The Role of Streamside Bufferstrips in the Ecology of Aquatic Biota[1]
Don L. Mahoney and Don C. Erman[2]
Abstract.—Riparian vegetation is important as a source of food to stream organisms, as shade over small-order streams, and as a bank-stabilizing force to prevent excessive sedimentation and to intercept pollutants. Logging may significantly affect each of these factors unless proper protective measures are employed. Current research is underway on the recovery of small northern California streams after logging. Analysis of algal samples from 30 streams shows light intensity and chlorophyll concentrations are major factors related to logging intensity that affect instream primary production. Transportable sediment from 24 streambeds has shown that this measure of sediment is higher (P = .001) in logged and narrow buffered streams than in controls 7 to 10 years after logging.
Introduction
Streamside vegetation is a source of food, provides shade which prevents excessive water temperatures, provides cover, and gives stability to the soil along the stream. If land-use activity on the slopes around a stream creates erosion or pollution hazards, streamside vegetation can intercept and filter sediments and contaminants before they enter the stream. Several studies have examined the value of streamside vegetation (bufferstrips) in protecting streams from timber management activities; we will emphasize these studies in this paper. In addition, preliminary results from our study of the recovery of logged streams in northern California will be presented.
Streamside Vegetation As a Source of Food
Streams are often classified according to the predominant source of energy used as a base for their food chains. Some streams receive energy primarily from leaffall and litter entering the stream, while others receive energy primarily from instream plant production. These two sources of energy can vary over the course of a year and from one section of stream to another. Recent emphasis has been placed on the role of outside energy sources to stream macroinvertebrates (Cummins 1974). Numerous studies (see Erman 1983; Knight and Bottorff 1983) have focused on the feeding habits of individual species of invertebrates, both as larvae and as adults. Much of this work has examined the functional aspects of the aquatic animals or the way that they obtain their food, rather than the origin of the food, the taxonomic nature of the species, or the trophic level they might occupy (Cummins 1973).
In a recent study, for example, Hawkins and Sedell (1981) have shown that the relative abundance of each functional group depends on the kind of food inputs into the stream system. They studied a relatively undisturbed stream system, from headwaters to lowlands, in the Oregon Cascades and found that shredders of large organic particles dominated upper shaded reaches; collectors of fine organic particles increased in abundance from the headwaters down; filterers of fine organic particles were only locally important; predators became equally distributed in the summer and fall, increasing in the lower reaches from a spring minimum; and algal scrapers became an important part of the fauna once the stream became wider and more open to light through the middle reaches. Obviously, if riparian vegetation is destroyed and a stream changes from a food web based on outside inputs to one based on internal production, then the various types of invertebrates will change in abundance also.
Another effect of streamside vegetation removal, although more indirect, is to alter
[1] Paper presented at the California Riparian Systems Conference. [University of California, Davis, Sept. 17–19, 1981].
[2] Don L. Mahoney is Ph.D. Candidate in Wildland Resource Science; Don C. Erman is Professor of Wildlife-Fisheries, both are in the Department of Forestry and Resource Management, University of California, Berkeley.
predation pressure from fish. Insects falling from the riparian zone are significant sources of food for stream fishes (Hunt 1975; Cadwallader etal . 1980), and when vegetation is removed, fish switch to feeding predominantly on instream invertebrates as the aerial source is diminished (Hess 1969; Cadwallader etal . 1980).
A compensating effect of the removal of the streamside shade is to increase the growth of certain algae, especially diatoms and filamentous green algae. Increased algal growth has been associated with increased biomass of some invertebrates and fish (Lyford and Gregory 1975; Murphy and Hall 1981), and may mask detrimental effects of increased sedimentation (Murphy etal . 1981), especially if adequate spawning sites for fish are available in nearby undisturbed sections of stream and if adequate care has been taken of the streambed. An increase in the amount of algae, however, may be at the expense of mosses and vascular macrophytes (Mahoney and Erman, unpublished), organisms which have been used as indicators of good water quality in Europe (Empain 1978; Wiegleb 1981).
Temperature Effects of Streamside Vegetation
Streamside vegetation has a marked effect on the temperature of stream water (Hall and Lantz 1970; Meehan 1970; Swift and Messer 1971; Brazier and Brown 1973). Temperature increases after removal of streamside vegetation can be expected to be greater where stream discharges are smaller, as in low-order, headwater mountain streams. Methods are now available for quantifying the relationship between the amount of streamside vegetation and the change in stream temperature (Brown 1972). Temperature change is related to the amount of solar radiation at critical periods that is intercepted by the canopy, the surface area of the stream, and stream discharge. In some cases the annual maximum temperature has increased 12°C by complete canopy removal in small streams (Brown and Krygier 1970). By way of comparison, the average increase in water temperature caused by once-through cooling of electric generation stations was 10–11°C a few years ago (Mittursky etal . 1970; Levin etal . 1972).
There are detrimental effects of temperature increases on fish survival (e.g., Paladino etal . 1980) including those caused by riparian vegetation removal (Swift and Messer 1971; Ringler and Hall 1975). But Hunt (1979) intentionally cleared upper-story vegetation from along a small Michigan stream in order to recreate a meadow environment and reported that temperature increases were ameliorated once meadow vegetation began overhanging the stream.
While many of these studies have shown that temperature increases affect fish populations, there have been fewer studies to determine the effect of temperature increases on invertebrates. The change in fish production would by itself change the predation pressure on the invertebrate community. Burton and Likens (1973) discussed possible changes in stream macroinvertebrate communities due to temperature increases from vegetation removal, and a number of other studies have demonstrated significant changes in populations and decreased diversity of macroinvertebrates in waters heated by power plant cooling systems (Howell and Gentry 1974; Ward 1976).
Streamside Vegetation as a Protection from Erosion and Pollution
One benefit of the herbaceous growth (including mosses and liverworts) along the banks of streams is to provide stability to the banks and thus prevent streambed erosion due to undercutting (e.g., Pfankuch 1975; Cederholm and Koski 1977). Taller, more woody growth along streams can prevent the debris produced by human activities or natural landslides from entering the channel (Benoit 1978). There are many reports of changes in the invertebrate biota that occur with increases in sedimentation (Cordone and Kelly 1961; Chutter 1964; Nuttal 1972; Rosenberg and Weins 1978). The exact mechanisms responsible for these changes are complex, involving smothering, loss of food or space, and substrate instability (Cederholm etal . 1978; Lenat etal . 1978; Williams and Mundie 1978).
When streams are buffered from the effect of road-building and logging by strips of vegetation, they have much lower increases in suspended sediment than comparable streams that are not protected by bufferstrips (Burns 1972; Moring 1975). Table 1 gives data from the Alsea watershed study in Oregon (Moring 1975), covering a period of 15 years; it clearly demonstrates the protective value of streamside vegetation. Similarly, in another study (Aubertin etal . 1974) only slight increases in stream turbidity occurred after clearcutting a 34-ha. watershed in West Virginia. The success in maintaining water quality was attributed to the retention of a 10-to 20-m. forest strip along the stream.
| ||||||||
Other studies, chiefly in the Midwest, have examined the value of vegetative bufferstrips as a protection from agricultural pollution. Vegetative strips around feedlots substantially reduced runoff, total solids, total nitrogen, and total phosphorus (Young etal . 1980). Asmussen
etal . (1977) found that vegetative bufferstrips effectively reduced pollution from agricultural chemicals. An extensive review of the impact of nearstream vegetation on water quality and stream biota (Karr and Schlosser 1977) concluded that "proper management of nearstream vegetation and stream morphology may produce substantial improvement in water quality and the stream biota of agricultural watersheds." A summary of this report is available in Karr and Schlosser (1978),
The exact extent of bufferstrip that is necessary to protect water quality has been calculated for some situations. Based on soil stability ratings and slope, Haupt (1959) developed guidelines for the width of bufferstrips necessary to protect streams from road-building activities. Benoit (1978) has done the same for timber management activities based on data from a number of streams in Oregon. Federal regulations for national forests, and the Forest Practices Act regulations on private lands in California, limit the extent of timber harvesting activities near watercourses, but they do not dictate specific buffer widths.
A major study of macroinvertebrates in streams in northern California was undertaken at the University of California, Berkeley, to see if macroinvertebrates could indicate water quality in streams associated with logging activities (Erman et al . 1977; Roby etal . 1977; Newbold etal . 1980). A total of 65 buffered, unbuffered, and control streams was sampled, mostly on national forest land. These investigations showed that invertebrate communities of logged or disturbed streams had a lower diversity index and higher populations (primarily of Chironomidae, Baetis , and Nemoura ) than streams in unlogged areas. Streams with bufferstrips wider than 30 m. had invertebrate communities no different than control streams; whereas, if bufferstrips were less than 30 m., some differences in communities were detected. The relationship between diversity and various land-use practice (no buffer, narrow buffer, wide buffer, control) is summarized in figure 1.
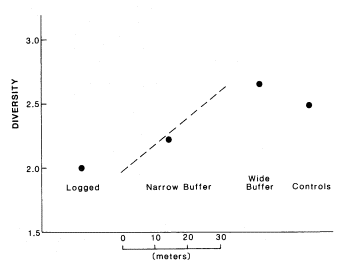
Figure l.
Diversity of macroinvertebrate communities in streams in northern
California as affected by varying forestry practices.
Similar work in New Zealand found changes in invertebrate abundances and diversity after streamside vegetation was cleared during logging operations (Graynoth 1979). Where a buffer of vegetation was left along the stream, changes in invertebrate populations did not occur.
A major concern of regulatory policy is whether fixed widths of vegetation along the streamside should be specified or land managers should make site-specific recommendations to balance resource use and stream protection. A fixed width (or some range of values) is appealing because of its simplicity of application and its provision of at least minimum stream protection. Site-specific recommendations theoretically allow for more precise adjustments to local risks (or lack of risks) and permit resource use (timber harvesting) to the limit of sound forest practices. Evidence is available to support either approach (Burns 1972; Erman etal . 1977). In the long run, we believe, use and protection must be balanced by the land manager, who needs to view land and water systems with equal concern.
Ongoing Study of Post-Logging Recovery
While the previous study of logging and bufferstrips in California demonstrated significant reductions in invertebrate diversity in unbuffered and narrow buffered streams, causal mechanisms were not investigated thoroughly. As a follow-up study, 30 of the streams in the original investigation are currently being studied to see the recovery rate of narrow and unbuffered streams and to investigate possible causal mechanisms. Data on invertebrate distributions and abundances, algal production, and transportable sediment stored in the substrate have been collected. Invertebrate analysis is not yet complete; preliminary data on algal production and sediment storage will be presented here.
Algae Production
As mentioned earlier, one of the effects of removing the stream canopy is to alter the instream algal community. There are many methods to quantify this community, and comparisons between methods have been attempted (e.g., Bott etal . 1978). While oxygen evolution methods may provide a more accurate measure of primary production under some circumstances, the ease with which chlorophyll and biomass estimates can be made has assured these methods a place in the literature, even though extraction methods for chlorophyll remain uncertain (Rai 1980). But before chlorophyll analyses can be used as indicators of perturbations, factors affecting differen-
ces in chlorophyll concentration must be fully understood.
We used an approach similar to that of Bannister (1974), where the amount of chlorophyll was weighted by the amount of available light. Higher chlorophyll concentrations are usually present in shade plants (Brown and Richardson 1968; Boardman 1977), but they do not necessarily mean higher production; this method takes that relationship into account in making estimates of potential production. The presence of more chlorophyll in shade plants has been known for some time to plant physiologists, but apparently is not appreciated by limnologists (Naiman and Sedell 1980). A full description of the methods used in this study will be available in a later publication.
Algae for this study was removed from constant rock sizes (2–4 cm.), size-classes that are moved by moderate storms. No noticeable differences were detected between logged, buffered, and control streams. In those streams that were not light-saturated (i.e., did not receive 10% of full sunlight), there was a relationship between the amount of chlorophyll per unit area and the amount of radiant energy—the less energy received the more chlorophyll present (fig. 2). Potential production for these streams was inversely related to the chlorophyll concentration present (fig. 2). Streams that were not lightlimited had a wide range of chlorophyll concentrations and production estimates. This range is due to a number of factors, the most prominent being the place in the bloom-dieoff cycle that was sampled. High values were obtained if samples were taken as a particular species of algae (usually filamentous) reached bloom proportions in streams modified to receive full sunlight. Low values were obtained from the same stream-types if samples were taken after a bloom of algae had begun to senesce. These results indicate some of the difficulties with, and hence limitations to, interpretation of algal chlorophyll data in evaluating stream perturbations.
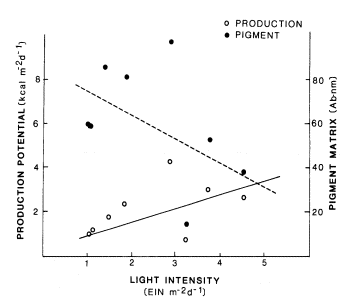
Figure 2.
Potential algal production and total pigment concentration
for 8 light-limited streams in northern California.
Changes in Transportable Sediment
The amount and size of sediments in stream substrates is a result of many processes. Inputs may come from human activities such as logging or road-building, or from natural causes such as landslides. How fast sediment is moved through the stream depends on such things as slope, instream vegetation, instream sediment traps, and the frequency of large storms for the period in question. It may easily take five years or more for a pulse of sediment to flow completely through a stream system (Dunn and Leopold 1978). Thus the amount of sediment that is currently in the process of being moved through a stretch of stream (including that temporarily stored) is a summation of all the land-use activity and weather patterns that have prevailed in the basin in question for the preceding several years.
For this study we measured the sediment that was present in a stream and could be easily moved (transportable sediment) by summer low flows. We stirred a small area of streambottom to a depth of 10 cm. and placed cylindrical containers into the substrate downstream to collect sediment (Beschta and Jackson 1979; Blomqvist and Hakanson 1981; Blomqvist and Kofoed 1981).
Figure 3 shows the percentage change in transportable sediment in logged or narrow-buffer treatment streams when compared with control streams. Each treatment (either logged or buffered) is compared with each control within that block of streams (a group of treatment and control streams from the same area). Transportable sediment was higher in 17 out of 20 comparisons of treatment to control (sign test: P = .001), even though several of the control streams were degraded by recent road-building activity. Figure 4 shows the change in transportable sediment, transportable detritus, and the ratio of the two for each block of streams. The average of treatment streams is compared against the average of control streams for each block. Comparisons between blocks are not completely analyzed. In all blocks, transportable sediment was higher in treatments than in controls (fig. 4). Transportable detritus was higher in five of the six blocks, while the ratio of detritus to sediment was lower in four of six blocks. These findings indicate that seven to ten years after logging, in unprotected streams and streams protected by buffers less than 30 m. wide, more sediment is being transported than in comparable controls. Detritus (from either out-of-stream or in-stream sources) is also higher in treatment streams, but this increase is not as great as the increase in sediments.
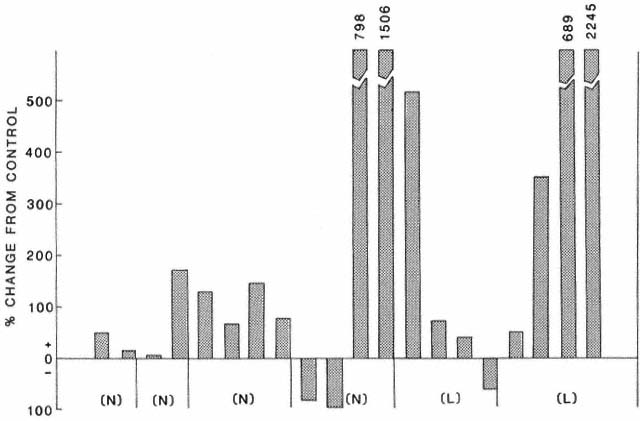
Figure 3.
Percentage change from control in transportable sediment in narrow-buffered
(N) and logged (L) streams in northern California.
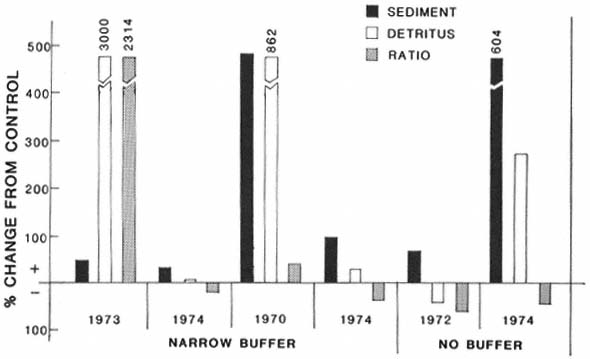
Figure 4.
Percentage change from control in transportable sediment, detritus, and the detritus: sediment ratio
in narrow-buffered and unbuffered streams in northern California (dates are year of initial logging).
Conclusion
Streamside vegetation has been shown to play an important role in the ecology of aquatic plants, macroinvertebrates, and fish. The sources cited in the review sections of this paper are meant solely as an introduction to a greater body of literature available on many aspects of the subject. Perhaps the best way to summarize the importance of streamside vegetation is to place it in the context of the river continuum concept (Vannote etal . 1980). In this concept, the biotic community is shaped by both the immediate physical environment and by inputs from further upstream. Furthermore, the stream biota, after an extended period of time, reaches a dynamic equilibrium with the physical environment which itself undergoes periodic fluctuations.
It is in the headwaters of a stream that riparian vegetation plays its most important role in the ecology of invertebrates, both as a predictable annual source of food and as protection from excessive heating. The influence of this vegetation, however, is felt not only in the headwaters but also further downstream, where much of it eventually drifts. In the middle reaches of stream systems, the immediate riparian vegetation becomes less important as food, relative to the instream algal production, and in the lowest reaches, in large rivers, it plays an even lesser role as food. In these lower reaches riparian vegetation has its greatest importance as a soilstabilizing force. This bank stabilization role is of course doubly important in the headwaters, as any sediment produced there will eventually find its way downstream.
Bufferstrips of vegetation left along streams affected by human activities have been shown to protect the integrity of stream systems. This vegetation is important in protecting not only the stream immediately adjacent to it, but also in protecting the biota further downstream since it prevents excessive sediment pulses from being flushed downstream. This downstream protective benefit of bufferstrips is usually not included in economic analyses of the costs and benefits of not harvesting the timber immediately adjacent to a stream (e.g., Gillick and Scott 1975). These downstream effects of riparian vegetation need more study so that they can be more realistically evaluated.
It is presently questionable how much riparian vegetation can be removed from a stream system without affecting the stability of the system. Natural resilience (e.g., increased algal blooms) may temporarily mask detrimental effects of this practice, but there is increasing evidence that decades may be necessary for the stream biota to return to normal. Since so much of the riparian vegetation in California has already been destroyed, it becomes even more imperative that what remains be managed wisely so that this important natural resource with all its diversity is not lost to posterity.
Acknowledgments
We wish to thank Nancy Erman for her help in preparing the manuscript and Brian Plant for help in collecting field data. This work has in part been funded by the University of California, Water Resources Center as part of Water Resources Center Project UCAL-WRC-W-586.
Literature Cited
Asmussen, L.E., A.W. White, Jr., E.W. Hauser, and J.M. Sheridan. 1977. Reduction of 2,4-d load in surfce runoff down a grassed waterway. J. Environ. Qual. 6:159–162.
Aubertin, G.M., and J.H. Patric. 1974. Water quality after clearcutting a small watershed in West Virginia. J. Environ. Qual. 3:243–249.
Bannister, T.T. 1974. Production equations in terms of chlorophyll concentration, quantum yield, and upper limit to production. Limnol. Oceanogr 19:1–12.
Benoit, C. 1978. Fluvial sediment delivery as percent of erosion. The relationship between landslope and effective streamside bufferstrip width. USDA Forest Service, Portland, Ore. Typescript.
Beschta, R.L. and W.L. Jackson. 1979. The intrusion of fine sediments into a stable gravel bed. J. Fish. Res. Board Can. 36:204–210.
Blomqvist, S. and L. Hakanson. 1981. A review on sediment traps in aquatic environments. Arch. Hydrobiol. 91:101–132.
Blomqvist, S. and C. Kofoed. 1981. Sediment trapping—a subaquatic in situ experiment. Limnol. Oceanogr. 26(3):585–590.
Boardman, N.K. 1977. Comparative photosynthesis of sun and shade plants. Ann. Rev. Plant Physiol. 28:355–377.
Bott, T.L., J.T. Brock, C.E. Cushing, S.V. Gregory, D. King, and R.C. Petersen. 1978. A comparison of methods for measuring primary productivity and community respiration in streams. Hydrobiologia 60:3–12.
Brazier, J.R., and G.W. Brown. 1973. Bufferstrips for stream temperature control. Research Paper 15, Forest Res. Lab., School of Forestry, Oregon State University, Corvallis. 9 p.
Brown, G.W. 1972. An improved temperature prediction model for small streams. 20 p. Dept. of For. Eng., Oregon State University, Corvallis, Water Resources Research Institute-16.
Brown, G.W., and J.T. Krygier. 1970. Effects of clear-cutting on stream temperature. Water Resour. Res. 6:1131–1139.
Brown, T.E., and F.L. Richardson. 1968. The effect of growth environment on the physiology of algae: Light intensity. J. Phycol. 4:38–54.
Burns, J.W. 1972. Some effects of logging and associated road construction on northern California streams. Trans. Amer. Fish. Soc. 101(1):1–17.
Burton, T.M., and G.E. Likens. 1973. The effect of strip-cutting on stream temperatures in the Hubbard Brook Experimental Forest, New Hampshire. Bioscience 23:433–435.
Cadwallader, P.L., A.K. Eden, and R.A. Hook. 1980. Role of streamside vegetation as a food source for GalaxiasolidusGunther (Pisces: Galaxiidae). Aust. J. Mar. Freshwater Res. 31:257–262.
Cederholm, C.J., and K.V. Koski. 1977. Effects of stream channelization on the salmonid habitat and populations of lower Big Beef Creek, Kitsap County, Washington 1969–1973. 31 p. Washington Cooperative Fish. Res. Unit, College of Fisheries, University of Washington, Seattle.
Cederholm, C.J., L.C. Lestelle, B.G. Edie, D.J. Martin, J.V. Tagart, and E.O. Salo. 1978. The effects of landslide siltation on the salmon and trout resources of Stequaleho Creek and the main Clearwater River, Jefferson County, Washington, 1972–1975. 53. University of Washington, College of Fisheries, Fish. Res. Institute.
Chutter, F.M. 1969. The effects of silt and sand on the invertebrate fauna of streams and rivers. Hydrobiologia 34:57–76.
Cordone, A.J. and D.W. Kelley. 1961. The influences of inorganic sediment on the aquatic life of streams. Calif. Fish and Game 47:189–228.
Cummins, K.W. 1973. Trophic relations of aquatic insects. Ann. Rev. Entomol. 18:183–206.
Cummins, K.W. 1974. Structure and function of stream ecosystems. Bioscience 24(1):631–641.
Dunn, T. and L.B. Leopold. 1978. Water in environmental planning. Freeman Company, San Francisco, Calif. 818 p.
Empain, A. 1978. Quantitative relationships between populations of aquatic bryophytes and pollution of streams. Definition of an index of water qualtity. (In French) Hydrobiologia 60:49–74.
Erman, D.C., J.D. Newbold, and K.R. Roby. 1977. Evaluation of streamside bufferstrips for protecting aquatic organisms. Contribution No. 165, California Water Resources Center, University of California, Davis. 48 p.
Erman, N.A. 1983. The use of riparian systems by aquatic insects. In : R.E. Warner and K.M. Hendrix (ed.). California Riparian Systems. [University of California, Davis, September 17–19, 1981]. University of California Press, Berkeley.
Gillick, T. and B.D. Scott. 1975. Bufferstrips and the protection of fishery resources: an economic analysis. Report No. 332, Washington Department of Natural Resources, Olympia. 30 p.
Graynoth, E. 1979. Effects of logging on stream environments and faunas in Nelson. New Zealand Journal of Marine and Freshwater Research 13(1):79–109.
Hall, J.D., and R.L. Lantz. 1970. Effects of logging on the habitat of Coho salmon and cutthroat trout in coastal streams. Technical Paper No. 2570, Oregon Agricultural Experiment Station, Corvallis. 21 p.
Haupt, H.F. 1959. A method for controlling sediment from logging roads. USDA Forest Service Intermountain Forest and Range Experiment Station Miscellaneous Paper 22, Ogdon, Utah. 22 p.
Hawkins, C.P., and J.R. Sedell. 1981. Longitudinal and seasonal changes in functional organization of macroinvertebrate communities in four Oregon streams. Ecol. 62(2): 387–397.
Hess, L.J. 1969. The effects of logging road construction on insect drop into a small coastal stream. M.S. Thesis, Humboldt State College, Arcata, Calif. 58 p.
Howell, F.G., and J.B. Gentry. 1974. Effects of thermal effluents from nuclear reactors on species diversity of aquatic insects. In : J.W. Gibbons and R.R. Scharitz (eds.). Thermal Ecology. U.S.A.E.C. Technical Information Center.
Hunt, R.L. 1975. Use of terrestrial invertebrates as food by salmonids. p. 137–151. In : A. D. Hasler (ed.). Coupling of land and water systems. Springer-Verlag, Berlin.
Hunt, R.L. 1979. Removal of woody streambank vegetation to improve trout habitat. Technical Bulletin No. 115, Wisconsin Department of Natural Resources, Madison. 36 p.
Karr, J.R., and I.J. Schlosser. 1977. Impact of nearstream vegetation and stream morphology on water quality and stream biota. Environ. Research Laboratory EPA-600/3-77-097, US Environmental Protection Agency, Athens, Georgia. 91 p.
Karr, J.R., and I.J. Schlosser. 1978. Water resources and the land-water interface. Science 201:229–234.
Knight, A.W., and R.L. Bottorff. 1983. The importance of riparian vegetation to stream ecosystems In : R.E. Warner and K.M. Hendrix (ed.). California Riparian Systems. [University of California, Davis, September 17–19, 1981]. University of California Press, Berkeley.
Lenat, D.R., D.L. Penrose, and K.W. Eagleson. 1981. Variable effects of sediment addition on stream benthos. Hydrobiologia 79: 187–194.
Levin, A.A., T.J. Birch, R.E. Hillman, and G.E. Raines. 1972. Thermal discharge: ecological effects. Env. Science and Technology 6(3):224–232.
Lyford, J.H., Jr., and S.V. Gregory. 1975. The dynamics and structure of periphyton communities in three Cascade mountain streams. Verh. Int. Verein. Theor. Angew. Limnol. 19:1610–1616.
Meehan, W.R. 1970. Some effects of shade cover on stream temperature in southeast Alaska. USDA Forest Service Pacific Northwest Forest and Range Experiment Station Research Note 113, Portland, Ore. 9 p.
Mittursky, J.A., A.J. McErlean, and V.S. Kennedy. 1970. Thermal pollution, aquaculture and pathobiology in aquatic systems. Journal of Wildlife Diseases 6:347–355.
Moring, J.R. 1975. The Alsea watershed study: effects of logging on the aquatic resources of three headwater streams of the Alsea River, Oregon. Part II. Fishery Research Report No. 9., Oregon Department of Fish and Wildlife, Corvallis. 39 p.
Murphy, M.L., and J.D. Hall. 1981. Varied effects of clear-cut logging on predators and their habitat in small streams of the Cascade mountains, Oregon. Can. J. Fish. Aquat. Sci. 38:137–145.
Murphy, M.L., C.P. Hawkins, and N.H. Anderson. 1981. Effects of canopy modification and accumulated sediment on stream communities. Trans. Amer. Fish Soc. 110:469–478.
Naiman, R.D., and J.R. Sedell. 1980. Relationships between metabolic parameters and stream order in Oregon. Can. J. Fish. Aquat. Sci. 37:834–847.
Newbold, J.D., D.C. Erman, and K.B. Roby. 1980. Effects of logging on macroinvertebrates in streams with and without bufferstrips. Can. J. Fish. Aquat. Sci. 37:1076–1085.
Nuttal, P.M. 1972. The effects of sand deposition upon the macroinvertebrate fauna of the River Camel, Cornwall. Freshwat. Biol. 2:181–186.
Paladino, F.V., J.R. Spotila, J.P. Schubauer, and K.T. Kowalski. 1980. The critical thermal maximum: a technique used to elucidate physiological stress and adaptation in fishes. Rev. Can. Biol. 39:115–122.
Pfankuch, D.J. 1975. Stream reach inventory and channel stability evaluation. USDA Forest Service, Northern Region, Missoula, Mont. 26 p.
Rai, H. 1980. (ed.). The measurement of photosynthetic pigments in freshwaters and standardization of methods. Arch. Hydrobiol. Beih. Ergebn. Limnol. 14. 106 p.
Ringler, N.H., and J.D. Hall. 1975. Effects of logging on water temperature and dissolved oxygen in spawning beds. Trans. Amer. Fish. Soc. 104:111–121.
Roby, K.B., D.C. Erman, and J.D. Newbold. 1977. Biological assessment of timber management activity impacts and bufferstrip effectiveness on National Forest streams of northern California. Earth Resources Monogr. 1, USDA Forest Service, Region 5, San Francisco, Calif. 170 .
Rosenberg, D.M., and A.P. Weins. 1978. Effects of sediment addition on macrobenthic invertebrates in a northern Canadian river. Water Research 12:753–763.
Swift, L.W., Jr., and J.B. Messer. 1971. Forest cuttings raise temperatures of small streams in the southern Appalachians. Jour. of Soil and Water Conservation 26:111–116.
Vannote, R.L., G.W. Minshall, K.W. Cummins, J.R. Sedell, and C.E. Cushing. 1980. The river continuum concept. Can. J. Fish. Aquat. Sci. 37:130–137.
Ward, J.V. 1976. Effects of thermal constancy and seasonal temperature displacement on community structure of stream macroinvertebrates. p. 302–307. In : G.W. Esch and R.W. McFarlane (ed.). Thermal ecology II, ERDA Symposium Series (Conf-740425).
Wiegleb, G. 1981. Application multiple discriminant analysis on the analysis of the correlation between macrophyte vegetation and water quality in running waters of Central Europe. Hydrobiologia 79:91–100.
Williams, D.D., and J.H. Mundie. 1978. Substrate size selection by stream invertebrates and the influence of sand. Limnol. Oceanogr. 23:1030–1033.
Young, R.A., T. Huntrods, and W. Anderson. 1980. Effectiveness of vegetated bufferstrips in controlling pollution from feedlot runoff. J. Environ. Qual. 9:483–487.