Physical and Organic Iodine
Theory of Trace Elements
A trace element's essentiality is difficult to demonstrate, for, in contrast to bulk and macro elements that are ingested and concentrated in living tissue at levels measured in grams and kilos, trace elements are ingested and concentrated in tissue at low concentrations and are measured in milligrams and micrograms. The biological role of only a few of these elements is known at present, but the list is expanding. An early definition of essentiality held that an element is essential if it is required for the maintenance of life and if the organism dies in its absence. The definition was problematic, however, for even in a laboratory experiment it is difficult to eliminate all traces of any particular element and hence to demonstrate that death follows from total deficiency. As a result, a more workable definition of what is essential has been proposed:
An element is essential when a deficient intake consistently results in an impairment of a function from optimal to suboptimal and when supplementation with physiological levels of this element, but not of others, prevents or cures this impairment. (Mertz 1981)
A trace element is now considered essential if on ingestion in suboptimal amounts, it impairs function and on supplementation, restores it. This change in definition is significant for health policy because the presence of apparently unafflicted individuals amid a population believed to be deficient posed, according to the old definition, a problem: their very presence cast doubt on the notion that the element was essential to the maintenance of life. A well-formed, intelligent individual amid a cretinous and goitrous population seemed, in the case of iodine, to call into question the whole idea of essentiality. The new definition disposes of that obstacle to prophylaxis.
The Dose Response Curve
The dose response curve (fig. 1) illustrates the new definition. It facilitates consideration of impaired function and deals with overintake as well as deficiency. Arsenic's toxic effects in large doses are well known, for example, but its deficiency effects are only beginning to be documented. Conversely, effects of molybdenum deficiency were well known before its toxic effects were even surmised.
The shaded area on the left in figure 1 shows impaired function below a certain threshold and adequate function above it. The shaded area on the right indicates the dysfunctional aspects of over-dosage. The intake of iodine at either extreme can produce a hypoor hyperfunctional thyroid gland. Optimal function takes place within a wide range of intake, allowing for daily and seasonal variation. People can take in most of their annual iodine requirement, for example, over the course of a fishing season. The breadth of that safe margin makes it unnecessary for policymakers to spend time pinpointing "locally ideal" levels of supplementation.
A trace element does not act by itself. Its efficacy depends on organification, that is, on its becoming part of a carbon compound within a living organism. It becomes effective only on forming part of larger molecules, such as the pair of thyroid hormones, T3 and T4.
Homeostatic mechanisms buffer the ends of the range of optimal intake. Supraoptimal amounts of a trace element may simply be excreted when intake far exceeds the required level. Suboptimal intake may be buffered, as in the case of iodine, by shifting production of hormone to T3, the generally more potent of the pair, which uses fewer atoms of iodine.
Below, I describe how iodine moves through the environment, the food chain, and the body; how certain factors impede its transformation into hormone; how the body responds to marginal intake; and the disorders in which iodine deficiency plays an important though often poorly appreciated role. This understanding of the cycle, and of iodine physiology and pathology comes from standard biomedical sources (Stanbury 1969, 1978; Matovinovic 1983; Fisher 1983; Utiger 1979; Tepperman, 1980; Petersdorf 1983; Netter 1965; Pitt-Rivers 1961; Thompson and Thompson 1980).[2]
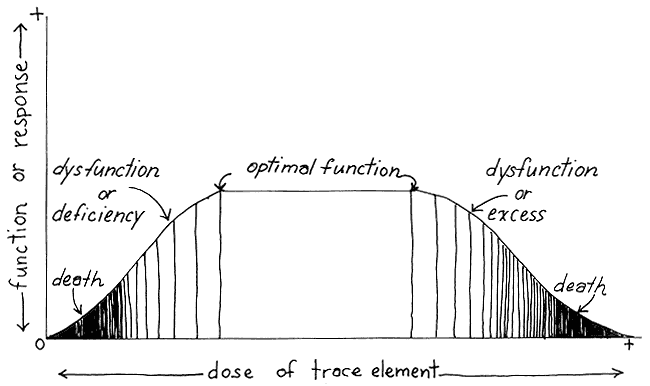
Fig. 1.
Dose Response Curve (based on Mertz 1981:1332)
My purpose in focusing on the element needs to be underscored and explained: conventional medical presentations leave clinicians and health officials without a proper appreciation for the movement of iodine through the physical and organic world, setting the stage for taking the presence and availability of iodine for granted. Prophylaxis may take a back seat to therapeutics when this movement fails to be appreciated.
The Cycle of Iodine in the Environment
Iodine makes up 0.4 percent of the earth's mass but is unevenly distributed. It is present in rock and earth in the form of soluble iodine salts that when taken up by plants, enter the food chain. Iodine's solubility makes it prone to being leached out of soil, especially in areas of heavy precipitation. In this way, it gravitates toward the sea where it becomes concentrated.
Oceanic evaporation permits iodine to become airborne and return to the land by way of atmospheric iodine transport. Climatic forces of glaciation and high precipitation leach iodine out of highlying mountain areas such as the Alps, Himalayas, and Andes, leaving many mountain populations severely iodine deficient.
Leaching is particularly severe where the parental rock is limestone, as in the Cantabrian range of central and eastern Asturias. Limestone lowlands, once glaciated, tend also to be poor in iodine. In such areas, problems of iodine deficiency are compounded, for limestone dissolves as water percolates through it, thus charging groundwater with minerals. As part of drinking water those minerals bind with iodine, making it less available for organification. The "goiter belt" of the United States, stretching from New York State to Minnesota and beyond, exemplifies such a case. As a general rule, the farther the area lies from the sea, the slower it is replenished by atmospheric transport.
Iodide is more abundant in rock and soil than in seawater, but the life forms that thrive in seawater concentrate it, for example, in kelp and fish thyroids. These substances themselves, or the ash derived from them, have long been used in China, the Andes, and Asturias as folk remedies for goiter.[3]
The largest natural storehouse and site of extraction of iodide is the Chilean nitrate bed, which was formed when ancient sea-
beds became mineralized. Until recently, most of the world's iodide production came from this deposit. With the multiplication of industrial uses of iodine in the twentieth century, iodide production has diversified, drawing on both minerals and plants for raw material. Kelp, for example, is harvested on Asturian shores and sent to other Spanish provinces for processing into gums and chemicals. Indeed, more than 99.5 percent of the world's current production of iodide and iodate is destined for industrial ends not related to nutrition. Supplementation of the world's human population with prophylactic iodine would annually take no more than 370 tons. However scarce iodine may be, even in the diets of people harvesting it from the sea for industrial purposes,[4] it cannot be considered a scarce world resource.
Dry salt mined from interior deposits may, before it is purified, be rich in iodide. But contrary to popular belief, solar salt and sea salt made from iodine-rich brine are not themselves rich in iodine, for brine contains impurities drawn off before the salt is harvested. Only artificial applications of iodide during later stages of salt manufacture ensure its iodine content.
Drinking water is frequently used as an indicator of local iodine status, though humans rarely receive more than 10 percent of their dietary iodine from drinking water. It may, however, be an appropriate indicator of intake if one recognizes that water draining the local environment generally reflects the iodine content of the vegetation, thus reflecting the iodine status of people subsisting chiefly on locally grown plant food. It is, however, a poor indicator of iodine status when the diet includes goitrogens (see below) or when the diet includes many foods of animal origin, since terrestrial iodine becomes concentrated at the top of the food chain. This means that people with greater access to milk, eggs, blood, and meat—to foods at the top of the food chain—are less likely to experience pathology than those subsisting almost exclusively on a diet of roots, nuts, and grain. A dual diet within a single zone can thus exempt the richer segment of society from symptoms while producing them in the poorer. Unfortunately, this differential effect props up belief in the innate vulnerability of the poor, while seeming to undermine the environmental hypothesis.
Iodine has been withdrawn or added to diets in unexpected ways. Disturbance of trade routes or a change in salt supply has
brought symptoms of iodine deficiency to populations formerly free of them. In Nepal, for example, newly available solar salt has supplanted the unrefined rock salt formerly transported by animal power over difficult mountain passes (Mumford pers. comm.). In New Guinea, noniodized commercial salt has suppressed traditional salt laboriously extracted from certain rare iodide-concentrating plants (Buchbinder 1977).
Commerce and industry have adventitiously introduced iodine in several ways. Subsistence agropastoralists turning to commercial feeds, for example, have inadvertently introduced iodine from outside the local ecosystem into their own food chain.[5] People have unknowingly absorbed iodine in medications and applied it as a first aid measure to the skin. The expanding food industry has introduced it into food, prompting the National Academy of Sciences to propose that "any additional increases should be viewed with concern. It is recommended that the many adventitious sources of iodine in the American food system, such as iodophores in the dairy industry, alginates, coloring dyes and dough conditioners, be replaced wherever possible by compounds containing less or no iodine" (National Academy of Sciences 1970). A more balanced statement by the academy would have addressed not only national surfeits but also global deficiencies, taking into account as well the dangers at the low end of the dose response curve. The academy thus displayed the unexamined assumption of "iodine affluence" characteristic of much of Western biomedicine. Health workers in the Midwest have recently reported the reappearance of goiter on farms (NYT Sept. 29, 1987:1), calling into question the assumption of iodine affluence even in the United States. In chapter 7, we will see how this assumption has been exported around the globe.