Accommodation and Defiance
Little wonder, then, that a note of panic appeared in Kolbe's October 1853 letter to Vieweg. Having adjusted his theories and devised what seemed to him to be decisive refutations, Kolbe wrote the critique discussed above, and incorporated it into the first installment of his textbook, which appeared in June 1854. In his introduction, he commented that a number of empirically shallow theories, proposed mostly by French chemists, threatened to destroy the true basis of organic chemistry, the electrochemical radical theory. It was therefore necessary to begin his text by treating these ideas in detail.
The task facing organic chemists today, Kolbe averred, is the elucidation of chemical constitutions by discerning the "rational compositions" of molecules, that is, the manner in which their proximate components are combined. Kolbe had nothing but contempt for those empiricists whose love of experimentation (Experimentirkunst ) was greater than that for true scientific research and who only sought to produce new substances as fast as possible without investigating their constitutions. He stated that we will "never" be able to gain a "clear view" of the spatial arrangements of the atoms in a molecule; but we are now and will be in the future able to discern the "functions" that atoms and proximate components perform with respect to each other.[39]
The only sure guide to rational constitutions, Kolbe continued, is Berzelius' electrochemical radical theory, founded on the conviction that organic chemical theory must be based on analogies to inorganic chemistry. There are problems with organic electrochemical theory, Kolbe conceded. Above all, we often cannot isolate the components of organic molecules, and even when we can, the determination of their electrochemical properties is problematic. But we should maintain a sense of perspective and not abandon an old, trusted, and useful theory at the first sign of difficulty. Have confidence in the flexibility of a good theory, he counseled, and trust to the future to work through the anomalies.[40]
Consciously or not, Kolbe illustrated this flexibility by the adjustments that he continued to make in his own version of the theory. In the 1840s he had viewed oxalic acid, HO.C2 O3 , as the progenitor of all organic acids. Beginning in 1848 he had preferred to view acids in a more general fashion, as deriving from an ultimate resolved radical C2 to which one could add methyl to form the more proximate acetic acid radical
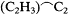
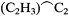
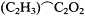
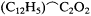
Thus, whereas in 1850 he had conceded to type theory the thesis that chlorine and other atoms may substitute in organic radicals with-
out necessarily causing major alterations of properties, he was now expanding this concession to include all-important oxygen. Moreover, he now viewed hydrogen substitution as the schematic means of constructing all organic acids and acid derivatives from formyl. Other systems were analogous: amines were copulated nitrogens produced by substitution of hydrogens by hydrocarbon radicals, and so on. The fact that halogen substitution does not much alter chemical properties of hydrocarbon radicals proves that "the grouping of their proximate components remains the same" and that their "types" are unaltered. This is true in some cases for inorganic substituting groups as well, such as amido, nitro, and sulfuryl groups.[42] Dumas could not have argued with greater force or clarity for the assumptions of type theory than Kolbe did here.
Despite these concessions, however, Kolbe remained resolutely faithful to dualistic organic chemistry. Although halogen substitution for hydrogen cannot be explained by means of electrochemical precepts, that does not mean that the precepts are wrong. Chlorine, perhaps even hydrogen, might undergo an alteration of electrochemical properties when it enters organic molecules. After all, elemental phosphorus has several chemically different states, and nascent hydrogen is very different from the stable gaseous element. Thus, he believed it would be "frivolous" to abandon dualism because of the single anomaly of substitution.[43]
Kolbe also retained the copula theory as the last major element of dualism left in organic theory. Copulas, he continued to affirm, have little influence on properties of the prototype acid. For example, formic, acetic, and propionic acids are quite similar despite the presence of hydrogen, methyl, and ethyl as copulas, respectively. Accepting Frankland's demolition of the copula theory would require conceding that atoms have fixed combining capacities, irrespective of the electrochemical properties of the adducts—a challenge directed to the very heart of dualism.
But Frankland's evidence was hard to deny. Although stressing that he did not agree with Frankland's conclusion, Kolbe admitted that there did seem to be a general law at work. One might be able to account for it, he thought, by a qualitative electrochemical argument relating to the varying affinities for oxygen of different elements. Since positive elements such as potassium and calcium combine with oxygen in only a small number of proportions, whereas negative elements such as halogens, sulfur, and nitrogen combine in a much larger number, perhaps the addition of alkyl groups to, say, nitrogen makes the nitrogen less negative, hence less capable of combining with additional oxygen. Thus, the total number of atoms or radicals combined with
nitrogen remains roughly the same. Even so, he noted that his tentative suggestion "in no way explains" the extreme regularity or exactness of the relationship revealed by Frankland.[44]
Although often sounding here much like a "typist" himself, in a historical section he poured scorn over the "games with formulas" that
. . . for many years the best chemists of France have been playing, with unbelievable self-deception. Rarely indeed has a new theory been introduced into science with such ostentation and such great confidence in its infallibility, as Dumas' type theory. Seldom has unscientific behavior and the desire immediately to derive general laws of nature from isolated facts been more severely punished as in this case. Of the numerous laws with which the type and substitution theories wanted to enrich science, one after another had to be retracted, and finally nothing more remained than the naked fact that in many organic compounds hydrogen can be substituted by chlorine without essentially altering the molecular grouping of the atoms or in many cases the characteristic properties of the compound.[45]
Kolbe noted the "remarkable fact" that it was almost exclusively German scientists who had developed the radical theory, and he ventured the opinion that the French had opposed the theory "because it had not developed on French soil." Laurent's theory of fundamental and derived nuclei, which some had considered a sort of radical theory, was nothing of the kind to Kolbe; it was a "painting of the imagination," reminiscent of Naturphilosophie. An example of Laurent's penchant for "paper-and-pencil laws of nature" was his notion that oxygen substituting within the nucleus does not alter the characteristic neutrality of organic nuclei (to form aldehydes, ketones, ethers, etc.), whereas if one, two, or three oxygen atoms are added to the exterior of the nucleus they create mono-, di-, or tribasic acids, respectively.[46]
Some, he noted, had criticized the radical theory for hypothesizing fictitious entities, just as he was criticizing Laurent's nuclei. These cavils no longer had any force since he and Frankland had isolated a number of hydrocarbon radicals. Others had suggested that methyl, ethyl, etc. were not real (monomeric) radicals but rather dimeric molecules. To the extent that this argument was based on the application of Laurent's even-number rule, it was just another example to Kolbe of a fictitious French law. Hofmann's claim that one ought to have expected extreme chemical lability from hydrocarbon radicals is also flawed; after all, the radicals are homologous with hydrogen, which is stable and fairly unreactive except regarding oxidation. Finally, the evidence from boiling points could also be countered. Kopp's laws were known to have many exceptions, such as comparing H-OH with
CH3 -OH, and it could well be that the new radicals represented further exceptions. (This despite the fact that in 1847 Kolbe himself had used boiling points to argue for his interpretation of the constitutions of the fatty acids.) In Kolbe's view, the case for monomer versus dimer formulas was simply unproved, and in such instances one must prefer the simpler hypothesis.[47]
At the end of the general section of his textbook, Kolbe printed a truncated version of his critique of the Williamson-Gerhardt ether theory. He chose to omit the Wrightson argument and the methodological critique of synthesis as providing access to constitutions, although this was probably not in reaction to Williamson's reply, which he had presumably not yet seen. However, the week after his critique and Williamson's rebuttal were read in London, Guthrie's experiments in Marburg cast further light on the matter, and Kolbe just had time to incorporate the consequent changes into his manuscript. If, as Williamson had argued, potassium ethoxide has a constitution analogous to water, then in electrolysis the constituents occupying the places of the hydrogen—potassium and ethyl—should share the latter's electrochemical behavior and emerge at the negative electrode. As Guthrie found that only hydrogen appeared there, the theory appeared to be weakened.[48]
Kolbe, however, put the matter more strongly. To Vieweg he declared Guthrie's evidence a "fundamental proof of the untenability of [Williamson's] hypotheses," and in his text he stated that this "fundamental experiment . . . completely refutes" the French-English theory.[49] Words such as "proof" and "refutation" leave no space for doubt or debate. It is more than a little ironic to see such strong wording coming from Kolbe, who here and elsewhere openly conceded the anomalous character of many electrolyses and the unpredictability of the electrochemical properties of organic compounds and their components. Furthermore, Heinrich Will was soon to note that Kolbe failed to see that his own formulas were fully as susceptible to his own critique as Williamson's. But even without such internal contradictions, the investment of perfect certainty in a purely analogical argument suggests methodological problems.