The Dalkon Shield
IUDs are probably the most controversial medical device in the United States. Chapter 4 discussed their entry and rapid distribution into the market in the early 1970s.[43]
See discussion in chapter 4.
By the mid-1980s, the technology had practically disappeared. The story of theDalkon Shield has been told elsewhere in great detail.[44]
See Morton Mintz, At Any Cost: Corporate Greed, Women, and the Dalkon Shield (New York: Pantheon, 1985); Susan Perry and Jim Dawson, Nightmare: Women and the Dalkon Shield (New York: Macmillan, 1985); and Sheldon Engelmayer and Robert Wagman, Lord's Justice: One Judge's Battle to Expose the Deadly Dalkon Shield IUD (New York: Doubleday, 1985).
It is discussed here to illustrate the impact of mass tort litigation on a firm.Few defend the actions of A. H. Robins Company, either in the marketing of the IUD or in its subsequent behavior after the product was withdrawn. It is generally agreed that Robins entered the contraceptive market without knowledge or experience. It relied on erroneous research data, ignored warnings of product risks, and denied the existence of evidence to the contrary. The court found serious wrongdoing on the part of the company, and an official court document affirmed "a strong prima facie case that [the] defendant, with the knowledge and participation of in-house counsel, has engaged in an ongoing fraud by knowingly misrepresenting the nature, quality, safety, and efficacy of the Dalkon Shield from 1970–1984."[45]
Hewitt v. A. H. Robins Co., No. 3-83-1291 (3rd Div. Minn., 21 February 1985).
The problem with the product has been traced to its multifilament tail, a string attached to the plastic shield (see figure 20). The string allowed women to check that the product was in place and facilitated removal by a physician. This string was not an impervious strand but was composed of many strands that allowed bacteria to be drawn from body fluids into the uterus. The result was inflammation and infection, leading to illness, sterility, and, in some instances, death.[46]
Subrata N. Chakravarty, "Tunnel Vision," Forbes, 21 May 1984, 214-215.
The product was removed from the market in 1974, after several deaths and 110 cases of septic abortion (miscarriage caused by infection in the uterus). Then the lawsuits began. By June 1985, there were 9,230 claims settled and 5,100 pending; Robins had paid out $378 million at that time.
The cases continued to pour in. The company filed for Chapter 11 bankruptcy protection in August 1985.[47]
New York Times, 22 August 1985, 36.
As part of the proceedings, a reorganization plan had to be approved, and it could not go into effect until it was clear that no legal challenges to it survived. A plan was finally approved at the end of 1989, more than four years after the initial bankruptcy filing.[48]Wall Street Journal, 7 November 1989, A3.
This action cleared the way for the acquisition of the company by American Home Products and the establishment of a trust fund of $2.3 billion to compensate women who had not yet settled their claims with Robins.The plan transferred all responsibility for the Dalkon Shield
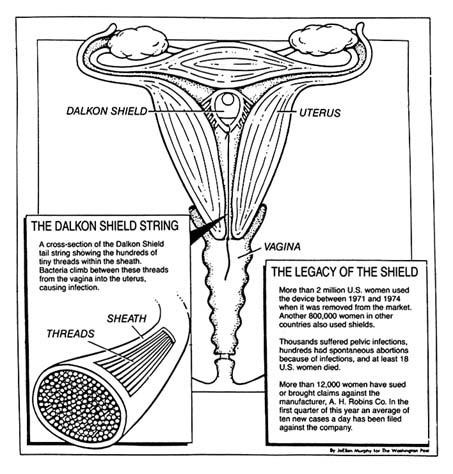
Figure 20. The Dalkon Shield.
Source: Washington Post National Weekly, 6 May 1985, 6.
claims to the trust. The trust funds were available to resolve the remaining 112,814 claims pending in 1989.[49]
Alan Cooper, "Way Is Cleared for Robins Trust," National Law Journal, 20 November 1989, 3, 30.
Twenty years after the product was marketed and sixteen years after it had been removed, injured claimants still awaited compensation. In late 1989, a federal grand jury began a criminal investigation into allegations that Robins concealed information and obstructed civil litigation.[50]Ibid., 30.
The course of this litigation raises questions about the efficiency of the tort system to accomplish its goal to compensate for
injuries. It also raises issues of deterrence. Who has been deterred? Ideally, of course, only unscrupulous companies or producers of unsafe products should be deterred by liability law. However, it appears that bona fide contraception innovators have generally abandoned the market in the wake of these lawsuits. It is difficult to justify a liability system when its primary goals—compensation and deterrence—are not met.