4—
Imported Physics
The year 1932 began at the Radiation Laboratory with the 11-inch cyclotron at 1.2 MeV, the 27-inch cyclotron little more than a magnet, and the Sloan x-ray tube a gleam in its inventor's eye. The staff of cyclotroneers had tripled. Besides Livingston there were now James Brady, who had recently completed his doctoral thesis on Lawrence's discontinued topic, the photoelectric effect in alkali vapor, and remained on, on the payroll of his new employer, Washington University of Saint Louis, to learn something about his professor's new lines of work; Milton White, a Berkeley graduate of 1931, who earned his Ph.D. in 1935; and Malcolm Henderson, from Yale, who already had a Ph.D. and, like Livingood, a private income, which allowed him to contribute his services in an unhurried way to the Laboratory. White worked on the 11-inch cyclotron; Brady, at first, on the 27-inch. When the big machine began to operate, Henderson replaced Brady, who then, with White, had the effrontery to check whether Lawrence and Livingston had, in fact, obtained high-speed resonant protons from the smaller machine. They did not find confirmation easy. By the summer of 1932, however, they had measured the penetrating power of the very meager beam and found it to answer expectations for protons of a million volts or so. This work did not enjoy the glory of publication because, according to Brady, he and
[98] T. Yasaki to Lawrence, 24 Oct 1951 (9/43), and F. Yamasaki et al. to Lawrence, 24 Oct 1951 (9/38), quote.
White thought it advisable not to give the impression that they had doubted their professor's claim.[99]
In their hurry to build accelerators, Lawrence and his associates did not find the time to provide themselves with the detectors needed to register the effects of any disintegrations they might provoke. That was only reasonable: the accelerators did give a beam that could be improved; there lay a proven road to progress. Lawrence's group therefore lagged behind Tuve's, which had developed excellent detectors—Geiger counters, linear amplifiers, a novel cloud chamber—during 1931. Not that Tuve's people preferred detection to acceleration. The dilatoriness of their overlords at the Carnegie Institution and their indifference to the possible market value of their x rays (the Carnegie did not pursue patents) had reduced them to small jobs while they awaited permission to rip out their Tesla coil and install their 1-meter Van de Graaff.[100] When the age of disintegration began, therefore, their combination of detectors and high-current accelerator made theirs the preeminent laboratory for nuclear physics in the country. Or so Johnson, a good friend of both Lawrence's and Tuve's, judged the situation early in 1932. "It looks as if he [Lawrence] has some nice work under way but as far as the general technique for working in the field is concerned I cannot help but feel that you are way ahead."[101]
Lawrence's old friends from Yale, Donald Cooksey and Franz Kurie, came to Berkeley in the summer of 1932 to help shorten the lead. They had been asked before—Kurie for the summer of 1931, Cooksey for the spring semester 1932—but had not been able to accept.[102] Both of them were accomplished performers on the sorts of instruments the Laboratory needed most. Kurie's instrument was the cloud chamber. Cooksey was a one-man band, curator of precision instruments in Yale's Sloan Laboratory, an
[99] Birge, History, 4 , xi, 14, 25; xii, 7, 26; Brady, interview with James Culp, Oct 1981, and PT, 36:3 (1983), 11, 13.
[100] Tuve to Lauritsen, 11 Nov 1932 (MAT, 8); Tuve, JFI, 216 (1933), 13–8; Johnson to Tuve, 6 June 1932 (MAT, 8); J.A. Fleming to Fleischmann Laboratories and to John Colt Bloodgood, 16 Feb 1931 (MAT, 9). The installation of the Van de Graaff was approved on 8 Oct 1932; it took about a month.
[101] Johnson to Tuve, 6 Jan 1932 (MAT, 8).
[102] Lawrence to Cooksey, 17 Jul [1931] (4/19); Birge to Cooksey, 2 Oct 1931 (Birge P, 33).
excellent machinist, and a connoisseur of fine design. They set up with the help of an improbable character, a retired officer of the Italian Navy, Telesio Lucci, who volunteered his services to the Laboratory. The reinforcements from Yale left the Laboratory with the rudiments—but only the rudiments—of the instrumentation of nuclear physics. Both later returned, Cooksey for a lifetime.[103]
Disintegration at Last
Lawrence's boys—he called all his staff, including his senior Cooksey, "boy," there being no women among them—pursued instrumentation with such urgency during the summer of 1932 because of the many great novelties in nuclear physics discovered that spring. In February, Cockcroft and Walton sent to the Royal Society of London a paper describing the acceleration of hydrogen ions down an eight-foot tube connected with their voltage multiplier. They managed to get around 700 kV across the tube and a current of perhaps 10 µA down it; and they mentioned preliminary experiments on fast protons entering a chamber separated from the evacuated tube by a thin mica window. The experiments were not very interesting. Cockcroft and Walton measured the range, or distance of penetration, of the protons through various gases. Their technique for detecting protons was identical to that perfected by Rutherford in the counting of alpha particles: they looked for scintillations where protons struck a fluorescent screen placed in the experimental chamber (plate 3.7). They promised more measurements of the same character and certain improvements in the apparatus by which they hoped to coax it to its design potential of 800 kV.[104]
Here we see again the compulsion of the instrument builder: to perfect the apparatus and to make it the subject of study. For two months after completing their apparatus, Cockcroft and Walton did not search for disintegration products. It is said that they did
[103] Birge, History, 4 , xi, 14, 23–4; xii, 8, 30, 32; Kurie to Cooksey, 4 Mar 1934 (10/21); Lawrence, recommendation for Kurie, 24 Feb 1932 (Simon Flexner P, B/F365/"NRC Fellowships"); Kurie, Naval res. rev., 9:2 (1954), 13; Lawrence to D.L. Webster, 13 Apr 1934 (18/2).
[104] Cockcroft and Walton, PRS, A136 (1932), 626–9; Nature, 129 (5 Feb 1932), 242, letter of 2 Feb.
not look immediately for material products of disintegration because they expected that much or all of the energy would be carried off by gamma rays, in keeping with the widespread preconception that (as will appear) had protected Joliot and Curie from discovering the neutron. Rutherford became impatient; Cockcroft and Walton should stop playing with their protons and look for disintegration products in the old way, with a fluorescent screen.
On April 14, 1932, they placed a thin film of lithium in the experimental chamber to receive protons and saw flashes of light on a screen that caught particles liberated from the target. A few elementary precautions ruled out the possibility that scattered protons caused the flashes. Two days later they knew the cause: a lithium nucleus that captures a proton can disintegrate into two alpha particles.[105] They had reached their goal: they had split the atom, by a reaction almost the inverse of the process discovered by Rutherford a dozen years before. He had then used alpha particles from natural sources to knock protons from nitrogen nuclei; they had used protons from an artificial source to make alpha particles from lithium. "When one learns that protons and lithium nuclei simply combine into alpha particles," Bohr wrote, on hearing the news from Rutherford, "one feels that it could not have been different although nobody has ventured to think so."[106]
Cockcroft and Walton subjected various targets to beams of various energies and tracked the disintegration particles with a fluorescent screen, a cloud chamber, and an advanced electronic detector developed at the Cavendish. They found that about half their beam was protons and the rest hydrogen-molecule ions; that the threshold for disintegration of lithium was astonishingly low, 125 kV; that the number of disintegrations increased rapidly with the energy of the incident protons, there being one for a billion at 250 kV, and ten per billion at 500 kV; and that the range of the emergent alpha particles, 8.4 cm of air, was consistent with the conservation of energy and momentum, the equivalence of mass and energy, and the reaction Li7 (p,a )a .
[105] Cockcroft and Walton, Nature, 129 (30 Apr 1932), 649, letter of 16 Apr, and PRS, A137 (1932), 229; Hartcup and Allibone, Cockcroft , 50–3.
[106] Letter of 2 May 1932, in Eve, Rutherford , 356, answering Rutherford to Bohr, 21 Apr 1932 (ER).
Boron and fluorine also gave off alpha particles under proton bombardment and other elements, even silver and uranium, seemed to yield a few. As for agreement with Gamow's theory, "which was largely responsible for stimulating the present investigation," it was not at all good. The theory gave as probability for the entry of a proton into a lithium nucleus one in six at 600 kV and three in a hundred at 300 kV; measurement suggested one in a million in the first case and five in a hundred million in the second.[107] But with elements heavier than iron, theory was much more stingy than fact: there should have been nothing from silver or uranium. In Tuve's opinion, it was this disintegration of heavier elements—"by far the most striking feature . . . , a feature which is distinctly disconcerting from the standpoint of all present-day theoretical ideas of the nucleus"—that had the first claim on the attention of physicists. Cockcroft also felt the difficulty. He appealed to Gamow, who could only suppose that the silver and uranium had contained some light impurity that gave the evidence of disintegration. Cockcroft did not like to lose a discovery or yield to theory so easily. "I always believed it possible for a really good theoretical physicist to explain any experimental result and now you fail me in the first test."[108]
The Berkeley group knew about Gamow's theory by the time Livingston got the 11-inch cyclotron going. He mentioned it in his thesis as the stimulant to efforts to reach high potentials and Lawrence understood that Livingston had given him what he needed to crack nuclei. "These protons have enough energy for nuclear disintegration experiments and it will not be long probably before we are disrupting atoms. But even more exciting are the possibilities of the large magnet, which at this very moment is being installed in our new radiation laboratory."[109] The moment was January 1932, four months before Cockcroft and Walton got their first counts.
The main reason that the Berkeley group did not succeed in splitting atoms before Cambridge is that they did not try. (As
[107] Cockcroft and Walton, PRS, A137 (1932), 229–42.
[108] Tuve, JFI, 216 (1933), 28–9; Gamow to Cockcroft, 7 Sep 1932, and reply, 29 Sep 1932, in Hartcup and Allibone, Cockcroft , 55. Cf. Bloch to Bohr, 12 Aug 1932 (BSC).
[109] Livingston, Production , 4; Lawrence to Akeley, 13 Jan 1932 (1/12).
much might be said about Lauritsen, then busy irradiating cancer victims, and Tuve, who had not yet set up his Van de Graaff.) Another reason, as Lawrence freely admitted, was that neither he nor Livingston "kn[e]w very much about nuclear theory."[110] They had not even a wrong idea about what to look for. The news that lithium yielded to particles with the piddling energies attainable at Cambridge was disappointing. "When Cockcroft and Walton . . . disintegrated lithium with only a few hundred thousand volts, the thought naturally suggested itself that perhaps our efforts to obtain very high voltages were hardly worth while." But soon enough, Lawrence wrote Poillon, in a curious mixture of apology and triumph, Berkeley had results more interesting than Cambridge's.[111]
Lawrence learned while preparing for his honeymoon that he had been anticipated by Cockcroft and Walton. He wired Brady, who turned the beam of the 11-inch cyclotron onto a crystal of LiF procured from the Chemistry Department.[112] He found only a few hints of disintegration: he had too weak a current and too insensitive a detector. As late as mid August, the Berkeley group, although enriched by Cooksey and Kurie, had nothing decisive. "Unfortunately [Lawrence wrote Cockcroft] our beam of protons is not nearly as intense as yours although of higher voltage."[113] Just before the group disintegrated—Brady to Saint Louis and Cooksey and Kurie to New Haven—they got counts with a beam of 700 kV protons originating in a filament source constructed by Cooksey and detected with his Geiger counter. In September a new group—Lawrence, Livingston, White, and Henderson—took over the experiments, increased the intensity of the proton beam, and got alpha particles in numbers that agreed, more or less, with the results of Cockcroft and Walton.[114] Thus Berkeley's labor-intensive effort, extending over six months, confirmed the discov-
[110] Lawrence to Slater, 19 Feb 1932 (12/12).
[111] Lawrence to Poillon, 3 June 1933 (15/16A).
[112] Childs, Genius , 170–1, 181, 191–3; Birge, History, 4 , xi, 14, and App. xvii (c); Birge to Lawrence, 7 May 1932 (Birge P, 33).
[113] Lawrence to Cockcroft, 20 Aug 1932 (5/4).
[114] Lawrence to Barton, 8 Sep 1932 (2/25), to Cooksey, 13 Sep [1932] (4/19), and to Cottrell, 22 Sep 1932 (5/3); Cooksey and Henderson, PR, 41 (1932), 392; Lawrence, Livingston, and White, PR, 42 (1932), 150–1 (letter of 15 Sep); Kurie, Naval res. rev., 9:2 (1956), 13.
ery made by a pair of Cambridge physicists in two days. The delay was not a consequence of too many cooks at the broth, but rather of the inexperience of the Berkeley group with detectors and of the difficulty of operating the cyclotron with a steady and useful current. Cockcroft and Walton forced the Laboratory to make its accelerators into instruments of research.
The Deuteron and the Neutron
The combined performance of several generations of chemists on the balance had resulted by World War I in a detailed knowledge of the relative weights of atoms. In particular they had fixed the weight of a hydrogen atom as 1.0077 on a scale defined by taking the weight of oxygen to be 16. The recognition of the existence of isotopes around 1914 opened the possibility that hydrogen or oxygen or both might be mixtures of atoms of different weights. The point was examined by Francis Aston, whose improved mass spectrograph of 1927 disclosed that the weight of a hydrogen atom was 1.00778 (O = 16).
That appeared to be quite satisfactory until Berkeley chemists challenged Aston's assumption that oxygen has but one isotope. Soon evidence from band spectra confirmed the challenge and allowed estimates of the relative abundance of O16 and O18 in ordinary oxygen. When the heavier isotope is taken into account, the weight of ordinary oxygen exceeds that of O16 . Aston's ratio between the weights of ordinary hydrogen and ordinary oxygen, 1.00778:16.00000, could be saved by supposing hydrogen too to be complex. Berkeley's specialist on physical constants, Birge, computed the consequences of the supposition based on the relative abundances of oxygen isotopes (O16 :O18 ::630:1); and he found that everything came into order if about one hydrogen atom in 4,500 has a weight twice that of the more plentiful type.[115]
A Ph.D. from Lewis's school of thermodynamics and chemistry at Berkeley, Harold C. Urey, had a system of classifying isotopes that required the existence of deuterium—to give heavy hydrogen
[115] Aston, Nature, 123 (1929), 488; Giauque and Johnston, ibid., 318, 831; Birge and Menzel, PR, 37 (1931), 1669–71; Bleakney and Gould, PR, 44 (1933), 265-8.
the name he gave it after a struggle with his colleagues and professors of Greek that lasted longer than his search for the new element.[116] Encouraged by Birge's numbers, he undertook to separate the graver from the lighter hydrogen atoms. Thermodynamic calculations suggested that evaporating liquid hydrogen around its critical point would enrich the liquid in heavy atoms at the cost of the vapor; a still set up at the low-temperature facilities of the National Bureau of Standards by F.G. Brickwedde effected a sufficient separation to allow detection of very faint spectral lines characteristic of deuterium from a fraction of the slightly enriched liquid. The detection made a stir and brought Urey a Nobel prize. "A great scoop for America," said Aston, who, like Birge, had graciously missed the discovery. Urey and Brickwedde gave a sample of the liquid to Aston's rival, K.T. Bainbridge, who worked to more than Cavendish accuracy. Bainbridge reported a mass for deuterium of 2.0126, where O16 = 16.[117]
While Urey was evaporating heavy hydrogen, his former professor Lewis was trying to separate the isotopes of oxygen. It was tedious and unrewarding work. The separation of hydrogen isotopes, with their pronounced difference in mass, appeared easier and, what was better, "much more interesting and important." Following up an observation by E.W. Washburn, chief of the Chemical Division of the National Bureau of Standards, that the water in old electrolytic cells was richer in deuterium than tap water, and guided by his feeling for the thermodynamics at work, Lewis effected a far better separation than did Urey or Washburn and estimated relative abundance at 1:6,500 as against his former student's modest 1:30,000.[118]
[116] Among the many rejected possibilities were pycnogen, barydrogen, barhydrogen (Urey's original preference), and diplogen; correspondence of Urey and Brickwedde, May and June 1933 (Urey P, 1), and Stuewer, AJP, 54 (1986), 206-18.
[117] Urey, JACS, 53 (1931), 2872; Urey, Brickwedde, and Murphy, PR, 40 (1932), 2–3, 6–7; Aston, quoted in Science service , press release, 20 June 1933 (Lewis P); Bainbridge, PR, 41 (1932), 115; Birge to Urey, 15 Mar 1932 (Urey P), offering congratulations, "even if I should have discovered it myself."
[118] Washburn and Urey, NAS, Proc., 18 (1932), 496; Lewis and Macdonald, Jl. chem. phys., 1 (June 1933), 341–4 (rec'd 15 Apr), quote on 341. Diffusion through metals, not electrolysis, had been Lewis's first plan for separation; Lewis to Polanyi, 13 June 1933, and to E.O. Kraemer, 5 Oct 1933 (Lewis P).
Some numbers speak louder than words. In January 1933 Urey's best sample of heavy water contained only 1.2 percent deuterium oxide, about four times the concentration Washburn had managed. They had nothing better in April, when Lewis had 30 percent. "I am very curious to know how G.N. Lewis succeeded . . . with so little effort," Urey wrote his collaborator. "I suppose that he used some electrolytic method and succeeded in hitting the correct conditions exactly."[119] Urey had had no luck with electrolysis. It was uncanny the way everything that Lewis tried succeeded, although he had no surer explicit knowledge than Urey of the electrochemistry at work. On February 28, 1933, the University of California News Service announced Lewis's discovery, which the national press advertised under the whimsical headline, "Record weight of water achieved by California chemist."[120]
The news brought requests from all over for samples to use in place of ordinary hydrogen in the favorite experiments of the petitioners. One could scarcely fail to find something publishable about the behavior of a brand-new substance with a familiar chemistry but unknown physical properties. Lewis gave deuterium freely, almost as fast as he could make it.[121] Several important experiments, as well as many trivial ones, sprang from his generosity. The earliest request came from Bainbridge. Then Otto Stern telegraphed from Hamburg for ammunition for his newly perfected beam machine for measuring magnetic moments. The sample arrived at the end of July. "It was really fabulously nice of you to have answered my cry of need for isotopic water so promptly," Stern wrote, especially since their own attempts to make heavy water had failed.[122] Then there was water for electrolytic studies, for the Stark effect, for spectroscopy, for overvoltage, and for Lewis's own investigations of the physical and chemical
[119] Urey to Washburn, 17 Jan and 3 Apr 1933, quote, and Washburn to Urey, 5 Dec 1932, 15 Apr and 10 May 1933 (Urey P, 5).
[120] Fowler to Rutherford, 5 Apr 1933 (ER); Urey to Lewis, 17 Apr 1933, and Fowler to Lewis, 23 Oct 1933 (Lewis P); releases of 28 Feb and 2 Mar 1933 (Lewis P, "Science service").
[121] Lewis to Fowler, 17 Jan 1934, and to Foster, 26 Jan 1934 (Lewis P).
[122] Bainbridge to Lewis, 13 Mar 1933 (Lewis P); Frisch to Meitner, 27 Mar, 14 May, 26 Jul 1933, and to Houtermans, 24 June 1933 (Frisch P); Stern to Lewis, 7 Aug 1933 (Lewis P). Cf. J. Rigden, HSPS, 13:2 (1983), 341–4.
properties of deuterium.[123]
The experiments to conjure with came from the mating of the deuteron and the accelerator. As Fowler watched a 50 percent solution of heavy water brew in Lewis's still, the Radiation Laboratory was planning "to repeat Cockcroft and Walton on Li and B with H particles [deuterons]—also to make He4 by shooting H2 at OH2 H2 ice. All nice and wild of course but exciting." With characteristic openness and generosity, Lewis sent Rutherford "some heavy water to play with," although the Cavendish physicists, with Lauritsen and Tuve, were the world's only competitors of the Berkeley bombardeers.[124] Their competition proved most fruitful.
The deuteron immediately attracted sustained theoretical interest. It was to nuclear physics what the hydrogen atom was to the old quantum theory: the simplest system available for calculation and experiment. According to the systematics of isotopes from which Urey had drawn evidence for the existence of deuterium, the deuteron should have consisted of two protons and an electron. Just before he announced his discovery, however, another intervention from Cambridge simplified the picture. The long-sought neutron, which Rutherford had believed in and which his students had looked for for a decade and more, had at last put in an appearance.[125]
The course of its discovery contains several points of general interest. To go back no further, in 1930 two physicists in Berlin, Walther Bothe and Hans Becker, discovered that beryllium gives off very penetrating rays, more penetrating even than the hardest gamma rays from radium, when hit by alpha particles from
[123] Requests from, resp., T. Heyrovsky (Prague), 24 May and 6 Jul (1933); J.S. Foster (Montreal), 7 Jul 1933, and Lewis's reply, 26 Jan 1934; L. Wertenstein (Warsaw), 8 Nov 1933; and T.M. Lowry (Cambridge), 12 Jan 1934 (Lewis P); Cabrera and Fahlenbrach (Madrid), Soc. esp. fis. quim., Anales, 32 (1934), 538–42 (magnetic susceptibility); Lewis et al.'s notes in JACS, 55 (1933), e.g., 3503–4 (biochemistry of heavy water), 3057–9 (density), 4730–1 (viscosity and dielectric constant). Cf. "Uses of deuterium" [1934] (Urey P, 5).
[124] Fowler to Rutherford, 22 Mar and 5 Apr 1933 (ER); J.A. Fleming to H.S. Taylor, 4 Jan 1934 (MAT, 13/"lab. letters").
[125] For prehistory, see Kröger, Physis, 22 (1980), 184–90, and Langer and Rosen, PR, 37 (1931), 1579–82, who assimilated a hypothetical neutron to a collapsed hydrogen atom, and the energy of collapse to Millikan's cosmic radiation.
polonium.[126] They supposed that this "beryllium radiation" consisted of gamma rays, for which, indeed, they had been looking. The discovery interested Irène Curie, who worked in her mother's Institut du radium in Paris, where there was more polonium, and less physics, than in Berlin or Cambridge. At the end of 1931 Curie had found beryllium rays so penetrating that, were they gamma rays, as she assumed, their energy would have been between 15 and 20 MeV, three times that of the alpha particles that brought them forth.
Meanwhile a student of Chadwick's at the Cavendish had found that the beryllium rays emitted in the direction of the incident alpha radiation were far more plentiful than those emitted in the opposite direction, an asymmetry suggestive of the collision of particles. As this asymmetry came to light in Cambridge, Curie and Joliot found that when they placed a screen of wax in the path of the beryllium radiation, a detector behind the screen registered protons with energies of around 4.5 MeV. That caused them to increase their estimate of the power of the radiation: were it electromagnetic and obedient to the equations of the Compton effect, it would require about 50 MeV to liberate protons of 4.5 MeV. The discrepancy of a factor of three in their estimates of the energy of the radiation did not encourage belief in their interpretation of its nature.[127]
In February 1932, a month after this second set of results from Curie's institute had come to hand, Chadwick declared the discovery of the neutron. A colleague, Norman Feather, had scavenged enough polonium from old radon tubes (mainly from the Kelly Hospital in Baltimore) to make a source nearly as powerful as the Parisian one. Excellent electronic detectors stood ready. With all this it was not difficult to show that the beryllium radiation knocked protons from other materials than wax and that everything made sense if it consisted of neutral particles of mass close to the proton's. As Fowler summed up for the jury of
[126] For the following story and references, see Feather in Hendry, Cambridge physics , 31–41; Badash, AJP, 51 (1983), 886; and the recent careful study by Six, Rev. d'hist. sci., 55 (1988) , 8–12, 18–22.
[127] Frisch to Meitner, 6 Mar 1932 (Frisch P), points to the factor of three as bothersome but "not so tragic" as the assumption by Curie and Joliot of an implausibly improbable mechanism, a Compton effect involving an entire atom.
theorists: "It seems to be absolutely correct and the case is good enough to hang anyone on, but perhaps not yet scientifically certain."[128]
By the time the news reached Berlin, Bothe and Becker had found that beryllium does emit gamma rays under alpha bombardment, but of a much more modest energy, some 5.1 MeV, than Curie and Joliot required. (In fact the energy is more modest still, since the gamma ray comes from the decay of an excited state of C12 , some 4.4 MeV above the ground state.) Thus in a year and a half the unknown beryllium radiation, which physicists first tried to absorb in a way that did the least damage to received ideas—hence problematic gamma rays rather than an entirely new particle—was unravelled to yield an important discovery and a warning about the complexity of nuclear reactions.
Because of its complexity, the Bothe-Becker radiation was not adapted to a clean determination of the neutron's mass. Chadwick therefore had recourse to the transformations of boron and lithium, which he supposed to occur as follows: B11 (a ,n)N14 and Li7 (a ,n)B10 . Chadwick set the velocity with which the neutrons leave the interaction equal to the maximum velocity of the protons they knocked out of the paraffin screen. With this assumption and the known energy of alpha particles from polonium, conservation of momentum gives the energy of the recoil atoms and conservation of relativistic energy the mass of the neutron. For the latter calculation the isotopic masses of the participating atoms must be known accurately. Here the then recent—and subsequently invalidated—work of Aston was essential. From the first transformation, of boron, mn = 1.0066 (O = 16) with a probable error of 0.001. From the second, of lithium, for which the neutron's velocity had not been measured precisely, mn = 1.0072. Hence the sum of the masses of neutron and proton (Aston's value) appeared to be 1.0072 + 1.0078 (mp ) - 0.0005 (me ) = 2.0139, or some 0.0013 mass units greater than Bainbridge's value for the mass of the deuteron. On the natural supposition
[128] Fowler to Bohr, 1 Mar 1932 (BSC); Curtis Bowman, Kelly Hospital, to Tuve, 10 Sep 1931 (MAT, 4). Cf. Segrè, Fermi , 69–70, on the independent unpublished theoretical discovery of the neutron by Ettore Majorana.
that d = p+n, the deuteron would have the meager binding energy of just over 1 MeV; should it consist of two protons and an electron, it would be more securely bound, by 0.0025 mass units or 2.5 MeV.[129]
Although, as soon appeared, Chadwick's estimate was based on a mistaken reaction and inaccurate isotopic masses, its consequence, that the deuteron possesses a positive binding energy, however meager, seemed an essential postulate. How else could the deuteron and nuclear physics hold together? Whatever one's views about the nature of the neutron—whether it is simple and singular, or a tight union of a proton and an electron—its weight could not be much less than the proton's if they were to join together in a stable deuterium nucleus.[130] Against the Cavendish's heavy neutron and its secure deuteron, Lawrence and his Laboratory placed a light neutron and an unstable nuclear physics.
Scurrying for Position
The discoveries of the neutron and deuteron and the demonstration of nuclear disintegration gave the few laboratories in the world able to do "high-energy physics" great scope for maneuver. They chose according to the opportunities afforded by their machines and circumstances, and by the temperaments of their directors. The Cavendish decided to work accurately and slowly, with very light elements and very modest energies. Carnegie's Department of Terrestrial Magnetism (DTM) worked from Cavendish energies up to a million volts or so and also concentrated on lighter elements; they sought to make their beams as homogeneous and their targets as clean as possible to provide a firm basis for further work. The Kellogg Laboratory at Caltech worked with a similar energy range, but not so energetically as the
[129] Chadwick, PRS, A136 (1932), 692, and Solvay, 1933, 100–2. Reviewing other evidence in February 1933, Bainbridge put the neutron's mass between 1.0057 and 1.0063; Bainbridge, PR, 43 (1933), 367–8, and PR, 41 (1932), 115.
[130] Theorists only gradually came to agree on the elementary character of the neutron, which, however, experimentalists appear to have assumed almost from the start. When theorists agreed to regulate the relations between neutron and proton by Fermi's theory of beta decay, they could not admit a neutron lighter (and hence stabler) than the proton. But at first this difficulty did not bother many people. Cf. Chadwick, Solvay, 1933, 102–3, and Bromberg, HSPS, 3 (1971), 309–23.
DTM. The Radiation Laboratory at Berkeley played its long suit, went to higher energies and heavier targets as quickly as it could, experimented sloppily, and opened and discovered vast new territory. Outclassed for exact measurement by high-tension machines with straight tubes and relatively strong currents, the cyclotron could compete only by shooting faster projectiles than the opposing artillery could field.
Following the detection of disintegration at Berkeley at the end of the summer of 1932, Lawrence's group, which then consisted of himself, Livingston, and White, thought that their minuscule beam of protons, a mµA (a billionth of an ampere), liberated more alpha particles from lithium than Cockcroft and Walton had reported.[131] This cornucopia, sanctioned by Oppenheimer's calculations from Gamow's theory, turned out to be a mistake, an error of reckoning, as Cockcroft observed, by a factor of sixty. With proper arithmetic, the Berkeley results came into rough agreement with Cambridge's. Meanwhile Henderson had taken over the 11-inch cyclotron and Cooksey's Geiger counter. He drove the measurements up to the energy limit, with protons of 1.23 MeV, and made a discovery: the probability of interaction of a proton with a lithium nucleus does not increase with energy of bombardment above 400 keV.[132]
Early in the new year, White came back on the machine, put boron in place of lithium, got a surprisingly high yield, and was anticipated by Cockcroft and Walton.[133] Then Livingston joined the game, with Lawrence. They took on aluminum, which, they reported, gave off alpha particles and, like lithium and boron, had an energy threshold above which the probability of proton absorption became constant. But they had run too fast and taken analogy, rather than Cockcroft and Walton, as their guide. Aluminum's "alpha particles" turned out to be soft x rays. "We are finding it a dickens of a job to make sure whether radiations are protons or alpha particles or gamma rays or the Lord knows
[131] Lawrence, Livingston, and White, PR, 42 (1932), 150–1.
[132] Cockcroft to Lawrence, 3 Oct 1932, and Lawrence to Cockcroft, 4 Nov 1932 (5/4); Henderson, PR, 43 (1933), 98–102 (letter of 10 Dec 1932).
[133] Lawrence to Henderson, 7 Jan 1933 (9/6); Cockcroft and Walton, Nature, 131 (7 Jan 1933), 23 (letter of 22 Dec 1932); White and Lawrence, PR, 43 (1933), 304–5 (letter of 27 Jan).
what," Lawrence had written Boyce a week before sending his misidentification of the aluminum x rays into print.[134] The error probably came to light when Livingston started "disintegrating 'to beat hell'" with the 27-inch cyclotron. In January 1933, having figured out how to shim the magnet, he knocked apart lithium and carried Henderson's curve up to 1.5 MeV.[135] But from aluminum the most conspicuous products were soft x rays.
For a month or so in February and March 1933, Lawrence believed that the initial era of machine design at the Laboratory had ended. "Therefore for some time in the future [we] shall devote most attention to the experimental study of the nucleus rather than the development of experimental technique." He was not looking forward to it. "We have decided not to be in any hurry about it and have settled down to patient, painstaking experimental study."[136] They were released from this uncongenial line of work by Lewis's method of collecting heavy water.
Tuve followed up the earliest of the discoveries, that of the neutron, as soon as he heard about it. He tried to assemble a polonium source equivalent to Chadwick's, but found it hard going. Feather had left few old radon tubes on the East Coast and was asking for more. "I have the greatest respect and warm regard for Cambridge physicists, and feel like a small boy speaking up in church when I deflect anything toward myself that might have helped them," Tuve wrote the radiologist at Kelly Hospital, insisting, however, that charity begin at home. His eagerness to confirm and extend Chadwick's discovery contrasts with Lawrence's initial reaction, that the neutron "has no particular effect on our experiment excepting to emphasize that there is a fascinating world of phenomena that will be accessible when we will have developed our method for producing swiftly moving protons."[137]
[134] Livingston and Lawrence, PR, 43 (1933), 369 (letter of 11 Feb); Lawrence to Cooksey, 2 Feb and 18 Mar 1933 (4/19), to Barton, 13 Mar 1933 (2/25), and to Boyce, 2 Feb 1933 (3/8); cf. McMillan and Lawrence, PR, 47 (1935), 348.
[135] Lawrence to Cooksey, 11 Dec 1932 (4/19), quote; to Henderson, 7 Jan 1933 (9/6). Livingston was using hydrogen-molecule ions, which he could get up to 4 MeV by February 1933, as projectiles; Lawrence to Cooksey, 2 Feb 1933 (4/19).
[136] Resp., Lawrence to G.G. Kratschmar, 9 Feb 1933 (10/8), and, almost verbatim, to Kurie, same date (10/21); Lawrence to Barton, 13 Mar 1938 (2/25).
[137] Tuve to F.W. West, 4 Mar 1932 (quote), and to G. Failla, Memorial Hospital, New York, 29 Feb 1932 (MAT, 4); Lawrence to G.W. Cattell, 16 Mar 1932 (25/2).
Tuve's group assembled enough polonium to develop a technique for detecting neutrons with their linear amplifier and improved cloud chamber. When the Van de Graaff came on line, Tuve searched for "artificial neutrons" using protons and alpha particles as projectiles, but found very little even at the maximum potential, some 750 kV, that he could reach in the spring of 1933. Nevertheless, he expected that even at moderate energies a Van de Graaff generator would be a very spectacular neutron source. On Gamow's theory, each µA of alpha particles on beryllium would give only 6 percent of Chadwick's yield (from 5.2-MeV polonium rays) at 800 kV; but twice that yield at 1,200 kV, 15 times it at 1,600 kV, over 50 times it at 2,000 kV.[138] Lauritsen's group checked the prediction as far as they could go; at 950 kV and 30 µA , their tube put out neutrons as copiously as the largest polonium source in use anywhere. Then they tried the same experiment with heavy water from Lewis. This time the artificial output exceeded the natural a hundredfold.[139] Simultaneously Lawrence was obtaining large yields of neutrons from deuterons shot at beryllium by the cyclotron.
When the 1-meter Van de Graaff started working, Tuve repeated Cockcroft and Walton's experiments using the target and detection scheme indicated in figure 3.5. He and his group confirmed the presence and the ranges of the alpha particles reported in the (p,a ) reactions investigated at Cambridge.[140] They then began a series of painstaking bombardments of light elements by protons that led them to a fine discovery. These measurements determined the "excitation function," the yield of a nuclear reaction as a function of the energy of the incident particles. As far as they could go, they found all Berkeley's thresholds wrong.[141] That was not their fine discovery. Their strong, homogeneous beam made possible detection of narrow reaches of energy at which
[138] Tuve to Lauritsen, 29 Mar 1933 (Lauritsen P, 1/8); Tuve, JFI, 216 (1933), 36–8.
[139] Crane, Lauritsen, and Soltan, PR, 44 (1933), 514, letter of 3 Sep, and ibid., 692–3, letter of 30 Sep.
[140] Tuve to Lawrence, 24 and 30 Jan 1933 (MAT, 4).
[141] Fleming to Robert D. Potter, New York Herald Tribune , 20 Jan 1933 (MAT, 8); Tuve and Hafstad, PR, 45 (1934), 651–3.
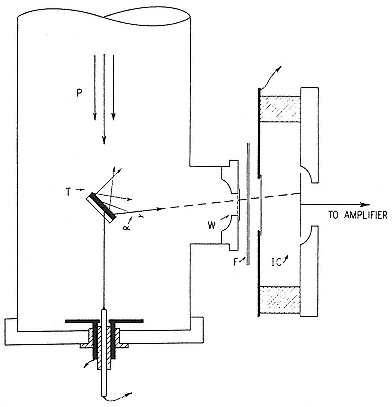
Fig. 3.5
The method of provoking and detecting nuclear reactions at the Carnegie
Institution. P is the incident proton beam; W, a window closing the
vacuum space; F, an absorber; IC, an ionization chamber. Tuve,
JFI, 216 (1933), 32.
impacting protons are particularly effective in exciting or disintegrating their targets. They found this "resonant excitation" in a characteristic way, by trying to clear up discrepancies between their excitation functions for carbon and Lauritsen's.[142] En route, they provided strong evidence that the apparent disintegrations of elements of medium atomic weight reported by Cockcroft and
[142] Tuve to W.H. Wells, 7 Feb 1935 (MAT, 14/"lab. letters"); Hafstad and Tuve, PR, 48 (1935), 306–9; Breit, RSI, 9 (1938), 69–70; Abelson, PT, 35:9 (1982), 92. Cf. Ofstrosky and Breit, PR, 49 (1936), 22.
Walton—the disintegrations that Tuve had regarded as eminently subversive of theory—originated, as Gamow had suggested, in contamination of the targets.[143] Their view proved correct.
Berkeley experimenters contributed little to this line of work or to the precise determination of the energies involved in nuclear reactions. These energies, measured primarily at the Cavendish and at Caltech, concerned hydrogen, helium, lithium, beryllium, and boron. The measurements established the basis of a system of isotopic masses more accurate than Aston's. One outcome of this system, which constantly underwent revision during the 1930s, was Hans Bethe's theory of the source of the sun's heat. None of this painstaking work will engage us further.[144] It was not the sort of thing that Lawrence's boys did.[145] As Bethe said of another sort of demanding measurement of importance in nuclear physics, the dependence of the range of a fast particle on its energy, "it is not likely to be solved in the ordinary course of research because it is too complex for a graduate student and would involve too much time for a more advanced scientist."[146]
Accuracy is not everything, even in science. In the first two years of its existence, the Rad Lab built a machine that outdid all others in the acceleration of charged particles. It made the most powerful commercial x-ray tube in the world. It developed a linear accelerator. It received national attention. The dilution of effort and the demands of institution building resulted in work that was scarcely fastidious. But they did not cost Lawrence what he valued most: victory in the race for high energy.
[143] Hafstad to Ladenburg, 8 June 1933 (MAT, 8).
[144] Bainbridge, PR, 44 (1933), 123; Bethe, correspondence with Bainbridge, Bonner, and Brubacker, and Cockcroft, 1935–37 (HAB, 3); Oliphant, Kempton, and Rutherford, PRS, A149 (1935), 406–16, and PRS, A150 (1935), 241–58, in Rutherford, CP, 3 , 397–406, 407–23; Bonner and Brubaker, PR, 49 (1936), 19–21; Oliphant, PT, 19:10 (1966), in Weart and Phillips, History 185–6.
[145] An exception is Lawrence, Henderson, and Livingston, PR, 46 (1934), 324–5.
[146] Bethe to Miss Skinner, American Philosophical Society, 23 Jan 1937 (HAB, 3).