertilization, as in vitro programs attempted to increase the likelihood of conception and successful pregnancy by removing more than one egg from the ovary and transferring more than one embryo per menstrual cycle. Normally, a single egg matures each month, bursting through the ovary's wall in the process of ovulation. To force maturation of several eggs in one cycle, the woman takes a combination of hormones, in most
cases by daily injections that she or her partner can give, in a regimen known as controlled ovarian hyperstimulation—also known as "superovulating" the woman.
Initially, IVF was intended for the relatively few women with blocked or absent fallopian tubes whose naturally ovulated egg cannot otherwise reach the uterus. Although the chances of a successful IVF pregnancy were always small for these women (hovering between 10 and 15 percent in the more experienced programs during earlier years, up to 15–20 percent by the mid-1990s), they were better than nothing. If the woman did become pregnant, the IVF procedure—bypassing the problematic fallopian tubes—was responsible. Birth of a healthy baby could rightfully be called an IVF "success." Once physicians and the public have access to a procedure like IVF, however, its use extends beyond the original target population. Doctors offer the new treatment for a broader range of conditions—wanting to "do something" when no better medical alternative exists—without systematically evaluating the outcomes. The number of patients and types of diagnoses indicating "need" for a treatment grow independently of biological rationale or scientific data. Doctors were soon trying IVF on women with conditions other than intractable tubal problems, prescribing this technique for patients with endometriosis, ovulation disorders, and "unexplained infertility." IVF even became a treatment aimed at male fertility problems that may lower a couple's chance of conceiving. By the 1990s, the American Society for Reproductive Medicine (ASRM) deemed IVF an appropriate treatment for anyone who had failed in attempts with conventional therapies (i.e., fertility drugs, surgery, inseminations). "The primary medical indication" for IVF, according to the society's rather broad definition-by-default, was "failure of conventional therapy to provide a pregnancy for the infertile couple."[2] One fertility specialist, chagrined but not surprised at the growth of IVF, put it succinctly: "If all I have is a hammer, the whole world looks like a nail."
The original in vitro fertilization process, with transfer of embryos to the uterus, has engendered variations that have, in turn, expanded in use (see Figure 1). For example, as with IVF, gamete intrafallopian transfer (GIFT) entails retrieving eggs from the ovary and combining them with sperm. In GIFT, however, the doctor places eggs and sperm (the female and male gametes) through a small incision near the woman's navel into the fallopian tube for fertilization, rather than into a laboratory dish. If conception occurs, the fertilized egg must then travel to the uterus in the usual, unassisted manner. GIFT is possible only if a woman has at least one open fallopian tube; the hope is that a more natural fertilization, within the tube, will allow for more successful embryo growth and
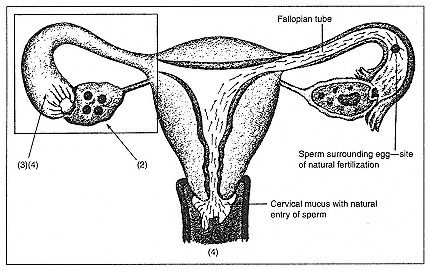
Figure 1
The assisted reproductive technologies. In this illustration of the female
reproductive tract, the natural process of fertilization is shown to the right,
with sperm entering at the cervix and proceeding through the uterus to meet a
released egg in the fallopian tube. On the left, the sites of ART interventions
are indicated, corresponding to the steps listed.
Step 1: (not shown) Ovarian hyperstimulation to mature several eggs.
Step 2: A needle puncture (usually ultrasound-guided, through the vaginal wall)
to aspirate eggs from mature follicles (usually 4–20 per cycle).
Step 3: Each egg mixed with prepared sperm for fertilization—done in individual
laboratory dishes for 1–5 days for IVF, TET, ZIFT; for GIFT, eggs retrieved by
laparoscopy are returned to fallopian tubes immediately, along with prepared sperm.
Step 4: Return of embryos vaginally to uterus (IVF) or during laparoscopy to
fallopian tube (TET, ZIFT). For intrauterine insemination with controlled ovarian
hyperstimulation (IUI/COHS) women undergo Step 1, followed by the injection of
the hormone human chorionic gonadotropin (hCG) to trigger ovulation; prepared
sperm are then placed through the cervix into the uterus. For intracytoplasmic
sperm injection (ICSI, a micromanipulation technique) a single prepared sperm is
injected directly into each mature egg (as adjunct to Step 3); if fertilization is
achieved, resulting embryo is then transferred to uterus and/or fallopian tubes.
(Illustration based on C. Harkness. 1992. The infertility book: A comprehensive
and emotional guide . Berkeley, Calif.: Celestial Arts.)
implantation. Less commonly used variations transfer eggs fertilized in vitro into a woman's fallopian tube. The specific timing of this transfer procedure aims at catching the most opportune embryonic stage for entering and implanting within the uterus. Zygote intrafallopian transfer (ZIFT) uses one or more fertilized but undivided eggs, and tubal embryo transfer (TET) uses fertilized
eggs that have undergone cell division. Additional spin-offs of the in vitro procedure include cryopreservation, the freezing and storing of unused embryos, which can be thawed and transferred during a later attempt at pregnancy; micromanipulation techniques, which assist the actual fertilization process by helping sperm enter an egg (see Chapter 4); the use of "donor eggs," usually from younger women, for transfer after fertilization into women nearing or beyond menopause (see Chapter 5); and combining donor sperm and/or eggs in various types of surrogate gestational relationships, during which one woman carries a pregnancy for another.
More Specialists, More ART
One decade after the first IVF birth, the American Society for Reproductive Medicine formally broadened the in vitro field to encompass numerous interventions employing laboratory-related conception techniques and direct retrieval of eggs from a woman's ovary; in the society's medical journal, Fertility and Sterility , the section previously entitled "In Vitro Fertilization" became "Assisted Reproductive Technology." Not long before, the same journal had published the editorial expressing concern over "exploitation" of infertile couples that was discussed in Chapter 2. This unusual editorial gave voice to increasing discomfort among some fertility specialists at how their colleagues were using fertility treatments, a discomfort magnified by the rapid growth of assisted reproduction.
By the late 1980s, concern over ART in particular spilled beyond editorial comment. At the society's annual convention in 1989 the professional dispute simmering in the journals gained greater exposure. True to medical convention form, the days were filled with presentations, honored speakers, prize papers, commercial exhibits, and hallway conversations. As participants packed into daily sessions on "clinical assisted reproductive technology," one could readily believe the society's description of infertility as the fastest growing subspecialty in medicine. Yet underlying all this activity was the disturbing sense of an Alice-in-Wonderland world in which the medical specialty was scrambling to catch up with events and people. Even as this conference churned out more physicians who would return home to practice newly learned procedures, one officer of the organization was decrying the steadily increasing number of doctors performing an expanding "alphabet soup" of untested IVF-related therapies.
In fact, anyone who graduates from medical school and obtains a state license can perform any reproductive procedure—IVF, GIFT, freezing embryos,
laser surgery—if he or she has the resources. Not only is any physician permitted to perform fertility treatments, but ob-gyns were attracted increasingly to these therapies by a peculiar set of incentives. The perception that no harm is done serves as one lure. In the words of another officer of ASRM, "People are more courageous in treating patients, since the ultimate side effect is not as obvious." In fact, injuries, and in rare cases deaths, have resulted from fertility treatments; but the perception of safety leads doctors to proceed more freely with these interventions. With assisted reproductive technologies, lack of success also encourages, paradoxically, more interventions. While critics were assailing deceptive, inflated pregnancy claims, a low rate of success—hovering around 10 percent nationwide for IVF—was becoming acceptable. As Dr. Martin Quigly, a specialist who performs these treatments, stated bluntly at the 1989 convention, "The expected result is failure." He went on to recommend counseling patients to cope with this reality.
With failure the norm, practitioners gained two significant payoffs. First, a physician's reputation and ability to attract patients could withstand considerable lack of success with IVF. Second, since failure could not easily be attributed to improper practice, physicians gained protection from a double-edged threat—malpractice lawsuits and soaring insurance rates. During the 1980s, according to fertility specialists, malpractice became a powerful economic force behind doctors' eagerness to perform new reproductive therapies. Malpractice insurance rates were rising most precipitously for obstetricians, since serious injury can occur during labor and delivery. To keep their insurance rates lower, many ob-gyns abandoned obstetrics altogether. To increase their gynecologic practices, they offered specialized fertility care, though few had special training. The costs of this venture—for example, investment in an IVF laboratory (or laser surgery machine)—could be recouped when patients were attracted and the equipment employed. This trend would be reinforced in the 1990s as managed care whittled down insured reimbursements for other ob-gyn services and physicians sought fertility patients, who generally pay out of their own pockets.
The sheer number of doctors hanging out fertility shingles with inadequate training exacerbated an absence of oversight typical of American medicine. No mechanism existed to oversee the explosion of fertility treatments performed in offices and clinics throughout the country, to evaluate whether doctors employ such procedures in appropriate cases and with the necessary skill. Unlike other types of medical laboratories, those handling embryos are subject to no mandatory regulations; federal legislation passed in 1992 set up a voluntary laboratory certification model, to be implemented by late 1994. (As of 1997, how-
ever, there was still no funding for implementation.) The American Society for Reproductive Medicine promulgated minimum standards for IVF and GIFT and published cautionary editorials, but these efforts carry no power of enforcement whatsoever. As the organization's officers made clear during their convention, this voluntary professional society is not a regulatory body and will not become one.
Like other physicians, fertility specialists hold tenaciously to their independence, resisting surveillance or regulation. With regard to one type of oversight, however, a novel chorus of medical grumbling about government inaction could be heard by 1989 over convention lunches, at press briefings, in hallway conversations. The government, complained these doctors, refused to fund research on IVF and related therapies. While the federal government is a major source of support for research on a range of medical therapies, it had removed itself from the area of assisted reproduction. Yet scientific study not only generates essential information about a medical treatment; it also provides more generally some degree of scrutiny, review, and discussion that can guide a treatment's development.
The government's position did not reflect a deliberate decision that reproductive technologies are not a medical research priority for limited government resources. Initial deliberations among government officials, medical professionals, ethicists, and lay citizens had occurred at the federal level more than a decade earlier, and the recommendation then was to go ahead with government funding. How the opposite prevailed, a story in itself, is told in Chapter 8. Suffice it to say here that the Reagan and Bush administrations, prodded by the anti-abortion movement, instituted and maintained a de facto moratorium on IVF research because embryos might be destroyed in the process. In a strange ideological alignment, "pro-life" forces were pitted against—and were triumphing over—women and men seeking to have babies. Stranger yet, these anti-abortion conservatives found themselves allied with those feminists who see the new technologies as robbing women of control over their reproductive lives rather than creating greater control and choice.[3]
Government noninvolvement did not, of course, halt the expansion of ART, though it did stymie research. The lack of government funding for research, coupled with lack of regulations, gave economic forces a freer rein, leaving development of these therapies exclusively to the private sector; this development would, in turn, directly affect what happened to individual patients.
"What you see here are two forces—entrepreneurs versus the academics," explained Dr. Robert Stillman, then a George Washington University fertility
specialist. As he surveyed participants spilling out of a 1989 convention session on "transfers of frozen-thawed human embryos," he worried that "everyone" was doing procedures that should be tested at a few special centers, to be improved or discarded. Proven procedures could then become more widely practiced at clinics throughout the country. Presenting the reality as a stark question, Stillman asked, "Why have them do forty patients wrong before they have a success?" And patients do bear the burden. They are the material on which a new IVF clinic develops its skill. Established IVF practitioners try new techniques on patients. Patients are not only paying for the cost of IVF equipment, personnel, and labor; they are essentially paying to be experimented upon, in a trial-and-error fashion, by individual doctors. Evaluation of these clinical experiments is impossible.
Stillman views his specialty's entrepreneurial direction as a "national tragedy," driving good scientists out of research as well as hurting patients. "The situation is grim," he commented in 1989. "Certain areas of medicine just shouldn't be free market." But free market it certainly was. In the absence of government-mandated quality control, fertility clinics proliferated and flourished. Rather than improve the product, stiff competition was increasing its quantity and cost, a dynamic that would become more apparent throughout the medical marketplace as the country embarked on health care reform efforts during the next decade.[4] As the 1987 Fertility and Sterility editorial on patient exploitation put it, "Unfortunately, in a consumer-driven medical care system, the physician is often in a precarious position of having to offer premature or indiscriminate therapy to those who demand it, or risk losing the patient to a more accommodating competitor."[5]
Certainly, some individual physicians saw their income swell as they performed more and more assisted reproductive treatments. Also profiting handsomely were manufacturers of various fertility drugs integral to the various new technologies. For-profit corporations, and their public relations agencies, soon entered the field of assisted reproduction. National and international chains of IVF clinics appeared, opening their doors and soliciting patients from local communities. Whether consumer- or provider-driven—or some combination thereof—women and men already susceptible to exploitation from physicians, for whom money is not supposed to be a primary motivation, entered a competition that required success to be measured in financial returns.
As the 1980s ended, fertility specialists faced an uncomfortable quandary, pushed largely by developments in assisted reproduction: How could medical
standards be monitored, improved, and maintained without stepping toward the precipitous slope of government regulation? Some ART practitioners perceived a need to rein in the technology, in part by restraining certain of their colleagues. The IVF industry could "self-destruct" without regulation and research, said the director of one prominent ART program. The profession's lack of accountability and tolerance of low success would "come back to haunt us," warned another. At the same time, the American Society for Reproductive Medicine concluded its 1989 convention with one officer, Dr. Quigly, thanking congressional critics for "the impetus to police ourselves," and the society's president asserting in his closing statement to gathered participants, "If we don't take care of our own business, someone else will do it for us."
By this time, IVF and GIFT had become common medical practices, with additional variations on these techniques not far behind. In the mid-1990s, 315 fertility clinics in the United States alone offered assisted reproductive technologies. Once a treatment is established as a common practice, moreover, attempts to study it become, by definition, all the more difficult: precisely because these techniques are considered to be standard practice—no longer "experimental"—they "should" be offered to patients, as well as paid for by their health insurance. Indeed, the latter concern reinforced a very loose definition of "standard," among some physicians, as any procedure the doctor or clinic performed more than one time; only the first attempt is "experimental." Missing from these definitions are evaluation and minimal standards for efficacy and safety. To deny patients an established treatment, even as part of a randomly assigned no-treatment control group in a clinical study, would not be medically ethical. Nor are patients always eager to participate in research if they are not receiving a treatment. Yet the increasingly widespread application of an untested "standard" therapy contributes to the urgent need for scientifically controlled studies. In 1990, the ASRM issued a formal statement attempting to delineate more specifically which assisted reproductive procedures are established, and which are experimental and therefore in need of scientifically controlled clinical trials—though again the statement carried no enforcement clout.[6]
The most vicious aspect of this circle is that inadequate research on reproductive technologies has increased the risks that come with any new medical intervention. Most prominently, inadequate research on IVF-related treatments increased reliance on hormonal superovulation. Harvesting many eggs, in the hope that several embryos could be transferred during each treatment
cycle, remained the easiest way to increase the likelihood of success. Yet there were no good data on outcomes of these technologies, especially compared to safer, less costly alternatives, and scientists could not determine the reasons assisted reproduction so often fails. Such data could guide development of reproductive interventions by identifying specific treatments that can provide benefits for particular groups of patients. Had the government funded research on IVF and its spin-offs, argue many specialists, large-scale, scientifically controlled studies could have helped identify what does and does not work, for whom, at what risk. Instead, the way reproductive technologies developed meant increased risks and complications—or "side effects"—for increased numbers of people.
No Harm Done?
Like other fertility treatments, assisted reproductive technologies are perceived by many doctors and patients to be generally safe, except in a few rare, unpredictable cases. Though the intervention may not result in pregnancy, they assume no permanent or life-threatening harm will be done in trying. However, these procedures are highly invasive. Beyond the physical, emotional, and financial costs inherent in any medical intervention, such fertility treatments entail certain unique risks. Chances for harm may be greater than commonly perceived.
Laypeople may understand that, basically, IVF includes removing eggs from a woman's ovary. They may not know that this procedure, repeated for each mature egg to be harvested during a particular treatment cycle, brings risks of injury and infection to the woman (for example, bleeding or infection at the puncture site in the vagina, abdominal wall, or ovaries). Initially, when IVF egg retrievals required pelvic surgery (laparoscopy) in order to see where the needle was going, the procedure also carried the risks attending all surgery. Later, IVF programs began switching to ultrasound-guided egg retrieval for most women, eliminating the pelvic surgery and its risks, as well as lowering the cost of treatment. However, the most commonly used IVF variations—transferring eggs and sperm (GIFT) or early embryos (ZIFT) into a woman's fallopian tube—do still rely on a laparoscopic surgical procedure.
More troublesome, in many ways, is the regimen of ovarian hyperstimulation characteristic of most assisted reproduction. The widespread use of potent fertility drugs on healthy women brings not only immediate risks for the
woman, but also considerable questions about long-term effects on her health. Moreover, risks from superovulation extend into the next generation.
Medically Controlled Menstrual Cycles Out of Control
Attempting to increase women's fertility is but one aspect of reproductive medicine's broader efforts to manipulate women's menstrual cycles. The controlled ovarian hyperstimulation of fertility treatments developed in tandem with its opposite—hormonal contraception, the Pill. Both superimpose exogenous (from outside the body) hormones upon a woman's natural hormonal cycle. In contraception, the intent is to shut down ovulation and/or prevent implantation, thereby preventing pregnancy. For assisted reproduction, the aim is to replace a woman's ongoing monthly interplay of hormones with a hyperstimulated, superovulating cycle. In this cycle, numerous eggs can mature, and the time of ovulation can be more accurately predicted and controlled. Since an egg must be fertilized within hours after ovulation, accurate timing contributes to successful egg retrievals and conception—not to mention convenient scheduling.
Most women undergoing controlled ovarian hyperstimulation receive daily or twice-daily injections of hormones to mature several eggs, each within an enlarged fluid-filled sac (the egg follicle); in some women the ovary responds to hormonal stimulation with maturation of one or two eggs, in others as many as thirty or forty. To monitor this process, women must also receive ultrasound scans of the ovaries and have blood drawn to test hormone levels. Immediate side effects of the drugs—which may include headaches, backaches, breast tenderness, bloating, nausea, insomnia, increased vaginal discharge—are generally considered a tolerable if unpleasant burden of fertility treatment.
Like the sorcerer's apprentice, however, the physician can lose control of the ovary's production of mature eggs. Instead of a single, raisin-sized egg follicle during a natural cycle, twenty or thirty or more can dangerously enlarge the ovary from golf-ball to grapefruit size and cause "weeping" of the ovaries, a condition in which the excess follicular fluid spills into the abdominal cavity. Estimates indicate that up to 2 percent of superovulated women suffer this severe form of ovarian hyperstimulation syndrome, with risk of liver damage, kidney or liver failure, rupture of the ovary and abdominal bleeding, compromised breathing or acute lung failure due to abdominal distention, shock, blood clots, and stroke. This syndrome, from which women have died, requires hospitalization and extremely careful monitoring. Another 3–4 percent experience less severe grades of the syndrome following "controlled" ovarian hyperstimulation.[7]
Beyond those side effects a woman feels in the present, beyond treatment complications her doctor must handle immediately, what of long-term threats to the woman's health? The most ominous threat lurking behind any manipulation of hormones is an increased risk of cancer. Scientists have long been aware that many cancers—particularly those affecting endocrine organs (breast, ovary, uterus, vagina, prostate, testicles) involve exposure to hormones, from both within and outside the body. Though understanding of the process remains incomplete, a biological relationship is well established. Scientists have, therefore, long been aware that cancer is a potential risk from fertility drugs. It was with good biological reason that Dr. Florence Haseltine, then director of the Center for Population Research at the National Institutes of Health, voiced this concern at the 1989 convention of fertility specialists: "Someone should be studying the women. . . . Everyone looks at risks of cancer from oral contraceptives, but what about these fertility drugs?"
These "unknown" risks, quietly discussed among a few researchers, became more plausible and more public in 1993 when the lay press reported findings of an ovarian cancer study suggesting that women who have taken fertility drugs are more likely to develop this disease than women who have not (see Chapter 6).[8] Whether a woman completes an IVF cycle or not, whether she becomes pregnant or not, most assisted reproductive technologies "prepare" women with some type of fertility drug; in fact, those who never became pregnant showed the greatest increase in risk, according to the ovarian cancer study. Nor is the danger limited to women who have undergone IVF-related treatments. Though this group grows ever larger and attracts the greatest attention, it still represents a minority of fertility patients, one segment of a much larger population of women given fertility drugs.
The Dangers of "Success"
The reliance of assisted reproduction on ovarian hyperstimulation has increased risks of a more evident "side effect," inhering in the very "success" of this one step in the process. Quadruplets and quintuplets, glimpsed now and then on our nightly news, are the public faces of an array of medical complications brought on by "multifetal" pregnancies. Development of IVF-related fertility treatments has centered on a technical dilemma: How many eggs retrieved and embryos transferred will give the best chance for ending up with a healthy baby? Practitioners of assisted reproductive technologies experiment with these numbers, aiming to improve their ultimate "take home baby" rate.
The trade-off is clear. Too few eggs retrieved and fertilized means fewer embryos transferred and lower chances of implantation, ongoing pregnancy, and delivery of a baby. For each embryo placed in the uterus during an IVF cycle, pregnancy rates rise about 8 percent—thus giving rates on average of 8, 16, 24, and 32 percent for one, two, three, or four embryos.[9] Reported increases are higher with GIFT and ZIFT. With many embryos transferred, however, too many may implant and grow; the average rate of multiple gestations resulting from assisted reproduction is about 25–30 percent of the total pregnancies.
For the pregnant woman, health risks increase with each fetus, jumping substantially with triplet and larger pregnancies. A typical journal article listing of maternal risks with multiple fetuses includes increased incidence of pre-term labor requiring medical treatment (which can itself bring life-threatening side effects such as cardiac failure and pulmonary edema), preeclampsia, pregnancy-induced hypertension, gestational diabetes, anemia, uterine rupture, placenta previa, abruptio placentae, thrombophlebitis, polyhydramnios, and serious postpartum hemorrhage, including the need for transfusion, which itself has carried risks of HIV and hepatitis.[10] In addition, women face a greatly increased risk of simply not being able to carry the pregnancy long enough, even with treatment of pre-term labor. The ultimate result may be no baby at all or several extremely premature babies risking the myriad problems of prematurity—including respiratory failure, brain hemorrhage, cerebral palsy, and eye damage. Other fetal risks include growth retardation, umbilical cord accidents, and congenital anomalies. Even twins, whether low birthweight or not, appear to have increased incidence of cerebral palsy—a risk that may not be explained to people considering fertility treatments.[11]
While an ART program may count these multiple newborns among their "successful" outcomes, many of the severely premature babies will die weeks or months later, or will survive with severely damaged health, a "side effect" of assisted reproduction that becomes the pediatricians'—and the parents'—problem. Media coverage of a septuplet birth in California in the mid-1980s did not follow the case. One year later, only three of the babies were still alive, at least two of them suffering severe, possibly long-term mental and physical handicaps. Less dramatic, but more typical, was a triplet birth, fifteen weeks pre-term, each baby weighing less than two pounds. After ten weeks of newborn intensive care, all the babies had vision problems; one, who suffered brain damage, was still attached to an oxygen tank at home nearly one year later. Dr. Louis Keith, director of the Chicago-based Center for the Study of Multiple
Births, describes the public picture as "buffed, toned, air-brushed, and made to look wonderful. What distorts the issue is that the press is very eager to do a story on a mother of quints all of whom are 4 pounds or more, but they don't find it very newsworthy to describe the cases where the babies are born very prematurely and die one by one."[12]
Nor does the public hear about high levels of stress and depression reported in parents caring for multiples when they do make it home from the hospital.[13] With fertility clinics pursuing higher success rates, the trade-off was not lost on some within the profession. A 1991 recommendation of the American College of Obstetricians and Gynecologists' Ethics Committee urged doctors to counsel fertility patients, prior to treatment, of the "dire consequences" of large multifetal pregnancies. As one specialist commented in the pages of a medical journal, "There is no question that grand multifetal pregnancy is a serious risk to the mother and fetuses. . . . [Such a] pregnancy secondary to poorly monitored reproductive techniques is not a therapeutic triumph."[14] Another notes that, contrary to depictions in the media, most of these pregnancies bring "suffering you can't imagine."[15]
By the mid-1980s, medical reports were beginning to reveal initial attempts to eliminate this major side effect of assisted conception—too many fetuses—by eliminating several of the fetuses conceived. The aim was to save a viable number of babies and protect the pregnant woman's health. The reports were soon appearing regularly, presenting hundreds of cases in which a woman pregnant with triplets, quadruplets, quintuplets, or more underwent "selective fetal reduction" (sometimes called "selective termination"). While a similar procedure had previously been used in spontaneous pregnancies with a fetal anomaly (e.g., one of a set of twins, especially when the abnormal fetus jeopardized survival of the other), the pressing concern now was an iatrogenic condition (i.e., caused by medical intervention) endangering mother and offspring. In these increasingly frequent instances, a number of normal fetuses must be terminated. The medical literature described various procedures and outcomes, as practitioners sought the best method for ending physiologic activity in some fetuses of a multifetal pregnancy without injuring or losing all. Early results were not encouraging; too often, the outcome was loss of the entire pregnancy. Practitioners altered their reduction techniques. They tried varying the number of fetuses eliminated, the number left to grow. Starting with six or eight, should they reduce to three or four or two? They discussed timing. Performing the procedure a few weeks later might allow spontaneous reduction to occur through miscarriage, a winnowing that brings fewer complications. However, if this nat-
ural process did not occur, delay might ultimately bring greater physical risk and psychological cost. And with six or more fetuses, two or three reduction sessions might be required.
Soon came the inevitable warnings to colleagues about the need for experience in order to perform this procedure successfully. Such warnings could only hint at the number of botched attempts becoming common knowledge within the medical community. "The literature fails to provide readers with appreciation of technical nuances this operation requires," one specialist cautioned with impressive medical tact, citing a "learning curve" of improved results as practitioners improve their skills.[16] By 1996, a report on 400 pregnant women would show that approximately 8 percent lost their entire pregnancy when fetal reduction was performed by experienced practitioners.[17]
The technical dilemma that created multifetal pregnancies through assisted reproduction translated immediately into an ethical debate. Although legal, abortion was a highly charged issue during the Reagan-Bush years—and remains so today. Beyond arguments against terminating any fetus, however, were concerns more pertinent to selective reduction. The idea of "choosing" which of several normal fetuses to eliminate was troublesome; with the choice determined by physiologic and technical considerations alone, many practitioners eliminated "selective" from the vocabulary. More difficult were questions of benefit and harm during these much-desired pregnancies, many of which followed years of fertility treatments. A 1988 Lancet editorial on selective fetal reduction identified as ethical issues "the risk of damaging the surviving fetuses; the degree to which complications of multiple pregnancy are resolved by selective reduction; the effect on the mother both during pregnancy and after the birth; social and economic considerations for the children's well-being; and the effects of information about selective termination on attitudes and feelings of the parents and surviving children." The authors note the "dearth of reports on the outcome of fetal reduction, particularly the occurrence of complications for the mother and remaining fetuses and any lingering mental or physical effects after birth." Not only is this procedure performed "more frequently than is apparent from published reports, "but the ethical issues "have not been discussed widely, and the public are largely unaware of the technique of fetal reduction or the medical reasons for it."[18]
The medical conundrum of fetal reduction gained sharpest focus at the hazy borderline of triplets. The threat of causing total pregnancy loss—and the agonizing balance of fetal and maternal well-being—hangs heaviest over three. Should large multifetal pregnancies be reduced to triplets or fewer? Should
triplet pregnancies be reduced to twins? Advances in high-risk obstetrics and newborn intensive care mean that triplets have a good chance of surviving, though with significant risks from prematurity and low birthweight. Risks to the woman pregnant with triplets remain considerable. One study in France, comparing triplet pregnancies that were and were not reduced, reported as "one of the most important results" the finding of "life-threatening maternal complications" among only the forty-eight women who chose not to undergo fetal reduction: spontaneous rupture of the liver complicating severe preeclampsia; hemiplegia (paralysis of one side of the body resulting from stroke) during the sixth month of pregnancy with incomplete recovery; and massive pulmonary embolism following cesarean section, requiring cardiac intensive care, despite preventive measures.[19]
Journal descriptions of fetal reduction portray in cool clinical detail the spiral of increasingly complex, invasive, and expensive technology spinning from those initial experiments with IVF. The medical literature documents the growth of this offshoot of a serious iatrogenic problem into a medical intervention in its own right, with its own specialists—and its own critics. Indeed, critics within the ranks began turning responsibility back onto the profession. Creating multifetal pregnancies in infertile patients is "a tragic irony of immense proportions," wrote one group of physicians and ethicists. "Many cases of iatrogenic multiple pregnancy are probably avoidable by more diligent management of infertility drugs. Many of the currently known instances of grand multiple pregnancies should never have happened. Selective termination should be viewed not as an end it itself, but as a provisional approach, appropriate until improved medical care obviates the need for its use."[20] A 1993 Belgian study of triplet pregnancies concluded: "The fundamental ethical problem the medical community has to solve is not whether to reduce triplet pregnancies but whether to continue to induce so many high-rank multiple pregnancies." The authors' warning of potential "banalization of embryo reduction" echoes the Lancet editorial's concern that fetal reduction "not become the management method for ovulation induction, IVF, and related techniques."[21]
More strongly and broadly put, a letter to Obstetrics and Gynecology asks: "Where is the duty to the woman before she is pregnant, a duty that must include protecting her from 'iatrogenic' multiple gestations that could threaten her life? Where is the duty to human life itself, a duty that must dictate not creating life that has little chance for a reasonable existence. . . . The ethics of selective termination must be paired with the ethics of infertility treatments: If obstetricians are willing to 'gamble' on the possibility of grand multiple gesta-
tions, they must be willing to provide some remedy for the harm they cause. Any ethical guidelines pertaining only to selective terminations further victimize the woman and her fetus(es). . . . If there is a clear 'wrong' . . . it lies in the unexamined enthusiasm for infertility treatments, not in the management of a woman's subsequent pregnancy."[22] Clearly, the ethical debate over selective reduction encompasses more than the imperative to minimize complications through more skillful fertility treatment. For women facing selective reduction, the added ethical irony is that better research might have meant less reliance on superovulation and transfer of multiple embryos—research stymied still by anti-abortion politics. Given that IVF and related technologies are often used inappropriately, on women who do not need them, the additional question is: How many women are undergoing this attempt to correct a "complication" of treatment that should never have been performed in the first place?
The Next Round
Amid the swell of journal reports describing the latest variations of ART and the increasing number of categories of patients on whom these variations were tried, a different type of argument appeared. With mounting evidence of serious side effects—actual and potential, immediate and long-term—and with persistent concern for cost, physicians began acknowledging that assisted reproduction literally reaches the point of diminishing returns. Creeping into the fray were titles such as "A Simplified Approach to In Vitro Fertilization," and "In Vitro Fertilization in Unstimulated Cycles." Practitioners of ART were now experimenting with less invasive protocols, suggesting fewer fertility drugs, describing nonsurgical techniques. Depending on which fertility specialist you were seeing, you might now be offered a much less onerous—and less risky—option than just a year or two before.
The pattern is familiar in fertility medicine, yet patients rarely perceive this medical progression that can so greatly alter the course of their treatment. Obtaining the latest information about a particular fertility condition—important as such knowledge is—reveals only a slice of the medical here and now. The current state of the art reflects developments that reach back into medical history and across specific medical conditions. Assisted reproductive technologies illustrate more vividly than most fertility treatments that the professional "learning curve" is more convoluted than a simple progression toward increased experience, skill, and, therefore, success. This curve may eventually turn downward,
toward moderation in therapeutic zeal. With assisted reproduction now a possibility for all fertility patients, they need to stand back and ask where these treatments fit into medical trends. They need to view this option—or any fertility treatment—as an intervention with a history that could significantly affect their care.
Consider the trends in development of assisted reproductive technologies and the potential impact on individual patients. Announcement of the first IVF baby unleashed an explosion of attempts throughout the world to create more "high-tech" babies. A 1989 World Health Organization survey identified 708 IVF-ET clinics in 53 countries.[23] In such clinics, large and small, physicians and technicians experimented with various steps of assisted reproduction, seeking to bump up stubbornly low success rates. Initial reports focused on quantity. They showed an increase in eggs retrieved and embryos transferred, along with some increase in pregnancies conceived, thus forging the link between ART and ovarian stimulation. However, the increase in egg fertilization and pregnancy did not translate as successfully into healthy "take home babies." Fertility specialists began to speak of limits, both on the number of eggs and embryos and on the number of attempts.
The 1988 Lancet editorial on selective fetal reduction was one of the first calls for less aggressive fertility treatment:
Given that the problems of premature delivery, very low birthweight, and perinatal mortality in IVF pregnancies are exacerbated by the high frequency of multiple pregnancies, there is a good case to reduce further [below three or four] the number of eggs and pre-embryos replaced. Some IVF and GIFT clinics continue to replace large numbers of eggs and pre-embryos in their patients. The reasons for this practice vary . . . [but none] is a reasonable excuse for putting a woman or her babies at risk of the severe complications of quintuplet or larger multiple pregnancies, and there are even grounds for concern about triplet and quadruplet pregnancy. . . . Instead of replacing large numbers of eggs and pre-embryos, IVF practitioners should carefully consider the reverse trend of replacing fewer eggs and embryos.[24]
During the ensuing years, more physicians reassessed their procedures. ART programs more commonly reported outcomes with fewer rather than more embryos, searching for an optimal number that would better balance increased births with decreased medical complications, psychological stress, and financial
cost. By the early 1990s, many doctors recommended transferring no more than three or four embryos in any one attempt. By 1997 the large number of triplet pregnancies, and their risks, led Dutch specialists to argue that transferring only two embryos results in acceptable pregnancy rates. Other European specialists suggest this limit is particularly warranted for women younger than thirty-seven who respond well to ovarian stimulation.[25]
In the same years, physicians began suggesting another type of limit—on the number of unsuccessful ART attempts patients should endure. A 1989 letter to the New England Journal of Medicine raised the question, "How Much Is Enough?" The authors write, "Medical technology offers almost endless hope for infertile couples; however, when to stop has become a difficult question to answer. When the treatment offered is in vitro fertilization, determined couples may initiate many cycles with the hope that with one more try they will succeed in having a child." However, among the first fifty women who conceived and delivered a baby in their IVF program, 84 percent of the births occurred after two IVF cycles, and "births were extremely unlikely after the fourth IVF cycle. . . . We conclude that the overwhelming majority of couples who will achieve pregnancy as a result of IVF do so within a relatively short period of time. Couples who do not achieve a viable pregnancy after four to six IVF cycles should be counseled that success with this technique is unlikely and should not be encouraged to pursue IVF further."[26]
Again, the concern was physical and psychological hardship, as well as expense. These IVF practitioners were seeking a cost-effective number of in vitro cycles, particularly as compared with such alternatives as major abdominal surgery for tubal abnormalities (most of which have low success rates) or extended non-IVF treatment. Three or four IVF cycles might keep stress and cost at reasonable levels while offering at least equal chances for success as other methods.
In addition to seeking limits, a second trend evident by the early 1990s was toward reducing invasiveness. One approach was to wean assisted reproduction from ovarian stimulation. Some doctors began prescribing a lighter fertility drug regimen for their patients or use of only clomiphene citrate instead of the more potent Pergonal (brand name for hMG, human menopausal gonadotropin), particularly in women younger than forty with fertile partners. A 1993 study described "a novel ovarian stimulation protocol," using reduced drug dosages on fewer days of an ART cycle,[27] in order "to lower the escalating costs of assisted reproduction and decrease the extent of patient discomfort and disruption of life-style without sacrificing success rates." Commenting that "the
history of ART illustrates how the pendulum of medical therapy can swing from one extreme to the other," the study's authors conclude that their "minimal stimulation" protocol "is easy to administer, requires less intensive monitoring, fewer medications, and virtually eliminates the risk of ovarian hyperstimulation syndrome."
By the mid-1990s, physicians would also acknowledge fertility patients' and egg donors' concern about ovarian cancer risk. In 1996, one group of specialists stated their own concern about this danger and about "as yet unrecognized factors in these complex and powerful endocrine treatments" that could, for example, adversely affect women's menopause; they communicated with other physicians in an editorial written "as practitioners in assisted reproduction who are increasingly concerned about current approaches to ovarian stimulation"—particularly "increasing reliance on complex treatment protocols resulting in large numbers of oocytes."[28] In this echo of the editorial on exploiting fertility patients written nearly a decade before, these specialists contend that such protocols "may help to organize the activities of the clinic," but "could be injurious to women's health." Fertility treatments should entail milder stimulations based on greater understanding of—and connection to—a woman's natural menstrual cycle. Simpler and milder stimulations that produce "relatively minor modifications of the natural cycle" could be tailored to individual patients "who could self-administer two or three injections per cycle rather than the daily injections that have become routine." They criticize especially the many doctors who emphasize the sheer number of eggs and embryos as a sign of successful ovarian stimulation when the goal should be to stimulate the fewest follicles necessary for the individual patient's treatment needs.
Compared to earlier years of reproductive technologies, patients now had new options and trade-offs. The pregnancy rate following milder ovarian stimulation is lower for each retrieval cycle, but so are the health risks and emotional stress. And, as fertility specialists admitted, the lowered cost achieved by minimizing use of fertility drugs provided many infertile couples with "their only financially sound access to ART."[29] Other ART programs backtracked even further, eliminating fertility drugs altogether. They retrieved patients' naturally ovulated eggs during unstimulated cycles, to be mixed in vitro with sperm. This latter approach was not "novel" at all, but rather swung the pendulum back to the original IVF birth in England, achieved during the mother's natural menstrual cycle.[30]
Further modification of IVF downgraded the high technology by eliminating both fertility drugs and fertilization in a laboratory. In 1992, a Harvard-affiliated
ART program described a treatment tried on forty-five women that combined natural cycle egg retrieval with "intravaginal fertilization." In this "simplified approach" doctors retrieve a spontaneously matured egg from the ovary. The egg is then placed with sperm and nutrients into a sealed capsule, rather than a laboratory dish as in standard IVF. The capsule goes into a special sealed envelope, which is inserted into the woman's vagina for two days. If fertilization occurs, doctors transfer the embryo to the woman's uterus. Advantages, according to the physicians, include elimination of fertility drugs, simplicity of monitoring egg maturation and retrieval, and lack of need for expensive laboratory equipment. This method, they suggest, "may prove appropriate for those women requiring IVF who fear multiple pregnancies, have side effects from controlled ovarian hyperstimulation, or cannot afford standard IVF." Their report concludes, "Pregnancy rates . . . may never equal those achieved with standard IVF. However, for some patients the marked advantages and reduced costs [approximately one-third standard IVF] . . . may outweigh the small reduction in the percentage of success."[31] They speculate that patients might be willing to repeat this easier and less expensive process more often than standard IVF, resulting in nearly the same number of women who eventually become pregnant.
Finally, the assisted reproduction trend revolved full circle. For some women with open fallopian tubes, egg retrieval itself could be eliminated, along with laboratory fertilization. Instead, the doctor might suggest trying less invasive and less costly intrauterine insemination of sperm, but with controlled ovarian hyperstimulation (called stimulated IUI). The theory was that something about superovulation—perhaps increased numbers of egg and sperm at the fertilization site—contributes to the success of assisted reproductive technologies; as with many fertility treatments, just what that something is could, perhaps, be determined in future studies. The trade-off here, of course, is that women still face the risks of ovarian hyperstimulation.
The journal article that proposed this alternative raised a stir among fertility specialists. Describing 148 stimulated IUI cycles in 85 couples, the authors reported pregnancy rates "approaching that of normal women and comparable to reported results with GIFT and IVF-ET in couples with . . . endometriosis, idiopathic [unexplained] infertility, or cervical factors." Of pregnancies conceived, 29 percent were multiples—five sets of twins, one of triplets—a proportion similar to IVF and GIFT.[32] Tagged onto the end of this article was an unusual, italicized comment from the journal's editor: "The decision to publish this controversial manuscript has been made with the intent of stimulating debate. The referees feel strongly that, before advocating IUI during hMG-stimulated cycles,
a prospective controlled study with critically evaluated infertile patients is mandatory. The advocating of this essentially empiric therapy cannot be supported by the rather meager retrospective data presented. . . . Clinicians should be discouraged from applying this therapy until controlled prospective studies can support this approach." While the editor may have been legitimately concerned that an unproven treatment with proven risks would become widely used, the comment failed to acknowledge what the article's authors point out—that GIFT and IVF in women with these same diagnoses had themselves never been properly evaluated. Yet clinicians were surely applying these therapies "empirically"—that is, based on trial and error and their personal observations, rather than on systematic, scientifically controlled studies.
Proposing superovulated intrauterine insemination as an alternative to egg retrieval, fertilization, and transfer of IVF or GIFT blurred the very definition of "assisted reproduction." Amidst the turmoil engendered by IVF-related treatments, however, one thing was clear: ART had become a fact of life. Though most fertility patients initially undergo "conventional" treatments involving medications, insemination, and/or surgery, assisted reproductive technologies have grown to be far more than a last resort for patients and doctors. These treatments are now the ever-present backdrop for doctors' recommendations and patients' decisions. ART is a benchmark within fertility medicine, an option with which conventional alternatives are compared if not combined. Moreover, these techniques are vehicles for future reproductive developments, particularly in combination with new genetic tools. At the same time, public interest in ART, the technology's media appeal and relative visibility, would eventually help throw light on inadequacies with fertility medicine more generally—the preponderance of unproven therapies, the questionable value of many diagnostic procedures, the potential for patient exploitation and harm, and the absence of guidelines or regulations for reproductive interventions. In addition, there is another fact about these interventions: disagreements among doctors translate directly into treatments of differing invasiveness, risk, and benefit for their patients. To the individual woman and her partner, these differences may make all the difference in the world.
Claims of Success
Among many nagging questions surrounding expansion of assisted reproduction, none has been more persistent than claims about success. None is more
important for fertility patients to understand: Will these substantial medical interventions increase their chances of having a healthy baby? For women with completely blocked or absent fallopian tubes, in vitro fertilization can be an effective treatment. Without treatment, the chance of pregnancy is virtually zero; through the IVF procedure, some of these women will conceive. The proportion of treated women who end up with a baby provides a fairly good measure of IVF success in overcoming this particular fertility problem.
As assisted reproduction expanded beyond this original use of IVF, however, the question of effectiveness became less clear-cut. The development of ART has been accompanied by controversy over just how successful these therapies are. The main complication is that most of the newer "indications"—the conditions for which a doctor might prescribe ART—bring respectable rates of spontaneous pregnancy; that is, the women thus diagnosed can conceive without treatment, though they may require a longer than average time to do so. As the uses of ART grew, so too did the numbers of women who became pregnant while on an ART waiting list.[33] To determine the success of medical intervention for this broader range of fertility problems, the spontaneous pregnancy rate—the "background rate"—becomes a key comparison. The question becomes whether the pregnancies result from the treatments. For the individual woman considering assisted reproduction, the question is whether her likelihood of pregnancy is appreciably greater with treatment than with no treatment or with a less invasive and costly one.
Success rates reported for GIFT provide a good example. If a woman has at least one functioning fallopian tube in which to place eggs and sperm for fertilization, her doctor may recommend GIFT, pointing to higher success rates than with standard IVF. However, because she does have at least one good fallopian tube, she also has a better chance of becoming pregnant without treatment. One ART program's description of "the ideal GIFT candidate" seems also to describe women who may well conceive spontaneously: "a woman who has previously proven her ability to fertilize her eggs (by IVF or a previous pregnancy), who has no tubal disease, is free of sperm antibodies [an immunologic factor that some doctors think reduces fertility], and who has a fertile partner; prime indications therefore include 'unexplained infertility' and non-immunologic cervical mucus hostility." At least a portion of the greater success attributed to GIFT, therefore, may result from its use on women who are relatively more fertile to begin with and will conceive more easily than, say, women advised to undergo IVF. And if these women have sexual intercourse during a GIFT cycle, there simply is no way of knowing whether a pregnancy was a result of treatment.
Aside from the very basic question of whether a treatment was responsible for a subsequent pregnancy, controversy over assisted reproduction reflects a confusion of definitions and profusion of methods for calculating success rates. Attempts to evaluate IVF-related procedures have been plagued, first of all, by misleading definitions of "success." The ultimate measure of a successful fertility treatment is birth of a healthy baby who would not otherwise have been born. While this definition may seem obvious, many IVF clinics stretched the meaning of success to improve their reported track record. As with surgery declared "a success, but the patient died," assisted reproductive treatments have too often been labeled "successes," but without, in the end, a baby.
A clinic might boast an impressive pregnancy rate, for example, but include in its tally biochemical pregnancies —transient elevations of the "pregnancy hormone" beta-hCG, detected through a very early blood test. A rise in this hormone level can indicate first stages of a pregnancy; however, results may also be only a temporary "positive" from the effect of hormones administered around the time of ovulation as part of the fertility treatment itself. In contrast, an actual pregnancy, known as a clinical pregnancy , can be more directly observed. To be counted as an established, clinical pregnancy, at least one gestational sac within the uterus must be seen by ultrasound examination (a count that thus excludes ectopic pregnancy, which must be removed). This pregnancy rate will, of course, be lower than the tally of biochemical pregnancies.
ART programs sometimes report their implantation rate —the proportion of embryos transferred that appear as gestational sacs during the initial ultrasound exam. Patients may want to know how successful an ART program is at getting embryos to implant, as one measure of technical ability. However, a relatively low implantation rate may instead reflect a relatively older patient population, since this step appears to be where pregnancies frequently fail in women nearing forty.
A fertility program's reported pregnancy rate will be higher than its success in producing live, healthy babies. Of ART pregnancies confirmed through ultrasound, the number of living newborns is approximately one-quarter to one-third lower, due to miscarriage and complications of multiple births. Even definitions surrounding births can be unclear. ART programs report live deliveries, defined as the birth of at least one living infant. Again, since multiple pregnancies are more common with ART, the number of deliveries will differ from the number of babies born alive. Some premature newborns never make it home from the hospital's intensive care unit; the number of babies born alive, therefore, will be higher than the total of "take home babies." Unfortunately, the de-
scription "healthy" too often disappears entirely from medical definitions and calculations of success. The rate of successful live deliveries tells us only that at least one baby was born alive, not that a baby went home in good health.
As IVF clinics proliferated during the 1980s, prospective patients needed also to be wary of an additional confusion—whose success rate were they hearing? Over half the clinics performing IVF in 1987 had failed to produce even one live birth. To inquiring patients, these clinics might quote a nationwide success rate or, more egregious still, rates for the country's most experienced programs; the patients would never know that for this particular clinic, the success rate was zero. The new Boston branch of a worldwide chain—IVF Australia—advertised "the program's" 236 live births, these impressive numbers culled from the entire IVF chain. By the 1990s, freezing extra embryos (cryopreservation) had become a common procedure, allowing women to attempt additional embryo transfers without undergoing additional risk and cost of hyperstimulation and egg retrieval. On the one hand, a clinic might boast that it offers cryopreservation, without mentioning to inquiring patients that not one of this program's frozen embryos has ever thawed into a successful pregnancy. On the other hand, clinics that limit the number of fresh embryos transferred—for example, to minimize multiple gestations—may achieve successful pregnancies from later transfers of patients' thawed embryos that are not reflected in standard success reports.[34]
Claims of success for ART have not been clouded solely by deceptive jiggling of numbers. Researchers do not always agree on how best to evaluate and compare outcomes of ART programs. What patients considering assisted reproductive technologies most need to know is the "take home baby" rate following treatment; yet this information can be more or less optimistic—intentionally or not—depending on how the proportion of live infants is calculated. Pressured by undeniably deceptive success claims and by growing congressional awareness of them, specialists in assisted reproduction began searching in the late 1980s for a precise measure of the likelihood that treatment would result in a living baby. The American Society for Reproductive Medicine helped establish a voluntary registry that gathers yearly reports from ART programs in the United States and Canada. The registry's first report, published in 1988, included overall data accumulated from forty different clinics. In 1991, after much internal argument, the registry began documenting success rates on a clinic-by-clinic basis—essential information for people choosing an ART program.[35] By mid-decade, this registry was reporting success rates for 267 ART programs. The most recent years show small increases in success overall, with women's age and male fertility problems as key factors
affecting rates of success. However, among ART practitioners debate about these reports continued; now that individual programs could be identified, registry participants were particularly concerned with protecting their own program's favorable standing in the growing ART competition.[36]
What Patients Should Know
While physicians argued over competing definitions of success, a "best" measure remained elusive. The need that emerged most convincingly was for a broad accumulation of information that could shed light on two separate but interrelated questions: What do the numbers about a particular reproductive technology reveal about its effectiveness for treating a particular fertility problem, when performed at its best? And where is this treatment performed at its best? That is, what do numbers from specific ART programs reflect about their skill and success with this intervention, compared to other programs?
For fertility patients, these general questions translate directly into decisions about whether a treatment is worth trying and, if so, where to seek care—a choice (assuming they have a choice; this issue is discussed in chapter 8) of obvious concern to providers of these treatments as well. In attempting to answer these questions, different calculations provide information about different aspects of a multistep process. Patients should understand that each method of calculation—while contributing to the overall picture—has limitations in what it can reflect about treatment success. Adding to the complexity of reported success rates are the varying characteristics of patients and of the assisted reproduction process that influence success or failure at each step along the way; some of these characteristics are known, others mysterious.
Most success rates now reported by ART programs present the number of live deliveries (i.e., at least one live newborn) as a percentage of a total number of attempts to achieve this goal during one year. This proportion of successful outcomes, however, depends on which step in the ART process is used to define the number of "attempts." Some calculations inflate the degree of success because they do not include all of the women who began a treatment. In fact, a crucial dimension underlying measures of success is an ART "attrition rate." Patients drop out at each stage of an ART cycle for a variety of reasons, physical, financial, and/or the stress of it all.
Some patients drop out after the first step of egg maturation and monitoring for the approach of ovulation. The vast majority of ART programs use hormonal
hyperstimulation, which can cause side effects requiring the treatment be stopped. Whether the cycle is stimulated or natural, the treatment cycle is canceled if ultrasound monitoring shows no enlarged egg follicle. Measures of follicle size (indicating egg maturation) and hormone levels (indicating readiness of endometrium for an embryo) may not meet requirements doctors consider to be properly synchronized for successful implantation, resulting in cancellation of the egg retrieval. If at least one egg does mature, the practitioner may not be successful in the second step, aspirating it (or them) from the woman's ovary. The ART process may falter at step three, the meeting of egg and sperm; fertilization may fail to occur or may not produce an embryo deemed suitable for transfer.
Since at least one embryo must be transferred in order to complete an ART cycle (although with GIFT, it is the eggs and sperm that are transferred to the fallopian tube), medical reports often use this fourth step—the embryo transfer procedure (referred to as ET)—as the basis for determining the likelihood of a live delivery if a woman completes the treatment. This success rate, then, reflects the proportion of live deliveries out of all embryo (or gamete) transfer procedures performed, but it does not include women who started treatment but never got as far as the transfer stage. Patients considering assisted reproduction, however, also want to know the likelihood of success for a woman who starts treatment. This calculation would compare the number of live deliveries to the number of egg maturation and monitoring procedures (or, if no hormonal stimulation, the total number of natural treatment cycles started).
|
|
A somewhat different calculation is also important: the number of clinical pregnancies (i.e., observable through ultrasound) out of all treatment attempts or embryo transfer procedures. Comparing this proportion to the eventual rate of
live deliveries helps determine the likelihood of pregnancy loss after successfully conceiving. This information, in the words of one group of ART evaluators, "provides couples with a realistic understanding that not every pregnancy progresses to delivery."[37] In reality, 20–25 percent of ART pregnancies end in miscarriage. Approximately 5 percent of ART attempts result in ectopic pregnancies and must be removed immediately; these should not be counted as clinical pregnancies.[38]
Understanding of ART success rates is not likely to be "realistic" if based solely on percentages of outcomes and procedures. Any such proportions can be only gross measures that require considerable explanation and that should be accompanied by data describing additional aspects of a complex picture. A multitude of characteristics about the patients treated and the process performed complicates all success calculations. Only the composite of many small pieces allows some grasp of relevant likelihoods—the likelihood of pregnancy, of pregnancy loss, of multiple births, of time and money, of a healthy "take-home baby." Despite the risk of information overload, this composite approach may avoid what the director of one prominent IVF clinic describes as "the major risk in interpreting reported outcome data . . . that of oversimplification."[39]
What prominent factors emerge, then, from ongoing medical debate as significant influences on success of an assisted reproductive technology? First, are the characteristics of patients undergoing treatment—most significantly, the woman's age and the couple's diagnosis . Fertility in women generally declines after the mid-thirties, a pattern reflected in both natural and assisted reproduction. As ART programs extended their age range and experimented with various egg donor–recipient combinations, the age of forty for women using their own eggs became a rough boundary, beyond which success rates fall to extremely low levels. For women of any age undergoing ART, the type and number of conditions that lower the couple's fertility also have an impact on success. "Male factor" problems and the diagnosis of several fertility problems in the woman and/or man bring lowest rates of success. Second, are the characteristics of the ART process—most significantly, the number of eggs and embryos used during a treatment cycle. Transferring more embryos (or eggs for GIFT) increases the pregnancy rate, but it also increases multiple gestations (possibly necessitating selective reduction procedures), pregnancy loss, and obstetric and neonatal complications. Reports of success need to include whether the treatment used ovarian stimulation, what particular hormone regimen was used, how many eggs or embryos (fresh or cryopreserved) were transferred per cycle, and whether procedures augmenting standard IVF were used (for example, directly injecting sperm into eggs, "assisted hatching," egg donation).
With the above characteristics in mind, additional questions help assess the amount of time, cost, and stress successful treatment may entail. Since individual patients often undergo many treatment attempts, which can extend over many years, reports of live deliveries also need to indicate which cycle resulted in this outcome. That is, how many attempts did a woman go through before the success? In addition to annual data on procedures performed for all patients, what proportion of women who entered an ART program eventually had a baby, and with how many treatment cycles over how many months? What is the "cumulative" likelihood that a woman will have a successful pregnancy after three treatment cycles? After six? At what point does success become very unlikely? When presented with a treatment's or program's cumulative success rate (i.e., chances for a live-born infant after a certain number of months or treatment cycles), patients need to ask themselves, moreover, whether they are willing and/or able to undergo that particular number of attempts. A cumulative rate after six IVF cycles is overly optimistic for a woman who will attempt no more than two treatment cycles. After fewer cycles, fewer successful pregnancies have accumulated, giving a lower cumulative rate.
Finally, comparisons among specific ART programs are crucial for patients considering treatment as well as for quality evaluations in general. Selecting and comparing only one number—such as the percentage of deliveries out of all embryos transferred—does not provide a valid evaluation of a fertility clinic. Although few clinics are significantly better or worse than average, those that perform only a small number of procedures cannot provide reliable success rates.[40] Aside from these relatively small programs (the SART Registry suggests thirty initiated cycles per year may be a minimal number for useful results), the number of live deliveries for all treatment attempts, for egg retrieval procedures, and for embryo transfers can provide a basis for comparing the degree of experience and success of different practitioners at different steps of the ART process. These numbers can help identify a clinic that performs far less well than most; however, the numbers are not useful for ranking ART programs from best to worst.[41] Differences in the patients treated—the type and severity of fertility problems that predominate, especially the women's age and couples' diagnoses—influence that program's success rate. A "highly successful" ART clinic may be treating the easiest patients. This clinic may offer IVF to many young women with relatively mild problems who have tried to conceive without treatment for only a short time—patients who stand a relatively good chance of pregnancy with or without treatment. Although clinics now report success rates for different age groups, they do not report how long their patients had been
trying to conceive before beginning treatment. A clinic may select primarily patients for whom a treatment is most effective—for instance, IVF for women younger than thirty-five whose only fertility problem is blocked fallopian tubes. A clinic may offer special payment plans to "medically eligible" patients—that is, those with the best prognosis for pregnancy. Such a program's impressive success may reflect in good measure what physicians call the "patient selection factor."[42] Another clinic, with a lower success rate, might advise the "easy" subfertile patients to wait another year in order to see whether a spontaneous pregnancy occurs, and then begin with a less invasive therapy that succeeds. This clinic may be willing to treat relatively large numbers of women who are over forty or couples with severe and several problems in the woman and man, patients with previous treatment failures referred by other clinics.
Patients who do require assisted reproduction in order to become pregnant must be certain a program has qualified, experienced personnel and a track record open to public scrutiny. However, an individual patient may well receive the best care from an ART program with somewhat lower success rates than others. Individuals need to find the best match for their particular needs. Among fertility programs with longest experience and generally similar success rates, characteristics of a program's patients and procedures are essential considerations. How does the program select and reject patients? For what reasons do its practitioners advise patients to drop out before embryo transfer? At what point do they suggest escalating treatment (for example, use of donor eggs)? After how many failed attempts do they advise patients not to try again? How aggressive are they about superovulation, the number of embryos transferred, and use of selective reduction if large multiple pregnancies occur? In cases of selective reduction, what are the rates of healthy newborns, of complications, of total pregnancy loss? In addition, patients must ask themselves how willing they are to rely on fetal reduction if they choose a more "aggressive" ART program—and how much disruption to their daily life treatment with this program will mean. (Chapter 9 discusses additional considerations in choosing a physician or ART program.)
Unfortunately, information needed to complete the ART picture has not been systematically gathered and reported. Patients and their doctors could benefit from more numbers, sharper calculations, more insight into the medical complexities that affect an individual's outcome. Even more disturbing than the lack of adequate statistics is the absence of regulation, oversight, internal checks, and public scrutiny—with outcomes that became apparent only years after flagrant medical practices were occurring.
There is yet another complication, an additional inadequacy in the data available and in the medical treatments themselves. These technologies proceed in spite of gaping holes in basic knowledge about the reproductive process and intervention in it. For example, individual patients often try assisted reproduction many times—yet no one understands the impact of such repetition on a woman's chances for having a baby. Evidence suggests that successful pregnancy becomes less likely during later cycles, but the reasons are not well understood. Will a treatment work well for particular women early in the process if it works at all? Does increasing age, inevitable as the number of attempts increases, make later success unlikely? Does a physiological reaction to repeated ovarian stimulation and puncture for egg retrieval compromise a woman's chances in later attempts to become pregnant? Claims of consistent success rates after as many as six attempts are questionable, since they are based on the small number of couples who persist through so many failures rather than stopping out of physical, emotional, or financial exhaustion; many studies, in fact, suggest very few successes beyond four cycles.[43] More generally, fertility specialists have not learned enough about why ART has not been more successful, why progress has indeed been so slow.[44] They try new hormonal stimulations, or change the timing of embryo transfers or alter nutrients in the fertilization dish; they select embryos for transfer that "look good" in the absence of ways to more accurately identify those embryos most likely to implant and develop successfully. For the most part, however, fertility specialists have not systematically studied, and do not know, why these interventions so often fail.
Inadequate knowledge about the safety and efficacy of ART—as well as professional dispute over the use and reported success of these technologies—has continued into the 1990s. Among physicians, calls for change in the performance and evaluation of assisted reproduction ranged from mild, even congratulatory, to angered. One eminent specialist, Dr. Howard W. Jones Jr., director of the Jones Institute for Reproductive Medicine, lambasted the specialty's efforts at reporting clinic-specific success rates. Writing in late 1993, he and three colleagues noted that "in the mid and later 1980s, there was considerable consumer disquiet about what was perceived as inadequate and misleading information about pregnancy rates." This disquiet, they add, was picked up by politicians, resulting in congressional pressure to publish clinic success rates. These specialists then cited "at least 10 serious variables that cloud the Clinic Specific Report in its present form and provide an opportunity to manipulate the variables . . . with the intention of providing superior data for the [report] at the expense of what is best for the individual patient." Their conclusion: the
profession's response to dissatisfaction about misleading information was generating "different but equally misleading information."[45]
By the early 1990s this specialty's arguments shifted increasingly to the issue of costs, to the need for evaluating diagnostic tests and treatments, especially expensive procedures such as ART. With the election of the Clinton administration, national health insurance loomed suddenly, if momentarily, on the horizon. Concerns about expense of tests, treatments, and resulting complications came to a head, overshadowing worries about the actual harm treatment might cause women and offspring. The prospect of limits dictated by cost and effectiveness did serve to heighten pressures to demonstrate what procedures work, for whom. But fertility patients did not as a result gain real protection from uncontrolled and controversial procedures. As the next chapter shows, the hottest areas of current clinical activity are usually connected in some manner to ART and continue to exhibit an unsettling eagerness to intervene with questionable reproductive experiments.