Twenty-one—
Hormones and Fuel Regulation in Fasting Elephant Seals
Vicky Lee Kirby and C. Leo Ortiz
ABSTRACT. This chapter summarizes current knowledge about fasting physiology in the northern elephant seal, Mirounga angustirostris . Changes in metabolic fuel distribution and plasma hormone levels as well as changes in insulin secretion and peripheral tissue sensitivity to plasma insulin are addressed. Pups at weaning and during an eight-week postweaning fast were hyperglycemic, hyperlipidemic, hypoinsulinemic with impaired glucose tolerance, and relatively insulin insensitive. Fasting northern elephant seal weanlings did not closely regulate their blood glucose.
It is suggested that the suckling elephant seal pup is preadapted to the postweaning fasting period because of the lack of carbohydrate in the milk, its high fat content (85–95% of the calories), and the large increase in body fat (up to 50% of the mass at weaning). All of these contribute to impaired insulin secretion and action in other mammals. Blood glucose could only be maintained by hepatic gluconeogenesis because of the lack of dietary carbohydrate in this species at all stages of its life history. Adaptations to a low carbohydrate, high fat diet are similar to those necessary for adaptation to fasting. Low plasma insulin and relative tissue insensitivity to insulin are normal adaptations to low carbohydrate diets and fasting and would not be clinically abnormal for carnivores.
The northern elephant seal, M. angustirostris , provides the physiologist with a model for studying the basic physiological, biochemical, and anatomical mechanisms underlying the ability to undergo natural extended periods of complete food and water abstinence in large nonhibernating mammals. With the exception of nursing pups, individuals of all ages and both sexes fast entirely during the terrestrial phase of their life cycle, notably, the reproductive and molting phases. This rigorous life-style begins early in life when pups of the year are weaned abruptly at one month of age (Le Boeuf, Whiting, and Gantt 1972; Reiter, Stinson, and Le Boeuf 1978). During this
period, young animals not only cope with the rigors of zero nutritional and water input but do so while continuing normal neonatal development. This entails substantial intertissue reorganization of protein, minerals, and other cellular components.
Over the past several years, we have investigated aspects of the physiology of spontaneous fasting in these young weanlings with two basic objectives. First, we wanted to understand the physiology of integrated biochemical processes underlying these prolonged fasts. Second, we wanted to determine the control mechanisms that simultaneously integrate catabolic processes involved in meeting energy needs with the anabolic processes required for protein recruitment and synthesis during development.
Our initial studies focused on the major regulatory hormones, insulin and glucagon, in fasting weanlings (Kirby and Ortiz 1989; Kirby 1990). In this chapter, we summarize our current understanding of fasting physiology during the postweaning fast, including a discussion of (1) fuel depots and fuel turnover studies; (2) plasma metabolite and hormone levels during the postweaning fast; and (3) changes in pancreatic and peripheral tissue responsiveness to glucose and insulin tolerance tests.
Fuel Depots:
Storage and Utilization
Northern elephant seals undergo dramatic changes in body composition during their first year of life. Unlike terrestrial mammals, the accumulation of adipose tissue occurs early in the neonatal period (Bryden 1968). Throughout the nursing period, pups gain on average 2 kg of adipose and 1 kg of lean tissue daily, and at weaning, the fat mass averages 48% in healthy pups (Ortiz, Costa, and Le Boeuf 1978). The fat mass gained during nursing is important for survival during the postweaning fast, as evidenced by the correlation between duration of the postweaning fast and the relative level of body fat at weaning (Kirby 1992).
In fasting animals, changes in body mass compartments can be used to calculate how much lean and adipose tissue contribute to metabolism. Newly weaned pups lose 1 to 2 kg of tissue/day in the first 2 weeks of fasting as compared to .65 kg/day during the rest of the 8- to 10-week fast (Kretzmann 1990; Rea 1990). This progressive sparing of tissue reserves is accomplished by a reduction of resting metabolic rate during the postweaning fast (Rea 1990). Although fasting seals catabolize both lean and adipose tissue at an equivalent rate, these tissues do not have equal energy content. Hydrated proteinaceous tissue has a lower energy content than an equivalent weight of adipose tissue. Thus, the size of the fat depot is important because energy mobilized from adipose tissue has to supply more than 85% of the total energy needs of the pup.
Normally, fuel stores important in carbohydrate metabolism are muscle and hepatic glycogen, amino acid stores in lean tissue, and triglycerides in adipose tissue. Although glycogen levels have not been measured specifically in elephant seal tissues, it has been shown in other species of pinnipeds that glycogen is not an important energy store. Therefore, glucose must be made from noncarbohydrate precursors, such as the glycerol moiety derived by triacylglycerol oxidation and the glucogenic amino acids derived from lean tissue. However, the relative contributions of lean and fat tissue to glucose formation cannot be determined from just monitoring changes in fuel depot size.
Fuel Turnover Studies
The relative contributions of lean and fat tissue to total energy needs and to glucose formation have been examined by isotope-labeled fuel metabolite studies in fasting weanlings. Fatty acid oxidation studies confirmed that lipid is the main energy source and suggested that sufficient glycerol was liberated by lipolysis to meet all glucose precursor needs (Castellini, Costa, and Huntley 1987). In fact, the direct contribution of glucose to the total metabolic rate was shown to be less than 1% in seals fasting longer than one month (Keith and Ortiz 1989). Although glucose turnover rates were within mammalian norms, most of the glucose carbon appeared to be recycled, possibly by futile cycling, and was not oxidized. This has also been observed in harbor seals (Davis 1983) and grey seals (Nordoy and Blix 1991).
Similar results from urea, albumin, and leucine turnover studies in fasting pups confirmed that protein oxidation contributed to less than 3% of total energy needs (Ortiz 1990; Pernia 1984; Pernia, Hill, and Ortiz 1980). Since it appears that protein and glucose oxidation, together, provide less than 10% of total energy needs, the physiological role of active turnover of these substrates remains unclear. It is possible that recycling protein and glucose carbon may serve as an important carbon shuttle mechanism, for example, glucose synthesis or synthesis of nonessential amino acids or other cellular components.
Plasma Metabolite Levels
Plasma levels of metabolites from carbohydrate, protein, and lipid metabolism are common parameters in characterizing in vivo fuel homeostasis. These metabolites usually include glucose, blood urea nitrogen (BUN), creatinine, nonesterified fatty acids (NEFA), and b -hydroxybutryate (BOHB).
Early studies reported that elephant seal pups may be hyperglycemic
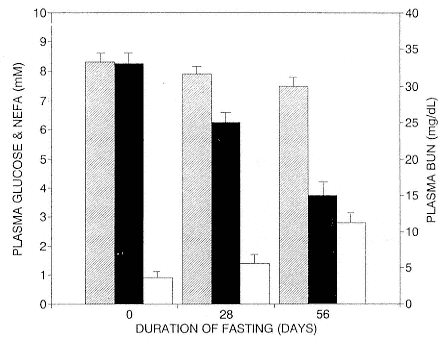
Fig. 21.1
Plasma levels (mean ± SD) of glucose (cross-hatched bars), blood urea nitrogen
(solid bars), and nonesterified fatty acids (open bars) in eight elephant seal pups
before fasting (at weaning), after 28 days of fasting, and after 56 days of fasting.
because plasma glucose levels were rarely seen below 6 mM, which is substantially higher than predicted by body size (Umminger 1975; Costa and Ortiz 1982). Plasma levels of glucose as high as 200 mg/dL have been reported for other species of pinnipeds (Englehardt and Ferguson 1980; Hochachka et al. 1979; Worthy and Lavigne 1982). Subsequent studies (Kirby 1990) confirmed that plasma glucose levels remain elevated above 6.5 mM throughout the postweaning fast, although there was a small but steady decrease in plasma glucose from 8 mM at the start of the fast to 7mM by the end of the fast (fig. 21.1).
Plasma BUN levels are an indirect measure of protein oxidation and demonstrate predictable changes associated with fasting. They were highest at weaning (33 mg/dL) and decreased significantly over the fasting period to 15 mg/dL, as shown in figure 21.1 (Adams 1991; Kirby 1992; Costa and Ortiz 1982). Another index of lean tissue catabolism is creatinine (C), which is released into plasma when muscle tissue is catabolized. The decrease in the BUN:C ratio from 37 to 16 by the end of the fast was consistent with protein sparing adaptations.
Conversely, plasma levels of NEFA progressively increased from < 1.0 mM at the beginning of the fast (at weaning) to ³ 2.6 mM after 8 weeks of fasting (fig. 21.1; Kirby 1992). The ketone body BOHB, a by-product of fat oxidation, also increased slowly during the fast (Castellini and Costa 1990). After 8 weeks of fasting, there was a sharp decrease in BOHB, and the seals departed within 10 days. This suggests that the seals terminated their land-based fast because adipose reserves had become too low to support ketogenesis and still provide enough insulation to aid thermoregulation while feeding at sea.
All of these changes in plasma fuel levels were consistent with the general animal fasting model (Felig et al. 1969; Cahill 1970) in which there is a metabolic shift from protein oxidation to lipid oxidation early in the fasting period. Similar adaptations have been observed in other animals in which a spontaneous fast is part of their life cycle, for example, the king penguin, Aptenodytes patagonica (Cherel, Stahl, and Le Maho 1987).
Plasma Hormone Levels
The shift to a lipid-based metabolism occurs in conjunction with specific changes in insulin and glucagon levels (Cahill et al. 1966; Felig et al. 1979). A decrease in insulin secretion is vital for fuel homeostasis during fasting because insulin normally inhibits lipolysis. In fasting elephant seals, mean plasma insulin levels decreased throughout the fast from 11 ± 4 µU/ml at weaning, to 9 ± 3 µU/ml after 4 weeks of fasting and 8 ± 2 µU/ml after 8 weeks. Low plasma insulin levels (5–15 µU/ml) were also observed in lactating and molting adult females, molting yearlings, and weanlings captured just after their return from feeding at sea (Kirby 1990; Kirby and Ortiz 1990). Similar low levels have also been reported for feeding harbor seals (Robin et al. 1981) and Weddell seals (Hochachka et al. 1979).
Glucagon levels were highly variable, especially in nursing pups. Different glucagon antibodies in the glucagon radioimmunoassay suggested the possibility that nonpancreatic glucagon cross-reacted with the antibody for pancreatic glucagon, thus overestimating glucagon. Extrapancreatic alpha cells have been observed in the gut of the South African fur seal, Arctocephalus pusillus (Van Aswegan and Viljoen 1987). The lowest total glucagon levels measured contributed to a molar I:G ratio £ 1.0 in all animals studied. In other mammals, an I:G ratio of less than 1 is correlated with high rates of hepatic gluconeogenesis (Unger, Eisentraut, and Madison 1963; Unger 1985).
In general, changes in plasma levels of insulin and glucagon as well as in their ratios were consistent with fasting adaptations observed in animals as diverse as humans and penguins (Marliss et al. 1970; Cahill and Aoki 1977; Cherel, Leloup, and Le Maho 1988). Decreased levels of insulin and a low
I:G ratio allowed for the increased mobilization of lipid from fat stores as protein and glucose were conserved. It was not expected that the prefasting insulin levels in newly weaned seals would be so low. However, potential explanations are that (1) the high fat milk diet (Le Boeuf and Ortiz 1977; Riedman and Ortiz 1979; Kretzmann 1990) suppressed insulin secretion; (2) the high body fat levels impaired insulin secretion (Kirby 1990); (3) 1-month-old seals were still too young developmentally to regulate glucose normally (Tieran 1970); or (4) insulin secretion was low because of its minor role in glucose homeostasis in this species (Kirby 1992).
Glucose Tolerance Tests
Glucose tolerance tests (GTT) can provide an index of pancreatic islet sensitivity to changes in plasma glucose. If insulin does not regulate blood glucose levels in these animals, one would expect an impaired response in which animals do not closely regulate their blood glucose levels. To test this, glucose was administered intravenously (IV) as a 50% solution over a 2- to 4-minute period in a standard dose of 25 g or 0.5 g glucose/kg body weight, and the disappearance rate (K) of glucose was measured over time. Feeding mammals normally have K values of 2.3 or greater in contrast to fasting animals, which have K values of 1.0 or less. The glucose tolerance profile for five seals at weaning and after 8 weeks of fasting is shown in figure 21.2. K values were £ 1.0 for all seals. This profile is similar to that seen in obese and diabetic humans (Salans, Knittle, and Hirsch 1983). Normal (nonobese) mammals would restore euglycemia within 60 to 90 minutes of the glucose injection due to an acute insulin response.
Impaired glucose clearance (K £ 1.0) and the total lack of an insulin response to a glucose injection were virtually identical for pups at weaning and after fasting 8 to 11 weeks. These data indicated that these seals were adapted for fasting, that is, they switched to fat oxidation metabolism, prior to weaning.
In other mammalian neonates, the transition from the glucose-based metabolism of the fetus to the high fat milk diet of the neonate is accompanied by an increase in gluconeogenesis and fatty acid oxidation and a decrease in hepatic lipogenesis in the perinatal period (Kalhan 1992). As carbohydrate is introduced into the diet, the neonate decreases its reliance on de novo synthesis of glucose and increases insulin secretion. Elephant seal milk does not contain carbohydrate; in fact, the fat content is at its highest just prior to weaning. Thus, the suckling elephant seal neonate cannot develop the ability to secrete or utilize insulin for carbohydrate uptake into tissues.
Impaired insulin secretion and tissue insensitivity to insulin are correlated with high body fat levels (and high fat diets) in other mammals. We
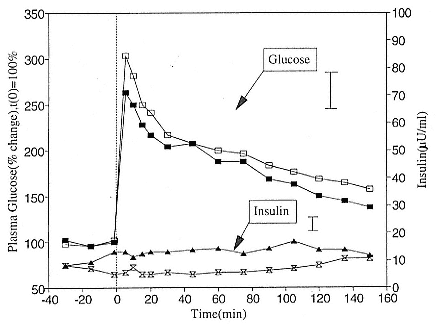
Fig. 21.2
Glucose tolerance test for five elephant seal pups just prior to weaning (closed
symbols) and after 8 weeks of fasting (open symbols). The mean glucose
response is represented by the square symbols (solid line). The mean insulin
response is represented by the triangle and hourglass symbols. At time zero,
a bolus injection of 25 g glucose in a 50% saline solution was injected
intravenously. For purposes of clarity, the largest standard deviations for
glucose and insulin are shown as vertical bars.
investigated the effect of body fat on the insulin response to a glucose challenge by comparing glucose clearance rates (K) in pups with known body weights, body composition, and age (Kirby and Ortiz 1989). It proved to be very difficult to separate changes in body fat levels from changes in age. Virtually all seals larger than 100 kg body weight at weaning had a fat mass of 48 to 50%. Seals smaller than 90 kg at weaning had lower body fat levels (< 40%). However, all seals, regardless of weaning mass, age, and duration of fasting, had impaired glucose clearance values less than 1.0 with little or no insulin response to the glucose injection. Glucose clearance values estimated from figures in harbor seal and Weddell seal studies were also less than 1.0 (Hochachka et al. 1979; Robin et al. 1981).
Insulin Tolerance Tests
As a mammal adapts to fasting, there should be a decrease in tissue sensitivity to insulin concomitant with decreased insulin secretion (Cahill et al.
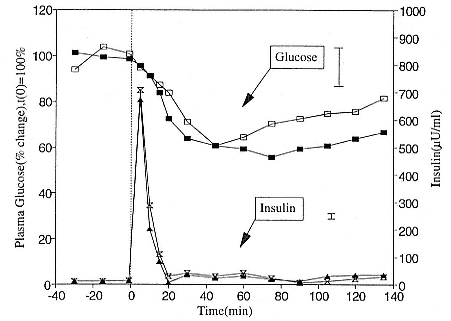
Fig. 21.3
Insulin tolerance test for five elephant seal pups just prior to weaning (closed
symbols) and after 8 weeks of fasting (open symbols). The mean glucose
response is represented by the square symbols (solid line). The mean insulin
response is represented by the triangle and hourglass symbols. At time zero,
a bolus injection of 0.1 U insulin/kg body weight was injected intravenously. For
purposes of clarity, the largest standard deviations for glucose and insulin are
shown as vertical bars. Note change of scale from fig. 21.2.
1966). Changes in peripheral tissue sensitivity to insulin were assessed by intravenous injections of 0.05 to 0.1 U insulin per kg body weight. In response to insulin, plasma glucose levels decreased in all seals studied. Although the exogenous insulin was cleared from the blood within 20 to 30 minutes postinjection, blood glucose levels did not reach a nadir until 40 to 90 minutes postinjection.
A typical insulin tolerance test (ITT) profile is shown in figure 21.3 for five seals prior to and after 8 weeks of fasting. Blood glucose was not restored within 135 minutes postinjection even though some seals tolerated blood glucose levels as low as 3.5 to 4 mM for almost an hour without any behavioral changes. There was no difference in glucose recovery rates for nursing and fasting pups. This pattern of impaired response has been observed in fasting and feeding obese humans as well as in diabetics (Drenick et al. 1972). In contrast, most mammals restore euglycemia within two hours of the insulin injection. The maximal hypoglycemia in response to insulin is reached within 30 minutes of the injection.
Elephant seal pups tolerated a reduction of plasma glucose of 50% of basal level glucose. This suggests that plasma glucose levels were not closely regulated by the standard insulin-glucagon push-pull model. It is possible that glucose control was not as important due to adipose tissue insensitivity to insulin. We measured changes in the by-products of lipolysis, plasma NEFA and BOHB, in response to an insulin injection in four seals after an 8-week fast. Although NEFA and BOHB both decreased in response to insulin, plasma NEFA levels began to recover immediately and returned to preinjection levels 20 minutes after the exogenous insulin was cleared from the plasma. Ketone levels did not start to recover until 60 minutes after the injection and, like glucose, did not return to preinjection levels within the 150-minute experimental period. Thus, insulin had an effect on lipolysis, although in this experiment we could not determine whether fatty acid levels decreased due to insulin suppression of adipocyte lipolysis or due to facilitated muscle cell uptake of fatty acids.
Summary and Conclusions
Overall, fasting elephant seal pups conformed to the general mammalian model of fuel homeostasis for fasting animals in which protein conservation is paralleled by increased mobilization and utilization of lipid. It is surprising that these same adaptations are also seen in suckling pups just prior to weaning. However, when we examine the nutritional life history of elephant seals, we find that fat is the major energy source throughout development whether individuals are consuming high fat milk, high fat fish, or body fat stores. Since elephant seals consume only a minimal amount of dietary carbohydrate, all glucose must be made from precursors derived from the diet or from tissue stores. To date, changes in fuel distribution, fuel turnover rates, and plasma metabolites confirm that lipolysis is the major source of energy—and possibly glucose—in northern elephant seals.
The insulin and glucose studies show that nursing elephant seals are preadapted for fasting. The lack of pancreatic islet response and the impaired glucose clearance (K £ 1; fig. 21.2), the tissue insensitivity to insulin (fig. 21.3), and low plasma insulin and low ratio of I:G all contributed to maximizing the release of lipid from adipose tissue and were not a function of age, body fat, or nutritional status in seals. This emphasis on lipid mobilization concurrent with minimal oxidation of glucose and protein suggests that glucose may be synthesized from glycerol while the fatty acids are oxidized to meet the total energy needs of feeding and fasting seals. The important difference between the feeding and fasting state would be the partitioning of amino acids into protein synthesis or into gluconeogenesis.
Despite the fact that insulin is considered essential to protein synthesis in muscle tissue, suckling pups can gain a kilogram of lean tissue daily, even
with plasma insulin levels of less than 12 µU/ml. We suggest that this same anabolic condition exists for young fasting seals who are actively recycling protein and glucose as they reorganize their tissues. The observation that active synthesis and reorganization of tissue protein occurs throughout the postweaning period of fasting and development is supported by the previously mentioned protein turnover studies. Although we do not understand how these seals balance anabolic and catabolic processes during the post-weaning fast, the hormonal data suggest that we reevaluate the importance of insulin and glucagon in fuel regulation in these animals in particular and in other carnivores in general.
If insulin is not important in fuel homeostasis in this species, then other factors may play a more important role in glucose homeostasis. The influence of diving hypoxia on various tissues as well as the brain may change the influence that insulin has on muscle. It has been observed that hypoxia enhances muscle tissue sensitivity to insulin, reducing the amount needed to stimulate tissue growth (King et al. 1987). Diving may also enhance plasma fuel availability because exercise induces a release in epinephrine, which then increases glucose and free fatty acid release (Hamburg, Hendler, and Sherwin 1979). Increases in epinephrine and glucocorticoid plasma levels can also inhibit glucose-stimulated insulin release (Ploug, Galbo, and Richter 1984; Porte, Smith, and Ensinck 1979).
Alternative speculations suggest that insulin may not be necessary for protein synthesis in muscle tissue because seals may have high plasma levels of growth hormone or growth factors. The cellular requirements for energy are also regulated in part by thyroid hormone and glucocorticoids. These hormones are important for phocid molting (Ashwell-Erickson et al. 1986) and could be important to overall fuel homeostasis in seals. However anabolic processes are regulated, the glucose needs of the brain and other glucose obligate tissues must first be met so that seals can survive as diving carnivores who routinely experience apnea, tissue hypoxia, extreme exercise (as in deep diving), and periodic fasting. While fuel homeostasis in fasting seals is consistent with the general mammalian model of fasting, adipose tissue may be far more important to the elephant seal for glucose homeostasis than it is in other mammals.
References
Adams, S. H. 1991. Changes in protein metabolism and water conservation in northern elephant seals during the postweaning fast. M.Sc. thesis, University of California, Santa Cruz.
Ashwell-Erickson, S., F. Fay, R. Elsner, and D. Wartzok. 1986. Metabolic correlates of molting and regeneration of pelage in Alaskan harbor and spotted seals. Canadian Journal of Zoology 64: 1086–1092.
Bailey, B. A., R. G. H. Downer, and D. M. Lavigne. 1980. Neonatal changes in tissue levels of carbohydrate and lipid in the harp seal. Comparative Biochemistry and Physiology 67B: 179–182.
Bryden, M. M. 1968. Growth of the southern elephant seal, Mirounga leonina (Linn.). Growth 33: 69–82.
Cahill, G. F., Jr. 1970. Starvation in man. New England Journal of Medicine 282: 668–675.
Cahill, G. F., and T. T. Aoki. 1977. The role of glucagon in amino acid homeostasis. In Glucagon: Its Role in Physiology and Clinical Medicine , ed. P. Foa, J. Bajaj, and N. Foa, 487–494. New York: Springer Verlag.
Cahill, G. F., M. G. Herrera, A. P. Morgan, J. S. Soeldner, J. Teinke, P. L. Levy, G. A. Reichard, and D. M. Kipnis. 1966. Hormone-fuel interrelationships during fasting. Journal of Clinical Investigation 45: 1751–1769.
Castellini, M. A., and D. P. Costa. 1990. Relationships between plasma ketones and fasting duration in neonatal elephant seals. American Journal of Physiology 259: R1086–R1089.
Castellini, M. A., D. P. Costa, and A. C. Huntley. 1987. Fatty acid metabolism in fasting elephant seal pups. Journal of Comparative Physiology 157B: 445–449.
Cherel, Y., J. Leloup, and Y. Le Maho. 1988. Fasting in king penguins. II. Hormones and metabolite changes during molting. American Journal of Physiology 254: R178–R184.
Cherel, Y., J. Stahl, and Y. Le Maho. 1987. Ecology and physiology of fasting in king penguin chicks. Auk 104: 254–262.
Costa, D. P., and C. L. Ortiz. 1982. Blood chemistry homeostasis during prolonged fasting in the northern elephant seal. American Journal of Physiology 242: R591–R595.
Davis, R. W. 1983. Lactate and glucose metabolism in the resting and diving harbor seal. Journal of Comparative Physiology 153: 275–288.
Davis, R. W., M. A. Castellini, T. H. Williams, and G. L. Kooyman. 1991. Fuel homeostasis in the harbor seal during submerged swimming. Journal of Comparative Physiology 160B: 627–635.
Drenick, E. J., L. C. Alvarez, G. C. Tamasi, and A. S. Brickman. 1972. Resistance to symptomatic insulin reactions after fasting. Journal of Clinical Investigation 51: 2757–2762.
Englehardt, F. R., and J. M Ferguson. 1980. Adaptive hormonal changes in harp seals and grey seals during the post-natal period. General Comparative Endocrinology 40: 434–445.
Felig, P., O. E. Owen, J. Wahren, and G. F. Cahill, Jr. 1969. Amino acid metabolism during prolonged starvation. Journal of Clinical Investigations 48: 584–594.
Felig, P., R. S. Sherwin et al. 1979. Hormonal interactions in the regulation of blood glucose. Recent Progress in Hormone Research 35: 501–511.
Hamburg, S., R. Hendler, and R. S. Sherwin. 1979. Epinephrine: Exquisite sensitivity to its diabetogenic effects in normal man. Clinical Research 27: 252A.
Hochachka, P. W., B. Murphy, G. C. Liggins et al. 1979. Unusual maternal-fetal blood glucose changes in the Weddell seal. Nature 277: 388–389.
Kalhan, S. C. 1992. Metabolism of glucose and methods of investigation in fetus
and newborn. In Fetal and Neonatal Physiology , vol. 1, eds. R. A. Polin and W. W. Fox, 357–372. Philadelphia: W. B. Saunders.
Keith, E. O., and C. L. Ortiz. 1989. Glucose kinetics in neonatal elephant seals during postweaning aphagia. Marine Mammal Science 5(2): 99–115.
Kerem, D., D. Hammond, and R. Elsner. 1973. Tissue glycogen levels in the Weddell seal: A possible adaptation to asphyxial hypoxia. Comparative Biochemistry and Physiology 45A: 731–736.
King, D. S., G. P. Dalsky, M. A. Staten, W. E. Clutter, D. R. Canhouten, and J. O. Holloszy. 1987. Insulin action and secretion in endurance-trained and untrained humans. Journal of Applied Physiology 63: 2247–2252.
Kirby, V. L. 1990. Marine Mammal Endocrinology. In The CRC Handbook of Marine Mammal Medicine , ed. L. Dieruf, 303–352. Boca Raton: CRC Press.
———. 1992. The regulation of fuel homeostasis in young northern elephant seals. Ph.D. dissertation, University of California, Santa Cruz.
Kirby, V. L., and C. L. Ortiz. 1989. Body fat and glucose insulin response in fasting and feeding northern elephant seals during the first year of life. In Proceedings of Eighth Biennial Conference on the Biology of Marine Mammals , December 7–11, Pacific Grove, Calif.
———. 1990. Glucose regulation in fasting and feeding northern elephant seals less than one year of age. Physiologist 33: A–108.
Kretzmann, M. B. 1990. Milk intake, metabolic rate and mass change in northern elephant seal pups: Evidence for sexual equality. M.Sc. thesis, University of California, Santa Cruz.
Le Boeuf, B. J., and C. L. Ortiz. 1977. Composition of elephant seal milk. Journal of Mammalogy 58: 683–685.
Le Boeuf, B. J., R. J. Whiting, and R. F. Gantt. 1972. Perinatal behavior of northern elephant seal females and their young. Behaviour 43: 121–156.
Marliss, E. B., T. T. Aoki, R. H. Unger, J. S. Soeldner, and G. F. Cahill, Jr. 1970. Glucagon levels and metabolic effects in fasting man. Journal of Clinical Investigations 49: 2256–2270.
Nordoy, E. S., and A. S. Blix. 1991. Glucose and ketone body turnover in fasting grey seal pups. Acta Physiologica Scandinavica 41: 565–571.
Ortiz, C. L. 1990. Protein metabolism during the prolonged natural postweaning fast in rapidly developing elephant seal pups. Physiologist 33: A–56.
Ortiz, C. L., D. P. Costa, and B. J. Le Boeuf. 1978. Water and energy flux in elephant seal pups fasting under natural conditions. Physiological Zoology 51: 166–178.
Pernia, S. D. 1984. Protein turnover and nitrogen metabolism during long-term fasting in northern elephant seal pups. Ph.D. dissertation, University of California, Santa Cruz.
Pernia, S. D., A. Hill, and C. L. Ortiz. 1980. Urea turnover during prolonged fasting in the northern elephant seal. Comparative Biochemistry and Physiology 65B: 731–734.
Ploug, T., H. Galbo, and E. A. Richter. 1984. Increased muscle glucose uptake during contraction: No need for insulin. American Journal of Physiology 247: E726–E731.
Porte, D., Jr., P. H. Smith, and J. W. Ensinck. 1979. Neurohormonal regulation of the pancreatic islet. Metabolism 25 (Suppl. 1): 1453–1460.
Rea, L. 1990. Changes in resting metabolic rate during long-term fasting in northern elephant seal pups (Mirounga angustriostris ). M.Sc. thesis, University of California, Santa Cruz.
Reiter, J., N. L. Stinson, and B. J. Le Boeuf. 1978. Northern elephant seal development: The transition from weaning to nutritional independence. Behavioral Ecology and Sociobiology 3: 337–367.
Riedman, M., and C. L. Ortiz. 1979. Changes in milk composition during lactation in the northern elephant seal. Physiological Zoology 52: 240–249.
Robin, E. D., J. Ensinck, A. J. Hance et al. 1981. Glucoregulation and simulated diving in the harbor seal. American Journal of Physiology 241: R293–R300.
Salans, L. B., J. R. Knittle, and J. H. Hirsch. 1983. Obesity, glucose tolerance, and diabetes mellitus. In Diabetes Mellitus: Theory and Practice , 3 ed., ed. M. Ellenberg and H. Rifkin, 469–479. New York: Medical Examination Publishing Co.
Tieran, J. R. 1970. Insulin in fetal and neonatal metabolism. In Physiology of the Perinatal Period , vol. 2, ed. U. Stave. New York: Plenum Medical.
Umminger, B. L. 1975. Body size and whole blood sugar concentrations in mammals. Comparative Biochemistry and Physiology 52A: 455–458.
Unger, R. H., 1985. Glucagon physiology and pathophysiology in the light of new advances. Diabetologia 28: 574–579.
Unger, R. H., A. M. Eisentraut, and L. L. Madison. 1963. The effects of total starvation upon the levels of circulating glucagon and insulin in man. Journal of Clinical Investigations 42: 1031.
Van Aswegen, G., and A. T. Viljoen. 1987. Endocrine cells in the gut of the South African fur seal, Arctocephalus pusillus. Acta Anatomica 130: 93–95.
Worthy, G. A., and D. M. Lavigne. 1982. Changes in blood properties of fasting and feeding harp seals after weaning. Canadian Journal of Zoology 60: 586–592.
Twenty-two—
Endocrine Changes in Newborn Southern Elephant Seals
Michael M. Bryden
ABSTRACT. The structure and function of some endocrine organs (hypophysis, thyroid, and pineal gland) of newborn southern elephant seals and their role in maintaining homeothermy are reviewed briefly. The adrenal gland is probably important, particularly in the immediate perinatal period, but it has not been examined yet.
The predominant cell in the hypophysis of the newborn seal is the somatotroph, suggesting that secretion of growth hormone is a major function of the gland during suckling, when pups grow rapidly. The degree of control of other endocrine organs by the hypophysis in newborn seals is unknown.
The thyroid gland is large, with histological evidence of activity at and soon after birth. The thyroid hormones T3 and T4, present in circulating blood at birth, are elevated within the subsequent two hours and remain high for approximately the first week postpartum. As circulating thyroid hormones decline, metabolic rate probably declines also, but direct measurements have not been made.
The pineal gland is very large and active in newborn southern elephant seals and remains so until 7 to 10 days postpartum. Circumstantial evidence suggests it is involved in the control of thermogenesis in early postnatal life.
Newborn phocid seals have a characteristically short lactation period, when the young undergo dynamic change (Bryden 1969; Oftedal, Boness, and Tedman 1987). Growth is rapid; most species at least treble their birth weight in just a few days or weeks (Laws 1959; Bowen, Oftedal, and Boness 1985). Most of the increase in weight is due to deposition of fat, although growth of the musculature and many other organs does occur. There is little increase in bones during this phase of growth (Bryden 1969). Dynamic shifts in the relative size of different tissues and organs occur during this stage; for example, the muscles that are used in terrestrial locomotion grow relatively more quickly than those that are not. This situation changes
when the seals begin swimming, when those muscles responsible for aquatic propulsion grow relatively more quickly (ibid.).
Elephant seals are subjected to severe environmental conditions at and soon after birth. Although they experience a less extreme temperature change in passing from the uterus to the external environment than do polar seals such as Weddell and ringed seals, it is conceivable that they experience greater cold stress at birth. The coat of polar seals dries very quickly after birth, as the placental fluids on the coat quickly freeze and fall off or can be shaken off. In addition, the natal fur of these species is thicker than that of the newborn elephant seal and is a more effective insulator.
Southern elephant seals are born on subantarctic islands, where the environmental temperature is usually in the range of –5°C to +5°C. Rain, sleet, or snow is common, and the coat of the newborn seal may remain wet for several hours. This combined with the relatively ineffective insulation of the coat means that the newborn elephant seal can be subjected to extreme cold stress at birth, exacerbated by average wind speed on many subantarctic beaches of 40 km/hour.
As a rule, newborn pups do not adopt behavioral means of reducing heat loss, such as curling up, seeking shelter, or seeking to huddle close to neighbors. Neither does the mother protect the newborn young from heat loss: she does not attempt to shelter it or draw it close to her body.
These observations suggest that physiological mechanisms are predominant in maintaining body temperature in early postnatal life and play an important role in other body mechanisms during this dynamic part of the seal's postnatal life. Shivering is observed rarely (Laws 1953; Little 1989) and does not appear to be an important means of thermogenesis except in the most extreme circumstances.
My group has been studying aspects of the endocrine changes in early postnatal life for several years. Major aims of the work have been to examine the endocrine control of early postnatal development and gain some insight into possible means of thermogenesis in the first hours and days after birth.
The Endocrine Glands in Newborn Seals
It was first observed about forty years ago that in newborn seals certain organs, notably, the gonads, are very large and appear to be subjected to endocrine stimulus at about the time of birth (Harrison, Matthews, and Roberts 1952; Bonner 1955). E. C. Amoroso, G. H. Bourne, R. J. Harrison, L. H. Matthews, I. W. Rowlands, and J. C. Sloper (1965) described the histological appearance of several endocrine organs in fetal, newborn, and adult gray and common seals and noted particularly that the hypophysis (pituitary gland) was well developed at birth. However, they concluded
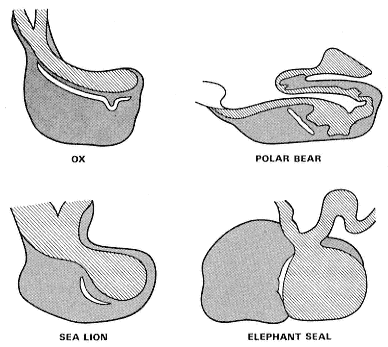
Fig. 22.1
Comparative median sections of the hypophyses of some mammals.
The shaded structure is the adenohypophysis; the cross-hatched
structures are the neurohypophysis and adnexa.
that the rapid decrease of endocrine influence on the gonads, which involute rapidly, suggested that the placenta rather than the fetal hypophysis is implicated. The large size of the gonads is due mainly to proliferation of interstitial cells and does not reflect functional status of the organs.
The Hypophysis
The hypophysis of the southern elephant seal conforms to the general mammalian pattern. Its form is compared with that of some other mammalian species in figure 22.1, which shows variations in shape but not of general arrangement. The significance of the intraglandular cleft, expansive in the newborn hypophysis but greatly reduced in the adult, is unknown.
Cell types in the pars distalis were differentiated by staining with a Periodic Acid Schiff–Alcian blue—orange G method modified from M. F. El Etreby, K.-D. Richter, and P. Günzel (1973). Four clearly distinct chromophilic cells were visible following staining, namely, gonadotrophs, thyrotrophs, lactotrophs, and somatotrophs. The relative frequencies of the different cell types in adult cows and young pups are shown in table 22.1.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
A preponderance of chromophobic cells in the hypophysis at birth contrasts with the situation in the adult, in which chromophilic cells suggest activity in controlling various physiological events and reproductive events in particular (Griffiths and Bryden 1986). A similar, relatively immature appearance of the adenohypophysis of the newborn animal, with the presence of many chromophobic cells, was observed in the harp seal by J. F. Leatherland and K. Ronald (1979). This contrasts with the finding in the common seal that the maturity of the adenohypophysis at birth is striking (Amoroso et al. 1965).
By far the most abundant chromophilic cell in the hypophysis of the elephant seal pup is the somatotroph, suggesting that the adenohypophysis may be actively secreting growth hormone during the rapid early postnatal growth of the pup (see table 22.1). Attempts to assay growth hormone in the plasma of elephant seal pups were unsuccessful, but the morphological evidence suggests that plasma concentrations of growth hormone may be quite high.
Immunocytochemical methods would improve the sensitivity of identifying the different cell types and would enable one to identify corticotrophic cells. They would also differentiate between luteotrophin-secreting and FSH-secreting cells, not possible with the histochemical staining regime used here.
The Thyroid
The hormones secreted by the thyroid gland, thyroxine (T4) and triiodothyronine (T3), are responsible for normal growth and maintenance of homeothermy through their role in controlling body metabolism. We have examined both histological and endocrinological indicators of thyroid function. A detailed description of the thyroid gland and its function in southern elephant seals has been provided by G. J. Little (1991).
Histology
Squamous thyroid epithelial cells are indicative of low or reduced thyroid activity, whereas cuboidal or columnar epithelium signifies increased activity. In southern elephant seal pups, thyroid epithelial cell height is significantly greater in the first two days of life than subsequently (Little 1991).
Ultrastructurally, dramatic morphological changes occur in the thyroid epithelial cells in the first 24 to 48 hours after birth and have been illustrated by Little (1991). Pseudopodia protrude into the thyroid follicular lumen from the surface of the epithelial cells. The pseudopodia engulf colloid within the follicular lumen and take it up into the epithelial cells. For the first few hours of postnatal life, colloid is confined to the apical portion of the epithelial cell, but after 6 hours, colloid droplets become distributed throughout the cytoplasm. This indicates that thyroglobulin, absorbed from
the lumen following engulfment by the pseudopodia, is being hydrolyzed and that T4 and T3 are being released.
Endocrinology
The plasma concentrations of T4 and T3 increased in the first 24 hours or so of postnatal life (Little 1991). Plasma T4 concentration showed about a twofold increase in the first 2 hours after birth, which is similar to the situation in many other mammals. Plasma T3 concentration, however, increased about eightfold at 24 hours after birth, the most dramatic increase of any newborn mammal. The concentration remained elevated until 7 days of age. Little (1991) concluded from these observations that the hypophysis-thyroid axis is functional at birth in the southern elephant seal and that the thyroid gland is the source of circulating T4 and T3 in the pup.
T3 is physiologically much more active than T4, and in newborn elephant seals, it seems to play an important role in thermogenesis, at least in the first week of postnatal life. It probably does so by increasing the metabolic rate. The growth of fat increases markedly after the first week (Bryden 1969), so the physical insulation it provides probably reduces heat loss, leading to lowered metabolic rate. We have not confirmed this in elephant seals, but it has been shown to be the case in common seals and harp seals (Davydov and Makarova 1964).
In summary, there is morphological and physiological evidence that the thyroid gland is very active within a few hours of birth and is responsible for increased levels of circulating T4 and T3 for the first days of life. It was not possible to confirm whether thyroid function was initiated and maintained by the hypophysis, but the presence of mature thyrotrophs in reasonable numbers in the hypophysis of the newborn seal (table 22.1) suggests this is probably the case.
The Pineal
It has been known for almost twenty years that polar animals tend to have a very large pineal gland. Southern elephant seals are no exception. A remarkable feature of this species, however, like the Weddell seal and other polar seal species, is that the pineal gland is particularly large at birth (Bryden et al. 1986; see fig. 22.2).
Histologically, the pineal contains many pinealocytes at birth, indicating that the gland is potentially functional (ibid.). This contrasts with the gonads, whose enlargement at birth results from the great proliferation of interstitial tissue.
Ultrastructurally, the pinealocytes appear relatively immature (Little and Bryden 1990). This suggests that the pineal, although enlarged, may not be very active at birth. However, the density of pinealocytes is similar
Fig. 22.2
Median section of the brain of a dog (top) and a newborn
elephant seal (bottom). The pineal of each is marked (P).
in pups and adults, and the pup pineal contains 50 to 100 times more pinealocytes than the adult (Bryden et al. 1986).
We tested for pineal activity by assay of plasma for the hormone secreted by the pineal gland, melatonin. Details of the assay method are given in D. J. Kennaway, T. A. Gilmore, and R. F. Seamark (1982) and C. R. Earl, M. J. D'Occhio, D. J. Kennaway, and R. F. Seamark (1985).
The assays revealed extremely high concentrations of circulating melatonin in the first days of postnatal life. Concentrations in excess of 60,000 picomoles per liter (pM/l) were measured. (This contrasts with levels in most adult mammals of 100–300 pM/l.) It is possible that at least some of the melatonin may originate from organs other than the pineal, for example, the retina. However, the fact that the pineals of newborn pups also con-
tained high concentrations of melatonin (Little and Bryden 1990) argues for a high level of production of melatonin in the pineal itself.
The pineal and its secretory product, melatonin, have been implicated in the control of several physiological mechanisms, in particular, reproduction, by mediating the influence of the photoperiod on the neuroendocrine-reproductive axis. As currently perceived, the function of the pineal is a rather general one, serving as an intermediary between the external environment (particularly the photoperiod) and the organism as a whole (Reiter 1981). We have observed that it is large and active in the newborn elephant seal and suggest it is involved in thermoregulation at this stage of the seal's life.
The profiles of plasma melatonin and T3 concentration are somewhat similar. They increase in the first hours of postnatal life and decline after about the first week.
Comparison of Southern and Northern Elephant Seals
If our conclusion that the pineal gland in newborn pups is involved in thermoregulation is correct, we should expect to see a lesser role for the pineal in early postnatal life in the northern elephant seal as compared to the subantarctic southern elephant seal. Plasma has been assayed for melatonin concentration in northern elephant seals (fig. 22.3). We see that although the concentration of melatonin in northern elephant seals is very high, and it follows a similar pattern to that in the southern elephant seal, the concentrations for the most part are substantially lower than in the southern elephant seal. This needs to be looked at in more detail, but these results tentatively support the notion that the pineal gland is involved in thermogenesis in elephant seals in early postnatal life.
Adrenal
An important omission so far is the adrenal gland, which has not been examined in elephant seals. F. R. Engelhardt and J. M. Ferguson (1980) reported high concentrations of cortisol and aldosterone immediately after birth in gray seal and harp seal pups. They discussed the possible role of these hormones acting synergistically to promote gluconeogenesis and the role of cortisol in increasing cold tolerance in the newborn. The extreme cold to which newborn southern elephant seals are subjected induces stress almost certainly, and the role of the adrenal needs to be addressed.
Conclusions
Endocrine glands of newborn southern elephant seals are active and almost certainly vital to survival in the first hours and days of postnatal life. Morphological evidence suggests the hypophysis secretes growth hormone, in-
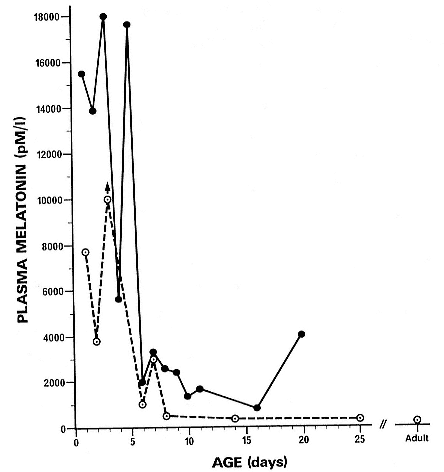
Fig. 22.3
Plasma melatonin concentration (pM/l) in neonatal southern (closed circles,
solid line) and northern (open circles, broken line) elephant seals.
volved in the very rapid growth during the lactation period. It has not been possible to quantify the degree of hypophyseal control of the thyroid gland in early postnatal life by the methods used so far.
The thyroid gland is large and active within about 2 hours of birth in the production of thyroid hormones. Increased amounts of thyroid hormones are released into the bloodstream during the first day, and high circulating levels are maintained for the first week of life. The rate of metabolism probably declines in the second week as a consequence of reduction in the levels of thyroid hormones, when the rate of blubber deposition increases and physical insulation is enhanced.
The pineal gland is very large and active in newborn elephant seals. Circumstantial evidence suggests that it plays an important role in thermoregulation in the first hours and days of postnatal life.
Acknowledgments
I thank the Director, Antarctic Division, Hobart, and the Director, Tasmanian National Parks and Wildlife Service, without whose support over many years this work would not have been possible. Many people have assisted in many ways, but in particular, I wish to acknowledge the contributions and stimulating discussions of David Griffiths, Gerald Little, David Kennaway, Raymond Tedman, and Jean Ledingham. I extend thanks to Ms. B. Jantulik for illustrations and Mrs. L. Hicks for preparing the manuscript.
References
Amoroso, E. C., G. H. Bourne, R. J. Harrison, L. H. Matthews, I. W. Rowlands, and J. C. Sloper. 1965. Reproductive and endocrine organs of foetal, newborn and adult males. Journal of Zoology, London 147: 430–486.
Bonner, W. N. 1955. Reproductive organs of foetal and juvenile elephant seals. Nature 176: 982–983.
Bowen, W. D., O. T. Oftedal, and D. J. Boness. 1985. Birth to weaning in 4 days: Remarkable growth in the hooded seal, Cystophora cristata. Canadian Journal of Zoology 63: 2841–2846.
Bryden, M. M. 1969. Relative growth of the major body components of the southern elephant seal, Mirounga leonina (L.). Australian Journal of Zoology 17: 153–177.
Bryden, M. M., D. J. Griffiths, D. J. Kennaway, and J. Ledingham. 1986. The pineal gland is very large and active in newborn antarctic seals. Experientia 42: 564–566.
Davydov, A. F., and A. R. Makarova. 1964. Changes in heat regulation and circulation in newborn seals on transition to aquatic form of life. Fiziol. Zhur. SSSR 50: 894–897.
Earl, C. R., M. J. D'Occhio, D. J. Kennaway, and R. F. Seamark. 1985. Serum melatonin profiles and endocrine responses of ewes exposed to a pulse of light late in the dark phase. Endocrinology 117: 226–230.
El Etreby, M. F., K.-D. Richter, and P. Günzel. 1973. Histological and histochemical differentiation of the glandular cells of the anterior pituitary in various experimental animals. Excerpta medica (Amsterdam), International Congress Series 288: 270–281.
Engelhardt, F. R., and J. M. Ferguson. 1980. Adaptive hormone changes in harp seals, Phoca groenlandica , and gray seals, Halichoerus grypus , during the postnatal period. General and Comparative Endocrinology 40: 434–445.
Griffiths, D. J. 1980. The control of the annual reproductive cycle of male elephant
seals (Mirounga leonina ) at Macquarie Island. Ph.D. dissertation, University of Queensland, Australia.
Griffiths, D. J., and M. M. Bryden. 1986. Adenohypophysis of the elephant seal (Mirounga leonina ): Morphology and seasonal histological changes. American Journal of Anatomy 176: 483–495.
Harrison, R. J., L. H. Matthews, and J. M. Roberts. 1952. Reproduction in some pinnipedia. Transactions of the Zoological Society of London 27: 437–540.
Kennaway, D. J., T. A. Gilmore, and R. F. Seamark. 1982. The effect of melatonin feeding on serum prolactin and gonadotropin levels at the onset of estrous cyclicity in sheep. Endocrinology 110: 1766–1772.
Laws, R. M. 1953. The elephant seal (Mirounga leonina Linn.). I. Growth and age. Falkland Islands Dependencies Survey, Scientific Reports 8: 1–62.
———. 1959. Accelerated growth in seals, with specific reference to Phocidae. Norsk Hvalfangst-Tidende 48: 425–452.
Leatherland, J. F., and K. Ronald. 1979. Thyroid activity in adult and neonate harp seals, Pagophilus groenlandicus. Journal of Zoology, London 189: 399–405.
Little, G. J. 1989. Thermoregulation in the newborn southern elephant seal, Mirounga leonina (L.). Ph.D. dissertation, University of Queensland, Australia.
———. 1991. Thyroid morphology and function and its role in thermoregulation in the newborn southern elephant seal (Mirounga leonina ) at Macquarie Island. Journal of Anatomy 176: 55–69.
Little, G. J., and M. M. Bryden. 1990. The pineal gland in newborn southern elephant seals, Mirounga leonina. Journal of Pineal Research 9: 139–148.
Oftedal, O. T., D. J. Boness, and R. A. Tedman. 1987. The behavior, physiology, and anatomy of lactation in the pinnipedia. In Current Mammalogy , vol. 1, ed. H. H. Genoways, 175–245. New York: Plenum.
Reiter, R. J. 1981. The mammalian pineal gland: Structure and function. American Journal of Anatomy 162: 287–313.