California Riparian Forests
Deciduous Islands in an Evergreen Sea[1]
Glen Holstein[2]
Abstract.—California riparian forests are dominated by deciduous trees and are thus anomolous in a state where most dominant woody plants are evergreen. Riparian zones provided refuges where riparian elements of the Arcto-Tertiary Geoflora could survive when its upland elements were decimated by the development of California's mediterranean-type climate. Water and nutrients imported to California's dry lowlands from wetter mountains by perennial streams permit high summer primary productivity in riparian communities while adjacent upland vegetation is severely drought stressed. High riparian productivity makes the cost of annual replacement of deciduous foliage affordable because such foliage is more photosynthetically efficient than that of evergreen upland dominants. Bird abundance and diversity in riparian communities are related to this high riparian productivity.
Introduction
Much of California has a mediterranean-type climate. In such climates rainfall and snowfall are maximal in winter, when minimal solar radiation limits plant growth. When the long days of summer potentially maximize growth, rainfall is minimal or nil and many plants are dormant or under severe drought stress. Thus moisture and solar radiation, two necessities for plant growth, are exactly out of phase (Major 1977).
Even near the moist northwest coast of California, fields of annual grasses in the hills above Redwood National Park (Humboldt County) are dead by late summer. Summer drought is also a major factor contributing to the uniqueness of California's alpine flora (Chabot and Billings 1972). Only at a few desert localities in California do some summer months have more rain than any single winter month, but here rainfall is so scanty and unpredictable at all times that vegetation is sparse and the flora limited to specialized drought resisters or evaders.
Most California vegetation is maximally productive in spring, when days are longer and warmer than in winter, and some moisture is still available. Stressful winter and summer conditions are thus both avoided. Productivity is less, however, than in ecosystems where light, warmth, and water are all simultaneously available in abundance. The productivity potential which is frequently unfulfilled in California because of summer drought stress is revealed by the increase in crop yield obtained there with irrigation, and by the productivity of the riparian vegetation which lines or once lined perennial streams. These streams carry the part of the winter water surplus which is slowly released from deep aquifers and melting mountain snow, making it available to lowland riparian vegetation in summer when little water is provided by the local climate. The resultant greater productivity and biomass of this vegetation is frequently obvious when contrasted with that of nearby communities which lack imported water. Riparian forests in central Asia ecologically similar to those of California's Central Valley are among the world's most productive natural ecosystems (Major 1977). When the current vacuum in California riparian research is filled, it is likely that riparian systems here will be found to be comparably productive.
Biogeography of Riparian Forest Components
Axelrod (1973) has provided compelling paleobotanical evidence that California's mediterranean-type climate is a relatively late phenomenon which first appeared in the upper Pliocene. This and other climatic perturbations caused the Arcto-Tertiary Geoflora, a zone of rich and diverse forest which was once continuous around the Northern Hemisphere, to retreat and become impoverished. Destructive impoverishment
[1] Paper presented at the California Riparian Systems Conference. [University of California, Davis, September 17–19, 1981].
[2] Glen Holstein is Lecturer, Botany Department, University of California, Davis.
of the Arcto-Tertiary Geoflora by spreading drought and cold was severe in western North America and Europe, but many of its elements survived in major refuges in eastern Asia, the Pontic region of southwest Asia, the Mexican highlands, and the southeastern United States. California's expanding mediterranean-type climate caused the replacement of many Arcto-Tertiary communities by drought-resistant vegetation known as the Madro-Tertiary Geoflora, which had long been adapted to local dry habitats (Axelrod 1975).
Riparian forests, as ecosystems in but not under the control of a mediterranean-type climate, seem likely refuges for Arcto-Tertiary elements within California, and Robichaux (1977) has shown in a review of their fossil record that most dominant California riparian forest taxa have modern ranges reduced from more widespread Tertiary distributions. These dominants all have relatives which are common in the Arcto-Tertiary derived deciduous forests of eastern North America (Axelrod 1960), and their dominance by deciduous trees and shrubs gives these vegetation types a similar aspect. Examination of the evolution and biology of the taxa dominant in California riparian forests provides further clues to the origin, evolution, and relationships of western North American riparian vegetation.
Acer (Maple)
This large genus, with 200 species of mostly deciduous trees, is one of the most important components of the temperate deciduous forests of the Northern Hemisphere, and its modern range coincides closely with those communities which are predominantly derived from the Arcto-Tertiary Geoflora (Hora 1981). It is by far the largest of the two genera in the Aceraceae and is the only one occurring naturally in North America, where it includes major upland dominants such as A . saccharum and widespread riparian species such as A . saccharinum and A . negundo .
The Aceraceae are part of the Sapindales (Cronquist 1968; Dahlgren 1975) or the essentially equivalent suborder Sapindineae of Thorne (1976), taxa which are otherwise largely dominated by entomophilous, evergreen, or drought-deciduous tropical to subtropical woody plants with compound leaves. The Hippocastanaceae are probably the closest relatives of the Aceraceae, and both of these families are unusual within the Sapindales because of their winter dormancy and largely north temperate distributions.
Acer consists mostly of winter-deciduous trees, but it is otherwise quite diverse and includes morphoclines both from compound to simple leaves and from flowers which are corollate and entomophilous to those which are reduced, apetalous, and wind pollinated. In both cases these cines reflect a shift from the primitive Sapindalean condition to a derived condition typical of the majority of dominant north temperate forest trees.
The four California species of Acer , A . glabrum , A . circinatum , A . macrophyllum , and A . negundo var. californicum , all can occur along streams, but only A . negundo (box elder) is primarily riparian. The other species are more common in mesic upland sites in the wetter parts of montane and coastal California, where gradients between riparian and upland vegetation are much more diffuse and less distinct than in the drier areas of the state.
A . macrophyllum is a particularly common and important tree throughout much of coastal and montane California, and it is listed by Roberts etal . (1977) along with Sequoiasempervirens , Umbellularia californica , and several more strictly riparian species as one of the common trees of California's north coastal riparian forests. In this region A . macrophyllum , S . sempervirens , and U . californica all occur from streambanks to the shaded, moist upland sites where they are most abundant.
A . negundo is frequent in riparian zones throughout the Mississippi basin and the Great Plains. Locally, it extends to the Atlantic coast and occurs along scattered streams and rivers in the southern Rocky Mountains, the Southwest, and California (fig. 1). Few other North American trees are transcontinental.
Within California A . negundo is locally common in riparian communities in the drier parts of the Coast Ranges and in the lower parts of the Sacramento and San Joaquin Valleys, where marine airflow through the Carquinez Straits somewhat moderates summer temperatures. Virtually nowhere in California, however, is it dominant. It is
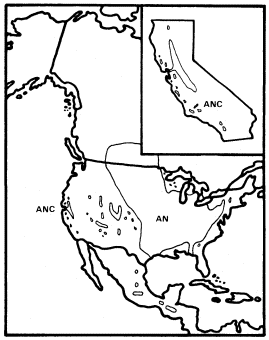
Figure 1.
Range of Acer negundo (AN) including A . negundo
var. californicum (ANC) (after Little 1971).
normally a shade-tolerant subordinate tree in dense riparian vegetation dominated by Populus fremontii , P . trichocarpa , Salixgooddingii , S . laevigata , S . lasiandra , or S . lasiolepis . Association with P . fremontii is especially frequent.
Dispersal of A . negundo is by wind dispersed samaras, but these are produced in smaller quantities and are less easily dispersed than the lighter, comose seeds of Salix and Populus . As a result, box elder is less efficient at colonizing the new riparian habitats which are frequently created on sandbars and along ditches and canals.
A . negundo appears to be in decline in California since it is a relatively poor competitor which has been restricted to the most highly competitive riparian zones. It may now be at an early stage of the process by which climatic vicissitudes eliminated (e.g., A . saccharinum and Ulmusamericana ) (Axelrod 1977) or almost eliminated (e.g., Juglanshindsii ) other riparian taxa from California whose relatives are still common in the much more extensive riparian systems of eastern North America, which receive summer rain.
A . negundo is taxonomically isolated among North American maples since it is the only member of section Negundo on this continent. This section is distinct enough to be segregated as the genus Negundo Boehm. by some (Willis and Airy Shaw 1973). Its other species are Asian, and it combines the putatively primitive character (within Acer ) of compound leaves with dioecy and inflorescences which are apetalous and anemophilous in A . negundo but corollate in at least some Asian species (Rehder 1940).
Alnus (Alder)
Alnus is a morphologically homogeneous genus of 35 species of deciduous trees and shrubs of the Northern Hemisphere and the Andes, and it has monoecious, anemophilous catkins and nutlets which vary among species in the degree of development of marginal wings and the resultant relative importance of wind, water, and gravity dispersal (Sudworth 1908; Fowells 1965).
It shares a distinctive pattern of ecological adaptation with some other important California riparian genera like Salix . These consist primarily of large, obligately riparian trees in warm temperate climates but are increasingly dominated by widespread shrubs which are only facultatively riparian in colder boreal and montane regions. Unlike other genera with large California riparian trees, however, Alnus contributes no important trees to North America's eastern deciduous forest. The commonest alder there is the shrubby A . serrulata . The adaptations needed by alders which are temperate riparian trees and those which are boreal and montane shrubs may not be greatly different since snowmelt frequently saturates soils of cold regions during the brief growing season and creates conditions similar to those found only along streambanks and lakeshores in warmer climates. Alders can symbiotically fix nitrogen, which otherwise may be limiting in forest environments and elsewhere (Spurr and Barnes 1980).
Alnus is a member of the Betulaceae, a family of mostly north temperate deciduous and anemophilous trees and shrubs which is in the Fagales (Cronquist 1968; Dahlgren 1975; Thorne 1976), an order it shares with the Fagaceae, to which it is linked by several intermediate genera (Corylus , Carpinus , Ostrya , and Ostryopsis ) sometimes placed in Betulaceae and sometimes in the segregate families Corylaceae and Carpinaceae (Willis and Airy Shaw 1973). The Fagales are the most important single order of angiosperm trees in temperate regions and most member taxa have Arcto-Tertiary distributions.
Alnus has four California species: A . sinuata , A . tenuifolia , A . rubra , and A . rhombifolia . The first two are largely shrubs of the boreal/montane type mentioned previously, but they can occasionally grow large enough along streams to be riparian trees (Sudworth 1908). The second two are among California's most important riparian trees. All California alders are in subgenus Alnus except A . sinuata which is in Alnaster .
Alnusrubra (A . oregona ), the red alder, is associated with the North Coastal Coniferous Forest (Munz 1959) from the Alaska panhandle to the coast of San Luis Obispo County (Little 1971; Griffin and Critchfield 1972). Within its range (fig. 2) it is frequently the dominant riparian tree. This largest of American alders (Elias 1980) is also very common on moist slopes, especially after conifers have been removed by logging operations, but it is much more likely to form dense and distinctive riparian gallery forests which it overwhelmingly dominates than are trees such as Acermacrophyllum , which are also found from moist slopes to streamsides. A fine example of such a gallery forest is protected along Prairie Creek in Humboldt County at Prairie Creek Redwoods State Park.
Alnusrhombifolia (white alder) forms similar gallery forests throughout much of the rest of California south and east of the range of A . rubra (fig. 2), but it much more obligately restricted to streamsides than its coastal relative. As a result, it is the most reliable indicator of permanent water among California's riparian trees (Jepson 1910). A . rhombifolia is the usual dominant in California's montane riparian forests up to about 1,600 m., but it also is dominant near sea level along Alameda Creek in Alameda County's Niles Canyon and numerous other similar places. It is most common along fast-flowing mountain streams west of the crest of the Sierra Nevada and near the coast south of Sonoma County (Griffin and Critchfield 1972). White alders are absent from much of the Central Valley floor but are common along the Sacramento River in Shasta County (ibid .) and further south (Conard et al . 1977). Such
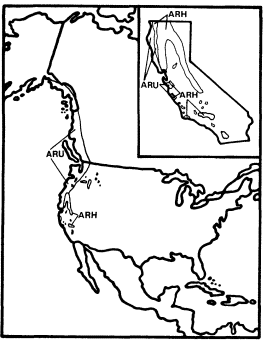
Figure 2.
Ranges of Alnus rubra (ARU) and A . rhombifolia
(ARH) (after Little 1971, 1976).
montane-coastal distributions suggest intolerance for summer heat (e.g., Populustrichocarpa ), but this is unlikely in the case of A . rhombifolia since it is common in the vicinity of Redding, where summer temperatures are as high or higher than in most of the area where it is absent. The ecological factor which most controls the distribution of A . rhombifolia seems to be a need for constant saturation of its root zone by cool, well-aerated water.
The total range of A . rhombifolia extends from southern California to central Washington in the Peninsular, Transverse, Coast, Sierra Nevada, Klamath, and Cascade ranges, with an extension through the Columbia River Basin to northwestern Idaho (Little 1976). It is most common and its range most continuous in the Sierra Nevada, Klamath Mountains, and northern Coast Ranges of California. A . rhombifolia and A . rubra are morphologically similar and closely related, but hybrids between them do not seem to have been reported. They apparently diverged from a common ancestor at an unknown time in the Tertiary or Quaternary Periods and adapted to wet and riparian sites within the Sierran-Klamath and north coastal forests, respectively.
Betula (Birch)
Betula is the second genus in the Betulaceae when that family is narrowly defined to exclude the Corylaceae and Carpinaceae (Willis and Airy Shaw 1973). Its characters are similar to those of its sister genus, Alnus , which it resembles in its deciduous habit, its anemophilous catkins, its north temperate range, and its nutlets, which are more consistently winged and wind dispersed than those of Alnus . Betula also shares a similar range of adaptations with Alnus since it includes both temperate zone trees and arctic and montane shrubs, but Betula contributes many more important upland trees to the deciduous forests of eastern North America than Alnus , which is more important as a source of dominant trees in western riparian forests than Betula .
California has two species of Betula : B . occidentalis (B . fontinalis ) and B . glandulosa , but only the former reaches tree size. B . occidentalis (water birch) is a large shrub or small tree of riparian sites which is widespread in the cordilleran region of western North America (Little 1976) but is restricted to just a few parts of California. It is relatively frequent in the Klamath Mountains and on the east slope of the southern Sierra Nevada, but much less so in the Warner, White, and Panamint Mountains (fig. 3). The Klamath and Warner Mountains have an abbreviated summer drought because of their northern locations, and the southern Sierra Nevada's east slope and the White and Panamint Mountains all regularly receive summer thunderstorms of tropical origin. As a result, all California populations of B . occidentalis receive quantities of summer rain which are unusual for that state and which approach the greater amounts received by the much larger populations in states to the east and north. Consequently, lack of summer rain must be suspected as an ecological factor limiting the range of this species despite its adaptation to riparian zones. Non-riparian Pinus balfouriana and other taxa have similar distributions for similar reasons (Raven and Axelrod 1978). Seedlings can be much more sensitive than mature
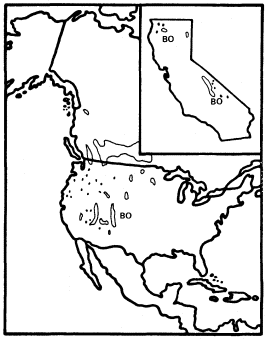
Figure 3.
Range of Betula occidentalis (BO) (after Little 1976).
plants to environmental stresses like summer drought (Grime 1979).
B . occidentalis is in series Albae within Betula (Rehder 1940) and is thus a close relatie of the white briches such as B . papyrifera , B . pendula , and B . pubescens , which are very important early successional trees throughout the upland boreal forests of North America and Eurasia.
Cephalanthus (Button Bush or Button Willow)
Cephalanthusoccidentalis , an obligately riaprian small tree or shrub, is the single California representative of this genus of 17 species which is widespread in the warm regions of the world and is one of only three California genera in the very large (500 genera and 7,000 species) family Rubiaceae, best known in temperate regions for the large and usually herbaceous genus Galium . Most of the Rubiaceae, however, are understory trees and shrubs in tropical forests. The family is clearly of tropical derivation and its placement in the Gentianales by Dahlgren (1975) and Thorne (1976) and in the related Rubiales by Cronquist (1968) reflects considerable consensus concerning its evolutionary relationships. Cephalanthus is in the subfamily Cinchonoideae, which is largely woody and tropical, rather than in the Rubioideae, which includes most of the family's temperate herbs and its other California genera.
C . occidentalis is primarily a deciduous shrub and only rarely reaches tree size in California. Its flowers are small but corollate and probably entomophilous like those of most Rubiaceae, and the fruit is a dry schizocarpic mericarp which lacks obvious adaptations for dispersal.
Like A . negundo , C . occidentalis is found naturally in both Atlantic and Pacific coast states (fig. 4). It is widespread in the East and ranges south through Mexico to Honduras, but it is restricted to a few Arizona stations and to the floor and adjacent watershed of California's Central Valley in the West (Little 1976). In California and in most of the rest of its range it is limited to areas with mean July temperatures above 20 C where most of the root zone is reliably saturated with water throughout the year. Relatively poor dispersal has made it an inefficient colonizer of the banks of artificial ditches and canals, but it is still common along many permanent natural streams. In backwaters where still, poorly oxygenated water stands throughout the year, C . occidentalis is best developed and can be dominant (Conard etal . 1977), but such habitats in California have been almost entirely destroyed by water resource and agricultural development. Their Button Bush Swamp Forest vegetation type is thus among the rarest and most endangered in the state. A particularly fine example of this vegetation is still extant along the Cosumnes River in southern Sacramento County west of Galt, and its continued preservation should receive high priority from California's conservation community.
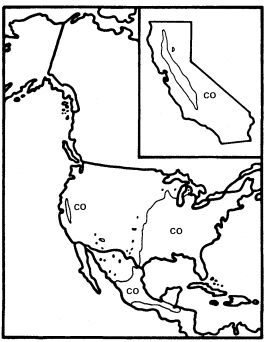
Figure 4.
Range of Cephalanthus occidentalis (CO) (after Little 1976).
It is clear that C . occidentalis in California is a relict which has survived the loss of a warmer and wetter climate because of the fortuitous juxtaposition of the hot Central Valley and the high mountains which surround it and keep it continuously supplied with abundant water.
Fraxinus (Ash)
This genus has a range which matches almost exactly that of the Arcto-Tertiary Geoflora, and it shows a range of adaptations including deciduousness, anemophilous catkins, and wind-dispersed samaras which is typical of the flowering trees in the modern forests derived from it. Fraxinus clearly evolved these characters by convergence, however, since its probable ancestors had few if any of them.
It is a member of the Oleaceae, whose placement in the Oleales by Dahlgren (1975) and Thorne (1976) and in the Scrophulariales by Cronquist (1968) only hints at the lack of consensus among plant evolutionists about its origin. Most other members of the family have entomophilous flowers with well-developed corollas, and many are tropical species with evergreen leaves and fleshy fruits adapted to internal dispersal by birds. Fraxinus itself includes a floral reduction series which suggests the mode of evolution of its apetalous, wind pollinated catkins since several species along the southern periphery of its range, including F . cuspidata in the southwestern United States and northern Mexico and F . ornus in southeastern Europe, have
fragrant entomophilous flowers with conspicuous corollas. Evergreen leaves are less common, but they occur in F . gooddingii of southern Arizona and northern Sonora (Elias 1980).
Most ash species like F . cuspidata and F . ornus which have primitive characters typical of the Oleaceae are included in section Ornus . Section Fraxinaster , however, includes many species of upland (F . americana , F . excelsior , F . quadrangulata ) and riparian (F . nigra , F . pennsylvanica ) trees which are important in north temperate deciduous forests and share many characters with the trees most highly adapted to that ecosystem in other families (Rehder 1940).
California is usually considered to have four Fraxinus species (F . dipetala , F . anomala , F . latifolia , and F . velutina ) (Munz 1959). F . dipetala is interesting among these as a Fraxinaster species with a corolla (Rehder 1940) and F . anomala for its frequently simple leaves, but only F . latifolia and F . velutina are important riparian trees in California (F . anomala is a riparian species of the Colorado Plateau with a few relict populations in the mountains of the eastern Mojave Desert). These are only nominally species, however, since the riparian ashes of California (fig. 5) are part of an attenuated but essentially continuous cine between more important ash populations in Arizona (F . velutina ) and in Oregon and Washington (F . latifolia ) along which species can be separated only artificially and arbitrarily (Griffin and Critchfield 1972).
The Pacific Northwest has a relatively short summer drought because of its northerly latitude, and Arizona regularly receives heavy summer thunderstorms of tropical origin, so both areas have much more summer rain than California. There ashes must rely almost entirely on riparian water during the growing season, and probably as a result, they are a very subordinate component of the state's riparian forests except near its northern border, where F . latifolia becomes more important as the climate becomes more like that of Oregon. In the rest of California F . latifolia /F . velutina occurs sparsely as a non-dominant riparian tree in the northern Coast Ranges, the Central Valley, the west slope of the Sierra Nevada, and in southern California, but it is rare or absent in the southern Coast Ranges, where summer drought is especially strongly developed and very few streams are naturally permanent. The Tehachapi Mountains and the western Transverse Range are conventionally used to separate these "species" in California (Griffin and Critchfield 1972).
The biology and distribution of California's riparian ashes suggest that they are declining relicts like Acer negundo var. californicum which are probably somewhat more tolerant of heat and low humidity but less tolerant of summer soil moisture deficits than that taxon. On a larger scale, Arizona and the Pacific Northwest appear to be relict nodes where populations of a once more widespread (Robichaux 1977) and probably continuously transcontinental riparian ash have successfully survived Quaternary climatic perturbations that eliminated it in much of the West and greatly reduced it in California. F . velutina and F . latifolia are, in fact, both similar enough to riparian F . pennsylvanica of the eastern United States (fig. 5) to be considered its subspecies (Miller 1955).
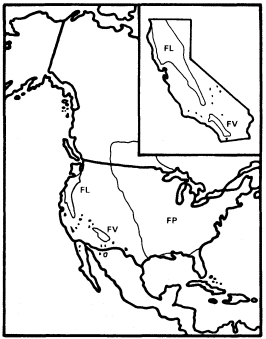
Figure 5.
Ranges of Fraxinus latifolia (FL), F. velutina (FV),
and F . pennsylvanica (FP) (after Little 1971, 1976).
Juglans (Walnut)
This genus of deciduous trees has a largely Arcto-Tertiary distribution like many of the other important California riparian genera, but its distribution within the Arcto-Tertiary zone is incomplete because of its absence from large areas, including much of Europe. It is best developed along the southern margins of this zone and extends far south of it to Argentina along the Andes.
Juglans and Carya are the only genera in their family, the Juglandaceae, which still include widespread and important north temperate forest trees. Both have distributions which suggest reductions from formerly more complete Arcto-Tertiary ranges. The other genera of the family, Pterocarya , Engelhardtia , Oreomunnea , Platycarya , and Alfaroa , are all restricted to much smaller warm temperate to tropical Arcto-Tertiary refuges in Middle America or Asia.
The Juglandaceae have often been treated as a distinctive order, the Juglandales, and associated (in the subclass Hamamelidae or Amentiferae)
with other families of temperate trees which share their characters of large-seeded woody fruits and anemophilous catkins (Cronquist 1968), but much current opinion (Dahlgren 1975; Thorne 1976) interprets these similarities as convergence and places the Juglandales close to or in the Sapindales/Rutales, the order of compound-leaved tropical trees from which the Aceraceae were also derived through a separate lineage.
The United States has six of the world's 15 species of Juglans , but J . cinerea is the only strongly distinctive species among these six. The other five include J . nigra , an important upland to weakly riparian forest tree of the eastern deciduous forest (Fowells 1965), and four species of various refuge areas in the West (fig. 6). The western species closely resemble J . nigra and suggest the same pattern of Late Tertiary to Quaternary reduction in the range of a formerly transcontinental species which we have seen in Fraxinus . These four include the two California species of Juglans , J . californica and J . hindsii , both of which are endemic to the state.
J . californica is a mostly non-riparian tree of southern California which is depauperate relative to J . nigra and J . hindsii but is not greatly different from them morphologically. It is restricted to deep, friable Tertiary marine shales with high water-holding capacity which permit it to survive as a local dominant on upland sites since the warm spring temperatures of southern California allow summerwet conditions to be simulated earlier in the year wherever soil storage capacity is adequate to hold surplus water from winter rains.
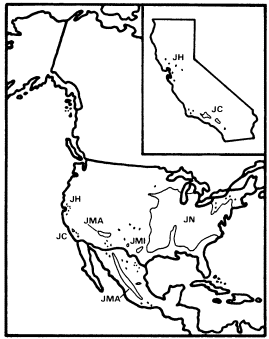
Figure 6.
Ranges of Juglans hindsii (JH), J . californica (JC),
J . major (JMA), J . microcarpa (JMI), and
J . nigra (JN) (after Little 1971, 1976).
J . hindsii was apparently restricted to a very few sites in central California when European settlement began there, and at least some of these sites were riparian (Griffin and Critchfield 1972). Since this species was probably derived from ancestors which were adapted to a summer-wet climate and were only weakly riparian, it is likely that J . hindsii was escaping a mediterranean-type climate to which it was completely unadapted in a riparian zone to which it was poorly adapted. Its large, nutrient-rich seeds in heavy nuts with little obvious capacity for dispersal are a more appropriate adaptation for reproduction in a stable forest (Grime 1979) than in the highly unstable, frequently floodprone riparian environment that existed in California before most of its streams were dammed.
J . hindsii was clearly on the verge of natural extinction when California was first settled by Europeans and was at the midpoint on a continuum between those taxa with similar histories such as Nyssa and Ulmus which are now known only from California's fossil record (Axelrod 1973) and those such as Acernegundo and Fraxinuslatifolia which survived until the settlement period with greater but still declining ranges and abundances (Robichaux 1977). Ironically, since European settlement, J . hindsii has been widely planted and subsequently commonly naturalized in California's now largely stabilized riparian systems at a time when its once much more abundant congener J . californica is declining rapidly because of the urban expansion of Los Angeles.
Platanus (Sycamore)
Platanus , even more than Juglans , is an example of an old, declining Arcto-Tertiary genus, since it is now largely restricted to warm temperate to tropical refuges along the southern periphery of the Arcto-Tertiary zone despite an extensive and diverse fossil record from as far north as Greenland (Engler and Melchior 1964). Only P . occidentalis of the eastern United States is still an important forest tree in a major north temperate forest biome.
Platanus is traditionally placed in the monotypic family Platanaceae, which is widely agreed to belong in the order Hamamelidales (Cronquist 1968; Dahlgren 1975; Thorne 1976), a relationship which links it to several other old families of Arcto-Tertiary trees and shrubs. The Hamamelidales show ancient tendencies toward the deciduous tree habit, unisexual wind pollinated flowers, and wind dispersed fruits, characters which are typical of the modern dominants of north temperate forests and of all 10 living species of Platanus , a taxon which culminates one of probably several floral reduction series within the order and its relatives (Thorne 1976).
Platanus in the United States includes P . occidentalis and two closely related species of the Southwest, P . wrightii of Arizona, New Mexico, and northwestern Mexico, and P . racemosa of California (fig. 7). All three species are strongly riparian, but the two southwestern species are more similar to P . orientalis , a riparian tree native from southeastern Europe to the Himalayas, than to the geographically closer P . occidentalis (Hsiao 1973). This may reflect ancient relationships and patterns of extinction within Platanus , but the very close relationship between P . racemosa and P . wrightii suggests they were separated relatively recently when expanding deserts separated woodlands that were continuous between Arizona and California in the Miocene (Axelrod 1975; Raven and Axelrod 1978).
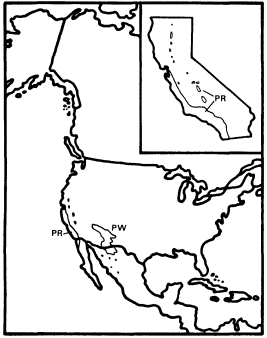
Figure 7.
Ranges of Platanus racemosa (PR) and
P . wrightii (PW) (after Little 1976).
P . racemosa is a common riparian tree in northwestern Baja California, southern California, the southern Coast Ranges, the southern Sierra Nevada foothills, and the Sacramento Valley, but it is scarce in the San Joaquin Valley and absent from the northern Coast Ranges, where much seemingly suitable habitat occurs (Griffin and Critchfield 1972). P . racemosa is an important secondary component of the mixed riparian forests of the Sacramento Valley, where it is often associated with sites higher and drier than those where dominant Populusfremontii is found (Conard et al . 1977), but it is particularly conspicuous as frequently the single dominant tree along the intermittent streams of the southern Coast Ranges and southern California. Very large sycamores form an open woodland along such streams, and fine examples of this distinctive vegetation-type can be seen along Pacheco Creek in southern Santa Clara County and along Orestimba Creek in Stanislaus County.
Intermittent streams are general in southern California and the southern Coast Ranges because of their mediterranean-type climate and lack of extensive highland snowfields to provide summer runoff, and sycamore woodlands are particularly characteristic of those with beds of coarse, porous sand and gravel. Such substrates are common in the region where sycamore woodlands occur because it has been subjected to very rapid and recent Plio-Pleistocene uplift which may still be continuing and to massive erosion which has inevitably followed (Page 1981). The small, comose achenes of P . racemosa are easily carried long distances by wind, enabling the rapid reestablishment of these woodlands after the flood damage which was frequent before California streams were dammed.
The obligate restriction of P . racemosa to riparian zones indicates a need for access to groundwater within its root zone, but its preference for dry, porous sites within riparian zones suggests that for a riparian species it also has a rather high requirement for aeration of at least part of its root zone. The reasons for its complete absence from the northern Coast Ranges are not obvious since seemingly suitable intermittent streams with coarse beds are fairly common there. Along some of these streams on the dry east slope of the northern Coast Ranges native riparian trees are replaced by halophytic graminoids and an introduced Tamarix species, probably because their flows are made brackish by salts leached from the Cretaceous marine sediments which dominate their watersheds,[3] but many other northern Coast Ranges streams have fine riparian gallery forests along their banks. The clay-rich Franciscan Formation dominates the northern Coast Ranges, but the same formation yields enough sand and gravel to support major stands of sycamore woodland in parts of the southern Coast Ranges. Temperature does not provide a simple explanation for the absence of sycamores from the northern Coast Ranges because summers there are not necessarily hotter or cooler nor winters colder or milder than those at places well within the range of P . racemosa .
A possibly significant factor limiting the capacity of sycamores to invade the northern Coast Ranges is that region's cool, wet spring. The collective mean precipitation for May is 43 mm. among the climatic stations of both the northern Coast Ranges and the Sacramento Valley, but that month is 1ºC warmer at the Valley sta-
[3] A high Mg/Ca ratio may be important in these streams as well since many have much serpentinite in their watersheds. This ratio is 1.6 in Cache Creek, which has many tributaries which have always lacked riparian trees. The Mg/Ca ratio, electrical conductivity, and dissolved Na are, respectively, 87%, 628%, and 975% greater in Cache Creek than in the Sacramento River into which it flows.
tions. In the southern Coast Ranges the collective May mean temperature is also 1°C warmer than in the northern Coast Ranges, but the collective mean precipitation for that month is 28 mm. less (US Department of Commerce 1970). Anthracnose (Gnomoniaplatani ) is a very serious and prevalent disease which can cause complete spring defoliation of Platanus species including P . racemosa , and it is known to be promoted by cool and wet spring weather (Fowells 1965; Collingwood etal . 1974; Pirone 1970). This fungus is currently severely stressing wild populations of P . racemosa in Alameda and Contra Costa Counties at the northwestern limit of the range of that species, and it must be suspected of limiting the further expansion of sycamores northwestward in California.
Populus (Cottonwood)
Populus is the most important riparian genus in California, and one of the two major genera of the Salicaceae, probably the most important riparian family in the world. This family was traditionally placed close to the Hamamelidales and Fagales in the artificial taxon Amentiferae because of its unisexual catkins, but it is now recognized to be misplaced there. Its characters, which include capsular fruit with many seeds, suggest a much closer relationship to the small, largely halophytic Tamaricales and to the large and diverse assemblage of plants variously known as the Violales or Cistales. It is distinctive enough, however, to be retained in its own monotypic order, the Salicales (Cronquist 1968; Dahlgren 1975; Thorne 1976).
The Salicaceae, which include the large and widespread genus Salix and the monotypic East Asian Chosenia in addition to Populus , are deciduous woody plants which are dioecious and have very light and easily wind dispersed comose seeds. They share the pattern seen in the Betulaceae of adaptation to riparian zones in temperate climates (as well as tropical in the case of Salix ), with much wider extension into upland habitats in boreal, montane, and arctic climates where late snowmelt saturates the soil during part or all of the growing season. Unlike Alnus , Betula , and Salix , however, Populus consists entirely of trees. Unlike Salix , which is secondarily entomophilous (Thorne 1976), it is entirely wind pollinated.
Populus consists of 35 species and ranges throughout the North Temperate Zone into parts of the Arctic. Four species (P . tremuloides , P . trichocarpa , P . fremontii , and P . angustifolia ) are native to California. P . tremuloides (aspen) is a largely upland species which is widespread in the boreal and montane parts of North Amnerica, and extends south to some of the higher California mountains. P . angustifolia is a riparian species of the Rocky Mountains, which occurs in a few colonies in the area east of the southern Sierra Nevada crest where summer thunderstorms of tropical origin also permit larger but still relict populations of Betulaoccidentalis to survive.
P . tremuloides is the most taxonomically distinctive Populus species in California, and is set off from the others in section Leuce . P . trichocarpa and P . angustifolia are close enough to share placement in section Tacamahaca , but even though P . fremontii is set off from these species in section Aegeiros (Rehder 1940), it can hybridize with P . trichocarpa (Little 1953). Such hybrids seem to be rare in California, but hybrids between P . angustifolia of Tacamahaca and P . sargentii of Aegeiros are abundant in the Great Plains (Elias 1980).
P . fremontii (Fremont cottonwood) and P . trichocarpa (black cottonwood) are the two principal riparian species of Populus in California, and P . fremontii is the single most important riparian species in the state since it dominates the great riparian forests of the Central Valley (Conard etal . 1977) as well as many of those elsewhere in cismontane and transmontane California (Roberts etal . 1977). Most of these magnificent forests have been destroyed (Thompson 1977), but small good examples have been preserved by the Nature Conservancy on the Kern River, by the California Department of Parks and Recreation at Caswell State Park, and by a few other groups and agencies elsewhere. Because of their great ecological significance (Sands 1977; Hehnke and Stone 1978) every effort should be made to preserve what remains of these rapidly vanishing natural communities. Particular attention should be given to those in the Sacramento and lower San Joaquin Valleys, where preservation opportunities are greatest, and to what remains of those along the Colorado River, where destruction has been most complete.
P . trichocarpa is one of the largest broad-leaved trees in North America, but it is a less conspicuous riparian tree than P . fremontii in California because it is most common in the cooler, wetter parts of the state, where it frequently associates with highly competitive riparian species such as Alnusrhombifolia , and often grows near upland forests dominated by giant conifers. Magnificent riparian forests dominated by P . trichocarpa do occur in coastal and montane California, however, and a fine example remains along the Carmel River in Monterey County.
The California ranges of P . trichocarpa and P . fremontii overlap (Griffin and Critchfield 1972), and individuals of the two species frequently grow sympatrically without hybridization. P . trichocarpa is essentially limited to those parts of California with July mean temperatures cooler than 25°C, while California populations of P . fremontii grow at sites with a range of July means approximately between 17°C and 36°C. The cooler P . fremontii sites are limited to coastal areas around San Francisco Bay and from San Luis Obispo County south where winters are mild and the growing season long.
The total ranges of these species and their closest relatives outside California (fig. 8 and 9) reflect a pattern similar to that of their ecological relationships in the state. P . trichocarpa is common throughout the cool, wet Pacific Northwest north to southern Alaska and east to the northern Rocky Mountains (Little 1971), and its closest North American relatives are P . angustifolia of the Rocky Mountains and P . balsamifera , a frequently upland species which is widespread in the continent's boreal regions (Rehder 1940). P . trichocarpa is considered conspecific with the latter by Eckenwalder (1980).
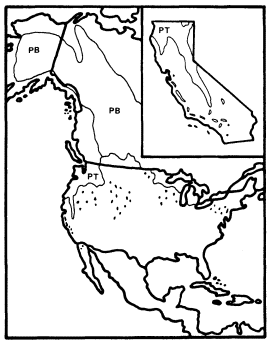
Figure 8.
Ranges of P . trichocarpa (PT) and
P . balsamifera (PB) (after Little 1971).
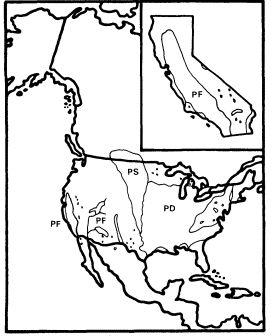
Figure 9.
Ranges of Populus fremontii (PF), P . deltoides
(PD), and P . sargentii (PS) (after Little 1971).
P . fremontii , in contrast, is found east to Trans-Pecos Texas in riparian sites throughout the Southwest, and it is most closely related to the riparian species P . arizonica of the Southwest, P . deltoides and P . sargentii of warm temperate eastern North America, and P . nigra of warm temperate Europe (Axelrod 1975). Macrofossils intermediate between P . fremontii and P . deltoides are known from the then warmer and wetter Miocene Ellensburg Flora of Washington, where neither species occurs today (Robichaux 1977).
These relationships suggest that both P . trichocarpa and P . fremontii are western derivatives of formerly transcontinental ArctoTertiary entities within Populus . P . trichocarpa and its relatives within section Tacamahaca are derivatives of a cool temperate to boreal species complex, and P . fremontii and the other species of section Aegeiros are of warm temperate ancestry.
Quercus (Oak)
This huge genus of 450 species dominates the upland deciduous forests of the North Temperate Zone, but it also includes many riparian, shrubby, and evergreen species, and extends south through the American tropics as far as the Colombian Andes. Quercus is in the Fagaceae, which it shares with several other major tree genera, and thus in the Fagales, which it shares with the Betulaceae, Corylaceae, and Carpinaceae (Willis and Airy Shaw 1973).
Quercus and other Fagaceae are wind pollinated and frequently deciduous like the Betulaceae, but their heavy, nutrient-rich fruits, like those of Juglans , are poorly dispersable and better adapted to germination in mature forests than in the regularly disturbed riparian zones to which the light, easily wind dispersed fruits of Alnus and Betula are well adapted (Grime 1979).
The 16 California species of Quercus include most of the range of morphological diversity in the genus, and all of its three subgenera (Quercus , Erythrobalanus , and Protobalanus ), but Q . lobata (valley oak) is the state's only major riparian oak. The other species are primarily or exclusively upland trees and shrubs, and even Q . lobata is somewhat more common in upland than in riparian zones. In the upland oak woodlands of the Coast Ranges it is common in sites which have heavy, poorly aerated soils with high water-holding capacity. Such ecological conditions are similar to those of the riparian sites where it occurs since these tend to be both drier and less well aerated than sites dominated by the other principal riparian trees.
In addition to its occasional dominance of upland oak woodland in the Coast Ranges when ecological conditions are suitable, Q . lobata can dominate two kinds of riparian communities: 1) riparian forest adjacent to streams when aeration is too poor for Populusfremontii , the typical riparian forest dominant. Q . lobata can form gallery forests in a matrix of treeless grassland in such situations, which were once common in the area of excessively heavy soil in the Sacramento Valley where rice is now extensively grown. The grasslands have almost entirely been converted to rice fields in this area, and many gallery forests of Q . lobata lost as well, but a fine example is still extant along Honcut Creek in northern Yuba County; 2) forest, woodland, and savanna on alluvial plains and terraces, usually above and toward the upland edge of typical riparian forest dominated by Populus fremontii (Conard etal . 1977). In these communities oaks must have access to water throughout the growing season, and their field relationships suggest that the accessibility of their water supply determines their density. Closed forests overwhelmingly dominated by Q . lobata can occur where water is abundant at relatively shallow depths, but progressively more open woodland and savanna communities in which oaks are scattered in a matrix of grassland are found as water apparently becomes more limiting. One of the very few field studies of the water relations of California oaks was done at such a Q . lobata community in Monterey County. It suggested that while Q . lobata had access to a reliable water table, Q . douglasii of adjacent uplands probably did not (Griffin 1973). However, since Q . lobata can also occur on upland sites similar to those of Q . douglasii (Griffin 1977), it should not be assumed that all its populations have the ready access to groundwater of its alluvial communities.
California's alluvial Q . lobata communities were once fairly common in parts of the Central Valley and in many Coast Ranges valleys as well, but since they were indicators of some of the world's best agricultural soils, most have long since been converted to farmland (Rossi 1980). A very few small but good examples of California's alluvial Q . lobata forests are still extant in Mendocino, Butte, Yolo, and Sacramento Counties, and perhaps elsewhere. Every effort should be made to permanently preserve them while the opportunity still exists.
All oaks need water, and several other upland species share some of Q . lobata's tolerance of poor soil aeration, so it is not surprising that they occasionally dominate riparian communities as well. Riparian Q . douglasii occurs along Mitchell Creek in Contra Costa County; riparian Q . engelmannii is found along Pala and other similar creeks in San Diego County; and Q . agrifolia forms a fine but very unusual riparian forest along the lower Mokelumne River on the floor of the Central Valley. Since the latter species is ordinarily restricted to the Coast Ranges and southern California, it must be assumed that marine airflow through the Carquinez Straits permits its survival in this part of the lower San Joaquin Valley. It is hoped that this rare and unstudied natural community will also soon receive adequate protection.
Q . lobata is a distinctive species endemic to California (fig. 10) of subgenus Quercus , the only one which extends to Eurasia (Tucker 1980). This subgenus is quite diverse and includes many large deciduous and evergreen trees as well as a number of evergreen shrubs in the Southwest and northern Mexico, but Q . lobata does not seem to be particularly closely related to any of these. As a large deciduous tree it is superficially similar to Q . garryana , but it stands somewhat apart from the series of increasingly drought-adapted species which Q . garryana forms with Q . douglasii and Q . engelmannii . It can occasionally hybridize with each of these, however. Surprisingly, it naturally hybridizes most abundantly with the shrub Q . dumosa (ibid .), and it is interesting but perhaps not significant that several other small white oaks of the Southwest have similarly elongated acorns with shallow cups. Since it is not particularly close to any of the eastern North American white oaks either, it is perhaps best to view Q . lobata as a distinctive derivative of subgenus Quercus which shares a complex pattern of interrelationships with other species of that taxon, and which has been a recognizable entity at least since the Miocene, when it was more widespread in western North America (Robichaux 1977).
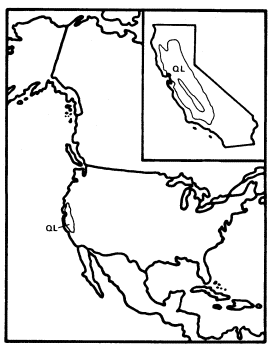
Figure 10.
Range of Quercus lobata (QL) (after Little 1971).
Salix (Willow)
Salix , the second major genus of the Salicaceae and Salicales, consists of about 500 species of trees and shrubs. The trees are very important components of riparian communities of the North Temperate Zone, and have invaded similar habitats in the tropics and South Temperate Zone as well, but the shrubs are largely restricted to the Northern Hemisphere and tend to be smaller and more associated with upland zones as high latitudes and altitudes are approached, a pattern of adaptation similar to but more welldeveloped than that seen in Alnus and Betula . Willows are typically deciduous and share the dioecious catkins and easily dispersed comose seeds of other Salicaceae, but they are usually secondarily entomophilous (Thorne 1976), and can be almost evergreen in the tropics (e.g., S . bonplandiana ).
California has about 32 Salix species (Munz 1959, 1968); the exact number is uncertain because of the difficult and controversial taxonomy of the genus. Which of these species should be considered trees is almost as controversial since the state's willow species are a continuum between large trees and dwarf shrubs, and several of the species which are usually shrubs can develop into small trees when conditions are favorable. The California willows which most frequently reach tree size are S . exigua , gooddingii , hindsiana , hookeriana , laevigata , lasiandra , lasiolepis , rigida (mackenziana ), scouleriana , sitchensis (coulteri ), and tracyi . All of these are riparian, but S . hookeriana , rigida , scouleriana , and tracyi are small local or northern species largely limited to riparian zones within montane or northwest coast coniferous forests, and are of little importance in the riparian communities of the rest of California.
Salixgooddingii is the most important willow of the great riparian forests of California's Central Valley, and it frequently shares dominance there with Populus fremontii , particularly at intermediate successional stages. It is a better pioneer than P . fremontii , if not as good as S . hindsiana , and it usually dominates the new riparian forests which often form in the Valley along neglected ditches and canals. Since it is generally more weedy and tolerant of stress than P . fremontii as long as water is abundantly available, it is particulaly important in the depauperate riparian forests along the lower San Joaquin River, where high salinity and poor development of natural levees have probably long limited maximal riparian community development (Kahrl 1979). S . gooddingii is limited to riparian zones of the Central Valley, southern California, and the desert Southwest (Little 1976; Elias 1980), a distribution which suggests a need for long, hot growing seasons as well as abundant groundwater (fig. 11). The relationships of this species are not in doubt since it is so similar to S . nigra , the most important large willow of eastern North America, that it is still some-times included within it as S . nigra var. vallicola (Little 1953, 1976).
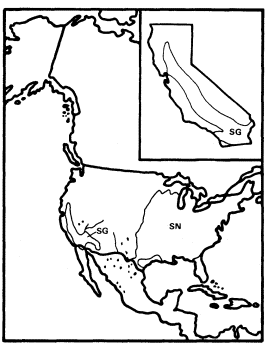
Figure 11.
Ranges of Salix gooddingii (SG) and
S. nigra (SN) (after Little 1971, 1976).
S . lasiandra and S . laevigata are similar enough to one another that Hoover (1970) doubted their distinctness. Their ecological niche seems similar as well since both are large riparian willows which grow along streams in the Coast Ranges and the lower foothills of the Sierra Nevada. S . laevigata is reported to prefer well-aerated, rapidly flowing streams (Elias 1980), and it has been mapped as absent from most of the Central Valley by Little (1976), who also excludes S . lasiandra from most of the San Joaquin Valley. Conard etal . (1977) and Roberts etal . (1977) report both species to be common components of Central Valley riparian forests, however. What is clear about these two species at this time is: 1) they are more common along streams in the highlands surrounding the Valley than along the Valley floor; 2) they occur on the Valley floor; and 3) the details of their distribution and ecological relationships in California need to be much better understood. S . lasiandra may have less tolerance for habitats along intermittent streams than S . laevigata and thus may have a greater need for permanent water, but this observation needs verification.
The total ranges of these species do suggest that S . laevigata may be the more drought-adapted of the two since S . lasiandra extends down the mountains and coast of California from a wide range in the cool and wet parts of the Pacific Northwest, the northern Rocky Mountains, western Canada, and central Alaska while S . laevigata is restricted to mediterranean Cali-
fornia and a few relict stations in Arizona, Nevada, and Utah (fig 12 and 13) (Little 1976). S . lasiandra is reported to be closely related to S . lucida of the boreal forests of eastern North America (Rehder 1940), and S . laevigata is similarly reported to be related to S . bonplandiana , a semi-evergreen species of southern Arizona and tropical western Mexico (Elias 1980), but a critical reexamination of their relationships to each other and to other species which they resemble would be desirable.
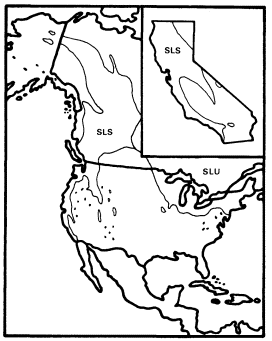
Figure 12.
Ranges of Salix lasiandra (SL) and
S . lucida (SLU) (after Little 1976).
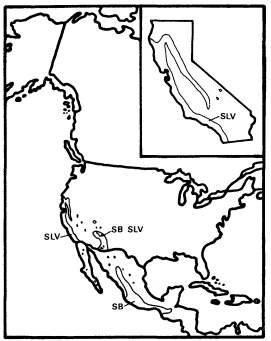
Figure 13.
Ranges of Salix laevigata (SLV) and
S. bonplandiana (SB) (after Little 1976).
S . hindsiana and S . exigua (fig. 14) are largely shrubs, but they are both major components of California riparian vegetation because they are usually the first woody plants to colonize sandbars and other newly-formed riparian habitats when these are relatively fine-grained and shallow to groundwater. The dominance of such sites by these willows produces a distinctive and common riparian shrub community (Conard etal . 1977), but Baccharisviminea and B . glutinosa can become more important when alluvium is coarser and the water table deeper.
S . hindsiana is largely cismontane and endemic to the California Floristic Province while S . exigua is mostly transmontane in California and widespread in the rest of North America (fig. 14) (Little 1976). Both are ecologically and morphologically similar, however, and difficult to distinguish when sympatric (Hoover 1970; Smith 1970). They are part of a complex of closely related and ecologically similar western willows which also includes S . fluviatilis , S . sessilifolia , and S . melanopsis .
S . lasiolepis is a common small willow of much of the California Floristic Province, and it also occurs at scattered, possibly relict stations throughout the West (fig. 15) (Little 1976). It becomes an important vegetation component in the fog belt of the California coast, however, because there it dominates a distinctive forest community on alluvial bottomlands and in dune slacks. Myricacalifornica is an important associate in these forests, and a rare thistle, Cirsium loncholepis , is entirely restricted to them. Most of this apparently previously undescribed community was lost early
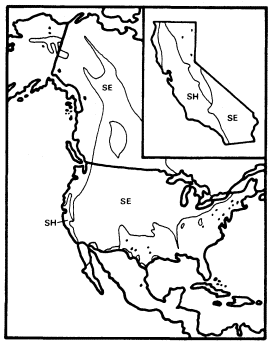
Figure 14.
Ranges of Salix hindsiana (SH)
and S . exigua (SE) (after Little 1976).
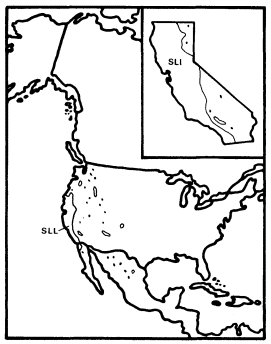
Figure 15.
Range of Salix lasiolepis (SLL) (after Little 1976).
to agriculture since it frequently dominated wide coastal plains in the Arroyo Grande, Oso Flaco, and Santa Maria Valleys, which are now rich vegetable districts. A small remnant of it is now protected at Pismo State Beach in San Luis Obispo County, and a larger stand, which includes critical habitat of the endangered Unarmored Threespine Stickleback (Gasterosteusaculeatus williamsoni ), could be saved along San Antonio Creek in Santa Barbara County.
S . lasiolepis is closely related to S . hookeriana and S . tracyi (Elias 1980), two small trees of north coastal riparian communities, and probably to a number of northern and montane shrubs as well. It is less close to S . sitchensis , a small tree which also grows along fog belt streams as far south as Santa Barbara County (Smith 1976) but seldom forms extensive forests.
Riparian Origins
When the Arcto-Tertiary Geoflora was decimated throughout western North America in the Late Tertiary and Quaternary by spreading drought and cold, it is clear that some of its elements which were already strongly adapted to riparian conditions were able to survive along California's permanent and intermittent streams (Axelrod 1977). Their descendents dominate the state's riparian forests today. Some Arcto-Tertiary elements which were weakly adapted to riparian conditions such as Juglans hindsii were also able to precariously survive to the present in riparian refuges. Riparian forests are not among California's major refuges for Arcto-Tertiary and other relicts, however (Stebbins and Major 1965). This is partly because most riparian taxa are still too successful to be considered relictual but primarily because riparian environments are too competitive and frequently disturbed to promote high plant species richness and thus the survival of many marginal, relict species. Grime (1979) has shown that plant species density is highest in environments of intermediate productivity in which neither competition nor stress are excessive.
Before California streams were dammed, floods periodically disturbed riparian communities, renewing nutrients and understory light in an environment where warmth and soil moisture were already ideal for maximal productivity. Such conditions of regular but relatively infrequent disturbance when resources are not limiting are conducive to maximum plant competition and thus low species richness (ibid .). In contrast, California's concentrations of relictual species are in areas of intermediate productivity where plant species density is not limited by extremes of either stress or competition.
Riparian Dominance and Environment
Populusfremontii is the most common dominant of central California's riparian forests, but as discussed previously, environmental factors can shift dominance to other species. The most obvious of these factors are frequency of disturbance, air temperature, root zone aeration, and depth to groundwater, but others are probably important as well. It has been shown that when disturbance is high, dominance is shifted to Salix hindsiana and when somewhat less severe to S . gooddingii . Cool growing seasons favor P . trichocarpa , and hot environments sites with ahigh water table and low root aeration promote Cephalanthus occidentalis , but when turbulent, well-aerated water is close to the surface, Alnusrhombifolia can become dominant. When water tables are relatively deep, Platanusracemosa is the usual dominant when aeration of the intervening soil is high and Quercuslobata when it is low.
Q . lobata , the only major California riparian tree which is probably not of riparian origin, is most frequently dominant in riparian systems when two stressing factors, deep groundwater and low soil oxygen, are both present. This suggests that it is a relatively more stress-tolerant competitor than other riparian dominants. It is interesting that this species is probably derived from drought-stressed upland climax forests and woodlands, where trees with the stress-tolerant competitor adaptive strategy would be expected to dominate, rather than from riparian communities, which are more frequently dominated by trees with a simply competitive strategy (Grime 1979).
Deciduousness and Productivity
Mediterranean-type climates, with their mild, wet winters and dry summers, are well known for their sclerophyllous evergreen vegetation (Mooney etal . 1977; Cody and Mooney 1978; Walter 1979), but the presence of seemingly anomalous winter-deciduous riparian vegetation well within such a climate in California has generated relatively little comment. Air temperature obviously does not exclude evergreens from the Central Valley since they are common there in non-riparian zones. This led Stebbins (1974) to suggest that winter-deciduousness is favored when a highly productive growing season alternates with a cool season which is much less favorable but not necessarily extremely cold. He further postulated that it arose in riparian zones because wet roots aggravate winter cold stress and thus deciduousness.
The first hypothesis is undoubtedly correct, but the second is unlikely since evergreen woody angiosperms are more common in riparian than in upland zones on the Atlantic Coastal Plain in South Carolina, where summers are hot, wet, and highly productive, and winters are about as cold as those of the Central Valley. Evergreen angiosperm trees common in some riparian zones on the South Carolina Coastal Plain include Gordonia lasianthus , Ilexcassine , I . coriacea , I . myrtifolia , I . opaca , I . vomitoria , Magnolia virginiana , Myricacerifera , M . heterophylla , Perseaborbonia , P . palustris , and Quercuslaurifolia (Elias 1980).
In January Columbia, South Carolina, at the inner edge of the coastal plain, has a mean temperature of 7°C and a mean minimum temperature of 1°C. Sacramento in January has the same mean temperature and a mean minimum of 3°C. Despite the similarity of these temperatures, however, Sacramento has a mean frost-free growing season of 307 days and Columbia of only 248. Charleston, on the coast at the outer edge of the coastal plain, has milder winters than Columbia or Sacramento since it has a January mean of 9°C and mean minimum of 3°C, but even here the mean frost-free growing season is only 285 days, fully 22 days shorter than at Sacramento (US Department of Agriculture 1941).
The shorter growing season and greater spread between winter mean and mean minimum temperatures of these South Carolina cities relative to Sacramento are caused by the much more frequent penetration of outbreaks of cold arctic air to the coastal plain than to the Central Valley, which is usually protected from them by the Rocky Mountains and the Sierra Nevada and Cascade Ranges. The riparian evergreens of South Carolina must cope with these regular cold outbreaks as well as with their mean winter climate, so it is unlikely that the winter-deciduousness of the California riparian community is a general phenomenon which occurs wherever winter cold and wet soil interact.
Rundel (1980), in a comparative study of the adaptive strategies of mediterranean-climate oaks, supported Stebbins' view that winter-deciduousness is favored when highly productive summers alternate with winters which are much less productive but not necessarily highly stressful. Rundel assembled evidence which showed that deciduous oak leaves are considerably more photosynthetically efficient than those of evergreen oak species. For this extra efficiency to be profitable, however, a growing season which is sufficiently long, warm, and stress-free must be predictably present to compensate for the energetic cost of producing a new crop of leaves each year.
There is every reason to believe that Rundel's generalizations about the adaptive strategies of oaks apply equally well to those of other taxa. Deciduousness is clearly an example of the ability to rapidly respond to environmental change, a characteristic which Grime (1979) considered central to the competitive adaptive strategy. As discussed above, riparian environments, with their high productivity, minimal stress, and regular but infrequent disturbance, provide just the conditions which Grime predicted would most favor this strategy.
Major (1963) has shown that the activity of upland plant communities can be estimated from readily obtainable climatic data by using Thornthwaite's water balance concept (Thornthwaite 1948; Thornthwaite and Mather 1955). Thornthwaite's potential evapotranspiration (PE) is a function of temperature and day length and thus of warmth and light. His actual evapotranspiration (AE) predicts the evapotranspiration which can take place at a site if no more water is available to it than that received from precipitation. Since AE is a function of warmth, light, and precipitation (as well as soil water storage) and these are three of the most essential ingredients of plant productivity, Rosenzweig (1968) pointed out that AE can provide a reasonably good estimate of this as well.
In mediterranean-type climates AE is depressed far below PE because lack of summer rain causes severe drought stress and suppresses plant productivity just at the time when warmth and light are maximal. At Sacramento, for example, in the heart of California's mediterranean-type climate, annual PE is 815 mm. but annual AE is only 458 mm. (Mather etal . 1964). AE and PE are both 15 mm. in January, but while PE climbs as high as 140 mm. in July, AE peaks at 72 mm. in May and then declines because of summer drought. If less soil moisture storage were assumed than the 300 mm. used to calculate these figures, AE would peak even earlier and its annual sum would be even less.
Grassland is the dominant upland vegetation type at Sacramento. Upland broad-leaved trees are sparse and consist almost entirely of two species, evergreen sclerophyllous Quercus wislizenii and deciduous but semi-sclerophyllous Q . douglasii .
At Columbia, South Carolina, however, summer rain is abundant, and the annual AE of 915 mm. almost matches the annual PE of 952 mm. Both AE and PE are 13 mm. in January, and both rise to similar peaks in July, when PE is 172 mm. and AE is 162 mm. Columbia is surrounded by a rich upland forest which can be dominated by pines or by several species of deciduous broad-leaved trees.
When rivers flowing from distant mountains import far more water to an area than would be available from local precipitation, keeping soil saturated at shallow depths throughout the year, AE, in effect, becomes equal to PE. This is exactly what happens in the riparian forests of California's Central Valley. As a result their defacto AE, their activity cycle, their productivity, and even their physiognomy are all very similar to those of the upland deciduous forests of South Carolina's coastal plain.
It can be seen that a gigantic natural experiment has confirmed Stebbins' and Rundel's hypothesis. Deciduousness is promoted wherever a long, very productive growing season is paired with a minimally productive but not necessarily very stressful cool or cold season. Like human beings with high incomes and low expenditures, deciduous trees can afford to rest during the season when their income would otherwise be lowest. The poorer evergreens do not have this luxury.
Quercuskelloggii (black oak) is found from San Diego County to central Oregon and is one of California's most abundant broad-leaved trees (Bolsinger 1980). Since Q . kelloggii is a non-riparian tree with deciduous, nonsclerophyllous leaves, theory predicts that it should occur where the local climate promotes maximal productivity during a well-defined growing season. Table 1 validates this prediction. Maximum monthly AE, and thus seasonal productivity, is considerably higher within the black oak's California range than in those parts of the state where it does not occur.
| ||||||||
AE is high within the range of Q . kelloggii because this species is limited to mountain slopes too low and too far inland to have cool summers but of relief sufficient for high orographic precipitation. Summers are dry, but where soil storage is good, surplus water from winter and early spring rains can promote high productivity during the warm days of late spring and early summer and thus favor this upland tree's deciduous habit.
Mineral Nutrition
Monk (1966) found that forests in north-central Florida are much more likely to be dominated by evergreen than deciduous trees when mineral nutrients and pH are low, and he generalized that relative soil sterility promotes evergreenness. Rundel (1980) also noted that evergreen leaves are more nutrient-use efficient. It is clear that nutrient losses from the regular shedding of deciduous leaves represent a cost which can only be made up during a long and productive growing season in a reasonably fertile environment. Since nutrient deficiencies lower productivity (Kramer and Kozlowski 1979) they, in effect, lower AE and promote evergreenness indirectly as well as directly.
California riparian forests receive imported nutrients as well as water from their rivers and streams (or did before dams stopped floods and became nutrient traps). Thus their productivity and deciduousness are doubly promoted. It is interesting that the Sacramento and the Stanislaus, the two Central Valley rivers with the most limestone in their watersheds and thus the most calcium, are also those with the best developed riparian forests along the banks of their lower reaches.
It was noted earlier, however, that some riparian communities on the Atlantic Coastal Plain are evergreen while surrounding upland communities are deciduous. These riparian communities occur because the low relief of the Coastal Plain does not permit rapid drainage of the heavy precipitation which it receives. They are nutrient-poor because their water is of local, meteorological origin, and can only leach and carry away mineral nutrients, not deposit them (Wharton and Brinson 1979). In this climate, where abundant summer rain makes AE maximal, the water available to riparian communities can not raise their productivity much above that of adjacent upland vegetation. It can only lower it through leaching. The result is evergreen vegetation in riparian communities on the Coastal Plain when the water is of local origin, but deciduous riparian forests dominated by species of Salix , Populus , Quercus , Platanus , and Fraxinus similar to those of California occur along the major rivers. These flow out of the Appalachians and import nutrients to the Coastal Plain to replace those lost by local leaching (Braun 1950; Hosner 1962; Wharton and Brinson 1979).
Productivity and Community Ecology
California's riparian communities are its most productive because they receive abundant water during hot, cloudless summers which are
ideal for maximum photosynthesis. Their high PE thus becomes a high AE. Everywhere else in California except on the highest mountain summits summer drought suppresses AE below PE and plant productivity below its potential. Outside of alpine areas AE approaches PE most closely among California's upland communities in the coastal redwood belt of Del Norte and northwestern Humboldt Counties, but summers are too cool and cloudy there for productivity and either AE or PE to be extremely high. Crescent City, in the center of this area, has an AE of 597 mm. and a PE of 650 mm. while Davis, in the Central Valley, has an upland AE of 420 mm. and a PE and thus riparian AE of 810 mm. (300 mm. soil storage assumed).[4]
The riparian systems of California are clearly far more productive than any of that state's communities which are dependent on their local climate can be. Maximal riparian productivity more closely approaches that of eastern deciduous forests in summer and of tropical rain forests throughout the year. It is not surprising, then, that California's riparian forests share some features with exotic ecosystems which are absent, rare, or poorly developed elsewhere in the state.
Herbs with tropical affinities, such as Hibiscus californicus and Fimbristylisvahlii , occur in California riparian communities, and riparian Vitis californica is a well-developed liana, a growth form 90% confined to tropical forests (Walter 1979) and very poorly developed in upland California. Abundant warmth and water are apparently essential to large lianas, and the inability to tolerate even a brief winter rest period is probably what confines most taxa to the tropics. Notoriously high root pressures have undoubtedly helped Vitis cope with this problem and survive in seasonally highly productive temperate habitats like California riparian zones (Kramer and Kozlowski 1979).
Despite their small overall area California's riparian forests are especially well known for the abundance and diversity of their bird fauna (Small 1974; Gaines 1977). Their breeding avifauna is particularly important because it includes many species which occur in virtually no other California habitat. Gaines (ibid .) has shown that these birds are very frequently insectivorous foliage gleaners which winter in tropical forests and have vicariant populations in eastern deciduous forests, two habitats which share the high productivity of western riparian communities.
Insects, as poikilotherms which are largely primary consumers, are expected to increase in abundance with increasing warmth and primary productivity, and in California upland vegetation insect biomass does, in fact, peak in spring and closely fluctuate with primary productivity throughout the year (Cody etal . 1977). Comparable data do not seem to be available for any California riparian community, but the extremely high summer productivity of such communities undoubtedly induces similarly high summer peaks of insect biomass. These in turn act as magnets for insectivorous migratory birds. Gaines (1977) was ambivalent about this since at more than one point he noted the connection between riparian insect and insectivorous bird abundance but also yielded to Willson's (1974) view that bird density is not dependent on habitat productivity. Willson reached this conclusion after field work in what were apparently mostly various successional stages of upland forest and woodland vegetation within a single local climate in east central Illinois. Her studies showed that avian biomass was similar at all these successional stages and bird density greatest at intermediate ones. Willson concluded that her data showed these parameters of bird populations are unrelated to either plant or insect productivity since she assumed plant productivity is highest in early successional stages and insect productivity highest in late stages. Her first assumption ignored the very important stem and root components of primary productivity, however, and the second was the result of a literature review which, in effect, assumed that vegetation in different climates—and thus productivity regimes—can represent stages of and thus show the ecological effects of a single successional sequence. Her data do not support her conclusion that bird populations are unrelated to community productivity, but they do suggest that bird density may be highest at intermediate successional stages. These are just the successional stages when primary productivity is greatest (Larcher 1975).
Bird abundance does appear to be positively related to community productivity, and riparian bird populations can be expected to be augmented most relative to those of upland habitats when contrasts between upland and riparian productitivity are greatest. Such contrasts occur whenever perennial streams reliably bring water to arid or semiarid lands. In deserts with summer rainy seasons, however, vegetation along ephemeral streams which merely carry away the floodwaters of rare storms may actually be less productive of plants and animals than nearby uplands which are less disturbed and no drier (Wauer 1978). The biotic effects of riparian environments are much less conspicuous in areas such as the Atlantic Coastal Plain where upland vegetation is highly productive, but even here the variety of drainage conditions typical of riparian zones produces edaphic diversity and resultant habitat and species diversity. (Hosner 1962; Hair etal . 1974).
Bird populations are not simply a function of primary productivity, however, since highly productive irrigated cropland supports bird populations which are less diverse and frequently less abundant than those of natural riparian
[4] Major, J. No date. Water balances for California climatic stations. Unpublished manuscript.
communities within the same local climate (Hehnke and Stone 1978). MacArthur and MacArthur (1961) demonstrated that bird species diversity is greatest in tall, highly stratified vegetation, and this can only occur when community phytomass is high. High phytomass is not universal in productive environments, but it is limited to them (Walter 1978).
It is tragic that the rich bird fauna of California's riparian communities has declined drastically within just the last few decades. Gaines (1977) cited reports which attribute this decline to brood parasitism by the recently introduced Brown-headed Cowbird, but he also noted that its introduction to Arizona occurred long before a similar decline in the riparian avifauna there. The cowbird has been especially frequently linked to the virtual extirpation of the riparian and insectivorous Bell's Vireo from California and Arizona, and yet these two species coexist in abundance in the less agricultural Rio Grande Valley of Texas (Wauer 1977). The riparian communities of Califorina and Arizona are frequently surrounded by agricultural areas where massive quantities of insecticides are used, but there seems to have been little comment about or investigation of their potential impact on a largely insectivorous riparian avifauna.
Literature Cited
Axelrod, D. 1960. The evolution of flowering plants. p. 227–305. In : S. Tax (ed.). Evolution after Darwin. Volume 1: the evolution of life. 629 p. University of Chicago Press, Chicago, Ill.
Axelrod, D. 1973. History of the mediterranean ecosystem in California. p. 225–277. In : F. diCastri and H. Mooney (ed.). Mediterranean type ecosystems: origin and structure. 405 p. Springer Verlag, New York, N.Y.
Axelrod, D. 1975. Evolution and biogeography of Madrean-Tethyan sclerophyll vegetation. Ann. Missouri Bot. Garden 62:280–334.
Axelrod, D. 1977. Outline history of California vegetation. p. 139–193. In : M. Barbour and J. Major (ed.). Terestrial vegetation of California. 1002 p. John Wiley, New York, N.Y.
Bolsinger, C. 1980. Oaks in California's commercial forests—volume, stand structure, and defect characteristics. p. 101–106. In : T. Plumb (tech. coord.). Proceedings of the symposium on the ecology, management, and utilization of California oaks. [Claremont, Calif., June 26–28, 1979]. USDA Forest Service GTR-PSW-44. 368 p. Berkeley, California.
Braun, L. 1950. Deciduous forests of eastern North America. 596 p. Hafner, New York, N.Y.
Chabot, B., and W. Billings. 1974. Origins and ecology of the Sierran alpine flora and vegetation. Ecol. Monog. 42:163–199.
Cody, M., E. Fuentes, W. Glanz, J. Hunt, and A. Moldenke. 1977. Convergent evolution in the consumer organisms of mediterranean Chile and California. p. 144–192. In : H. Mooney (ed.). Convergent evolution in Chile and California mediterranean climate ecosystems. 224 p. Dowden, Hutchinson & Ross, Stroudsburg, Penn.
Cody, M., and H. Mooney. 1978. Convergence versus nonconvergence in mediterranean climate ecosystems. Ann. Rev. of Ecol. and Syst. 9:265–321.
Collingwood, G., W. Brush, and D. Butcher. 1974. Knowing your trees. 374 p. American Forestry Association, Washington, D.C.
Conard, S., R. MacDonald, and R. Holland. 1977. Riparian vegetation and flora of the Sacramento Valley. p. 47–55. In : A. Sands (ed.). Riparian forests in California: their ecology and conservation. Institute of Ecology Pub. No. 15. 122 p. University of California, Davis.
Cronquist, A. 1968. The evolution and classification of flowering plants. 396 p. Houghton Mifflin, Boston, Mass.
Dahlgren, R. 1975. A system of classification of the angiosperms to be used to demonstrate the distribution of characters. Bot. Notiser. 128:119–147.
Eckenwalder, J. 1980. Populus . p. 420–421. In : J. Kartesz and R. Kartesz (ed.). A synonymized checklist of the vascular flora of the United States, Canada, and Greenland. Volume II: the biota of North America. 500 p. University of North Carolina Press, Chapel Hill, N.C.
Elias, T. 1980. The complete trees of North America: field guide and natural history. 948 p. Van Nostrand Reinhold, New York, N.Y.
Engler, A., and H. Melchior. 1964. Syllabus der pflanzenfamilien, Band II. 666 p. Gebruder Borntraeger, Berlin-Nikolassee.
Fowells, H. 1965. Silvics of forest trees of the United States. USDA Forest Service Agriculture Handbook No. 271. 762 p.
Gaines, D. 1977. The valley riparian forests of California: their importance to bird populations. p. 57–85. In : A. Sands (ed.). Riparian forests in California: their ecology and conservation. Institute of Ecology Pub. No. 15. 122 p. University of California, Davis.
Griffin, J. 1973. Xylem sap tension in three woodland oaks of central California. Ecology 54:152–159.
Griffin, J. 1977. Oak woodland. p. 383–415. In : M. Barbour and J. Major (ed.). Terestrial vegetation of California. 1002 p. John Wiley, New York, N.Y.
Griffin, J., and W. Critchfield. 1972. The distribution of forest trees in California. USDA Forest Service Research Paper PSW-82. 114 p.
Grime, J. 1979. Plant strategies and vegetation processes. 222 p. John Wiley, New York, N.Y.
Hair, J., G. Hepp, L. Luekett, K. Reese, and D. Woodward. 1979. Beaver pond ecosystems and their relationships to multi-use natural resource management. p. 80–92. In : R.R. Johnson and J.F. McCormack (tech. coord.). Strategies for protection and management of floodplain wetlands and other riparian ecosystems. [Callaway Gardens, Georgia, December 11–13, 1978]. USDA Forest Service GTR-WO-12. 410 p. Washington, D.C.
Hehnke, M., and C. Stone. 1979. Value of riparian vegetation to avian populations along the Sacramento River system. p. 228–235. In : R.R. Johnson and J.F. McCormack (tech. coord.). Strategies for protection and management of floodplain wetlands and other riparian ecosystems. [Callaway Gardens, Georgia, December 11–13, 1978]. USDA Forest Service GTR-WO-12. 410 p. Washington, D.C.
Hoover, R. 1970. The vascular plants of San Luis Obispo County, California. 350 p. University of California Press, Berkeley.
Hora, B. (ed). 1981. The Oxford encyclopedia of trees of the world. 288 p. Oxford University Press, New York, N.Y.
Hosner, J. 1962. The southern bottomland hardwood region. p. 296–333. In : J. Barrett (ed.). Regional silviculture of the United States. 610 p. Ronald, New York, N.Y.
Hsiao, J. 1973. A numerical taxonomic study of the genus Platanus based on morphological and phenolic characters. Amer. Journ. Bot. 60:678–684.
Jepson, W. 1910. The silva of California. University of California Mem. Vol. 2. 480 p. University of California, Berkeley.
Kahrl, W. 1979. The California water atlas. Prepared by the Governor's Office of Planning and Research in cooperation with the California Department of Water Resources. 118 p. Sacramento, Calif.
Kramer, P., and T. Kozlowski. 1979. Physiology of woody plants. 811 p. Academic Press, New York, N.Y.
Larcher, W. 1975. Physiological plant ecology. 252 p. Springer Verlag, New York, N.Y.
Little, E., Jr. 1953. Check list of native and naturalized trees of the United States (including Alaska). USDA Forest Service Agriculture Handbook No. 41. 472 p.
Little, E., Jr. 1971. Atlas of United States trees. Volume 1: conifers and important hardwoods. USDA Forest Service, Misc. Publ. No. 1146. 9 p., 200 maps.
Little, E., Jr. 1976. Atlas of United States trees. Volume 3: minor western hardwoods. USDA Forest Service, Misc. Publ. No. 1314. 13 p., 210 maps.
MacArthur, R. and J. MacArthur. 1961. On bird species diversity. Ecology 43:594–598.
Major, J. 1963. A climatic index to vascular plant activity. Ecology 44:485–498.
Major, J. 1977. California climate in relation to vegetation. p. 11–74. In : M. Barbour and J. Major (ed.). Terestrial vegetation of California. 1002 p. John Wiley, New York, N.Y.
Mather, J., D. Carter, and F. Hare. 1964. Average climatic water balance data of the continents. Part VII: United States. C.W. Thornthwaite Associates Lab. Climatol. Pub. Climatol. 17:419–615.
Miller, G. 1955. The genus Fraxinus , the ashes in North America, north of Mexico. Cornell Agri. Exp. Sta. Mem. 335. 64 p.
Monk, C. 1966. An ecological significance of evergreenness. Ecology 47:504–505.
Mooney, H., J. Kummerow, A. Johnson, D. Parsons, S. Keeley, A. Hoffman, R. Hays, J. Giliberto, and C. Chu. 1977. The producers—their resources and adaptive responses. p. 85–143. In : H. Mooney (ed.). Convergent evolution in Chile and California mediterranean climate ecosystems. 224 p. Dowden, Hutchinson & Ross, Stroudsburg, Penn.
Munz, P. 1959. A California flora. 1681 p. University of California Press, Berkeley.
Munz, P. 1968. Supplement to a California flora. 224 p. University of California Press, Berkeley.
Page, B. 1981. The southern Coast Ranges. p. 329–417. In : W. Ernst (ed.). The geotectonic development of California. 706 p. Prentice-Hall, Englewood Cliffs, N.J.
Pirone, P. 1970. Diseases and pests of ornamental plants. Fourth edition. 546 p. Ronald, New York, N.Y.
Raven, P., and D. Axelrod. 1978. Origin and relationships of the California flora. Uniiversity of California Publ. in Botany. Volume 72. 134 p. University of California Press, Berkeley.
Rehder, A. 1940. Manual of cultivated trees and shrubs. Second edition. 996 p. Macmillan, New York, N.Y.
Roberts, W., J. Howe, and J. Major. 1977. A survey of riparian forest flora and fauna in California. p. 3–19. In : A. Sands (ed.). Riparian forests in California: their ecology and conservation. Institute of Ecology Pub. No. 15. 122 p. University of California, Davis.
Robichaux, R. 1977. Geologic history of the riparian forests of California. p. 21–34. In : A. Sands (ed.). Riparian forests in California: their ecology and conservation. Institute of Ecology Pub. No. 15. 122 p. University of California, Davis.
Rosenzweig, M. 1968. Net primary productivity of terrestrial communities: prediction from climatological data. Amer. Nat. 102:67–74.
Rossi, R. 1980. History of cultural influences on the distribution and reproduction of oaks in California. p. 7–18. In : T. Plumb (tech. coord.). Proceedings of the symposium on the ecology, management, and utilization of California oaks. [Claremont, Calif., June 26–28, 1979]. USDA Forest Service GTR-PSW-44. 368 p. Berkeley, California.
Rundel, P. 1980. Adaptations of mediterranean-climate oaks to environmental stress. p. 43–54. In : T. Plumb (tech. coord.). Proceedings of the symposium on the ecology, management, and utilization of California oaks. [Claremont, Calif., June 26–28, 1979]. USDA Forest Service GTR-PSW-44. 368 p. Berkeley, California.
Sands, A. (ed.). 1977. Riparian forests in California: their ecology and conservation. Institute of Ecology Pub. No. 15. 122 p. University of California, Davis.
Small, A. 1974. The birds of California. 310 p. Collier, New York, N.Y.
Smith, C. 1976. A flora of the Santa Barbara region, California. 331 p. Santa Barbara Museum of Natural History, Santa Barbara, Calif.
Spurr, S., and B. Barnes. 1980. Forest ecology. Third edition. 687 p. John Wiley, New York, N.Y.
Stebbins, G. 1974. Flowering plants: evolution above the species level. 399 p. Belknap Harvard, Cambridge, Mass.
Stebbins, G., and J. Major. 1965. Endemism and speciation in the California flora. Ecol. Monog. 34:1–35.
Sudworth, G. 1908. Forest trees of the Pacific Slope (1967 reprint of 1908 edition). 455 p. Dover, New York, N.Y.
Thompson, K. 1977. Riparian forests of the Sacramento Valley, California. p. 35–38. In : A. Sands (ed.). Riparian forests in California: their ecology and conservation. Institute of Ecology Pub. No. 15. 122 p. University of California, Davis.
Thorne, R. 1976. A phylogenetic classification of the Angiospermae. p. 35–106. In : M. Hecht, W. Steere, and B. Wallace (ed.). Evolutionary biology. Volume 9. Plenum, New York, N.Y.
Thornthwaite, C. 1948. An approach toward a rational classification of climate. Geogr. Rev. 38:55–94.
Thornthwaite, C., and J. Mather. 1955. The water balance. Drexel Inst. Technol. Pub. Climatol. 8:1–104.
Tucker, J. 1980. Taxonomy of California oaks. p. 19–29. In : T. Plumb (tech. coord.). Proceedings of the symposium on the ecology, management, and utilization of California oaks. [Claremont, Calif., June 26–28, 1979]. USDA Forest Service GTR-PSW-44. 368 p. Berkeley, California.
US Department of Agriculture. 1941. Climate and man: yearbook of agriculture. 1248 p. US Department of Agriculture, Washington, D.C.
US Department of Commerce. 1970. Climate of California. In : Climates of the states: climatography of the United States, 60–4. 57 p. US Depatrment of Commerce, Washington, D.C.
US Department of Interior, Geological Survey. 1977. Water resources data for California. Water year 1977. Volume 4. USGS Water-data Report CA-77-4.
Walter, H. 1979. Vegetation of the earth and ecological systems of the geo-biosphere. Second edition. 274 p. Springer Verlag, New York, N.Y.
Wauer, R. 1977. Significance of Rio Grande riparian systems upon the avifauna. p. 165–174. In : R.R. Johnson and D.A. Jones (tech. coord.). Importance, preservation, and management of riparian habitat: a symposium. [Tuscon, Ariz., July 9, 1977]. USDA Forest Service GTR-RM-43. 217 p. USDA Forest Service Range and Experiment Station, Fort Collins, Colorado.
Wauer, R. 1978. The breeding avifauna of Isla Tiburon, Sonora, Mexico. Unpublished manuscript. 75 p. USDA Fish and Wildlife Service, Washington, D.C.
Wharton, C, and M. Brinson. 1979. Characteristics of southeastern river systems. p. 3240. In : R.R. Johnson and J.F. McCormack (tech. coord.). Strategies for protection and management of floodplain wetlands and other riparian ecosystems. [Callaway Gardens, Georgia, December 11–13, 1978]. USDA Forest Service GTR-WO-12. 410 p. Washington, D.C.
Willis, J., and H. Airy Shaw. 1973. A dictionary of the flowering plants and ferns. Eighth edition. 1245 p. Cambridge University Press, London.
Willson, M. 1974. Avian community organization and habitat structure. Ecology 55:1017–1029.