Fifteen—
Developmental Aspects of Diving in Northern Elephant Seal Pups
Philip H. Thorson and Burney J. Le Boeuf
ABSTRACT. The aim of this study was to describe the development of diving and measure concomitant changes in physiological correlates that enable this behavior in northern elephant seals, Mirounga angustirostris , during the first nine months of life.
Fifty-seven known-age juvenile seals born at Año Nuevo, California, were studied during the period 1988–1990. We measured (1) time spent in the water and changes in dive depth near the natal rookery during the 2½-month period following weaning, before the seals went to sea for the first time; (2) changes in blood volume, hematocrit, hemoglobin, and myoglobin in seals from near birth to eight months of age; (3) metabolic rate, from oxygen consumption, in 1½- to 3½- month-old juveniles diving in a hooded saltwater tank; and (4) the free-ranging dive pattern of 4-month-old juveniles during part of the first trip to sea.
Diving performance improved quickly during the 10-week period between weaning and going to sea, as reflected by increases in time spent in the water to 12½ hours per day, mean dive duration to 5.9 minutes, and mean dive depth to 16 m. Concurrently, blood and muscle oxygen stores increased, leading to a 46.7% elevation in mass specific oxygen stores, and diving metabolic rate decreased by approximately 50%. Metabolic rate also declined with increasing length of dives and total time submerged. The diving behavior of two 4-month-old seals during the first 12 to 26 days at sea resembled the continuous, deep, and long diving pattern of adults. Mean dive duration was 10 minutes (maximum = 22.3 min), mean surface interval was in the range 1.4 to 1.8 minutes, and mean dive depth was 206 m (maximum = 553 m); approximately 85% of the time at sea was spent submerged.
In only 10 weeks, newly weaned elephant seals undergo profound changes in mass specific blood volume, oxygen stores, and diving metabolic rate while learning to swim and dive near the rookery. These developments prepare them for long-duration deep diving over several months at sea.
Phocid seals suckle out of the water on the substrate where they are born. Consequently, in most species, pups get little or no experience swimming or
diving prior to weaning. Newly weaned pups must develop these skills before they can forage on their own, often over long periods in the open ocean. Despite the critical importance for survival of the transition from a terrestrial to a marine existence, this period has not been studied in depth in any diving mammal.
Our aim was to study behavioral and physiological changes that accompany the development of diving in the northern elephant seal, M. angustirostris , a seal in which the move from land to sea is especially abrupt and demanding, requiring extreme adaptations for breath holding and withstanding high pressure. Adults lead a pelagic existence characterized by continuous, long duration, deep diving (Le Boeuf et al. 1986, 1988, 1989; DeLong and Stewart 1991; Hindell, Slip, and Burton 1991).
Northern elephant seal pups are nursed daily for up to 28 days before being weaned when the mother goes to sea (Le Boeuf, Whiting, and Gantt 1972). They remain on the natal rookery for the next 2½ months, fasting from food and water while learning to swim and dive. Within 2 weeks after weaning, the weanling enters the water for the first time, usually standing freshwater ponds, tide pools, or shallow water in protected coves or beaches (Rasa 1971; Reiter, Stinson, and Le Boeuf 1978). Initial attempts at swimming and diving are awkward and uncoordinated, but improvement is rapid. During this time, mean sleep apnea duration on land doubles from 4 to 8 minutes (Blackwell and Le Boeuf 1993). Within 8 to 10 weeks of weaning, these juveniles make the first pelagic trip to sea to forage, a journey that lasts approximately 4 months (Reiter et al. 1978).
The first trip to sea is a critical period in the life of elephant seals, as only a mean 46.0 ± 7.7% that depart in the late spring survive and return to the rookery in the fall (Le Boeuf, Morris, and Reiter, this volume). Little is known of the behavior of elephant pups during this period. Tag resight records reveal a general dispersal to the north, with pups from the most northerly rookeries in central California being observed as far north as northern California and Vancouver Island, British Columbia (Bonnell et al. 1979; Condit and Le Boeuf 1984). Examination of the stomach contents of juveniles reveals the remains of species common at depths of 200 m or more (Condit and Le Boeuf 1984; Antonelis et al. 1987).
The development of diving ability during the postweaning fast is important both for initial foraging success and for avoidance of white sharks, Carcharodon carcharias , a near-surface predator (Ainley et al. 1981; Le Boeuf, Riedman, and Keyes 1982). Critical to understanding diving performance is knowledge of the amount of oxygen stored, the rate that oxygen is utilized, and its effect on diving behavior. Changes in hemoglobin concentration, blood volume, and myoglobin concentration directly affect oxygen storage capacity (Snyder 1983; Kooyman 1989). Changes in metabolic rate
with age affect the rate that oxygen stores are used. Oxygen storage capacity and diving metabolic rate determine the aerobic dive limit (ADL), the amount of time that a seal can remain submerged while diving aerobically (Kooyman et al. 1983).
The specific objectives of this study were to do the following: (1) describe the development of swimming and diving behavior of free-ranging juveniles near the natal rookery during the 2½-month period from weaning to departure from the rookery; (2) measure changes in blood volume, hematocrit, hemoglobin, and myoglobin in developing seals, from near birth to 8 months of age; (3) determine the metabolic rate of juveniles, 1½ to 3½ months old, during the course of diving, swimming, sleeping, and resting at the surface of a seawater tank; and (4) record the free-ranging dive pattern of juveniles, 3½ to 9 months of age, during the first pelagic trip to sea.
Methods
Diving Behavior near the Rookery during the Postweaning Fast
Diving behavior of juveniles, 1 to 3½ months of age, was studied in the waters surrounding their natal rookery at Año Nuevo, California, from February to May during the years 1988 to 1990. This encompasses the period from initial water entry to departure from the rookery on the first pelagic foraging trip to sea. All seals were known-age, having been marked with cattle ear tags (Dalton Jumbo Rototags, Oxon, England) in the inter-digital webbing of the hind flippers a few days after weaning.
Three types of instruments were used to record changes in time per day spent in the water, dive duration, and dive depth. The amount of time spent in the water was recorded with modified digital watches (Cairns et al. 1987), which were attached to 15 juveniles at 1½ months of age. The watches were glued to the hair on the back of a sleeping juvenile, using 5-minute epoxy (Devcon, Danvers, Mass.). When the seal entered the water, the watch shorted out, and time on the watch did not advance during the period in the water. When the pup exited, the watch began to operate. Watches were read at least twice a week.
Dive duration data were obtained with radio transmitters (Titley Micro-electronics, Blenheim, New Zealand) glued to the hair on the heads of 7 juveniles with 5-minute epoxy as they slept. Dive duration was measured using a stopwatch to record the time between when the radio signal was lost (submerged) and when the signal was recovered (surface). The range of the radio signal was approximately 4 km and was received with a Telonics TR-4 receiver (Mesa, Ariz.).
Maximum dive depth of 17 juveniles was measured with capillary tube depth recorders (Burger and Wilson 1988). Teflon tubing, 100 cm in
length, was sealed at one end and dusted with blue dye powder. As the seal dived, pressure forced water part way up the tube, washing out the dye. The distance the water traveled through the tube was determined by pressure that is proportional to the maximum depth the seal attained. Maximum depth recorders (MDRs) were glued to the hair on the back of sleeping juveniles with 5-minute epoxy. After several days, the MDRs were recovered and maximum depth calculated from the equation of A. E. Burger and R. P. Wilson (1988).
Oxygen Storage Capacity
Total oxygen storage capacity was calculated as the sum of the blood, muscle, and lung oxygen stores, based on the equations of G. L. Kooyman et al. (1983).
Mass determinations, blood samples, and estimates of blood volume were obtained from 25 juveniles (11 males and 14 females) ranging in age from 2 days to 8 months old. The seals were restrained using a mixture of ketamine hydrochloride and diazepam at a dose of 4 mg/kg of body weight (Briggs, Hendrickson, and Le Boeuf 1975). Mass was determined for all seals by hoisting them in a modified canvas bag (Pernia, Hill, and Ortiz 1980) suspended from a 450 ± .5 kg spring scale (Chatillon, New York, N.Y.) attached to a tripod.
Blood samples were drawn from the extradural intravertebral vein using an 8.0-cm, 18-ga spinal needle (Geraci and Smith 1975). Samples were placed into sodium heparin Vacutainers (Becton-Dickson, Rutherford, N.J.). Blood volume was measured by first taking a blood sample, then injecting Evans blue dye into the extradural vein (4 mg/kg of body weight) (Linden and Mary 1983). A final blood sample was taken at least 20 minutes later, after allowing for equilibration.
Hematocrit was determined in duplicate from aliquots of whole blood that had been centrifuged at 11,500 rpm (IEC Micro Hematocrit, Needham Heights, Mass.). Hemoglobin (Hb) determinations were made using the cyanomethohemoglobin conversion method (Sigma Chemical Co., Assay Kit 525, St. Louis, Mo.). Blood volume was determined using the protocol of R. J. Linden and D. A. S. G. Mary (1983).
Myoglobin Assay
Muscle samples were obtained from 13 seals (8 females and 5 males) varying in age from stillborn pups to 8-month-old juveniles. For both fresh carcasses and live animals, muscle samples were taken from the latissimus dorsi just lateral to the pelvis. For biopsies of live seals, the site was cleaned with Betadyne solution, and a local anesthetic, lidocaine hydrochloride, was injected. A sterile 8 mm suction biopsy needle was inserted to obtain a tissue sample weighing approximately 75 mg (Dubowitz and Brooke 1973;
Evans, Phinney, and Young 1982). The assay for myoglobin concentration was performed using the protocol of B. Reynafarje (1963).
Metabolic Studies
Twelve juveniles ranging in age from 1½ to 3½ months old were transported from Año Nuevo to the Long Marine Laboratory where they were held in outdoor seawater tanks. Metabolic rate was determined using an open-circuit respirometry system to measure oxygen consumption in a manner similar to the method of T. M. Williams (1987).
This system consisted of a metabolic hood (a plexiglass dome measuring 2 × 1 × 0.5 m) with an intake and exhaust port placed over a seawater tank which measured 2.5 by 1.8 by 2 m, the only area from which the seal could breathe. Ambient air was pulled through a dry gas meter (Singer, American Meter Division) and then through the metabolic hood by vacuum pump. An aliquot of the air exiting the dome was continually driven through a Baralyme column to remove carbon dioxide, and a Drierite column removed water before passing through an S-3A oxygen analyzer (Ametek, Sunnyvale, Calif.) to measure the fractional oxygen content. The analog output of the oxygen analyzer was converted to a digital signal (Sable Systems, Los Angeles, Calif.) and transferred to a computer. Equation 4b from P. C. Withers (1977) was used to calculate metabolic rate from the fractional change of oxygen.
Seals were weighed in the laboratory prior to experiments using a load cell platform scale (Senstek 2000, Canada). Water temperature of the metabolic tank ranged between 12 and 16°C, which is within the thermoneutral zone of weaned elephant seal pups (P. Thorson, unpubl. data).
Free-ranging Dive Pattern at Sea
Time-depth recorders (TDRs) were attached to 5 juveniles (4 females and 1 male) during 1989 and 3 juveniles (2 males and 1 female) in 1990 at Año Nuevo just prior to departing on their first foraging trip to sea when they were 3½ months old. Two types of TDRs were used, one mechanical and the other a microprocessor system (Wildlife Computers, Woodinville, Wash.). The mechanical TDR measured 2.5 cm in diameter by 8.5 cm long with a mass of 70 (Naito, Asaga, and Ohyama 1990). The TDR used a mechanical pressure transducing system with a timing circuit to record time and depth on pressure-sensitive paper. The pressure transducing system had a threshold limit of 227 m. When recovered, the record was enlarged and digitized for analysis. The microprocessor TDR measured 15 cm long by 2.5 cm wide with a mass of 100 g. The TDR had 256 kilobytes of memory and was programmed to sample depth every 10 seconds (maximum depth limit = 2,000 m) and temperature every 10 minutes. At recovery, the data in the TDR were downloaded to a computer and analyzed.
The seals were chemically restrained, weighed, and blood sampled as mentioned above. TDRs were attached to the hair with 10-minute epoxy (Fibre Glass Evercoat, Cincinnati, Ohio), using the method of Le Boeuf et al. (1988, 1989).
Results
Diving Behavior near the Rookery during the Postweaning Fast
Newly weaned pups began entering the water at approximately 2 weeks postweaning at 1½ months of age. Time per day spent in the water was less than 2% at first and was concentrated at dawn and dusk. It increased to about 52% per day by 10 weeks postweaning, being concentrated at night, and remained at this level until departure on the first foraging trip to sea (fig. 15.1a).
The mean duration of dives in the waters surrounding the rookery increased from 1.9 minutes at initial water entry to 6.1 minutes at the end of the postweaning fast (fig. 15.1b). During this time, the seals did not venture far from the rookery and remained in water less than 12 m deep (except for one 16 m dive by seal H113 who was carrying a TDR; see below); mean dive depth increased with age (fig. 15.1c).
Changes in Physiological Variables over the First Eight Months of Life
Table 15.1 shows that as mass decreased during the postweaning fast, hematocrit, hemoglobin concentration, mass specific blood volume, and myoglobin concentration increased significantly (t-tests, all significant at p < .005). Consequently, mass specific oxygen stores increased by 46.7% over the postweaning fast, or 69.4% from the suckling period to the time when the seals were ready to go to sea. The highest levels of myoglobin concentration, mass specific blood volume, and total oxygen stores were reached in seals returning from their first trip to sea.
Metabolic Studies in Seawater Tanks during the Postweaning Fast
Diving metabolic rate decreased significantly (t-test = 5.43, df = 234, p < .001), by about 50%, over the course of the postweaning fast (fig. 15.2a). Metabolic rate also declined as a function of increasing dive duration (fig. 15.2b) and increasing percentage of time spent submerged, when observed in 30-minute blocks (fig. 15.2c). This trend was seen throughout the postweaning fast, although it was more pronounced in the later period.
Diving Behavior during the First Foraging Trip to Sea
Two of the eight TDRs deployed on juveniles before their first trip to sea were recovered and contained diving data; both of them were carried by
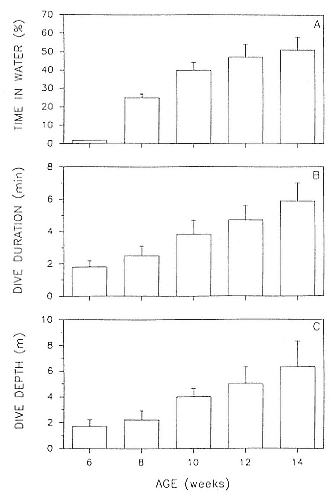
Fig. 15.1
(A) Percentage of time spent in water during the postweaning
fast as a function of age based on 57 observations, from 15
individuals (9 females, 6 males). (B) Dive duration as a
function of age during the postweaning fast, based on 121
observations of 7 individuals (4 males, 3 females). (C) Changes
in dive depth during the postweaning fast as a function
of age, based on 110 observations of 17 individuals
(10 females, 7 males).
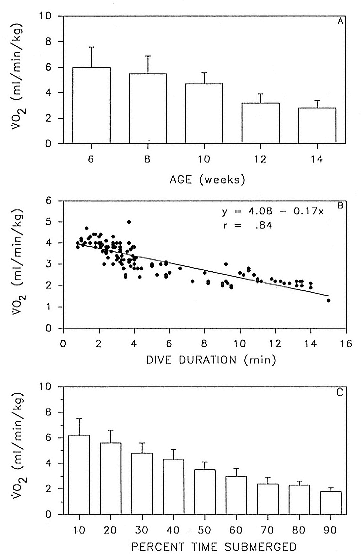
Fig. 15.2
(A) Changes in diving metabolic rate, measured as oxygen
consumption, during the postweaning fast, based on 235
observations from 11 individuals (7 males, 4 females).
(B) Change in metabolic rate, measured as oxygen consumption,
as a function of dive duration. Data based on 137 observations
from 6 individuals (4 males, 2 females). (C) Relation between
metabolic rate, measured as oxygen consumption, and the
percentage of time spent underwater. Data based on 115 30-min
periods from 6 individuals (4 males, 2 females). Numbers in bars
represent the number of observations for that interval.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
females. The TDR of seal G372 was recovered in December 1990 at Año Nuevo, 7½ months after deployment; it recorded the first 12 days at sea, May 12–13, 1990. The TDR of seal H113 was recovered in March 1991 at Half Moon Bay, 45 km north of Año Nuevo, 10 months after deployment; it recorded the first 26 days at sea, May 14 to June 10, 1990.
A summary of the diving data for these two seals is shown in table 15.2. Both seals exhibited similar dive durations with a mean of about 10 minutes; seal H113 exhibited the longest dives, with the maximum lasting 22.3 minutes (fig. 15.3a). Approximately 90% of the surface intervals of both seals were between 0.5 and 1.75 minutes; only 5% were longer than 3 minutes (fig. 15.3b). Both seals had one extended surface interval longer than one hour, and both spent the majority of their time at sea underwater.
The mean depth of the dives of seal H113 was 206 m (median depth = 160 m), and its maximum dive depth was 553 m (table 15.2). Twenty-nine percent of the dives of seal G372 exceeded the depth limit of its TDR, 227 m. By comparison, 42% of the dives of the other seal exceeded 227 m.
Mean dive duration increased as a function of the time at sea for the first
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
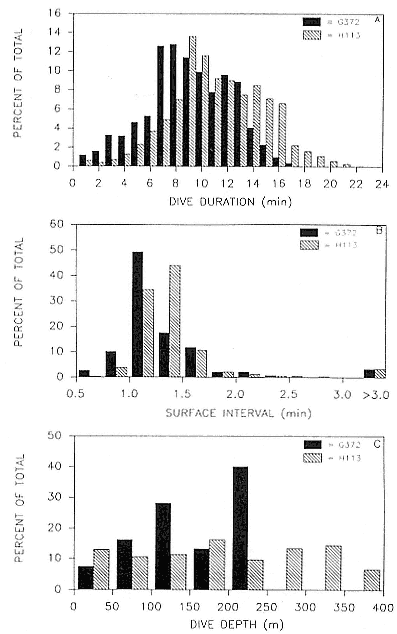
Fig. 15.3
(A) Frequency distributions of dive durations of 2 juvenile northern
elephant seals during the first 12 to 26 days at sea. (B) Frequency
distributions of surface intervals of 2 juvenile elephant seals during
the first trip to sea. (C) Frequency distributions of dive depth of 2
juvenile northern elephant seals during the first trip to sea.
The TDR of seal G372 had a depth limitation of 227 m.
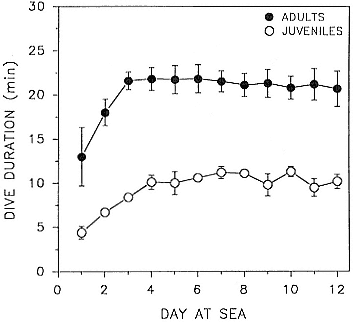
Fig. 15.4
Changes in mean dive duration per day (± 1 SD) of 2 juvenile
elephant seals and 4 adult female elephant seals during the
first 12 days at sea following lactation. Data on adult
females from unpublished records.
four days, but acclimation to diving deeply was as rapid as that of adult females with much more diving experience (fig. 15.4). There was no correlation between dive duration and postdive surface interval (r = .01).
Discussion
During the 10-week period following weaning, young elephant seals undergo behavioral and physiological changes that prepare them to forage for several months at sea, a period characterized by virtually continuous, deep, and long duration diving. The diving performance of 3½-month-old elephant seals on the first trip to sea is similar to that of adults and exceeds that of most other adult pinnipeds, in terms of dive duration and dive depth (Kooyman 1989; Le Boeuf, this volume). Perhaps no other marine mammal must make such a drastic transition in such a short time.
We summarize and discuss the principal changes during this critical period of development that prepare the animal to be a deep and long duration diver. We emphasize that concomitant with improvements in swim-
ming and diving performance, there occur significant increases in oxygen storage capacity and decreases in diving metabolic rate.
Oxygen Storage Capacity
Blood is the most important storage site of oxygen in phocid seals, as it contains approximately 65% of the total oxygen stores, followed by the muscle (30%) and the lungs (5%) (Kooyman 1985, 1989). Blood oxygen storage capacity is determined by hemoglobin concentration and blood volume (Snyder 1983). Diving animals tend to have higher hemoglobin concentrations and blood volumes than terrestrial animals. Among marine mammals, the deeper and longer duration divers have the highest hemoglobin concentrations and blood volumes (Ridgway and Johnston 1966; Sleet, Sumich, and Weber 1981; Duffield, Ridgway, and Cornell 1983; Snyder 1983; Kooyman 1985, 1989), and as we would expect from adult diving performance, these values have been reported to be high for northern elephant seal pups (Simpson, Gilmartin, and Ridgway 1970; Castellini, Costa, and Huntley 1986; Hedrick, Duffield, and Cornell 1986; Wickham 1989). Changes in these values with development have received little attention. A. M. Kodama, R. Elsner, and N. Pace (1977) reported that mass specific blood and hemoglobin concentrations increased during the first year in the harbor seal, Phoca vitulina . M. M. Bryden and G. H. K. Lim (1972) reported that southern elephant seals, M. leonina , increased their mass specific blood volume during the postweaning fast and during the first trip to sea, an increment that is similar to what we observed in northern elephant seal pups (table 15.1). By the time juvenile elephant seals are ready to go to sea for the first time, their body is 14.4% blood, as compared to 21.2% blood for an adult female (P. Thorson, unpubl. data).
Total oxygen stores increase rapidly to a high level in developing elephant seals, due to increases in blood and muscle oxygen stores (table 15.1). When it is time to make the first trip to sea, these young juveniles have already amassed mass specific oxygen stores of 60.3 ml/kg, 73.5% of the mass specific oxygen stores recorded in adult females (82.1 ml/kg) (P. Thorson, unpubl. data). The oxygen stores in juvenile elephant seals are similar to those found in adult phocids of other species (Kooyman 1985, 1989).
Metabolic Rate
By 3 months of age, elephant seals had significantly lower metabolic rates and longer dive durations than they exhibited at weaning (figs. 15.1B, 15.2A). This is not surprising, as it is known that metabolic rate decreases with age (Brody 1945; Miller and Irving 1975; Ashwell-Erickson and Elsner 1981) and with increasing time spent fasting (Brody 1945; Kleiber 1975; Ashwell-Erickson and Elsner 1981; Worthy and Lavigne 1987; Rea and
Costa 1992). Age and fasting effects alone, however, may not be responsible for the metabolic decrease we observed. During the postweaning fast, juvenile elephant seals spent an increasing amount of time swimming and diving, in the process utilizing protein and redistributing muscle mass (fig. 15.1; Bryden 1969). Using protein increases the metabolic rate, while diving decreases it.
The oxygen consumption pattern of juveniles was less consistent early in the postweaning fast than later on. This suggests that developing seals gained increasing control over their metabolic rate as their mass specific oxygen stores and diving experience increased. In addition, metabolic rate was inversely correlated with dive duration and the total amount of time spent diving (fig. 15.2), an effect that was most pronounced late in the postweaning fast. M. A. Fedak (1986) reported a similar trend for gray seals, Halichoerus grypus , swimming and diving in a water flume.
Diving Behavior on the First Trip to Sea
The diving pattern of juveniles during the first part of their first trip to sea suggests that they were diving aerobically. Postdive surface intervals are brief regardless of the duration of the previous dive (table 15.2). The percentage of dives that exceeded the ADL for the two juveniles in this study and for two juvenile Weddell seals is shown in table 15.3. With a diving metabolic rate of 1.5 times resting (Kooyman et al. 1973, 1983), 30.5 to 46.9% of the dives of the two juvenile elephant seals exceeded the ADL. This stands in marked contrast to the performance of immature Weddell seals who exceeded the ADL on only 4% of their dives, despite a 28% weight advantage. Moreover, for the immature Weddell seals, lactic acid and postdive surface intervals increased after dives above 10 minutes (Kooyman et al. 1983).
If the swim velocities of juveniles are relatively low on long duration dives, as preliminary data indicate (P. Thorson, unpubl. data) and as is the case in adult females (Le Boeuf et al. 1992; Crocker et al., this volume), the diving metabolic rate should be relatively low. Metabolic rate is highly correlated with swim velocity (Davis, Williams, and Kooyman, 1985; Ponganis et al. 1990; Williams, Kooyman, and Croll 1991); therefore, a decrease in swim velocity would decrease the diving metabolic rate and the ADL would increase. A diving metabolic rate of 0.36 to 0.39 1/min is 0.8 times the predicted basal metabolic rate (BMR) and would include even the longest dives of each pup at sea (table 15.3). Because diving metabolic rate in the laboratory decreases with increasing dive duration to levels below the predicted BMR (fig. 15.2b), it is possible for a seal to have a diving metabolic rate near resting when swim speed is low. Direct measurements of diving metabolic rate of Weddell seals have been recorded at near resting levels (Kooyman 1973; Castellini, Kooyman, and Ponganis 1992).
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The depth of dives attained by juvenile elephant seals during the first 2 to 3 weeks at sea is remarkable in two respects. The dives are deeper than those of most other adult pinnipeds (see Le Boeuf et al. 1988, for review). Second, great depths are reached despite a body composition that averages 48% lipids, double the mean lipid composition of nonpregnant adult females and adult males (Kretzmann 1990; Rea and Costa 1992; unpubl. data). Because of the high ratio of fat to lean body mass, juveniles going to sea for the first time are positively buoyant. Thus, it would seem that juveniles would require greater effort to reach depths than adults.
In conclusion, the 10-week period following weaning that northern elephant seal juveniles spend swimming and diving near the natal rookery provides critical preparation for life at sea. Increases in blood volume, hematocrit, hemoglobin concentration, and myoglobin concentration result in large oxygen storage capacity, which, combined with an increased ability to decrease metabolic rate while diving, enables them to maximize time underwater for travel, foraging, rest, and predator avoidance.
Acknowledgments
We thank V. Kirby, M. Kretzmann, P. Morris, L. Starke, and E. Theiss for assistance in the laboratory and in the field. M. A. Castellini, D. P. Costa, G. L. Kooyman, P. J. Ponganis, and G. A. J. Worthy provided invaluable advice on many aspects of this project. We are indebted to Y. Naito for providing four of the mechanical time-depth recorders as well as for digitizing the record of seal G372. We thank G. Strachan and the rangers at Año
Nuevo State Reserve for their cooperation. This work was supported by grants from the Earl and Ethyl Myers Oceanographic and Marine Biology Trust, Friends of the Long Marine Laboratory, Biology and Marine Sciences boards of the University of California, Santa Cruz, the G. MacGowan estate, and the National Science Foundation. This work was conducted under federal permit number 496 issued by the National Marine Fisheries Service.
References
Ainley, D. G., C. S. Strong, H. P. Huber, T. J. Lewis, and S. H. Morrell. 1981. Predation by sharks on pinnipeds at the Farallon Islands. Fishery Bulletin 78: 941–945.
Antonelis, G. A., M. S. Lowry, D. P. De Master, and C. H. Ficus. 1987. Assessing northern elephant seal feeding habits by stomach lavage. Marine Mammal Science 3: 308–322.
Ashwell-Erickson, S., and R. Elsner. 1981. The energy cost of free existence for Bering Sea harbor and spotted seals. In The Eastern Bering Sea Shelf: Oceanography and Resources , ed. D. W. Hood and J. A. Calder, 869–899. Washington, D.C.: U.S. Department of Commerce.
Blackwell, S. B., and B. J. Le Boeuf. 1993. Developmental aspects of sleep apnoea in northern elephant seals. Journal of Zoology, London 231: 437–447.
Bonnell, M. L., B. J. Le Boeuf, M. O. Pierson, D. H. Dettman, and G. D. Farrens. 1979. Summary Report 1975–1978. Marine Mammal and Seabird Surveys of the Southern California Bight Area . III Pinnipeds . Bureau of Land Management, Department of the Interior, Contract AA550-CT7-36 to Principal Investigators: Kenneth S. Norris (Cetacea), Burney J. Le Boeuf (Pinnipeds), and George L. Hunt, Jr. (Birds).
Briggs, G. D., R. V. Hendrickson, and B. J. Le Boeuf. 1975. Ketamine immobilization of northern elephant seals. Journal of the American Veterinary Medicine Association 167: 546–548.
Brody, S. 1945. Bioenergetics and Growth with Special Reference to the Efficiency Complex in Domestic Animals . New York: Hafner Press.
Bryden, M. M. 1969. Relative growth of the major body components of the southern elephant seal, Mirounga leonina (L.). Australian Journal of Zoology 17: 153–177.
Bryden, M. M., and G. H. K. Lim. 1972. Blood parameters of the southern elephant seal (Mirounga angustirostris , Linn.) in relation to diving. Comparative Biochemistry and Physiology 28: 139–148.
Burger, A. E., and R. P. Wilson. 1988. Capillary-tube depth gauges for diving animals: An assessment of their accuracy and capability. Journal of Field Ornithology 59: 345–354.
Cairns, D. K., K. A. Bredin, V. L. Bert, and W. A. Montevecchi. 1987. Electronic activity recorders for aquatic wildlife. Journal of Wildlife Management 51: 395–399.
Castellini, M. A., D. P. Costa, and A. C. Huntley. 1986. Hematocrit variation during sleep apnea in elephant seal pups. American Journal of Physiology 251: R429–R431.
Castellini, M. A., G. L. Kooyman, and P. J. Ponganis. 1992. Metabolic rates of
freely diving Weddell seals: Correlations with oxygen stores, swim velocity, and diving duration. Journal of Experimental Biology 165: 181–194.
Condit, R., and B. J. Le Boeuf. 1984. Feeding habits and feeding grounds of the northern elephant seal. Journal of Mammalogy 65: 281–290.
Davis, R. W., T. M. Williams, and G. L. Kooyman. 1985. Swimming metabolism of yearling and adult harbor seals, Phoca vitulina. Physiological Zoology 58: 590–596.
DeLong, R. L., and B. S. Stewart. 1991. Diving patterns of northern elephant seal bulls. Marine Mammal Science 7: 369–384.
Dubowitz, V., and M. Brooke. 1973. Muscle Biopsy: A Modern Approach . Philadelphia: W. B. Saunders Co.
Duffield, D. A., S. H. Ridgway, and L. H. Cornell. 1983. Hematology distinguishes coastal and offshore forms of dolphins (Tursiops ). Canadian Journal of Zoology 61: 930–933.
Evans, W. J., S. D. Phinney, and V. R. Young. 1982. Suction applied to a muscle biopsy maximizes sample size. Medicine and Science in Sports and Exercise 14: 101–102.
Fedak, M. A. 1986. Diving and exercise in seals: A benthic perspective. In Diving in Animals and Man , ed. A. Brubakk, J. W. Kanwisher, and G. Sundnes, 11–32. Tronheim: Tapir.
Fedak, M. A., L. Rome, and H. J. Seeherman. 1981. One-step-N2 dilution technique for calibrating open-circuit VO2 measuring systems. Journal of Applied Physiology 51: 772–776.
Geraci, J. R., and T. G. Smith. 1975. Functional hematology of ringed seals (Phoca hispida ) in the Canadian arctic. Journal of the Fisheries Research Board of Canada 32: 2559–2564.
Hedrick, M. S., D. A. Duffield, and L. H. Cornell. 1986. Blood viscosity and optimal hematocrit in a deep-diving mammal, the northern elephant seal (Mirounga angustirostris ). Canadian Journal of Zoology 64: 2081–2085.
Hindell, M. A., D. J. Slip, and H. R. Burton. 1991. The diving behaviour of adult male and female southern elephant seals, Mirounga leonina (Pinnipedia: Phocidae). Australian Journal of Zoology 39: 595–619.
Kleiber, M. 1975. The Fire of Life: An Introduction to Animal Bioenergetics . Huntington, N. Y.: Robert E. Kreiger Publishing.
Kodama, A. M., R. Elsner, and N. Pace. 1977. Effects of growth, diving history, and high altitude on blood oxygen capacity in harbor seals. Journal of Applied Physiology 42: 852–858.
Kooyman, G. L. 1973. Respiratory adaptation in marine mammals. American Zoologist 13: 457–178.
———. 1985. Physiology without restraint in diving mammals. Marine Mammal Science 1: 166–178.
———. 1989. Diverse Divers: Physiology and Behavior . Berlin: Springer Verlag.
Kooyman, G. L., M. A. Castellini, R. W. Davis, and R. A. Maue. 1983. Aerobic diving limits of immature Weddell seals. Journal of Comparative Physiology 151: 171–174.
Kooyman, G. L., D. H. Karem, W. B. Campbell, and J. J. Wright. 1973. Pulmonary gas exchange in freely diving Weddell seals, Leptonychotes weddelli. Respiratory Physiology 17: 283–290.
Kooyman, G. L., E. A. Wahrenbrock, M. A. Castellini, R. W. Davis, and E. E. Sin-
nett. 1980. Aerobic and anaerobic metabolism during voluntary diving in Weddell seals: Evidence of preferred pathways from blood chemistry and behavior. Journal of Comparative Physiology 138: 335–346.
Kretzmann, M. B. 1990. Maternal investment and the post-weaning fast in northern elephant seals: Evidence for sexual equality. Master's thesis, University of California, Santa Cruz.
Lane, R. A. B., R. J. H. Morris, and J. W. Sheedy. 1972. A haematological study of the southern elephant seal, Mirounga leonina (Linn.). Comparative Biochemistry and Physiology 42A: 841–850.
Le Boeuf, B. J., D. P. Costa, A. C. Huntley, and S. D. Feldkamp. 1988. Continuous, deep diving in female northern elephant seals, Mirounga angustirostris. Canadian Journal of Zoology 66: 446–458.
Le Boeuf, B. J., D. P. Costa, A. C. Huntley, G. L. Kooyman, and R. W. Davis. 1986. Pattern and depth of dives in northern elephant seals, Mirounga angustirostris. Journal of Zoology, London 208: 1–7.
Le Boeuf, B. J., Y. Naito, T. Asaga, D. Crocker, and D. P. Costa. 1992. Swim speed in a female elephant seal: Metabolic and foraging implications. Canadian Journal of Zoology 70: 786–795.
Le Boeuf, B. J., Y. Naito, A. C. Huntley, and T. Asaga. 1989. Prolonged, continuous, deep diving by northern elephant seals. Canadian Journal of Zoology 67: 2514–2519.
Le Boeuf, B. J., M. Riedman, and R. S. Keyes. 1982. White shark predation on pinnipeds in California waters. Fishery Bulletin 80: 891–895.
Le Boeuf, B. J., R. J. Whiting, and R. F. Gantt. 1972. Perinatal behaviour of northern elephant seal females and their young. Behaviour 43: 121–156.
Lenfant, C., K. Johansen, and J. D. Torrance. 1970. Gas transport and oxygen storage capacity in some pinnipeds and the sea otter. Respiratory Physiology 9: 277–286.
Linden, R. J., and D. A. S. G. Mary. 1983. The measurement of blood volume. Techniques in the Life Sciences: Cardiovascular Physiology P305: 25.
Miller, K., and L. Irving. 1975. Metabolism and temperature regulation in young harbor seals, Phoca vitulina richardsi in water. American Journal of Physiology 229: 506–511.
Naito Y., T. Asaga, and Y. Ohyama. 1990. Diving behavior of Adélie penguins determined by time-depth recorder. Condor 92: 582–586.
Pernia, S., A. Hill, and C. L. Ortiz. 1980. Urea turnover during prolonged fasting in the northern elephant seal. Comparative Biochemistry and Physiology 65B: 731–734.
Ponganis, P. J., G. L. Kooyman, M. H. Zornow, M. A. Castellini, and D. A. Croll. 1990. Cardiac output and stroke volume in swimming harbor seals. Journal of Comparative Physiology 160B: 473–482.
Rasa, O. A. 1971. Social interaction and object manipulation in weaned pups of the northern elephant seal Mirounga angustirostris. Zeitschrift für Tierpsychologie 29: 82–102.
Rea, L. D., and D. P. Costa. 1992. Changes in standard metabolism during long-term fasting in northern elephant seal pups (Mirounga angustirostris). Physiological Zoology 65: 97–111.
Reiter, J., N. L. Stinson, and B. J. Le Boeuf. 1978. Northern elephant seal development: The transition from weaning to nutritional independence. Behavioral Ecology and Sociobiology 3: 337–367.
Reynafarje, B. 1963. Simplified method for the determination of myoglobin. Journal of Laboratory and Clinical Medicine 61: 139–145.
Ridgway, S. H., and D. G. Johnston. 1966. Blood oxygen and ecology of porpoises. Science 151: 456–457.
Simpson, J. G., W. G. Gilmartin, and S. H. Ridgway. 1970. Blood volume and other hematological values in young elephant seals (Mirounga angustirostris ). American Journal of Veterinary Research 31: 1449–1452.
Sleet, R. B., J. L. Sumich, and L. J. Weber. 1981. Estimates of total blood volume and total body weight of a sperm whale (Physeter catodon ). Canadian Journal of Zoology 59: 567–570.
Snyder, G. K. 1983. Respiratory adaptations in diving mammals. Respiratory Physiology 54: 269–294.
Wickham, L. L. 1989. Blood viscosity in phocid seals: Possible adaptations to diving. Journal of Comparative Physiology 159B: 153–158.
Williams, T. M. 1987. Approaches for the study of exercise physiology and hydrodynamics in marine mammals. In Approaches to Marine Mammal Energetics , ed. A. C. Huntley, D. P. Costa, G. A. J. Worthy, and M. A. Castellini, 127–145. Lawrence, Kan.: Allen Press.
Williams, T. M., G. L. Kooyman, and D. A. Croll. 1991. The effect of submergence on heart rate and oxygen consumption of swimming seals and sea lions. Journal of Comparative Physiology 160B: 637–644.
Withers, P. C. 1977. Measurement of VO2 , VCO2 , and evaporative water loss with a flow-through mask. Journal of Applied Physiology 42: 120–123.
Worthy, G. A. J., and D. M. Lavigne. 1987. Mass loss, metabolic rate, and energy utilization by harp and gray seal pups during the postweaning fast. Physiological Zoology 60: 352–364.