Sixteen—
Postbreeding Foraging Migrations of Northern Elephant Seals
Brent S. Stewart and Robert L. DeLong
ABSTRACT. Adult northern elephant seals depart southern California Channel Islands rookeries in February and early March to forage and replenish body reserves that were depleted during intensive breeding season fasts. Females remain at sea for around 66 days and males for around 120 days before they return to the Channel Islands to molt. During that period seals dive—and presumably forage—deeply and continually while migrating between southern California rookeries and haulouts and offshore, northern foraging areas between 40° and 48°N latitude (females) and the Gulf of Alaska and eastern Aleutian Islands (males); females cover over 5,500 km and males over 11,100 km during these round-trip postbreeding migrations. Males and females differ in their vertical and geographic distributions during these migrations, but the reasons for that segregation are unknown.
Adult northern elephant seals depart terrestrial rookeries from late January through early March and remain at sea, diving continually, for around 2 (females) to 4 (males) months before returning to land to molt (Le Boeuf et al. 1989; DeLong and Stewart 1991). Their behaviors while at sea during those postbreeding periods have been documented in extraordinary detail in recent years (e.g., Le Boeuf et al. 1988, 1989; DeLong and Stewart 1991), though the geographic locations of the seals have been unknown. In 1987, we began, in collaboration with colleague R. Hill, to develop and test a microprocessor-based event recorder that would allow simultaneous documentation of the locations and diving patterns of foraging northern elephant seals throughout their long periods at sea (DeLong, Stewart, and Hill 1992). Using data collected with those instruments, we describe here the postbreeding migrations of 8 adult male and 5 adult female northern elephant seals between San Miguel Island, in southern California, and pelagic foraging areas in the North Pacific, and we interpret intra- and in-
tersexual variation in dive patterns in light of this new information on seal dispersion.
Methods
We instrumented 8 lactating females in 1990 and 16 adult males in 1989 and 1990 with microprocessor-based, geographic-location-time-depth recorders (termed geolocation recorders, or GLTDRs; DeLong, Stewart, and Hill 1992) at San Miguel Island (34°02¢ N, 120°23¢ W) at the end of the breeding season (February–March). We recovered the instruments when the seals returned to land to molt several months later (females, April–May; males, June–July). The design and function of the GLTDRs are described in detail by R. L. DeLong and B. S. Stewart (1991) and DeLong, Stewart, and Hill (1992). Briefly, we programmed the instruments to sample hydrostatic pressure ( = depth ± 2 m) at 30- or 60-second intervals for the entire periods the seals were at sea. Measurements of sea-surface temperature (SST) and ambient light levels were made and stored during the seals' brief interdive surface periods. We estimated each seal's latitudinal location each day by calculating day length and longitude from local apparent noon using daylight profiles stored in the GLTDRs and computer algorithms developed by DeLong, Stewart, and Hill (1992). Determination of location from daylight profiles and the factors that affect location accuracy are discussed in detail elsewhere (DeLong, Stewart, and Hill 1992; Hill, this volume). The most important influences on accurate calculation of latitude are the durations of seals' dives near twilight and the equinox, when day length does not vary substantially with latitude. Near the vernal equinox (March 22) we compared GLTDR sea-surface temperature measurements to latitudinal distributions of SST from other sources to determine seals' latitudinal locations (see DeLong, Stewart, and Hill 1992). We also used SST comparisons to validate all other locations. Our field calibration studies of these instruments indicate that locations that are calculated and corrected using this technique are accurate to around 60 nm or better (ibid.; Stewart and DeLong, unpubl. data). We determined the number of days that each seal was at sea by direct inspection of the dive records, which indicated departure and return dates and times.
Statistical analyses of dive parameters were performed using Systat. We report sample statistics (i.e.,
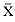
Results
We recovered instruments from 5 females and 9 males. The light-level sensor in one male's instrument failed, but all other GLTDRs contained depth, light-level, and SST data for the seals' entire periods at sea.
Females were at sea for around 66 days and males for around 120 days before returning to San Miguel Island to molt (tables 16.1, 16.2). All seals dove continually while at sea (figs. 16.1, 16.2, 16.3). Females' dives averaged 520 m deep (SEM = 15 m) and 22.3 minutes long (SEM = 1.3 min.), and males' dives averaged 367 m deep (SEM = 34 m) and 22.6 minutes long (SEM = 1 min.); interdive periods at the surface were routinely brief (females, µ = 2.1 min., SEM = 0.05 min.; males, µ = 3.2 min., SEM = 0.1 min.; fig. 16.4).
Seals spent little time at depths shallower than 200 m, except when rapidly descending or ascending to preferred depths, or greater than 800 m (figs. 16.1, 16.2, 16.3). As 20 to 50% of most dives were spent within a range of 30 m of maximum depth (DeLong and Stewart 1991; Stewart and DeLong, unpubl. data), we use maximum depth of each dive as an index of seals' water depth preference and presumably foraging habitat. Females dove deeper, on average, than males did (p < .01; tables 16.1, 16.2), although the greatest depths reached during their migrations were similar (females, 983–1,567 m; males, 831–1,581 m).
All seals began traveling north immediately upon entering the water in late February and March, covering about 90 to 100 km/day for approximately 16 (females, SD = 7.6 days) to 38 days (males, SD = 5.7 days) before travel speeds slowed (figs. 16.5, 16.6, 16.7). Seals then remained in somewhat more defined geographic areas for periods of around 36 (females, SD = 5.2 days) to 51 days (males, SD = 6.4 days). We refer to those areas as foraging areas and define them according to periods when distances covered between days during three or more consecutive days were less than 32 km, to distinguish them from rapid northward movements away from San Miguel Island in March and similar southward movements when seals were returning to the island to molt. We refer to the latter as north and south transits, respectively. South transits to molting beaches from foraging areas took females around 15 days and males around 31 days (table 16.2), traveling at minimum speeds of 90 to 100 km/day ( = 1.04–1.15 m/sec.). Foraging areas of females (figs. 16.5, 16.6) were less obvious than those of males, whose day-to-day movements in northern areas were small and highly concentrated (fig. 16.7). Female foraging areas therefore appear to be series of high-density clusters of daily locations rather than the single clusters characteristic of males (figs. 16.5, 16.6, 16.7).
Females covered at least 5,500 km during their postbreeding migrations and males at least 11,100 km. Seals remained in deep water (from one to
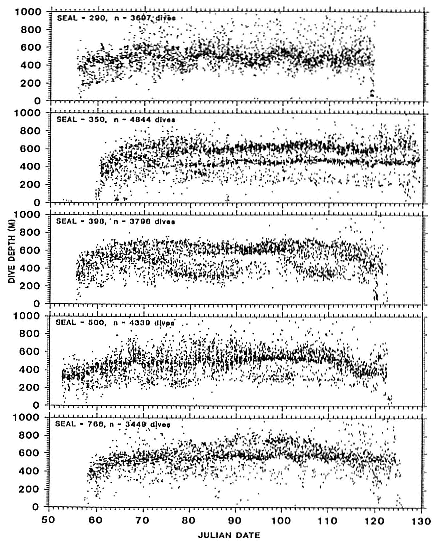
Fig. 16.1
Depths of all dives made each day for 5 northern elephant seal
females during their postbreeding migrations in 1990.
several thousand or more meters) throughout their postbreeding migrations. Although males traveled through female foraging areas, between 40° and 48°N latitude, they did not linger there but continued rapidly north to the Gulf of Alaska and the eastern Aleutian Islands. Although the 1989 and 1990 foraging areas of some males overlapped, dives made in those areas
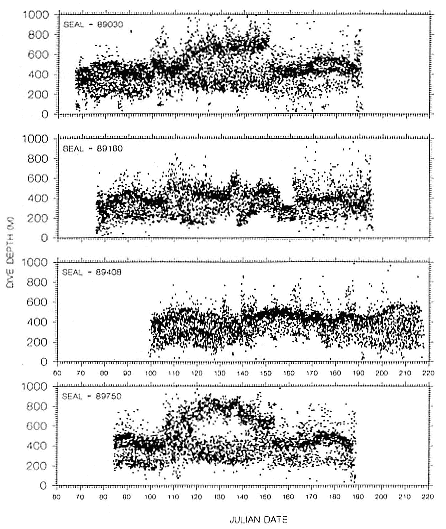
Fig. 16.2
Depths of all dives made each day for 4 adult northern elephant
seal males during their postbreeding migrations in 1989.
were shallower in 1990 (µ = 318 m, SEM = 56.2) than they were in 1989 (µ = 415.7 m, SEM = 24.4; p < .01).
Dive depths were similar among all females, and dives made in foraging areas were deeper than those made during north or south transits (table 16.1). Similarly, depths of foraging area dives of males were usually deeper than transit area dives (table 16.2). Dives of 3 males in 1990 were notable exceptions; seals 90640 and particularly 90480 spent substantial periods at
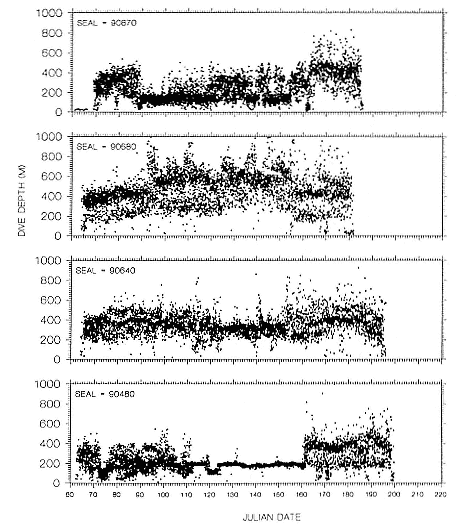
Fig. 16.3
Depths of all dives made each day for 4 adult northern elephant
seal males during their postbreeding migrations in 1990.
shallow depths, and there was much less variation around maximum depth during those periods compared to the records of other males (figs. 16.2, 16.3). The patterns of these 2 males while in foraging areas differed fundamentally in this way from those of females. But the dive depths of 3 other males (89030, 89750, 90680) in their foraging areas south of the eastern Aleutian Islands were similar to dive depths of females that foraged in areas far to the south (tables 16.1, 16.2; figs. 16.2, 16.3, 16.4).
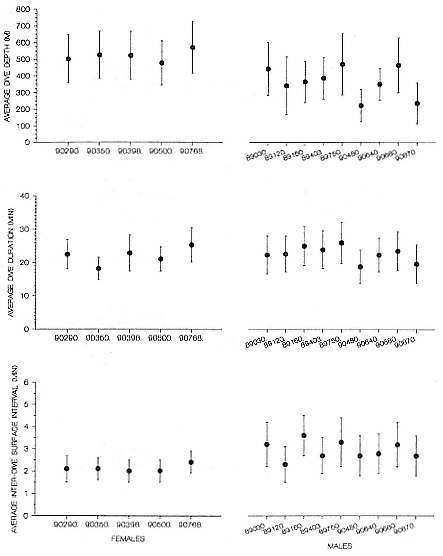
Fig. 16.4
Postbreeding season dive statistics for adult female and male northern elephant
seals ranging from San Miguel Island, California (individual seal identification
numbers are along the x axis; bars are standard deviations; there
are no location records for male 89120).
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Discussion
DeLong and Stewart (1991) reported significant diurnal and seasonal variation in dive parameters of adult northern elephant seal males. By simultaneously documenting dive patterns and geographic locations of seals, we have shown that location and migratory behavior (transiting vs. stationary) can explain such apparent changes. The dive patterns that we report here for adult males in 1989 and 1990 are similar to those reported for postbreeding males in 1988 (DeLong and Stewart 1991). Similarly, the dive patterns of females from San Miguel Island in 1990 are similar to those reported by Le Boeuf et al. (1988, 1989) and Le Boeuf (this volume) for females that breed at Año Nuevo. Females appear to be more consistent in their patterns of dive depths, as shown in figure 16.1, than are males, who show greater seasonal and interindividual variability in preferred dive depths (figs. 16.2, 16.3). We are uncertain about the reasons for this difference, but we suspect that the greater physical oceanographic complexity
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
near the Aleutian Islands compared with the central Pacific Subarctic Transition Zone (e.g., Favorite, Diomead, and Nasu 1976; Wetherall 1991) may result in greater regional variability in thermocline depth and vertical water mass discontinuities in the foraging areas of males.
The foraging area dives of seal 90480 are particularly interesting because of his shallow, narrow depth range of dives during a 50-day period. D. E. Crocker et al. (this volume) proposed that this type of diving is indicative of foraging in benthic or epibenthic habitats. However, our location records for seal 90480 indicate that he was feeding in an area where water depths exceeded several thousand meters and that the nearest shallow water areas (i.e., < 300 m) were 241 to 322 km to the north. Further, sea-surface temperatures from other sources compared to the seal's GLTDR SST data confirm the location records. Although some seamounts do rise from the Aleutian Trench near where the seal was foraging, the charted tops of those seamounts are at least several hundred meters below the seal's foraging depths. We attribute the prolonged shallow diving of this seal to his preference for feeding at that depth rather than epibenthic foraging in shallow coastal habitats or on near-surface seamounts or guyots. Similar depth preferences can be seen throughout his north transit period in the high-density depth bands in his dive records (fig. 16.3), although there is greater variability around the high-density bands during those times. Similar clustering of dive depths, which appear to represent interindividual differences in depth preferences, can be seen in the records of other males, although they are most obvious as shallow depth preferences in the records of seals 90870, 90640, and 89160 for portions of their migrations. We also attribute the inter-annual differences in dive depths among males that foraged in similar areas to differences in individual preferences, as the similarities in SSTs in those areas in 1989 and 1990 (compare figs. 16.6 and 16.7 with figs. 16.9 and 16.10) do not suggest any yearly differences in oceanographic conditions there. Canyon, seamount, or current divergence and convergence influences could cause sharp temperature discontinuities between vertical water masses, which would result in substantial local variation in thermocline depth. Such local physical variability would have strong influences on prey distributions. I. L. Boyd and T. Arnbom (1991) showed that the dive depths of one female southern elephant seal were closely linked to temperature characteristics of the water column that were likely influencing prey concentrations. Simultaneous collection of oceanographic data in areas where elephant seals are known to be foraging would be invaluable during future research on elephant seal foraging dynamics.
Adult female and male northern elephant seals that breed at San Miguel Island evidently migrate to different areas of the North Pacific to forage and recover the substantial body mass they lost during breeding season fasts. Females are at sea about half as long as males in spring before they must
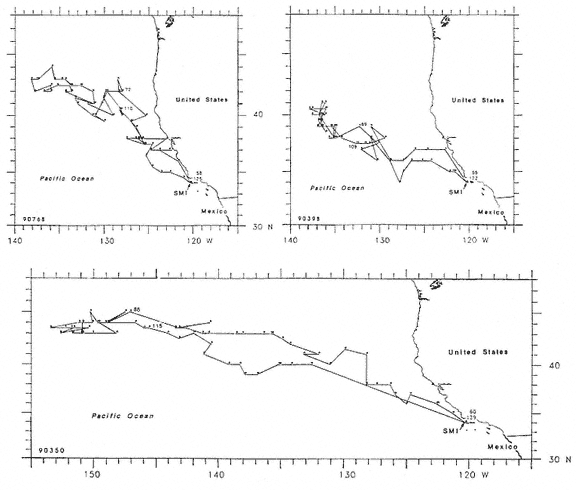
Fig. 16.5
Migratory routes of 3 northern elephant seal females from San Miguel Island, California, in 1990
(dots represent daily locations for all days that seals were at sea, except for seal 90350
whose instrument's memory filled up as it returned to San Miguel Island;
numbers in lower left corners are seal identification numbers).
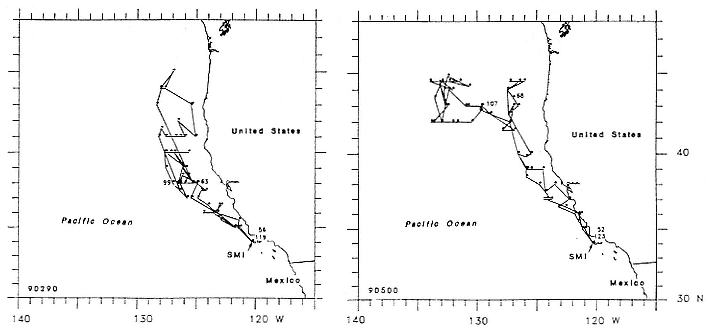
Fig. 16.6
Migratory routes of 2 northern elephant seal females from San Miguel Island, California, in 1990
(dots represent daily locations for all days that seals were at sea).
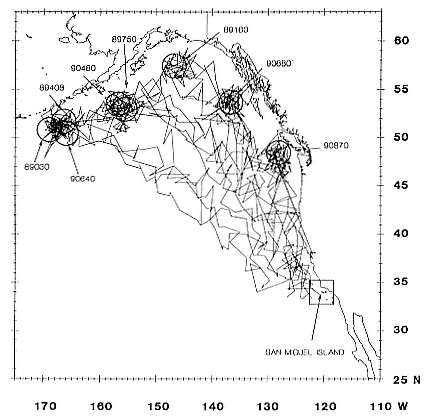
Fig. 16.7
Postbreeding migratory routes and foraging areas of 8 northern elephant seal
males in 1989 and 1990 (all days that seals were at sea are plotted).
return to land to molt (tables 16.1, 16.2). This difference may explain why females do not continue to travel northward to the areas where males forage. A round-trip alone to those areas without lingering there would take around 60 days. It is intriguing, though, that males do not remain in the southern areas where females forage even though there appear to be adequate prey resources. Latitudinal differences in prey quality or size may constrain males to continue north in pursuit of more rewarding food resources. Sea-surface temperatures differ by 3 to 5° between the foraging areas of males and females (figs. 16.8, 16.9, 16.10), but we do not think that temperature alone would constrain the distribution of elephant seal adults in the North Pacific. Records from southern elephant seals (Fedak et al., this volume; Slip, Hindell, and Burton, this volume) show that adult males and females may occupy the same coastal Antarctic waters where SSTs are
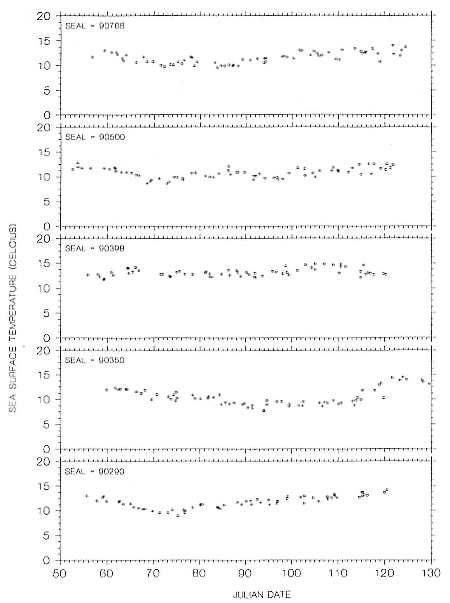
Fig. 16.8
Sea-surface temperature measurements recorded by GLTDRs attached to 5 adult
northern elephant seal females during their postbreeding migrations in 1990.
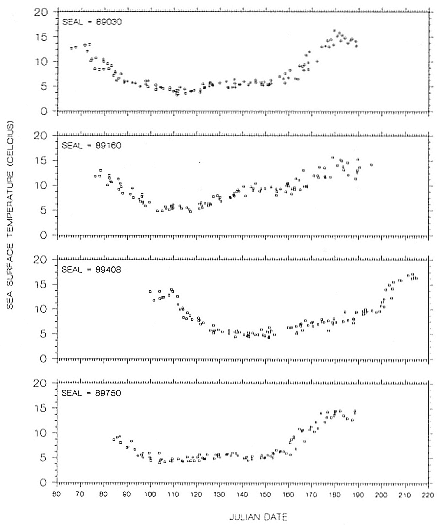
Fig. 16.9
Sea-surface temperature measurements recorded by GLTDRs attached to 4
adult northern elephant seal males during their postbreeding migrations in 1989.
several degrees colder than in the Gulf of Alaska and the eastern Aleutian Islands where male northern elephant seals forage.
Le Boeuf (this volume) reported that some females from Año Nuevo Island traveled into offshore British Columbia waters to feed, an area of overlap with adult males. We can think of no reason other than time constraints that females from the Channel Islands do not migrate that far north. Certainly, additional studies are needed to examine the biotic and abiotic
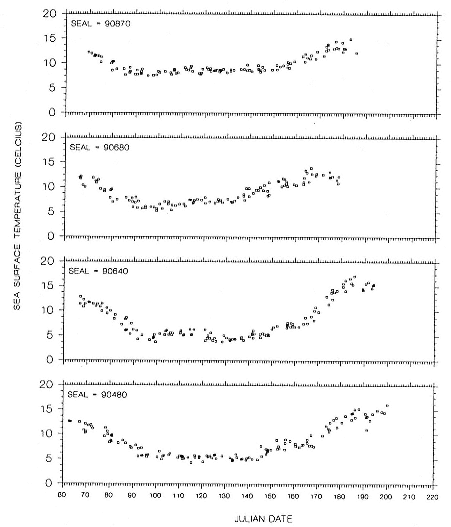
Fig. 16.10
Sea-surface temperature measurements recorded by GLTDRs attached to 4 adult
northern elephant seal males during their postbreeding migrations in 1990.
factors that influence the differential distributions of foraging northern elephant seals during their seasonal migrations.
Our studies indicate that northern elephant seals that breed on San Miguel Island feed in offshore waters in the mesopelagic zone while pursuing vertically migrating prey. Studies of the diets of these seals (Condit and Le Boeuf 1984; Antonelis et al., this volume; Stewart and DeLong
1993) indicate that those prey are principally squid of several families that are known to be vertical migrators inhabiting mesopelagic habitats (Jefferts 1983; Roper and Young 1975). While in the California Current north of San Miguel Island, male and female elephant seals eat similar prey (Antonelis et al., this volume; Stewart and DeLong, unpubl. data). While transiting through that area, however, males and females are diving to different depths (tables 16.1, 16.2); thus they may be foraging on different size classes of those common prey, but we are unable to confirm this with the available data. Data on the diet of elephant seals in their offshore foraging areas are lacking, but we do know from the stomach contents of other predators (e.g., sperm whales, beaked whales, northern right whale dolphins, Pacific white-sided dolphins, Dall's porpoise) and midwater trawls (R. L. DeLong, unpubl. data) that squid species that are eaten by elephant seals in the southern part of the California Current are also present in the Subarctic Transition Zone, near the eastern Aleutian Islands and in the Gulf of Alaska. We presume that elephant seals are pursuing those prey during most of their periods at sea, but latitudinal differences in squid age and sex composition and behavior could explain the apparent differences in dive depths between male and female northern elephant seals.
Elephant seals depart breeding beaches in lean condition, having lost 40% or more of their body mass during breeding season fasts and lactation (Costa et al. 1986; Deutsch, Haley, and Le Boeuf 1990). We presume that they begin feeding immediately on entering the water, a hypothesis that is supported by diving records and the large increases in mass of males and females by the time they haul out to molt (Le Boeuf et al. 1988, 1989; DeLong and Stewart, unpubl. data). The continuity and characteristics (e.g., diurnal variation in dive depth to presumed depths of the deep scattering layer) of diving also suggest that the seals forage continuously while at sea. The identification of specific destinations where seals, particularly males, spend several weeks or more suggests that some areas of the North Pacific are more productive and energetically more rewarding to northern elephant seals than others. Satellite imagery has revealed dynamic primary productivity south of the eastern Aleutian Islands and in parts of the Gulf of Alaska, particularly in spring and summer when ocean warming and stabilization and longer days promote explosive phytoplankton blooms (Lewis 1989). Elephant seal males are evidently attracted to the deeper biological communities that respond to that heightened surface productivity. Southern elephant seals also appear to be attracted to similar areas of seasonally enhanced biological productivity along the Antarctic Peninsula and to other coastal Antarctic regions (Fedak et al., this volume; Slip, Hindell, and Burton, this volume). Elucidating the cues that attract, guide, and drive elephant seals to those areas will be challenging topics for future research.
The California Current and the North Pacific Transition Zone (NPTZ)
and Subarctic Frontal Zone in the central North Pacific, where northern elephant seal females migrate to forage, are also known to be highly productive areas. The NPTZ, in particular, attracts large numbers of seabird, turtle, and other marine mammal predators during some seasons (e.g., Wetherall 1991). The near-surface communities of that area have been relatively well studied in recent years, especially since the explosive growth of the drift net squid fishery in the central North Pacific (ibid.). But little is known about the mesopelagic communities in that area which appear to play an important role in the foraging dynamics of female northern elephant seals.
The abilities of northern elephant seals to make long-distance, deep-water, foraging migrations appear to be important adaptations that permit them to range widely during most of the year to accumulate substantial resources that are essential to the energetically demanding terrestrial-bound activities of breeding and molt. Because elephant seals from the San Miguel Island and Año Nuevo rookeries evidently mix in foraging areas yet show strong fidelity to molting and breeding sites, continued study of intercolony differences and similarities in foraging patterns and migrations will be important to understanding the mechanisms that will limit and regulate the growth of each colony and of the still-increasing northern elephant seal population (see Stewart et al., this volume).
Acknowledgments
We thank P. Yochem, B. DeLong, D. DeLong, S. Melin, G. Antonelis, S. Osmek, H. Huber, T. Ragen, and J. Francine for field assistance, the Channel Islands National Park Service for facilitating our research at San Miguel Island, and G. Antonelis, J. Baker, I. Boyd, R. Gentry, J. R. Jehl, B. Le Boeuf, and P. Yochem for comments on earlier drafts of the manuscript. We thank Clairol Research Laboratories for supplying hair dye and bleach products used for marking seals and R. Hill, S. Hill, and M. Braun for consultation during development and field testing of the GLTDRs. GLTDRs were manufactured by Wildlife Computers (Woodinville, Wash.), and VHF transmitters were manufactured by ATS (Isanti, Minn.). BSS was supported by a contract to the U.S. Air Force, Space Systems Division. The research was conducted under Marine Mammal Permit No. 579 to BSS.
References
Boyd, I. L., and T. Arnbom. 1991. Diving behaviour in relation to water temperature in the southern elephant seal: Foraging implications. Polar Biology 11: 259–266.
Condit, R., and B. J. Le Boeuf. 1984. Feeding habits and feeding grounds of the northern elephant seal. Journal of Mammalogy 65: 281–290.
Costa, D. P., B. J. Le Boeuf, A. C. Huntley, and C. L. Ortiz. 1986. The energetics of lactation in the northern elephant seal. Journal of Zoology 209: 21–33.
DeLong, R. L., and B. S. Stewart, 1991. Diving patterns of northern elephant seal bulls. Marine Mammal Science 7: 369–384.
DeLong, R. L., B. S. Stewart, and R. D. Hill. 1992. Documenting migrations of northern elephant seals using day length. Marine Mammal Science 8: 155–159.
Deutsch, C. J., M. P. Haley, and B. J. Le Boeuf. 1990. Reproductive effort of male northern elephant seals: Estimates from mass loss. Canadian Journal of Zoology 12: 2580–2593.
Favorite, F., A. J. Diomead, and K. Nasu. 1976. Oceanography of the Subarctic Pacific Region, 1960–1971. International North Pacific Fish Commission Bulletin 33: 1–187.
Hindell, M. A., H. R. Burton, and D. J. Slip. 1991. Foraging areas of southern elephant seals, Mirounga leonina , as inferred from water temperature data. Australian Journal of Marine and Freshwater Research 42: 115–128.
Jefferts, K. 1983. Zoogeography and systematics of cephalopods of the northeastern Pacific Ocean. Ph.D. dissertation, Oregon State University, Corvallis.
Le Boeuf, B. J., D. P. Costa, A. C. Huntley, and S. D. Feldkamp. 1988. Continuous, deep diving in female northern elephant seals, Mirounga angustirostris. Canadian Journal of Zoology 66: 446–458.
Le Boeuf, B. J., Y. Naito, A. C. Huntley, and T. Asaga. 1989. Prolonged, continuous, deep diving by northern elephant seals. Canadian Journal of Zoology 67: 2514–2519.
Lewis, M. R. 1989. The variegated ocean: A view from space. New Scientist 1685: 1–4.
Roper, C. F. E., and R. E. Young. 1975. Vertical distribution of pelagic cephalopods. Smithsonian Contributions to Zoology 209: 1–51.
Stewart, B. S., and R. L. DeLong. 1990. Sexual differences in migrations and foraging behavior northern elephant seals. American Zoologist 30: 44A.
———. 1993. Seasonal dispersion and habitat use of foraging northern elephant seals. In Marine Mammals: Advances in Behavioral and Population Biology , ed. I. L. Boyd, 179–194. Symposia of the Zoological Society of London no. 66. London: Oxford University Press.
Wetherall, J. A., ed. 1991. Biology, oceanography, and fisheries in the North Pacific Transition Zone and Subarctic Frontal Zone. NOAA Technical Report NMFS 105: 1–110.
Zar, J. 1984. Biostatistical Analysis . Englewood Cliffs, N.J.: Prentice-Hall.