Seventeen—
Functional Analysis of Dive Types of Female Northern Elephant Seals
Tomohiro Asaga, Yasuhiko Naito, Burney J. Le Boeuf, and Haruo Sakurai
ABSTRACT. The aim of this study was to elucidate the function of individual dive types observed in the dive records of female elephant seals, Mirounga angustirostris . Free-ranging dive records spanning 29 to 81 days were obtained from three adult females from Año Nuevo, California, in 1990, using time-depth recorders glued to the pelage on their backs.
Type D dives, assumed to serve pelagic foraging, (1) accounted for 75 to 80% of all dives, (2) occurred in series with a mean length of 10.1 to 22.9 dives, (3) had a bottom of dive element that accounted for 28 to 44% of the total dive duration, and (4) exhibited longer durations and deeper depths during the day than at night. Type A, or "transit," dives were sparsely and widely distributed in the record, accounting for only 1.7 to 7.1% of all dives; they rarely occurred in a series and were the deepest dives observed in all records. Type C dives accounted for 2.6 to 6.8% of all dives, and they were the shallowest dives. They occurred in series with a mean of 3.6 to 4.7 dives. The second descent segment of these dives, which accounted for 52 to 58% of the total dive duration, showed a 30 to 83% reduction in descent rate over the preceding descent segment. There was an inverse relationship between Type D and Type C dive frequency of occurrence as a function of time of day; C dives peaked between 0400 and 1000 hours, the time interval when D dives were least frequent.
The result of this study are consistent with the hypothesis derived from swim speed analysis that Type A, D, and C dives serve transit, pelagic foraging, and physiological processing functions, respectively.
By providing a continuous record of the duration and depth of dives, time-depth recorders (TDRs) provide insights into the at-sea behavior of marine mammals. Data from TDRs allowed us to focus on the individual dives of northern elephant seals, M. angustirostris , with the aim of elucidating their function and role in foraging.
The diving pattern of northern elephant seals differs in many respects
from that of other pinnipeds. After lactation and weaning their pups, adult females go to sea to feed for 2½ months. During this period, their dives are long and deep. Mean dive depth is 446 to 544 m, and mean dive duration is 17.1 to 22.5 minutes (Naito et al. 1989; Le Boeuf et al. 1989). Sea lions (Zalophus californianus and Z. c. wollabaeki ) and fur seals (Callorhinus ursinus, Arctocephalus gazella, A. pusillus, A. australis , and A. galapagoensis ) dive to mean depths of less than 100 m, and mean dive durations are less than 3 minutes (Feldkamp, DeLong, and Antonelis 1989; Gentry, Kooyman, and Goebel 1986). Weddell seals, Leptonychotes weddelli , dive to less than 200 m most of the time, and more than half of their dives are less than 10 minutes in duration (Kooyman 1989). Elephant seals dive deeper than sperm whales, Physeter catodon , who dive to mean depths of 314 to 382 m, with most dives being less than 500 m (Papastavrou, Smith, and Whitehead 1989). These comparisons suggest that northern elephant seals dive longer and deeper than other pinnipeds and most whales.
Several studies have showed that many diving mammals dive in bouts, a series of dives over a certain period of time; diving bouts are followed by rest (Gentry, Kooyman, and Goebel 1986). Northern elephant seals do not dive in bouts but rather dive continuously for the duration that they are at sea. The dive bouts of Weddell seals, California sea lions, and fur seals last for several hours (Feldkamp, DeLong, and Antonelis 1989; Kooyman 1989; Kooyman et al. 1980), and so do those of blue-eyed shags, Paracrocorax atriceps , and Adélie penguins, Pygoscelis adeliae (Croxall et al. 1991; Naito et al. 1989). Female northern elephant seals dive continuously, sometimes up to 2½ months at a stretch during which the seal may exhibit more than 5,000 dives with only short surface intervals of less than 3 minutes between dives (Le Boeuf et al. 1989). Thus, an analysis of dive bouts, the usual approach in studying otariid diving behavior, is not applicable to the study of elephant seals. This discrepancy suggests that elephant seal diving differs fundamentally from the diving behavior of otariids.
Among California sea lions and northern fur seals, there may be rest at the surface, rest on land, or transit to foraging areas between dive bouts (Feldkamp, DeLong, and Antonelis 1989; Kooyman and Gentry 1986). These activities are also observed in gentoo penguins, Pygoscelis papua , and chinstrap penguins, P. antarctica (Trivelpiece et al. 1986). The diving behavior of northern elephant seals is devoid of swimming at the surface (Le Boeuf et al. 1992; see chap. 10), and it is not likely that they sleep at the surface (Le Boeuf et al. 1988). This is important for understanding their diving behavior, their migrations to foraging areas, and their rest or sleep activities at sea. There are temporal and frequency differences in dive types that we think elucidate the function of their unusual diving behavior.
In this study, we analyze the distribution of distinguishable dive types of free-ranging female northern elephant seals during the 2½-month period at
sea after breeding. Our investigation is aimed at understanding the function of dives in relation to foraging and in elucidating their physiological basis.
Methods
The field aspects of this study were conducted at Año Nuevo Point, California, in 1988. Three adult female northern elephant seals were immobilized with ketamine hydrochloride (Briggs et al. 1975) during mid-February near the end of their lactation periods. A TDR and a radio transmitter (Advanced Telemetry Systems, Bethel, Minn.) were attached to the pelage of the back above the shoulders of each female with marine epoxy (Evercoat Ten-Set, Fibre-Evercoat Co., Cincinnati, Ohio). Each seal was weighed at this time (see Le Boeuf et al. 1988 for method).
The TDR (Naito et al. 1989) was 52 mm in diameter and 193 mm in length and weighed 980 g in air. It was housed in an aluminum casing that withstood pressures to 3,000 m in depth. The instrument contained a diamond stylus that inscribed a line on aluminum-coated paper (20 µ thick) proportional to water pressure. The motor was capable of running for 130 days, being powered with two 1.5 v lithium batteries. The depth range was 0 to 900 m. Recording error was estimated at less than 2% of depth and duration.
When the seals returned to the rookery in May, each one was weighed and the TDRs recovered. The recording paper was subsequently enlarged 14½ times with a reader-printer (Minolta PR507). From the strip chart records, the dives for each female were classified into five dive types based on their time-depth profiles (fig. 17.1). This classification is based on that of B. J. Le Boeuf et al. (1988) with little modification and is the same classification used in Le Boeuf et al. (1988) with little modification and is the same classification used in Le Boeuf et al. (1992) and D. E. Crocker et al. (this vol.). Type A dives have a straight descent to a sharp point, then direct ascent to the surface. Type B dives are similar except that the bottom of the dive is rounded. Both A and B dives have no bottom time. Type C dives have direct descent to a depth, at which point the descent rate decreases dramatically until the bottom of the dive, then following a rather sharp inflection point, ascent to the surface is direct. Type D dives are characterized by direct descent to a depth, at which point there occur 2 to 12 vertical excursions or "wiggles," ending in direct ascent to the surface. Type E dives show direct descent to the bottom of the dive, which is flat, and end in direct ascent to the surface. Dives that could not be put into one of these categories were excluded from analysis. Type E dives in this study correspond to Type E and F dives in Le Boeuf et al. (1988).
A Hitachi H-F8844-65 was used to digitize the dives. Each classifiable dive was digitized with 3 to 5 points (fig. 17.1), which gave a measure of dive depth, duration, and descent and ascent rates. The minimum mea-
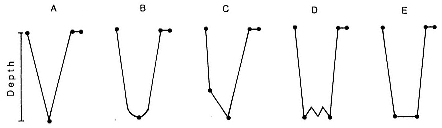
Fig. 17.1
Schematic profiles of five dive types observed in the dive records of northern
elephant seals (adapted from Le Boeuf et al. 1992). The points of each dive
type were digitized to determine dive elements.
sured value of surface intervals (SIs) was 0.4 minutes, set by the time resolution of the instrument. SIs greater than 10 minutes, extended surface intervals (ESIs), were excluded from statistical analysis.
In an attempt to elucidate the temporal patterning of dive types and their function, we analyzed the temporal patterning of each dive type. For analytical purposes, we defined a dive sequence as a series of dives of the same type bordered by a different dive type or an ESI. That is, we regarded a change in dive type or a surface interval of 10 minutes or more as an interruption signaling the end of a dive type sequence and the dives following a change in dive type or an ESI as the beginning of a new dive type sequence. An additional criterion was that a dive type sequence contain a minimum of three dives.
Results
All females returned to the breeding site. Their TDRs were recovered, and they were weighed. All females gained a mean of 1.0 kg/day, an indication that they succeeded in foraging and were not impeded by the instruments.
Summary Statistics
The entire dive records of two females, Tuf (81 days at sea) and Tow (64 days at sea), were obtained. For the third subject, Vi, the first 48 days of 77 days at sea were recorded; the last 29 days of the record were lost due to a tear in the recording paper. Summary statistics for all females are shown in table 17.1. Diving performance was similar to that of eight females reported in Le Boeuf et al. (1988).
Dive Type Distribution and Temporal Pattern
To clarify the temporal frequency of each dive type, we divided each record into three periods: (1) beginning—the first five days; (2) end—the last five days; and (3) middle—the entire rest of the record. Half of the Type B
| ||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
dives and all Type E dives occurred during the beginning and end periods (table 17.2). The mean depth of dives during these periods was not stable compared to dives in the middle period. Because dive depth is constrained by the shallow ocean floor between the rookery and the continental slope (Le Boeuf et al. 1988; Le Boeuf et al. 1989), the dive records for the beginning and end periods of each record were excluded from analysis. That is, all analysis here excludes the first five days of all records and the last five days of the records of females Tuf and Tow.
D dives were the dominant dive type in the middle period of all records (fig. 17.2). These dives occurred in long series. Type C dives occurred more frequently than Type A dives, except for female Tuf, and Type C dives occurred several times every day, except for female Tow. Type C dives were the majority of dives (68%) following ESIs. Type A dives occurred unpredictably. There were no Type E dives, and Type B dives were rare (2.1–2.7% of all dives); consequently, these dive types are excluded from further analysis.
Characteristics of Dive Parameters
Dive Depth-Duration Ratio
We tested the dive depth-duration difference among dive types. The mean dive depth-duration ratio of A, C, and D dives was 29.5 to 35.9, 13.7 to 21.2, and 22.4 to 31.3, respectively. Differences in these ratios among dive types were significant for all females (Mann-Whitney U = 13.3, p < .05). This indicates that the classification by dive depth-duration profiles was suitable for dive type analysis.
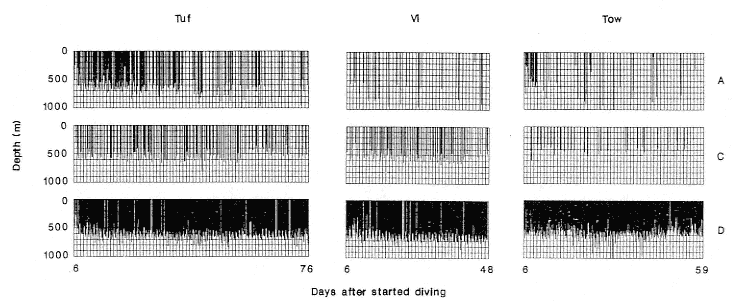
Fig. 17.2
The dive records of 3 females showing the distribution of A, C, and D dive types during the middle period at sea.
This excludes the first five days of each record, when the animal is going from shallow to deep water, and the
last five days, when the reverse occurred.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Dive Depth, Dive Duration, and Surface Intervals
Table 17.3 shows that the mean depths of Type A dives were significantly deeper in all records than other dive types (t = 3.4, p < .05). Indeed, most dives (64–92%) deeper than 800 m were Type A dives, and these were the maximum depth dives for all females. In contrast, Type C dives were significantly shallower than other dive types (t = 13.3, p < .05). The longest mean dive durations of all females were Type C dives (t = 6.5, p < .05).
There were no significant differences in SIs among dive types in all females except for SIs following Tow's Type A dives, which were significantly longer than those of other females (t = 2.5, p < .05).
Bottom Time and Descent and Ascent Rates
The mean bottom duration of Type D dives accounted for 28 to 44% of the total duration of dives. The mean duration of the second descent segment of Type C dives (fig. 17.1) took up 52 to 58% of the total duration of the dives.
The mean descent rate during the second descent segment of Type C dives was significantly slower than in any other dive type (t = 8.8, p < .05). The mean descent rate during this segment represents a 30 to 83% reduction over the descent during the first descent segment of Type C dives. In addition, the mean descent rates between A and D dives, except for Vi, were statistically significant (t = 5.8, p < .05). The mean ascent rate of Type C dives was slower than that of other dive types (t = 5.2, p < .05).
Interrelationships between Dive Elements
Correlations between dive depth, dive duration, bottom time, and descent and ascent rates are shown in table 17.4. Significant correlations were observed between dive depth and dive duration for all dive types and all females. The positive relationship between these two variables was highest for Type C dives and lowest for Type D dives. The relationship between transit time and depth of Type D dives was strong for all females.
For Type C dives, there were high and positive correlations between dive depth and the duration of the second descent segment, as well as between total dive duration and the second descent segment. For Type D dives, there were weak negative correlations between dive depth and bottom duration. There were positive correlations between total dive duration and duration at the bottom of dives. Weak relationships were in evidence between dive depth and descent rate, total duration and descent rate, and total duration and ascent rate.
Diel Pattern
Number of Dives
There was an inverse relationship in the daily frequency pattern of C and D dives in all females (fig. 17.3). Type C dives peaked
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
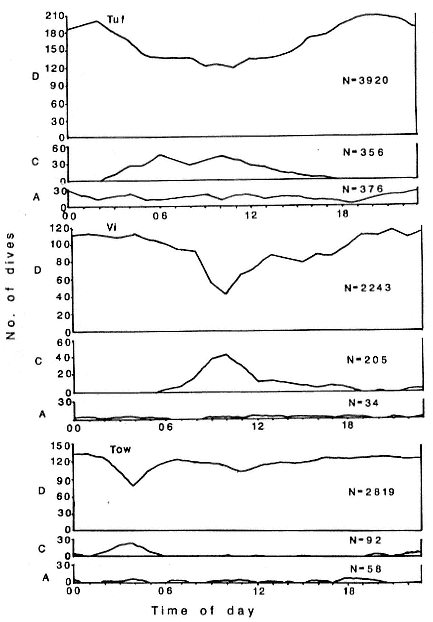
Fig. 17.3
A frequency distribution of three dive types as a function of time
of day for each of 3 females.
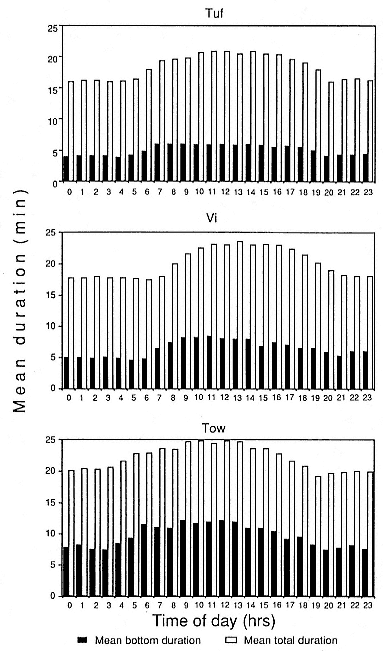
Fig. 17.4
Mean dive duration and mean bottom time of D-type dives
as a function of time of day for 3 females.
in frequency between 0400 and 1000 hours, when Type D dives were most infrequent. Type A dives did not vary systematically as a function of time of day.
Dive Elements
We describe the daily rhythm of dive elements of Type D dives only because the frequency of other dive types was low and did not vary with time of day.
Mean dive duration and mean bottom duration of Type D dives were longer during the day than at night (fig. 17.4). The time when these durations were longest corresponded with the modal frequency of Type C dives. To further investigate this association, we selected 10 Type A, C, and D dives at random, each of which was preceded and followed by a series of 10 consecutive Type D dives. We then tested for differences in dive durations of the Type D dives as a function of the preceding dive type. The majority of dive durations of Type D dives, 70 to 100% of them, increased following Type C dives but showed little increase after Type A dives (0–10%) and Type D dives (0–30%).
The mean dive depths of Tuf and Vi during the day (0600–2000 hrs) were deeper than at night (2100–0500 hrs), but this was not the case for female Tow. Mean SI, descent rate, and ascent rate for Type D dives did not vary with hour of the day for all females.
Dive Type Sequence
Type A dives showed the lowest number of dives in a sequence among the three dive types (table 17.5). Except for female Tow, 80% or more of Type C dives occurred in series. The mean duration of the second descent segment of Type C dives was about half of the dive sequence duration. The depth where the second descent segment began in Type C dives was constant in a dive sequence regardless of its maximum depth (F = 3.41, p < .01). The dive duration of Type C dives gradually increased toward the end of a dive sequence (50–80% of total dive sequence number).
More than 90% of Type D dives occurred in dive sequences. The total duration of these dive sequences in a day was 15.5 to 20.5 hours. The bottom duration corresponded to 24 to 39% of dive sequence duration, indicating that females remained at the maximum depth of dives for 3.6 to 8 hours every day.
Discussion
This study provides additional information on the putative function of dive types in elephant seals. Le Boeuf et al. (1988) hypothesized that Type D dives serve foraging. Using information provided by measurement of swim
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
speed, Le Boeuf et al. (1992) attributed a similar function to Type D dives and added that they served pelagic foraging. They further hypothesized that Type E dives served benthic foraging, especially in males, that Type A and B dives functioned primarily as transit dives during migrations, and that Type C dives were processing dives that facilitated digestion, were instrumental in the removal of anaerobic metabolites, or served rest or sleep. We discuss the relevance of the present data to the presumed function of dive types.
Type D dives made up 75% or more of the dive types in each of the dive records in this study, a percentage similar to that reported for females in an earlier study (Le Boeuf et al. 1988). The predominance of Type D dives—coupled with the large proportion of time spent at the dive bottom (34.0 ± 8.7% of the total dive duration), the up-and-down activity of the seal at the dive bottom, the rapid descent and ascent rates, and the length and duration of a series of these dives—is consistent with the hypothesis that they serve foraging (Le Boeuf et al. 1988; Le Boeuf et al. 1992).
This hypothesis is bolstered by a number of ancillary findings regarding Type D dives.
1. They occurred regularly throughout the period at sea with the exception of the first few days at sea and the last few days at sea when the animal was moving across the continental shelf. This suggests that feeding is not restricted to foraging areas but occurs throughout migration (Le Boeuf et al. 1992; see Le Boeuf, this volume).
2. The mean dive duration, mean bottom-of-dive duration, and mean dive depth of these dives are greater during the day than at night. This is consistent with the hypothesis that Type D dives reflect foraging in the deep scattering layer, which contains prey that come closer to the surface at night and retreat to depths at midday (Le Boeuf et al. 1988).
3. The frequency of Type D dives was lowest during the day when the frequency of Type C dives peaked. This is consistent with the idea that the latter type are processing dives and that the best time for the seal to reduce its dive rate and rest, digest food, or get rid of anaerobic metabolites is when it must dive deepest for prey. Data from ongoing studies show that Type D dives occur with great frequency in both sexes and in seals ranging in age from weanlings on their first trip to sea to adults (Le Boeuf, this volume).
Le Boeuf et al. (1992) hypothesized that A and B dives were primarily transit dives. This was based in part on the fact that the mean horizontal distance covered, 1.3 km, was greater for this dive than for other dive types (0.6–1.1 km), and they argued further that this dive shape was most
efficient for horizontal travel. Our data show that Type A dives are on average deeper and of shorter duration than Type D dives. The shorter duration of Type A dives might be due to the greater energy expenditure required to go deeper, which puts a lower limit on dive duration. The swim speed data indicate that foraging may occur during transit. The consistency of Type A dives in females throughout the length of the periods at sea supports this idea. We suggest further that the dives might have an exploratory function. This is consistent with the dive's depth and its spiked bottom; at depth, the seal may decide to abort the dive and ascend when it does not find suitable prey.
With the exception of M. A. Hindell's dive analysis on southern elephant seals, M. leonina (Hindell 1990; Hindell, Slip, and Burton 1991), no other investigators have attempted detailed descriptions of dive types in other species. Dives resembling the A-type dives of elephant seals, however, have been observed in northern fur seals and South African fur seals (Gentry, Kooyman, and Goebel 1986; Kooyman and Gentry 1986); 90% of the dives of these animals had spiked bottoms resembling the shape of A-type dives of elephant seals. The investigators suggested that these dives served foraging. G. L. Kooyman (1981) came to the same conclusion about some of the dives of Weddell seals.
Several findings in this study are consistent with the hypothesis that Type C dives have a processing function and that the animal is drifting down during the second segment of descent (Le Boeuf et al. 1992). First, Type C dives are as long as the dive durations of the other dive types, even though reduced energy expenditure is expected due to the animal drifting down during the second segment of descent rather than powering down as in Type A and D dives. Second, the mean distance traveled during these dives (0.6 km) is less than for any other dive type (ibid.). Third, Type C dives were the most common dive type following an extended surface interval. Since we know from the swim speed study (ibid.) that the animal is not swimming, this is a strong argument that it is resting. The contiguity of ESIs and Type C dives suggests that the latter may have a "processing" function similar to that of ESIs. Fourth, Type D dives that followed Type C dives had a longer mean duration than normal. This suggests that the animal was now rested or in some way better prepared to dive long. Last, the peak frequency of Type C dives, coupled with the low frequency of Type D dives, suggests that these two dive types have opposite functions.
We conclude that our analysis of dive types is consistent with the hypotheses advanced regarding their function (ibid.). The ultimate tests of these hypotheses will be empirical and will require more sophisticated diving instruments than the ones currently in use.
Acknowledgments
We thank D. Costa and P. Thorson for their support with fieldwork at Año Nuevo Point, California, and N. Satoh, head of the computer center of the National Institute of Polar Research in Tokyo, and his staff for their advice and help with data processing. This study was financed in part by a grant from the National Science Foundation.
References
Briggs, G. D., R. V. Hendrickson, and B. J. Le Boeuf. 1975. Ketamine immobilization of northern elephant seals. Journal of the American Veterinary Medicine Association 167: 546–548.
Croxall, J. P., Y. Naito, A. Kato, P. Rothery, and D. R. Briggs. 1991. Diving patterns and performance in the Antarctic blue-eyed shag, Phalacrocorax atriceps. Journal of Zoology, London 225: 177–199.
Feldkamp, S. D., R. L. DeLong, and G. A. Antonelis. 1989. Diving patterns of California sea lions, Zalophus californianus. Canadian Journal of Zoology 67: 872–883.
Gentry, R. L., G. L. Kooyman, and M. E. Goebel. 1986. Feeding and diving behavior of northern fur seals. In Fur Seals: Maternal Strategies on Land , ed. R. L. Gentry and G. L. Kooyman, 61–78. Princeton University Press.
Hindell, M. A. 1990. Population dynamics and diving behaviour of a declining population of southern elephant seals. Ph.D. dissertation, University of Queensland, Australia.
Hindell, M. A., D. J. Slip, and H. R. Burton. 1991. The diving behaviour of adult male and female southern elephant seals, Mirounga leonina (Pinnipedia: Phocidae). Australian Journal of Zoology 39: 595–619.
Kooyman, G. L. 1981. Weddell Seal: Consummate Diver . Cambridge: Cambridge University Press.
———. 1989. Diverse Divers: Physiology and Behavior . Berlin: Springer Verlag.
Kooyman, G. L., and R. L. Gentry. 1986. Diving behavior of South African fur seals. In Fur Seals: Maternal Strategies on Land , ed. R. L. Gentry and G. L. Kooyman, 142–152. Princeton: Princeton University Press.
Kooyman, G. L., E. A. Wahrenbrock, M. A. Castellini, R. W. Davis, and E. E. Sinnett. 1980. Aerobic and anaerobic metabolism during voluntary diving in Weddell seals: Evidence of preferred pathways from blood chemistry and behavior. Journal of Comparative Physiology 138: 335–346.
Le Boeuf, B. J., D. P. Costa, A. C. Huntley, and S. D. Feldkamp. 1988. Continuous, deep diving in female northern elephant seals, Mirounga angustirostris. Canadian Journal of Zoology 66: 446–458.
Le Boeuf, B. J., Y. Naito, T. Asaga, D. Crocker, and D. P. Costa. 1992. Swim speed in a female northern elephant seal: Metabolic and foraging implications. Canadian Journal of Zoology 70: 786–795.
Le Boeuf, B. J., Y. Naito, A. C. Huntley, and T. Asaga. 1989. Prolonged, continuous, deep diving by northern elephant seals. Canadian Journal of Zoology 67: 2514–2519.
Naito, Y., B. J. Le Boeuf, T. Asaga, and A. C. Huntley. 1989. Long-term diving records of an adult female northern elephant seal. Nankyoku Shiryo (Antarctic Record ) 33(1): 1–9.
Papastavrou, V., S. C. Smith, and H. Whitehead. 1989. Diving behavior of sperm whale, Physeter macrocephalus , off the Galápagos Islands. Canadian Journal of Zoology 67: 839–846.
Trivelpiece, W. Z., J. L. Bengtson, S. G. Trivelpiece, and N. J. Volkman. 1986. Foraging behavior of gentoo and chinstrap penguins as determined by new radiotelemetry techniques. Auk 103: 777–781.