Chapter 1—
Plant Cell Walls
1.1—
Introduction
Plant cell walls establish a home, and indeed a city, for plant protoplasts. They serve many specialized functions in plant tissues, and form the skin, the skeleton, and the circulatory system of plants.
There are many variations in the form and substance of plant cell walls. The walls may be plastic or they may be rigid, permeable or impermeable, impregnated with plastics or coated with slime, cemented in layers to form fibres or dissolved in spots to form pores. These variations are of vital importance to the proper biological functioning of plant cells and organs, and thus the structure of the cell wall is often our best indication of the nature of the protoplast which dwells inside.
1.2—
The Molecular Structure of Plant Cell Walls
Polysaccharides are the principal components of all plant cell walls. The polysaccharides of the cell wall are made up of sugars which are linked to each other by glycosidic bonds to form the polymer chains. Each polysaccharide contains particular kinds of sugars which are joined to each other in characteristic patterns of linkage position and sequence. It is now known that the secondary, tertiary and quaternary structures of cell wall polysaccharides are determined by the structures of the component sugars and the linkages between them, just as the three dimensional structure of a protein is determined by the sequence and structures of its component amino acids (Rees, 1972).
The various polysaccharide chains of the plant cell wall are connected to each other in specific ways, and they form an integrated network. The properties of this network depend not only on the amounts, characteristic properties and orientations of the individual polysaccharides, but also on the nature and frequency of the interconnecting linkages between them.
The conformational structures of the nine sugars commonly found in plant cell walls are shown in Fig. 1.1. The three types of polysaccharide normally found in plant cell walls (cellulose, hemicelluloses, and pectic polysaccharides), and the structural protein of primary walls, are described briefly below.
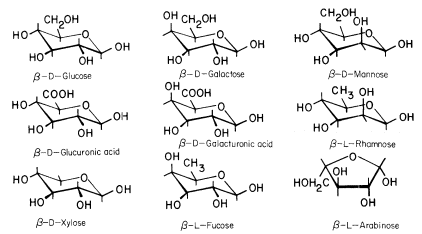
Figure 1.1
Sugars of plant cell walls.
Conformational line drawings indicate approximate bond angles. b -L arabinose is shown in its
preferred planar furanose ring form. The other sugars are shown in their most stable pyranose
chair form. Carbon atoms are numbered as indicated for b -D -glucose. Ring hydrogens are
indicated by bonds only. Note that groups attached to a ring may be either axial (projecting
above or below the ring) or equatorial (projecting to the side of the ring). Substituents at C1
project equatorially in the b configuration, but are axial in the a configuration. All 'bulky'
groups (–OH, –CH2 OH, & –COOH) are in equatorial positions in b -D -glucose, b -D -
glucuronic acid, and b -D-xylose. Note that these sugars differ only in the group
attached to C5 . Galactose, galacturonic acid and fucose are similarly related
(axial –OH group at C4 ), as are mannose and rhamnose (axial –OH group at C2 ).
1.2.1—
Cellulose
Cellulose occurs as a crystalline, fibrillar aggregate of b -1,4-linked glucan chains (Frey-Wyssling, 1969). Cellulose fibrils give plant cell walls most of their enormous strength, much as glass fibres embedded in an epoxy resin give strength to a fibreglass composite (Northcote, 1972).
The basic structure of the b -1,4-linked glucan chains of cellulose is illustrated in Fig. 1.2 by conformational line drawings and in Fig. 1.3 by molecular models. Residues of b -D -glucose (Fig. 1.1) are glycosidically linked to each other, from carbon 1 of one residue to carbon 4 of the adjacent residue. The upside-down inversion of every second residue in the chain minimizes contact between atoms of adjacent residues. Close inspection of the models in Fig. 1.3 shows that the –OH groups at carbon 3 are in very close proximity to the ring oxygens (O5 ) of adjacent residues. Hydrogen bonds between O3 and O'5 help to stabilize the flat, straight, ribbon-like structure of b -1,4-linked glucan chains.
The flat, ribbon-like structure allows the chains to fit closely together, one on top of the other, over their entire lengths. These interchain associations are stabilized by hydrogen bonds between O6 of a glucose residue in one chain and
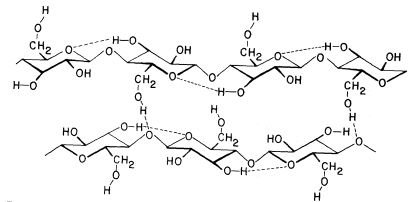
Figure 1.2
b -1,4–linked glucan chains of cellulose.
Portions of two associated chains are illustrated by
conformational line drawings. Distances between atoms
are not accurately indicated in this illustration, but see Fig. 1.3.
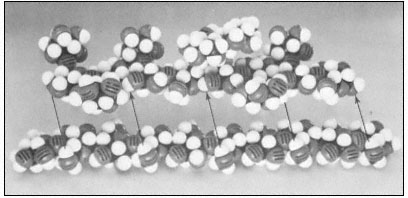
Figure 1.3
Hemicellulosic xyloglucan associated with cellulose.
The repeating subunit of a hemicellulosic xyloglucan is shown in
association with a portion of a b –1,4 –linked glucan chain of cellulose
(Bauer et al., 1973). Molecular models have been used to accurately indicate
interatomic distances and bond angles. Hydrogen bonds from the cellulosic
glucan chain to the glucan backbone of the hemicellulose are indicated by arrows.
the oxygen of the glycosidic bond (O1 ) between glucose residues in an adjacent chain. Since the glucan chains of cellulose are very long (8,000 to 15,000 residues), the number of hydrogen bonds between adjacent chains is very large. The resultant crystal is extremely stable and so tightly packed that there is no room for water molecules in the crystal structure.
Although there is some controversy as to whether native cellulose fibrils are 3.5 nm or 10 nm in diameter, it is clear that the glucan chains of cellulose
aggregate to form stiff crystalline rods of very considerable length and mechanical strength. The molecular structure of b -1 ,4-linked glucose thus neatly determines the secondary and tertiary structures of cellulosic glucan chains, and establishes the quaternary, interchain associations—although not the dimensions—of the microfibrils. The stiff, crystalline rods of cellulose are clearly well suited to their biological function in the plant cell wall.
1.2.2—
Hemicelluloses
Xylans, arabinoxylans, galactomannans, glucomannans and xyloglucans are common types of hemicelluloses (Timell, 1965). The basic repeating sequence of a hemicellulosic xyloglucan molecule is shown in Fig. 1.3, adjacent to a cellulosic glucan chain.
Although different hemicelluloses have different component sugars, all have two structural features in common which bear importantly on their biological function. (1) All hemicelluloses have straight, flat b -1,4-linked backbones. Any side chains attached to the backbone are short—usually just one sugar long—and stick out to the sides of the backbone (cf. Fig. 1.3). (2) All hemicelluloses have some structural feature which prevents the chains from extended self-aggregation of the type which exists between the b -1,4-linked glucan chains of cellulose. Xyloglucans, for example, have a b -1,4-linked glucan backbone (just as cellulose), but most of the glucose –CH2 OH groups (C6 ) are substituted with xylose side chains (see Fig. 1.3) and are thus not available for the formation of interchain hydrogen bonds. Similarly, since xylose is a pentose (i.e. a five carbon sugar), the b -1,4-linked xylose backbones of xylans and arabinoxylans have no–CH2 OH groups available for interchain hydrogen bonding. The glucomannans have b -1,4-linked backbones containing both glucose and mannose. The –CH2 OH groups of these sugars are unsubstituted, but the axial conformation of the –OH groups at carbon 2 of the mannose residues (cf. Fig. 1.1) prevent close interchain associations wherever mannose residues occur in the backbone.
Although hemicelluloses cannot self-aggregate to form long, close-packed crystalline fibrils in the manner of cellulose, the chains of hemicellulosic polysaccharides can form important hydrogen bonded associations with each other in the cell wall, particularly between regions of the chains which have few side branches or axial hydroxyl groups (Blake & Richards, 1971; McNiel et al., 1974). Even more importantly, hemicelluloses can co-crystallize with cellulosic glucan chains at the surface of the cellulose microfibrils (Bauer et al., 1973, Northcote, 1972). The cocrystallization probably involves the formation of hydrogen bonds from the –CH2 OH groups present in cellulose chains to the glycosidic oxygens in the adjacent hemicellulose chains (see Fig. 1.3). This association would form a tightly bound monolayer of the hemicellulose on the surface of the cellulose microfibril, and would function as part of the 'glue' which holds the microfibrils together in the cell wall.
1.2.3—
The Pectic Polysaccharides and Structural Protein
1.2.3.1—
Rhamnogalacturonans
The rhamnoglacturonans are long polymers of a -1,4-linked galacturonic acid interspersed with a few residues of 1,2-linked rhamnose (Aspinall, 1973). There is some evidence that the rhamnosyl residues may occur at definite positions in the galacturonan chain, giving a subunit structure to the rhamnogalacturonan polymer (Talmadge et al., 1973; see Fig. 1.4).
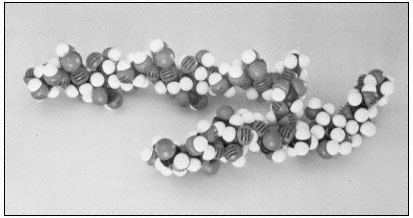
Figure 1.4
Pectic rhamnogalacturonan.
CPK models illustrate the repeating subunit of a rhamnogalacturonan,
with a short sequence of b -1,4-linked galactan attached to C4 of one
of the rhamnosyl residues (Talmadge et al., 1973). The sequence
of the subunit is GalUA8 Rha GalUA Rha GalUA4 .
The diaxial conformation of the a -1,4 glycosidic linkages between galacturonic acid residues (see Fig. 1.1) causes the orientation of adjacent rings to be twisted. Thus, the galacturonan polymer forms a tight, stiff, rod-like helix with three residues per turn (Rees & Wight, 1971; Fig. 1.4). The insertion of 1,2-linked rhamnosyl residues in the galacturonan chain creates 'kinks' or right angle bends.
Divalent cations, particularly calcium, form complexes with the carboxyl and hydroxyl groups of galacturonic acid residues in the polymer. Complex formation of this type occurs primarily between adjacent residues in a galacturonan polymer, but could serve to create ionic ligand bridges between adjacent galacturonan chains.
1.2.3.2—
Arabinogalactans
Two distinct types of arabinogalactans are known to occur in plant cell walls (Aspinall, 1973). The first type has a b -1,4-linked galactan backbone with
highly branched arabinose side chains. The second type has a b -1,3-linked galactan backbone with many short side chains containing galactose and arabinose.
1.2.3.3—
Structural Protein
Primary cells walls (i.e. the type of walls characteristic of actively growing cells) contain a structural protein component. While the structural protein of these walls has not yet been isolated as an intact molecule, the analysis of peptide fragments from the primary walls of dicotyledonous plants has revealed several interesting characteristics (Lamport, 1970; Lamport et al., 1973). This structural protein contains over 25% hydroxyproline, which is an unusual amino acid known to break the continuity of a -helical structures. In animals, hydroxyproline occurs almost exclusively in the proteins of connective tissue (collagen and gelatin). Several tryptic peptides of the structural protein have been isolated,
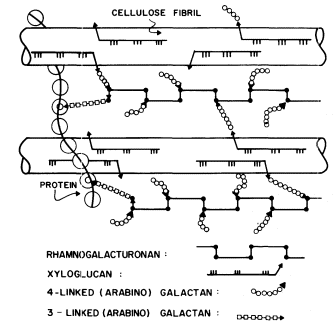
Figure 1.5
Interconnections between cell wall components.
Schematic representation of the polymeric components of
sycamore primary cell walls and their interconnections (Keegstra et al.,
1973). Hemicellulosic xyloglucan polymers are cocrystalized with cellulosic
glucan chains on the surface of the (two) microfibrils. The reducing ends of some
—but not all—of the xyloglucan chains are glycosidically attached to some—but not
all—of the b -1,4-linked (arabino) galactan side chains of the rhamnogalacturonan polymers.
The rhamnogalacturonan polymers and the structural protein are interconnected by 1,3-linked
(arabino) galactan bridges. Arrowheads indicate the reducing ends of polysaccharide chains.
and all appear to contain the sequence Ser-Hyp4 . Arabinose tetrasaccharides are glycosidically linked to virtually all of the hydroxyproline residues in the protein. In addition, many of the serine residues are glycosylated with galactose. As a result of this extensive glycosylation, the structural protein is very resistant to degradation by proteases, and is likely to have an extended, rod-like shape.
1.3—
Interconnections between Cell Wall Components
The polysaccharide components of the primary cell walls of dicotyledonous plants are specifically attached to one another to form an interconnected network (Keegstra et al., 1973; see Fig. 1.5). The b -1,4-linked glucan chains of cellulose are aggregated to form crystalline microfibrils. The hemicellulosic xyloglucan chains cocrystallize with the cellulose chains on the surface of the microfibrils as described above (1.2.2). The reducing ends of the xyloglucan chains are covalently (glycosidically) attached to b -1,4-linked arabinogalactan. The arabinogalactan chains, in turn, are glycosidically linked to the backbone of the rhamnogalacturonan, probably to the 4 positions of the rhamnosyl residues. The 1,4 arabinogalactan is thus a side chain of the rhamnogalacturonan, and forms an interconnecting bridge between the xyloglucan and the rhamnogalacturonan. The other type of arabinogalactan (b -1,3-linked) also seems to serve as an interconnecting bridge, glycosidically linking the reducing ends of rhamnogalacturonan chains to amino acid residues in the structural protein of the cell wall.
1.4—
The Universality of Plant Cell Wall Structures
Studies of cell walls isolated from suspension-cultured plant cells have demonstrated quite clearly that dicotyledonous plants from taxonomically diverse species have very similar primary walls (Albersheim, 1974). Similar studies on the primary walls of aspen cambial tissue and potato tuber tissue have shown that these walls are quite similar to the walls of suspension-cultured cells (Timell, personal communication; Bauer, unpublished results). Thus, all dicotyledons probably have the same basic primary cell wall.
The primary cell walls of monocotyledons have a structure which is somewhat different from that of dicotyledons, although the walls from various monocotyledon species appear to be quite similar to each other. The hemicellulose of the primary monocotyledon walls is an arabinoxylan instead of a xyloglucan, and the structural protein appears to contain very little hydroxyproline (Burke et al., 1974).
Most cells, after they stop active growth, synthesize 'secondary' cell wall material which is quite different from the primary wall. Secondary cell walls
consist almost exclusively of large amounts of cellulose and one (or more) of the hemicellulosic polysaccharides. Lignification is very common for cells with secondary walls.
Within broad taxonomic groups, related plants appear to have very similar cell walls. However, it should be recognized that relatively few types of cell walls from relatively few plant species have been carefully analysed. The diversity of specialized cell wall functions is likely to be reflected in a diversity of cell wall structures.
1.5—
Cell Wall Plastics
1.5.1—
Lignin
Lignin is a biological plastic formed in plant cell walls by the enzymic dehydrogenation of coumaryl, coniferyl and synapyl alcohols (Fig. 1.6) followed by a free radical polymerization (Freundenberg, 1968; Northcote, 1972). Since the polymerization is not enzymically controlled, and the monomeric free radicals can react with each other in a variety of ways, lignin does not have a unique structure. However, the lignin from a particular plant species or tissue does usually have a characteristic monomer composition. Some covalent linkages are formed between lignin and the polysaccharides of the cell wall during lignin biosynthesis (Morrison, 1974).
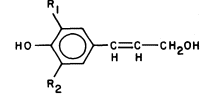
Figure 1.6
Lignin precursors.
R1 = H, R2 = H, coumaryl alcohol.
R1 = H, R2 = OCH3 , coniferyl alcohol.
R1 = OCH3 , R2 = OCH3 , synapyl alcohol.
Lignin formation is initiated very soon after secondary wall synthesis begins, and proceeds from the region of the middle lamella (the pectin-rich layer between adjacent cell walls) inward towards the plasmalemma. Thus, both the primary and secondary walls become fully impregnated with a rigid, hydrophobic plastic which is covalently linked to the polysaccharide matrix. The resultant structure is extremely strong and resistant to degradation.
1.5.2—
Cutin
Cutin is a biological plastic which coats the cell walls on the outer surface of plant epidermal cells. Although little is known about the detailed structure of
cutin, it is clear that the principal monomeric components of this material are mono-, di-, and trihydroxyfatty acids (C16 –C18 ). These hydroxyfatty acids are linked to each other mainly through ester bonds, although the presence of both ether and peroxide bonds have been reported (Kolattukudy & Walton, 1972). The formation of ester linkages between monomeric hydroxyfatty acids and preformed cutin appears to involve the enzymic transacylation of the hydroxyfatty acids from a Coenzyme A intermediate to free hydroxyl groups in cutin (Croteau & Kolattukudy, 1974).
The film of cutin polymer is impregnated—and frequently coated—with a complex mixture of waxes. The cutin-wax structure (cuticle) merges gradually with the normal polysaccharide components of the epidermal cell wall. There is evidence for the existence of hydrophylic channels (ectodesmata) in the hydrophobic cuticle (Franke, 1967). These channels may occur in morphologically distinct patterns on the epidermal surface.
The cuticular waxes and the cutin polymer form a tough hydrophobic skin over the surface of the plant which is important in minimizing water loss and preventing mechanical injury or invasion by pathogens.
1.6—
Biosynthesis of Plant Cell Walls
It is generally believed that cell wall polysaccharides are synthesized from the appropriate sugar nucleotides (e.g. UDP-glucose) by specific enzymes or enzyme complexes. Many—though not necessarily all—cell wall polysaccharides are synthesized in the golgi bodies (dictyosomes) and transported to the wall in golgi-derived vesicles which are able to fuse with the plasmalemma (O'Brien, 1972). The cell wall polysaccharides are then presumably interconnected while in the wall.
There are many reports of isolated enzymes or particulate complexes which incorporate sugars from the sugar nucleotides into polysaccharide material. However, the amount of polysaccharide material formed by these in vitro enzyme systems is often a miniscule fraction of the amount formed in vivo. Thus the relationship between these isolated enzymes and cell wall biosynthesis is uncertain.
Isolated enzyme preparations have been reported to synthesize alkaliinsoluble b -1,4 glucan ('cellulose') at rates comparable to the rate of in vivo cellulose synthesis (Rollit & Maclachlan, 1974). However, these enzymes have not as yet been shown to form the microfibrils characteristic of cellulose. There is some evidence that cellulose microfibrils are synthesized at the plasmalemma rather than in the golgi (Bowles & Northcote, 1972), perhaps by plasmalemmafused vesicles which contain rows of membrane-bound, microfibril-synthesizing particles (Kiermayer & Dobberstein, 1973).
1.7—
Cell Wall Ultrastructure
1.7.1—
Cellulose Microfibrils
One of the most important and intriguing aspects of plant cell wall ultrastructure is the orientation of the cellulose microfibrils within the wall (Albersheim, 1965). Cellulose microfibrils are deposited (or possibly synthesized) at the inner surface of the wall, adjacent to the plasmalemma. They have a particular orientation when deposited. On the other side of the plasmalemma there is a thin layer of microtubules. The orientation of the microtubules in this layer exactly parallels the orientation of the cellulose microfibrils being deposited on the opposite side of the plasmalemma (Newcomb, 1969). Disruption of the microtubules (e.g. by colchicine) affects the orientation of the microfibrils.
In the primary walls of elongating cells, the most recently deposited cellulose microfibrils (those closest to the plasmalemma) lie parallel to the plasmalemma and are oriented perperndicular to the long (growth) axis of the cell, like the hoops which hold a barrel together. Cellulose microfibrils deposited at earlier times (and thus further from the plasmalemma) are still parallel to the plasmalemma, but are oriented at decreasing angles (i.e. more nearly parallel) to the growth axis of the cell. It is as though the process of cell wall elongation pulls the ends of the microfibrils towards the ends of the cell. In the end walls of a cell, or in the newly forming cell wall created by cell division (i.e. the cell plate), the cellulose microfibrils again lie parallel to the plasmalemma, but are randomly crossed and not parallel to each other.
The cellulose microfibrils in secondary walls are usually deposited in discrete, concentric layers. The microfibrils in a given layer are all parallel to each other, but are oriented at a considerable angle to both the microfibrils in adjacent layers and to the long axis of the cell. This cross-hatching of microfibrils in adjacent layers of secondary walls adds considerably to the overall strength of the wall, and is the same principle used in the manufacture of fibreglass-epoxy fishing rods, where great strength for weight is essential.
Secondary wall material may also be deposited in very localized regions to form highly specialized structures such as the rings or spirals of secondary wall found in xylem cells. In such cases the microtubules on the inside of the plasmalemma have the same localization and orientation as the cellulose microfibrils being deposited on the opposite side of the plasmalemma.
1.7.2—
Specialized Structures
Many of the modifications of cell wall structure which occur are related to communication or transport between cells. In cells with primary walls, intercellular communication is facilitated by plasmadesmata. Plasmadesmata are small pores of approximately 40 nm diameter which extend through the wall between two adjacent cells (Ledbetter & Porter, 1970; Fig. 1.7). The plasmalemma
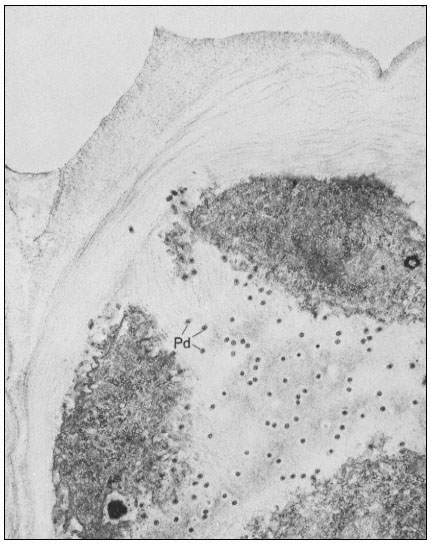
Figure 1.7
Plasmadesmata of plant cell walls.
Plasmadesmata (Pd) in the end walls of collenchymal cells of a wheat
stamen filament. The 'core' material in the plasmadesmata can be seen
as an electron-dense dot in the centre of the plasmadesmatal pores.
(See Ledbetter & Porter, 1970. Reproduced courtesy of the authors.)
of the cell(s) also extends through these pores, so that the cytoplasm of one cell is continuous with that of adjacent cells. There appears to be a core of electron dense material in the center of the plasmadesmatal tubes (see Fig. 1.7). This core may be a specific (though as yet speculative) structure capable
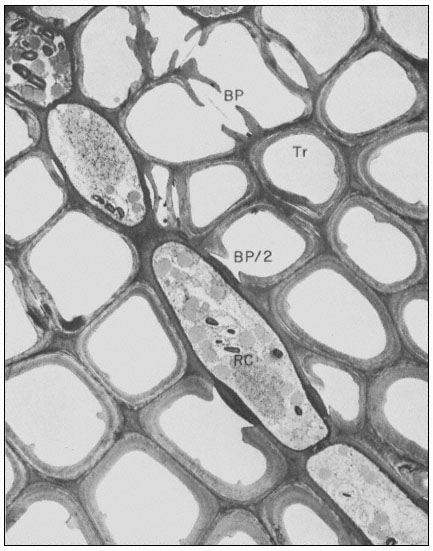
Figure 1.8
Pits in tracheids of Taxus canadensus.
A full bordered pit (BP) can be seen in cross-section in the upper left portion of the figure.
The faint line in the centre of the pit is the disc. The pits lie between tracheids (Tr), which are
long, cylindrical, cytoplasmless tubes that carry water from the roots to the leaves. The primary
cell walls and middle lamella can be seen as electron dense material running in a thin line between
cells and in the corners where 3 or 4 cells meet. Layering of the thick secondary wall material is
evident. Several half-bordered pits (BP/2) between tracheids and ray cells (RC) can be seen.
(See Ledbetter & Porter, 1970. Reporduced courtesy of the authors.)
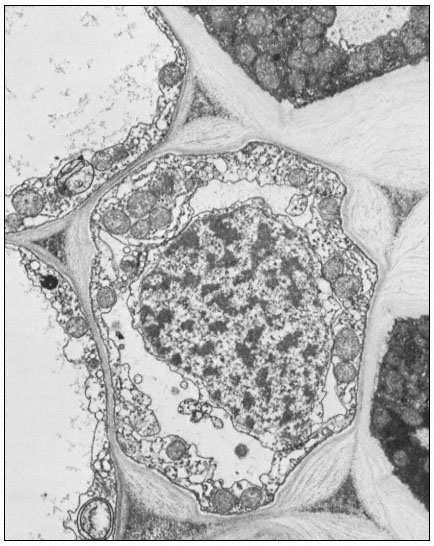
Figure 1.9
Collenchymal cells.
The walls of these cells are unusually thick primary walls, heavily
hydrated and unlignified. Cellulose microfibrils can be seen throughout
the walls. These cells elongate very rapidly, and the walls are quite plastic.
(See Ledbetter & Porter, 1970. Reproduced courtesy of the authors.)
of regulating what can and cannot be passed between cells. Regardless of the nature of the core, it is clear that the plasmadesmata are an aspect of plant biology which has no obvious counterpart in animal tissues. Each cell may have thousands of plasmadesmata, either randomly scattered as individual
pores or grouped in distinct fields where the primary wall is often thinner than usual.
In cells with secondary walls, and particularly in tracheids, intercellular communication and transport is facilitated by pits (Ledbetter & Porter, 1970; Fig. 1.8). In the full, bordered type of pit, the primary wall and middle lamella are largely dissolved. The hole thus formed is surrounded and partly overgrown by depositions of secondary wall material which create a raised, overhanging border. The primary wall and middle lamella in the pit is replaced by a relatively impermeable disc or torus of thickened wall material. The disc is suspended by an easily permeable, radial network of cellulose microfibrils. The diameter of the disc is slightly larger than the aperture of the overhanging pit border. Thus the disc can act as a valve to close the pit when pressed against the border by a large pressure differential (Albersheim, 1965). Gas bubbles, which would break the flow of water through the tracheids, can be sealed off by the action of such valves.
Where the tracheids are adjacent to ray cells, the pits are differentiated only on the tracheid side, giving half-bordered pits (Fig. 1.8). The walls of the ray cells in the pit region appear to remain intact or only slightly modified.
A further important example of a cell wall modification which facilitates intercellular communication or transport is the sieve plate of primary phloem. The phloem cells have primary cell walls with many plasmadesmata. Some of the plasmadesmata of the crosswalls between adjacent phloem cells enlarge considerably and become lined with callose (a b -1,3 glucan) on the wall side of the plasmalemma. The pores of the sieve plate thus formed become filled with a fibrous protein (which may be analogous to the core material of plasmadesmata).
Many other specialized cell wall structures or modifications could be described (see also Fig. 1.9). Several examples may be mentioned just to indicate the range of possibilities: the walls of pollen cells, which are so indestructible that they are used by archeologists to characterize the flora of dwelling sites many thousands of years old; the walls of bark cells, made largely of suberin, a wall material similar to cutin that can form a protective coating over wounds so as to prevent water loss or pathogen invasion; and the walls between adjacent endodermal cells, which have very dense Casparian strips which prevent the movement of ions through the walls and into the xylem stream.
The wall structures and modifications described in this section may all be seen with the microscope—there are undoubtedly many other important differentiations of the cell wall which we cannot see.
1.8—
Hormonal Control of Cell Wall Biosynthesis and Differentiation
We know that cell wall biosynthesis and the modification of cell walls are important aspects of cellular differentiation, and that cellular differentiation can
be controlled—or at least affected—by plant hormones. However, the mechanisms by which particular plant hormones affect the form, substance and synthesis of particular cell walls are almost wholly unknown.
Significant progress has been made recently in elucidating the role of auxins in cell wall elongation. Indoleacetic acid and several similar compounds cause a marked increase in the rate of coleoptile cell elongation (Cleland, 1971). Coleoptiles normally receive auxin from the apical region of the shoot. However, excised coleoptiles can respond to auxin exogenously supplied in solution, elongating at a rate of 10–30% per hour. The mechanism of auxin-stimulated elongation of coleoptile cells has been proposed to involve the activation of a hydrogen ion 'pump' in the plasmalemma (Cleland, 1973; Rayle, 1973). The hormonal activation of this pump results in a lower pH in the cell walls which, in turn, appears to activate enzymes in the cell wall which are capable of selectively 'loosening' the polysaccharide network so that the walls can elongate more rapidly. Auxin must also stimulate cell wall biosynthesis (by some unknown mechanism) since the walls retain a constant thickness while more than doubling their length during prolonged auxin treatment.
A further important aspect of hormonal effects on plant cell wall biosynthesis and differentiation has been revealed by studies on the changes in microtubule orientation caused by exogenously supplied hormones (Shibaoka, 1974). In bean epicotyl segments, kinetin inhibits elongation and promotes a thickening or lateral expansion of the cells. Gibberellins, on the other hand, promote elongation and inhibit lateral expansion. The microtubules adjacent to the plasmalemma in cells of epicotyl sections supplied with kinetin and auxin are found to be oriented parallel to the cell axis. However, in sections supplied with gibberellin and auxin the microtubules are oriented transverse to the cell axis. The microtubules are randomly oriented in sections supplied with auxin alone. Thus, kinetin and gibberellins (but not auxins) appear to be able to control the direction of cell growth by somehow determining the orientation of the microtubules. The orientation of the microtubules, in turn, determines the orientation of the cellulose microfibrils being deposited in the cell wall—which determines the direction in which the walls can most easily expand. (See Chapter 13 for further discussion of hormone action.)
1.9—
The Role of the Plant Cell Wall in Interactions with Other Organisms
Plants are beset by many pests and pathogens and helped by a variety of symbionts. The walls of epidermal cells may be specialized in a number of ways to form a protective skin over the entire plant (e.g. cutin, wax, gums and mucilages, bark and thorns, etc.). Many cell walls throughout the plant can become resistant to degradation by pathogens by means of lignification.
Quite apart from such modifications, however, the structure of the cell wall
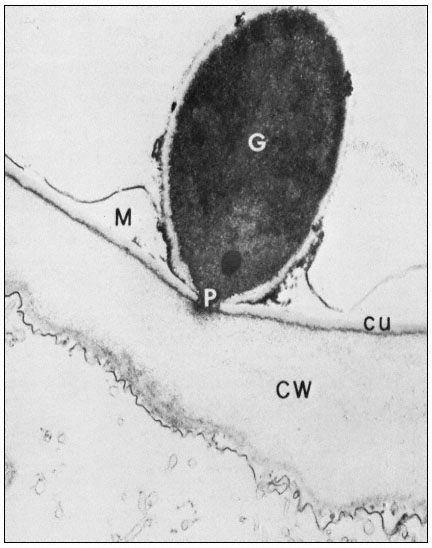
Figure 1.10
Penetration of epidermal cell walls of broad bean by Botrytis cinerea.
The fungal germ tube (G) is attached to the surface of the cuticle (CU) by
a layer of mucilage (M) secreted by the fungus. The fungus appears to
have dissolved a hole through the cuticle and to have begun dissolving
the plant cell wall (CW) beneath this hole. A membrane-bound infection
peg (P) penetrates through the pore in the fungal cell wall and the cuticle.
(Courtesy Prof. W. E. McKeen. See Phytopathology (1974), 64, 461–67.)
can itself present a formidable barrier to pathogens. In order to penetrate the cell wall and utilize its component sugars, pathogenic fungi and bacteria have evolved sophisticated, inducible batteries of enzymes which can hydrolyze components of the plant cell wall (Bateman & Basham, 1975). Figure 1.10 illustrates the initial stages of cell wall penetration by a fungal pathogen. The pathogen appears to have dissolved a hole through the cuticle and to have started degrading the cell wall.
Plants, in turn, have evolved countermeasures to the hydrolytic enzymes of the pathogens. In primary walls, the molecular architecture is such that only the pectic polysaccharides are accessible to hydrolytic enzymes (Bauer et al., 1973). It is probably for this reason that the first enzymes to be secreted by an invading pathogen are the pectin-hydrolyzing enzymes (Bateman & Basham, 1975). This is also likely to be the reason why the walls of many plants contain proteins which specifically inhibit the pectin-degrading enzymes (and only the pectin-degrading enzymes) of pathogenic microorganisms (Anderson & Albersheim, 1971). In other plants the pectic polysaccharides are heavily acetylated, and thus resistant to enzymic attack.
Plants have evolved mechanisms for counterattack as well as defence. Inducible enzymes are present in the cell walls of several plants which hydrolyze the wall polysaccharides of invading fungal pathogens (Ables et al., 1970; Pegg & Vessey, 1973). The pathogens react by secreting proteins which specifically inhibit the attacking plant enzymes (Albersheim & Valent, 1974). The cell walls of a variety of other plants contain glycoside-hydrolyzing enzymes which can release hydrogen cyanide from cyanogenic glycosides. The release of hydrogen cyanide by the plant occurs in response to attack by a pathogen. The pathogen (sometimes) avoids cyanide poisoning by an inducible enzyme which converts the cyanide to harmless formamide (Fry & Munch, 1975).
From these and other examples it is clear that the cell wall is a most important battleground in the contest between plants and their pathogens. The plant cell wall is not just a strong but passive barrier to invasion. It is impregnated with a host of molecules which can recognize a pathogen, modify the defences, or mount a counterattack.
Further Reading
Albersheim P. (1965) Substructure and function of the cell wall. In Plant Biochemistry (Ed J.E. Varner) pp. 151–186. Academic Press, New York.
Aspinall G.O. (1973) Carbohydrate polymers of plant cell walls. In Biogenesis of Plant Cell Wall Polysaccharides (Ed. F. Loewus) pp. 95–115, Academic Press, New York.
Bateman D.F. & Basham H.G. (1975) Degradation of plant cell walls and membranes by microbial enzymes. In Physiological Plant Pathology Vol. I (Ed. P.H. Williams & R. Heitifus) Springer-Verlag, Berlin. In press.
Frey-Wyssling A. (1969) The ultrastructure and biogenesis of native cellulose. Fortschr. Chem. Organ. Naturst.27, 1–30.
Keegstra K., Talmadge K.W., Bauer W.D. & Albersheim P. (1973) The structure of plant cell walls III. A model of the walls of suspension-cultured sycamore cells based on the interconnections of the macromolecular components. Plant Physiol.51, 188–96.
Lamport D.T.A. (1970) Cell wall metabolism. Ann. Rev. Plant Physiol.21, 235–70.
Ledbetter M.C. & Porter K.R. (1970) Introduction to the Fine Structure of Plant Cells. Springer-Verlag, Berlin.
Northcote D.H. (1972) Chemistry of the plant cell wall. Ann. Rev. Plant Physiol.23, 113–32.
Rees D.A. (1972) Shapely polysaccharides. Biochem. J.126, 257–73.
Timell T.E. (1965) Wood hemicelluloses. Advan. Carbohyd. Chem.20, 409–83.