7—
Frankland and the Chemists, 1866–85
month after month, with a process of analysis that is faulty in the extreme, and with speculations that admit of no proof, we are either frightened or amused [by figures] . . . showing that such water . . . had been contaminated with sewage or manure matter equivalent to so many hundred parts of average filtered London sewage. Gentlemen, if this were not put forth in the garb of science, and, moreover, in a semi-official form, it would be regarded as a burlesque, and would excite nothing but ridicule.[1]
H Letheby
To make sense of Frankland's career as a water analyst we must view it in two contexts. The first is of water policy-making and of the participation of scientists in that process. The second is of the growth and changing structure of the profession of chemistry. During the period in which Frankland came into government service, government-by-experts was coming to the fore. According to Oliver Macdonagh and those who have followed him they came in through a common pattern.[2] Entering government initially to cope with crisis or as a result of tepid legislative initiative, inspector-experts expanded their mandates, discovered ever more that needed doing, and by the presentation of undeniable fact, prevailed upon the legislature to grant them expanded powers. At first glance Frankland's career seems to conform to this pattern. He had stepped into a marginal position and expanded its importance. He inspected, found things wanting, and reported, calling for major new legislative initiatives. Yet the divergence from this pattern is more important. Despite his official appointments, Frankland retained his independence as a consultant. And his policy recommendations were not so much the outcome of his investigations, but the result of principles of water
supply he had adopted early in his career as a water analyst. His subsequent investigations and reports, from 1870 onward, were more important in maintaining his reputation as a well-informed expert than in advancing his views, which changed little until the mid '90s. His monthly reports on London's water had their greatest impact directly on public opinion through their publication in newspapers; those officials to whom they were directed ignored his recommendations on those occasions when they were not actively trying to moderate his extreme interpretations.
Frankland's stature as a chemist, his skilful exploitation of his official status, and his adroit circumvention of the limits of empirical investigation profoundly affected British water analysis for the remainder of the century. To understand how we need to understand the structure of British chemistry. For present purposes we can divide British chemists into three groups. First, there were several other eminent chemists, holders of professorships, recipients of government patronage with interests in sanitary matters—chemists, that is, who would seem to have been well enough placed to have acquired prominence in water matters equal to Frankland's but didn't. Second were other prominent chemists who for various reasons came to form a loosely knit faction in opposition to Frankland. Most important were William Crookes, James Alfred Wanklyn, and Henry Letheby and Charles Meymott Tidy, the latter two the principal consultants to the London water companies. Third was the large (and growing) mass of practicing chemists, often with training from the Royal College of Chemistry or another technical school, and working for the most part quietly in the provinces in industry and in private analytical practice, which might occasionally include water analysis. The great range of competence and ethical standards among these chemists led the leaders of the profession, including Frankland, to establish the Institute of Chemistry in the early 1880s, an organization which would certify competence and set professional standards.[3] The first two of these groups are considered in this chapter, while the public analysts, those of the rank-and-file chemists who took most interest in water analysis, are considered in the next.
Potential Rivals
During the years following the cholera of 1866 many of Frankland's colleagues were wrestling with the same problems of purification
and analysis, and many of them came to share his belief that chemistry could neither discover the poisons of cholera nor guarantee any method of removing them. The range of opinion (and bewilderment) is evident in the three nights of discussion that took place at the Institution of Civil Engineers following a short paper on water filtration by Edward Byrne in 1867. At issue was whether water was harmful, what it was in water that was harmful (changing organic matter, disease germs, organic and inorganic poisons of some kind), how it acted (by predisposition, infection, or neither), how to analyse the organic matter in water (ignition and permanganate processes were both objectionable), and what, if anything, filters did to purify water. Well respected experts (including arch enemies Thomas Hawksley and Edwin Chadwick) could still be found who dismissed the notion of water-borne disease. There was a great variety of dogmatic opinion on particular points at issue, but no clear route to consensus, either in matters of analytical technique or water policy.[4]
Among the most prominent of Frankland's contemporaries taking an interest in such issues were W A Miller, William Odling, and Robert Angus Smith. Miller, professor of chemistry at Kings College, had been with Hofmann and Graham one of the three 'government chemists' to investigate London's water in 1851. He later served as consulting analyst to Britain's senior public health official, John Simon. In June 1865 Miller presented the Chemical Society with his 'Observations on Some Points in the Analysis of Potable Waters.'[5] Before joining Frankland as one of the consultant chemists to the Water Supply Commission, William Odling had gained experience in sanitary matters as medical officer to the Parish of St Mary's Lambeth. Trained as a medical man, he had studied chemistry with A S Taylor at Guy's Hospital and was among the most successful in combining medicine with chemistry. Odling became one of the leading chemical theorists in Britain and succeeded Brodie in the Oxford chemistry chair in 1872. In the early '80s he became one of the analysts for the London water companies and the most astute of Frankland's critics on water analysis.[6] Chadwick's ally Robert Angus Smith had been one of the Cattle Plague Commissioners who in 1865 had tentatively considered the implications of a germ theory of disease. In taking a serious interest in the work of Pasteur he was unusual among British chemists, and tried to take water analysis away from a narrow focus on the quantity of organic matter. All three testified to the Water Supply Commission on the state of
water analysis.
These three chemists shared most of the elements of the Frankland-Brodie perspective. Miller agreed that chemists did not know exactly what in water was harmful or potentially harmful and agreed that both analysis and purification were untrustworthy, but he thought that if chemists would only adopt uniform procedure, it would be possible to correlate composition with health. On the key question of whether contaminated water could be safely consumed, Miller replied that 'in the majority of instances' it could though 'there may be cases in which danger is produced.'[7] Odling, similarly vague, saw drinking water purity as simply 'a practical question'—the purer the better, but water might still be acceptable even if it were not quite so pure. In 1860 and again in 1868 he expressed general satisfaction with the water of the London companies, yet admitted great inadequacies in water analysis.[8] Smith believed cholera to be caused by some sort of living germ, which if 'carefully nursed' would be visible through the microscope.[9] He still held that because not all types of organic matter were equally harmful, the population of microscopic creatures in a water rather than its chemical composition was the best indicator of the type of organic matter present, yet he retained the old and vague Hassallian standard; it was the number of organisms, their size, and disgustingness that remained the measure of water quality.[10] On the vexed issue of previous sewage contamination all three acknowledged that inorganic forms of nitrogen sometimes came from sewage but denied that they afforded grounds for calculation of Frankland's 'previous sewage contamination'. They all expressed great admiration for the combustion process—indeed Odling was its most ardent early defender—yet none of them followed Frankland in insisting on the 'utter untrustworthiness' of alternative processes.[11] Odling, like Miller and Smith, used the permanganate process, Wanklyn's new process, and Frankland's process, acknowledging that each offered useful information.
Each of these three was a chemist of stature, each held some manner of official appointment relating to sanitary chemistry and water quality; any one of the three might well have become the dominant figure in water analysis. Yet though each recognized much that was problematic in water analysis none of them saw the need for such thoroughgoing reformulation as Frankland did, and consequently none was able to provide the leadership that he did. Satisfied that science, however inadequate the existing state of its art might
be, provided the best guide to better water, they saw no need for Frankland's manipulations of public opinion.
These three, however, remained on the margins of water matters. They analysed, consulted, testified, yet avoided being drawn into the maelstrom of controversy that surrounded Frankland. Such was not the case with William Crookes, Henry Letheby (and his successor Charles Meymott Tidy), and James Alfred Wanklyn. The careers of these men, at least in matters of water quality, came to be dominated by opposition to Frankland.
Crookes and Chemical News
As editor of the Chemical News , the profession's chief trade paper, William Crookes had a significant impact on the reception of Frankland's ideas. Crookes' pages were open to controversy, and through editorials and book reviews he took part. Crookes vacillated for several years before finally coming to an anti-Frankland stance on water matters. Although in 1865 he had been one of the developers of a germ theory of the cattle plague, he initially had little patience with Frankland's undetectable and unremovable water-borne cholera particles. In Crookes' view germs were air-borne: they were in sewer gas and they infected cisterns, cholera therefore had more to do with local unsanitary conditions than with the systematic distribution of bad water. Basing his conclusions in part on the 1851 Graham, Miller, Hofmann, study, Crookes maintained that 'there is no evidence, physical or chemical, to show that this [organic matter in London water] is otherwise than harmless.'[12]
By April 1867, however, Crookes had admitted that the East London company's polluted supply and poor management had caused the epidemic there, though he was relieved to learn that the fault had been the engineers', not the chemists'.[13] The spring of 1869 marked the high point of his support for Frankland. He defended 'previous sewage contamination': 'the phrase simply states an indubitable fact.' He recognized that Frankland was not denying that bad water might become pure, only challenging the claim that purification was inevitable. The burden of proof, Crookes argued, properly lay with those who put their faith in purification.
We know as an absolute fact that tons upon tons of human excrement are thrown into the waters of the Thames and . . . Lea before we drink them, and we have a right to look for absolute demonstration of the complete destruction of all this filth; and not only
of the filth itself—of the lifeless organic matter;—we must also be convinced that it is impossible, at all times and under all circumstances, for living matter—the low forms of life and their germs with which the processes of disease and putrefaction appear to be so closely connected—to retain their vitality.[14]
But growing differences caused a rift between the two in the early '70s. While in 1869 he had praised the combustion process as 'very greatly more accurate than any that has hitherto been suggested,' by 1872 he was urging a ban on its use in official investigations.[15]
The grounds for Crookes' opposition were threefold. The first was personal. The two disagreed about the best means of sewage treatment. Crookes favoured the sewage precipitation approach in which chemicals were added to sewage to coagulate suspended matters into a form that could be marketed as a dry fertilizer. Frankland regarded such schemes as unworkable, even downright fraudulent. In 1868–69 his Rivers Commission investigated the ABC process of the Native Guano Company in which Crookes was deeply involved as a director, publicist, chemical advisor, salesman, and even for a time as chairman. Frankland made an example of the process, hinting that Native Guano was trying to fool its manure customers by sending out doctored samples and even trying to fool his investigators by secretly diluting its effluent.[16] Throughout the 1870s Chemical News championed precipitation and attacked Frankland's alternative of land treatment as a rarely practicable technique advocated by muddle-headed idealists.[17]
The other grounds for opposition were more clearly professional. Chemical News was a mouthpiece both for professional chemists and for the British chemical industry. Industrialists rejected Frankland's claim that industrial wastes could be profitably recycled and maintained that the Commission's proposed effluent standards would be impossible to meet. Crookes took up their case: the Commission's effluent standards were harsh, inconsistent, and arbitrary.[18] Parliament did drop these standards from unsuccessful bills presented in 1872 and 1873. Much to Crookes' chagrin, the Rivers Pollution Prevention Act which passed in 1876 had no standards whatever.[19]
Finally, Crookes was concerned with the implications of Frankland's system for the practice of water analysis. The time, expense, and experience required to use the combustion process successfully meant that for practical purposes it was unavailable to most analytical chemists, who would only occasionally be analysing waters. According to Cornelius Fox, a leading medical officer of health, it
would require six months for an ordinary medical man to learn the process while the apparatus would cost at least 13 guineas.[20] What concerned Crookes and others was that the effluent standards proposed by the Rivers Commission were expressed in terms of organic carbon and organic nitrogen, variables that could only be measured by the combustion process. If these standards became law water analysis would become centralized. Chemists would be forced either to adopt Frankland's costly techniques or abandon water analysis. The rank and file of analytical chemists who made up the Chemical News constituency did not use combustion, Crookes pointed out repeatedly. The issue was thus one of professional democracy: chemists looked upon Frankland's process as 'impracticable and fallacious'; in the name of professional unity he ought to give it up.[21]
These concerns were inter-related and the issue of the validity of Frankland's water analysis underlay all of them. If Frankland's analysis were discredited the rest of his water science would fall with it. Crookes was an able polemicist and rarely neglected an opportunity to make a scornful aside about Frankland's water science. A review of Pettenkofer's work on cholera or of an annual report by the Massachusetts State Board of Health was occasion to snipe at PSC or the Rivers Commission.[22]
The Wanklyn Affair
Crookes' criticisms were occasional; he had other polemics to occupy him. Such was not the case with James Alfred Wanklyn, for whom revenge on Frankland became a raison d'être . Wanklyn's criticisms of Frankland were the longest sustained, the bitterest, and yet the most substantive. Between 1868 and 1877 the relative merits of Frankland's combustion and Wanklyn's 'ammonia' processes dominated discussions of water analysis in Britain. In Wanklyn's view the dispute was over which process better measured the harmfulness of a water. To Frankland such a quest was futile; analysis was to shed light on a water's history. Nonetheless, the debate was conducted largely on Wanklyn's terms, it being assumed by most chemists that the process that more accurately determined the quantity of putrescible matter gave the better measure of harmfulness. Indeed, quibbling about parts per million of organic nitrogen or 'albuminoid ammonia' completely displaced the more important questions of what, if anything, these entities signified. Still, Wanklyn's
criticisms are important: his attacks on Frankland's character damaged Frankland's credibility; his substantive criticisms of the combustion process were extensively used by others who knew little of chemistry and whose reasons for attacking Frankland lay elsewhere.
In its early stages Wanklyn's career had paralleled that of Frankland, his senior by nearly a decade. A Lancastrian like Frankland, he had been apprenticed to a Manchester doctor and later studied with Frankland at Owens College in the early '50s, where he was Frankland's assistant. Like Frankland he took advanced training at Bunsen's laboratory at Marburg and with Frankland's assistance secured appointment as a demonstrator to Lyon Playfair in 1859. From 1863 to 1870 Wanklyn was a lecturer at the London Institution; he spent the rest of his career as public analyst to a number of authorities, as a lecturer at St George's Hospital, and in private practice as an analyst.[23]
In June 1867 Wanklyn described to the Chemical Society the new 'ammonia' process that he had developed with E T Chapman and Miles H Smith. The process was based on the belief that albumin was the dangerous material in water since albuminoid substances putrefied unusually rapidly. Wanklyn believed that a definite proportion of a water's albumin was converted to ammonia when the water was distilled with a caustic solution of potassium permanganate. The amount of this 'albuminoid ammonia' could be determined by the Nessler colour test, a common and well accepted approach for quantitative determination of ammonia. This quantity was to be the main index of water quality.[24]
Wanklyn's process was thus made public in the middle of the same year that Frankland was developing the combustion approach. Both were working in the Hofmann tradition and were deeply concerned about the types of organic nitrogenous matter that might undergo dangerous decomposition. Like Frankland, Wanklyn rejected existing evaporation techniques. He ridiculed Miller's call for uniformity in analytical procedures and in the statement of analytical results, arguing that to pursue uniformity on Miller's terms would merely be to multiply comparable results of doubtful accuracy.[25]
Initially British chemists were not impressed and Wanklyn and his collaborators quickly found it necessary to moderate their claims.[26] The first Wanklyn–Chapman–Smith paper had reported trials on natural waters. When Wanklyn and associates tried to verify the process by using it on artificial solutions of pure organic compounds they ran into some surprises. In early experiments pure albumen
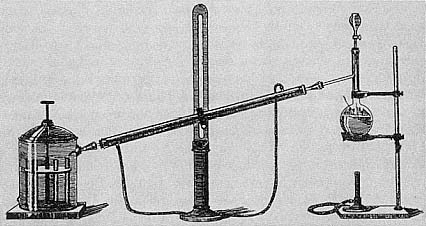
Figure 7.1
Wanklyn's ammonia process was for most British chemists the central technique
for determining the salubrity of potable water during the '70s and '80s
(From T B Stillman, Engineering Chemistry [Easton, PA: Chemical
Publishing Co, 1897], p 75).
(from egg white) gave up two/thirds of its nitrogen as ammonia. But other nitrogenous compounds apparently gave up all, or a half, or a quarter, or even a seventh of their nitrogen as ammonia. When the empirical results were multiplied by the proper factor there was close correspondence between 'theory' and observation, and to Wanklyn this was proof of the soundness of the process: substances were breaking down in an orderly way and yielding a definite proportion of their nitrogen as ammonia. Yet he was able to offer a truly theoretical explanation in only one case. To other chemists the process looked arbitrary. Wanklyn's 'theory' seemed nothing more than selecting the nearest whole number which when multiplied by his empirical result would give a result close to the calculated level of ammonia. Moreover, the question remained of what trials on pure substances implied for natural waters. In Wanklyn's view there was nothing to worry about: natural waters could be relied upon to yield a definite and unchanging proportion (two-thirds) of their nitrogen as ammonia.[27]
It may seem that the rationale for the ammonia process was flimsy. It was. The so-called 'albuminoid ammonia' was not a natural substance but an artefact of the analysis. Nor was it clear whether its quantity bore any relation whatever to harmfulness, potential for harmfulness, or even putrescibility. (In practice sanitari-
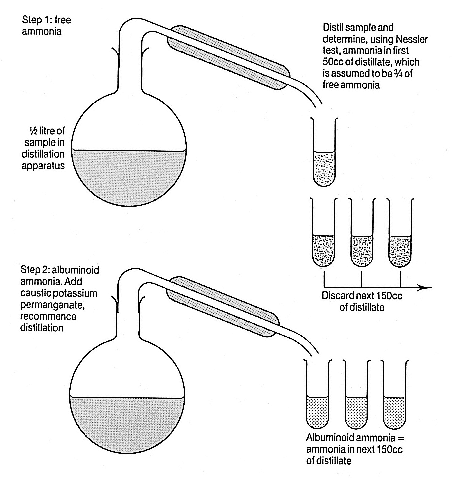
Figure 7.1
(continued)
ans would come to minimize these problems; as Fox put it, the yield of albuminoid ammonia was seen 'to keep pace with the purity or impurity' of waters.[28] )
Indeed, it is not too much to say that the launching of the ammonia process was a disaster and that Wanklyn and his colleagues made fools of themselves in it. Chemical News published a savage review of Wanklyn and Chapman's handbook on water analysis, whose authors suffered from
an excess of scientific fervour, a sort of scientific afflatus , which forms a part of the unconscious poetry of these gentlemen's natures, and which impels them to burst forth in paeans at the Chemical Society whenever any one of them conceives a new idea. We may admire such gushing enthusiasm, but we cannot but regret that the scientific
fame which these gentlemen are acquiring . . . should be sullied by the publication of raw, incomplete, and sometimes inaccurate results.[29]
Even had there been no Frankland and no combustion process to contend with, Wanklyn would have been in for rough treatment from his peers. But for him it was Frankland who came to symbolize the injustice that could be expected from the elite of chemistry.
Their dispute began in June 1867. On the same day that he formally presented the ammonia process to the Chemical Society Wanklyn testified about it to the Royal Commission on Water Supply. He said that he had been in frequent contact with Frankland, who 'had admitted . . . the extreme value of the [ammonia] process' and was testing it. He was familiar with Frankland's attempts to measure organic nitrogen through combustion and claimed that Frankland had now come to recognize that that approach was 'unsatisfactory to the last degree.'[30]
Given Wanklyn's bravado on June 20, the events of ensuing months must have been deeply humiliating. Frankland and Odling did test the ammonia process in their work for the Water Supply Commission, but in a way that was completely unfair in Wanklyn's view. They compared results obtained with the new and unverified ammonia process with those obtained with the new and unverified combustion process. Finding the two processes incompatible they rejected the ammonia process as unreliable. The rejection was not in fact quite so high-handed as it may seem—the combustion process was intended to measure organic nitrogen directly according to a simple and well-accepted principle while the ammonia process depended on the novel and somewhat dubious (and soon discarded) claim that the caustic permanganate would convert all organic nitrogen to ammonia.[31]
Matters came to a head in January 1868 when Frankland publicly introduced the combustion process in a Chemical Society lecture. He made short work of the ammonia process: it had been surpassed. The discussion took up the merits of the rival processes. Campbell, Odling, and Frederick Abel spoke against Wanklyn. Wanklyn maintained that in Frankland's own trials the combustion process had produced errors sometimes larger than the amount of organic nitrogen likely to be in the water. He and Chapman raised what seemed insuperable problems in Frankland's procedure. If combustion were to measure organic nitrogen accurately, it was necessary that all nitrates be destroyed during the initial evaporative step, yet Chapman still found nitrates in the residues of samples evaporated according
to Frankland's instructions. Further, any volatile organic materials would be lost during evaporation. Any attempt to eliminate one of these errors would exacerbate the other. Efforts to ensure total destruction of nitrates such as adding more sulphurous acid would likely increase volatilization.[32]
Wanklyn's points were well taken: 'one of the most formidable pieces of criticism which we have ever met,' noted the British Medical Journal . Throughout the early 1870s others of Frankland's critics reiterated these objections and Frankland himself devoted great attention to resolving these problems.[33] But substantive criticism was not Wanklyn's style. The ad hominem attacks made on him during the early days of the ammonia process, and what he saw as the ruthlessness and arbitrariness of Frankland's rejection of the process, played upon his paranoia. Chemical objections became secondary; he came to believe that he was a victim of a conspiracy: Frankland was the head of a washed-up elite of 'chemists whose activity does not take the direction of . . . original research.'[34] In 1871 he wrote a 'History of the Ammonia Process,' partly to warn 'younger chemists . . . [of] the sort of reception which awaits them at the hands of their chemical brethren in this country, should they be so unfortunate as to make any notable advance in chemical methods.'[35]
Ironically, as Wanklyn became increasingly bitter and increasingly isolated, the ammonia process was becoming more widely used. In 1876 Frankland himself acknowledged that the ammonia process was 'now almost [as] generally used by analytical chemists as were formerly the incineration and permanganate processes.'[36] Using the process did not necessarily mean sympathy with Wanklyn, however; many who used it had little concern with the feud or even with the validity of the two processes. The ammonia process was adopted because it was easy to learn, easy and cheap to use, and because however weak its rationale, chemists saw it as a reliable way to distinguish good water from bad. So long as one did not attach too much significance to absolute quantities of free and albuminoid ammonia, but used the results to suggest further investigations or discover changes in the condition of a particular water supply, the ammonia test was useful. Thus, ironically, the test came to have exactly the significance Frankland thought water analysis ought to have—its results were to complement other information, not to dictate whether water was safe.[37]
By the early 1870s Wanklyn had attacked most aspects of Frankland's water science and had systematically taken positions opposing
Frankland's. He had allied himself with the London water companies, defending them from Frankland's criticisms, and reversing his own earlier position.[38] In 1872 he ridiculed 'previous sewage contamination' and accused Frankland of using it for political purposes. In 1872 and again in 1879 he began programs of alternative analyses of London's waters. By employing a chemist (Frankland) who defiantly used 'illusory and defective methods,' the government had shirked its duty; Wanklyn would selflessly perform that duty.[39] He repeated the analyses of the Cumberland and Welsh waters examined by the Water Supply Commission and found them no better than the Thames.[40] In 1878 he accused Frankland of having stolen his work in the years during which he had been Frankland's assistant and noted that Frankland's great discoveries had ceased when he had left Frankland's lab.[41]
However much social factors may have kept Wanklyn from fulfilling his potential as a chemist, it is hard to grant credence to the accusations or to sympathize with the accuser: the complaints are inconsistent, incoherent, and hysterical. Even as chemists were adopting the ammonia process as an easy and useful component in a broadly based empirical approach to water analysis, Wanklyn continued to insist that it was the ultimate in water analysis and that albuminoid ammonia should be regarded as the harmful material in the water (no matter what form this was presumed to take). As W H Brock has pointed out, Wanklyn still did not accept the germ theory of disease as late as 1906; it was too much a part of Frankland's system.[42]
In the long run Frankland prevailed. He, not Wanklyn, was internationally acknowledged as the greatest expert on water quality. Many analysts continued to use the ammonia process, but in the early 1880s a few, notably Charles Meymott Tidy (who would replace Wanklyn as Frankland's main adversary during the decade) did adopt combustion, if only to put themselves in a better position to attack Frankland's conclusions about water quality.
Letheby, Tidy, and the London Water Companies
The third great challenge to Frankland's water analysis came from a group of chemists employed by the London water companies as analysts, consultants, expert witnesses, and publicists, very much the sort of multiple role Pearson and Gardner had played in 1828 and Brande and Taylor in 1850–52. There was one difference: owing to
the establishment of the position of a water analyst in the Registrar General's Office the companies now faced continual scrutiny of their supplies. They therefore had need of their own analysts to ensure that the public's knowledge of the water did not come solely from some minion of William Farr, no friend of the companies. Among the companies' chemists were men of high reputation in the world of science (it was of course precisely that reputation that made them useful to the companies) such as William Crookes, William Odling, and James Dewar. Others, like Henry Letheby and Charles Meymott Tidy, were of lesser stature, yet willing to tailor their statements to the companies' needs, no matter how far this might depart from current scientific consensus.
The first of these regular analysts was Henry Letheby, medical officer for the City of London and professor of chemistry at the London Hospital. In 1856 Letheby had succeeded John Simon as medical officer of the City of London and continued Simon's policies. In 1861 he began monthly analyses of the water of the New River, Kent, and East London companies, and by 1864 he was analysing waters of all the companies. Letheby continued these analyses for the rest of his life (he died in 1876). Ostensibly they were sponsored by the Association of Metropolitan Medical Officers of Health, but the expenses of analysis were met by the companies. However 'uninspired' and 'plodding' he may have been as a sanitary administrator, Letheby the water analyst was outspoken and aggressive.[43]
By the early 1860s Letheby had recognized specifically polluted water as a route of zymotic disease transmission and was campaigning to close the City's cesspool-polluted shallow wells. During the 1866 cholera he, like Frankland, found existing methods of purification and analysis inadequate. Even the best filtration would 'never be sufficient to . . . [secure] the complete removal of those subtle agents of disease, which even the most refined appliances of the chemist have failed to discover.' And worse, if living germs were responsible for cholera—and 'unquestionably' they were—oxidative purification, whether in rivers or by Condy's fluid (potassium permanganate), might not destroy these presumably resistant organisms. He was skeptical of chemistry: a chemist 'would be putting forth very dangerous propositions, . . . if by relying on his science alone he ventured to dogmatise on so difficult a subject.'[44] His assessment differed from Frankland's in one striking particular however: whereas Frankland believed the water companies were distributing dangerously polluted water, Letheby believed contamina-
tion occurred in household cisterns, and was therefore the responsibility of the individual.
By the end of 1866 Letheby had begun to back away from the strong stand he had taken during the epidemic. He testified as a representative of the East London Water Company at several of the official inquiries on the epidemic.[45] Letheby insisted that not only had the company's water not caused the cholera, but that its customers had possessed a 'singular' exemption from the cholera that surrounded them.[46] Among his sharpest turnabouts was with regard to analysis. In February 1867 he affirmed that analysis did accurately reflect the sanitary quality of water.[47] Since the water had been analytically good during the epidemic it could not have been the source of the cholera.
Through 1867 and 1868 the split between Letheby and Frankland widened as it became clear how pervasively Frankland was using his monthly reports to serve the cause of water reform. Letheby and others of the companies' allies were particularly troubled by the 'previous sewage contamination' calculation, which they rightly saw as a means of undermining public confidence in the water. In an attack on Frankland in the Saturday Review , PSC was described as 'an imponderable and imaginary element . . . entirely delusive.' Writing in the Quarterly Review , the engineer William Pole noted:
the companies complain, and we think with reason [that] . . . the Registrar's analysis [Frankland's] is calculated to produce needless alarm and groundless popular prejudice. . . . The prominent reiteration, month after month, of the terrifying charge of an enormous 'sewage contamination,' must produce an impression in the great mass of the public (who know nothing of the doubtful and disputed reasoning on which the statement is founded, or the far-fetched and metaphorical interpretation it is intended to bear), that it refers to some well-ascertained and offensive and unwholesome present state of the water.[48]
From the presidency of the Institution of Civil Engineers, Thomas Hawksley likewise thundered against 'theoretical chemists' (Frankland) who used unwarranted phrases 'invented to frighten Her Majesty's subjects from the use of some of the purest and most harmless . . . of waters the world can furnish.'[49]
Letheby took his stand against Frankland in April 1869 in an address to the Association of Metropolitan Medical Officers. His title was 'Methods of Estimating Nitrogenous Matter in Potable Water,' yet the focus of the address was his subtitle: 'On the Value
of the Expression "Previous Sewage Contamination," as used by the Registrar-General in his Reports of the Metropolitan Waters.' Coming slightly a year after the Frankland-Wanklyn clash of January 1868, the paper occasioned another round of heated discussion and editorials and letters to editors. Letheby recounted the enormous improvement in Thames water in the previous two decades. Organic matter had been reduced from four to six grains per gallon to about one-half grain. Yet 'great as the improvement is, the public mind continues to be agitated and alarmed by vague fears . . . unnecessarily excited by the persistent use of certain expressions of an improper kind by those who have taken upon themselves to report in a pseudo-official manner of the quality of the London waters.' As the 'custodians of the public health' medical officers should be particularly concerned about 'undue excitement and alarm of the public mind,' Letheby maintained.[50]
In the discussion Frankland replied that he had not sought the position of water analyst, but otherwise sidestepped water politics to deal with the scientific issues at hand. He upheld the propriety of his analysis. Some standard was necessary to replace the unreliable incineration (ignition) and permanganate methods, he insisted. He maintained that 'previous sewage contamination' was now an empirically verified construct: nitrates, at levels greater than could be accounted for by rainfall, were found only in waters contaminated with sewage or manure. He was strongly supported by B H Paul and also by Charles Heaton, a medical officer, and J M Rendel, an engineer. But most of the meeting was sympathetic to Letheby, and to Wanklyn, though Letheby was pressed to explain his close relationship with the water companies.[51]
An Infusion of Neutrality:
The Office of the Water Examiner, 1871
Beginning in 1871, however, Frankland was no longer the sole government official having regular oversight of water quality. The Metropolis Water Act of 1871 (Parliament's response to the Royal Commission on Water Supply) called for establishment of the post of a water examiner who was to ensure that the filtration required by law was effectively carried out. The first occupant of the post was Colonel Francis Bolton, a military engineer with experience in signalling apparatus and lighthouses.[52] Bolton was not to be an analyst; he was simply an inspector working within the revolution-in-
government tradition as it is usually understood. Bolton ieant well and he was industrious, but he was politically naive and knew little of sanitary chemistry.
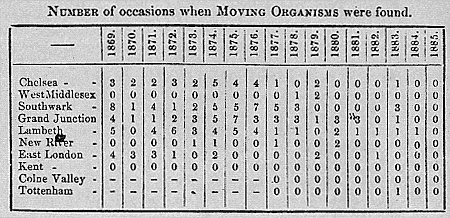
Figure 7.2
Among the least sophisticated but most effective of Frankland's propaganda
techniques was the listing of the 'living and moving organisms' detected in the
monthly water samples. Their presence ostensibly signified ineffective filtration
(15th Annual Report of the LGB , app B, p 96).
On taking up the post Bolton found himself in the midst of the furor over 'previous sewage contamination' and the other Frankland stratagems. In December 1871 he received a petition from the Vestry of St Mary, Newington, complaining of their wretched water supply. The petition was studded with extracts from Frankland's reports, including references to 'living and moving organisms' that Frankland had found in the water. Bolton examined the water microscopically, and found in it paramecia, confervae, fungi, and organic debris. It deposited a brownish sediment on standing. Nevertheless he rejected Frankland's claim that the water was bad: E A Parkes' Manual of Hygiene set a maximum safe limit of three grains per gallon of 'organic or volatile matter' and the water in question contained only 1.9 grains. Misunderstanding Frankland's views on water analysis, Bolton insisted that the water was also safe according to Frankland's standards. He took issue with Frankland's listing of 'living and moving organisms,' arguing that microscopic life was ubiquitous in waters.[53]
Frankland was indignant. Bolton received a chilling rebuke from S J Smith, secretary of the Rivers Commission. Having pointed
to Bolton's misreadings, errors, and reliance on obsolete analytical methods, Smith informed him that 'Her Majesty's Commissioners' had no objection to his stating his opinion but thought that
in a matter of such importance . . . it would have been a proper course for the Water Examiner [Bolton] to have put himself in communication with them before using their experimental data and conclusions in a mutilated form to support his own opinions, and, as an engineer, review the work of a chemist: in so doing he has shown his limited acquaintance with physics and chemistry, and consequent misunderstanding of chemical language and results.[54]
Poor Bolton! As if holding one's 'own opinions' were not sin enough, he had the misfortune to be an engineer. Bolton sputtered, but there was little to say.
With the coming of Bolton, questions arose of what Frankland's future status was to be. Inasmuch as it was Bolton's responsibility to certify that London's water was being 'effectually filtered' and inasmuch as Frankland was analysing the water at government expense in a way that would show the adequacy of filtration, there was some thought that Frankland ought to submit his results to Bolton, who would then decide whether they represented effectual filtration. This would have made Frankland a glorified technician, working under someone who knew far less about water quality and analysis than he did, but it might have been attractive to those uncomfortable with Frankland's activism. Exactly what was proposed is not clear, but George Graham, the Registrar General, vigorously (and successfully) resisted any major change in Frankland's status. On 8 May 1872 Graham wrote to the Local Government Board, not to claim that Frankland was impartial, but to praise Frankland's sanitary activism:
nothing has had so great an effect upon them [water companies] as Prof. E. Frankland's undisguised description of the impurities discovered, published by the authority of the government, and his comparison of what we are here compelled to drink, with what is supplied to Glasgow, Manchester, etc.[55]
Graham admitted that he himself knew nothing of the chemistry at issue. But he thought it was perfectly normal that Frankland would be criticized, for 'the higher the individual attacked may be, the greater glory will be their fate who demolishes his reputation.' The water companies might well find 'rival chemists glad to dispute the accuracy of the analyses,' but one ought not to be misled by
them. He added an ultimatum:
Of course upon receiving monthly reports from Prof. E. Frankland framed conscientiously on analyses which with his large experience and acknowledged learning he considers the best and truest that can be made, it is not wished that before publishing them I should expurgate what may be likely to offend any company and their hired chemical advisor? It cannot be contemplated that the professor would submit to such treatment; still less can it be thought that I should publish garbled statements in a matter of such importance.
The reports in future must be published as I receive them on the responsibility of Prof. E. Frankland, or not be published at all.[56]
Graham professed not to care which option was taken, but he had left the LGB in a difficult position. Frankland's reports continued to appear independently, under aegis of the Registrar General, until 1875 at which point the water analyst's position was transferred to the LGB which was coordinating more and more aspects of public health policy. In most respects this was a more suitable arrangement since the Board was responsible for most aspects of sanitary policy, but it did mean that Frankland's monthly analyses and his often inflammatory interpretations were published (without editing) in Bolton's monthly report, leaving Bolton with the problem of how to reconcile Frankland's views with his own.
The administrative arrangement was troublesome in other ways. As water examiner Bolton was the closest thing to a public regulator of privately owned public utilities. He was a specialized civil servant, a subordinate in the LGB hierarchy. Frankland, on the other hand, was an unsalaried consultant, paid directly for his analyses and not subject to the bureaucrats. From time to time there was talk in the LGB of getting Frankland to restrain his speculations about the water danger, but it was realized that even the appearance of censorship would be politically embarrassing.[57]
Matters were further complicated by Bolton's political naiveté. He failed to appreciate the symbolic power that an official publication conferred on any partisan reports included in it. At the end of 1873 Bolton began including reports of analyses by Letheby and J K Bamber (the companies' analyses). As it came to appear that Bolton's pages were open, more of those with anti-Frankland axes to grind found excuses for the inclusion of their analyses. Always there were good reasons: a need for more data to confirm, complement, or supplement Frankland. Always these programmes of analyses were made under the sponsorship of some ostensibly non-
partisan body; just as the Association of Medical Officers of Health received Letheby's analyses (though the companies paid for them), the metropolitan vestries sponsored a series begun in 1879 by Frankland's nemesis J A Wanklyn and his associate William Cooper. But the intent of the programmes was invariably to dilute Frankland's influence; to give members of the public free choice to take truth from whichever official expert most closely matched one's ideology.[58]
By the later '70s Bolton's superiors at the LGB had become concerned about this situation and in 1879 they advised against including the Wanklyn/Cooper analyses. Bolton disagreed, citing Wanklyn's eminence as a water analyst. He liked the simplicity of Wanklyn's format, noted that the analyses were expressly for the vestries, and that besides, there was extra white space on the page.[59] The issue came to a head in spring 1883 when a question was raised in the House of Commons as to the official status of the analyses done for the water companies (the Association of Medical Officers had withdrawn its sponsorship in 1879) by Letheby's successor Charles Meymott Tidy. Tidy (1843–92) was so central in water matters during the '80s that it is appropriate to digress to introduce him here. Son of a Hackney medical practitioner, Tidy studied medicine and chemistry with Letheby, qualifying in 1864 and taking an M B degree from Aberdeen in 1866. On Letheby's death in 1876 he became professor of chemistry, public health, and medical jurisprudence at the London Hospital and medical officer for Islington. He also lectured on medical jurisprudence at the Inns of Court and toward the end of his life qualified as a barrister. From 1876 until his death Tidy was Frankland's principal antagonist. He was far more tactful than Wanklyn, and cleverer than Letheby. Not only was he a defender of the London water companies, but Tidy (like Wanklyn) opposed Frankland on almost every water issue: he believed chemical methods of water analysis were adequate to pronounce waters safe, doubted germs caused disease, and maintained that polluted rivers purified rapidly. He was an adept publicist, and a talented expert witness. Scientists did not take him seriously, among sanitarians he lacked Letheby's stature, yet he was effective and influential nevertheless.[60]
In November 1880 the Association of Medical Officers of Health had informed Tidy that he need no longer supply them with monthly analyses of the London waters.[61] Without the Association to legitimate the analyses it became even more important that they be included in Bolton's reports. In early 1881 the companies took steps to

Figure 7.3
The printing of analyses done for the water companies in official LGB reports
gave these analyses some claim to official status, which the analysts William
Crookes, William Odling, and Charles Meymott Tidy attempted to expand, much
to the distress of the LGB. As these examples show, even when they changed
wording in response to LGB complaints, their reports still looked official|
(PRO MH 29 5).

Figure 7.3
(continued)
strengthen the credibility of their analyses, commissioning William Odling and William Crookes, chemists of substantial reputation, to collaborate with Tidy. Moreover the reports were henceforth to be based on daily analyses of the waters of each company, not just
the once-a-month samples that Frankland took. The three chemists took the offensive when the question arose in 1883 of the status and impartiality of the analyses they sent Bolton. Theirs weren't the partisan analyses, Frankland's were, they maintained. Further, a public report, like Bolton's, ought to present a balanced view.[62]
From the LGB viewpoint the problem was not simply one of the publication of the Crookes—Odling—Tidy analyses in Bolton's reports. What the Board received each month was a copy of a printed report, addressed to the Board but distributed to the public and the press as well. The format of this privately published report implied that the analyses were commissioned by the Board. It was addressed (in large capitals in a font similar to that used in official blue books) 'To The RIGHT HONOURABLE THE PRESIDENT OF THE LOCAL GOVERNMENT BOARD.' When LGB staff tried to persuade the three chemists to make it clear that their analyses were not official, the three responded with formats that met the Board's specific complaints yet conveyed the impression of being official in ingenious new ways. Their June 1883 report was addressed to the Board's secretary (in small letters), and admitted that the analyses were 'for the information of the Local Government Board' and had been 'made at the expense of the Water Companies.' Bolton, however, insisted the analyses be addressed to the companies. This Tidy refused to do, and beginning in August the cover page of the report (again with font and layout mimicking a blue book) announced that the analyses were 'addressed to the Official Water Examiner for the Metropolis.'[63]
Bolton and his superiors had better luck dealing with the Wanklyn—Cooper analyses. Bolton's September 1884 report did not include a Wanklyn—Cooper analysis; their contribution had not come in, Bolton maintained. His reports through January 1885 carried similar statements, even though the analyses were being received, in some cases several days before Frankland's (which were included) were received, and in all cases but one in time for publication. Somewhere in the LGB Wanklyn's reports were going astray; whether Bolton knew what was happening is not clear. By August Wanklyn and Cooper had stopped sending reports.[64]
These episodes indicate how pervasively politicized analyses of London's waters had become. Bolton's monthly reports, ostensibly the record of an impartial investigator appointed to safeguard public health, in fact represented the struggles of conflicting interests, each of which sought to gain power by gaining control of the icons of an
official report. On one side was Frankland, possessing 'official' status, yet not being responsible to any official. On the other was the triumvirate of Crookes, Odling, and Tidy, all able polemicists and masters in the construction and manipulation of images of authority. In the middle was poor Bolton, holding a marginal position in the LGB bureaucracy, trying unsuccessfully to pretend that supervising London's filters could be based on straightforward empirical investigations. It was an impossible job. The financial and political issues involved were too large, the scientific issues shrouded in too much uncertainty.
The Companies' Critique
The reports of Tidy, Crookes, and Odling were clearly undertaken to undermine Frankland's credibility. Were it possible to regard their writings only as political propaganda it would be unnecessary to consider them. Yet the sorts of arguments they made were equally those of the science of water analysis. There was no independent fount of truth, free of the taint of politics, which might then be prostituted into the service of some vested interest. Instead, the issues of water analysis were inextricably mixed with social and political conflict. The science developed as a dialectic between those who were trying either to prove the sure and certain safety of public water supplies or to incite public action to make those supplies far more trustworthy than they currently were. Hence the work of Tidy and his colleagues deserves scrutiny not because it was disinterested, but because in the period before the development of bacteriology, they put the case that analysis and purification could be trusted more thoroughly and thoughtfully than had any others.
Shortly after taking over the analyses Tidy began to solidify the scientific foundations of his attack on Frankland. In 1879 he reopened the vexed issue of which process was best for the determination of organic matter, delivering a long paper on the subject to the Chemical Society in early February.[65] Like most of Tidy's presentations, this one was a rhetorical masterpiece. It presented little in the way of new or significant scientific findings, and served a larger strategic end in providing a foundation for claims and arguments Tidy would make in subsequent years. Setting himself up at the outset as an impartial authority, Tidy based his criticism of the combustion process on Wanklyn's arguments and his criticism of the ammonia process on Frankland's. At the level of chemical
principles, both sets of criticisms were unanswerable. Having used Frankland and Wanklyn to neutralize one another, he turned to the oxygen-absorbed (permanganate) process, which Letheby had long championed and which he had inherited. Frankland and Wanklyn had represented the process as naive in conception, meaningless in results. Tidy admitted as much, but claimed that he and Letheby had greatly improved the process. It had been objected that the process failed to distinguish between harmless and harmful organic matter, so Tidy began the practice of taking two readings, treating the oxygen absorbed after one hour as representing the oxidation of the dangerous highly putrescible, animal organic matter, with the three-hour reading representing relatively unobjectionable vegetable organic matter.[66]
Throughout the paper Tidy maintained a tone of superiority. To the argument that combustion was too tricky he retorted that if chemists could not do it they ought not to be chemists (it might help weed incompetents from the profession). He made much of his own medical training, which gave him an authority in such matters of life and death that those trained only as chemists could not pretend to have.[67] The most important of Tidy's stratagems was the stance he took toward the rival analyses he and Frankland were doing of the London waters. Though highly critical of both combustion and ammonia, Tidy ended up supporting Frankland against Wanklyn. He based his support on his own trials on the combustion, ammonia, and permanganate processes. Tidy found a significant concordance between combustion and permanganate and on this basis condescended to endorse combustion, however objectionable it might be in theory. He still favoured the permanganate process as the more prudent for its errors lay in the direction of yielding false positives (which would induce the analyst sometimes to condemn safe waters), while combustion might give false negatives in the case of volatile organic matters that would be lost during evaporation.[68]
These moves enabled Tidy to represent his disagreements with Frankland in a much different light than had Letheby. For Letheby the conflict had been as much about which analytical process was better as about whether London's water was safe. Endorsing combustion allowed Tidy to focus on interpretation. He soon adopted combustion in his analyses (keeping the oxygen process as well) and attempted, unsuccessfully, to have his analyses and Frankland's done on duplicate samples so that there might be no equivocation about changes in composition. The strategy was thus to remove any pre-
tense that his differences with Frankland could be ascribed either to their competence or to their samples. If both used the same methods, and Frankland continued to come up with inflammatory conclusions, it could mean only that Frankland was an irresponsible rabble rouser. Hence while Tidy might claim that the analyses were 'not made . . . in any spirit of antagonism or opposition,' he had simply found a far stronger basis for opposition. (In fact, as W C Young would point out in 1895, the levels of impurities discovered by the companies' chemists were generally higher than those on which Frankland based his condemnations of London's waters.)[69] The 1879 paper on analysis set up Tidy's next production, a similarly lengthy paper on 'River Water' presented a little over a year later. Here he challenged Frankland's claim that rivers did not self-purify to any significant degree and Frankland's belief that the morbid poisons of water-borne diseases were resistant living germs.[70]
A lengthy and acrimonious exchange ensued. Frankland's exhaustive and sometimes sarcastic response appeared in the Journal of the Chemical Society as a research report (on the grounds that it was the first public presentation of self-purification experiments done by the Rivers Commission). A second 'River Water' by Tidy in March 1881 added nothing new. W Noel Hartley of the College of Science in Dublin and Charles Folkard, a Frankland student, published lengthy papers supporting Frankland, as did Frankland's son Percy, in a savage 1884 attack on 'The Upper Thames as a Source of Water Supply.'[71] In all these forums Tidy was thoroughly taken to task. His meagre experiments, numerous unwarranted assumptions, and self-serving concepts of morbid poisons were repeatedly pointed out. Nonetheless, Tidy had made himself a celebrity, the champion of all those who felt Frankland was too unrelentingly (and perhaps unrealistically) hard on existing water supplies. All the while Tidy was taking a beating in the learned societies, he and Odling and Crookes were taking their case to the public. Their monthly reports became a forum for denouncing Frankland and extolling the unexceptionable yet constantly improving London waters. As in the early '50s and the mid '60s, the fierceness of the battle reflected a new round in the struggle for control of the supply. Again there was grumbling in the vestries about high cost and poor service. In 1879 the government had called for public takeover and a Select Committee had taken up the problem in 1880. The companies were willing to sell; the political problem remained how much they would receive for their fixed assets and in recompense for future dividends, and there would be
no resolution of this problem for another two decades.[72]
In the main, Tidy, Odling, and Crookes followed the strategy of ignoring all the unanswered questions (e.g. those of the identity and characteristics of the morbid poisons of water-borne diseases) and returning the debate to the validity of Frankland's inferences and the propriety of his methods of presenting his results. With a confidence rarely seen since the days of Brande, they reaffirmed a simple and absolute faith in the reliability of chemical water analysis. When they wrote that 'mere chemistry, however refined, has not been able to distinguish a difference, or even to establish a presumption of a difference' between the water taken from the headwaters of the Thames and that taken from near the companies' intakes (contaminated with sewage from upstream towns) they were, in effect, asserting that no difference existed.[73] With their assumption of the unassailability of chemistry, the three went on to chide Frankland for intentionally misleading the public. In May 1883 they brought up Frankland's periodic allusions to 'moving organisms' in the water. They called upon him to state what kinds of organisms these were and noted that Frankland himself had stated (in a lecture to the Royal Institution 22 years earlier, no less) that such organisms were safe.[74] In August they took issue with Frankland's 'Table E.' Here Frankland compared the organic contamination in the water supplied by the river-water companies with what the Kent Company took from deep wells. The table used the Kent's level as unity and hence represented how many times dirtier the river-derived waters were than what London might have if it converted to the deep wells that Frankland now advocated. Crookes, Odling, and Tidy complained that comparing waters from different types of sources was unfair, and noted that the organic matter in Thames-derived water was roughly the same as that in Glasgow's pure Loch Katrine supply.[75]
The campaign of Crookes, Odling, and Tidy and the contemporaneous analytical programme of the Society of Public Analysts that will be considered in the next chapter were the last great hurrahs of chemical water analysis. On strictly chemical grounds the points the three chemists made were defensible—using chemical methods Frankland could not prove that there were significant differences in waters from various places along the Thames; he was guilty of inappropriate comparisons and misleading use of terms. Yet what the three chemists did not fully realize was the extent to which determinations of water quality were escaping (and indeed had escaped) the
province of chemistry. They were defending an empty orthodoxy.
Frankland:
The Triumph of the Symbolic
It is as well to take stock here, to assess the transformation Frankland had wrought on the community of analysts. In 1866 most analysts would have looked upon water analysis as an empirical issue. They might have admitted that their processes were not yet very good for making fine qualitative distinctions or even terribly accurate quantitatively. Yet they did believe that in measuring organic matter, 'animal' organic matter, or hydrogen sulphide in water they were measuring the harmful matter, or at least approximating it as closely as possible. They might admit that this matter did not always lead immediately to acute disease, yet it was still possible to conceive that one was measuring the harmful substance: according to contemporary medical theory one might believe that bad water only debilitated health, acted, that is, as a predisposing cause. Or one might regard the effect of bad water as cumulative and as having no effect until a threshold was reached. Or one might argue that the water would become lethal only when epidemic conditions were present. Underlying any of these interpretations, however, was the belief that the analyst really was measuring the harmful matter—its harmfulness simply might not be quickly, conveniently, or obviously manifested.
This is the empiricism: one solves the problem of whether the water is harmful by measuring the harmful material in it. In such a perspective claims about health effects were subject to the findings of chemistry and such an outlook was a natural outgrowth of chemists' long-standing claims that their science provided the ultimate authority in matters of water quality. If now we regard this claim as over-reaching its warrant, or even as unfounded, we must remember that it was regarded at the time as a prudent claim: even if not all organic matter was harmful, it was organic matter (at measurable levels) that was harmful. Hence the analyst could only go wrong by making false positives, by advising caution when there might be no need for it.
Taking this empiricist perspective involved minimizing or conveniently overlooking Hofmann's observation that what one could measure was really very different from what one ought to be worrying about. It was different in category; it was process not substance. It was characterized not by its quantity but by its transitoriness: the
matter which underwent the dangerous process might constantly be present, yet the deadly process might only occasionally occur in it. Such a perspective was not logically incompatible with the empiricism of traditional chemists, for they could still maintain that their processes identified and quantified an essential antecedent to the development of water-borne disease. But if it did not exclude empiricism, Hofmann's new perspective did strain it: if the important distinctions were really unmeasurable qualitative distinctions, knowledge of the amount of organic matter was virtually irrelevant. If all nitrogenous organic matter in water were to be accepted as capable of undergoing pathogenic putrefaction false positives would become so frequent that it would become questionable whether analysis was of any service at all.
The final blow to this philosophy came from Frankland's discovery of false negatives during the 1866 London cholera. On precisely the occasion when water analysis was most needed—the occasion of an epidemic—it failed. The cholera germs, whatever they were, were so minute that a water which would be regarded as safe according to any set of chemist's standards might actually be lethal. Hence empiricism might lead not to prudence but to folly. But what to do then? Frankland had pretty well falsified traditional conceptions of morbid poison but he had nothing—at least nothing analytically tangible—with which to replace them. Hence the campaign of what might best be called counter-analyses that he embarked on in the following two years. They were counter analyses in the sense that they were effective because they were presented in an environment (and in a form) in which they were viewed in traditional empiricist terms. All the while the conclusions Frankland was coming to derived from a few prudent and simple principles about what waters could be trusted—the main one being 'don't trust any water unless you know where it's been.' What Frankland was doing then was to reverse the direction of argument, but he did so behind the scenes. Now chemistry was to be subject to epidemiological demonstrations, not the other way round. And since the phenomena of concern were epidemiological, not chemical, chemical information was really irrelevant. Yet it was, as Frankland astutely realized, the only form of information policy makers were likely to pay attention to. Brande had done his job well; chemistry was the unquestioned source of authority in such matters, and besides, Frankland was a chemist.
In removing chemical analysis from centre to periphery, in using it mainly to legitimate conclusions arrived at through other ratio-
nales and to persuade others to accept those conclusions, Frankland was liberating himself from certain modes of accountability as well as from certain assumptions. The initial reactions of colleagues and competitors, Letheby for example, convey a sense of shock, outrage, and exasperation—Frankland had broken unwritten rules in an unexpected way. By using reductio ad absurdum arguments about undetectable, resistant germs, he was no longer subjecting himself to the arguments chemists could make, no longer was he accountable to the profession. But Frankland had not so much broken rules as changed them, and Tidy, Odling, and perhaps Wanklyn had by the early '80s become skilled at playing the game according to Frankland's rules. Frankland's rules were much looser. They permitted one to arrive at an opinion of a water's quality by whatever route and on whatever basis one chose, and to defend it by whatever means seemed most effective. On the one hand the adoption of this sensibility by at least the elite London chemists can be taken as a sign of maturity: no longer were they perpetuating an instrumental rationality by taking refuge in simplistic and arbitrary chemical standards which for years had been becoming increasingly fraudulent and hypocritical. By the same token, the realization that the proper end of water analysis was not knowing parts per million of organic nitrogen but securing better water, i.e. that water analysis must be subject to water policy, meant that it was hard to take the statements of analysts at face value. A statement by Tidy that London's waters were safe, or by Frankland that they were unsafe, had immediately to be translated into the political message it symbolized, either that existing arrangements were adequate or that great change was needed.
As it turned out this abandonment of empiricism was to be short-lived. In the mid '80s empiricism returned to water analysis in two forms. One of these was bacteriology, which engendered as much naive optimism as chemistry once had. The other was a more modest, and ultimately more successful empiricist programme. It emerged from the reaction of rank-and-file water analysts to the controversies over analytical methods between Frankland and Wanklyn and Frankland and Letheby. Especially important are the responses of two emerging groups of public health officials: the local medical officers of health (mandated by the sanitary legislation of 1875), and the local public analysts, appointed under the adulteration acts to ensure that consumers could be assured of safe and pure foods and drugs. Neither medical officers nor public analysts were specifically required to inquire into water quality, but a great many did and
found the processes of the London chemists wholly unsuited to their needs.