PART III—
ANIMALS
7—
Common Insects and Other Arthropods
John Smiley and Derham Giuliani
Insects, members of the Phylum Arthropoda (arthropods, or "jointed-legged" creatures), are small animals that usually have six legs and whose adult forms usually bear wings. They are the most varied group of organisms on Earth; nearly a million species have been described. Insects are primarily terrestrial, although many inhabit freshwater streams, ponds, and lakes. In the oceans, insects are replaced by their relatives, the crustaceans. Other arthropods common in terrestrial habitats are the spiders, mites, ticks, and scorpions (collectively known as arachnids); a few species of terrestrial crustaceans, such as pillbugs and sowbugs; and the centipedes and millipedes. This chapter emphasizes species of insects but also lists and illustrates other types of arthropods commonly encountered in the vicinity of the White Mountains.
The small size and great diversity of arthropods make determination of species difficult; therefore, it is usually a job for experts. However, recognition of major groups is not difficult, and one can obtain as much satisfaction from recognizing a particular family or order as from identifying an interesting bird species. For example, identifying an insect family provides information about the intricate (and commonly fascinating) life cycle of the insect in question, its relations with its environment, and its interactions with other species of plants and animals. In our discussions of major groups, we encapsulate some of the more interesting facets of arthropod life history along with species distributions and aids to identification. Names are given for some of the more common, large species, most of which are illustrated as well. Our selection is necessarily somewhat arbitrary, because we have neither the space nor the collected material to systematically list species or even genera. Useful books to aid in insect identification are California Insects by J. A. Powell and C. H. Hogue, the Peterson Field Grade to the Study of Insects by D. J. Borror and C. L. White, and Introduction to the Study of Insects by D. J. Borror, D. M. Delong, and C. A. Triplehorn.
Most terrestrial arthropods are small, but adult individuals range considerably in size, depending on the amount of food available during the juvenile stages. For this reason, and because we wish to emphasize major groups rather than species, we do not report sizes for most species. Instead, we indicate size in general terms. "Large" generally refers to insects over 0.4 in (10 mm) in length, and "small" refers to insects under 0.2 in (4 mm) long.
Most of the species discussed in this chapter occur along the road from Big Pine to Westgard Pass, or from there up the road to the Bristlecone Pine forest and
the Barcroft facilities of the White Mountain Research Station (WMRS). Many of the species occur in the vicinity of the Crooked Creek facilities (WMRS), at 10,150 ft (3,094 m), Barcroft Laboratory, at 12,470 ft (3,801 m), and White Mountain Peak, at 14,250 ft (4,343 m). Most species listed as occurring at low elevations are present in the vicinity of the Owens Valley facilities (WMRS), 3 mi (5 km) east of Bishop, California.
Spiders, Mites, Ticks, Scorpions, and Others:
(Class Arachnida)
Arthropods of the Class Arachnida generally bear eight legs, and the head and thorax are usually fused into a single body section, the cephalothorax. The arachnids generally prey on other arthropods (e.g., spiders), but some are herbivorous (e.g., Spider Mites) and others are parasitic (e.g., ticks). Still other types feed on decaying matter.
Scorpion (Order Scorpiones)
Scorpions (Fig. 7.1) are common at lower elevations and are generally nocturnal. These predaceous arthropods have a stinger at the tip of the tail-like abdomen and should be handled with care. Smaller forms are common around houses and woodpiles.
Spiders (Order Araneae)
The spiders are a diverse group of predatory arthropods. In many habitats they are extremely abundant and consume vast numbers of tiny insects and other small prey. They are also a favorite food of many birds and other insectivorous vertebrates.
Tarantulas (Family Theraphosidae). (Fig. 7.1) Large spiders with very hairy bodies. Although the Tarantula can bite, North American species are relatively harmless. Tarantulas are nocturnal and serve as prey for several large spider-killing wasps, including those of the genus Pepsis . During the day they hide in silk-lined burrows in the ground.
Black Widow Spider,Latrodectus hesperus(Family Theridiidae). (Fig. 7.1) Produces tangled webs made of exceptionally strong, thick silk, commonly occur in buildings and sheds. The female is black with an hourglass-shaped red patch on the underside. The venom is very toxic, and bites should be treated medically. Black Widows are known to catch their prey by "spitting" strands of silk, entangling it.
Orb-weaving Spiders (Family Araneidae). (Fig. 7.1) A large family of spiders that weave the familiar circular "orb" web, which is used to catch flying insect prey.
Wolf Spiders,Lycosaspp. (Family Lycosidae). (Fig 7.1) Terrestrial hunters, often abroad by day. The females of these hairy brown spiders are commonly seen carrying a large, spherical egg case or newly hatched young on their back. Small Wolf Spiders
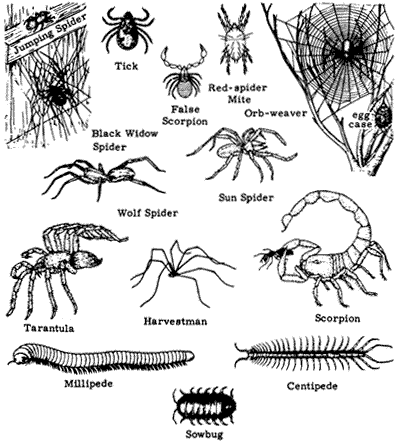
Figure 7.1
Non-insect arthropods: arachnids, sowbugs, centipedes, and millipedes.
may be extremely abundant in grasslands and meadows up to 14,000 ft (4,300 m) elevation.
Jumping Spiders (Family Salticidae). (Fig. 7.1) An easily recognized family of spiders with very short legs, which they use to rapidly run and jump in search of their prey. Diurnal, they have large eyes and hunt their prey visually, jumping on it and delivering a paralyzing bite. Some species are brightly colored with red, metallic green, or blue.
Harvestmen (Order Opiliones)
Harvestmen have the entire body fused into a circular or oval shape and commonly have very long legs. Most species are predaceous.
Daddy Longlegs (Suborder Palpatores). (Fig. 7.1) Some Daddy Longlegs huddle together in masses of hundreds in cool, damp spots, their legs resembling masses of brown hair.
Mites and Ticks (Order Acarina)
Mites and ticks are usually small, with very short legs as compared with spiders. They have sucking mouthparts.
Ticks (Families Ixodidae and Argasidae). (Fig. 7.1) Ticks are blood-sucking parasites of vertebrates, to which they attach themselves and feed until they are engorged. In large numbers they may cause temporary paralysis until removed. Tick bites can become infected, particularly when a feeding tick is removed carelessly, its head broken off and left in the wound. Ticks also serve as vectors for disease.
Red-spider Mites (Family Tetranychidae). (Fig. 7.1) An important group of extremely tiny arthropods (less than 0.04 in or 1.0 mm) that are herbivorous, causing severe economic damage to certain crops and cultivated plants, particularly indoors. They are slow-moving and sedentary. Other mites, larger and more active, are predaceous and commonly seen at all elevations on the ground and in foliage. They are usually red or orange.
False Scorpions, or Pseudoscorpions (Order Pseudoscorpiones)
False Scorpions (Fig. 7.1) are small arthropods that lack the tail-like stinger of true scorpions but possess a pair of pincers and otherwise look very much like true scorpions. These animals overwinter under stones and logs, in a silken case.
Wind Scorpions (Order Solifugidae)
Wind Scorpions are a group of large, conspicuous athropods. They are fast-running predators chiefly active at night.
Sun Spiders. (Fig. 7.1) Yellow-brown arachnids that look like large spiders except that the abdomen is segmented, like that of a scorpion, and the head bears large, foreward-facing jaws. They do not produce venom.
Isopods:
(Class Crustacea)
The Class Crustacea is an extremely diverse group of arthropods found primarily in aquatic or marine habitats, including such forms as shrimp, crabs, lobsters, and barnacles. Only one group, the isopods, have invaded terrestrial habitats.
Sowbugs (Order Isopoda). (Fig. 7.1) The Sowbug is a widespread, extremely successful crustacean. Sowbugs have a gray-black segmented carapace, have seven
pairs of legs, and are commonly seen around habitations or where the ground is watered. Some are capable of rolling up into a ball and are known as pillbugs.
Millipedes:
(Class Diplopoda)
Millipedes are long, wormlike arthropods with two pairs of legs on each body segment and commonly 60–100 legs overall. Usually occurring in damp places, millipedes are generally scavengers and do not bite.
Cylindrical Millipedes (Order Opisthospermophora). Cylindrical millipedes (Fig. 7.1) are relatively large and have a hard shell. They occur in leaf litter and on the ground.
Centipedes:
(Class Chilopoda)
Like millipedes, centipedes are long and slender and composed of many segments. Unlike millipedes, centipedes bear only one pair of legs per segment and are predaceous, actively running and seeking out prey. Larger individuals are capable of delivering a painful bite.
Centipedes (Several orders). Centipedes (Fig. 7.1) range from small to very large (up to 6 in long) and are capable of biting or pinching with both their mandibles and their tail appendage. The venom is generally not toxic in North American species.
Insects:
(Class Insecta)
Insects have an inelastic, external skeleton that must be shed as the animal grows. After hatching from the egg, a typical insect undergoes a fixed number of moults until it reaches adulthood; the stages between moults are called instars. When the insect reaches the adult stage, it may develop wings; this change is called metamorphosis. Juvenile insects never have wings. Adult insects are capable of reproduction but not of growth since no further moults are possible. Thus, a small insect with wings is a fully formed adult and will grow no further, whereas a large insect without wings may be a juvenile or, in some cases, a wingless adult. In insects with incomplete metamorphosis, the juveniles resemble the adults and are usually called nymphs. In species with complete metamorphosis, the juveniles differ greatly from the adult and are usually called larvae (singular: larva). Figure 7.2 illustrates these differences.
Because they are small, insects cannot store large amounts of water, food, or heat in their bodies. Under ideal growth conditions insects grow and multiply rapidly, but they must pass seasons of cold or drought by hiding in a state of dormancy, or
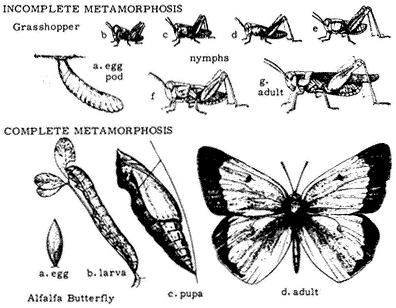
Figure 7.2
Complete and incomplete metamorphosis.
diapause. In the White Mountains region, most insects are active in the springtime and dormant during the dry summers and the cold winters. Other insects are active in the summer and fall, and a few species are adapted to cold conditions, active on melting snow or under the snow during the winter months. Owing to differences in the regimes of temperature and moisture at different elevations, the period of spring activity begins in March or April in the Owens Valley, but this period does not begin at Barcroft or White Mountain Peak until July or August. There "spring" commonly lasts until the first snows in September or October.
Figure 7.3 illustrates the external anatomy of a typical insect, a grasshopper. An insect has a head, thorax, and abdomen. The head contains the eyes, which may be simple or compound (i.e., composed of hundreds of simple eyes). Also on the head is a single pair of segmented antennae. They are primarily used for detecting odors, although some species with long antennae may use them as "feelers" for detecting objects or vibrations. The head also bears the mouthparts, including mandibles (jaws), maxillae, and various jointed palps for manipulating food. During the course of evolution, insect mouthparts have taken many different forms, including piercing "syringes," beaks, and coiled tubes. Each group of insects has characteristic mouthparts that are very useful in identification.
The thorax consists of three segments: the prothorax, mesothorax, and metathorax from front to back, each of which bears a pair of legs. The top of each segment is protected by a hardened plate. In addition to legs, the meso- and metathorax each bear a pair of wings in most adult insects. The anterior wing is called the
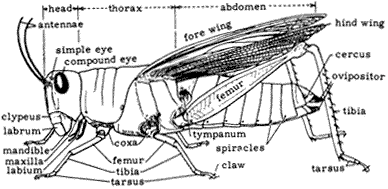
Figure 7.3
External anatomy of a grasshopper.
forewing, and the posterior the hindwing. The wings of insects are membranous, with a framework of rigid veins. The arrangement of these veins is very important in insect identification.
A typical insect leg has four visible jointed segments: the coxa, femur, tibia, and tarsus. The coxa is usually rounded and attached to the body. The femur usually extends out from the body and is the first long segment. The tibia usually extends downward from the femur, nearly touching the substrate. The tarsus functions like a foot, with claws, hairs, and pads for clinging to surfaces. The tarsus is itself composed of several segments, the number of which is useful in identification.
The abdomen of insects usually has 6 to 10 visible segments. The upper (dorsal) and lower (ventral) surfaces of each segment may bear hardened plates, but in some species the abdomen is soft. The posterior end of the abdomen bears the anal opening, appendages for copulation, and, in female insects, the ovipositor for laying eggs. In a few insects, such as grasshoppers, roaches, and related groups, a pair of sense organs are borne on the upper surface of the posterior end of the abdomen. These are called cerci (singular: cercus).
The Class Insecta is divided into approximately 20 orders and 750 families, 500 of which occur in North America. In this natural history guide we introduce most of these orders and a few of the most easily distinguished families occurring in the White Mountains. Because there may be as many as 5,000 to 10,000 insect species present in the region, we can only present a sampling of the most common and interesting species. The only large group that is nearly completely represented here is that of the butterflies, for which identification may be accomplished by reference to the color plates.
Dragonflies and Damselflies (Order Odonata)
Members of the Order Odonata are easily recognized in the adult form. Species that hold their wings extended laterally when resting are known as Dragonflies (Suborder Anisoptera), and species that hold their wings pressed together dorsally at rest are
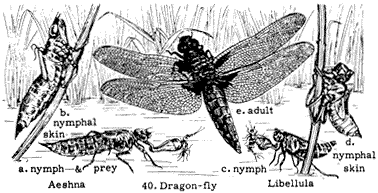
Figure 7.4
Life stages of a dragonfly.
known as Damselflies (Suborder Zygoptera). These insects are predators, capturing other insects in flight with their basketlike legs. Individuals copulate in flight and frequently are seen flying in tandem, with the male clasping the female behind the head using appendages at the tip of his abdomen. The female may also lay eggs in the water while in tandem with the male. The nymphs live in fresh water, where they are predators of small animals. Adults are common on the floor of the Owens Valley and have been recorded as high as White Mountain Peak, at over 14,000 ft (4,300 m). Figure 7.4 illustrates the life stages of dragonflies.
Green Jacket Dragonfly,Erythemis simplicicollis(Family Libellulidae). Occurs from the Owens Valley floor to Crooked Creek (10,000 ft, or 3,100 m). The large, green adults fly in July and August, commonly at some distance from water.
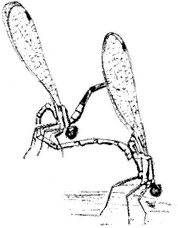
Figure 7.5
Dancer Damselfly (Argia sp.).
Dancers,Argiaspp. (Family Coenagrionidae). (Fig. 7.5) Damselfly genus occurs from the Owens Valley Floor to Crooked Creek (10,000 ft, or 3,100 m), usually near water.
Termites (Order Isoptera)
Termites are highly social insects. Some species can be very destructive due to their feeding activities on wooden structures. They are soft-bodied, are whitish in color, and live in large colonies with a well-defined caste system. Their gut contains diverse symbiotic microorganisms that aid in the digestion of wood.
Dampwood Termites,Zootermopsisspp. (Family Hodotermitidae). (Fig. 7.6) Occupy dead logs and stumps, especially places where wood is damp. Another termite, the Subterranean Termite (Reticulotermes spp.), does not occur at relatively high elevations in the White Mountains but is present in the Owens Valley, where it can damage structural wood in contact with the soil.
Grasshoppers, Roaches, Mantids, and Related Groups (Order Orthoptera)
The Order Orthoptera is a highly diverse group, including many large, common species. The crickets, katydids (Family Tettigoniidae), and grasshoppers (Family Acrididae) are primarily herbivorous and have enlarged, powerful hind legs that enable them to jump (see Fig. 7.3). Many grasshoppers take flight during their jump and display brightly colored wings, which carry them up to 100 ft (30 m). The roaches, which have long antennae and long abdominal cerci, are primarily nocturnal scavengers. The phasmids, or walking sticks, are herbivorous. The mantids are highly
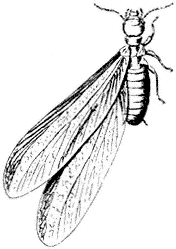
Figure 7.6
Dampwood Termite
(Zootermopsis sp.).
predaceous, with forelegs modified into striking claws that can grab prey with lightning speed.
Most adult orthopterans are winged, including roaches, but some species of montane grasshoppers and crickets are flightless. Crickets, katydids, and grasshoppers commonly possess sound-producing organs on their legs and wings; in most species the males produce trilling, buzzing, or clicking sounds that attract females for mating. Grasshoppers are common in open, dry habitats and are well-represented in the White Mountain region. Here is also present a flightless species (Agnostokasia sublima ) that occurs nowhere else.
American Cockroach,Periplaneta americana(Family Blattidae). (Fig. 7.7) A house and yard pest species that occurs at lower elevations in the Owens Valley. They apparently do not reach Crooked Creek, at 10,000 ft (3,100 m).
Short-horned Grasshoppers (Family Acrididae). Short-horned Grasshoppers may be recognized by their antennae, which are much shorter than the body. Many species have brightly colored wings, visible in flight.
Migratory Grasshopper, Melanoplus sanguinipes . (Plate 7.1) Commonly present in swarms between 11,000 and 13,000 ft (3,300 and 4,000 m). At lower elevations it rarely occurs in large numbers but can be a devastating crop pest when it does.
Pallid-winged Grasshopper, Trimerotropis pallidipennis . (Plate 7.1) Occurs in the Owens Valley at elevations as high as 5,000 ft (1,500 m).
Sierran Blue-winged Grasshopper, Circotettix thalassinus . Common at middle elevations (6,000–9,000 ft, 1,800–2,700 m), where males display by flying up and making loud popping or crackling noises.
Agnostokasia sublima . (Plate 7.1) Known only from 12,000 to 13,000 ft (3,600 to 4,000 m) in the White Mountains, including Barcroft area. A flightless species.
Mormon Cricket,Anabrus simplex(Family Tettigoniidae). (Plate 7.1) Occurring near Crooked Creek and near Barcroft, 10,000–12,000 ft (3,000–3,600 m), always associated with sagebrush (Artemisia ). Flightless, the males call in late morning with a loud stridulation. This species is a member of a subfamily of crickets (the Decticinae)

Figure 7.7
American Cockroach (Periplaneta americana ).
that is very diverse in the Owens Valley region. Known as the Shield-backed Katydids, Decticinae are commonly flightless, especially the females.
Camel Cricket,Ceuthophilus lamellipes (Family Gryllacrididae). (Fig. 7.8) Occurs from the Owens Valley to elevations of 11,000 ft (3,400 m), usually present under stones and logs.
California Mantid,Stagmomantis californica(Family Mantidae). (Fig. 7.9) Occurs in Owens Valley up to higher elevations. Mantids have been collected at Crooked Creek, at 10,150 ft (3,100 m), but not at higher sites.
True Bugs (Order Hemiptera)
Members of the Order Hemiptera, or true bugs, possess a slender, segmented beak, arising from the anterior tip ("forehead") of the insect, and front wings divided into two parts, with the basal portion thick and leathery and the rest transparent. The antennae are fairly long and consist of four or five segments. Many hemipterans have scent glands, which give off an acrid scent when the insect is disturbed. Juvenile hemipterans look like the adults, except they lack wings. Juveniles eat the same food as the adults and occur in the same habitats. Most species feed on plant juices, and some are considered serious pests. Other hemipterans are considered beneficial to man because they attack harmful insects. Still other hemipterans suck blood, and a few of these insects act as vectors for disease. Some hemipterans resemble ants in appearance and mode of walking, presumably to deceive their predators.
Say's Stink Bug,Chlorochroa sayi (Family Pentatomidae). (Fig. 7.10) Green bugs widespread and common in the White Mountains from 4,000–14,000 ft (1,200 to 4,300 m). They can be found feeding on a variety of plants from spring to fall.
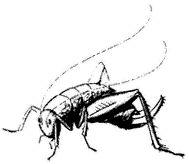
Figure 7.8
Camel Cricket (Ceuthophilus
lamellipes ).
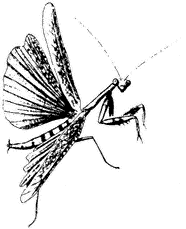
Figure 7.9
California Mantid (Stagmomantis
californica ).
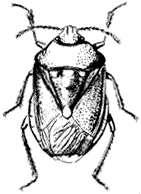
Figure 7.10
Say's Stink Bug
(Chlorochroa sayi ).
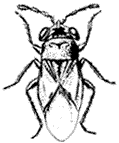
Figure 7.11
Big-eyed Bug
(Geocoris bullatus ).
Big-eyed Bug,Geocoris bullatus(Family Lygaeidae). (Fig. 7.11) Abundant, very small insect occurring in the Mount Barcroft area under low vegetation, where it probably eats seeds and soft-bodied insects.
Alydus pluto(Family Alydidae). (Plate 7.1) The bright red-orange abdomen shows in flight but is covered by dark wings when the insect is at rest. Common at middle elevations (6,000–10,000 ft; 1,800–3,100 m). Nymphs resemble ants and feed on plants.
Common Milkweed Bug,Lygaeus kalmii (Family Lygaeidae). (Plate 7.1) A common member of the Seed Bug Family that occurs at elevations as high as 11,000 ft (3,400 m) in the White Mountains. Red with black markings, this bug prefers milkweed seeds for its food but will also feed on a wide range of other plants.
Cicadas, Leafhoppers, Aphids, Scale Insects, and Others (Order Homoptera)
The large and diverse Order Homoptera is related to the Order Hemiptera. Homopterans exhibit a large variation in body structure, but the most distinguishing characteristic is the beak, which is located near the insect's neck (on the "chin"). The antennae are very short and bristlelike, and the compound eyes are usually large. Winged homopterans usually have four membranous wings, which at rest are usually held rooflike over the body.
All homopterans feed on plant sap, and many species are serious pests of cultivated plants. A few homopteran species are beneficial, used to make shellac, dyes, and other materials.
Giant Willow Aphid,Tuberolachnus salignus(Family Aphididae). (Fig. 7.12) An aphid common on willows (Salix spp.) in the region, feeding in large colonies on the trunks and branches. Also known as Plant Lice, members of the Aphid Family
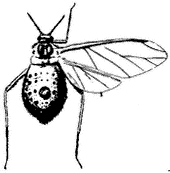
Figure 7.12
Grant Willow Aphid
(Tuberolachnus salignus ).
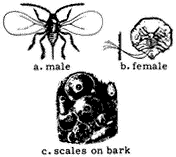
Figure 7.13
Scale insect.
are sedentary insects that feed on plant sap. Many aphids are "tended" by ants, which obtain sugary "honeydew" excretion in return for protection from enemies such a Ladybird Beetles and maggots (larvae) of Syrphid Flies. Adult aphids may be winged sexual reproductives or wingless females that reproduce parthenogenically (i.e., without mating). Aphids may occur at all elevations in the White Mountains.
Scale insects (Family Coccidae). (Fig. 7.13) The body of a scale insects is hidden under a waxy shell that resembles fish Scales attached to the bark of trees and shrubs. Some species secrete honeydew and are tended by ants. They occur in the Owens Valley, and probably on trees and shrubs at elevations below 12,000 ft (3,700 m).
Leafhoppers (Family Cicadellidae). (Fig. 7.14) Occurring at all elevations in the White Mountains, Leafhoppers are highly active homopterans that live on twigs of trees, shrubs, and herbs. Usually cylindrical and bullet-shaped, many Leafhoppers have the habit of quickly moving to the opposite side of the twig when approached so as to be shielded from view.
Cicadas (Family Cicadidae). (Fig. 7.15) Occur at elevations below 12,000 ft (3,700 m). Usually heard but not seen, most male cicadas produce a loud, musical buzz or whine that is easily distinguished from the trills and intermittent buzzes/clicks of most orthopterans. Cicadas are large-bodied, and the juveniles feed underground on

Figure 7.14
Leafhopper.

Figure 7.15
Cicada.
plant roots for two to five years. One species, Okanagana cruentifera , is locally very abundant in sagebrush (Artemisia ).
Beetles (Order Coleoptera)
The Order Coleoptera contains about 40% of all known insect species. Beetles occur in virtually every habitat except the ocean. The wings are the most distinguishing characteristic of Coleoptera. The hard, shell-like front pair, called elytra, are used mainly for protection. The second pair of wings, membranous and longer than the elytra, are used for flying and are folded under the elytra when not in use. Beetles have chewing mouthparts and undergo complete metamorphosis. Overwintering can occur at any stage of development, depending on the species. The larvae vary considerably in form among different families. Beetles and beetle larvae are known to feed on all types of plant and animal materials. Many beetles are considered valuable because they destroy injurious insects or act as scavengers.
Tiger Beetles,Cicindelaspp. (Family Cicindelidae). (Fig. 7.16) Very active, predaceous beetles commonly seen at elevations up to 12,000 ft (3,700 m). They may be metallic green, blue, or plain brown, but their colors are difficult to see because they move so rapidly. Two species, C. plutonica and C. montana, are common at high elevations (11,000–12,000 ft, 3,300–3,700 m).
Ground Beetles,Pterostichusspp. (Family Carabidae). (Fig. 7.17) Occurs near 9,000 ft (2,700 m) in July. Ground Beetles are common insects that forage for prey mainly on the ground. Species are often similar in shape but may differ greatly in size. They are usually black or brown and have fine "lines" on the wing covers parallel to the margins. There are very many species. Another genus that occurs in the White Mountains is Callisthenes, near 10,000 ft (3,100 m) in July and August.
River Beetle,Agabus lutosus(Family Dytiscidae). (Fig. 7.18) A water beetle that commonly occurs in small ponds at elevations up to 12,500 ft (3,800 m). Both the larvae and the adults are predaceous.

Figure 7.16
Tiger Beetle (Cicindela sp.).

Figure 7.17
Ground Beetle
(Pterostichus sp.).

Figure 7.18
River Beetle
(Agabus lutosus ).

Figure 7.19
Serica sp.
Burying Beetle,Nicrophorus hecate (Family Silphidae). (Plate 7.1) Two varieties — one black, the other red and black — are found on carrion from 4,000 to 10,000 ft (1,200 to 3,100 m), from spring to fall. These beetles bury small carrion, remove the hair, and feed and lay eggs in an underground chamber.
Sericaspp. (Family Scarabidae). (Fig. 7.19) Occurs near 8,000 ft (2,400 m) elevation in summer, commonly on trees and shrubs.
Twig Girdler,Agrilus walshinghami (Family Buprestidae). (Fig. 7.20) Occurs from 4,000 to 6,000 ft (1,200–1,800 m) in August and September on flowers of Rabbit Brush Chrysothamnus nauseosus .
Cactus Flower Beetles,Carpophilusspp. (Family Nitulidae). (Fig. 7.21) Occur in cactus flowers at lower elevations (4,000–9,000 ft, 1,200–2,700 m) in spring.
Common Checkered Beetle,Trichodes ornatus(Family Cleridae). (Fig. 7.22) Commonly occurs on flowers, including cactus flowers, and probably occurs up to 10,000 ft (3,100 m).
Blister Beetles (Family Meloidae). A family of beetles common and diverse in the low-elevation areas of the Owens Valley. Many are brightly colored. Eggs are laid on
Figure 7.20
Twig Girdler
(Agrilus
walshinghami ).

Figure 7.21
Cactus Flower Beetle
(Carpophilus sp.).

Figure 7.22
Common Checkered
Beetle (Trichodes
ornatus ).
the ground or on flowers, and the first instar larvae actively seek out host insects such as bees or grasshopper eggs, feeding on the host eggs and larvae.
Meloe spp. May occur up to 12,000 ft (3,700 m). The larvae are parasites of bees.
Epicauta oregona . A small and grey species with black spots that occurs in the Owens Valley. Its larvae probably feed on grasshopper eggs.
Soldier Blister Beetle, Tegrodera latecincta . (Plate 7.1) A large meloid occurring in the Owens Valley and up into the foothills to 4,500 ft (1,400 m). This and the following two species may be seen feeding on flowers in summer.
Pleurospasta mirabilis . (Plate 7.1) Occurs below 5,000 ft (1,500 m) in the Owens Valley.
Phodaga alticeps . (Plate 7.1) Occurs below 5,000 ft (1,500 m) in the Owens Valley.
Armored Stink Beetle,Eleodes armatus (Family Tenebrionidae). (Plate 7.1) A large, black beetle that occurs commonly during all seasons from 4,000 to 7,000 ft (1,200 to 2,100 m), where it is usually found walking on open ground. When disturbed, it raises the tip of its abdomen into the air, sometimes emitting a foul-smelling vapor. These beetles superficially resemble Ground Beetles (Family Carabidae), but their behavior and the lack of prominent jaws are usually sufficient to distinguish the two families. Other common species include Eleodes obscura, which occurs from 5,000 to 8,000 ft (1,500 to 2,400 m), spring to fall; Eleodes pilosa, at 10,000 ft (3,100 m) in July; and Eleodes (Blapylus) species, which occur from 10,000 to 13,000 ft (3,100 to 4,000 m) in summer and fall.
Convergent Ladybird Beetle,Hippodamia convergens(Family Coccinellidae). (Plate 7.1) A familiar beetle occurring at all elevations. Thousands of "ladybugs" may locally occur clustered in watered canyons or on mountain peaks, especially in fall and winter. This and the following species are important predators of other insects, especially homopterans.
Neomysiaspp. (Family Coccinellidae). Occurring from 6,000 to 9,000 ft (1,800 to 2,700 m), pale, faintly striped species montane in distribution and common on Pinyon Pine.
Leaf Beetles,Alticaspp. (Family Chrysomelidae). Small (0.1 in, 3mm) metallic blue or purple beetles common at higher elevations (10,000–12,000 ft, 3,100–3,700 m). Members of this abundant, diverse family are commonly green, blue, or black and resemble the ladybird beetles in shape. They are commonly very small and feed primarily on the leaves of plants. Also common are the Trirhabda species, whose irridescent green larvae feed on sagebrush.
Long-horn Beetles (Family Cerambycidae). Adult beetles of the Family Cerambycidae have thick, long antennae and so are collectively known as "long-horn beetles." Larvae of most species bore into wood.
Figure 7.23
Banded Alder Borer
(Rosalia funebris ).
Banded Alder Borer, Rosalia funebris . (Fig. 7.23) A large, conspicuous beetle that occurs along Owens River, where its larvae bore into alder, willow, and other hardwood trees.
Crossidius hertipes nubilis . (Plate 7.1) Occurs from 7,000 to 8,000 ft (2,100 to 2,400 m), where it is commonly seen feeding on flowers of Rabbit Brush Chrysothamnus viscidiflorus .
Arhopalus asperatus . A large, black cerambycid whose larvae bore into pine and other conifers.
Judolia instabilis . (Plate 7.1) Commonly occur on flowers, especially lupine. Larvae bore into pine species. Adults of this species may be yellow and black, or all black. Occurs from 7,000 to 13,000 ft (2,100 to 4,000 m).
Butterflies and Moths (Order Lepidoptera)
Lepidoptera is a familiar group of insects commonly known as butterflies and moths. The adults possess minute scales on their wings that, in many species, produce brilliant color patterns. The mouthparts are modified into a thin, coiled tube through which they drink fluids. Most butterflies have six functional legs, but adults of one family, the Nymphalidae, possess only four.
Lepidoptera are almost entirely herbivorous: only in the larvae stage, when they are known as caterpillars, do they eat solid food. They are voracious eaters and can quickly defoliate a plant, particularly when feeding in groups. Lepidopteran caterpillars can be recognized by the gap (of at least two segments) between their thoracic "true" legs and their abdominal "prolegs," which are not jointed legs but simply extensions of the abdominal body wall. Other insect larvae that might be mistaken for Lepidoptera
caterpillars either: (1) have prolegs on every abdominal segment (Sawflies, of the Order Hymenoptera), (2) lack prolegs except for an anal "clasper" (Ladybird and Leaf Beetles, of the families Coccinellidae and Chrysomelidae, respectively), or (3) lack thoracic legs entirely (Hover flies, of the Family Syrphidae).
Most adult moths are gray or brown and are easily distinguished from butterflies, which are usually brightly colored. However, there are some exceptions. In such cases, butterflies may usually be distinguished by the presence of clubbed antennae, unlike the usual hairlike or plumose antennae of moths.
Lepidoptera, particularly the butterflies, are a favorite of collectors. Consequently, their habits and distributions are relatively well known. Most species of butterfly are brightly colored and may be recognized by comparison with drawings or photographs; we have included most of the species known from the White Mountain area in Plates 7.1–7.5. Good specimens of most butterflies may be obtained by locating the caterpillars and raising them to adults in a jar or plastic bag, feeding them leaves of the host plant they were found upon.
Tent Caterpillar,Malacosoma americana (Family Lasiocampidae). Commonly weaves large, conspicuous "tents" of silk in shrubs belonging to the Rose Family (locally plants in other families are used). Each tent contains 50 or more caterpillars. The adults, plain brown moths, occur in desert scrub up to 9,000 ft (2,700 m).
White-lined Sphinx Moth,Hyles lineata (Family Sphingidae). (Plate 7.2) Occurs up to 13,000 ft (4,000 m) in spring, summer, and fall. These moths are commonly attracted to lights at night, sometimes in large numbers, and may be seen visiting flowers at dusk or on overcast days.
Skippers (Family Hesperiidae). Skippers derive their common name from their rapid, "skipping" flight, usually seen when they visit flowers. At rest, many male skippers do not fold their wings together over their back but hold them at an angle, with the fore- and hindwings separated. Many skipper caterpillars feed on monocot plants, such as grasses, and are green or brown with very large heads.
Common Sootywing, Pholisora catullus . (Plate 7.2) Found at 6,000 ft (1,800 m) in May. Larvae feed on pigweeds such as Chenopodium and Amaranthus .
Great Basin Sootywing, Pholisora libya lena . (Plate 7.2) Adults occur at 6,000 ft (1,800 m) in June.
Sandhill Skipper, Polites sabuleti tecumseh . (Plate 7.2) Adults occur from 10,000 to 13,000 ft (3,100 to 4,000 m) in the summer. Larvae feed on grasses such as Distichlis and lawn grass.
Sierra Skipper, Hesperia miriamae . (Plate 7.2) Adults occur from 13,000 to 14,000 ft (4,000 to 4,300 m) during the summer. Larvae of this high-altitude species probably feed on grasses.
Uncas Skipper, Hesperia uncas macswaini . Looks like H. miriamae but with a dusky border on the upper surface of the hindwing. It flies at very high elevations in the
White Mountains, in grassy areas in June and July. Larvae feed on Needlegrass Stipa nevadensis .
Common Banded Skipper, Hesperia comma harpalus . (Plate 7.2) Occurs from 4,000 to 14,000 ft (1,200 to 4,300 m) from spring to fall. Larvae feed on grasses and sedges.
Juba Skipper, Hesperia juba . (Plate 7.2) May occur from 6,000 to 10,000 ft (1,800 to 3,100 m) in summer. Larvae feed on grasses.
Mexican Cloudy-wing, Thorybes mexicana blanca . A skipper medium brown in color, with black-bordered white spots on the forewing. It occurs above 7,000 ft (2,100 m) in June–August. Larval food plants are unknown.
Whites and Sulfurs (Family Pieridae). As their names suggest, Whites and Sulfurs are butterflies that are usually white or yellow in coloration. They are typically fragile animals, losing their legs and scales easily. They have six functional legs. The caterpillars are mostly green or yellow-green, lack long spines or hairs, and feed on plants in the Mustard and Caper families (White Butterflies) or in the Legume Family (Sulfur Butterflies).
Cabbage White, Pieris rapae . A species common around gardens and fields on the Owens Valley floor and occasionally at higher elevations. It has whitish or yellowish underwings, in contrast to the other whites of the region, which have dark markings below.
Checkered White Butterfly, Pontia protodice . (Plate 7.2) The underside has relatively few brownish markings. Occurs from 4,000 to 14,000 ft (1,200 to 4,300 m) from spring to fall. Larvae feed on plants in the Mustard Family (Brassicaceae).
Becker's White Butterfly, Pontia beckerii . (Plate 7.2) A white with black spotting on the tips and margins of the forewings; the undersides of the wings have greenish brown markings. Adults occur from 4,000 to 8,000 ft (1,200 to 2,400 m) in June and July. Larvae feed on mustard plants such as the Black Mustard (Brassica nigra ).
Hyantis Marble Butterfly, Euchloe hyantis lotta . (Plate 7.2) A small white with green markings on the underside of the wings. The adults occur from 4,000 to 8,000 ft (1,200 to 2,400 m) in May. Larvae feed on several species of mustard.
Orange Sulfur, Colias eurytheme . (Plate 7.2) Occurs from 4,000 to 14,000 ft (1,200 to 4,300 m) from spring to fall. Larvae feed on several species of plants in the Legume Family, including alfalfa (Medicago spp.), clover (Trifolium spp.), vetches and locoweeds (Astragalus spp.), and lupines (Lupinus spp.).
Swallowtails (Family Papilionidae). Members of the Family Papilionidae derive their common name from the "tails" attached to the hindwing. Swallowtails are large, sturdy butterflies, usually black and yellow. They are commonly seen gliding

Figure 7.24
Western Tiger Swallowtail
(Papilio rutulus ).
effortlessly across open ground, visiting flowers and searching for host plants. Adults have six legs.
Western Tiger Swallowtail, Papilio rutulus . (Fig. 7.24) Commonly occur in watered canyons and around towns. Larvae feed on willow, poplar, and sycamore trees.
Short-tailed Black Swallowtail, Papilio indra nevadensis . (Plate 7.3) Adults occur from 6,000 to 9,000 ft (1,800 to 2,700 m) in June. Larvae usually feed on plants in the Carrot Family (Apiaceae), but they are also known to feed on sagebrush (e.g., Artemisia tridentata ).
Blues and Coppers (Family Lycaenidae). Lycaenidae is a family of butterflies diverse in the White Mountains region, and many of its members are associated with ants in various ways. The caterpillars are sluglike, with a thick skin that is resistant to ant attack. Some species have glands and pores that secrete nectar and amino acids. This functions to attract a protective "guard" of ants, much in the way that many homopterans attract ants. For unknown reasons, male lycaenids have somewhat reduced forelegs compared with the females, which have six functional legs.
Mormon Metalmark Butterfly, Apodemia mormo . (Plate 7.3) Occurs from 4,000 to 7,000 ft (1,200 to 2,100 m) in the fall. Larvae feed on buckwheat (Eriogonum spp.).
Edward's Blue, Plebejus (Lycaeides) melissa paradoxa . (Plate 7.3) Occurs from 4,000 to 11,000 ft (1,200 to 3,400 m) in the summer. The larvae feed on species in the Legume Family, including lupines, vetches, and locoweed (Oxytropis spp.).
Greenish Blue, Plebejus saepiolus . (Plate 7.3) Occurs at 12,000 ft (3,700 m) throughout the summer. Larvae feed on clover (Trifolium spp.).
Boisduval's Blue, Plebejus (Icaricia) icarioides . (Plate 7.3) Occurs from 9,000 to 12,000 ft (2,700 to 3,700 m) during the summer. Larvae feed on lupine (Lupinus spp.).
Alpine Blue, Plebejus shasta . (Plate 7.3) Occurs from 10,000 to 13,000 ft (3,100 to 4,000 m) during the summer. Larvae feed on lupines, clover, and locoweed (e.g., Astragalus ).
Lupine Blue, Plebejus lupini . (Plate 7.3) Occurs from 10,000 to 13,000 ft (3,100 to 4,000 m) during the summer.
Acmon Blue, Plebejus acmon . (Plate 7.3) Adults occur from 4,000 to 11,000 ft (1,200 to 3,400 m) in July. Larvae feed on buckwheat (Eriogonum spp.).
Arrowhead Blue, Glaucopsyche piasus . (Plate 7.4) The underside of the wing bears white "arrowhead" markings. The adults occur in June at 9,000–11,000 ft (2,700–3,400 m). Larvae feed on lupine (Lupinus spp.).
Silvery Blue, Glaucopsyche lygdamus . (Plate 7.4) The underside of the wing bears a single jagged row of small black dots ringed with white. The adults occur in May between 6,000 and 10,000 ft (1,800 and 3,000 m). Larvae feed on lupine (Lupinus ), and locoweed (e.g., Oxytropis and Astragalus ).
Spring Azure, Celastrina ladon . (Plate 7.4) Occurs from 5,000 to 10,000 ft (1,500 to 3,100 m) during the spring and summer.
Pygmy Blue, Brephidium exilis . (Plate 7.4) Small, with a wingspread less than 3/4 in (1.9 cm). Occurs between 4,000 and 11,000 ft (1,200 and 3,400 m) from spring to fall. Larvae feed on pigweed (Chenopodium spp. and Amaranthus spp.) and saltbush (Atriplex spp.).
Blue Copper, Lycaena (Chalceria) heteronea . (Plate 7.4) Occurs from 10,000 to 12,000 ft (3,100 to 3,700 m) during the summer. Larvae feed on buckwheat (Eriogonum spp.).
Edith's Copper, Lycaena (Gaeides) editha . (Plate 7.4) Occurs from 10,000 to 13,000 ft (3,100 to 4,000 m) during the summer. Larval food plants are unknown.
Cupreus Copper, Lycaena cuprea . (Plate 7.4) Occurs from 10,000 to 13,000 ft (3,100 to 4,000 m) during the summer and fall. Larvae feed on Mountain Sorrel (Oxyria ).
Thicket Hairstreak, Mitoura spinetorum . (Plate 7.4) A species with short "tails" on the hindwings. Wings have dull blue upper surfaces and brown lower surfaces. It occurs from 6,000 to 9,000 ft (1,800 to 2,700 m) during the summer. Larvae are known to feed on mistletoe (Arceuthobium ) species that parasitize conifers.
Siva Hairstreak, Mitoura siva . (Plate 7.4) Has "tails" like M. spinetorum , but wings have brown upper surfaces and greenish lower surfaces. Ir occurs from 6,000 to 8,000 ft (1,800 to 2,400 m) during the spring. Larvae resemble the twigs of their host plant, juniper (Juniperus spp.).
Comstock's Green Hairstreak, Callophrys comstocki . A tailless hairstreak light grey above with a broken white band on the underside of the hindwing, edged inwardly with black. The undersurface of the wing is greenish. It occurs from 5,000 to 7,000 ft (1,500 to 2,100 m) in the spring. Larvae feed on buckwheat (Eriogonum ).
Brush-footed Butterflies (Family Nymphalidae). Nymphalids are commonly large and showy, and may be easily recognized by the presence of only four functional legs, the forelegs being modified into "drumming" organs used by the females to "taste" the host plants. Nymphalid caterpillars are commonly spiny or brightly colored, although the grass feeders tend to be green and difficult to see.
Monarch Butterfly, Danaus plexippus . (Plate 7.5) Occurs from 4,000 to 14,000 ft (1,200 to 4,300 m) from spring to fall. Larvae feed on milkweed (Asclepias spp.).
Dark Wood Nymph, Cercyonis oetus . (Plate 7.5) Occurs from 10,000 to 12,000 ft (3,100 to 3,700 m) in the summer. Larvae feed on grass.
Riding's Satyr, Neominois ridingsii . (Plate 7.1) Occurs from 9,000 to 11,000 ft (2,700 to 3,100 m). It "flushes" when disturbed, flies a short distance, and quickly alights, camouflaging itself on rocks or lichens. Its larvae probably feed on grass.
Neumoegen's Checkerspot, Chlosyne (Charidryas) neumoegeni . (Plate 7.5) Occurs from 4,000 to 6,000 ft (1,200 to 1,800 m) in the spring. Larvae feed on plants in the Sunflower Family (Asteraceae).
Leanira Checkerspot, Chlosyne (Thessalia) leanira alma . (Plate 7.5) Occurs from 5,000 to 8,000 ft (1,500 to 2,400 m) in the spring. Larvae feed on Indian Paintbrush (Castelleja spp.).
Anicia Checkerspot, Euphydryas (Occidryas) anicia wheeleri . (Plate 7.5) Occurs from 9,000 to 11,000 ft (2,700 to 3,400 m) in summer. Larvae feed on plants in the Scrophulariaceae, including species of Penstemon and Castelleja .
Milbert's Tortoiseshell, Nymphalis (Aglais) milberti . (Plate 7.5) Occurs from 10,000 to 13,000 ft (3,100 to 4,000 m) in the summer and fall. Larvae usually feed on nettles (Urtica spp.) but have been reported on species of willow (Salix spp.) and sunflower (Helianthus spp.).
Mourning Cloak, Nymphalis antiopa . (Plate 7.1) A large, easily recognized species that occurs along streams and in the Owens Valley. The larvae feed on willow (Salix spp.) and other trees.
Painted Lady, Vanessa cardui . (Plate 7.5) Occurs from 4,000 to 14,000 ft (1,200 to 4,300 m), spring to fall. Larvae feed on plants in the Sunflower Family (Asteraceae).
West Coast Lady, Vanessa annabella . (Plate 7.5) Adults occur in June near 7,000 ft (2,100 m). Larvae feed on plants in the Mallow Family (Malvaceae).
Red Admiral, Vanessa atalanta . (Plate 7.1) Occurs from 4,000 to 11,000 ft (1,200 to 3,400 m), spring to fall. This large species feeds on nettle (Urtica spp.).
Lorquin's Admiral, Limenitis lorquini . (Plate 7.1) Larvae feed on willow, poplar, and other trees along streams and in the Owens Valley. The adults fly with quick wingbeats interspersed with gliding and are large and striking.
Flies, Gnats, and Mosquitoes (Order Diptera)
Dipterans, also known as the "true flies," may be recognized by the possession of only two wings instead of the four that other adult insects have. In Diptera, the hindwings have been modified into tiny, clublike halteres, which are used as organs of balance. Most dipterans lack mandibles, and adults are common visitors to flowers, where they drink nectar with their spongelike or sucking mouthparts. Adult female dipterans of many species are blood suckers and require one or more blood meals for egg production. The larvae of many dipterans are aquatic or feed in anaerobic decaying matter; others feed on plant material or are predaceous on other insects. One family (Tachinidae) is particularly important as parasites of caterpillars; the larvae feed internally until the entire host is consumed. Dipteran larvae are legless and maggotlike except for specialized aquatic forms, such as mosquito larvae.
Crane flies,Tipulaspp. (Family Tipulidae). (Fig. 7.25) Crane flies have the appearance of very large mosquitoes but are completely harmless. They occur at elevations up to 13,000 ft (4,000 m).
Mosquitoes,Anophelesspp. (Family Culicidae). (Fig. 7.26) Mosquitoes are common in wet areas, such as along the Owens River or boggy areas at higher elevations. Members of this genus are the principal vectors of malaria in California.
Robber Flies, (Family Asilidae). (Fig. 7.27) Robber Flies are common and easily recognized by their stout thorax, wings, and legs, and by their behavior of perching in exposed positions and sallying forth after flying prey. They are very diverse in the White Mountains at all elevations.
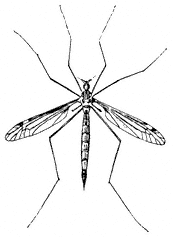
Figure 7.25
Crane Fly (Tipula sp.).
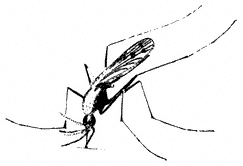
Figure 7.26
Mosquito (Anopheles sp.).

Figure 7.27
Robber Fly.
Hover Flies (Family Syrphidae). Hover Flies are commonly seen visiting flowers. Many species look very much like bees or wasps. They also commonly hover in midair, holding a fixed position relative to the surrounding vegetation, and abruptly changing position when approached. Larvae of some species are caterpillarlike and feed on aphids and small herbivorous insects. Some species occur at elevations as high as the top of White Mountain Peak.
Common Hover Fly, Metasyrphus aberrantis . (Plate 7.1) Occurs on flowers from 6,000 to 10,000 ft (1,800 to 3,100 m). The larvae crawl on plants and eat aphids and other small insects. They are legless, yet manage to cling to vegetation very efficiently.
Sawflies, Wasps, Bees, and Ants (Order Hymenoptera)
Adult hymenopterans possess four wings, with the hindwings smaller than the forewings. Except for the Sawflies, hymenopterans also have a constricted "waist" between the first and second abdominal segments and, in females, an ovipositor modified into a stinger. The larvae of Sawflies are mainly herbivorous and greatly resemble lepidopteran caterpillars. The wasps are mainly predaceous or parasitic on other insects and are very important in controlling their numbers. Some wasps and Sawflies parasitize plants by laying their eggs in plant tissues. The egg or resulting larvae then induces the plant to form a gall, which surrounds the insect. These galls are particularly common on oaks and willows. The bees feed primarily on pollen and nectar and are probably the most important agents of flower pollination.
Many hymenopterans live in tightly coordinated social groups usually dominated by one or more reproductive "queens." The queen's efforts at reproduction are aided by numerous "workers," which may be temporarily or permanently sterile. Ants represent the extreme of this type of social evolution, and the sterile, wingless workers are among the most common, visible insects in many habitats. They feed on small insects and obtain sugary "honeydew" secretions from Homoptera. They are very important in controlling the numbers of other insects in many habitats.
Sawflies,Tenthredospp. (Family Tenthredinidae). (Fig. 7.28) Adult Sawflies resemble wasps, except that they have a stout "waist" instead of the threadlike waist of the true wasps and bees. Sawflies are commonly encountered as larvae feeding on the leaves of plants. Many Sawfly larvae look very much like caterpillars of Lepidoptera,
Figure 7.28
Sawfly (Tenthredo sp.).
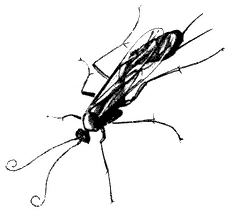
Figure 7.29
Ichneumon (Ophion sp.).
except that they possess prolegs on every abdominal segment rather than on five or fewer segments (as seen in Lepidoptera). Many Sawflies lay their eggs internally in the host plant leaves rather than externally, as in most Lepidoptera. Some Sawflies make galls in their host plants. Sawflies occur as high as Crooked Creek in the White Mountains (10,150 ft, 3,100 m).
Ichneumons,Ophionspp. (Family Ichneumonidae). (Fig. 7.29) Ichneumons are elongated wasps, the larvae of which are parasitic on other insects. They are frequently seen searching vegetation for their hosts by quivering their long, curled antennae and making short flights between branches or plants. They occur at all elevations below 13,000 ft (4,000 m).
Cuckoo Wasps,Chrysisspp. (Family Chrysididae). Cuckoo Wasps are brillant green and reproduce by parasitizing larvae of other wasps in their burrows. They occur at all elevations below 13,000 ft (4,000 m), where they may be seen drinking from flowers.
Tarantula Hawks,Pepsisspp. (Family Pompilidae). (Plate 7.1) Easily recognized wasps because of their very large size and black and orange wings, they occur from 4,000 to 8,000 ft (1,200 to 2,400 m) in summer and fall. These and other spider wasps actively hunt spiders, which they paralyze by stinging and drag to a burrow. Then they deposit an egg and seal up the burrow. Eventually the egg hatches, and the wasp larva consumes the spider. Smaller spider wasps in the same family occur at elevations up to 13,000 ft (4,000 m).
Resin Bees,Dianthidiumspp. (Family Megachilidae). (Fig. 7.30) Occur at elevations below 8,000 ft (2,400 m), and possibly higher. Commonly found in deserts and foothills in California, they nest alone rather than in colonies. Resin Bees are common pollinators of Phacelia and other desert flowers.

Figure 7.30
Resin Bee (Anthidium sp.).

Figure 7.31
Honey Bee (Apis mellifera ).
Bumble Bees,Bombus centralisandB. morrisoni(Family Apidae). (Plate 7.1) Bumble bees occur at elevations up to 11,000 ft (3,400 m). Commonly seen visiting flowers, these bees live in nests underground.
Honey Bee,Apis mellifera(Family Apidae). (Fig. 7.31) Abundant at lower elevations, an introduced bee that has established itself in the wild, nesting in hollows in trees, caves, and underground.
Carpenter Bee,Xylocopa californica arizonensis(Family Anthophoridae). (Fig. 7.32) Adults are large, black bees that bore into dead wood to make nest chambers. They occur between 6,000 and 8,000 ft (1,800 and 2,400 m).
Ants (Family Formicidae). Ants are familiar organisms at all elevations in the White Mountains. Protected underground and by numerous worker individuals willing to sacrifice their lives in defense of the colony, ants are among the most successful creatures on earth. Perhaps because of the ants' abundance, many insects have evolved specific adaptations to survive with them. For example, aphids and many butterflies of the Family Lycaenidae secrete sugary honeydew, which attracts ants, which in return protect the insects from enemies such as parasitic wasps and Ladybird Beetles.

Figure 7.32
Carpenter Bee (Xylocopa sp.).

Figure 7.33
Mound Ant (Formica sp.).

Figure 7.34
Carpenter Ant (Camponotus sp.).
Mound Ants, Formica spp. (Fig. 7.33) Occur from the Owens Valley to 12,500 ft (3,800 m) in large, mound-shaped nests on bare ground. These ants may be very aggressive. The species F. subpolita occurs at Barcroft Laboratory at 12,500 ft (3,800 m).
Carpenter ants, Camponotus spp. (Fig. 7.34) Large, black ants that usually nest in or near dead wood, which they hollow out. In spite of their large size, these ants are commonly timid and nonaggressive. They occur in dead wood to 11,000 ft (3,400 m). C. sansabeanus and C. ochreatus nest in dry wood near Westgard Pass.
References
Borror, D. J., D. Delong, and C. A. Triplehorn. 1976. Introduction to the Study of Insects . Holt, Rinehart, and Winston, New York.
Borror, D. J., and R. E. White. 1970. A Field Guide to the Insects of America North of Mexico . Peterson Field Guide Series, Houghton Mifflin, Boston.
Emmel, T. C., and J. F. Emmel. 1973. The Butterflies of Southern California . Natural History Museum, Los Angeles.
Garth, J. S., and J. W. Tilden. 1986. California Butterflies . University of California Press, Berkeley.
Powell, J. A., and C. L. Hogue. 1979. California Insects . University of California Press, Berkeley.
8—
Fishes
Edwin P. Pister
Introduction
For all practical purposes, the fishes of the White Mountains are introduced species. It is unlikely that native species ever existed in these mountains except in the very lowest reaches of either easterly or westerly drainages.
Perhaps the terms introduced species and native species should be clarified. When Europeans first arrived in the Owens Valley, only four fishes were found there, all of them considered nongame species: the Owens Pupfish (Cyprinodon radiosus Miller), Owens Chub (Gila bicolor snyderi Miller), Owens Dace (Rhinichthys osculus sp.), and Owens Sucker (Catostomus fumeiventris Miller). On the east side of the range, in Fish Lake Valley, only a chub (Gila sp.) was found. These constitute the native fish fauna of the White Mountains area, and through many years of neglect only one of them, the Owens Sucker, now occurs in abundance. The others are endangered, threatened, or of undetermined status. Recovery programs are in progress for the endangered and threatened fauna, under the provisions of the federal and state endangered species acts.
The streams flowing from the White Mountains are spring-fed. Being a desert range, the White Mountains seldom hold the summer snowbanks that typify the Sierra Nevada, just a few miles to the west. Once the winter snows have melted, the stream flows quickly stabilize except during occasional summer thundershowers.
A glance at a map of the Inyo National Forest reveals a large number of streams flowing from the crest of the White Mountains. However, only a few of them contain significant fish populations, and these generally only in the upper reaches. The vast majority of these streams, whether they flow east or west, have for many years been diverted for irrigation.
Early Trout Planting
The exact history of fish introductions into the White Mountains is unknown. Accurate records of early fish planting, unfortunately, were not kept. It is safe to assume, however, that most introductions occurred in this century prior to World War II. Some introductions were made by the California Division (now Department) of Fish and Game, and others were made by the Rainbow Club, an early sportsmen's club headquartered in Bishop. The club has planted no fish since before World War II.
Most White Mountain streams have been planted with trout, and one may expect to find Eastern Brook Trout, Salvelinus fontinalis (Mitchill), and Rainbow Trout, Oncorhynchus mykiss gairdnerii (Richardson), in the higher elevations and Brown Trout,
Salmo trutta Linnaeus, in the lower elevations. However, the California streams are no longer being stocked with trout; all populations are self-sustaining. The Nevada Department of Wildlife, however, occasionally stocks Rainbow Trout in streams flowing into northern Fish Lake Valley.
Paiute Trout
An exception to the general rule concerning trout species composition and distribution is found in Cottonwood Creek, which flows easterly into Fish Lake Valley from near White Mountain Peak. In 1946, when Department of Fish and Game and U.S. Forest Service biologists noted that the Paiute Cutthroat Trout, Oncorhynchus clarki seleniris (Snyder), populations of the upper East Fork Carson River (Alpine County, California) were seriously threatened, a limited number were planted in the previously fishless upper North Fork Cottonwood Creek. This stream has been generally closed to angling since that time. The subspecies is currently listed as threatened under the federal Endangered Species Act.
The North Fork Cottonwood Creek Paiute Cutthroat population is perhaps the only genetically pure population left in the world, and it is being protected as part of the recovery plan for the subspecies. Should the subspecies recover sufficiently, management plans may allow a very limited harvest. Until that time, however, the North Fork Cottonwood Creek will remain closed to angling. To accelerate the recovery of the Paiute Cutthroat Trout, it may be spread to other suitable streams as part of a plan under consideration by the Department of Fish and Game and the U.S. Forest Service. At present, however, the only stream area in the White Mountains with a special restriction is the North Fork Cottonwood Creek. Anglers planning to fish anywhere in California should check current fishing regulations, which are available at most sporting goods stores.
Species Descriptions
Rainbow Trout,Oncorhynchus mykiss gairdneri(Richardson). (Fig. 8.1) Other than the Paiute Trout, the Rainbow Trout is the only trout species in the White Mountains that is native to California. It may be distinguished from the other species by its generally silvery appearance and profusion of spots, including a spotted tail. It also commonly has a reddish lateral band extending from head to tail. However, look for the spotted tail as the definitive characteristic. None of the other trout species in the White Mountains has a heavily spotted tail.
Eastern Brook Trout,Salvelinus fontinalis(Mitchill). (Fig. 8.2) Although called a trout, the Eastern Brook Trout is actually a char. It is native to the eastern United States and was brought to California many years ago. It has been introduced widely throughout the Sierra Nevada and occurs in several streams in the White Mountains.
Eastern Brook Trout are generally olive green with light spots on the sides. They may be distinguished from the trout species by vermiculations, or wavy lines, along

Figure 8.1
Rainbow Trout, Oncorhynchus mykiss gairdnerii (Richardson).
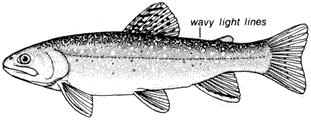
Figure 8.2
Eastern Brook Trout, Salvelinus fontinalis (Mitchill).

Figure 8.3
Paiute Cutthroat Trout, Oncorhynchus clarki seleniris
(Snyder).

Figure 8.4
Brown Trout, Salmo trutta Linnaeus.
the back from head to tail. They also have distinct white borders along the anterior margins of the ventral and anal fins.
Paiute Cutthroat Trout,Oncorhynchus clarki seleniris(Snyder). (Fig. 8.3) The Paiute Cutthroat Trout, which is native to the Great Basin, was introduced into the North Fork Cottonwood Creek in 1946 in one of the first recorded efforts toward species preservation in California. It has survived in Cottonwood Creek in limited numbers, but the North Fork is closed to angling to ensure its protection. The Paiute Cutthroat Trout evolved through geographic isolation from the Lahontan Cutthroat Trout, Oncorhynchus clarki henshawi (Gill and Jordan), in upper Silver King Creek, Alpine County.
This trout normally has an overall purplish hue, which distinguishes it from the other trout species in the White Mountains. It also has very few (if any) spots and possesses the distinctive reddish cutthroat marks under the jaw.
Brown Trout,Salmo truttaLinnaeus. (Fig. 8.4) The Brown Trout, a native of Europe, was brought into California many years ago and eventually found its way into the White Mountains. It is common there, especially in the lower stream areas. It is an excellent game fish and is sought after by anglers, especially fly fishermen.
The Brown Trout is usually dark or olive brown on the back and golden brown on the sides. It generally has red spots surrounded by a halo on its sides. It may have spots on the tail, but they are sparse in contrast to the profusion of tail spotting present in Rainbow Trout.
Conclusion
Some researchers are now questioning the general advisability of introducing nonnative species into a naturally balanced ecosystem. The trout stocking that occurred in the early 1900s was a result of resource management procedures in practice at that time, but current research is beginning to recognize the long-term implications of stocking to the ecosystem. Such introduction invariably harms native organisms and is now considered unacceptable ecological practice.
References
California Department of Fish and Game. 1969. Trout of California . Calif. Dept. of Fish and Game, Sacramento.
Hubbs, C. L., and R. R. Miller. 1948. Correlation between fish distribution and hydrographic history in the desert basins of western United States. In The Great Basin, with emphasis on glacial and postglacial times . Bulletin of the University of Utah, no. 38, pp. 17–166.
Miller, R. R. 1948. The cyprinodont fishes of the Death Valley system of eastern California and southwestern Nevada . Miscellaneous Publications of the Museum of Zoology, University of Michigan, no. 68.
Miller, R. R. 1973. Two new fishes , Gila bicolor snyderi and Catostomus fumeiventris, from the Owens River basin, California . Occasional Papers, Museum of Zoology, University of Michigan, no. 667.
Miller, R. R., and E. P. Pister. 1971. Management of the Owens pupfish, Cyprinodon radiosus , in Mono County, California. Transactions of the American Fisheries Society 100:502–509.
Pister, E. P. 1974. Desert fishes and their habitats. Transactions of the American Fisheries Society 103:531–540.
Pister, E. P. 1976. A rationale for the management of nongame fish and wildlife. Fisheries 1:11–14.
Pister, E. P. 1979. Endangered species: Costs and benefits. Environmental Ethics 1:341–352.
Pister, E. P. 1981. The conservation of desert fishes. In R. J. Naiman and D. L. Soltz (eds.). Fishes in North American deserts , pp. 411–455. John Wiley & Sons, New York.
Schumacher, Genny (ed.). 1969. Deepest valley: Guide to Owens Valley and its mountain lakes, roadsides, and trails . Wilderness Press, Berkeley, Calif.
Soltz, D. L., and R. J. Naiman. 1978. The natural history of native fishes in the Death Valley system . Natural History Museum of Los Angeles County, Science Series, no. 30.
Vestal, E. H. 1947. A new transplant of the Paiute trout (Salmo clarkii seleuiris ) from Silver King Creek, Alpine County, California. California Fish and Game 33(2):89–95.
9—
Amphibians
J. Robert Macey and Theodore J. Papenfuss
Introduction
Amphibians are cold-blooded vertebrates with moist, scaleless skin. These animals either occur in close association with permanent water or are active only during wet times of the year. There are approximately 3,200 species in the world, and amphibians are present on all continents except Antarctica.
Amphibians are divided into three groups: caecilians, salamanders, and frogs and toads. Caecilians are elongate, "wormlike," burrowing or aquatic forms that lack limbs and functional eyes. The approximately 165 species are restricted to the tropical regions of the world; no species occur in the United States. Most caecilians are less than 2 ft (0.6m) long; however, one species in South America reaches a length of 5 ft (1.5m).
Most salamanders have a lizardlike shape with four legs and a tail. They have smooth, moist skin that lacks scales. In the New World there are three areas with high species diversity. One is the mountainous region of southern Mexico and northern Central America, with some 50 species. Another is the Appalachian Mountains of the eastern United States, with about 45 species. The third region comprises the Pacific states of California, Oregon, and Washington, with about 35 species.
The species that are present in California have two very different developmental patterns. Some breed in water, and the adults return to streams and ponds during the rainy season to lay eggs. Gilled larvae develop in the water and later transform into terrestrial adult salamanders. Other salamanders are completely terrestrial. The eggs are laid on land in moist places. The larval stage takes place in the egg, and small, fully transformed salamanders emerge. All western fully terrestrial salamanders lack lungs, and respiration takes place through the skin.
Three species of salamanders are known to occur in the California portion of the White-Inyo mountains region. The only state with no native salamanders is Nevada. There have been several reports of salamanders in Nevada, but none have been confirmed. Although the species accounts included here for salamanders have been brought up to date, they are probably incomplete and may even be lacking a species. We would appreciate receiving any additional information, such as a new distribution or a salamander that does not fit the descriptions.
The frogs and toads are the largest group of amphibians. Approximately 2,600 species of frogs and toads are known, and they occur on every continent except Antarctica. The greatest species diversity is in the tropics, but frogs and toads are
Map 9.1
Map 9.2
well represented in temperate regions. These amphibians occur in a great variety of habitats. Some species occur in arid regions, where they spend most of their lives underground, coming to the surface only during occasional rains. Others are fully aquatic and never leave the water. A few species in tropical regions spend their entire lives in the tree canopy. There are 23 families of frogs and toads worldwide, of which 5 occur in California and Nevada. The families that occur in the area under study are the True Toads (Family Bufonidae), Treefrogs (Hylidae), Spadefoot Toads (Pelobatidae), and True Frogs (Ranidae).
Species Accounts
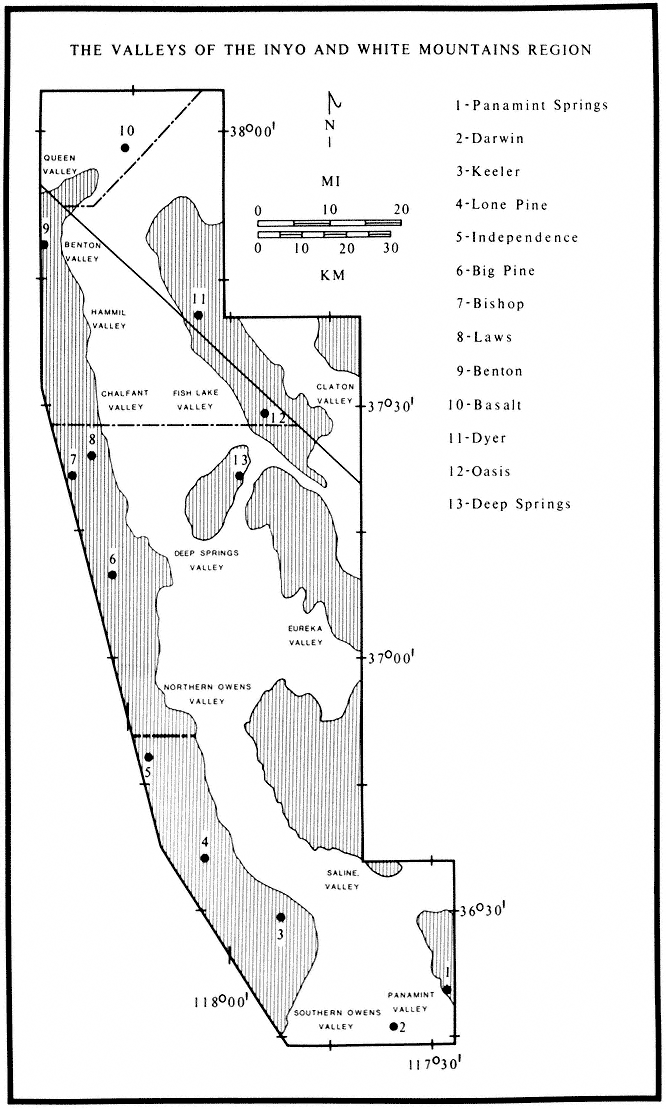
The following species accounts cover the three salamanders and seven frogs and toads that occur in the White-Inyo mountains region. Accounts are ordered by main group, then alphabetically by family name, and finally alphabetically by species name. Each account consists of a brief identification, information on habitat, and a remarks section, which treats natural history. A brief summary of the range and a list of the exact localities corresponding to the dots on the range maps are also included. When the locality is vague, it is not plotted on a map. In these lists the first reference to a place name is listed in its entirety, and all other references to that place name follow it as separate localities. These localities are based on museum specimens. The majority of specimens from this region are deposited in the Museum of Vertebrate Zoology, University of California at Berkeley (MVZ). Other museums represented in the localities sections are: American Museum of Natural History, New York (AMNH); California Academy of Sciences, San Francisco (CAS); Carnegie Museum of Natural History, Pittsburgh (CMNH); Field Museum of Natural History, Chicago (FMNH); University of Kansas (KU); Natural History Museum of Los Angeles County (LACM); Museum of Comparative Zoology, Harvard University (MCZ); San Diego Natural History Museum (SDSNH); University of Michigan Museum of Zoology (UMMZ); University of Nevada, Reno (UN); and United States National Museum, Washington, D.C. (USNM). The acronym of the museum follows each locality. Absence of an acronym indicates that the locality is based on a specimen from the Museum of Vertebrate Zoology. For accuracy, elevations and distances of localities are cited as recorded by the collector and have not been converted to or from the English or metric system.
The color plates of species will aid in identification. All amphibians in the color plates are from the White-Inyo mountains region except the Northern Leopard Frog (Rana pipiens ), which is from Churchill County, Nevada. For each species, pertinent literature references are listed; the complete citations are at the end of the chapter. General references to the amphibians of the region are: Macey (1986), Papenfuss (1986), Stebbins (1951), and Stebbins (1985). All scientific names are after Collins (1990). In addition, the following chapter of this book, on reptiles, has two sections that contain general information on amphibians of the White-Inyo mountains region.
Lungless Salamanders (Family Plethodontidae)
Kern Plateau Slender Salamander, Batrachosepssp.(no author, not yet described). (Plate 9.1) 3–4 in (7.5–10 cm); dorsal pattern variable; black with a brown middorsal stripe or black with an overlay of silver specks; only four toes on each foot. Habitat: This species occurs around permanent springs and creeks with riparian vegetation. The salamanders live in the daytime under cover objects such as rocks and logs. Remarks: This salamander is related to the Inyo Mountains Slender Salamander (B. campi ) and was the second salamander to be discovered in the White-Inyo mountains region. The Kern Plateau Slender Salamander has a narrower head and shorter legs than the Inyo Mountains Slender Salamander. The ranges of the two species do not overlap. Species of the genus Batrachoseps are the only salamanders in California that have just four toes on each hind foot. Other species of Batrachoseps , known as Slender Salamanders, are widely distributed throughout California. They commonly occur in urban areas under rocks and boards. This species is currently known from the Kern Plateau and the southeastern slopes of the Sierra Nevada, but it may have a more extensive range. Range: Kern Plateau and the eastern slopes of the Sierra Nevada south and west of Owens Lake. Reference: Stebbins (1985).
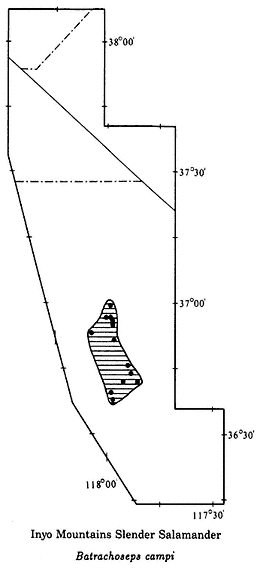
Inyo Mountains Slender Salamander,Batrachoseps campi(Marlow, Brode & Wake, 1979). (Plates 9.2 [from Hunter Canyon] and 9.3 [from French Spring], Map 9.3) 2–3 in (5–7.5 cm); dorsal pattern variable; background color dark brown to black; in some populations scattered green, lichenlike spots on body; in others the background color is completely overlain with silver flecks; only four toes on each foot. Habitat: Occurs only around permanent springs and seeps that provide a riparian habitat. Inyo Mountains Slender Salamanders are active at night. During the day they take shelter under moist rocks or in damp crevices. They have been found at springs as low as 1,800 ft (550 m) and as high as 8,600 ft (2,600 m). Remarks: The discovery in 1973 of these salamanders in the arid Inyo Mountains was unexpected. In the past, numerous biologists had visited many of the springs where these salamanders live without detecting them, because salamanders are not considered desert animals. Doubtless, additional populations will be found. These salamanders are relicts that entered the Inyo Mountains prior to the rise of the Sierra Nevada and the formation of the Mojave and Great Basin deserts. In some instances these isolated populations may consist of only a few hundred individuals. They are protected by state law and should not be collected. At the present time, capping of springs is the major threat to these populations. Range: Both east and west slopes of the Inyo Mountains. References: Marlow, Brode, and Wake (1979); Yanev and Wake (1981).
Localities: California, Inyo Co.: 6,800–7,000, 8,000–8,300 ft, Addie Canyon; 6,400 ft, Barrel Springs; 6,400 ft, Cove Springs; 3,500 ft, Craig Canyon; 6,000 ft, French Spring (LACM, MVZ); 1,800 ft, 2,200 ft, Hunter Canyon; 4,000 ft, Keynot Canyon; 6,500 ft, Lead Canyon (LACM, MVZ); 5,600 ft, Long John Canyon (LACM, MVZ); 3,500 ft, McElvey Canyon; 7,500 ft, top of ridge between Lead and Addie canyons; 7,200 ft, Waucoba Canyon; 4,700 ft, Willow Creek Canyon.
Map 9.3
Map 9.4
Owens Valley Web-toed Salamander, Hydromantessp. (no author, not yet described). (Plate 9.4) 3 1/2–4 1/2 in (9–11.5 cm); body and head very flattened; dorsal pattern variable; greenish brown to silver with scattered black spots; scattered lichenlike silver spots on ventral surface; feet with extensive webbing; four toes on front feet and five toes on hind feet. Habitat: The Owens Valley Web-toed Salamander occurs in the vicinity of permanent springs and mountain streams with riparian vegetation. Although this salamander is nocturnal, it can be encountered under wood and rocks in areas with moist soil. Remarks: The Genus Hydromantes is distributed in California, Italy, and Sardinia. The species in Europe are the only members of the lungless salamander family, Plethodontidae, in the Old World. The Owens Valley Web-toed Salamander was discovered in 1985 during field work in preparation for this chapter.
Range: Eastern slopes of the Sierra Nevada, at least from the area around Owens Lake to Big Pine. Reference: Wake, Maxson, and Wurst (1978).
True Toads (Family Bufonidae)
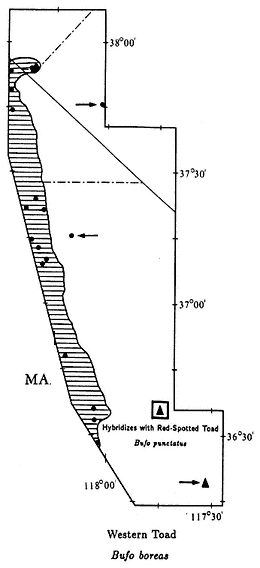
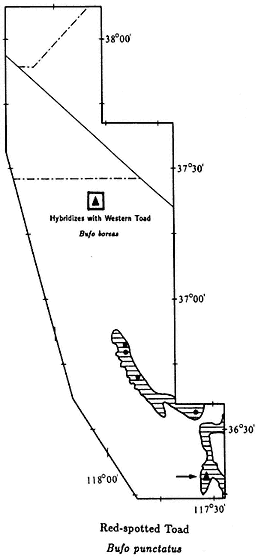
Western Toad,Bufo boreas(Baird & Girard, 1852). (Plate 9.5, Map 9.4) 3–5 1/2 in (7.5–13.75 cm); marbled dorsal pattern of brown, gray, and green in equal proportions; distinct white or light yellow line down middle of back; ventral background color cream with black spots; small warts scattered over back; oblong gland (parotoid gland) behind eye is longer than upper eyelid. Habitat: In White-Inyo Range, occurs around permanent ponds and slow-moving streams. Western Toads are generally nocturnal; however, recently discovered populations in the northern White Mountains (see Map 9.4) are also active during the day, at least in spring and summer. Remarks: Western Toads are a common, wide-ranging species in much of the western United States, occurring from sea level to above 9,000 ft (2,740 m). They are often seen at night on roads and in yards in rural areas. In the White-Inyo mountains region, this species is generally restricted to valleys, but it has been found above 7,000 ft (2,130 m) in the Pinyon-juniper Woodland of the northern White Mountains. A Creosote Bush Scrub outpost for this species is Darwin Falls Canyon in the northern Argus Mountains. Here the Western Toad and the Red-spotted Toad (B. punctatus ) coexist and occasionally hybridize (see Red-spotted Toad account). At a second isolated locality, Fish Lake in Fish Lake Valley, Western Toads may be extinct due to the introduction of Bullfrogs (Rana catesbeiana ) (see Bullfrog account). Range: Darwin Falls Canyon, northern Argus Mountains; Owens Valley; vicinity of Fish Lake, Fish Lake Valley; extreme northern White Mountains. Reference: Karlstrom (1962).
Localities: California, Inyo Co.: Alvord (USNM); 3.0 mi S Bartlet; Batchelder Spring, Westgard Pass; Big Pine; 1.5 mi SW; 4 mi NW (JACM); Bishop; 0.5 mi NW (UMMZ); 5 mi E (LACM); 4000 ft, Bishop Creek (USNM); Darwin Falls, Argus Mtns.; Diaz Lake, Owens Valley (CAS); Fish Lake Spring (CAS); Independence; Laws (CAS, MVZ); near (UMNZ); Lone Pine (MCZ, MVZ, USNM); 2.9 mi S (CAS). Mono Co.: Benton; 5 mi N (SDSNH); 3 mi from Nevada State line, spring, Taylor Ranch, 5 mi N Benton (UMMZ). Nevada, Esmeralda Co.: Fish Lake (MVZ, UMMZ); 7,820 ft, Buffalo Canyon. Mineral Co.: Orchard Spring, Buffalo Canyon; Queen Canyon.
Black Toad,Bufo exsul(Myers, 1942). (Plate 9.6, Map 9.5) 1 1/2–2 1/2 in (3.75–6.25 cm); dorsal color predominantly black; faint white line down middle of back; ventral color black, with white mottling becoming more extensive on chin; small warts scattered over back; oblong gland (parotoid gland) behind eye is longer than upper eyelid. Habitat: This species is the most aquatic toad in the region. It never occurs far from permanent water. Black Toads prefer marshy areas around pools or slow-moving streams. They are generally diurnal but may be active at night during the summer. Remarks: The Black Toad along with the three salamanders are the only amphibians endemic to the White-Inyo mountains region. It is native only to the Deep Springs Valley but has been introduced to Batchelder Spring on the west side of
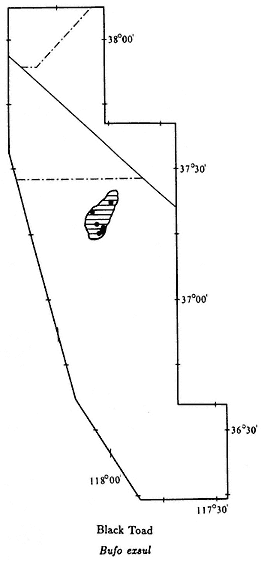
Map 9.5
Map 9.6
Westgard Pass. However, no toads have been seen at Batchelder Spring in recent years, and that population may be extinct. The Black Toad is closely related to the Western Toad (B. boreas ). It has been isolated from the Western Toad for at least 12,000 years, since the last Pleistocene moist period. The Black Toad, and the Amargosa Toad (B. nelsoni ) in Nye County, Nevada, have commonly been regarded as subspecies of the Western Toad. However, a recent genetic study suggests that these two forms be recognized as full species. The only other amphibian in Deep Springs Valley is the Great Basin Spadefoot (Spea intermontana ). Spadefoots have light-colored, smooth skin and spades on the hind feet (see Great Basin Spadefoot Toad account). Black Toads are protected by California law and should not be collected. Range: Areas of permanent
water in the Deep Springs Valley; introduced to Batchelder Spring, Westgard Pass, but may be extinct. References: Myers (1942), Schuierer (1962).
Localities: California, Inyo Co.: Antelope Spring; Deep Springs Valley (CAS, LACM); Batchelder Spring, Westgard Pass; Bog Mounds Spring, Deep Springs Valley; Buckhorn Spring, Deep Springs Valley (CAS, MVZ); Deep Springs (CAS, CMNH, FMNH, SDSNH, USNM, KU, MCZ); 6 mi S (LACM, MVZ); 7 mi S; 7.5 mi S (AMNH, UMMZ); 8 mi S (LACM); Deep Springs Lake (CAS, MCZ, USNM); 0.5 mi E; Warm Spring at Deep Springs Lake.
Red-spotted Toad,Bufo punctatus(Baird & Girard, 1852). (Plate 9.7, Map 9.6) 2–3 in (5–7.5 cm); greenish gray background color; small warts scattered over back, arms, and legs; each wart is reddish brown and surrounded by a black circle; round gland (parotoid gland) slightly smaller than upper eyelid, located behind each eye. Habitat: This toad occurs near springs and semipermanent streams in and around Creosote Bush Scrub. Red-spotted Toads are nocturnal but may be found during the day under rocks adjacent to streams. Remarks: This species can be distinguished from other toads in the area by the small red spots and the round parotoid gland that is smaller than the eyelid. It is a wide-ranging, predominantly Creosote Bush Scrub species that reaches the northern limits of its distribution in our area. At Darwin Falls in the northern Argus Mountains (see Map 9.6), Red-spotted Toads and Western Toads (B. boreas ) occur together. Locally, hybrids between the two species have been found; this is the only reported case in California of hybridization between Red-spotted Toads and other toads. Range: East side of the Argus and Inyo mountains in canyons draining into the Panamint and Saline valleys. Reference: Feder (1979).
Localities: California, Inyo Co.: Darwin Falls, Argus Mtns. (CAS, MVZ); Grapevine Canyon, Nelson Mtns.; Hunter Creek, Saline Valley (CAS, MVZ); below Jackass Spring, Panamint Mtns. (E of study area) (USNM); Pat Keyes Canyon, Saline Valley; Willow Creek, Saline Valley.
Treefrogs (Family Hylidae)
Pacific Treefrog,Pseudacris regilla (Baird & Girard, 1852). (Plate 9.8, Map 9.7) 1–2 in (2.5–5 cm); dorsal pattern variable, gray with brown blotches or uniform green; individual frogs can change color; dark stripe extends from nose through eye and beyond jaw; smooth skin; toe tips enlarged to form small adhesive pads. Habitat: In the White-Inyo Range this species is restricted to permanent streams and marshy areas. Treefrogs are mainly nocturnal and form breeding choruses during the spring and early summer. Some live during the day next to streams and on vegetation in marshes. The adhesive pads on their toes allow them to climb. Remarks: The Pacific Treefrog is a widespread and common frog in California. However, in the White-Inyo mountains region its distribution is limited. It appears to be restricted to the southern Owens Valley from Owens Lake to Independence, and it is present in the Queen Valley. It seems to be absent not only from the rest of the Owens Valley, but also from the White-Inyo Range. Suitable habitat is present in both areas, but
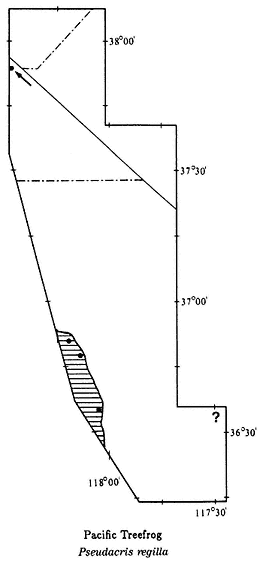
Map 9.7
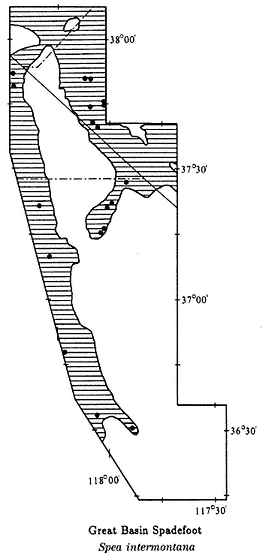
Map 9.8
no specimens have been reported. It is common, however, in several canyons in the Panamint Mountains. The 1891 U.S. Department of Agriculture Death Valley expedition collected two specimens in Cottonwood Canyon, in the northern Panamint Mountains. This locality is just east of the area covered in this chapter, and it is possible that Pacific Treefrogs may occur in the Panamint Mountain portion (see ? on Map 9.7). Range: Owens Lake to Independence in the southern Owens Valley; Queen Valley. Expected in the northwestern Panamint Mountains. References: Hedges (1986); Jameson, Mackey, and Richmond (1966).
Localities: California, Inyo Co.: 3,800 ft, Cottonwood Canyon, below Jackass Spring, Panamint Mtns. (E of study area) (USNM); 3 mi E Independence; 3 mi N; 0.8 mi S Lone Pine. Mono Co.: 40 N Bishop [ = Bramlett-Taylor Ranch Springs, 8 mi N Benton] (UMMZ).
Spadefoot Toads (Family Pelobatidae)
Great Basin Spadefoot Toad,Spea intermontana(Cope, 1883). (Plate 9.9, Map 9.8) 1–2 1/2 in (2.5–6.25 cm); dorsal background light gray with dark gray spots; skin smooth; a jet black oval protrudes from the bottom of each hind foot; eyes relatively large; fleshy bump; known as a boss, between eyes. Habitat: This species is the only amphibian in the area that is not restricted to the vicinity of permanent water. Typical habitat in our area is Great Basin Scrub below 6,500 ft (1,980 m). However, on the east side of Owens Lake they have been found in Creosote Bush Scrub (see Map 9.8). Spadefoots are strictly nocturnal. They are most active in the fall, in the spring, and during summer rains. Remarks: The smooth skin, black spades, and boss between the eyes distinguish this toad from all others in the region. Spadefoots may be observed on roads at night, most commonly during or after rains. In the day and during dry times of the year, they use their spades to dig backward into the soil. These toads are very resistant to water loss and may remain underground for long periods of time. A related species in southeastern California, the Couch's Spadefoot Toad (S. couchii ), has been known to remain underground for two years waiting for rains. The Great Basin Spadefoot Toad usually breeds during the spring in temporary ponds. The hatching of eggs into tadpoles and the transformation of tadpoles into toads may take place in a few weeks. Although Great Basin Spadefoots have not been observed in the White-Inyo mountains region above 6,500 ft (1,980 m), they have been found at an elevation of 9,200 ft (2,800 m) in the Bodie Hills north of Mono Lake. Range: West side of the White-Inyo Range in the entire Owens Valley, Chalfant Valley, Hammil Valley, Benton Valley, and Queen Valley; south and east side of the White Mountains in the Deep Springs and Fish Lake valleys. Reference: Mayhew (1962).
Localities: California, Inyo Co.: Big Pine; 0.5 mi NE; 5.6 mi NE; 27.8 mi NE; 0.3 mi E; Bishop; 3.0 mi N; Deep Springs (CAS); 7 mi S; Deep Springs Lake; NE end (LACM); Diaz Lake (CAS); 0.5 mi SE of Hwy. 168 on Eureka Valley Rd.; 1.4 mi W Independence; 2.0 mi SE; Lone Pine; 8.1 mi SE; 10.0 mi SE; 1.0 mi S Mono County line on Hwy. 168. Mono Co.: Benton; 3.2 mi N (LACM); 1.6 mi W (LACM). Nevada, Esmeralda Co.: 1 mi W Hwy. 264 along Chiatovich Creek, Fish Lake Valley; 5,000 ft, Chiatovich Ranch, Fish Lake Valley; Dyer; 1.8 mi S; 4.0 mi N; Fish Lake (MVZ, UMMZ); 1 mi S.
True Frogs (Family Ranidae)
Bullfrog,Rana catesbeiana(Shaw, 1802). (Plate 9.10, Map 9.9) 4–8 in (10–20 cm); background color light to dark green, uniform on back with crossbars on legs; conspicuous large eardrum behind eye. Habitat: This frog prefers slow-moving streams and rivers. It is also common in marshes associated with permanent ponds and lakes. Bullfrogs are active both during the day and at night. They are very wary, and when approached they commonly escape by jumping from the bank into the water. Remarks: Bullfrogs are not native to the area. They were originally introduced from the eastern United States to parts of western North America to provide a fresh source of meat. Natural expansion of this frog's range and further introductions have greatly
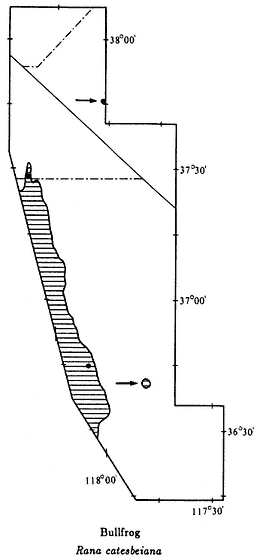
Map 9.9
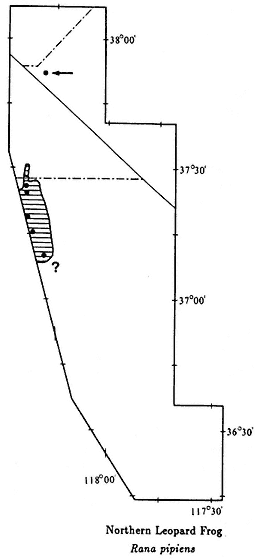
Map 9.10
increased the area in which Bullfrogs live. Unfortunately, they have had a disastrous effect on native amphibians because they prey on them. A large Bullfrog is capable of eating the young and even adults of the native frogs and toads. Red-legged Frogs (R. aurora ) and Foothill Yellow-legged Frogs (R. boylei ) are now extinct in many areas of central California due to the presence of Bullfrogs. In our area, they were recently introduced into the Fish Lake Valley, where Western Toads (Bufo boreas ) were formerly common. During the preparation of this chapter, extensive field work was conducted in the Fish Lake Valley, but no Western Toads were seen. Range: Introduced into the Owens Valley; Fish Lake, Fish Lake Valley; marshes around Salt Works, Saline Valley. References: Moyle (1973).
Localities: California, Inyo Co.: Owens River, 2 mi S Independence. Mono Co.: Fish Slough, 0.4 mi N Inyo County line. Nevada, Esmeralda Co.: Fish Lake.
Northern Leopard Frog,Rana pipiens (Schreber, 1782). (Plate 9.11, Map 9.10) 2 1/2–4 in (6.25–11.25 cm); background color gray or tan; distinct dark-brown or black spots over back and sides; spots on legs forming bars; pale-colored ridge runs down each side of the back from top of eyelid to hind leg; ventral surface uniformly pale. Habitat: Northern Leopard Frogs occur around permanent ponds and streams. They are active both during the day and at night. This is a wary species that usually jumps into the water when approached. Remarks: Leopard Frogs, which range from Panama to the Northwest Territories, Canada, are the most widely distributed group of frogs in North America. Until recently they were all considered one species. The Northern Leopard Frog is the widest-ranging species currently recognized in this group; it occurs from New Foundland to the western Great Basin. This species' wide range is presumed to be due to major expansions since the recession of the last glacial period, which started 18,000 years ago. Leopard Frogs are commonly used in biology class dissections and laboratories around the country. This practice has led to many introductions of this species where they did not formerly occur. Range: Northern Owens Valley from Big Pine to north of Bishop; may occur further south in the Owens Valley (see ? on Map 9.10); reported from the east side of the White Mountains below Boundary Peak. References: Hillis, Frost, and Wright (1983); Sage and Selander (1979).
Localities: California, Inyo Co.: 1 mi W Big Pine; 4 mi W Bishop (W of study area); 3.2 mi N (UMMZ); 5 mi N (LACM, MVZ); 8 mi S. Nevada, Esmeralda Co.: 7,100 ft, east side of White Mountains below Boundary Peak (UN).
References
Collins, J. T. 1990. Standard common and current scientific names for North American amphibians and reptiles, 3d ed. Society for the Study of Amphibians and Reptiles, Herpetological Circular no. 19.
Feder, J. H. 1979. Natural hybridization and genetic divergence between the toads Bufo boreas and Bufo punctatus. Evolution 33:1089–1097.
Hedges, B. S. 1986. An electrophoretic analysis of Holarctic hylid frog evolution. Systematic Zoology 35:1–21.
Hillis, D. M., J. S. Frost, and D. A. Wright. 1983. Phylogeny and biogeography of the Rana pipiens complex: A biochemical evaluation. Systematic Zoology 32:132–143.
Jameson, D. L., J. P. Mackey, and R. C. Richmond. 1966. The systematics of the pacific tree frog, Hyla regilla. Proceedings of the California Academy of Sciences 19:551–620.
Karlstrom, E. L. 1962. The toad genus Bufo in the Sierra Nevada of California. University of California Publications in Zoology 62:1–104.
Macey, J. R. 1986. The biogeography of a herpetofaunal transition between the Great Basin and Mojave deserts. In C. A. Hall, Jr. and D. J. Young (eds.), Natural history of the White-Inyo Range, eastern California and western Nevada, and high altitude physiology, pp. 119–128. University of California White Mountain Research Station Symposium, August 23–25, 1985, Bishop, Calif. vol. 1.
Marlow, R. W., J. M. Brode, and D. B. Wake. 1979. A new salamander, genus Batrachoseps, from the Inyo Mountains of California with a discussion of relationships in the genus. Natural History Museum of Los Angeles County, Contributions in Science, no 308.
Mayhew, W. W. 1962. Scaphiopus couchi in California's Colorado Desert. Herpetologica 18:153–61.
Moyle, D. B. 1973. Effects of introduced Bullfrogs, Rana catesbeiana, on the native frogs of the San Joaquin Valley, California. Copeia 1973:18–22.
Myers, G. S. 1942. The Black Toad of Deep Springs Valley, Inyo County, California. Occasional Papers, Museum of Zoology, University of Michigan, no. 469.
Papenfuss, T. J. 1986. Amphibian and reptile diversity along elevational transects in the White-Inyo Range. In C. A. Hall, Jr. and D. J. Young (eds.), Natural history of the White-Inyo Range, eastern California and western Nevada and high altitude physiology, pp. 129–136. University of California White Mountain Research Station Symposium, August 23–25, 1985, Bishop, Calif. vol. 1.
Sage, R. D., and R. K. Selander. 1979. Hybridization between species of the Rana pipiens complex in central Texas. Evolution 33:1069–1088.
Schuierer, F. W. 1962. Remarks upon the natural history of Bufo exsul Myers, the endemic toad of Deep Springs Valley, Inyo County, California. Herpetologica 17:260–266.
Stebbins, R. C. 1951. Amphibians of western North America . University of California Press, Berkeley and Los Angeles.
Stebbins, R. C. 1985. A field guide to western amphibians and reptiles . Houghton Mifflin, Boston.
Wake, D. B., L. R. Maxson, and G. Z. Wurst. 1978. Genetic differentiation, albumin evolution, and their biogeographic implications in Plethodontid salamanders of California and southern Europe. Evolution 32:529–539.
Yanev, K. P., and D. B. Wake. 1981. Genic differentiation in a relic desert salamander, Batrachoseps campi. Herpetologica 37:16–28.
10—
Reptiles
J. Robert Macey and Theodore J. Papenfuss
Introduction
The great majority of living reptiles are in the Order Squamata, which includes three major groups. The smallest is the Suborder Amphisbaenia. These are elongate, blind, burrowing reptiles. Most of the 200 or so species live in South America and Africa. All are limbless except for three species of the Genus Bipes in Mexico that have front legs. Only one amphisbaenian, the Florida Worm Lizard (Rhineura floridana ), occurs in the United States. The largest groups of squamate (scaly) reptiles are the snakes and lizards, with about 2,500 and 3,000 species, respectively. Snakes and lizards are the only reptiles present in the White-Inyo mountains region. By western North American standards, the White-Inyo mountains region, accommodating 37 known species, is relatively rich in reptiles.
Using the Chapter
Each species in the region is discussed in a species account, listed alphabetically by species name within each family, and illustrated with a color plate. All reptiles in the color plates are from the White-Inyo mountains region except the juvenile Southern Alligator Lizard (Elgaria multicarinata ), which is from Kern County, California; the juvenile Western Whiptail (Cnemidophorus tigris ), which is from Nye County, Nevada; and the Mojave Shovel-nosed Snake (Chionactis occipitalis occipitalis ), Striped Whipsnake (Masticophis taeniatus ), Spotted Leaf-nosed Snake (Phyllorhynchus decurtatus ), and Lyre Snake (Trimorphodon biscutatus ), which are from San Bernardino County, California. A range map shows the distribution of each species in the area. The species account gives a brief description that should be used in conjunction with the color plate for positive identification. Sizes are given for adult specimens; naturally, juveniles and hatchlings will be much smaller. The size stated is total length, from head to tip of tail. In most lizards the actual body length is only one-third to one-half the total length. The remarks section provides information of general biological interest for each species. The range of the species is summarized for the region, and this summary should be used in conjunction with the range map for the species. One or more literature references are listed for each species; the complete citations are at the end of the chapter. Two references, Stebbins (1954) and Stebbins (1985), provide general information on western reptiles. All scientific names are after Collins (1990). Five references that discuss detailed studies on the reptiles of this region or nearby desert areas are: Banta (1962) (Saline Valley), Macey (1986) (White-Inyo region), Miller and Stebbins (1964) (Joshua Tree National Monument), Papenfuss
(1986) (White-Inyo region), and Tanner and Jorgensen (1963) (Nevada Test Site, Nye County).
Finally, a list of exact localities based on museum specimens is provided. These localities correspond to dots on each range map. When the locality is vague, it is not plotted on a map. In these lists the first reference to a place name is listed in whole, and other references to that place name follow it as separate localities. Refer to maps 9.1 and 9.2 for a guide to place names, valleys, and elevation. The majority of the specimens are housed at the Museum of Vertebrate Zoology, University of California at Berkeley. The acronym for this institution (MVZ) does not follow the locality unless one or more other institutions have specimens from the same locality. In order to assist biologists who may wish to examine specimens from the region, the localities for specimens in other institutions are followed by the museum acronyms. The institutions with specimens from the area include: American Museum of Natural History, New York (AMNH); Brigham Young University (BYU); California Academy of Sciences, San Francisco (CAS); Carnegie Museum of Natural History, Pittsburgh (CMNH); Field Museum of Natural History, Chicago (MNH); University of Kansas (KU); Natural History Museum of Los Angeles County (LACM); Museum of Comparative Zoology, Harvard University (MCZ); Nevada State Museum (NSM); San Diego Natural History Museum (SDSNH); University of California at Santa Barbara (UCSB); University of Colorado Museum (UCM); University of Michigan Museum of Zoology (UMMZ); University of New Mexico (UNM); and the National Museum of Natural History (USNM). For accuracy, elevations and distances of localities from reference points are cited as recorded by the collector and have not been converted to or from the English or metric system.
Two additional sections are included. "Amphibian and Reptile Diversity in Selected Habitats" provides outlined information on how to find the species that occur in selected areas. This will aid in finding and observing amphibians and reptiles in the region. "Amphibian and Reptile Biogeography," which follows this introduction, presents a synthesis of the distributional data in this chapter and in Chapter 9, on amphibians. This section will help the observer understand why a particular species occurs in an area.
Observing Reptiles
Exact localities and habitats are listed in each account. Using this information along with the section "Amphibian and Reptile Diversity in Selected Habitats," at the end of the chapter, will facilitate the observation of reptiles.
Most of the lizard species are easy to observe. Except for the Western Banded Gecko (Coleonyx variegatus ), all are diurnal, and many are common in preferred habitats. Lizards are most easily seen when they are basking in the morning during the spring and early summer. In low-elevation desert areas, they are not active during the hot, dry midsummer. There is a second period of activity during September and October, followed by hibernation from November through March. At intermediate and high elevations, lizards come out of hibernation in May or June and are active throughout
the summer. Lizards tend to be wary, but by walking slowly and quietly, the observer can approach most species within a few feet.
Snakes are more difficult to find than lizards. Ten of the 19 species in the area are always active at night, and 5 more are nocturnal during hot weather. These species are best found by "night driving," a technique used by herpetologists that involves driving slowly at night along deserted paved roads and watching for snakes either lying on or crossing the road. This method works best on warm nights when the moon is not full. Snakes tend to be much less active during the full moon, perhaps because they are more easily seen by predators then. When snakes are on the road, they can be approached and even picked up with ease. Rattlesnakes, of course, should be avoided. Several of the little-traveled roads in the region are good for night driving. These include the Lone Pine-Death Valley road (Hwy. 136), the Big Pine-Eureka Valley road (Eureka Valley Road), the Big Pine-Westgard Pass road (Hwy. 168) below 6,000 ft (1,830 m), the road across Deep Springs Valley (Hwy. 168), the Fish Lake Valley roads (east-west Hwy. 168 and Hwy. 266, north-south Hwy. 266 and Hwy. 264), and the Benton-Lee Vining road (Hwy. 120). Highway 395 through the Owens Valley and Hwy. 6 through the Chalfant, Hammil, Benton, and Queen valleys have too much traffic, but short side roads in these valleys are alternatives.
Diurnal snakes are occasionally seen by hikers, but they are more commonly seen on the road in the morning and late afternoon. During the daytime snakes tend to be more alert and wary. Species such as the Coachwhip (Masticophis flagellum ), the Striped Whipsnake (M. taeniatus ), and the Western Patch-nosed Snake (Salvadora hexalepis ) are surprisingly quick and will flee when approached. Aquatic species such as the Sierra Garter Snake (Thamnophis couchii ) and the Western Terrestrial Garter Snake (T. elegans ) are slow on land, but once in the water they can escape with ease.
Amphibian and Reptile Biogeography
The amphibians and reptiles that occur in the White-Inyo mountains region show several different distributional patterns. This is due in part to changes in desert vegetation in the area. The southern valleys, which are relatively low in elevation, have Creosote Bush (Larrea tridentata ) as the dominant plant. This is typical of the Mojave Desert as well as other southern deserts in North America. Panamint Valley, Saline Valley, Eureka Valley, and the western slopes of the Inyo Mountains in southern Owens Valley all have Creosote Bush Scrub. The higher, northern valleys, where Creosote Bush is absent, have other dominant plants, such as Shadscale (Atriplex confertifolia ). Fish Lake Valley, Deep Springs Valley, Owens Valley (except the southwestern part), Chalfant Valley, Hammil Valley, Benton Valley, and Queen Valley all feature this Great Basin Scrub desert. The change in desert foliation causes a south-to-north transition in species of amphibians and reptiles.
The amphibians and reptiles can be grouped into five distributional patterns (see Table 10.1). The southern group consists of species whose ranges are primarily south of the area in Creosote Bush Scrub; these species typically occur in the Mojave Desert. In the White-Inyo mountains region, two species appear to follow the distribution
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
of Creosote Bush closely: the Desert Iguana (Dipsosaurus dorsalis ) and the Western Blind Snake (Leptotyphlops humilis ). However, the Western Banded Gecko (Coleonyx variegatus ), Common Chuckwalla (Sauromalus obesus ), Desert Night Lizard (Xantusia vigilis ), Southwestern Black-headed Snake (Tantilla hobartsmithi ), Sidewinder (Crotalus cerastes ), and Speckled Rattlesnake (Crotalus mitchellii ), extend north of the Creosote Bush Scrub into Great Basin Scrub. The Red-spotted Toad (Bufo punctatus ), the only southern amphibian in the area, requires a moist habitat with permanent water. It is absent from the Eureka and southern Owens valleys. The Glossy Snake (Arizona elegans ) and Sidewinder (Crotalus cerastes ) seem to be absent from the Saline and Eureka valleys. The Western Shovel-nosed Snake (Chionactis occipitalis ) has not been found in the Eureka Valley. The Rosy Boa (Lichanura trivirgata ), Spotted Leaf-nosed Snake (Phyllorhynchus decurtatus ), and Lyre Snake (Trimorphodon biscutatus ) have yet to be recorded from any valley except the Panamint.
The wide-ranging species have distributions in both the Mojave Desert and the Great Basin Desert. These species occur in every valley, with the exception of the Zebra-tailed Lizard (Callisaurus draconoides ), which appears to be absent from Deep Springs Valley.
Species in the northern group generally avoid Creosote Bush Scrub. They have distributions to the north in the Great Basin Desert. Many of these species also occur to the west of the Sierra Nevada, and some are found isolated on high mountains in the Mojave Desert. The Northern Leopard Frog (Rana pipiens ) is restricted to the Owens River and its tributaries in the northern Owens Valley, and apparently along creeks draining the eastern White Mountains, since there is one record from 7,100 ft (2,160 m). The Western Toad (Bufo boreas ), Pacific Treefrog (Pseudacris regilla ), and Great Basin Spadefoot Toad (Spea intermontana ), which tend to be near water, each have one record in the range of Creosote Bush. The Western Skink (Eumeces skiltonianus ) is mainly distributed in mountain areas in the vicinity of water and avoids Creosote Bush Scrub in this area. The Sagebrush Lizard (Sceloporus graciosus ) mainly occurs in high mountain areas but it also occurs on the valley floors of the southern Owens, northern Owens, Chalfant, Hammil, Benton, Queen, and Fish Lake valleys. The Western Fence Lizard (Sceloporus occidentalis ) occurs in or around all valleys, but at higher elevations around the Panamint Valley. The Western Rattlesnake (Crotalus viridis ) is present only in the Queen and Fish Lake valleys, where it may be hybridizing with the Speckled Rattlesnake (Crotalus mitchellii ). The Striped Whipsnake (Masticophis taeniatus ) is present in all northern valleys, as is the Western Terrestrial Garter Snake (Thamnophis elegans ). The latter is also in the southern Owens Valley.
The five endemic species occur only in the White-Inyo mountains region and directly adjacent areas. The Inyo Mountains Salamander (Batrachoseps campi ) occurs at 13 springs in the Inyo Mountains, and is in the Saline Valley and southern and northern portions of the Owens Valley. The Kern Plateau Salamander (Batrachoseps sp.) occurs along creeks draining the Sierra Nevada in the southern Owens Valley. The Owens Valley Web-toed Salamander (Hydromantes sp.) is present along creeks draining the Sierra Nevada in the southern and northern portions of the Owens Valley. The Black Toad (Bufo exsul ), a close relative of the Western Toad (Bufo boreas ), occurs
only in the Deep Springs Valley, where the Western Toad (Bufo boreas ) is absent. The Panamint Alligator Lizard (Elgaria panamintina ) is recorded from the Panamint and Saline valleys and throughout the eastern portion of the Owens valley. A site record for the west side of the White Mountains in the Hammil Valley also exists.
Species in the western group have distributions mainly to the west of the Sierra Nevada. The Southern Alligator Lizard (Elgaria multicarinata ) is distributed along the east side of the Sierra Nevada at least from Olancha to Grant Lake, Mono County. The Gilbert Skink (Eumeces gilberti ) is present in springs at low elevations and in a variety of habitats at higher elevations. It occurs in many mountain ranges throughout the Mojave Desert and in all Creosote Bush Scrub valleys, but it appears to be absent from the western slopes of the Inyo Mountains in the southern Owens Valley. It also occurs on the western slopes of the White Mountains in the northern Owens Valley and in the southeastern slopes of the White Mountains in the Deep Springs and Fish Lake valleys. The Sierra Garter Snake (Thamnophis couchii ) is present in the southern Owens, northern Owens, and Chalfant valleys.
Figures 10.1 and 10.2 show the percentages of southern, wide-ranging, northern, endemic, and western species in each of the valleys. In general, as southern species decline in number, northern species increase. The valleys to the east of the White-Inyo Range show an abrupt transition between the northern fauna of the Great Basin Desert and the southern fauna of the Mojave Desert. In the Panamint, Saline, and Eureka valleys, which contain Creosote Bush, a few northern species occur. In contrast, the Fish Lake Valley to the north, in which no Creosote Bush is present, has 8 northern species. Additionally, the only southern species in the Fish Lake Valley is the Speckled Rattlesnake (Crotalus mitchellii ). To the south in the Eureka Valley there are 6 southern species, in the Saline Valley 9, and in the Panamint Valley 14.
On the west side of the White-Inyo Range a more gradual transition between the two desert fauna exists. The southern Owens Valley has 7 northern species, although it contains Creosote Bush desert. The northern Owens Valley contains 8, and the Chalfant, Hammil, Benton and Queen valleys together have 9 northern species. In the latter grouping of valleys only 3 southern species — the Desert Night Lizard (Xantusia vigilis ), Sidewinder (Crotalus cerastes ), and Speckled Rattlesnake (Crotalus mitchellii ) — occur, yet the northern Owens Valley, which lacks Creosote Bush, has 6 species. In the southern Owens Valley there are 10 southern species.
The Deep Springs Valley between the Inyo and White mountains contains Great Basin Scrub desert. The Speckled Rattlesnake (Crotalus mitchellii ) is the only southern species present in the valley. The wide ranging Zebra-tailed Lizard (Callisaurus draconoides ) appears to be absent from this valley. In addition, only four northern species occur here, along with one western species and one endemic.
To illustrate the relationships among valleys, two trees are included. These trees were derived from two formulas, called faunal resemblance factors. The Braun-Blanquet faunal resemblance factor (BFRF) emphasizes the differences between the species compositions of different geographic regions (Braun-Blanquet, 1932). It is calculated by taking the number of species in common (C ) to two sites and dividing it by the total number present at the site with the larger number of species (NL ).
Figure 10.1
Amphibian and reptile distributional patterns on the two sides of the White-Inyo Range. Above are the valleys to the east
of the White-Inyo Range. Below are the valleys to the west of the White-Inyo Range. Note that the Chalfant Valley is
included with Hammil, Benton, and Queen valleys.
Figure 10.2
Amphibian and reptile distributional patterns across the White-Inyo Range.
The resulting value is multiplied by 100 to take the form of a percentage.
BFRF = (C/NL )100
Alternatively, the Simpson faunal resemblance factor (SFRF) emphasizes the similarities between the species compositions of different geographic regions (Simpson, 1960). It is calculated by taking the number of species in common (C ) to two sites and dividing it by the total number present at the site with the smaller number of species (N S ).
SFRF = (C/NS )100
The numbers that were derived are presented in Table 10.2. To show the relation between the valleys in the White-Inyo mountains region, two sites outside the area were included. In the Mojave Desert, the Granite Mountains south of Kelso, San
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Figure 10.3
The Baun-Blanquet faunal resemblance factor (BFRF) percent similarity tree, which
emphasizes differences.
Bernardino County, California, was used. In the Great Basin Desert, Pyramid Lake, Washoe County, Nevada, was used.
The trees are presented in Figs. 10.3 and 10.4. They illustrate the abrupt transition between the two desert fauna present on the east side of the White-Inyo Range. Both trees cluster the Panamint, Saline, and Eureka valleys with the Granite Mountains in the Mojave Desert. Both trees also cluster the Chalfant (including the Hammil, Benton, and Queen valleys), Deep Springs, and Fish Lake valleys with Pyramid Lake
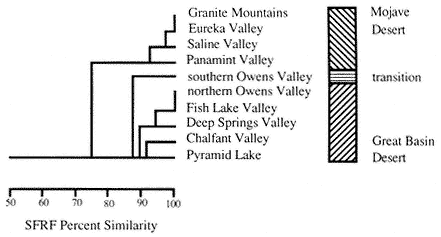
Figure 10.4
The Simpson faunal resemblance factor (SFRF) percent similarity tree, which
emphasizes similarities.
in the Great Basin Desert. The two trees differ on the positioning of the southern Owens and northern Owens valleys. The BFRF, emphasizing differences, places the southern and northern portions of the Owens Valley together in a subcluster of the Mojave desert cluster. The SFRF, emphasizing similarities, places the southern Owens Valley furthest out from the Great Basin Desert cluster and puts the northern Owens Valley with the Fish Lake Valley. This illustrates a more gradual and broad transition between the two fauna in the Owens Valley to the west of the White-Inyo Range. This is due to a wider range of habitats present in the Owens Valley. The Owens River and the creeks that drain the eastern Sierra Nevada provide riparian habitat and cooler conditions for northern species. The Creosote Bush Scrub in the southern Owens Valley and the gradual elevational increase from Owens Lake to the Queen Valley provide a corridor for southern species to range further north (Macey, 1986; Murphy, 1983).
Species Accounts
Alligator Lizards (Family Anguidae)
Southern Alligator Lizard,Elgaria multicarinata(Blainville, 1835). (Plates 10.1 and 10.2, Map 10.1) 8–16 in (20–40 cm); brown to gray dorsal background color; narrow light brown bands, about one scale wide, across top of back, turning darker on sides; white fleck at end of each scale in dark brown bands on side; distinct fold with granular scales between side and belly; up to two-thirds of total length may be tail. Habitat: In the White-Inyo mountains region this species seems to be restricted to the vicinity of permanent water. Southern Alligator Lizards are active during the day and early evening, but they are seldom seen because they are secretive and spend much of their time in leaf litter. Remarks: The subspecies of Southern Alligator Lizard occurring in this area is the San Diego Alligator Lizard (E. m. webbii ), which is known from a single locality in the Alabama Hills near Lone Pine (see Map 10.1). It is a wide-ranging species in California west of the Sierra Nevada that enters the area from the south and extends north along the east side of the Sierra Nevada. The five localities (four outside of our area) in Inyo County are all west of the Owens River. The only species that could be confused with this lizard is the Panamint Alligator Lizard (E. panamintina ), which has body bands three to four scales wide rather than one scale wide. Although the two species have not yet been found together, it is possible that they coexist in the Owens Valley. Range: Owens Valley west of the Owens River and canyons draining the east slope of the Sierra Nevada at least between Olancha, Inyo County, and Grant Lake, Mono County. References: Fitch (1935), Fitch (1938), Good (1988a), Good (1988b), Stebbins (1985).
Localities: California, Inyo Co.: 1,730 m, Division Creek, 13 mi NNW Independence (W of area); 6,000 ft, 5 mi W and 1.25 mi S Independence (W of area); 4,400 ft, 2.75 mi NW Lone Pine, Alabama Hills; 5,700 ft, south fork of Oak Creek, 5.0 mi W Independence (W of area); 5,200 ft, Walker Creek, 4 mi SW Olancha (S of area).
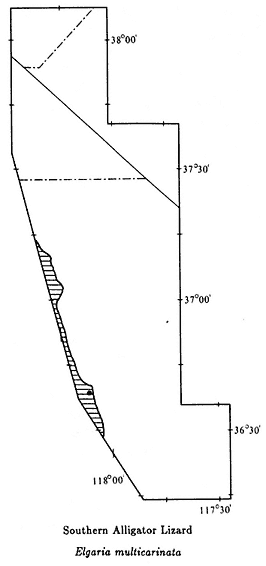
Map 10.1
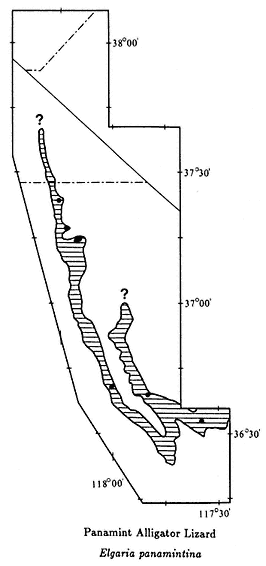
Map 10.2
Panamint Alligator Lizard,Elgaria panamintina(Stebbins, 1958). (Plates 10.3 and 10.4, Map 10.2) 7–14 in (17.5–35 cm); gray dorsal background with light to dark-brown bands three to four scales wide, circling the top and sides; white flecks on sides behind each band; ventral coloration cream with gray flecks; banded tail longer than body. Habitat: This lizard is most common in rocky canyons in the vicinity of springs and streams. Panamint Alligator Lizards are not commonly encountered because they spend much of their time in rock piles or brush. They can sometimes be seen during the late afternoon and early evening basking in the open. Remarks: This species was first discovered in 1954 in Surprise Canyon, Panamint Mountains. It has since been found in the Argus, Nelson, Inyo, and southern White mountains. Panamint Alligator Lizards at French Spring in the Inyo Mountains occur less than
10 mi (16 km) east of the Alabama Hills, where Southern Alligator Lizards (E. multicarinata ) live (see Maps 10.1 and 10.2). Although it is geographically close to the Southern Alligator Lizard, its nearest relative is the Madrean Alligator Lizard (E. kingii ), which occurs 300 mi (480 km) to the southeast, in Arizona. At one time a single species of alligator lizard ranged from Arizona to the White-Inyo mountains region. The formation of the Great Basin Desert and the drying of the Mojave Desert separated this population, leaving the Panamint Alligator Lizard isolated. Since its discovery, fewer than 30 specimens have been deposited in the collections of major museums. This species is now protected by state law and should not be collected. Range: Panamint, eastern Argus, Nelson, Inyo, and western White mountains below 7,500 ft (2,290 m); site record for the western slopes of the White Mountains above Hammil Valley; may occur more widely (see ? on Map 10.2). References: Banta (1962), Good (1988a), Good (1988b), Stebbins (1958).
Localities: California, Inyo Co.: 1 mi E Batchelder Spring, Westgard Pass (LACM); 1.5 mi NE; 9.8 mi NE Big Pine; 6,650 ft, 10.1 mi NE; Daisy Canyon, Saline Valley (CAS); 6,000 ft, French Spring, Inyo Mtns.; 4,850 ft (CAS, LACM), 5,000 ft (CAS), 5,030 ft (CAS), 5,100 ft (CAS), Grapevine Canyon, Nelson Mtns.; 6,800 ft, Marble Canyon, White Mtns.; Silver Creek Canyon, White Mtns.
Geckos (Family Gekkonidae)
Western Banded Gecko,Coleonyx variegatus(Baird, 1858). (Plate 10.5, Map 10.3) 3–6 in (7.5–15 cm); pale yellow or cream background with brown bands on body and tail; in large individuals body bands may separate into blotches; regenerated tails short, fat, and unbanded; eyes large and catlike with vertical pupils. Habitat: This species is most abundant in rocky areas where Creosote Bush is present, but it has also been found in Great Basin Desert Scrub below 6,000 ft (1,830 m). These geckos are active only at night. During the day they take shelter under rocks, in cracks, or in rodent burrows. Remarks: The subspecies of the Western Banded Gecko in this area is the Desert Banded Gecko (C. v. variegatus ). Geckos from the Owens Valley have a more distinct banded pattern than those found in the other valleys, which tend to have more broken bands and speckling. This is the only member of the gecko family in our area. Geckos are primarily a tropical group. Over 800 species are known worldwide, only 5 of which are native to the southwestern United States. The northernmost known locality for this species is in the Eureka Valley. They can commonly be seen at night on roads that go through rocky areas. They are especially abundant during the late spring and early summer on the Eureka Valley Road. Banded Geckos readily lose their tails as a defense mechanism against predators, and many adults have regenerated tails. Range: Throughout the Panamint, Saline, and Eureka valleys and extending into the mountains surrounding those valleys; Owens Valley to at least Big Pine and Westgard Pass; a site record for east of Bishop (see ? on Map 10.3). References: Congdon, Vitt, and King (1974); Klauber (1945).
Localities: California, Inyo Co.: Big Pine; 4,500 ft, 4.7 mi NE; 4,850 ft, 5.5 mi NE; 6.0 mi NE; 7.0 mi NE; Darwin Falls; 0.4 mi SE of Hwy. 168 on Eureka Valley
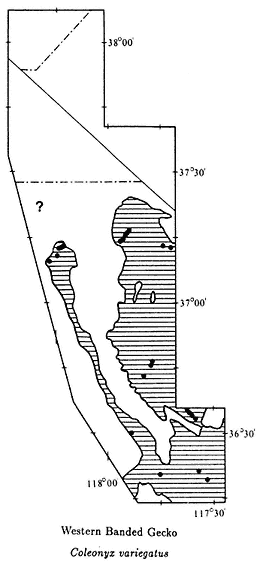
Map 10.3
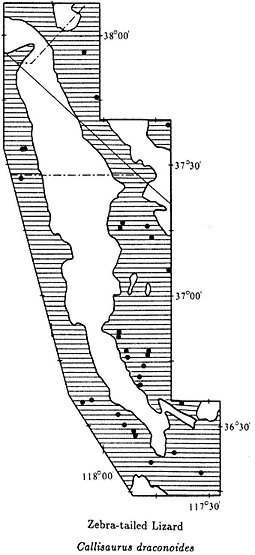
Map 10.4
Rd.; 5,750 ft, 22.3 mi SE; 5,600 ft, 22.6 mi SE; 24.0 mi SE; 24.4 mi SE; 24.5 mi SE; 24.6 mi SE; 24.8 mi SE; 24.9 mi SE; 25.2 mi SE; 25.5 mi SE; 25.9 mi SE; 26.0 mi SE; 26.3 mi SE; 26.7 mi SE; 27.5 mi SE; 27.7 mi SE; 27.8 mi SE; 28.2 mi SE; 37.3 mi SE; 40.3 mi SE; 2,300–4,030 ft, Grapevine Canyon, Nelson Range (CAS); 8.9 mi SE of Hwy. 136 on Hwy. 190; 7.5 mi W Panamint Valley Rd. on Hwy. 190 (CAS); 37.2 mi W; 1,060–1,200 ft along Warm Springs Rd., Saline Valley (CAS).
Iguanids (Family Iguanidae)
Zebra-tailed Lizard,Callisaurus draconoides(Blainville, 1835). (Plates 10.6 and 10.7, Map 10.4) 5–9 in (12.5–22.5 cm); dorsal pattern gray, mottled with white
flecks; faint black blotches down middle of back, forming bands on tail; ventral surface white with paired black bars on each side; in males the bars are surrounded by blue; underside of tail black- and white-banded. Habitat: Zebra-tailed Lizards are most common in valley floors and along sandy washes below 5,000 ft (1,520 m). This diurnal lizard has a high tolerance to heat and may be active in the middle of the day, even during the hot summer. Remarks: The subspecies of Zebra-tailed Lizard in the area is the Common Zebra-tailed Lizard (C. d. draconoides ). This species, which ranges to the tip of Baja California and Sinoloa, Mexico, is common throughout much of the western deserts. The dorsal pattern provides good camouflage for these animals. They commonly are not seen until they run. This is an extremely fast and wary lizard that has been clocked at nearly 20 miles (30 km) per hour for short distances. Just before they run, they usually curl and wag their banded tail over their back. Range: On the west side of the White-Inyo Range, in the entire length of the Owens Valley, and the Chalfant, Hammil, Benton, and Queen valleys; on the east side of the White-Inyo Range, in the Panamint, Saline, Eureka, and Fish Lake valleys below 6,000 ft (1,830 m). Appears to be absent from the Deep Springs Valley. References: Kay, Miller, and Miller (1970); Pianka and Parker (1972).
Localities: California, Inyo Co.: Aeolian Sand Dunes, Saline Valley (CAS); 5.5 mi N Bishop (UCSB); 4,600 ft, 12.5 mi N, 0.5 mi W (LACM); Darwin Falls (CAS, LACM, SDSNH); Darwin Wash; 1,900 ft, Daisy Canyon, Saline Valley (CAS); 27.7 mi SE of Hwy. 168 on Eureka Valley Rd.; 29.1 mi SE; jct. Eureka Valley Rd. and North Eureka Valley Rd.; 3.9 mi N of Eureka Valley Rd. on North Eureka Valley Rd.; E side of Eureka Valley sand dunes; 2,280–3,225 ft, Grapevine Canyon, Nelson Range (CAS); Grapevine Canyon, Nelson Range (SDSNH); Keeler; 1 mi S (LACM); between Lone Pine and Independence (CAS); Lone Pine (MVZ, USNM); 2.3 mi W (LACM); 2.9 mi W (LACM); 1.5 mi S, 7 mi E (UCM); 10.0 mi SE; 23 mi E; 5.6 mi N of Hwy. 190 on Saline Valley Rd.; 1,100 ft, center along dirt rd., Saline Valley (CAS); 1, 175–2,350 ft, N end along dirt rd. (CAS); Seven Springs, Saline Valley (CAS); 1,090 ft, Tramway, Saline Valley (CAS); 890–1,200 ft, Warm Springs Rd., Saline Valley (CAS); Willow Creek, Saline Valley (MVZ, SDSNH). Mono Co.: Chalfant (SDSNH). Nevada, Esmeralda Co.: Fish Lake; Jct. Hwy. 264 and Hwy. 774; 7.9 mi SSW Silver Peak.
Great Basin Collard Lizard,Crotaphytus bicinctores(Smith & Tanner, 1972). (Plate 10.8, Map 10.5) 8–14 in (20–35 cm); tan background color with narrow, broken yellow bands and small gray spots on back; a distinct black collar around neck, which is split in the middle with a white and gray band; head appears very large in proportion to body; tail long, about twice body length. Habitat: Collard Lizards occur in rocky areas throughout the study area up to an elevation of at least 6,500 ft (1,980 m) at the lower extent of the Pinyon-juniper Woodland. They are commonly observed basking on boulders. Remarks: The diet of this species consists mainly of insects and other lizards. The collared lizards (Genus Crotaphytus ) and the leopard lizards (Genus Gambelia ) are closely related and have considerable dietary overlap. They avoid competing by living in different habitats (see Long-nosed Leopard Lizard,
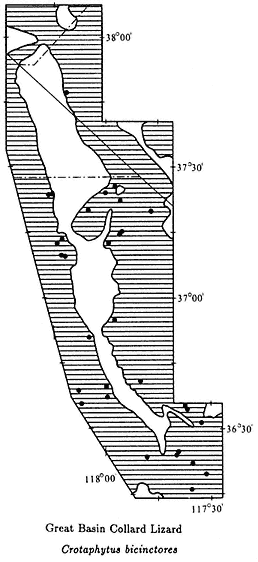
Map 10.5
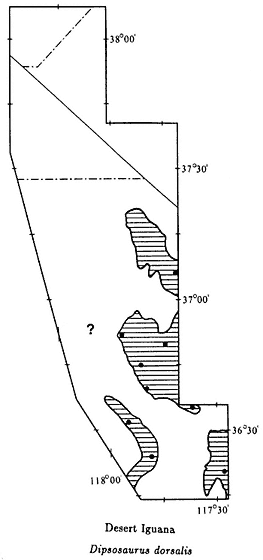
Map 10.6
G. wislizenii ). Until recently, only two species of collard lizards were recognized: the Reticulate Collard Lizard (C. reticulatus ), occurring in a limited area of southern Texas and northern Mexico, and a wide-ranging species occurring from Idaho to southern Baja California and from California to eastern Missouri. Recent studies suggest that the latter species be partitioned into three species: the Common Collard Lizard (C. collaris ), ranging from western Arizona to eastern Missouri; the Baja California Collard Lizard (C. insularis ), occurring from southern California to southern Baja California; and the Great Basin Collard Lizard, which lives from southern California and western Arizona to Idaho. Some workers consider C. bicinctores a subspecies of C. insularis . Range: Throughout the White-Inyo mountains region below about 6,500 ft (1,980 m). References: Axtell (1972); Montanucci (1983); Montanucci, Axtell, and Dessauer (1975); Sanborn and Loomis (1979); Smith and Tanner (1974).
Localities: California, Inyo Co.: Antelope Spring, Deep Springs Valley (LACM); 5 mi E Big Pine; 6,000 ft, 9 mi NE (AMNH); Darwin (CAS); Darwin Falls (CAS, LACM, MVZ); 6,200 ft, 4.0 mi NE Deep Springs (LACM); NW end Deep Springs Valley (LACM); 3 mi SE Hwy. 168 on Eureka Valley Rd., (BYU); 4.5 mi SE; 19.8 mi SE; 24.1 mi SE; 25.2 mi SE; 9.1 mi N Eureka Valley Rd. on North Eureka Valley Rd.; French Spring, Inyo Mtns.; 2,300–4,480 ft, Grapevine Canyon, Nelson Range (CAS); 0.5 mi W Inyo National Forest on Hwy. 168; 8 mi SE Keeler (CAS, SDSNH); 4.0 mi E Laws; 4,200 ft, Long John Canyon, Inyo Mtns.; 4.5 mi NE Lone Pine; Lone Pine Creek, ridge S of Wilson Ranch; Mazourka Canyon, Inyo Mtns. (SDSNH); 7.5 mi W Panamint Springs (CAS); Pearly Gates, 7 mi S Saline Valley; S end Saline Valley (CAS, BYU); W end Saline Valley (CAS); 5.6 mi N of Hwy. 190 on Saline Valley Rd.; 7 mi N; 1 mi W Salt Lake, Saline Valley (LACM); 4,600 ft, 7,000 ft, Silver Creek Canyon, White Mtns.; Waucoba Wash area, 33.3 mi from Big Pine, W end Saline Valley (CAS). Nevada, Esmeralda Co.: Indian Canyon, White Mtns.
Desert Iguana,Dipsosaurus dorsalis (Baird & Girard, 1852). (Plate 10.9, Map 10.6) 12–16 in (30–40 cm); dorsal coloration light brown to gray with darker reddish brown mottling; mottling forms bands on tail; sides and belly cream with some reddish flecks on sides; dorsal pattern fades during heat of day, giving this species a bleached appearance; row of enlarged scales runs down middle of back. Habitat: Desert Iguanas almost always occur near Creosote Bush. Although this species is a habitat generalist, it prefers flat, sandy washes below 5,000 ft (1,520 m). These lizards are active on warm days and can be seen basking when it is too hot for other lizards. Remarks: The diet of Desert Iguanas consists mainly of plants. The flowers and leaves of the Creosote Bush provide a major source of food. These lizards are active at very high temperatures and have been recorded with body temperatures as great as 115°F (64°C), which is higher than for any other species of lizard in the area. Desert Iguanas are widely distributed in the southwestern United States and northwestern Mexico. This species, along with the Chuckwalla (Sauromalus ), is related to the giant iguanas of Mexico and Central and South America, including the well-known Galapagos Marine Iguana. Desert Iguanas are very common in the Panamint and Saline valleys, where they may be observed basking on small rocks or under Creosote Bush along roads. They are not very wary and can be approached closely. Range: On the east side of the Inyo Mountains in the Panamint, Saline, and Eureka valleys; on the west side of the Inyo Mountains in the southern Owens Valley, north to at least the vicinity of Lone Pine (see ? on Map 10.6). References: Norris (1953), Pianka (1971).
Localities: California, Inyo Co.: Near Big Horn Mine, Hunter Canyon, Saline Valley (USNM); Darwin Falls; sand dunes, S end Eureka Valley; 8 mi SE Keeler (SDSNH); 2,280 ft, 3,150 ft, Grapevine Canyon, Nelson Range (CAS); 10.0 mi SE Lone Pine; 4,100 ft, 3 mi E Owens Lake (USNM); Panamint Springs; N Saline Valley (CAS); 5 mi N Salt Works, Saline Valley; upper Warm Springs Valley, Saline Valley (LACM); Willow Creek, Saline Valley.
Long-nosed Leopard Lizard,Gambelia wislizenii(Baird & Girard, 1852). (Plate 10.10, Map 10.7) 10–16 in (25–40 cm); narrow cream-colored bands across back, extending to the tail; areas between bands are brown with cream rings, making back appear spotted; tail very long, about twice body length; brown stripes on chin fading toward throat; belly uniform cream or with small gray spots. Habitat: In our area, leopard lizards occur in all habitats up to at least 7,500 ft (2,290 m) in the White Mountains. They are most common in flat, sandy Great Basin Scrub and Creosote Bush Scrub areas but also occur in rocky areas and well into the Pinyon-juniper zone. They are very numerous in the Eureka Valley, where they can be seen in the early morning basking on rocks along the side of the road. Remarks: The subspecies of Long-nosed Leopard Lizard occurring in the White-Inyo mountains region is the Large-spotted Leopard Lizard (G. w. wislizenii ). In addition to feeding on insects, this large, aggressive lizard feeds on other lizards, occasionally even members of its own species. They are sit-and-wait predators that perch on rocks or lie under bushes until a prey item wanders by. This is a species with a wide range that occurs from Idaho to southern Baja California and from California to Texas. The only other member of the genus is the Blunt-nosed Leopard Lizard (G. sila ), which occurs in the San Joaquin Valley of California. It is on the Federal Endangered Species List due to extensive habitat loss from agricultural development. Range: Throughout the White-Inyo mountains region below 7,500 ft (2,290 m). References: Parker and Pianka (1976), Tollestrup (1982).
Localities: California, Inyo Co.: Aberdeen (LACM); 5.6 mi N Bishop (UCSB); Darwin (USNM); 15 mi N; Darwin Falls, Argus Mtns. (CAS, MVZ); Deep Springs; 4 mi SW; NW end Deep Springs Valley (LACM); 6.3 mi SE of Hwy. 168 on Eureka Valley Rd.; within 1.0 mi W of North Eureka Valley Rd. on Eureka Valley Rd.; 1.6 mi E; 10.4 mi N Eureka Valley Rd. on North Eureka Valley Rd.; Fish Lake Valley; French Spring, Inyo Mtns.; 2,300–5,570 ft, Grapevine Canyon, Nelson Range (CAS); Independence (MVZ, USNM); 0.5 mi W (AMNH); Keeler; 1 mi S (LACM); Lone Pine (LACM, MCZ, USNM); Lone Pine Creek; between Lone Pine and Independence (CAS); Mazourka Canyon, Inyo Mtns.; 14.6 mi NE Olancha (LACM); N Saline Valley (CAS); 1 mi W Salt Lake, Saline Valley (LACM); 1,190–1,300 ft, Warm Springs Rd., Saline Valley (CAS). Mono Co.: Benton; 4,840 ft, Cinnamon Ranch, 10 mi SSE; 6.0 mi S Nevada state line on Hwy. 266. Nevada. Esmeralda Co.: 8 mi S Hwy. 6 on Hwy. 264; 7,000 ft, Chiatovich Creek, White Mtns.; 4,900 ft, Chiatovich Ranch, Fish Lake Valley; 4,800 ft, 7 mi E (E of area); Fish Lake; 7,400 ft, Indian Canyon, White Mtns.; 3.7 mi S of Leidy Creek Rd. on Hwy. 264, Fish Lake Valley (BYU); 6,000 ft, 9 mi W Lida Summit; jct. of rd. to Middle Creek and Hwy. 264, Fish Lake Valley; 8.4 mi SSW Silver Peak. Mineral Co.: 1.6 mi NE of California state line on Hwy. 6.
Desert Horned Lizard,Phrynosoma platyrhinos(Girard, 1852). (Plate 10.11, Map 10.8) 4–6 in (10–15 cm); dorsal background gray to light brown with broken black stripe running down back; ventral color white; flattened oval body; horns at back of head; tail short. Habitat: In this area Desert Horned Lizards are present in
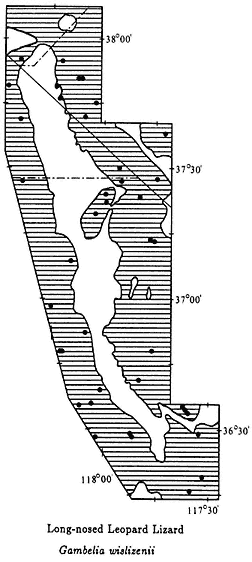
Map 10.7
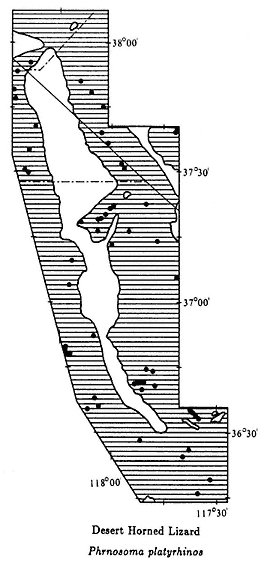
Map 10.8
all habitats up to about 7,000 ft (2,130 m) in the Pinyon-juniper Woodland. They are most common in valley floors and sandy washes. These lizards are often seen in the early morning and late afternoon basking on small rocks at the edge of roads. They are especially abundant in low-elevation Creosote Bush Scrub valleys. Remarks: The White-Inyo mountains region is an area of intergradation between the Southern Desert Horned Lizard (P. p. calidiarum ) and the Northern Desert Horned Lizard (P. p. platyrhinos ). The northern form has shorter horns than the southern form. Horned lizards, commonly called "horned toads," are one of the most specialized groups of lizards in North America. There are 14 species in the genus Phrynosoma , which occurs from southern Canada to northern Guatemala. They are all similar in body shape and feed in large part on ants. Horned lizards are easily approached and can be picked
up by hand because they rely on camouflage and their horns for protection. Range: Throughout the White-Inyo mountains region below 7,000 ft (2,130 m). References: Pianka and Parker (1975), Tanner and Krogh (1973).
Localities: California, Inyo Co.: 2,200 ft, 3,800 ft, 6,200–6,400 ft, near Big Horn Mine, Hunter Canyon, Saline Valley (USNM); 20.8 mi NE Big Pine; 23.3 mi NE; 26.3 mi NE; Bog Mound Spring, Deep Springs Valley; 4,500 ft, Daisy Canyon, Inyo Mtns. (CAS); Darwin; Darwin Falls; Deep Springs (BYU, LACM); 5.0 mi W (UNM); 5.2 mi W (UNM); 7.5 mi S (UMMZ); 12 mi W (UNM); Deep Springs Lake (LACM); 2,800 ft, 2,930 ft S end Eureka Valley (LACM); 3.3 mi SE Hwy. 168 on Eureka Valley Rd.; 27.7 mi SE; Jct. of North Eureka Valley Rd. and Eureka Valley Rd.; 1.3 mi S of Canyon Rd. on North Eureka Valley Rd.; Fish Lake Valley; 2,950–5,570 ft, Grapevine Canyon, Nelson Range (CAS); Independence (MVZ, USNM); 4,000 ft, 0.5 mi W (AMNH); 2 mi NE; Joshua Flats, 21.2 mi SE of Hwy. 168 on Eureka Valley Rd., Inyo Mtns.; Keeler; Lone Pine (USNM); 2.0 mi ENE; 3 mi W; 6,000 ft, Mazourka Canyon, Inyo Mtns.; 1,200 ft, Mesquite Sand Dunes, Saline Valley (CAS); Oak Creek, Owens Valley (UMMZ); 5.6 mi N Hwy. 190 on Saline Valley Rd.; Saline Valley 1 mi W Salt Lake (LACM); Santa Rosa Hills, 25 mi NE Olancha (LACM); E side Tinemaha Reservoir, Owens Valley; 1,090 ft, 2 mi ESE Tramway, Saline Valley (CAS); 860 ft, 990 ft, 1,000 ft, Warm Springs Rd., Saline Valley (CAS), Mono Co.: Benton; 2 mi S; 5 mi N (SDSNH); 5,100 ft, Cinnamon Ranch, 10 mi SSE; 10 mi N Bishop (USNM); 10.5 mi N, 1 mi W (SDSNH); Arrowhead Knoll, 15 mi N (UCSB); 3.2 mi S Nevada state line on Hwy. 266; 4.6 mi S. Nevada, Esmeralda Co.: 1.5 mi N California state line on Hwy. 264, Fish Lake Valley (NSM); 2 mi W of Hwy. 264 along Chiatovich Creek; 5,500 ft, 7 mi W Chiatovich (E of area); 2.5 mi SE Dyer; 8.6 mi N; Fish Lake; 6.3 mi SSW Silver Peak; 6.5 mi SSW; 6.7 mi SSW; 6.8 mi SSW; 6.9 mi SSW; 7.2 mi SSW; 7.5 mi SSW; 7.7 mi SSW. Mineral Co.: 1.4 mi E Janes Ranch at Hwy. 6, Queen Valley.
Common Chuckwalla,Sauromalus obesus (Baird, 1858). (Plate 10.12, Map 10.9) 12–18 in (30–45 cm); heavy-bodied; dorsal coloration generally brown or black; small granular scales; tail uniform tan or banded with black; juvenile body coloration brown to green. Habitat: This lizard is restricted to rock outcrops, where it is commonly seen basking on large boulders during the day. It is most common in Creosote Bush Scrub below 5,000 ft (1,520 m). Remarks: The subspecies of Common Chuckwalla present in the area is the Western Chuckwalla (S. o. obesus ). This is the largest species of lizard in the region and, next to the Gila Monster (Heloderma suspectum ), the largest in the United States. The leaves and flowers of Creosote Bush are one of the primary foods of this mainly plant-eating lizard. Most of the range of this species overlaps with Creosote Bush. An exception is in the foothills of the Inyo Mountains between Independence and Big Pine, where Creosote Bush is absent. Chuckwallas live in cracks in boulders, where they readily take shelter when approached. Once in a crack, they inflate their lungs and tightly wedge themselves in, making it virtually impossible for a predator to extract them. This is a highly social species; a large, dominant male controls a harem of several females. It has been estimated that it takes
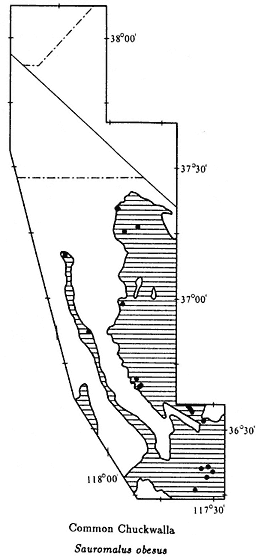
Map 10.9
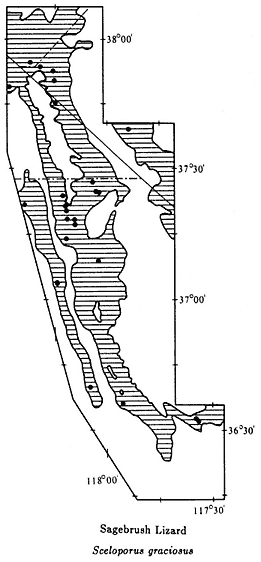
Map 10.10
a male 10 or more years to reach this status. Range: In the Owens Valley, restricted to the Alabama Hills and the lower slopes of the Inyo Mountains from Big Pine south; rocky areas in the Panamint, Saline, and Eureka valleys. Reference: Berry (1974).
Localities: California, Inyo Co.: 3,800 ft, near Big Horn Mine, Hunter Canyon, Saline Valley (USNM); 3,500–4,300 ft, Daisy Canyon, Inyo Mtns. (CAS); Darwin; Darwin Falls (CAS, LACM, MVZ); 25.2 mi SE of Hwy. 168 on Eureka Valley Rd.; 29.2 mi SE; 2,250–4,630 ft, Grapevine Canyon, Nelson Range (CAS); Mazourka Canyon, Inyo Mtns.; 2.5 mi SE of Hwy. 168 on Eureka Valley Rd., Owens Valley; 1.8 mi NW Panamint Springs, 4 mi NW (CAS); 6 mi NW (CAS); Pearly Gates, 7 mi S, Saline Valley; 4,500 ft, Soldier Pass Canyon, Eureka Valley; Waucoba Wash area, 37 mi from Big Pine, N Saline Valley (CAS).
Sagebrush Lizard,Sceloporus graciosus (Baird & Girard, 1852). (Plate 10.13, Map 10.10) 4–6 in (10–15 cm); dorsal coloration brown with turquois-blue stripe from top of head to base of tail; two white stripes on each side; throat and belly dark blue in males, pale blue in females; midventral region black, commonly divided by white in males, lacking black in females. Habitat: Sagebrush Lizards occur in a variety of habitats mostly below about 9,000 ft (2,740 m). They are most common in the Pinyon-juniper Woodland. This species is absent from desert areas and is usually not present below 5,000 ft (1,520 m), except in the Owens Valley. These lizards are commonly seen basking on small rocks or on the ground at the edges of bushes. Remarks: The subspecies of Sagebrush Lizard present in the area is the Northern Sagebrush Lizard (S. g. graciosus ). The Sagebrush Lizard is one of three species of the genus Sceloporus that occur in the White-Inyo mountains region. It can be distinguished from the Desert Spiny Lizard (S. magister ) by its striped dorsal pattern and small size, and from the Western Fence Lizard (S. occidentalis ) by the striped dorsal pattern rather than irregular black bands. All three species occur together in the Owens Valley and at the lower edge of the Pinyon-juniper zone. Sagebrush Lizards reach higher elevations than any other species of reptile in the region. Range: Owens Valley, White-Inyo Range, Nelson Range, Last Chance Range, Silver Peak Range. References: Stebbins (1944), Stebbins and Robinson (1946), Tinkle (1973).
Localities: California, Inyo Co.: 6,000 ft, 6 mi E [NE] Big Pine; 7.5 mi S (LACM); 13 mi NE; Bishop (LACM); Dead Horse Meadow, White Mtns. (LACM); Pass over Inyo Mtns. on Eureka Valley Rd.; 8,200 ft, Grandview Campground, White Mtns. (LACM); 5,480–5,977 ft, Grapevine Canyon, Nelson Range (CAS); 4 mi N Lone Pine (LACM); 7.0 mi E; Sierra View Point, White Mtns. (LACM); between old and new Silver Creek Canyon (LACM); 7,500 ft, Waucoba Pass, Inyo Mtns.; Westgard Pass (LACM, MVZ); 6,500 ft, White Mtn. Rd. (AMNH); 8,000 ft sign, White Mtn. Rd. (LACM); 9,100 ft, White Mtn. Rd. (AMNH); Wyman Canyon, White Mtns. (LACM); 7,500 ft; Roberts Ranch, Wyman Canyon, White Mtns.; 1 mi E. Mono Co.: Benton (LACM, MVZ); 8,400 ft, Indian Creek, White Mtns. Nevada, Esmeralda Co.: Chiatovich Creek, White Mtns.; 7,700 ft, 2 mi S Piper Peak, Silver Peak Range; Alberta Mine, Queen Canyon, White Mtns.; 2,430 m, Trail Canyon, White Mtns. Mineral Co.: Orchard Spring, Buffalo Canyon, White Mtns.
Desert Spiny Lizard,Sceloporus magister(Hallowell, 1854). (Plates 10.14 and 10.15, Map 10.11) 8–12 in (20–30 cm); body covered with large spiny scales; dorsal background gray to pale yellow with six or seven transverse crossbars, which are more pronounced in females; black neck collar; females with uniform cream ventral coloration; males have extensive blue and black ventral coloration with green and yellow flecks. Habitat: This species occurs in a variety of vegetation types, from low-elevation Creosote Bush Scrub up to the lower edge of the Pinyon-juniper Woodland. It is most common in wooded or rocky areas below 7,000 ft (2,130 m). This wary lizard can be observed basking on rocks and tree trunks. Remarks: The subspecies of Desert Spiny Lizard present in this area is the Barred Spiny Lizard (S. m. transversus ). Other subspecies range from northern Nevada to the tip of Baja California, Sinoloa,
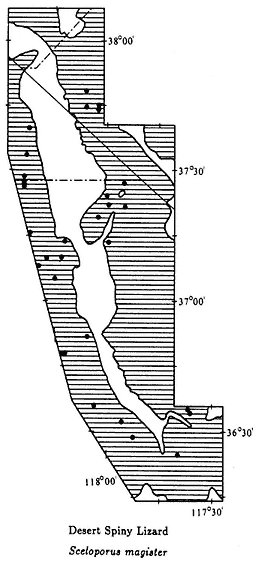
Map 10.11
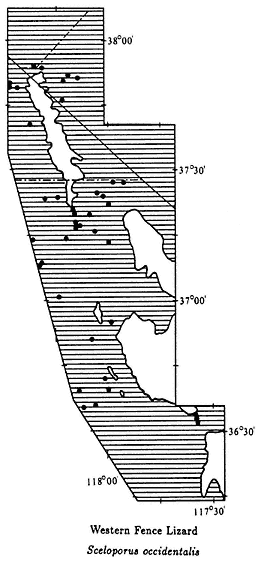
Map 10.12
and northeastern Mexico, and from the Coast Range of central California to western Texas. In our area Desert Spiny Lizards are very common at Joshua Flats in the Inyo Mountains on the road from Big Pine to Eureka Valley. The spiny leaves of the Joshua Tree provide protection from predators. This large, heavy-bodied lizard feeds mainly on insects but will also eat small lizards. Range: Entire White-Inyo mountains region below 7,000 ft (2,150 m). References: Parker and Pianka (1973), Phelan and Brattstrom (1955).
Localities: California, Inyo Co.: Batchelder Spring, 9.8 mi E Big Pine (CMNH, UNM); Big Pine (CAS, MVZ); 4 mi W (SDSNH); 6 mi E; 1,620 m, 21.3 mi NE; Deep Springs (CAS); NW end Deep Springs Valley (LACM); 4,400 ft, NW end Eureka Valley; 2.0 mi SE of Hwy. 168 on Eureka Valley Rd.; Fish Slough, 5 mi N Bishop
(UCSB); 3,430–4,850 ft, Grapevine Canyon, Nelson Range (CAS); Independence; 0.5 mi W (UMMZ); 1.4 mi W; 21.5 mi N (LACM); Joshua Flats, 21.2 mi SE of Hwy. 168 on Eureka Valley Rd.; Keeler (SDSNH); Keough's Hot Springs, 7 mi S Bishop (CAS, LACM); Lone Pine (CAS, USNM); 8.1 mi SE; Mazourka Canyon, 7.0 mi ENE Independence; 1.0 mi S Mono County line on Hwy. 168; 5.6 mi N of Hwy. 190 on Saline Valley Rd.; 1,380 m, Silver Canyon, 2.0 mi E Laws; 4,500 ft, Soldier Pass Canyon, Eureka Valley. Mono Co.: 5,040 ft, Cinnamon Ranch, 10 mi SSE Benton; Fish Slough, 8 mi N Bishop (UMMZ). Nevada, Esmeralda Co.: 1,570 m, along Chiatovich Creek, 1.5 mi W of Hwy. 264; 4.6 mi N Dyer; 8.2 mi N; Fish Lake (MVZ, UMMZ): 0.5 mi S; 1 mi S.
Western Fence Lizard,Sceloporus occidentalis(Baird & Girard, 1852). (Plate 10.16, Map 10.12) 5–9 in (12.5–22.5 cm); dorsal coloration gray to brown with irregular black bands across top of back; blue patch on each side of belly, outlined with black; throat blue, some are outlined with black. Habitat: This lizard occurs in a variety of habitats from below 4,000 ft (1,220 m) to 9,000 ft (2,740 m) but is absent from Creosote Bush Scrub areas. It is the most abundant lizard in the pinyon-juniper belt, where it is commonly seen basking on rocks or logs. Remarks: The subspecies of Western Fence Lizard present in the area is the Great Basin Fence Lizard (S. o. biseriatus ). The Western Fence Lizard, commonly called the "blue-belly lizard," is the most wide-ranging species of lizard in California, occurring in all habitats except alpine regions and Creosote Bush Scrub. There are two similar species in the area. The Desert Spiny Lizard (S. magister ) is larger and has distinct black patches on each side of the neck. The Sagebrush Lizard (S. graciosus ) has stripes running down the back. Over 60 species of Sceloporus , ranging from the United States to Panama, are known. It is the largest genus of lizards present exclusively in North America. Most of the species occur in Mexico, where as many as six are known to live together at a single place. Range: On the west side of the White-Inyo Range in the entire Owens, Chalfant, Hammil, Benton, and Queen valleys; White-Inyo Range, Nelson Range, Panamint Mountains (at higher elevations), Last Chance Range, Silver Peak Range, and Deep Springs and Fish Lake valleys. References: Fitch (1940a), Tanner and Hopkin (1972).
Localities: California, Inyo Co.: 2.6 mi W of Hwy. 395 on Hogback Rd., Alabama Hills (SBMNH); Antelope Spring, Deep Springs Valley (LACM); Batchelder Spring, 9.8 mi E Big Pine (CMNH, MVZ); 3 mi W Big Pine (LACM); 4 mi W (SDSNH); 6,000 ft, 9 mi NE (AMNH); 4 mi NW Black Rock Springs; Cedar Flat, White Mtns.; Cottonwood Creek, Mono County line, Fish Lake Valley (UMMZ); Deep Springs (CAS, MVZ, UMMZ); Deep Springs Lake (LACM); French Spring, Inyo Mtns.; 4,000–5,977 ft, Grapevine Canyon, Nelson Range (CAS); Joshua Flats, 21.2 mi SE of Hwy. 168 on Eureka Valley Rd.; Keough Hot Springs, 7.0 mi S Bishop (SDSNH); Lone Pine (MCZ); 2.9 mi W (LACM); 3 mi W (LACM); 7.5 mi NE; 1.0 mi S Mono County line on Hwy. 168; Payson Canyon, 2 mi W Deep Springs Valley; Pinyon Picnic Area, White Mtns.; Roberts Ranch, Wyman Canyon; 1 mi E; Sierra View Point, White Mtn. Rd. (LACM); 4,600 ft, Silver Creek Canyon; 2,060 m,
Silver Creek Canyon, 7.2 mi E Laws; 7,300 ft, E base Waucoba Mtn., Inyo Mtns.; 8,000 ft, White Mtn. Rd. (LACM, MVZ); Willow Springs Canyon, 8.0 mi ENE Independence; Wyman Canyon (LACM); 6,400 ft; 6,750 ft. Mono Co.: Benton; 1.25 mi N, 2.5 mi E; 5,400 ft, Cinnamon Ranch, 10 mi SSE Benton. Nevada, Esmeralda Co.: Albert Mine, Queen Canyon; 1,680 m, Chiatovich Creek, Fish Lake Valley; 7,000 ft; 2,080 m, Dry Creek, White Mtns.; 7,400 ft, south fork Indian Creek, White Mtns. Mineral Co.: Orchard Spring, Buffalo Canyon, White Mtns.
Side-blotched Lizard,Uta stansburiana (Baird & Girard, 1852). (Plate 10.17, Map 10.13) 4–6 in (10–15 cm); variable dorsal pattern of spots, speckles, or bands; ventral color pale, distinct small dark blue to black patch on side behind each arm. Habitat: In this area, Side-blotched Lizards occur in all habitats up to at least 7,000 ft (2,130 m). This is the most common species of lizard in the region, but because of its small size, it is easily overlooked. Remarks: This wide-ranging species occurs in arid and semi-arid regions of the western United States and Mexico. Side-blotched Lizards occur over a broader geographic range than any other species of desert lizard in western North America. They occur from Washington to the tip of Baja California and northern Mexico, and from the coast of central California to Oklahoma. The number of individuals may exceed 20 per acre in some areas, and females may lay two to four clutches of eggs in a single season. Studies have shown that this species has a short life span and that there may be an almost complete turnover in the adult population from one year to the next. Side-blotched Lizards are a major source of food for lizard-eating predators because of their abundance. Range: Throughout the White-Inyo mountains region below 8,000 ft (2,440 m). References: Parker and Pianka (1975), Tinkle (1967).
Localities: California, Inyo Co.: Antelope Spring, Deep Springs Valley (LACM); Batchelder Spring, 9.8 mi NE Big Pine (CMNH, MVZ); near Bighorn Mine, Hunter Canyon, Inyo Mtns. (USNM); 4 mi W Big Pine (SDSNH); 9.7 mi N (LACM); 16.6 mi S (UMMZ); 1,620 m, 21.3 mi NE; 5.4 mi S Bishop (UMMZ); 6 mi S (SDSNH); Cedar Flat, 11 mi NE Big Pine; 2,250–4,800 ft, Daisy Canyon, Inyo Mtns. (CAS); Darwin (CAS); 8 mi N (SDSNH); 15 mi N; Darwin Falls (LACM); Deep Springs (CAS, UMMZ); 7.5 mi S (UMMZ); 8.2 mi W (UMMZ); Deep Springs Lake (LACM); W end Deep Springs Valley (LACM); 3.4 mi SE of Hwy. 168 on Eureka Valley Rd.; Eureka Valley sand dunes; 2,800 ft, near (LACM); Fish Slough pond, 5.4 mi N Bishop (UMMZ); Fish Slough, 6.25 mi N Bishop (UCSB); 2,250–5,977 ft, Grapevine Canyon, Nelson Range (CAS); 3,800 ft (LACM); Independence; 4,000 ft, 0.5 mi W (AMNH); 1.4 mi W; Joshua Flats, 21.2 mi SE of Hwy. 168 on Eureka Valley Rd.; Keeler (MVZ, USNM); Keough's Hot Springs, 7 mi S Bishop (LACM); Laws; Lee Flat, 15 mi E Keeler; Lone Pine; 2.3 mi W (LACM); 4,000 ft, 3.0 mi W (LACM); 8.1 mi SE; 10.0 mi SE (MVZ, UMMZ); 4,200 ft, Long John Canyon, Inyo Mtns.; Mesquite Sand Dunes, Saline Valley (CAS); 1.0 mi S Mono County line on Hwy. 168; 7.5 mi W Panamint Springs (CAS); 11.7 mi W (UMMZ); Payson Canyon, 2 mi W Deep Springs Valley; N end Saline Valley (CAS); 4,450 ft, Silver Creek Canyon, White Mtns.; 1,380 m, Silver Creek Canyon, 7.2 mi E.
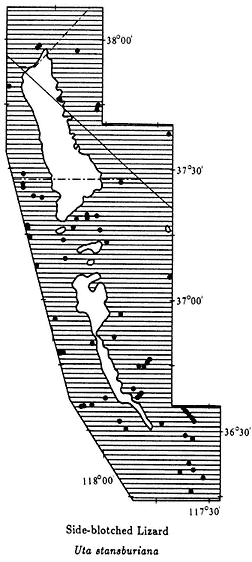
Map 10.13
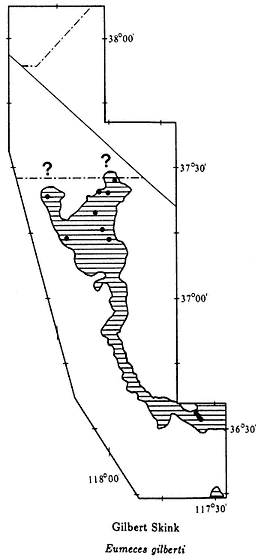
Map 10.14
Laws; 1,080–1,350 ft, Warm Springs Rd., Saline Valley (CAS); Willow Creek, Saline Valley; Willow Springs Canyon, 8.0 mi ENE Independence; mouth, Wyman Canyon, White Mtns.; 6,750 ft. Mono Co.: Benton; Fish Slough, 8 mi N Bishop (UMMZ). Nevada, Esmeralda Co.: Fish Lake (MVZ, UMMZ); 0.75 mi SSW; 7,400 ft, Indian Creek, White Mtns.; 9.1 mi SSW Silver Peak. Mineral Co.: 7,000 ft, 0.7 mi W Montgomery Pass (CAS).
Skinks (Family Scincidae)
Gilbert Skink,Eumeces gilberti(Van Denburgh, 1896). (Plates 10.18 and 10.19, Map 10.14) 6–10 in (15–25 cm); dorsal coloration uniform brown in adults; juveniles with two white lateral stripes running from top of head to base of tail,
dividing brown dorsal coloration into three stripes; a middorsal brown stripe extending onto tail, and a brown stripe on each side stopping at hind legs, not extending onto base of tail; adults with brown tails; juveniles with pink tails. Habitat: In this area Gilbert Skinks are most common around springs and streams between 4,000 and 8,000 ft (1,220 and 2,440 m). They also occur in habitats away from water between 6,000 and 8,000 ft (1,830 and 2,440 m). This secretive lizard is rarely observed but can sometimes be seen in leaf litter or at the base of bushes during the midmorning or late afternoon. It is most easily found by turning rocks near springs or streams. Remarks: The subspecies of Gilbert Skink present in the area is the Western Red-tailed Skink (E. g. rubricaudatus ). A juvenile Gilbert Skink can be distinguished from the Western Skink (E. skiltonianus ) by differences in tail coloration. In the former, the tail color is predominantly pink, and in the latter, it is blue. One exception is that juvenile Gilbert Skinks in the Panamint Mountains have blue tails. In both subadult Gilbert Skinks and adult Western Skinks the tail coloration fades. They can still be distinguished by the length of the brown stripes on the sides, which extend onto the base of the tail in the Western Skink and stop at the hind legs in the Gilbert Skink (Fig. 10.5). (See Western Skink, E. skiltonianus , account for a discussion on distributional interactions between the two species.) Range: Mid-elevations of Argus Mountains, Nelson Range, Panamint Mountains, Inyo Mountains, and White Mountains
Figure 10.5
Gilbert Skink (Eumeces gilberti ): Brown stripes on the sides of the body stop at hind
legs (above). Western Skink (Eumeces skiltonianus ): Brown stripes on the sides of
the body extend onto the base of the tail (below).
at least as far north as the Mono County line (see ? on Map 10.14). Reference: Rodgers and Fitch (1947).
Localities: California, Inyo Co.: Batchelder Spring, 9.8 mi NE Big Pine; 1,840 m, Cottonwood Canyon, 3.7 mi W of Hwy. 168 on rd., White Mtns.; Deep Springs Lake (LACM); NW end, Deep Springs Valley (LACM); 4,000–5,977 ft, Grapevine Canyon, Nelson Range (CAS); 4,850 ft (BYU); Joshua Flats, 21.2 mi SE of Hwy. 168 on Eureka Valley Rd.; 2,060 m, Silver Creek Canyon, 7.2 mi E Laws; sec. 18, T. 6 S., R. 36 E., Wyman Canyon, White Mtns. (CMNH).
Western Skink,Eumeces skiltonianus (Baird & Girard, 1852). (Plate 10.20, Map 10.15) 5–8 in (12.5–20 cm); dorsal coloration brown with two white lateral stripes running from top of head onto tail; two lateral brown stripes below white stripes extend beyond hind legs onto base of tail; tail blue, sometimes fading in adults, especially in individuals with regenerated tails. Habitat: In this area, the Western Skink is a high-elevation species that has not been found below 7,000 ft (2,130 m) and may occur as high as 10,000 ft (3,050 m). An exception to this are populations on the eastern slope of the Sierra Nevada that occur a little lower. It appears to inhabit only Pinyon-juniper Woodland. These lizards are rarely seen because they spend most of their time under rocks and logs and in leaf litter. Remarks: The subspecies of Western Skink that occurs in the area is the Great Basin Skink (E. s. utahensis ). See Gilbert Skink (E. gilberti ) account for distinguishing characteristics between these two species. The Western Skink and the Gilbert Skink are very closely related species. It is possible that they interbreed where their ranges contact in the White-Inyo mountains region, but not enough specimens have been obtained to come to any definitive conclusions. Only a single Western Skink is recorded from the White Mountains. A skink with a blue tail was seen at 6,300 ft (1,920 m) in Beveridge Canyon, Inyo Mountains (see small ? on Map 10.15). A young specimen from 6,100 ft (1,860 m) in Cottonwood Canyon on the east side of the White Mountains has a pink tail with a blue tip. An adult from the same area has typical Gilbert Skink coloration. Range: Sight record from Inyo Mountains (see small ? on Map 10.15); White Mountains above 7,000 ft (2,130 m); Silver Peak Range above 7,000 ft (2,130 m); expected in the mountains north of Queen Valley (see large ? on Map 10.15); eastern slope of the Sierra Nevada. References: Rodgers and Fitch (1947), Tanner (1957).
Localities: California, Inyo Co.: 8,000–8,200 ft, corner Secs. 17, 18, 19, 20, R. 35 E, T. 7 S, N of Westgard Pass, White Mtns. Nevada, Esmeralda Co.: 7,120 ft, Birch Creek, Spring, Palmetto Mtns. (E of area); 7,300 ft, Valcalda Spring, Silver Peak Range (NE of area).
Whiptails (Family Teiidae)
Western Whiptail,Cnemidophorus tigris (Baird & Girard, 1852). (Plates 10.21 and 10.22, Map 10.16) 8–14 in (20–35 cm); four middorsal pale yellow stripes, distinct on front part of body, less distinct on back part; reticulate pattern of black and gray between stripes, on sides of body, and on limbs; reticulate pattern on underside of body, with neck and chest nearly completely black in some individuals; tail very
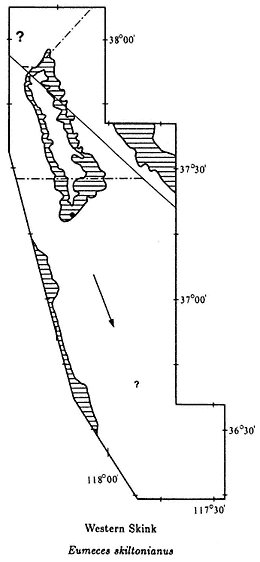
Map 10.15
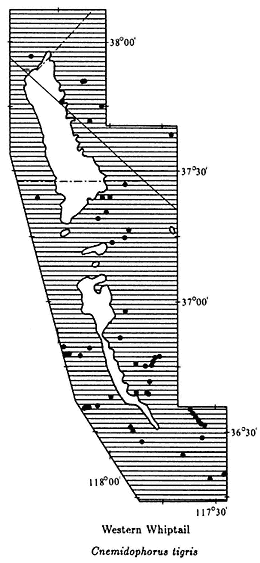
Map 10.16
long, usually more than twice body length; in juveniles pale yellow stripes continuous and tail blue (see Plate 10.22). Habitat: In this area, Western Whiptails occur in all terrestrial habitats up to about 7,500 ft (2,290 m) in the Pinyon-juniper Woodland. This species is most common in washes and sandy areas. They are active lizards and are commonly seen moving from bush to bush in search of food. These lizards feed on invertebrates, which are captured on the surface or dug our of the ground. Remarks: The subspecies of Western Whiptail present in the area is the Great Basin Whiptail (C. t. tigris ). Members of the genus Cnemidophorus range from Minnesota to Argentina and from California to Maryland. There are about 45 species, of which 15 are all-female. These species reproduce asexually, from eggs laid by the female and each offspring is a clone of the mother. These all female species have a larger-than-normal chromosome number (polyploidy) because of the original hybridization of two
different species and the incorporation of chromosomes from both parent species into the new asexual species. The Western Whiptail is not an all-female species, but it is an ancestral parent for some of the all-female species that occur in Arizona, New Mexico, Texas, and northern Mexico. Range: Throughout the White-Inyo mountains region below 7,500 ft (2,290 m). References: Bezy and Sites (1987), Cole (1984), Parker (1972), Wright and Low (1968).
Localities: California, Inyo Co.: 3,800 ft, 6,600–6,800 ft, Near Big Horn Mine, Hunter Canyon, Inyo Mtns. (CAS); 1,620 m, 21.3 mi NE Big Pine; 5,000 ft, 24 mi NE; 2,400–4,850 ft, Daisy Canyon, Inyo Mtns. (CAS); 5,200 ft, 15 mi N Darwin; Darwin Falls (CAS, MVZ); NW end Deep Springs Valley (LACM); 23.6 mi SE of Hwy. 168 on Eureka Valley Rd.; 27.7 mi SE; 2,250–5,977 ft, Grapevine Canyon, Nelson Range (CAS); Independence; 4,000 ft, 0.5 mi W (AMNH); 1.4 mi W; 2 mi N; 3,800 ft, 2.5 mi E; 6,000–6,100 ft, 2 mi W Jackass Spring, Nelson Range; Joshua Flats, 21.2 mi SE of Hwy. 168 on Eureka Valley Rd.; 1 mi S Keeler (JACM); Laws; 4,200 ft, Long John Canyon, Inyo Mtns.; Lone Pine (FMNH, USNM); 4,300 ft, 2.3 mi W (LACM); 2.9 mi W (LACM); 4,700 ft, 3 mi W, 0.5 mi S; 4 mi W; 10.0 mi SE; 4,000 ft, Mazourka Canyon, Inyo Mtns.; 1 mi S Mono County line on Hwy. 168; 1,200 ft, Mesquite Sand Dunes, Saline Valley (CAS); Panamint Springs (CAS, MVZ); 5.6 mi N of Hwy. 190 on Saline Valley Rd.; near Freshwater Pond, W side, Saline Valley (CAS); NW end, Saline Valley (CAS); 5 mi N Salt Works; near Seven Springs, S of Lower Warm Spring, Saline Valley (CAS); 850–1,400 ft, Warm Springs Rd., Saline Valley (CAS); Willow Creek, Saline Valley; 6,750 ft, Wyman Creek, White Mtns. Mono Co.: Benton. Nevada, Esmeralda Co.: 0.25 mi W of Hwy. 264 along Chiatovich Creek, Fish Lake Valley; 5,000 ft, 0.75 mi W; 4,800 ft, 1.5 mi N Dyer; Fish Lake; 7,400 ft, Indian Creek, White Mtns.; 9.1 mi SSW Silver Peak; Mineral Co.: 3.9 mi NE California state line on Hwy. 6.
Night Lizards (Family Xantusiidae)
Desert Night Lizard,Xantusia vigilis (Baird, 1858). (Plate 10.23, Map 10.17) 2–4 in (5–10 cm); background coloration gray or brown with tiny black flecks that form indistinct stripes; belly uniform cream with scales arranged in transverse rows; eyelids absent. Habitat: This secretive, rarely seen lizard occurs in a variety of habitats up to at least 6,800 ft (2,070 m) in the White-Inyo mountains region. However, it has been found above 9,000 ft (2,750 m) in the Panamint Mountains of Death Valley National Monument. Specimens are most commonly found under the rubble of fallen Joshua Trees but have also been taken from under rocks, logs, and piles of brush. At Joshua Flats and Lee Flat in the Inyo Mountains, where no specimens have been seen, they appear not to live in Joshua Trees. Remarks: The subspecies of Desert Night Lizard present in the area is the Common Night Lizard (X. v. vigilis ). Although the common name implies that this species is nocturnal, they are in fact active during the day under cover objects. This is the smallest species of lizard in the area and is easily overlooked. There are very few records of Desert Night Lizards from north of the Panamint Valley, and the range of this species is certainly more extensive than is
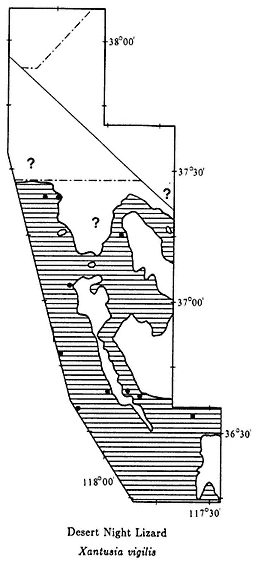
Map 10.17
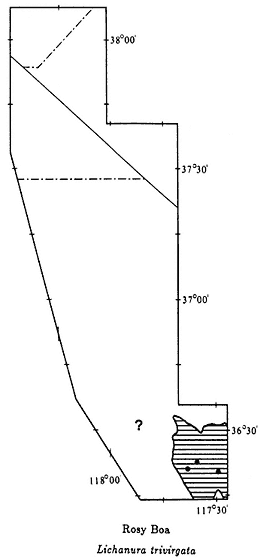
Map 10.18
presently known. The farthest north that Desert Night Lizards are known to occur in the west is the vicinity of Bishop. In the east, they are known from southeastern Utah. One record, for 6,800 ft (2,070 m) in Silver Creek Canyon, east of Laws in the White Mountains, is at the northern edge of the known range of this species. This high-elevation locality indicates that this species should be expected further north (see ? on Map 10.17). The Night Lizard family (Xantusiidae) consists of about 12 species, most of which are present in Mexico. A single species occurs in Cuba, and one species is present on three of the Channel Islands of California. Range: Valleys and mountains of the southern White-Inyo mountains region up to at least 6,800 ft (2,070 m). Presently known from the Panamint Valley, Argus Mountains, Saline Valley, Last Chance Range, Eureka Valley, Owens Valley north to the Mono County
line, and the west side of the White Mountains. Reference: Bezy and Sites (1987), Zweifel and Lowe (1966).
Localities: California, Inyo Co.: Near Big Horn Mine, Hunter Canyon, Inyo Mtns. (USNM); 4,500 ft, 5 mi N, 2 mi W Bishop (W of area); BLM Ecology Center, 9 mi NW Bishop on Hwy. 395 (NW of area) (UNM); 4,500 ft, Daisy Canyon, Inyo Mtns. (CAS); 25.1 mi SW Hwy. 168 on Eureka Valley Rd.; French Spring, Inyo Mtns.; 4,200–4,400 ft, Grapevine Canyon, Nelson Range (CAS); 1.4 mi W Independence; 5,800 ft, Last Chance Spring, Last Chance Range; 5 mi W Lone Pine (LACM); Montezuma Mine, 1 mi NE Tinemaha Reservoir; 1,380 m, Silver Creek Canyon, 2.0 mi E Laws; 2,060 m, Silver Creek Canyon, 7.2 mi E Laws.
Boas (Family Boidae)
Rosy Boa,Lichanura trivirgata(Cope, 1861). (Plate 10.24, Map 10.18) 24–36 in (60–90 cm); heavy-bodied with head small in proportion to body; background color gray with three broad, distinct reddish stripes running the length of the body; tail short and blunt. Habitat: In our area, Rosy Boas are restricted to Creosote Bush Scrub below about 5,000 ft (1,500 m). They occur in rocky hills and canyons and are very rare or absent on valley floors. This species is mainly nocturnal but is occasionally active in the early morning and late afternoon during the spring. Remarks: The subspecies of Rosy Boa present in the area is the Desert Rosy Boa (L. t. gracia ). Two other subspecies occur in coastal southern California and in Baja California and Sonora, Mexico. The Rosy Boa and the Rubber Boa (Charina bottae ) are the only members of the Boa Family in the United States. These two species are tiny in comparison with the giant pythons of Africa and Asia, the Anaconda of the Amazon Basin, and the Boa Constrictor, which occurs from Mexico into South America. The Rosy Boa, like other boas and pythons, kills its prey by constriction. This species feeds on rodents, birds, and occasionally lizards. Rosy Boas are gentle, slow-moving snakes. They usually remain motionless when approached and never attempt to bite when picked up. Range: Known only from the foothills of the Panamint Mountains and Argus Range surrounding the Panamint Valley. Suitable habitat exists in the Owens Valley in the foothills of the Inyo Mountains east of Owens Lake, and in the hills surrounding the Saline and Eureka valleys, but no specimens have been recorded (see ? on Map 10.18). References: Gorman (1965), Klauber (1931).
Localities: California, Inyo Co.: 0.2 mi E Darwin Wash Rd. on Hwy. 190; 10 mi W; 8.9 mi W Panamint Valley Rd. on Hwy. 190.
Colubrids (Family Colubridae)
Glossy Snake,Arizona elegans (Kennicott, 1859). (Plate 10.25, Map 10.19) 24–28 in (60–95 cm); cream to light gray background with narrow light brown blotches running down back; black flecks around edges of blotches; ventral surface uniform white; scales smooth. Habitat: This nocturnal snake usually occurs in valley floors and sandy washes. In the area, Glossy Snakes have been found only in
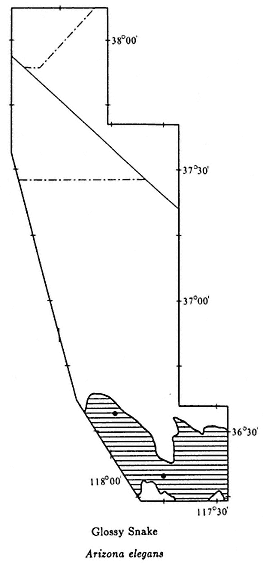
Map 10.19
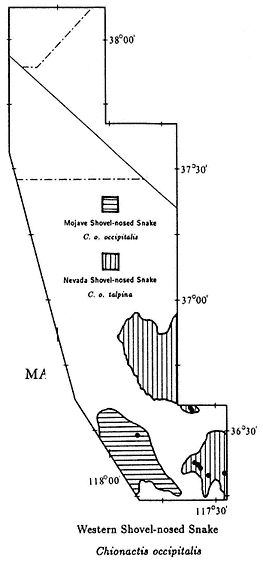
Map 10.20
and around Creosote Bush Scrub. These active snakes are rarely seen because they do not lie on roads at night, like many other species, but move rapidly across them. Remarks: The subspecies of Glossy Snake present in the area is the Mojave Glossy Snake (A. e. candida ). Other subspecies occur throughout much of the southwestern United States and northern Mexico. Glossy Snakes resemble juvenile Gopher Snakes (Pituophis catenifer ) but are readily distinguished by their smooth scales and lighter background color and blotches. This species has a flattened head, enabling it to bury in sand or loose soil. Food consists of small rodents, lizards, and reptile eggs. Range: Panamint Valley; foothills of northern Argus and southern Inyo Mountains; southern Owens Valley in the vicinity of Owens Lake; expected in the Saline and Eureka valleys. Reference: Klauber (1946).
Localities: California, Inyo Co.: 8.1 mi SE of Hwy. 136 on Hwy. 190; 4 mi SE Lone Pine.
Western Shovel-nosed Snake,Chionactis occipitalis(Hallowell, 1854). (Plates 10.26 and 10.27, Map 10.20) 10–16 in (25–40 cm); a small, banded snake; background yellow with brown bands; faint reddish-brown band in middle of each yellow band (absent in snakes from southern Owens Valley); pale ventral color, with brown bands crossing belly in some individuals; snout flattened. Habitat: This species occurs in Creosote Bush Scrub below about 5,000 ft (1,500 m). These generally nocturnal snakes are most common in sandy areas. Remarks: There are two subspecies of Western Shovel-nosed Snake in our area. The Mojave Shovel-nosed Snake (C. o. occipitalis ) is present in the extreme southern Owens Valley around Owens Lake. This subspecies lacks the faint reddish-brown band in the middle of each yellow band. The Nevada Shovel-nosed Snake (C. o. talpina ), which occurs in the Panamint and Saline valleys, has the faint reddish-brown band (see Map 10.20). This snake uses its flattened snout to aid in burying itself in the ground. In areas of loose sand they can crawl beneath the surface of the sand for great distances. In Arizona, where Western Shovel-nosed Snakes occur together with venomous Arizona Coral Snakes (Micruroides euryxanthus ), the Shovel-nosed Snakes have a similar color pattern with red bands. The two species resemble each other, with the harmless Western Shovel-nosed Snake mimicking the venomous Arizona Coral Snake. Range: Vicinity of Owens Lake in the southern Owens Valley (C. o. occipitalis ); Panamint and Saline valleys (C. o. talpina ). References: Elvin (1963), Klauber (1951), Norris and Kavanau (1966).
Localities: California, Inyo Co.: Darwin Falls; 6.8 mi W of Darwin Rd. on Hwy. 190; 9.2 mi W; 2,340–3,750 ft, Grapevine Canyon, Nelson Range (CAS); Owens Lake (dot on Map 10.20 is the position of an additional site record) (FMNH); 2.1 mi E Panamint Springs; 9.5 mi E of Saline Valley Rd. on Hwy. 190.
Night Snake,Hypsiglena torquata (Günther, 1860). (Plate 10.28, Map 10.21) 12–24 in (30–60 cm); a slender snake with a wide, flattened head; gray background coloration with numerous brown spots running the length of body; a series of paired dorsal spots may be fused or slightly offset, giving a checkered appearance; belly uniform cream; distinct dark brown neck band which extends along side of head to eye, commonly with a projection extending to middle of top of head. Habitat: Night Snakes occur in all habitats below about 7,000 ft (2,130 m). They are most common in rocky places in low- to moderate-elevation desert areas. This is a strictly nocturnal species that is sometimes seen crossing roads at night. They are fairly common on the Westgard Pass road (Hwy. 168) west of Tollhouse Spring and on the Eureka Valley Road between Joshua Flats and the Eureka Valley floor. Remarks: The subspecies of Night Snake present in the region is the Desert Night Snake (H. t. deserticola ). This wide-ranging species occurs from extreme southern British Columbia to southern Mexico and from coastal central California to Texas. These small snakes have enlarged, grooved teeth in the back of the upper jaw. These teeth are used in channeling venom into prey, which usually consists of small lizards. It is unlikely that this small species could envenomate a human, but care should be taken if these snakes
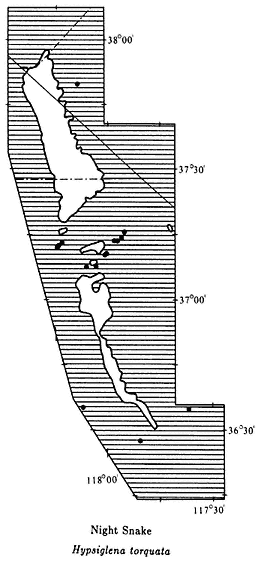
Map 10.21
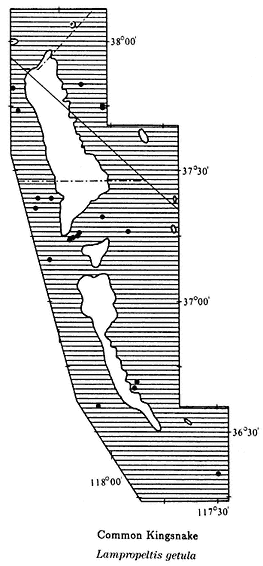
Map 10.22
are handled. Range: Throughout the White-Inyo mountains region below 7,000 ft (2,130 m). Reference: Tanner (1944).
Localities: California, Inyo Co.: 4.7 mi NE Big Pine; 5 mi NE; 6.4 mi NE; pass over Inyo Mtns. on Eureka Valley Rd.; 11.3 mi SE of Hwy. 168 on Eureka Valley Rd.; 1,920 m, 19.5 mi SE; 1,840 m, 19.9 mi SE; 24.1 mi SE; 24.9 mi SE; 26.3 mi SE; 27.4 mi SE; 3,630 ft, Grapevine Canyon, Nelson Range (CAS); 3 mi W Lone Pine; 15.3 mi SE. Nevada, Esmeralda Co.: 5,200 ft, 2 mi W of Hwy. 264 along Chiatovich Creek, Fish Lake Valley.
Common Kingsnake,Lampropeltis getula (Linnaeus, 1766). (Plate 10.29, Map 10.22) 30–48 in (75–120 cm); black- and white-banded; black bands cross belly; black bands wider on top than on sides and belly. Habitat: Kingsnakes occur in
all habitats below about 7,500 ft (2,290 m). They are active both during the day and on warm nights. Remarks: The subspecies of Common Kingsnake present in the area is the California Kingsnake (L. g. californiae ). Common Kingsnakes are one of the widest-ranging snakes in the United States. They occur across North America from the Pacific coast to the Atlantic coast. There are a number of subspecies with varying color patterns. Kingsnakes eat a variety of vertebrate animals, including mammals, small birds, and reptiles. They are well known for eating other snakes, even rattlesnakes. They are generally not affected by the venom and will continue eating a rattlesnake even if bitten. It has been shown that rattlesnakes sense the odor of kingsnakes and attempt to avoid them. The only similar-looking species in the area is the Western Long-nosed Snake (Rhinocheilus lecontei lecontei ). In the black- and white-banded color phase of this species, the black bands do not encircle the belly. In this region, Common Kingsnakes are relatively rare in Creosote Bush Scrub areas. They appear to be more common in Great Basin Scrub areas, especially in the vicinity of springs and streams. Range: Throughout the White-Inyo mountains region below 7,500 ft (2,290 m). Reference: Carpenter and Gillingham (1975).
Localities: California, Inyo Co.: Beveridge Canyon, Saline Valley (FMNH); Big Pine; 6,300 ft, 7 mi NE; 7.8 mi NE; 1,800 m, 8.2 mi NE; 9.0 mi NE; 22.0 mi NE; 2 mi E Bishop; 6,600–6,800 ft, Big Horn Mine, Hunter Canyon, Saline Valley (USNM); 0.3 mi E Darwin Wash Rd. on Hwy. 190; 27.7 mi SE of Hwy. 168 on Eureka Valley Rd.; Laws; Lone Pine; 5.9 mi S of Mono County line on Hwy. 6; 4.6 mi E Hwy. 6 on Silver Creek Canyon Rd.; White Mtns. Mono Co.: 1.0 mi W Benton; 18.9 mi N of Inyo County line on Hwy. 6. Nevada, Esmeralda Co.: 1,640 m, Chiatovich Creek, Fish Lake Valley; Fish Lake (LACM); 0.5 mi S.
Coachwhip,Masticophis flagellum(Shaw, 1802). (Plate 10.30, Map 10.23) 36–84 in (90–210 cm); a long, slender snake with large eyes; dorsal background red or reddish brown; indistinct narrow tan or cream crossbands on front half of body; between two and six distinct dark brown or black crossbands on neck; ventral surface pale with small black spots on chin and throat; juveniles are banded or blotched, usually less red than adults, with dark neck bands less distinct. Habitat: Coachwhips are diurnal snakes that occur in all habitats up to about 6,500 ft (1,980 m) in the lower Pinyon-juniper Woodland. They are relatively common in desert canyons where riparian vegetation is present. Remarks: The subspecies of Coachwhip present in the area is the Red Coachwhip (M. f. piceus ). The seven subspecies of this wide-ranging snake occur from coastal southern California across the southern half of the country to the Carolinas and Florida, and south in Mexico to the states of Veracruz, Sinoloa, and the tip of Baja California. Other subspecies are variable in color pattern and may not look like individuals from the White-Inyo mountains region. Coachwhips eat a variety of food, including lizards, snakes, birds, mammals, and even insects. This is the fastest-moving snake in the region. In the open, it will flee if approached and usually disappear into a clump of vegetation or down a rodent burrow. This evil-tempered species always tries to bite when picked up. Although it is not poisonous, the tiny,
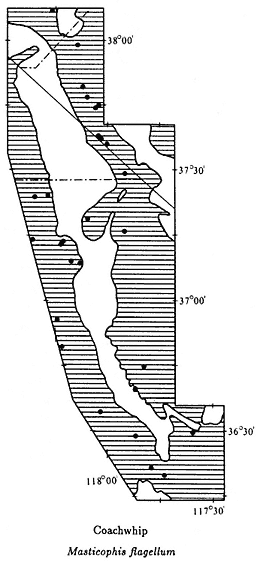
Map 10.23
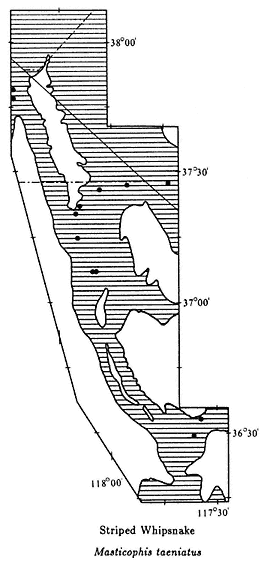
Map 10.24
razor-sharp teeth will draw blood. Range: Entire White-Inyo mountains region below about 6,500 ft (1,980 m). References: Ortenburger (1928), Wilson (1970).
Localities: California, Inyo Co.: 1.5 mi SW Batchelder Spring, Westgard Pass; near Big Horn Mine, Hunter Canyon, Inyo Mtns. (USNM); 5,500 ft, 6 mi E Big Pine; 19.9 mi NE; 31 mi S Bishop; Charcoal Kilns off Hwy. 395, N Independence (LACM); W end Deep Springs Valley (LACM); 5,000 ft, 4.9 mi SE of Hwy. 168 on Eureka Valley Rd.; 7.3 mi SE; 26.9 mi SE; 9.8 mi E of Hwy. 136 on Hwy. 190; 2 mi N Independence; 4 mi N (SDSNH); Keeler (USNM); 9 mi SE (SDSNH); Keough Hot Springs, 7 mi S Bishop (SDSNH); Laws; Lee Flat, 3.3 mi S of summit on Saline Valley Rd. (CAS); 2 mi SE Lone Pine; 1,200 ft, Mesquite Sand Dunes, Saline Valley (CAS); 6,000 ft, Silver Creek Canyon, White Mtns. Mono Co.: Oasis (BYU, MVZ).
Nevada, Esmeralda Co.: 1.7 mi E California state line on Hwy. 264 (LACM); 4.9 mi E; 1 mi W of Hwy. 264 on Chiatovich Creek, Fish Lake Valley; 2 mi S Chiatovich Creek on Hwy. 264, Fish Lake Valley; 4.8 mi S Dyer (LACM); Fish Lake; 1 mi SW. Mineral Co.: 8.1 mi N of Hwy. 6 on Hwy. 360.
Striped Whipsnake,Masticophis taeniatus(Hallowell, 1852). (Plate 10.31, Map 10.24) 30–60 in (75–150 cm); a slender snake with a narrow neck, wide head, and large eyes; dorsal background coloration brown, commonly with a bluish cast; white lateral stripe on each side divided in half by a narrow black line; two additional narrow black stripes on lower part of each side; midventral coloration yellow, becoming pale toward neck and pink toward tail; small black spots on chin and throat; black spot on the side of each ventral scale. Habitat: In this area, Striped Whipsnakes occur from mid-elevation Great Basin Scrub, above 5,000 ft (1,520 m), to at least 8,400 ft (2,560 m) in the Pinyon-juniper Woodland of the White Mountains. The species has been recorded at 9,400 ft (2,870 m) in the Panamint Mountains. This active, diurnal form is very alert and will flee when approached. Remarks: The subspecies of Striped Whipsnake present in the region is the Desert Striped Whipsnake (M. t. taeniatus ). The species ranges from eastern Washington south throughout the Great Basin and east into central Texas. It occurs in Mexico at least as far south as the state of Michoacán. A detailed study of Striped Whipsnakes was conducted between 1969 and 1973 in northern Utah, at a site where the snakes hibernate in communal dens. A total of 242 whipsnakes were marked and studied. It was found that young snakes ate only lizards, but adult snakes ate both lizards and small mammals. During the summer, individual snakes commonly moved 400–500 ft (122–152 m) in a day, and one snake traveled more than a mile (1.6 km) from the den. Range: Entire White-Inyo mountains region, between about 5,000 ft (1,520 m) and 9,000 ft (2,740 m) elevation. References: Bennion and Parker (1976), Ortenburger (1928), Parker and Brown (1980).
Localities: California, Inyo Co.: 6,620 ft, 9.8 mi NE Big Pine; Dead Horse Meadow, Crooked Creek, White Mtns. (LACM); 6,600 ft, 10 mi SE of Hwy. 168 on Eureka Valley Rd. (LACM); 6,750 ft, 10.7 mi SE (LACM); 5,400 ft, Grapevine Canyon, Nelson Range [Willow Creek] (USNM); Inyo Lodge Camp, Inyo Mtns. (LACM); 1.2 mi S Mono County line on Hwy. 168, White Mtns.; 15.5 mi NE of Hwy. 190 on Saline Valley Rd., Nelson Range (LACM); 3.1 mi N of Hwy. 168 on White Mtn. Rd. (LACM); 8,400 ft, 6.0 mi N. Mono Co.: Benton; 5,200 ft, 2 mi S. Nevada, Esmeralda Co.: 12 mi E Oasis, Silver Peak Range (KU).
Spotted Leaf-nosed Snake,Phyllorhynchus decurtatus(Cope, 1868). (Plate 10.32, Map 10.25) 12–20 in (30–50 cm); light reddish brown to gray dorsal background coloration with brown blotches running down back; uniform white ventral coloration; black stripe through each eye; enlarged, leaf-shaped rostral scale at tip of nose. Habitat: In the White-Inyo mountains region Spotted Leaf-nosed Snakes occur in Creosote Bush Scrub below about 3,000 ft (910 m). They are most common in sandy or gravelly areas. This strictly nocturnal species is commonly seen crossing roads on warm nights in the late spring and early summer. Remarks: The subspecies
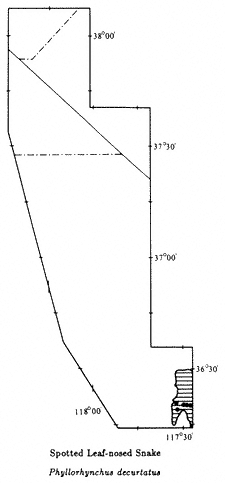
Map 10.25
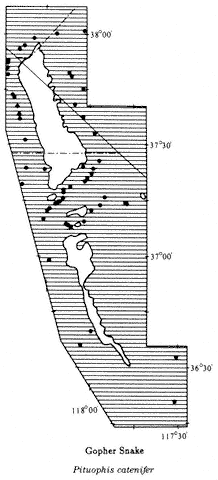
Map 10.26
of Spotted Leaf-nosed Snake present in the region is the Western Leaf-nosed Snake (P. d. perkinsi ). Until 1922, only six specimens were known to science, and it was considered one of the rarest North American snakes. At about this time, biologists discovered that nocturnal snakes could easily be collected by driving on paved roads at night. It was soon learned that Spotted Leaf-nosed Snakes were one of the most abundant snakes in the southern California deserts. The diet of this species is very specialized. Occasionally small lizards are eaten, but the majority of food taken consists of the eggs of other reptiles. The enlarged scale on the snout of this snake aids in digging in the sand in search of eggs. Range: Recorded only from the Panamint Valley. Suitable habitat is present in the extreme southern Owens Valley, Saline Valley, and Eureka Valley, but no specimens are recorded. References: Brattstrom (1953), Klauber (1935).
Localities: California, Inyo Co.: Darwin Falls; 3.9 mi W of Darwin Rd. on Hwy. 190; 0.5 mi E Panamint Springs; 1 mi NW; 1.3 mi E; 1.5 mi E; 1.7 mi E; 4.7 mi E; 5.7 mi E; 2.6 mi W of Panamint Valley Rd. on Hwy. 190; 4.3 mi W.
Gopher Snake,Pituophis catenifer (Blainville, 1835). (Plate 10.33, Map 10.26) 30–60 in (75–150 cm); large, blotched snake with distinctly keeled scales; dorsal coloration gray to tan; large black to brown blotches running down back; blotches often fused in neck region. Habitat: Gopher Snakes occur in all habitats below about 8,000 ft (2,440 m). They are active during the day and on warm nights, and they are commonly seen resting on paved roads during the late afternoon and at night. Remarks: The subspecies of Gopher Snake present in the area is the Great Basin Gopher Snake (P. c. deserticola ). Other subspecies of this wide-ranging snake occur from the Pacific coast to the Great Plains and from Canada to Mexico. In the past, many of these subspecies were recognized as distinct species. Some of the forms in the United States look very different from each other, and future studies may determine that they are, in fact, distinct species. This large, heavy-bodied snake feeds primarily on rodents, which are first killed by construction. The Gopher Snake is occasionally mistaken for a rattlesnake because it sometimes coils its body and vibrates its tail, making a rattling sound in dry leaves. Young Gopher Snakes are similar in color pattern to Glossy Snakes (Arizona elegans ). The former has keeled scales, and the latter has smooth scales. Range: Throughout the White-Inyo mountains region below 8,000 ft (2,440 m). References: Klauber (1947), Parker and Brown (1980).
Localities: California, Inyo Co.: 4,000 ft, 1 mi E Big Pine; 5.1 mi NE; 6.0 mi NE; 8.2 mi NE; 8.6 mi NE; 10.3 mi NE; 11.9 mi NE; 15.4 mi NE; 21.9 mi NE; 22.8 mi NE; 24.5 mi NE; 27.2 mi NE; 3.5 mi ESE Bishop; Hwy. 190 between Darwin Wash Rd. and 4,000 ft Vista Lookout; 6.9 mi SE of Hwy. 168 on Eureka Valley Rd.; 5,750 ft, 7.4 mi SE; 8.4 mi SE; 7,150 ft, 16.1 mi SE; 20.3 mi SE; 22.7 mi SE; 35.1 mi SE; 35.4 mi SE; French Spring, Inyo Mtns.; 5,750 ft, Grapevine Canyon, Nelson Range (CAS); 13 mi N Independence (LACM); Jackass Spring, 20 mi N Darwin, Panamint Mtns. (USNM); Lone Pine (CAS, USNM); 4.6 mi N; 3.1 mi S Mono County line on Hwy. 168; 6.8 mi S Mono County line on Hwy. 6; Westgard Pass (BYU); 6,000 ft; Wyman Canyon, 2 mi W Deep Springs Valley, White Mtns. Mono Co.: Benton; 5 mi N (SDSNH, UMMZ); 6 mi S (SDSNH); N end Fish Slough, 19 mi N Bishop (UCSB); 4.4 mi N Inyo County line on Hwy. 6; 10.9 mi N; 11.9 mi N; Silver Canyon, 4.5 mi E Laws; 18.6 mi N; 3.2 mi SW Nevada state line on Hwy. 6; 4.8 mi SW; Nevada state line on Hwy. 266, N Oasis. Nevada, Esmeralda Co.: 1 mi W of Hwy. 264 along Chiatovich Creek, Fish Lake Valley; 1 mi S Chiatovich Creek on Hwy. 264, Fish Lake Valley; 0.3 mi N Dyer; Fish Lake; jct. Hwy. 6 and Hwy. 264; 11.3 mi E Mineral County line on Hwy. 6; 5.1 mi N California state line on Hwy. 264. Mineral Co.: 1.6 mi E Montgomery Pass on Hwy 6; 2.4 mi NE California state line on Hwy. 6; 4.6 mi NE.
Long-nosed Snake,Rhinocheilus lecontei (Baird & Girard, 1853). (Plate 10.34, Map 10.27) 20–40 in (50–100 cm); a speckled snake with distinct black saddles across back and sides; white flecks on the sides of each saddle; in most individuals the area
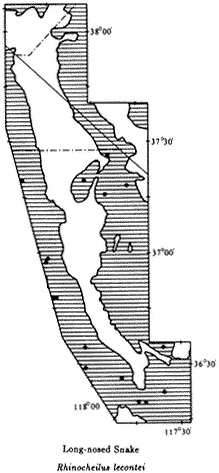
Map 10.27
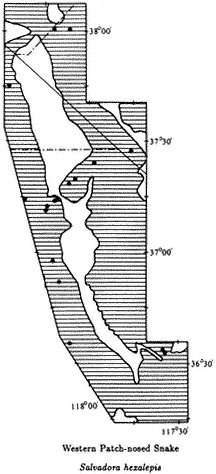
Map 10.28
between the saddles is white with red and black speckles; in some specimens the area between the saddles is uniform white; belly pale, some with black blotches; snout pointed. Habitat: Long-nosed Snakes occur throughout the White-Inyo mountains region below about 6,000 ft (1,830 m). They are most common in sandy Creosote Bush Scrub and Great Basin Scrub areas. This species is almost exclusively nocturnal and is usually seen crossing roads at night during the spring and summer. It is a fast-moving snake that rarely rests on the pavement as Gopher Snakes (Pituophis catenifer ) and rattlesnakes (Crotalus ) do. Remarks: The subspecies of Long-nosed Snake in the area is the Western Long-nosed Snake (R. l. lecontei ). At one time the black and white color phase was recognized as a distinct subspecies (R. l. clarus ). Owing, in part, to reports of both color phases being hatched from a single clutch of eggs, this taxonomic
distinction has been dropped for the present. However, more research is needed on the clarus phase. In some areas, such as parts of the Coachella Valley in Riverside County, California, all Long-nosed Snakes lack red speckles. The clarus phase may be distinguished from the similarly colored Common Kingsnake (Lampropeltis getula ) by the pointed snout and the absence of black bands connecting across the belly. Long-nosed Snakes feed on lizards and lizard eggs, small snakes, small mammals, and occasionally birds. Range: Throughout the White-Inyo mountains region below 6,000 ft (1,830 m). References: Klauber (1941), Shannon and Humphrey (1963).
Localities: California, Inyo Co.: 0.6 mi E Aberdeen; 1.3 mi S; Bartlett (SDSNH); 12 mi N Big Pine (LACM); 20.1 mi NE on Hwy. 168; 30.7 mi NE; 10 mi S Bishop; 5.7 mi N of Eureka Valley Rd. on North Eureka Valley Rd.; 26.9 mi SE of Hwy. 168 on Eureka Valley Rd.; 3,850 ft, Grapevine Canyon, Nelson Range (CAS); Independence; 1.4 mi W; 2 mi SE Lone Pine (SDSNH); 16.6 mi SE; 38 mi E; 1.3 mi W of Saline Valley Rd. on Hwy. 190; 0.8 mi E.
Western Patch-nosed Snake,Salvadora hexalepis(Cope, 1866). (Plate 10.35, Map 10.28) 24–36 in (60–90 cm); gray to light brown dorsal coloration with two narrow, broken stripes running down back; ventral coloration uniform cream; enlarged scale on tip of snout (rostral scale). Habitat: This snake occurs throughout the entire White-Inyo mountains region up to about 6,500 ft (1,980 m). It is most common in sandy valley floors. This is a fast-moving diurnal species. Patch-nosed snakes are sometimes seen in the late afternoon basking on roads. Remarks: The subspecies of Western Patch-nosed Snake in the area is the Mojave Patch-nosed Snake (S. h. mojavensis ). This genus contains eight species that range from northern Nevada to Guatemala. They occur in arid and semi-arid regions. The Western Patch-nosed Snake ranges the farthest north of any species, occurring from northern Nevada into central western Mexico, and from coastal southern California to western Texas. This wary, alert snake will attempt to escape when approached. In this area, it is more common in Great Basin Scrub habitats than in Creosote Bush Scrub habitats. Patch-nosed Snakes feed mainly on lizards and reptile eggs. Range: Throughout the White-Inyo mountains region below 6,500 ft (1,980 m). Reference: Bogert (1939).
Localities: California, Inyo Co.: Antelope Spring, Deep Springs Valley (LACM); 5,000 ft, 5 mi E Big Pine; 6,000 ft, 6 mi NE; 6,400 ft, 7 mi NE; 20.4 mi NE; 2 mi NE Deep Springs (LACM); 8.1 mi SW (LACM); 1 mi NE of Eureka Valley Rd. on Hwy. 168 (CMNH); 4,480 ft, 5,100 ft, Grapevine Canyon, Nelson Range (CAS); 5.0 mi NE Independence; 11.0 mi N; Keough Hot Springs, 7 mi S Bishop (LACM); 2.8 mi W Lone Pine (LACM); 9 mi W Panamint Springs on Hwy. 190; 6,000 ft, 3.1 mi S of summit on Saline Valley Rd. (CAS). Mono Co.: 4.4 mi S of Hwy. 120 at Benton on Hwy. 6. Nevada, Esmeralda Co.: 10 mi E Basalt (UMMZ); 4.0 mi E California state line on Hwy. 266; 0.5 mi E Mineral County line on Hwy. 6.
Ground Snake,Sonora semiannulata(Baird & Girard, 1853). (Plate 10.36, Map 10.29) 10–16 in (25–40 cm); black- and orange-banded; width of bands about equal; bands not sharply defined and not continuous ventrally except on tail. Habitat: The few specimens recorded from the region have been found in mountainous areas in
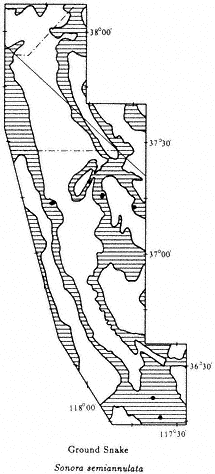
Map 10.29
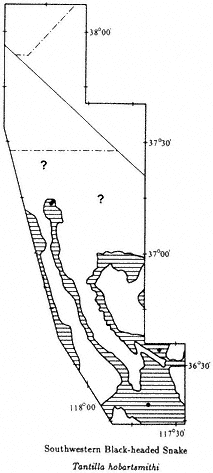
Map 10.30
both Creosote Bush Scrub and Great Basin Desert Scrub below 6,000 ft (1,830 m). Ground Snakes may occasionally be found under rocks near streams but are most commonly encountered on roads at dusk. Remarks: Ground Snakes range from Texas to California and from Idaho to the tip of Baja California and northeastern Mexico. They may be locally abundant but are seldom seen throughout most of their range. In some places striped or uniformly colored populations exist. In the past, some of these populations were regarded as separate species, but currently only a single species is recognized. Ground Snakes prey on spiders and other invertebrates. The only other snake in the region likely to be confused with this species is the Western Shovel-nosed Snake (Chionactis occipitalis ), which has a flattened snout and yellow rather than orange bands. There is also usually a faint brown saddle in each yellow band, in
the latter species. Range: Presumed to occur throughout the White-Inyo mountains region below 6,500 ft (1,980 m). May be absent from valley floors. Reference: Frost (1983).
Localities: California, Inyo Co.: 7 mi NE Big Pine; 7.7 mi NE; 4,000–5,000 ft, Darwin Vicinity on Hwy. 190; E end Deep Springs Valley (SDSNH); 27.3 mi SE of Hwy. 168 on Eureka Valley Rd.; 27.7 mi SE; 38.7 mi SE; 4,500 ft, Grapevine Canyon, Nelson Range (CAS); 6.8 mi E of Saline Valley Rd. on Hwy. 190.
Southwestern Black-headed Snake,Tantilla hobartsmithi(Taylor, 1936). (Plate 10.37, Map 10.30) 8–14 in (20–35 cm); small, thin brown snake with a black head; ventral color red. Habitat: In the White-Inyo mountains region, Black-headed Snakes have been found in rocky, mountainous places below 6,500 ft (1,980 m). These snakes occur both in Creosote Bush Scrub and Great Basin Scrub and into the Pinyon-juniper Woodland. They are most common around springs and streams. This is a nocturnal species that can occasionally be found by turning rocks. Remarks: This secretive, small snake is seldom observed. There are only four museum specimens from the White-Inyo mountains region. The northernmost known record for the species is on the Westgard Pass road (see Map 10.30), but it is probable that they occur farther north in suitable habitat. Black-headed Snakes have a very specialized diet, feeding almost exclusively on centipedes. Although they are not considered dangerous to humans, this species has enlarged rear fangs and venom to aid in killing prey. Outside of our area, the range of the Southwestern Black-headed Snake extends southeast into Nevada, Utah, Colorado, Arizona, New Mexico, Texas, and northern Mexico. Range: Northern Argus Mountains, Nelson Range above the Saline Valley, western slopes of the Inyo Mountains; may occur further north (see ? on Map 10.30). Reference: Cole and Hardy (1981).
Localities: California, Inyo Co.: 5,550 ft, 7.2 mi NE Big Pine; 8.5 mi NE; 4,020–4,030 ft, Grapevine Canyon, Nelson Range (CAS); Hogback Creek, 6 mi S Olancha (S of area); 3.4 mi E of Saline Valley Rd. on Hwy. 190.
Sierra Garter Snake,Thamnophis couchii (Kennicott, 1859). (Plate 10.38, Map 10.31) 24–42 in (60–105 cm); dorsal background color olive-brown with black spots arranged in four rows running down the body; pale chin; ventral surface light to dark brown, commonly with black line at posterior margin of each ventral scale; long, narrow, triangular head with black neck collar. Habitat: This highly aquatic snake is restricted to the vicinity of marshes, streams, and rivers. It is a wary diurnal species that readily enters the water if disturbed while basking. Once in the water, these snakes usually hide under rocks at the bottoms of pools. Remarks: This was formerly regarded as one wide-ranging species with six subspecies in California and western Nevada. It has now been split up into four species. In the western Sierra Nevada this snake has a typical striped garter snake pattern, but in our area it is spotted with no stripes. The snakes from the Owens Valley have not been studied yet and may actually represent a fifth species in this group. A second species of garter snake, the Western Terrestrial Garter Snake (T. elegans ) is also found here. Where the two species coexist
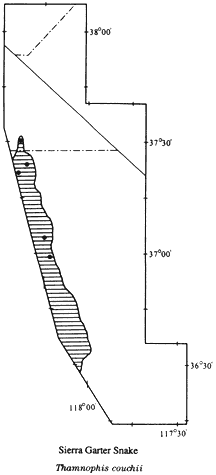
Map 10.31
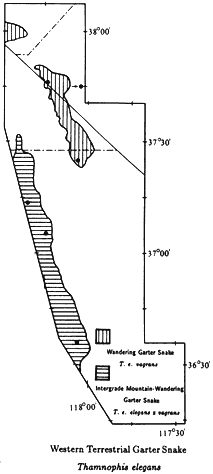
Map 10.32
in the Owens Valley, the Western Terrestrial Garter Snake has a middorsal stripe (see following account). Sierra Garter Snakes feed primarily on fish and frogs. Like other garter snakes, this species is live-bearing, and the young are born at a size of about 6 in (10 cm) in the late summer. Range: Owens Valley from Bishop to Lone Pine and streams draining the eastern slopes of the Sierra Nevada. References: Fitch (1940b), Fitch (1941), Rossman and Stewart (1987).
Localities: California, Inyo Co.: Owens River just above Aberdeen (UMMZ); 4,000 ft, Alvord, Owens Valley (USNM); Bishop; 4,200 ft, 2 mi W; 4, 100 ft, Laws; Lone Pine (USNM); Tinemaha Creek, 0.5 mi NW of Hwy. 395, 7 mi S Big Pine (LACM). Mono Co.: Fish Slough, 10 mi N Bishop (USNM).
Western Terrestrial Garter Snake,Thamnophis elegans(Baird & Girard, 1853). (Plate 10.39, Map 10.32) 18–36 in (45–90 cm); dorsal background light to dark brown with black spots forming irregular bands; in Owens Valley individuals, a middorsal yellow stripe is present; ventral surface light brown with irregular black patches, which sometimes completely cover the belly; black neck band may extend to top of head. Habitat: This species usually occurs in the vicinity of permanent water but may wander some distance. It frequents mountain streams, rivers, ponds, marshes, and lakes. These snakes are mainly diurnal but are occasionally active at night during the summer. Remarks: This variable species occurs throughout much of the western United States. In the northern portion of the White-Inyo mountains region, the Wandering Garter Snake (T. e. vagrans ) is present; this subspecies has no middorsal stripe. In the Owens Valley, intergrades between this subspecies and the Mountain Garter Snake (T. e. elegans ) occur (see Map 10.32); these intergrades have a middorsal stripe. The stripe will distinguish this species from the Sierra Garter Snake (T. couchii ), which also occurs in the Owens Valley. In addition to fish and frogs, Terrestrial Garter Snakes feed on lizards, mice, and even nestling birds. Young are born in the late summer. Range: Entire length of Owens Valley (T. e. eleganśvagrans );[1] Fish Lake Valley, streams on the eastern and southern slopes of the White Mountains to at least 8,200 ft (2,500 m), (T. elegans vagrans ). References: Fitch (1941), Fox (1951), White and Kolb (1974).
Localities: California, Inyo Co.: 4,000 ft, 5 mi NW Big Pine; 5.4 mi S (UMMZ); 4,200 ft, 2 mi W Bishop; Lone Pine (USNM); Roberts Ranch, Wyman Canyon, White Mtns. Nevada, Esmeralda Co.: 8,200 ft, Chiatovich Creek, White Mtns; Fish Lake.
Lyre Snake,Trimorphodon biscutatus (Duméril, Bibron & Duméril, 1854). (Plate 10.40, Map 10.33) 24–36 in (60–90 cm); gray dorsal coloration with brown blotches running down back; gray saddle within each brown blotch; white ventral coloration with small brown blotches fringing belly; head wide and flattened with blunt nose; brown V-shaped stripe behind eyes. Habitat: This nocturnal species occurs in rocky Creosote Bush Scrub areas below 5,000 ft (1,520 m). Lyre Snakes are uncommon but can occasionally be observed crossing roads at night. Remarks: The subspecies of Lyre Snake in this area is the California Lyre Snake (T. b. vandenburghi ). The Lyre Snake is a wide-ranging desert and tropical species occurring from Inyo County, California, southern Nevada, and southern Utah to Costa Rica, and from coastal southern California to western Texas. In the past, Lyre Snakes were classified as six distinct species, but recent work suggests that they represent a single, geographically variable species. In tropical Mexico, Lyre Snakes may reach a length of over 5 ft (1.5 m). This is a mildly venomous snake that has enlarged rear fangs. The venom is used to kill small lizards and perhaps small rodents. Lyre Snakes are not considered dangerous to humans but should be handled with caution. Range: In our area, known only from the Panamint Valley below 5,000 ft (1,520 m). Suitable
[1] ́ refers to intergrades between the subspecies T. e. elegans and T. e. vagrans
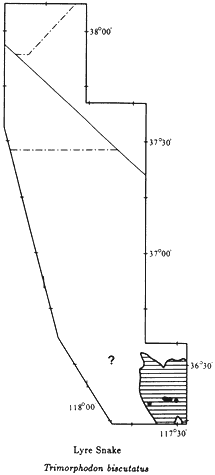
Map 10.33
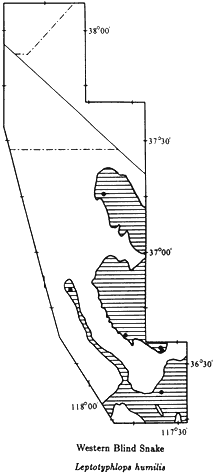
Map 10.34
habitat is present in the Saline, Eureka, and southern Owens valleys, but no specimens have been recorded (see ? on Map 10.33). References: Cowles and Bogert (1953), Gehlbach (1971), Klauber (1940a).
Localities: California, Inyo Co.: 1 mi W of Darwin Falls Rd. on Hwy. 190; 3 mi W; 3.2 mi W; 4.6 mi W; Hwy. 190, between Darwin Wash Rd. and 4,000 ft Vista Point; 4.7 mi W Panamint Springs (CAS); 3.5 mi E of Saline Valley Rd. on Hwy. 190 (LACM).
Slender Blind Snakes (Family Leptotyphlopidae)
Western Blind Snake,Leptotyphlops humilis(Baird & Girard, 1853). (Plate 10.41, Map 10.34) 6–12 in (15–30 cm); shiny in appearance; dorsal color silver or
light brown with a silver cast; eyes reduced to black spots covered by scales; head, body, and tail of uniform diameter. Habitat: In our area this species appears to be restricted to Creosote Bush Scrub below 5,000 ft (1,520 m). Most specimens have been found in rocky canyons where streams or springs are present. Worm Snakes, as they are commonly called, spend most of the time underground but occasionally are active on the surface at night. Remarks: Blind Snakes of the genus Leptotyphlops occur in Africa, Southwest Asia, South America, Central America, and North America. Most species occur in the tropics, and only two are present in the United States. Western Blind Snakes do not look like typical snakes and could be mistaken for worms because of their small size, uniform diameter, and lack of an obvious head. The greatly reduced eyes are covered by scales and function only to distinguish light intensity. They live underground, commonly in association with termite colonies. Termites, ants, and ant eggs are a major part of their diet. Blind snakes are rarely encountered in the White-Inyo mountains region but are commonly found in the Colorado River region of the Arizona-California border. Range: Canyons draining into the Panamint, Saline, Eureka, and Southern Owens valleys. References: Klauber (1940b), Brattstrom and Schwenkmeyer (1951).
Localities: California, Inyo Co.: 3,900 ft, Daisy Canyon, Saline Valley (CAS); 27.7 mi SE of Hwy. 168 on Eureka Valley Rd.; 4,000 ft, 4,480 ft, 4,500 ft, 4,630 ft, Grapevine Canyon, Nelson Range (CAS); 5 mi ENE Independence; 9.5 mi W Panamint Valley Rd. on Hwy. 190.
Vipers (Family Viperidae)
Sidewinder,Crotalus cerastes (Hallowell, 1854). (Plate 10.42, Map 10.35) 14–24 in (35–60 cm); light-gray background color with a single row of dark gray to brown oval blotches running down the center of the back; black speckling, occasionally forming indistinct blotches, along sides of body; no distinct black bands at base of rattle; enlarged scale forms a hornlike process above each eye; white stripe across top of each brown eye horn. Habitat: Sidewinders are most commonly encountered in sandy areas below 5,000 ft (1,520 m); however, individuals are occasionally found in rocky areas. They are primarily nocturnal but may be active in the late afternoon. Remarks: The subspecies of Sidewinder in the White-Inyo mountains region is the Mojave Desert Sidewinder (C. c. cerastes ). The Sidewinder reaches its northern distributional limits in the Hammil Valley. It probably ranges as far north as the Queen Valley in Mineral County, Nevada (see ? on Map 10.35). This species is unique among all New World snakes in that it moves sideways. This adaptation allows Sidewinders to move easily over sand dunes. Sidewinding has evolved independently in a few species of sand dune-dwelling vipers that occur in the deserts of northern and southern Africa. Sidewinders are the only rattlesnakes that bury themselves in the sand. It is possible to follow the distinctive S-shaped tracks of this snake and locate the site where the animal has buried itself for the day. Look for a circular depression in the sand, usually at the base of a bush, and watch for the top of the head protruding from the sand. Do not get too close because this is a venomous species. Range: On the west side
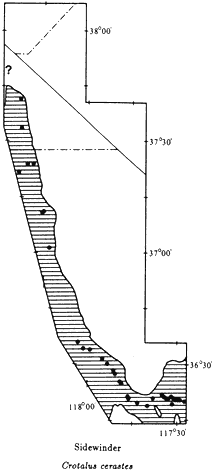
Map 10.35
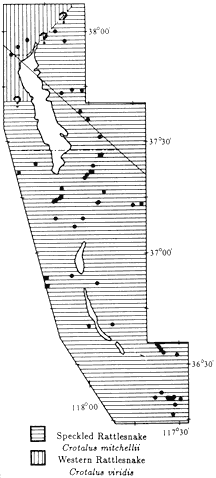
Map 10.36
of the Panamint Mountains in the Panamint Valley; on the west side of the White-Inyo Range in the Owens Valley, Chalfant Valley, and Hammil Valley; absent from the Deep Springs Valley and the eastern side of the White-Inyo Range. References: Klauber (1944), Klauber (1972).
Localities: California, Inyo Co.: 1.9 mi NE Big Pine; 2.4 mi NE; Bishop (SDSNH); Darwin Falls, Argus Mtns.; jct. of Darwin Falls Rd. and Hwy. 190; 0.5 mi W; 3.3 mi E; jct. Hwy. 136 and Hwy. 190; 5.5 mi SE of Hwy. 136 on Hwy. 190; 10.0 mi SE; Keeler; 1 mi S (CMNH); 4 mi SE (SDSNH); 8 mi SE; Laws; Lone Pine; 1.5 mi SE; 3.5 mi SE; 8.6 mi E; 10.0 mi SE; 3.4 mi NW Panamint Springs; 1.6 mi W; 4.1 mi W; 5.2 mi W; 6.1 mi W; 6.3 mi W; 6.5 mi W; 7 mi W; 8.0 mi W; 11.5 mi W; 2.6 mi E Saline Valley Rd. on Hwy. 190; 4,450 ft, Silver Creek Canyon, 5
mi ENE Bishop; E side Tinemaha Reservoir. Mono Co.: 4,840 ft, Cinnamon Ranch, 10 mi SSE Benton; 6.9 mi N Inyo County line on Hwy. 6.
Speckled Rattlesnake,Crotalus mitchellii(Cope, 1861). (Plate 10.43, Map 10.36) 24–36 in (60–100 cm); background color light gray to light brown with narrow gray to dark brown bands running from neck to rattle; bands wider on middle of back than on sides; commonly scattered black and white flecks at margin of bands; ventral coloration cream with scattered black flecks; end of tail and base of rattle black; top of head with two internasal scales in contact with the rostral scale, and supraocular scales pitted or with outer edges broken. Habitat: This snake prefers rocky canyons and rocky slopes surrounding valleys but is occasionally found on valley floors. Speckled Rattlesnakes occur as high as 9,000 ft (2,740 m) in the White Mountains. This species is active during the day in the spring and fall and at high elevations. At lower elevations, it is nocturnal during the summer. Remarks: The subspecies of Speckled Rattlesnake present in this area is the Panamint Rattlesnake (C. m. stephensi ). This snake may be confused with the Western Rattlesnake (C. viridis ) because the two species are similar in size and color pattern. They can be distinguished by careful examination of the scales on the top of the head. The Panamint Rattlesnake has two internasal scales in contact with the rostral scale and has supraocular scales that are pitted or with broken outer edges. The Western Rattlesnake has three or four internasal scales touching the rostral scale, and the supraocular scales are smooth and regular in shape. Speckled Rattlesnakes occur from the tip of Baja California through southern California, western Arizona, and southern Nevada. The northern known limit of its distribution is the Fish Lake Valley. A detailed discussion of the distributional interactions between this species and the Western Rattlesnake is found in the following account. Owing to their size and tendency to rattle when approached, Speckled Rattlesnakes are easily observed. These snakes feed primarily on rodents but will also eat birds and lizards. Range: On the west side of the White-Inyo Range north to at least the Mono County line (see ? on Map 10.36); on the east, in the Panamint, Saline, Eureka, Deep Springs, and Fish Lake valleys; surrounding mountains to around 9,000 ft (2,740 m). References: Klauber (1936), Klauber (1972).
Localities: California, Inyo Co.: 2 mi S Aberdeen [old Aberdeen at railroad] (SDSNH); Barrel Springs, 9 mi NE Independence; Batchelder Spring, 8 mi NE Big Pine; Beveridge Canyon, Saline Valley (FMNH); 6 mi NE Big Pine; 6.3 mi NE; 6.6 mi NE; 7.2 mi NE: 8.2 mi NE; 10.7 mi NE; 23.3 mi NE; 25.6 NE; 27.1 mi NE; 29.4 mi NE; 31.9 mi NE; near Bishop (SDSNH); Darwin Falls; 5.2 mi W of Darwin Falls Rd. on Hwy. 190; 7 mi S of Hwy. 190 on Darwin Rd.; Deep Springs (CMNH); near; E end Deep Springs Valley (SDSNH); 3.7 mi SE of Hwy. 168 on Eureka Valley Rd.; 2,070 m, 10.6 mi SE; 2,150 m, 14.5 mi SE; 1,790 m, 21.8 mi SE; 24.1 mi SE; 24.3 mi SE; 28.1 mi SE; 37.4 mi SE; 40.1 mi SE; 3,300–5,320 ft, Grapevine Canyon, Nelson Range (CAS); 5,500 ft, Grapevine Canyon, Nelson Range [Willow Creek] (USNM); 5–8 mi N Independence (SDSNH); Jackass Spring, 20 mi N Darwin, Panamint Mtns. (SDSNH); 39 mi E Lone Pine; 8.7 mi SE; 1.9 mi S Mono County line on Hwy. 168; 5 mi NW Panamint Springs; 6.5 mi W; 4.1
mi W of Panamint Valley Rd. on Hwy. 190; 10.1 mi W; 5.7 mi E of Saline Valley Rd. on Hwy. 190; Silver Creek Canyon, White Mtns.; 3.4 mi S Nevada state line on Hwy. 266; summit of Westgard Pass (CAS); Mt. Whitney Fish Hatchery, 5 mi N Independence (LACM); Willow Springs, Saline Valley (AMNH). Nevada, Esmeralda Co.: 3 mi S Dyer (LACM); 3.4 mi N; 7.2 mi S; 1 mi S Fish Lake; 4.0 mi S of Hwy. 6 on Hwy. 264; 2,150 m, Indian Creek, White Mtns.; 7,150 ft, Trail Canyon, White Mtns.
Western Rattlesnake,Crotalus viridis (Rafinesque, 1818). (Plate 10.44, Map 10.36) 28–50 in (70–125 cm); background color light gray to light brown, with narrow gray to dark brown bands running from neck to rattle; bands wider on middle of back than on sides; commonly scattered black and white flecks at margin of bands; ventral coloration cream with scattered black flecks; end of tail and base of rattle black; top of head with three or four prenasal scales in contact with rostral; supraocular scales smooth and regular in shape. Habitat: This snake occurs in valley floors, rocky canyons, and well into the mountains. Western Rattlesnakes are active during the day and are also nocturnal on warm nights. Remarks: The subspecies of Western Rattlesnake that occurs in the area is the Great Basin Rattlesnake (C. v. lutosus ). (See preceding account for characteristics that distinguish this species from the similar Speckled Rattlesnake, C. mitchellii. ) There are eight subspecies of the Western Rattlesnake, ranging from Canada to Mexico. In most places where Western Rattlesnakes occur together with or near other species of rattlesnakes, there are differences in size and color pattern that make it easy to tell the species apart. This is not the case in the White-Inyo mountains region. The Speckled Rattlesnake has been found as far north as Silver Creek Canyon, just south of the Mono County line in the Owens Valley, and at the northern edge of the Fish Lake Valley. The only three specimens of Western Rattlesnake known from the area were collected in the vicinity of the California-Nevada state line in the Queen Valley. These three specimens are similar in color pattern to specimens of Speckled Rattlesnakes taken in the region to the south. These two species may hybridize in the northern White Mountains and adjacent valleys. Unfortunately, no specimens have been obtained from these areas. Range: Northwestern slopes of White Mountains, Queen Valley, and areas north to edge of study area (see ? on Map 10.36). References: Fitch and Glading (1947), Klauber (1972).
Localities: California, Mono Co.: 0.5 mi SW Nevada state line on Hwy. 6. Nevada, Mineral Co.: 6,400 ft, 3.2 mi W Montgomery Pass; 2.3 mi NE California state line on Hwy. 6.
Amphibian and Reptile Diversity in Selected Habitats
A variety of amphibian and reptile habitats are present in the White-Inyo mountains region. In this section, eight sites that are representative of particular habitats have been selected. Each site is on or near a main road and is easily accessible to people who may wish to observe a particular species or the species assemblage at a specific habitat.
Southern Owens Valley
The rocky slopes at the base of the Inyo Mountains are drained by canyons. At the mouth of each canyon a rocky, boulder-strewn alluvial fan gradually merges into the sand dunes around Owens Lake (Fig. 10.6). Scattered Creosote Bushes are present at the base of the mountains.
Amphibians
Great Basin Spadefoot (Spea intermontana ). Nocturnal; breeds in temporary pools in sand dunes.
Lizards
Western Banded Gecko (Coleonyx variegatus ). Nocturnal; most common in rocky areas.
Zebra-tailed Lizard (Callisaurus draconoides ). Diurnal; very common on gravel flats and at bases of sand dunes.
Great Basin Collard Lizard (Crotaphytus bicinctores ). Diurnal; basks on boulders.
Desert Iguana (Dipsosaurus dorsalis ). Diurnal; uncommon on alluvial fans and around bushes in sand dunes.
Long-nosed Leopard Lizard (Gambelia wislizenii ). Diurnal; basks on small rocks and under bushes; rare on rocky slopes.
Desert Horned Lizard (Phrynosoma platyrhinos ). Diurnal; most common in sandy areas; well camouflaged except when basking on small rocks.
Chuckwalla (Sauromalus obesus ). Diurnal; basks on boulders on slopes and in canyons.
Desert Spiny Lizard (Sceloporus magister ). Diurnal; occurs in rocky areas and around large bushes in sand dunes.
Side-blotched Lizard (Uta stansburiana ). Diurnal; occurs in all habitats; most common lizard in the area.
Western Whiptail (Cnemidophorus tigris ). Diurnal; present in all habitats; less common in rocky areas.
Desert Night Lizard (Xantusia vigilis ). Diurnal; secretive; active under cover objects such as logs and brush piles.
Snakes
Glossy Snake (Arizona elegans ). Nocturnal; usually occurs in sandy areas.
Western Shovel-nosed Snake (Chionactis occipitalis ). Nocturnal; most common among sand dunes.
Night Snake (Hypsiglena torquata ). Nocturnal; usually occurs on rocky slopes and in canyons.

Figure 10.6
East side of Owens Lake, southern Owens Valley.
Common Kingsnake (Lampropeltis getula ). Nocturnal during summer, diurnal during spring and fall; occurs in all habitats but rare in sand dunes.
Coachwhip (Masticophis flagellum ). Diurnal; lives in all habitats but less common in rocky areas.
Gopher Snake (Pituophis catenifer ). Both nocturnal and diurnal; occurs in all habitats.
Long-nosed Snake (Rhinocheilus lecontei ). Nocturnal; most common in sandy areas.
Western Patch-nosed Snake (Salvadora hexalepis ). Diurnal; most common in sandy areas.
Ground Snake (Sonora semiannulata ). Nocturnal; active in the early evening; prefers rocky areas.
Southwestern Black-headed Snake (Tantilla hobartsmithi ). Nocturnal; occurs in rocky areas.
Western Blind Snake (Leptotyphlops humilis ). Nocturnal; occurs in washes of rocky areas; can be common in areas with permanent surface water.
Sidewinder (Crotalus cerastes ). Usually nocturnal; occurs in all habitats but most common in sandy areas.
Speckled Rattlesnake (Crotalus mitchellii ). Both nocturnal and diurnal; most common in rocky areas.
Southern White Mountains
Tollhouse Spring (Fig. 10.7) is the only source of permanent surface water along the Westgard Pass road (Hwy. 168). There is a fairly extensive area of riparian vegetation around the spring. The Pinyon-juniper Woodland starts just above the spring. A wide, gravelly, boulder-strewn wash below the spring separates the rocky lower slopes of the Inyo Mountains to the south from the White Mountains to the north.
Amphibians
Western Toad (Bufo boreas ). Both nocturnal and diurnal; breeds in stream below Tollhouse Spring; may be extinct.
Black Toad (Bufo exsul ). Diurnal except during heat of midsummer; introduced to Tollhouse Spring; may be extinct.
Great Basin Spadefoot (Spea intermontana ). Nocturnal; expected but not confirmed from Tollhouse Spring; known to occur nearby.
Lizards
Panamint Alligator Lizard (Elgaria panamintina ). Usually diurnal but sometimes active after dusk; may climb in vegetation in search of food; sometimes on road at dusk.
Western Banded Gecko (Coleonyx variegatus ). Nocturnal; commonly observed at night on road below spring at around 5,000 ft (1,520 m) elevation.
Zebra-tailed Lizard (Callisaurus draconoides ). Diurnal; occurs in sandy or gravelly washes.
Great Basin Collard Lizard (Crotaphytus bicinctores ). Diurnal; basks on boulders.
Long-nosed Leopard Lizard (Gambelia wislizenii ). Diurnal; basks on small rocks and under bushes in wash.
Desert Horned Lizard (Phrynosoma platyrhinos ). Diurnal; occurs in wash; may bask on small rocks.
Sagebrush Lizard (Sceloporus graciosus ). Diurnal; occurs from Tollhouse Spring up into Pinyon-juniper Woodland.
Desert Spiny Lizard (Sceloporus magister ). Diurnal; basks on rocks and tree trunks.
Western Fence Lizard (Sceloporus occidentalis ). Diurnal; basks on rocks, logs, and tree trunks; most common lizard from Tollhouse Spring up into Pinyon-juniper Woodland.
Side-blotched Lizard (Uta stansburiana ). Diurnal; most common in wash below spring.

Figure 10.7
Vicinity of Tollhouse Spring, Westgard Pass, southern White Mountains
Gilbert Skink (Eumeces gilberti ). Diurnal but secretive; occurs in riparian vegetation around Tollhouse Spring.
Western Whiptail (Cnemidophorus tigris ). Diurnal; most common around bushes in wash below spring.
Desert Night Lizard (Xantusis vigilis ). Diurnal but secretive; active under cover objects such as logs and rocks.
Snakes
Night Snake (Hypsiglena torquata ). Nocturnal; relatively common on road at night below Tollhouse Spring.
Common Kingsnake (Lampropeltis getula ). Diurnal during spring and fall, nocturnal in heat of midsummer; very common around Tollhouse Spring.
Coachwhip (Masticophis flagellum ). Diurnal; present in all habitats but most common in wash.
Striped Whipsnake (Masticophis taeniatus ). Diurnal; occurs in all habitats.
Gopher Snake (Pituophis catenifer ). Diurnal during spring and fall, nocturnal during heat of summer; commonly seen on road around Tollhouse Spring.
Long-nosed Snake (Rhinocheilus lecontei ). Nocturnal; occasionally seen crossing road below Tollhouse Spring.
Western Patch-nosed Snake (Salvadora hexalepis ). Diurnal; most common in sandy or gravelly washes.
Ground Snake (Sonora semiannulata ). Nocturnal; active in early evening; during the day, expected under rocks around spring.
Southern Black-headed Snake (Tantilla hobartsmithi ). Nocturnal; two records from about 5,500 ft (1,680 m) elevation on road below spring and 6,500 ft (1.980 m) on road above spring.
Sidewinder (Crotalus cerastes ). Usually nocturnal; occurs in all habitats at lower elevations below spring but most common in sandy areas.
Speckled Rattlesnake (Crotalus mitchellii ). Both nocturnal and diurnal; occurs in all habitats around Tollhouse Spring.
Central Owens Valley
Along the Owens River are extensive, large tree stands with other riparian vegetation. Such growth is also present around creeks draining the Sierra Nevada (Fig. 10.8). Along the Owens River many side pools provide breeding habitat for amphibians.
In this list only riparian-associated species are discussed. For other species in the area, refer to the southern White Mountains list (except for the Panamint Alligator Lizard, Elgaria panamintina , and the Gilbert Skink, Eumeces gilberti ).
Amphibians
Owens Valley Web-toed Salamander (Hydromantes sp.). Nocturnal; active in spring; occurs under wood and rocks along streams draining the Sierra Nevada.
Western Toad (Bufo boreas ). Both nocturnal and diurnal; breeds in side pools.
Pacific Treefrog (Pseudacris regilla ). Both nocturnal and diurnal; breeds in side pools; occurs along streams and in marsh areas.
Great Basin Spadefoot (Spea intermontana ). Nocturnal; breeds in temporary pools.
Lizards
Southern Alligator Lizard (Elgaria multicarinata ). Diurnal but secretive; active in the late afternoon; occurs under wood and rocks along streams draining the Sierra Nevada.
Western Skink (Eumeces skiltonianus ). Diurnal but secretive; occurs under wood and rocks along streams draining the Sierra Nevada.
Snakes
Sierra Garter Snake (Thamnophis couchii ). Diurnal and wary; highly aquatic; occurs along the Owens River and large streams draining the Sierra Nevada.

Figure 10.8
East side of the Sierra Nevada in the central Owens Valley.
Western Terrestrial Garter Snake (Thamnophis elegans ). Mainly diurnal but also nocturnal during warm weather; occurs along the Owens River and streams draining the Sierra Nevada; may wander from permanent water.
Queen Valley and Northern White Mountains
The floor of Queen Valley, at an elevation of about 6,000 ft (1,830 m), is covered with typical Great Basin Scrub vegetation (Fig. 10.9). On the southeast side of the valley, Pinyon-juniper Woodland starts on the slopes of the White Mountains at an elevation of 6,800 ft (2,070 m). Springs with dense riparian vegetation are present in Queen and Buffalo canyons.
Amphibians
Western Toad (Bufo boreas ). Both nocturnal and diurnal; occurs in riparian areas in Queen and Buffalo canyons.
Great Basin Spadefoot (Spea intermontana ). Nocturnal; breeds in temporary pools; occasionally seen on roads at night during and after rains.

Figure 10.9
Queen Valley and northern White Mountains.
Lizards
Zebra-tailed Lizard (Callisaurus draconoides ). Diurnal; expected in sandy and gravelly areas below Pinyon-juniper Woodland.
Great Basin Collard Lizard (Crotaphytus bicinctores ). Diurnal; occurs in rocky areas below about 6,500 ft (1,980 m).
Long-nosed Leopard Lizard (Gambeila wislizenii ). Diurnal; basks on small rocks and under bushes; rare on rocky slopes.
Desert Horned Lizard (Phrynosoma platyrhinos ). Diurnal; most common in sandy areas; not present above 7,000 ft (2,130 m).
Sagebrush Lizard (Sceloporus graciosus ). Diurnal; common in Pinyon-juniper Woodland.
Desert Spiny Lizard (Sceloporus magister ). Diurnal; occurs in rocky slopes and around large bushes below 7,000 ft (2,130 m).
Western Fence Lizard (Sceloporus occidentalis ). Diurnal; commonly seen basking on rocks and logs in Pinyon-juniper Woodland; may climb trees to escape when approached.
Side-blotched Lizard (Uta stansburiana ). Diurnal; most common in sandy areas on valley floor; absent above 7,000 ft (2,130 m).
Western Skink (Eumeces skiltonianus ). Diurnal but secretive; not confirmed but expected in canyons draining the White Mountains.
Western Whiptail (Cnemidophorus tigris ). Diurnal; occurs up to about 7,500 ft (2,290 m); most common in Great Basin Scrub areas.
Snakes
Night Snake (Hypsiglena torquata ). Nocturnal; expected in rocky foothills below 6,500 ft (1,980 m).
Common Kingsnake (Lampropeltis getula ). Nocturnal during summer, diurnal during spring and fall; occurs in all habitats below about 7,500 ft (2,290 m).
Coachwhip (Masticophis flagellum ). Diurnal; expected on valley floor; usually absent above 6,000 ft (1,830 m).
Striped Whipsnake (Masticophis taeniatus ). Diurnal; occurs from valley floor well into Pinyon-juniper Woodland in the foothills.
Gopher Snake (Pituophis catenifer ). Both nocturnal and diurnal; occurs in all habitats below about 8,000 ft (2,440 m).
Long-nosed Snake (Rhinocheilus lecontei ). Nocturnal; expected in sandy areas on valley floor up to about 6,000 ft (1,830 m).
Western Patch-nosed Snake (Salvadora hexalepis ). Diurnal; most common in sandy areas on valley floor; not expected above 6,500 ft (1,980 m).
Ground Snake (Sonora semiannulata ). Nocturnal; active in the early evening; expected in rocky areas up to about 6,000 ft (1,830 m).
Western Rattlesnake (Crotalus viridis ). Both nocturnal and diurnal; present on valley floor; expected in foothills.
Northeastern Argus Mountains
The Panamint Valley has the most Creosote Bush Scrub species. The slopes of the Argus Mountains provide a good habitat for species restricted to rocky Creosote Bush Scrub. Darwin Falls (Fig. 10.10) has an extensive riparian-vegetated area.
All species covered in the southern Owens Valley account occur here (except the Great Basin Spadefoot, Spea intermontana ) and are found in a similar manner. Additional species are discussed in the following list.
Amphibians
Western Toad (Bufo boreas ). Mostly nocturnal but also diurnal; occurs in riparian areas at Darwin Falls; hybridizes with the Red-spotted Toad (Bufo punctatus ).
Red-spotted Toad (Bufo punctatus ). Nocturnal; breeds in pools in Darwin Canyon; hides during the day in rodent burrows or under rocks; hybridizes with the Western Toad (Bufo boreas ).

Figure 10.10
Darwin Falls, northeastern Argus Mountains.
Snakes
Rosy Boa (Lichanura trivirgata ). Nocturnal; diurnal in late evening and morning during spring; during midsummer nocturnal only; in rocky areas to 5,000 ft (1,520 m).
Spotted Leaf-nosed Snake (Phyllorhynchus decurtatus ). Nocturnal; usually present in sandy or gravelly places.
Lyre Snake (Trimorphodon biscutatus ). Nocturnal; in rocky areas to 5,000 ft (1,520 m).
Base of East Side of Inyo Mountains
Hunter Canyon (Fig. 10.11) is one of several canyons draining the east side of the Inyo Mountains that contains extensive riparian vegetation and surface water all year. The rocky canyon mouth is located at an elevation of about 1,600 ft (490 m) in rocky Creosote Bush Scrub. Only riparian-restricted amphibians and reptiles are discussed here. General lowland Creosote Bush Scrub species that occur here (except for the Great Basin Spadefoot, Spea intermontana , the Glossy Snake, Arizona elegans , and the Sidewinder, Crotalus cerastes ) are covered in the Southern Owens Valley account.

Figure 10.11
Hunter Canyon, east side of the Inyo Mountains.
Amphibians
Inyo Mountains Slender Salamander (Batrachoseps campi ). Nocturnal; restricted to vicinity of water; lives in mossy, damp crevices and under rocks where ground is wet.
Red-spotted Toad (Bufo punctatus ). Nocturnal; breeds in pools in canyon; hides during the day in rodent burrows or under rocks.
Lizards
Panamint Alligator Lizard (Elgaria panamintina ). Usually diurnal but sometimes active after dusk; may climb in vegetation in search of food.
Gilbert Skink (Eumeces gilberti ). Diurnal but secretive; commonly forages in leaf litter.
Northeast Slopes of Inyo Mountains
Joshua Flats (Fig. 10.12), at an elevation of about 6,200 ft (1,890 m) on the Eureka Valley Road, is covered with an extensive stand of Joshua Trees. Pinyon-juniper Woodland is present on the surrounding hills. Several narrow, rocky canyons drain into the flats.

Figure 10.12
Joshua Flats, northeastern Inyo Mountains.
Lizards
Zebra-tailed Lizard (Callisaurus draconoides ). Diurnal; occurs in sandy or gravelly washes.
Great Basin Collard Lizard (Crotaphytus bicintores ). Diurnal; basks on boulders.
Long-nosed Leopard Lizard (Gambelia wislizenii ). Diurnal; basks on small rocks and under bushes.
Desert Horned Lizard (Phrynosoma platyrhinos ). Diurnal; most common in sandy parts of Joshua Flats.
Sagebrush Lizard (Sceloporus graciosus ). Diurnal; active on ground around bushes; basks on small rocks and logs; occurs in Pinyon-juniper Woodland above flats.
Desert Spiny Lizard (Sceloporus magister ). Diurnal; very common at Joshua Flats; climbs high in Joshua Trees.
Western Fence Lizard (Sceloporus occidentalis ). Diurnal; basks on rocks and fallen Joshua Trees.
Side-blotched Lizard (Uta stansburiana ). Diurnal; most common in sandy part of flats.
Gilbert Skink (Eumeces gilberti ). Diurnal but secretive; occurs under large rocks and in piles of Joshua Tree rubble.
Western Whiptail (Cnemidophorus tigris ). Diurnal; most common around bushes in sandy areas.
Desert Night Lizard (Xantusia vigilis ). Diurnal but secretive; should live in piles of Joshua Tree rubble but has not been found at this site.
Snakes
Night Snake (Hypsiglena torquata ). Nocturnal; can be found during the day under fallen Joshua Tree logs; common along road at night.
Common Kingsnake (Lampropeltis getula ). Diurnal during spring and fall, nocturnal during midsummer; occurs in all habitats.
Coachwhip (Masticophis flagellum ). Diurnal; occurs in all habitats but most common in sandy flats.
Striped Whipsnake (Masticophis taeniatus ). Diurnal; occurs in all habitats.
Gopher Snake (Pituophis catenifer ). Nocturnal during midsummer, diurnal during spring and fall; occurs in all habitats.
Long-nosed Snake (Rhinocheilus lecontei ). Nocturnal; expected in sandy areas of Joshua Flats.
Patched-nosed Snake (Salvadora hexalepis ). Diurnal; most common in sandy areas but present in all habitats.
Ground Snake (Sonora semiannulata ). Nocturnal; occasionally found by turning rocks and fallen logs.
Speckled Rattlesnake (Crotalus mitchellii ). Both nocturnal and diurnal but not active in heat of day during midsummer.
Deep Springs Valley
Most of the sandy floor of Deep Springs Valley is covered with Great Basin desert vegetation. The lowest point is about 5,000 ft (1,520 m) at Deep Springs Lake. At the southeastern edge of the valley, the flow from Deep Springs forms a marsh of a few acres around Deep Springs Valley (Fig. 10.13). The rocky foothills of the White-Inyo Range, which surround the valley, support stands of pinyon and juniper.
Amphibians
Black Toad (Bufo exsul ). Diurnal, becoming nocturnal during heat of summer; restricted to pools and marshes around springs.
Great Basin Spadefoot (Spea intermontana ). Nocturnal; breeds in pools; occasionally seen on road at night.

Figure 10.13
Deep Springs Valley
Lizards
Great Basin Collard Lizard (Crotaphytus bicinctores ). Diurnal; basks on boulders in foothills surrounding valley.
Long-nosed Leopard Lizard (Gambelia wislizenii ). Diurnal; basks on small rocks and under bushes; most common in sandy areas.
Desert Horned Lizard (Phrynosoma platyrhinos ). Diurnal; common throughout the valley; commonly seen along the highway.
Sagebrush Lizard (Sceloporus graciosus ). Diurnal; absent from valley floor; occurs in Pinyon-juniper Woodland of foothills.
Desert Spiny Lizard (Sceloporus magister ). Diurnal; occurs in rocky hills and around large bushes on valley floor.
Western Fence Lizard (Sceloporus occidentalis ). Diurnal; lives in rocky foothills; commonly seen basking on rocks along lower Wyman Canyon Road and along highway east of Deep Springs College.
Side-blotched Lizard (Uta stansburiana ). Diurnal; most common in sandy areas on valley floor.
Gilbert Skink (Eumeces gilberti ). Diurnal but secretive; rarely seen; appears to be restricted to the vicinity of springs.
Western Whiptail (Cnemidophorus tigris ). Diurnal; occurs throughout valley and surrounding foothills; very common around bushes on valley floor.
Snakes
Night Snake (Hypsiglena torquata ). Nocturnal; occurs in rocky foothills surrounding valley.
Common Kingsnake (Lampropeltis getula ). Nocturnal during summer, diurnal during spring and fall; occurs in all habitats.
Coachwhip (Masticophis flagellum ). Diurnal; most common on valley floor.
Striped Whipsnake (Masticophis taeniatus ). Diurnal; occurs from valley floor well into Pinyon-juniper Woodland.
Gopher Snake (Pituophis catenifer ). Both nocturnal and diurnal; occurs in all habitats.
Long-nosed Snake (Rhinocheilus lecontei ). Nocturnal; occurs on valley floor.
Western Patch-nosed Snake (Salvadora hexalepis ). Diurnal; most common in sandy areas on valley floor.
Ground Snake (Sonora semiannulata ). Nocturnal; active in the early evening; prefers rocky areas.
Speckled Rattlesnake (Crotalus mitchellii ). Both nocturnal and diurnal; most common in rocky areas but present on valley floor.
References
Axtell, R. W. 1972. Hybridization between western collard lizards with a proposed taxonomic rearrangement. Copeia 1972:707–727.
Banta, B. H. 1962. A preliminary account of the herpetofauna of the Saline Valley hydrographic basin, Inyo County, California. Wasmann Journal of Biology 20:161–251.
Bennion, R. S., and W. S. Parker. 1976. Field observations of courtship and aggressive behavior in desert striped whipsnakes, Masticophis t. taeniatus. Herpetologica 32:30–35.
Berry, K. 1974. The ecology and social behavior on the chuckwalla, Sauromalus obesus obesus Baird. University of California Publications in Zoology 101:1–60.
Bezy, R. L., and J. W. Sites, Jr. 1987. A preliminary study of allozyme evolution in the lizard family Xantusiidae. Herpetologica 43:280–292.
Bogert, C. M. 1939. A study of the genus Salvadora, the patch-nosed snakes. Publications of the University of California at Los Angeles in Biological Sciences 1:177–236.
Brattstrom, B. H. 1953. Notes on a population of leaf-nosed snakes Phyllorhynchus decurtatus perkinsi. Herpetologica 9:57–64.
Brattstrom, B. H., and R. C. Schwenkmeyer. 1951. Notes on the natural history of the worm snake, Leptotyphlops humilis. Herpetologica 7:193–196.
Braun-Blanquet, J. (translated by G. D. Fuller and H. S. Conrad). 1932. Plant sociology: The study of plant communities . McGraw-Hill, New York.
Carpenter, C. C., and J. C. Gillingham. 1975. Postural responses to kingsnakes by crotaline snakes. Herpetologica 31:293–302.
Cole, C. J. 1984. Unisexual lizards. Scientific American 250(1):94–100.
Cole, C. J., and L. M. Hardy. 1981. Systematics of North American colubrid snakes related to Tantilla planiceps (Blainville). Bulletin of the American Museum of Natural History 171:199–284.
Collins, J. T. 1990. Standard common and current scientific names for North American amphibians and reptiles, 3d ed. Society for the Study of Amphibians and Reptiles, Herpetological Circular no. 190.
Congdon, J. D., L. J. Vitt, and W. W. King. 1974. Geckos: Adaptive significance and energetics of tail autotomy. Science 184:1379–1380.
Cowles, R. B., and C. M. Bogert. 1935. Observations on the California lyre snake, Trimorphodon vandenburghi Klauber, with notes on the effectiveness of its venom. Copeia 1935:80–85.
Elvin D. W. 1963. Variation and distribution of the shovel-nosed snakes (Chionactis occipitalis ) in the northern Mojave Desert, California and Nevada. Herpetologica 19:73–76.
Fitch, H. S. 1935. Natural history of the alligator lizards. Transactions of the Academy of Science at Saint Louis 29:1–38.
Fitch, H. S. 1938. A systematic account of the alligator lizards (Gerrhonotus ) in the western United States and lower California. American Midland Naturalist 20:381–424.
Fitch, H. S. 1940a. A field study of the growth and behavior of the fence lizard. University of California Publications in Zoology 44:151–172.
Fitch, H. S. 1940b. A biogeographical study of the ordinoides artenkreis of garter snakes (genus Thamnophis ). University of California Publications in Zoology 44:1–150.
Fitch, H. S. 1941. The feeding habits of California garter snakes. California Fish and Game 27(2):2–32.
Fitch, H. S., and B. Glading. 1947. A field study of a rattlesnake population. California Fish and Game 33(2):103–123.
Fox, W. 1951. Relationships among the garter snakes of the Thamnophis elegans rassenkreis. University of California Publications in Zoology 50:485–530.
Frost, D. 1983. Relationships of the Baja California ground snakes, genus Sonora. Transactions of the Kansas Academy of Science 86:31–37.
Gehlbach, F. R. 1971. Lyre snakes of the Trimorphodon biscutatus complex: A taxonomic resume. Herpetologica 27:200–211.
Good, D. A. 1988a. Allozyme variation and phylogenetic relationships among the species of Elgaria (Squamata; Anguidae). Herpetologica 44:154–162.
Good, D. A. 1988b. Phylogenetic relationships among Gerrhonotine lizards: An analysis of external morphology. University of California Publications in Zoology . 121:1–139.
Gorman, G. C. 1965. The distribution of Lichanura trivirgata and the status of the species. Herpetologica 21(4): 283–287.
Kay, F. R., B. W. Miller, and C. L. Miller. 1970. Food habits and reproduction and Callisaurus draconoides in Death Valley, California. Herpetologica 26:431–436.
Klauber, L. M. 1931. A new subspecies of the California boa, with notes on the genus Lichanura. Transactions of the San Diego Society of Natural History 6:305–318.
Klauber, L. M. 1935. Phyllorhynchus, the leaf-nosed snake . Bulletins of the Zoological Society of San Diego, no. 12.
Klauber, L. M. 1936. Crotalus mitchellii, the speckled rattlesnake. Transactions of the San Diego Society of Natural History 8:49–184.
Klauber, L. M. 1940a. The lyre snakes (genus Trimorphodon ) of the United States. Transactions of the San Diego Society of Natural History 9:163–194.
Klauber, L. M. 1940b. The worm snakes of the genus Leptotyphlops in the United States and northern Mexico. Transactions of the San Diego Society of Natural History 9:87–162.
Klauber, L. M. 1941. The long-nosed snakes of the genus Rhinocheilus. Transactions of the San Diego Society of Natural History 9:289–332.
Klauber, L. M. 1944. The sidewinder, Crotalus cerastes, with description of a new subspecies. Transactions of the San Diego Society of Natural History 10:91–126.
Klauber, L. M. 1945. The geckos of the genus Coleonyx with descriptions of new subspecies. Transactions of the San Diego Society of Natural History 10:133–216.
Klauber, L. M. 1946. The glossy snake, Arizona, with descriptions of new subspecies. Transactions of the San Diego Society of Natural History 10:311–398.
Klauber, L. M. 1947. Classification and ranges of the gopher snakes of the genus Pituophis in the western United States . Bulletins of the Zoological Society of San Diego, no. 22.
Klauber, L. M. 1951. The shovel-nosed snake, Chionactis, with descriptions of two new subspecies. Transactions of the San Diego Society of Natural History 11:141–204.
Klauber, L. M. 1972. Rattlesnakes: Their habits, life histories, and influence on mankind, 2d ed. 2 vols. University of California Press, Berkeley.
Macey, J. R. 1986. The biogeography of a herpetofaunal transition between the Great Basin and Mojave deserts. In C. A. Hall, Jr. and D. J. Young (eds.). Natural history of the White-Inyo Range, eastern California and western Nevada, and high altitude physiology, pp. 119–128. University of California White Mountain Research Station Symposium, August 23–25, 1985, Bishop, Calif., vol. 1.
Miller, A. H., and R. C. Stebbins. 1964. The lives of desert animals in Joshua Tree National Monument . University of California Press, Berkeley.
Montanucci, R. R. 1983. Natural hybridization between two species of collard lizards (Crotaphytus ). Copeia 1983:1–11.
Montanucci, R. R., R. W. Axtell, and H. C. Dessauer. 1975. Evolutionary divergence among collard lizards (Crotaphytus ), with comments on the status of Gambelia. Herpetologica 31:336–347.
Murphy, R. W. 1983. Paleobiogeography and genetic differentiation of the Baja California herpetofauna . Occasional Papers of the California Academy of Sciences, no. 137.
Norris, K. S. 1953. The ecology of the desert iguana Dipsosaurus dorsalis. Ecology 34:265–287.
Norris, K. S., and S. L. Kavanau. 1966. The burrowing of the western shovel-nosed snake, Chionactis occipitalis Hallowell, and the undersand environment. Copeia 1966:650–664.
Ortenburger, A. I. 1928. The whip snakes and racers: Genera Masticophis and Coluber. Memoirs of the University of Michigan Museums, no. 1.
Papenfuss, T. J. 1986. Amphibian and reptile diversity along elevational transects in the White-Inyo Range. In C. A. Hall, Jr. and D. J. Young (eds.). Natural history of the White-Inyo Range, eastern California and western Nevada, and high altitude physiology, pp. 129–136. University of California White Mountain Research Station Symposium, August 23–25, 1985, Bishop, Calif., vol. 1.
Parker, W. S. 1972. Ecological study of the western whiptail lizard, Cnemidophorus tigris gracilis, in Arizona. Herpetologica 28:360–369.
Parker, W. S., and W. S. Brown. 1980. Comparative ecology of two colubrid snakes, Masticophis t. taeniatus and Pituophis deserticola, in northern Utah . Milwaukee Public Museum Publications in Biology and Geology, no. 7.
Parker, W. S., and E. R. Pianka. 1973. Notes on the ecology of the iguanid lizard, Sceloporus magister. Herpetologica 29:143–152.
Parker, W. S., and E. R. Pianka. 1975. Comparative ecology of populations of the lizard Uta stansburiana. Copeia 1975:615–632.
Parker, W. S., and E. R. Pianka. 1976. Ecological observations on the leopard lizard (Crotaphytus wislizeni ) in different parts of its range. Herpetologica 32:95–114.
Phelan, R. L., and B. H. Brattstrom. 1955. Geographic variation in Sceloporus magister. Herpetologica 11:1–14.
Pianka, E. R. 1971. Comparative ecology of two lizards. Copeia 1971:129–138.
Pianka, E. R., and W. S. Parker. 1972. Ecology of the iguanid lizard Callisaurus draconoides. Copeia 1972:493–508.
Pianka, E. R., and W. S. Parker. 1975. Ecology of horned lizards: A review with special reference to Phrynosoma platyrhinos. Copeia 1975:141–162.
Rodgers, T. L., and H. S. Fitch. 1947. Variation in the skinks (Reptilia: Lacertilia) of the skiltonianus group. University of California in Zoology 48:169–220.
Rossman, D. A., and G. R. Stewart. 1987. Taxonomic reevaluation of Thamnophis couchii (Serpentes: Colubridae ). Occasional Papers of the Museum of Zoology, Louisiana State University, no. 63.
Sanborn, S. R., and R. B. Loomis. 1979. Systematics and behavior of collard lizards (Crotaphytus, Iguanidae) in southern California. Herpetologica 35:101–106.
Shannon, F. A., and F. L. Humphrey. 1963. Analysis of color pattern polymorphism in the snake, Rhinocheilus lecontei. Herpetologica 19:153–160.
Simpson, G. G. 1960. Notes on the measurement of faunal resemblance. American Journal of Science 258:300–311.
Smith, N. M., and W. W. Tanner. 1974. A taxonomic study of the western collard lizards, Crotaphytus collaris and Crotaphytus insularis. Brigham Young University Science Bulletin, Biological Series, vol. 19, no. 4.
Stebbins, R. C. 1944. Field notes on a lizard, the mountain swift, with special reference to territorial behavior. Ecology 25:233–245.
Stebbins, R. C. 1954. Amphibians and reptiles of western North America . McGraw-Hill, New York.
Stebbins, R. C. 1958. A new alligator lizard from the Panamint Mountains, Inyo County California . American Museum Novitates, no. 1883.
Stebbins, R. C. 1985. A field guide to western amphibians and reptiles . Houghton Mifflin, Boston.
Stebbins, R. C., and H. B. Robinson. 1946. Further analysis of a population of the lizard Sceloporus graciosus gracilis. University of California Publications in Zoology 48:149–168.
Tanner, W. W. 1944. A taxonomic study of the genus Hypsiglena. Great Basin Naturalist 5:25–92.
Tanner, W. W. 1957. A taxonomic and ecological study of the western skink (Eumeces skiltonianus ). Great Basin Naturalist 17:59–94.
Tanner, W. W., and J. M. Hopkin. 1972. Ecology of Sceloporus occidentalis longipes Baird on Rainier Mesa, Nevada Test Site, Nye County, Nevada . Brigham Young University Science Bulletin, Biological Series, vol. 15, no. 4.
Tanner, W. W., and C. D. Jorgensen. 1963. Reptiles of the Nevada Test Site . Brigham Young University Science Bulletin, Biological Series, vol. 3, no. 31.
Tanner, W. W., and J. E. Krogh. 1973. Ecology of Phrynosoma platyrhinos at the Nevada Test Site, Nye County, Nevada. Herpetologica 29:327–342.
Tinkle, D. W. 1967. The life and demography of the side-blotched lizard, Uta Stansburiana. Miscellaneous Publications, Museum of Zoology, University of Michigan, no. 132.
Tinkle, D. W. 1973. A population analysis of the sagebrush lizard, Sceloporus graciosus, in southern Utah. Copeia 1973:284–296.
Tollestrup. K. 1982. Growth and reproduction in two closely related species of leopard lizards, Gambelia silus and Gambelia wislizenii. American Midland Naturalist 108:1–20.
White, M., and J. A. Kolb. 1974. A preliminary study of Thamnophis near Sagehen Creek, California. Copeia 1974:126–136.
Wilson, L. D. 1970. The coachwhip snake, Masticophis flagellum (Shaw): Taxonomy and distribution. Tulane Studies in Zoology and Botany 16:31–99.
Wright, J. W., and C. H. Lowe. 1968. Weeds, polyploids, parthenogenesis, and the geographical and ecological distribution of all-female species of Cnemidophorus. Copeia 1968:128–138.
Zweifel, R. G., and C. H. Lowe. 1966. The ecology of a population of Xantusia vigilis, the desert night lizard . American Museum Novitates, no. 2247.
11—
Breeding Birds
Ned K. Johnson and Carla Cicero
Illustrated by N. John Schmitt and Gene M. Christman
A rich variety of birds nest in the White-Inyo Range, including a number of species not widely distributed elsewhere in California. In this chapter, our chief objective is to introduce the reader to the most frequently seen nesting species and to supplement this list with comments on life history, habitats, and distribution. For reasons of space, we emphasize the montane species and exclude from treatment a host of other, equally interesting kinds of birds that breed in the lowland habitats of the region, for example, Owens Valley, Deep Springs Valley, and Fish Lake Valley. Furthermore, we pass over species known solely in the region as transients, winter visitants, or vagrants. The list of such species is long; both Oasis and Deep Springs Ranch, on the east side of the mountains, are popular birding localities where many unusual distributional records have been gathered in recent years. For an up-to-date summary of the status in the region of all excluded species, we refer the reader to Garrett and Dunn (1981). Johnson and Cicero (1986) have recently summarized records of all species of birds thought to breed in the White-Inyo mountains. Their analysis provided the basis for a quantitative comparison of the boreal avifaunas of the two ranges with those of several nearby mountain systems. Common names, scientific names, and the sequence of species follow the style of the American Ornithologists' Union (1983).
In each of the following species accounts, our goal is to provide an accurate statement on the relative abundance, seasonal status, local distribution, and known elevational range of each form. Descriptions of relative abundance (e.g., "common," "fairly common," "uncommon") are based on the definitions and philosophy offered by Grinnell and Miller (1944). Names of ecologic formations (capitalized) follow Miller (1951). Elevations were determined from specimen labels and field notes, and through direct measurement with an altimeter. Accurate determination of elevational range in this region is especially important because some of the highest known records for certain species are from the White Mountains. The data on lengths and weights are offered to allow gross estimates of body size. These estimates assist in field identification and enable rough comparisons of species. We hasten to note that the information on length is too imprecise to serve any other useful purpose. Figures on lengths and weights are averages of at least five individuals of each sex from study specimens in the Museum of Vertebrate Zoology, University of California, Berkeley (MVZ). Where possible, specimens from Mono County or Inyo County were used for these size data. Measurements in the metric system have been converted to English units; the former were taken directly from specimens and therefore are more accurate.
Each species account contains information on habitat occurrence, foraging and nesting habits, voice and other useful identifying features of behavior, and, occasionally, unusual characteristics of life history or ecology. Much of this information is derived from our own annual field experience in the Great Basin and eastern Sierra Nevada, which for the first author dates more than 30 years and includes many visits to the White-Inyo mountain region. For some species, however, we have drawn details from the literature cited at the end of each account. These citations also serve to introduce the serious student of birds to other sources of information for each species.
For general information on distribution, the notes and collections in MVZ were particularly useful. These were obtained by field parties that visited the White Mountains in 1917 and 1955. The notes of Joseph G. Hall, Ward C. Russell, and Francis S. L. Williamson were especially helpful. In addition, Ward C. Russell (pers. comm. ) provided information on the habitat of Blue Grouse in the White Mountains. We sincerely thank these individuals for the detailed permanent record they left on the status of the avifauna 30 years ago.
We do not emphasize field identification in the accounts because such information is now readily available in several excellent, new pocket guides. Thus, the beginner will want to carry a guide to the identification of North American birds, along with this book, when exploring these fascinating mountains.
Cathartidae (New World Vultures)
Turkey Vulture,Cathartes aura. (Fig. 11.1) Male length 25 1/2 in (65 cm), female length 27 in (68 cm); male weight 3 1/2 lb (1,559 g), female weight 3 2/3 lb (1,660 g). Uncommon summer resident in the White-Inyo Range; occurs at all elevations.
Turkey Vultures scavenge over open woods, brush, and grassland in varied terrain. Ranches and farming country are also visited. Although most common over valleys and foothills, they occasionally appear at higher elevations. The species is easily recognized by its very small head and broad wings held in a dihedral while soaring. Turkey Vultures forage singly, in pairs, or in loose groups. Odors wafting from carrion are picked up by their keen sense of smell, a rare trait in birds. Because vultures depend largely on thermals for uplift, they are less active on windless days or during stormy weather. This species generally roosts in two separate sites; the main one is used during the night. The second roost, used at dawn and dusk, serves mainly for sunning and preening. These bulky birds prefer to perch in open-branching live or dead trees, on poles, or even on the ground. Such perches enable easy takeoffs and landings. Nest sites are sheltered pockets in cliff faces or among large rocks on steep, brushy slopes. References: D. Davis (1979), Rea (1983).
Accipitridae (Hawks and Eagles)
Cooper's Hawk,Accipiter cooperi. (Fig. 11.2) Male length 15 1/2 in (39 cm), female length 17 1/2 in (45 cm); male weight 10 1/3 oz (291 g), female weight 16 1/2 oz (466

Figure 11.1
Turkey Vulture.

Figure 11.2
Cooper's Hawk.
g). Scarce permanent resident in the White Mountains from the valleys to 9,500 ft (2,896 m).
This hawk prefers to nest in aspens, willows, and cottonwoods in canyon bottoms. Foraging takes them over adjacent slopes grown to pines, broken woodland, and brush. A skilled hunter, the Cooper's Hawk maneuvers through openings in foliage, around trees, and over clearings in search of small birds and mammals. Reptiles, amphibians, and large insects are taken infrequently. Like other accipiters, it actively pursues its prey while using vegetation to screen its approach. It may also dash toward prey from a concealed perch in dense foliage. Prey are commonly seized near the ground, in shrubs, or in the canopy of low cover. The hawk usually returns with its kill to feed within 100 ft (30 m) of the nest tree. References: Snyder and Wiley (1976), Reynolds and Meslow (1984).
Red-tailed Hawk,Buteo jamaicensis. (Fig. 11.3) Male length 20 1/2 in (52 cm), female length 23 in (58 cm); male weight 2 lb (910 g), female weight 2 2/3 lb (1,223 g). Common permanent resident in the White-Inyo Range, at all elevations up to 10,500 ft (3,200 m).
Figure 11.3
Red-tailed Hawk.
Red-tailed Hawks are one of the most widespread species in the region, occurring throughout a variety of habitats. They hunt for small and medium-sized mammals over broken woodland, brushy slopes, and grassland. Invertebrates, birds, reptiles, and amphibians are taken less commonly. Red-tailed Hawks search for terrestrial prey while soaring high above the ground or while perching quietly in a tree or on a pole. They attack with long dives or sudden plunges. The bulky stick nest is built on a cliff shelf or in a tree and, once abandoned, may serve importantly as a nesting site for large owls. During the breeding season, male Red-tailed Hawks swoop and ascend in exaggerated flight while carrying a snake or other conspicuous prey item in their talons. This courtship display may signify to the female that he is a competent hunter. References: Fitch, Swenson, and Tillotson (1946); Weathers (1983).
Golden Eagle,Aquila chrysaetos. (Fig. 11.4) Male length 32 in (81 cm), female length 34 in (87 cm); male weight 7 1/4 lb (3,293 g), female weight 8 7/8 lb (4,030 g). Uncommon resident in the White-Inyo Range, from the valley floors to the summits of the highest peaks.
Golden Eagles soar throughout these mountains over all available habitats, including brushland, Pinyon-juniper Woodland, Subalpine Forest, riparian cover in canyon bottoms, and alpine steppes. Open country is preferred for foraging. Mammals are taken primarily, but birds and some snakes may also be eaten. The composition
Figure 11.4
Golden Eagle.
of the diet depends on prey availability, but all animals ranging in size from mice to fawns and songbirds to grouse are fair game. This species is notorious for killing domestic livestock, especially lambs, but such events occur uncommonly. Golden Eagles hunt either from a perch or from the air while near the ground or soaring at great heights. The large stick nest is built on an inaccessible cliff ledge or in tall trees. Early in the breeding season, pairs of eagles conduct elaborate aerial courtship displays that involve mutual dives and other acrobatics. References: Brown and Amadon (1968), Olendorff (1975).
Falconidae (Falcons)
American Kestrel,Falco sparverius. (Fig. 11.5) Male length 10 in (26 cm), female length 10 1/2 in (27 cm); male weight 3 3/8 oz (97 g), female weight 4 1/8 oz (116 g). Uncommon permanent resident in the White-Inyo Range at all elevations.
This species forages in open terrain where vegetation is sparse and low-growing, and where suitable perch sites are available; meadows, unforested slopes, sagebrush flats, and rocky outcrops are frequented. The American Kestrel is a generalized

Figure 11.5
American Kestrel
predator of invertebrates and small vertebrates. Grasshoppers, crickets, and other insects make up the bulk of their diet, but reptiles, mammals, and birds may also be taken. This small falcon typically searches for and attacks its prey from an exposed perch at a moderate height, striking on or near the ground surface. On windy days, however, the birds may hunt by hovering over the ground, especially where perches are sparse or lacking. American Kestrels also "hawk" insects on the wing or, uncommonly, forage directly on the ground for nonflying invertebrates. This species nests commonly in tree cavities, especially in old Northern Flicker holes, and rarely in earth banks or cliff crevices. Being highly maneuverable, American Kestrels harass larger birds of prey and Common Ravens by long stoops and swift ascents. Reference: Balgooyen (1976).
Prairie Falcon,Falco mexicanus. (Fig. 11.6) Male length 15 1/2 in (39 cm), female length 18 in (46 cm); male weight 1 1/8 lb (519 g), female weight 1 7/8 lb (849 g). Scarce resident in the White-Inyo Range at all elevations.
An arid-adapted species, the Prairie Falcon avoids heavily wooded areas. Open rocky canyons and ridgetops grown to Singleleaf Pinyon, sagebrush flats and slopes, alpine scrub, and grassland are preferred. There it searches for small mammals, birds, and invertebrates such as grasshoppers and crickets. Horned Larks are a common food item in open terrain. Prairie Falcons are swift and highly maneuverable in flight, and they deftly take prey from either the air or the ground. They also hunt while perched on a post or pole. Nests are built on rocky ledges or in cliff cavities, commonly far from water but near suitable foraging habitat. The Prairie Falcon can travel long distances daily away from the nest site in search of food. Reference: Cade (1982).
Figure 11.6
Prairie Falcon.
Phasianidae (Partridge, Grouse, and Quail)
Chukar,Alectoris chukar. (Fig. 11.7) Male length 13 in (33 cm), female length 13 in (33 cm); male weight 21 2/3 oz (614 g), female weight 17 2/3 oz (500 g). Introduced. Common resident from the foothills to at least 10,000 ft (3,050 m) in the Inyo Mountains and 13,400 ft (4,090 m) in the White Mountains. Most numerous between 4,000 and 8,000 ft (1,220 and 2,440 m). Scarce in the northern portion of the region and at very high elevations.
Initially released into California in 1932, this partridge is now widely established in many arid portions of the state. It favors rough and inhospitable canyonsides where scattered clumps of brush and cheatgrass are interspersed with rock outcrops and steep talus slopes. Water from tiny seeps, steadily flowing streams, or tanks must be accessible daily during warm periods and may dictate the local occurrence of birds. Chukars feed primarily on the blades, seeds, and buds of cheatgrass and annuals, although grasshoppers and caterpillars are taken in the spring. Seeds are most important in the summer diet. The birds move continually while feeding, walking up steep boulder faces with agility and skulking across openings. When flushed, the covey explodes into the air and typically heads downhill, alternating rapid wing beats with short glides. Shrill alarm notes accompany the departure of disturbed adults. Foraging adults utter a throaty, resonant chuckling call, which carries for great distances in mountain canyons. Reference: Christensen (1954).
Figure 11.7
Chukar.
Blue Grouse,Dendragapus obscurus. (Fig. 11.8) Male length 19 in (49 cm), female length 16 in (41 cm); male weight 2 5/8 lb (1,213 g), female weight 1 3/4 lb (803 g). Rare to fairly common, but local, permanent resident in the northern and central White Mountains; recorded between 8,000 and 10,200 ft (2,440 and 3,110 m) elevation. Numbers fluctuate annually. No known records from the Inyo Mountains.
Blue Grouse occur primarily on the east side of the White Mountains, inhabiting upper stream canyons lined with willow and aspen, as well as open Limber Pine forest on adjacent north- and northeast-facing slopes. The diet is well known for populations in the Sierra Nevada and other regions but has been poorly documented in the White Mountains. Spring and summer food in the Sierra Nevada reportedly consists of buds, twigs, catkins, and conifer needles, with fir needles becoming predominant during fall and winter. Because no native firs occur in the White Mountains, it is uncertain what the local grouse eat during those months. However, Limber Pine needles could be an important food. The species supplements its diet with willow leaves in the summer. Blue Grouse leave piles of characteristic droppings on the ground. Each dropping, approximately
Figure 11.8
Blue Grouse.
1 in (2.5 cm) long and 1/4 in (0.6 cm) in diameter, is slightly curved and composed of tightly stacked, coarse plant material that shears neatly into individual cylinders. Males "boom" from trees during the breeding season, amplifying these hoots by expelling air held in vivid orange neck sacs. Reference: Johnsgard (1973).
Sage Grouse,Centrocercus urophasianus . (Fig. 11.9) Male length 25 in (63 cm), female length 19 in (48 cm); male weight 4 1/4 lb (1,954 g), female weight 2 3/8 lb (1,085 g). Scarce summer resident in the White Mountains between 8,500 and 12,000 ft (2,590 and 3,660 m) elevation, especially in alpine sagebrush north of White Mountain Peak.
Sage Grouse are strictly tied to the sagebrush association, especially on flat or gently rolling terrain. Sage leaves constitute the main diet of adult birds, although herbaceous legumes and grasses are also eaten; chicks feed primarily on insects. The nest is usually well hidden underneath a sage bush. Prior to breeding, males congregate in large arenas or "leks" subdivided into individual territories. Here they conduct elaborate courtship displays for a group of females. The more dominant males typically occupy central territories in the lek and perform most of the matings. After courtship, the two sexes separate until the fall, when flocks are formed. The range and abundance of the Sage Grouse in California have declined dramatically during this century as a result of the devastation of sagebrush habitat by livestock and agriculture. Reference: Wiley (1973).
California Quail,Callipepla californica. (Fig. 11.10) Male length 8 1/2 in (22 cm), female length 9 in (23 cm); male weight 5 5/8 oz (159 g), female weight 5 1/2 oz (155 g). Relatively common resident in the White-Inyo Range; occurs between 3,300 and 8,400 ft (1,010 and 2,560 m), although scarce at higher elevations.
This species occurs in localized areas of favorable habitat near streams and springs. Willows and other riparian vegetation, as well as sagebrush and Pinyon-juniper Woodland on adjacent slopes, are frequented. The quail forage mainly in the drier habitats but prefer to nest and roost under the concealing thicket cover. Seeds of Rabbitbrush and sagebrush make up the bulk of their diet in the Great Basin, although in other regions legumes and annuals are a more important seed source. Grass or legume leaves and the fruits, buds, and catkins of brush and juniper are also eaten. In addition, adults take small quantities of animal food, especially during the spring and summer; chicks subsist mainly on such food for the first month. Nests are typically placed on the ground in tall, dense cover of herbaceous vegetation. The California Quail is highly gregarious except during the breeding season. In the fall and winter, coveys forage together in alleyways through brush or alongside roads and other corridors. The social structure of coveys is strictly organized. Reference: Leopold (1977).
Mountain Quail,Oreortyx pictus. (Fig. 11.11) Male length 10 in (25.5 cm), female length 9 1/2 in (24 cm); male weight 8 1/2 oz (242 g), female weight 7 5/8 oz (218 g). Scarce permanent resident in the White-Inyo Range; current status unclear.
Figure 11.9
Sage Grouse.
Figure 11.10
California Quail.
Figure 11.11
Mountain Quail.
Mountain Quail occur locally in the region on slopes and in canyon bottoms where dense brush is interspersed with open woodland. Sagebrush, Rabbitbrush, and currant within either broken Pinyon-jumper Woodland or Mountain Mahogany are favored. Water is required on a daily basis. Seeds of various perennial plants are taken year-round, with leaves and some fruits, flowers, and insects supplementing the diet. Young chicks subsist primarily on insects. Nests are well concealed under fallen branches, in tall weeds and dense shrubs, or near large, shaded rocks surrounded by thick vegetation. Like other species of quail, Mountain Quail spend the year in coveys except when breeding. Pairs form within coveys while these social groups are intact. In spring, males give a Far-reaching, single inflected whistle that resembles the call of the Northern Pygmy-Owl. Males call frequently in the morning during the early breeding season. This species is highly secretive and prefers to remain hidden in dense brushy cover. In other regions, especially where the birds summer at high elevations, postbreeding coveys are thought to migrate downslope on foot into snow-free, warmer environments. References: Gutiérrez (1980), Miller and Stebbins (1964).
Columbidae (Pigeons and Doves)
Mourning Dove,Zenaida macroura. (Plate 11.1) Male length 11 in (28 cm), female length 10 1/2 in (26.5 cm); male weight 4 1/4 oz (122 g), female weight 4 oz
(113 g). Fairly common summer (permanent?) resident in the White-Inyo Range; occurs up to approximately 10,000 ft (3,050 m).
This species inhabits a variety of habitats, including pinyon woodland, sagebrush, riparian thickets, and meadows. The birds travel long distances daily in search of both food and water. Mourning Doves feed primarily in open areas such as meadows and grassland, where they can find an ample supply of seeds from herbaceous plants. Grain can also constitute an important part of the diet; this is taken mainly in cultivated fields at lower elevations. Tiny pebbles and gravel are also consumed to help grind seeds in the muscular stomach. Nesting habitat requirements are flexible. The preferred nesting substrate consists of a horizontal branch in a tree or shrub, but the flimsy nest may also be built on the ground if woody vegetation is lacking. In addition, Mourning Doves commonly use abandoned nests, either of their own species or of others. Strongly monogamous, Mourning Doves are commonly seen in pairs. This species is easily detected by its characteristic call, a mournful awoo-coo-coo-coo . Reference: Johnsgard (1975).
Strigidae (Typical Owls)
Western Screech-Owl,Otus kennicottii. (Fig. 11.12) Male length 8 1/2 in (22 cm), female length 9 in (22.5 cm); male weight 4 3/4 oz (133 g), female weight 5 1/2 oz (155 g). Uncommon permanent resident in the White-Inyo Range; recorded from 7,200 to 8,200 ft (2,200 to 2,500 m) elevation.
Although this species is locally common in old-growth cottonwoods and tree willows in Owens Valley, only small numbers occur in the White-Inyo mountains. There they prefer the warmer portions of the Singleleaf Pinyon belt. Western Screech-Owls dwell in the lower stretches of canyons in mature trees on gently sloping ground. Natural or woodpecker-excavated cavities in large pinyon trunks are used for roosting and nesting. Foraging takes these owls into more open habitats, but at least some scattered pinyons or junipers seem essential for the presence of this species. Western Screech-Owls feed on a variety of insects, including grasshoppers, crickets, beetles, moths, caterpillars, and spiders. Vertebrates are occasionally eaten, especially during the winter months. The bouncing-ball song of the local subspecies (O. k. inyoensis ) is shorter and composed of fewer notes than the vocalizations of related forms. Like all small owls, the Western Screech-Owl has suffered from fuel-wood cutting in the Singleleaf Pinyon Woodland and from the removal of riparian trees generally. Numbers nearly everywhere have been declining over the past several decades. Reference: Miller and Stebbins (1964).
Great Horned Owl,Bubo virginianus. (Fig. 11.13) Male length 18 in (45.75 cm), female length 19 1/2 in (49.75 cm); male weight 2 lb (914 g), female weight 2 1/2 lb (1,142 g). Fairly common permanent resident in the White-Inyo Range; occurs at all elevations up to approximately 9,500 ft (2,896 m).
The Great Horned Owl is widespread through the region in a variety of habitats. Mammals of small to medium size predominate in their diet, but birds such as quail,

Figure 11.12
Western Screech-Owl.
woodpeckers, and passerines are also taken. To a lesser extent, the species may eat reptiles or amphibians, large insects (e.g., crickets, beetles), and carrion found along highways. Great Horned Owls are most active at night, but they can be seen abroad during the day under overcast conditions. However, most days are spent roosting in large pinyons, in other conifers or aspens, or in cavities in canyon walls. Stick nests abandoned by other raptors, either on cliff shelves or in trees, serve as the primary nesting sites. This species breeds in late winter or early spring. Toward the onset of the nesting season and during the breeding period, both sexes emit deep, resonant hoots. These hoots are deeper pitched in males despite their smaller size relative to females. Great Horned Owls are generally shy and difficult to approach, even at night. References: Burton (1973), Fitch (1947).
Long-eared Owl,Asio otus. (Plate 11.2) Male length 13 in (33.25 cm), female length 13 in (33.5 cm) male weight 8 5/8 oz (245 g), female weight 97/8 oz (279 g). Uncommon permanent resident throughout the White-Inyo region, occurring up to 9,500 ft (2,900 m).
Although in this region the Long-eared Owl favors dense pinyon forest, it also frequents stands of cottonwoods, tree willows, aspens, and Mountain Mahogany. At night, the owls hunt through woodland and along the edges of clearings in search of prey, which consists primarily of small mammals. These owls also feed occasionally on

Figure 11.13
Great Horned Owl.
small birds and, more rarely, on reptiles or amphibians. During the day, the species roosts in dense clumps of Singleleaf Pinyon, Mountain Mahogany, cottonwoods, or aspens. Long-eared Owls are generally solitary except during the winter, when they form loose roosting aggregations in riparian thickets in the foothills and lowlands. Breeding takes place in abandoned nests of other large birds, particularly Red-tailed Hawks, Common Ravens, and, in the lower valleys, Black-billed Magpies. The Long-eared Owl is shy and difficult to attract by means of imitated calls. References: Grinnell and Storer (1924), Marti (1976).
Northern Saw-whet Owl,Aegolius acadicus. (Fig. 11.14) Male length 7 in (17.75 cm), female length 7 1/2 in (18.75 cm); male weight 2 5/8 oz (75 g), female weight 3 1/4 oz (91 g). Uncommon resident in the White Mountains. Winter status is uncertain; at that time, individuals from other regions may augment the local population. Recorded up to approximately 9,000 ft (2,740 m).
This owl occurs in dense riparian thickets and on remote canyon slopes far from water. They forage at the edges of meadows or clearings, particularly among open

Figure 11.14
Northern Saw-whet Owl.
stands of small to medium-sized conifers. There, mice and an occasional bird are taken. Nest sites consist of abandoned woodpecker holes or natural cavities at medium heights in trees. During the day, individuals roost in the dense foliage of small trees, commonly within 10 ft (3.0 m) of the ground. Mated males call infrequently. In contrast, unmated Northern Saw-whet Owls call steadily in the late winter and spring. These calls carry up to 0.5 mi (0.8 km), and thus the owls can easily be detected. They are difficult to locate, however, because the steady whistles quaver in pitch and fade in and out in intensity. Once a Northern Saw-whet Owl is found, either during the day or at night, commonly it can be closely approached. Reference: Earhart and Johnson (1970).
Caprimulgidae (Nightjars)
Common Nighthawk,Chordeiles minor. (Fig. 11.15) Male length 9 in (22.75 cm), female length 9 in (22.75 cm); male weight 2 1/4 oz (63 g), female weight 2 1/2 oz (72 g). Scarce summer resident in the White Mountains; more common in the northern part of Owens Valley to the west. Recorded from 4,000 to 6,750 ft (1,220 to 2,060 m) elevation.
Figure 11.15
Common Nighthawk.
Common Nighthawks breed in the sagebrush-pinyon-covered alluvial fans on both sides of the White Mountains, and in adjacent ranch country with riparian growth in the valleys. Each evening in June and early July, they perform spectacular courtship dives in which the male plunges toward the ground and recovers suddenly with a loud, muffled vvrrrrr sound. The call commonly delivered during high aerial foraging flights is a nasal peent . Females lay their eggs directly on the ground among gravel, sticks, and other plant debris, either in brushland or in large openings in forest. If disturbed while incubating, she flops awkwardly across the ground with fanned wings and tail, luring the intruder away from the nest site. This species feeds exclusively on insects caught on the wing, commonly over water surfaces. References: Armstrong (1965), Caccamise (1974).
Common Poorwill,Phalaenoptilus nuttalli. (Fig. 11.16) Male length 7 1/2 in (18.75 cm), female length 7 in (18 cm); male weight 1 3/4 oz (48 g), female weight 1 3/4 oz (48 g). Fairly common summer resident in the White-Inyo Range; winter status uncertain. Recorded up to 9,500 ft (2,900 m).
Poorwills prefer warm, open terrain with scattered trees or brush where there is an abundance of exposed sand, gravel, and rocks. This habitat is common in both the sagebrush and Pinyon-juniper zones of the region. Mountain Mahogany is also

Figure 11.16
Common Poorwill.
visited, especially when mixed with pinyon. Poorwills commonly rest on gravel road beds, where in the evening their reflected eye shine can be seen in car headlights or with a flashlight. They feed at dusk and dawn, when they take short flights from the ground in search of flying insects such as moths. The whistled poor-will call is followed by a brief dup note, delivered at a somewhat lower pitch and audible only at very close range. This species can become torpid during cold weather; such dormant individuals hide in protected crannies in rocks. References: Jaeger (1948), Marshall (1957).
Apodidae (Swifts)
White-throated Swift,Aeronautes saxatalis. (Plate 11.3) Male length 5 3/4 in (14.5 cm), female length 5 1/2 in (14 cm); male weight 1 1/10 oz (31 g), female weight 1 oz (29 g). Locally common summer resident in the White-Inyo Range, between 6,000 and 10,300 ft (1,830 and 3,140 m).
White-throated Swifts inhabit deep, rocky canyons that offer steep cliff faces for nesting and roosting. Foraging birds fly over a wide variety of habitats where insects are taken on the wing. The daily cruising range of the White-throated Swift exceeds that of most other species of birds in the region. Nests of twigs are cemented together and attached to cliff crevices by means of saliva. Granitic rock is apparently preferred as a nest substrate because it provides for easier nest attachment. White-throated Swifts commonly nest and roost colonially. They forage singly or in groups, darting swiftly at heights from near the treetops to high overhead. A series of excited shrill notes is commonly uttered in flight. During cold snaps, this species becomes dormant and Individuals remain together in rocky crevices until warmer temperatures resume. Reference: Weathers (1983).
Trochilidae (Hummingbirds)
Broad-tailed Hummingbird,Selasphorus platycercus. (Fig. 11.17) Male length 3 1/2 in (9 cm), female length 3 3/4 in (9.5 cm); male weight 1/10 oz (3.8 g), female weight 1/8 oz (4.5 g). Relatively common summer resident locally in the White-Inyo Range; occurs between approximately 6,000 and 9,000 ft (1,829 and 2,743 m) elevation.
This hummingbird inhabits the pinyon-juniper-Mountain Mahogany belt, where it prefers riparian thickets along wet or dry watercourses or near springs. There it feeds primarily on nectar from a variety of plants. Insects and spiders on flowers, near sapsucker borings, or in the air are also taken. The small, cup-shaped nests are typically placed on low horizontal branches of streamside trees. Like most hummingbirds, the Broad-tailed Hummingbird constructs its nest with spider webs, fine leaves, shreds of bark, and cottony material. Prior to breeding, one or several males display with U-shaped dives to a perched female from 20 ft (6 m) or more above the ground. During this display, sharp clicking notes are commonly uttered. In ordinary flight, males produce with specialized wing feathers a characteristic cricketlike trill. Reference: Johnsgard (1983).
Picidae (Woodpeckers)
Red-naped Sapsucker,Sphyrapicus nuchalis. (Plate 11.4) Male length 7 in (18 cm), female length 7 1/2 in (19 cm); male weight 1 1/2 oz (44 g), female weight 1 1/2 oz (43 g). Locally fairly common summer resident in the White Mountains, between approximately 9,000 and 9,600 ft (2,740 and 2,930 m).

Figure 11.17
Broad-tailed Hummingbird.
This woodpecker lives in canyons on the east side of the range, in streamside growth of aspens and willows. Mountain Mahogany and Bristlecone Pine are visited to a lesser extent. The adults drill linear rows of rectangular holes in the trunks and limbs of these trees and dip up the exuding sap with their brushy-tipped tongues. Such borings commonly lure insects, which, in turn, attract other species of birds. The Red-naped Sapsucker usually excavates its nest cavity in a large dying aspen, where decaying wood provides for easy digging. As the nestlings mature, their loud begging cries can be heard up to 100 yd (91 m) from the nest. In response to these cries, the parents arrive independently every few minutes carrying billsfull of large Timber Ants and other insects. Other cavity-nesting species of birds commonly use abandoned sapsucker nests for raising their own young. Consequently, sapsuckers and other woodpeckers play an important role as primary cavity excavators for a significant group of secondary hole-nesting species. For decades this form was combined with the Yellow-bellied Sapsucker (S. varius ) and the Red-breasted Sapsucker (S. ruber ) as a single species. References: Johnson and Johnson (1985), Short (1982).
Hairy Woodpecker,Picoides villosus. (Fig. 11.18) Male length 8 in (20.25 cm), female length 8 1/4 in (21 cm) male weight 2 1/8 oz (60 g), female weight 2 1/5 oz (62 g). Fairly common permanent resident in the White-Inyo Range, between 6,750 and 9,600 ft (2,060 and 2,930 m).
This species is perhaps the most generalized of all North American woodpeckers. In the White-Inyo mountain region, it is at home in such diverse habitats as Pinyon-juniper Woodland, aspen-willow associations, and coniferous forest of Limber and Bristlecone pines. Pinyon is used less commonly toward the south; there the trees must be large and in relatively thick groves on cool slopes or in canyons. Nest cavities are excavated in dead trees of any kind. The species forages mostly in dead or dying trees, where adult and larval insects are obtained by digging into decaying wood. In these vast mountains, Hairy Woodpeckers are sparsely distributed, and usually only one or two pairs may be encountered in a single day. Reference: Short (1971).
Northern Flicker,Colaptes auratus. (Plate 11.1) Male length 11 1/4 in (28.5 cm), female length 11 in (28.25 cm); male weight 5 3/8 oz (151 g), female weight 5 oz (141 g). Relatively common permanent resident in the White-Inyo Range, up to 10,350 ft (3,155 m).
Northern Flickers breed throughout the region in all types of woodland habitats, preferably where there are extensive tracts of good-sized trees near openings. Large, dying pinyons, aspens, or pines are all used for excavating nest cavities. Similar flexibility is shown in foraging behavior. Flickers search for ants, caterpillars, grubs, termites, beetles, and other terrestrial or foliage insects in live and dead tree trunks, leaf litter, bare soil, and ant hills. Because of its unusual terrestrial habits, this woodpecker is likely to be flushed from the ground in meadows, sagebrush flats, or other openings in wooded country. The attractive salmon-red color underneath the wings and tail, clearly seen in flight, is based on red pigments obtained through the diet. Occasional individuals that exhibit yellowish wing or tail feathers have not

Figure 11.18
Hairy Woodpecker.
eaten sufficient pigment-containing food prior to and during feather growth. Reference: Short (1965).
Tyrannidae (Tyrant Flycatchers)
Olive-sided Flycatcher,Contopus borealis. (Fig. 11.19) Male length 6 1/2 in (16.5 cm), female length 6 1/2 in (16.5 cm); male weight 1 1/8 oz (32.6 g), female weight 1 1/8 oz (32.6 g). Fairly common summer resident locally in the White Mountains. Recorded from 8,200 to 10,500 ft (2,500 to 3,200 m).
This large flycatcher breeds only in the higher coniferous forests of Limber Pine, Bristlecone Pine, and Lodgepole Pine. It prefers open stands of mature timber, particularly where high perches and prominent song posts are available. Such perches are in the tops of tall dead and dying conifers. Olive-sided Flycatchers have the highest foraging beat of all North American members of their family, the Tyrannidae.
Figure 11.19
Olive-sided Flycatcher.
They feed mainly on large, flying insects. In contrast, nests are typically built at lower heights, on the outer live branches of Limber or Bristlecone pines. Males may sing continuously, but pairs are usually widely spaced on extensive territories. The easily recognizable song, quick-three-beers , accented on the second note, can be heard from up to 0.5 mi (0.8 km) away. Late migrants passing through the valleys and foothill canyons during late May and early June may also sing occasionally. Reference: Grinnell and Storer (1924).
Western Wood-Pewee,Contopus sordidulus . (Fig. 11.20) Male length 5 3/4 in (14.5 cm), female length 5 1/2 in (14 cm); male weight 1/2 oz (13 g), female weight 1/2 oz (13 g). Common summer resident in the White Mountains, between 7,400 and 9,000 ft (2,260 and 2,740 m) elevation.
This flycatcher is most common in woodland and coniferous forest along streams. Because it prefers to forage from tall trees of open branchwork, dense pinyon and Mountain Mahogany are shunned. Large Quaking Aspen, Bristlecone Pine, and Limber Pine are frequented most commonly. Western Wood-Pewees seek flying insects from moderate heights above the ground; their foraging beat is therefore between that of the Dusky Flycatcher and that of the Olive-sided Flycatcher, with overlap at both
Figure 11.20
Western Wood-Pewee.
the upper and lower levels. Wood-Pewees migrate well into June. Then they may occur in Pinyon-juniper Woodland, willow thickets, and other nonbreeding habitats. The nasal buzz of the Western Wood-Pewee is commonly given throughout the day, even on sunny summer afternoons when few other species of birds are calling. Reference: Marshall (1957).
Dusky Flycatcher,Empidonax oberholseri . (Fig. 11.21) Male length 5 1/4 in (13.5 cm), female length 5 in (13 cm); male weight 2/5 oz (11.6 g), female weight 2/5 oz (11.6 g). Common summer resident in the White-Inyo Range, between 8,200 and 10,500 ft (2,500 and 3,200 m).
Although this species avoids Singleleaf Pinyon except occasionally during migration, most other trees as well as willow thickets are used for breeding. In particular, it frequents Mountain Mahogany groves, streamside aspen woodland, and open forests of Bristlecone and Limber pines. Nests are usually built within 5 ft (1.5 m) of the ground in upright twigs of tall, dense shrubs (such as Wild Rose), aspen saplings, and willows. The adults feed near the nest and higher up in the openings between tall trees. The singing and sentry posts are often well above the ground. Dusky Flycatchers feed on miscellaneous flying insects such as small beetles, wasps, flies, and bugs, all of which are snapped from the air. Reference: Johnson (1963).
Gray Flycatcher,Empidonax wrightii . (Fig. 11.22) Male length 5 1/4 in (13.25 cm), female length 5 in (13 cm); male weight 2/5 oz (11.6 g), female weight 2/5 oz
Figure 11.21
Dusky Flycatcher.
(11.6 g). Common summer resident in the White-Inyo Range from 7,200 to 10,500 ft (2,200 to 3,200 m).
Gray Flycatchers are most numerous in the vast tracts of Singleleaf Pinyon growing on either flat or sloping terrain. They also breed sparingly in sunny stands of Limber and Bristlecone pines up to 10,500 ft (3,200 m) elevation in the Inyo Mountains. This is the highest known nesting station for the species. In other regions, such as near the south end of Mono Lake, they breed in stands of tall sagebrush and Bitterbrush. Such growth does not seem to be used extensively in the White-Inyo mountain area. Nesting pairs prefer medium to large Singleleaf Pinyons near openings with relatively sparse brush. The nest straddles a horizontal branch and is commonly placed right near the trunk. Males commonly perch on a bare twig near the nest tree, where they are conspicuous by virtue of their silvery white underparts and tail-dipping mannerism. Typical perch sites for singing or foraging are within 12 ft (3.7 m) of the ground. Insects are caught from bare or nearly bare patches of ground between sagebrush, Bitterbrush, lupine, and small Beavertail Cactus. Like its relative, the Dusky Flycatcher, the Gray Flycatcher catches small insects while in flight. Although they maintain separate territories, the two species commonly occur side by side where their preferred habitats meet in these mountains. Reference: Johnson (1966).
Figure 11.22
Gray Flycatcher.
Say's Phoebe,Sayornis saya. (Plate 11.1) Male length 6 3/4 in (17 cm), female length 6 3/4 in (17 cm); male weight 3/4 oz (21.6 g), female weight 7/10 oz (20.4 g). Sparse summer resident in the White-Inyo region, between 5,900 and 9,500 ft (1,800 and 2,900 m).
Say's Phoebes inhabit the pinyon-juniper-sagebrush zone in the region. Although the species is not numerous, nesting pairs can usually be seen in arid canyon mouths where sunny gravel banks and/or human constructs are present. Nests are placed on protected shelves in cliffs or buildings, and the adults forage nearby. Insect food is snapped from the air or the ground as the birds momentarily leave their low perches on bushtops or rocks, or as they hover. Postbreeding adults and independent young are widespread in shrubland or ranch country, where they perch singly on fenceposts, wires, or bushtops. Reference: Weathers (1983).
Ash-throated Flycatcher,Myiarchus cinerascens. (Fig. 11.23) Male length 7 1/2 in (18.75 cm), female length 7 in (18 cm); male weight 1 oz (28.6 g), female weight 9/10 oz (26.4 g). Uncommon summer resident in the White-Inyo Range, from the valley floors to approximately 7,500 ft (2,290 m).
This flycatcher inhabits Pinyon-juniper Woodland and brush on warm, dry slopes, and open riparian growth with at least scattered trees. Because much of the foraging
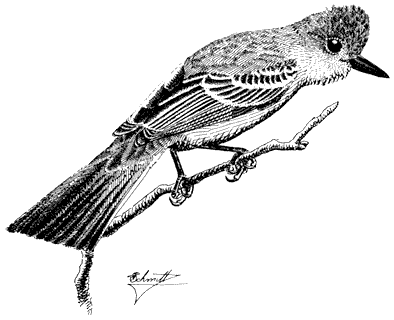
Figure 11.23
Ash-throated Flycatcher.
activity occurs within 20 ft (6 m) of the ground, low perching sites are essential. Bushtops or exposed dead twigs in a dead or dying tree are used most typically. The Ash-throated Flycatcher captures large wasps, flies, beetles, and bugs by means of aerial sorties. Nests are built of twigs and lined with grass in a natural or woodpecker-excavated cavity in an old Singleleaf Pinyon or juniper. Both parents are usually conspicuous near the nest site. Pairs are widely spaced through the habitat on large territories and announce their presence with a loud, rolling ka-brick or prit-wherr call (accented on the second syllable). Reference: Miller and Stebbins (1964).
Alaudidae (Larks)
Horned Lark, Eremophila alpestris . (Fig. 11.24) Male length 6 1/4 in (16 cm), female length 6 in (15 cm); male weight 1 oz (29.2 g), female weight 9/10 oz (26.5 g). Locally common summer resident in the White-Inyo Range. Occurs in two separate elevational belts: from the valley floors to 8,000 ft (2,440 m), and from 10,000 ft (3,050 m) to the summit of the White Mountains (14,246 ft or 4,340 m).
The Horned Lark nests over the widest elevational span of any bird species in the region. Because it prefers open and flat or gently sloping terrain, its local distribution is sharply divided into two main parts: lower valleys and flats covered with brush, up to 8,000 ft (2,440 m); and rolling upper sagebrush steppes and alpine fell-fields above timberline, from approximately 10,000 ft (3,050 m) to the highest peaks. This species is most at home in the skimpy grass, herbs, and rocks of windswept wastes,
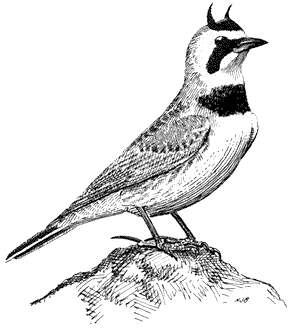
Figure 11.24
Horned Lark.
where all nesting and foraging activities take place. Grass seeds and insects compose the diet. Breeding males announce their territories by spectacular song flights above their nesting areas. Early in the breeding period, two or more males swiftly chase over bushtops and high overhead. After being aloft for several minutes, they plunge from the sky and perch on the ground, on the tops of rocks, or on prominent shrubs. Postbreeding flocks of coalesced family groups or scattered singles wheel over open country and then land in the rocks and grass, each individual vanishing by virtue of its remarkable concealing coloration. References: Behle (1942), Verbeek (1967).
Hirundinidae (Swallows)
Violet-green Swallow,Tachycineta thalassina. (Plate 11.3) Male length 4 3/4 in (12 cm), female length 4 1/2 in (11.5 cm); male weight 1/2 oz (15.6 g), female weight 1/2 oz (15.5 g). Common summer resident in the White-Inyo mountain region, between 6,750 and 10,300 ft (2,060 and 3,140 m).
The Violet-green Swallow, one of the most conspicuous birds in the mountains, can be seen overhead on virtually any day. It is most common near cliffs or rocky outcrops above 7,500 ft (2,290 m), with numbers declining in the warmer areas below. Several pairs may form loose colonies in the vicinity of such cliff sites. Unlike most other swallows, this species build nests in two distinct kinds of places: either in rock crevices or in abandoned woodpecker cavities in aspens and conifers. The adults
commonly perch on dead limbs of pines or aspens near the nest. This species feeds entirely on small insects caught on the wing. Reference: Grinnell and Storer (1924).
Corvidae (Crows and Jays)
Steller's Jay,Cyanocitta stelleri. (Fig. 11.25) Male length 10 3/4 in (27.5 cm), female length 10 1/2 in (26.5 cm); male weight 3 7/10 oz (105 g), female weight 3 1/2 oz (98 g). Fairly common permanent resident locally in the White-Inyo Range. Recorded between 6,200 and 9,500 ft (1,890 and 2,900 m).
Most records of this jay are from streamside willows and aspens, especially where the latter are mixed in canyon bottoms with large Singleleaf Pinyons or, at higher elevations, with Bristlecone and Limber pines. Diet is exceptionally varied; conifer seeds, fruits, large adult insects, caterpillars, and eggs and young of small birds are all devoured. Steller's Jays are also known to pilfer the seed stores of the Clark's Nutcracker. Nests in the White Mountains have been found in dense willow thickets. The breeding cycle starts in early spring so that stubby-tailed young, attended by
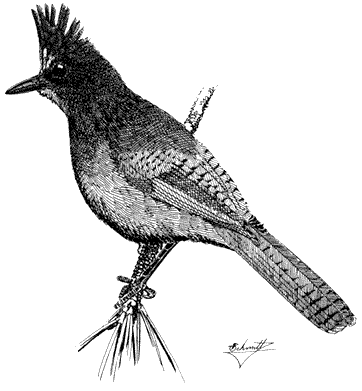
Figure 11.25
Steller's Jay.
parents, can be seen in late May. In other regions, Steller's Jays have been shown to defend group territories, where each pair is dominant to other members of the group on a portion of the total territory. These jays are therefore gregarious year-round. The handsome crest reveals the relative degree of aggressiveness (crest erect) or submissiveness (crest flattened) displayed during interactions with other members of their own species or with potential enemies. Reference: Brown (1964).
Scrub Jay,Aphelocoma coerulescens. (Fig. 11.26) Male length 11 1/2 in (29.3 cm), female length 10 3/4 in (27.3 cm); male weight 2 4/5 oz (79 g), female weight 2 3/4 oz (77 g). Fairly common permanent resident in the White-Inyo Range, from 6,000 to 9,500 ft (1,830 to 2,900 m).
This species occurs in Pinyon-juniper Woodland on the slopes of the White-Inyo Range. Willows in canyon bottoms and groves of Mountain Mahogany are also visited if the preferred Singleleaf Pinyon is nearby. Brush near pinyon is used sparingly. The Scrub Jay is omnivorous wherever it occurs: seeds, nuts, fruits, large insects, and eggs and nestlings of small birds are all taken when available. Pinyon nuts are a particularly important food in these mountains. Scrub Jays living in pinyon
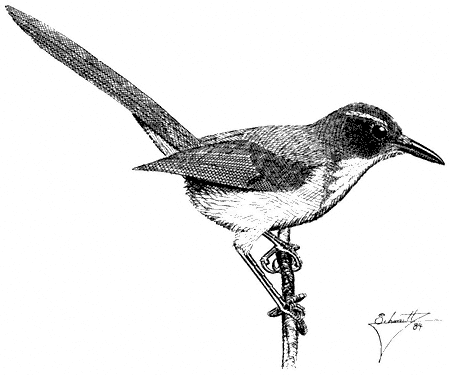
Figure 11.26
Scrub Jay.
woodlands of southeastern California are grayer and have longer, thinner bills than the oak-chaparral birds west of the Sierra Nevada. Presumably, the drabber coloration in the arid pinyon environment aids in concealment, and the bill shape is adapted for extracting pinyon seeds from cones. Reference: Ritter (1983).
Pinyon Jay,Gymnorhinus cyanocephalus . (Fig. 11.27) Male length 10 1/2 in (26.5 cm), female length 10 in (25 cm); male weight 4 oz (112 g), female weight 3 1/2 oz (97 g). Common permanent resident in the White-Inyo Range; recorded between 7,200 and 10,500 ft (2,195 and 3,200 m) elevation. Annual numbers fluctuate dramatically in any one place in concert with the availability of pinyon seeds.
Pinyon Jays are one of the most characteristic species of the interior Pinyon-juniper Woodland. The loud kraw call, given in chorus from the tops of trees or in flight, usually announces the presence of these conspicuous birds. When foraging, garrulous flocks, commonly numbering into the hundreds, sift through arid woodlands and over adjacent sagebrush country. Pinyon seeds are the major component of their diet and are also important in feeding displays and other courtship rituals. During the fall and early winter, large quantities of the seeds are harvested, transported in the throat, and stored on traditional nesting grounds. Because pinyon seed production is highly sporadic, the foraging range as well as the size of the flock depend on local seed availability. Pinyon Jays also eat some insects, which are taken from open ground by probing or from bark crevices. Pairs nest in loose colonies in large pinyons or junipers in fairly open woodland. Like other jays, the Pinyon Jay is very aggressive and will commonly harass hawks and other threatening birds of prey, especially when the latter are near the nesting area. Reference: Balda and Bateman (1973).
Clark's Nutcracker,Nucifraga columbiana. (Plate 11.2) Male length 11 1/2 in (29.5 cm), female length 11 1/4 in (28.7 cm); male weight 5 oz (139 g), female weight 4 5/8 oz (131 g). Common permanent resident in the White-Inyo Range; recorded from 6,800 to approximately 11,000 ft (2,073 to 3,353 m).
The Clark's Nutcracker is one of the more conspicuous species in the region and is commonly seen or heard overhead in a variety of habitats. The loud, grating kraaa call reveals its presence 0.5 mi (0.8 km) away. According to other studies, this species conducts seasonal altitudinal migrations in the Sierra Nevada, and it may show similar behavior in the White Mountains. Summers are commonly spent in the timber at higher elevations, and the onset of winter initiates a downslope movement into Pinyon-juniper Woodland. Although Clark's Nutcrackers are opportunistic feeders, they subsist mainly on the seeds of various conifers. Whitebark Pine and Jeffrey Pine are the primary trees utilized in the Sierra Nevada, but in the virtual absence of these conifers in the White Mountains, the local populations must eat the seeds of Limber pine and possibly Bristlecone Pine; little work has been done to document this, however. Nutcrackers also commonly visit pinyon in the summer, and when pinyon nuts are plentiful, they may be an important food source. This species is known to store vast seed reserves throughout the year in the Sierra Nevada. A small sac behind the tongue, unique to nutcrackers in the crow family, enables individuals
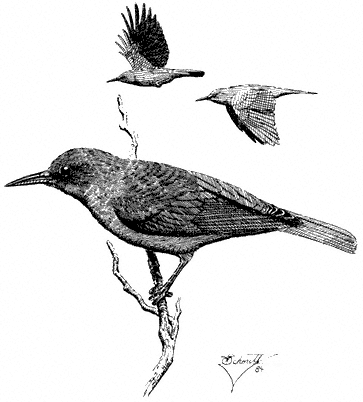
Figure 11.27
Pinyon Jay.
to carry seeds long distances between sources and storage sites. In addition to conifer seeds, Clark's Nutcrackers also eat insects and spiders, especially during the warmer months. Uncommonly, berries, freshly killed vertebrates (small mammals, nestling birds), and carrion are eaten. Nests are preferably built in fairly small conifers such as junipers. Typically, the birds begin nesting in late winter or early spring, so that by early summer the fully grown young are able to accompany their parents on long feeding flights. References: Tomback (1978a, 1978b).
Common Raven,Corvus corax. (Fig. 11.28) Male length 21 3/4 in (55.25 cm), female length 21 1/4 in (54 cm); male weight 2 lb (891 g), female weight 1 7/10 lb (767 g). Relatively common permanent resident at all elevations in the White-Inyo Range.
Although the species is conspicuous, total numbers in the region are probably small. Typically a bird of open country, the species is attracted to carrion of livestock and rabbits, large insects such as grasshoppers, and small mammals. Ravens move long distances daily in search of food. Postbreeding movements bring groups of ravens
Figure 11.28
Common Raven.
to largely barren alpine wastes. Nests are bulky piles of sticks placed on shelves in cliffs; buildings may also be used. The Common Raven is commonly harassed in the air by smaller birds such as American Kestrels and Violet-green Swallows. Our largest perching bird, the Common Raven soars like a hawk near windswept crags and over ridges. Reference: Goodwin (1976).
Paridae (Titmice)
Mountain Chickadee,Parus gambeli. (Plate 11.4) Male length 5 in (12.5 cm), female length 5 in (12.5 cm); male weight 2/5 oz (11.4 g), female weight 3/8 oz (10.7 g). Very common resident in the White-Inyo Range, from 6,750 to 10,500 ft (2,057 to 3,200 m).
This species is most at home in the coniferous forests of Limber Pine, Bristlecone Pine, and Lodgepole Pine occurring above 8,900 ft (2,710 m). However, it also uses lower-elevation habitats of pinyon woodland and streamside thickets of willow-rose-birch, particularly during the nonbreeding season. Nesting activities are concentrated in small cavities in dead conifers or aspen snags. The species forages in the lower foliage layers, where it takes caterpillars and various insects from needle clusters,
twigs, and small branches. When the birds are not breeding, they are gregarious and forage in flocks, either with other chickadees or in mixed groups with nuthatches, creepers, and other small insect-eating species. Mountain Chickadees utter a plaintive series of whistles, teeee-tee-tee , with the first note on a higher pitch and all three or four notes given in a minor key. This vocalization is a common sound in the White Mountains, especially early in the nesting period, and functions in territorial defense. The species also has a variety of buzzy and sputtering calls. References: Dixon (1965), Grinnell and Storer (1924).
Plain Titmouse,Parus inornatus. (Fig. 11.29) Male length 5 in (13 cm), female length 5 1/4 in (13.25 cm); male weight 5/8 oz (16.3 g), female weight 1/2 oz (15.4 g). Uncommon permanent resident in the White-Inyo Range; occurs from 7,200 to 7,900 ft (2,200 to 2,410 m).
This species is distributed sparsely through the warm, arid portion of mixed Pinyon-juniper Woodland; cool tracts of pure pinyon on northeast slopes are usually avoided. Plain Titmice favor open groves with at least some large, old trees that offer substantial limbs and trunks containing rotted cavities for nest placement. They hop
Figure 11.29
Plain Titmouse.
leisurely on large branches and twigs while searching the foliage for adult insects and caterpillars. Pinyon seeds are pounded open with the stout bill. The species commonly delivers a clear, ringing pee-two, pee-two, pee-two song while foraging. References: Dixon (1949); Johnson, Bryant, and Miller (1948).
Aegithalidae (Long-tailed Tits and Bushtits)
Bushtit,Psaltriparus minimus. (Fig. 11.30) Male length 4 1/3 in (11 cm), female length 4 1/4 in (10.75 cm); male weight 1/5 oz (5.5 g), female weight 1/5 oz (6.1 g). Fairly common resident in the White-Inyo Range, from 6,750 to 8,200 ft (2,060 to 2,500 m).
Despite their small size, Bushtits are conspicuous in the summer as they forage in areas of pinyon-juniper intermixed with sagebrush and Mountain Mahogany. Streamside willow thickets are also inhabited. During the breeding season, in early spring, pairs are generally quiet and fairly shy. By June, however, they join other Bushtits to form tight flocks commonly comprising 20 or more individuals. Such flocks move widely through tree canopies and tracts of tall brush, following lead birds from one foraging site to another while constantly conversing in soft, twittery notes. Small insects and some vegetable matter are eaten as the birds forage among outer leaves and twigs in an acrobatic manner, moving with agility in the fine foliage. The Bushtit nest is an elongated pouch woven from fine plant materials and usually suspended from the end of a pinyon bough. References: Ervin (1977), Marshall (1957).
Figure 11.30
Bushtit.
Sittidae (Nuthatches)
Red-breasted Nuthatch,Sitta canadensis . (Fig. 11.31) Male length 4 1/4 in (10.75 cm), female length 4 in (10.25 cm); male weight 2/5 oz (10.9 g), female weight 3/8 oz (10.3 g). Uncommon summer (permanent?) resident of the White Mountains; recorded from 8,900 to 9,500 ft (2,710 to 2,900 m).
This nuthatch occurs very sparingly and only in Subalpine Forest of Limber and Bristlecone pines. It is the least numerous of the three species of nuthatch that breed in the mountains. Red-breasted Nuthatches feed in the thickly foliaged tops of pines, where they can be extremely difficult to watch. They decoy readily, however, to imitated calls of small owls and will approach to within a few feet if one sits motionless next to a tree trunk. Like other nuthatches, this species excavates its own nest cavity in dead trunks or large limbs by digging vigorously with the bill. References: Anderson (1976), Kilham (1973).
White-breasted Nuthatch,Sitta carolinensis. (Plate 11.2) Male length 5 1/3 in (13.5 in), female length 5 1/4 in (13.25 cm); male weight 5/8 oz (16.6 g), female
Figure 11.31
Red-breasted Nuthatch.
weight 3/5 oz (16.3 g). Relatively common permanent resident in the White-Inyo Range, from 7,200 to 10,500 ft (2,200 to 3,200 m).
This species prefers relatively large Singleleaf Pinyon and Limber pines for foraging but will also use Bristlecone and Lodgepole pines to a lesser extent. An agile climber, the White-breasted Nuthatch ascends, descends, and circles large limbs and trunks in search of insects. Most prey is taken from the bark and from bark fissures. However, they also retrieve insects from deeper inside the wood by woodpeckerlike pounding and digging with their bills. Occasionally, this species drops to the ground to secure fallen items. During the breeding season, pairs of White-breasted nuthatches are widely spaced through the coniferous forest. At other times, they may join other nuthatches and insect-eating species in loose foraging groups. Like the Red-breasted and Pygmy nuthatches, the White-breasted Nuthatch nests in cavities in dead pine or aspen trees. References: Kilham (1972), McEllin (1979).
Pygmy Nuthatch,Sitta pygmaea. (Plate 11.4) Male length 4 in (10.25 cm), female length 4 in (10.25 cm); male weight 4/10 oz (10.1 g), female weight 3/8 oz (10.8 g). Relatively common resident in the White and northern Inyo mountains, between 9,500 and 10,500 ft (2,900 and 3,200 m).
The Pygmy Nuthatch typically inhabits Ponderosa Pines and other Yellow Pine associations throughout most of its range. In the White Mountains, in contrast, where Yellow Pines are virtually absent, it occurs almost exclusively in Bristlecone Pines. Aspen stands may be surveyed infrequently for possible nest sites, but the Bristlecone Pines largely fulfill requirements for food, nesting, and roosting. Pygmy Nuthatches feed on both plant and animal matter. Through the year, the bulk of their diet is composed of pine seeds, but insects predominate during the warmer months. Unlike the Red-breasted and White-breasted nuthatches, these small birds prefer to forage at upper levels in the pines, on the smallest, outermost twigs and needle clusters; trunks and large limbs are usually avoided. This species commonly forages in groups. Occasional individuals dart out after flying insects. The Pygmy Nuthatch uses its bill to dislodge insects from crevices in bark and also to wedge pine seeds into fissures before cracking them open. Some seeds are also stored in such crevices. Nest and roost sites are in tree cavities that they or other species excavate. Occasionally, several birds in addition to the nesting pair may assist in the digging. Some nests may also be shared by three nuthatches instead of two, the third individual typically being an unmated male helper. This species is gregarious when roosting in cavities. This occurs year-round, from before sunset to well after sunrise. Reference: Norris (1958).
Certhiidae (Creepers)
Brown Creeper,Certhia americana. (Fig. 11.32) Male length 4 1/2 in (11.75 cm), female length 4 3/4 in (12 cm); male weight 3/10 oz (8 g), female weight 3/10 oz (7.9 g). Uncommon permanent resident in the White-Inyo Range; recorded from 7,700 to 10,000 ft (2,350 to 3,200 m).

Figure 11.32
Brown Creeper.
Brown Creepers inhabit Subalpine Forest of Limber Pine, Bristlecone Pine, and Lodgepole Pine and woodlands of large Singleleaf Pinyon. They prefer mature timber in fairly dense stands for both foraging and nesting. These birds are excellent climbers, spiraling up tree trunks while probing into bark fissures for insects with their long, decurved bills. Their pale white throats may aid in reflecting light into these dark cracks. After attaining a certain height in the tree, creepers drop to the base of the same or another tree and continue their foraging. Brown Creepers nest in crevices or spaces underneath slabs of loosened tree bark. Although the back is camouflaged and resembles bark, the species can be detected by its constant movement. Reference: C. Davis (1979).
Troglodytidae (Wrens)
Rock Wren,Salpinctus obsoletus. (Plate 11.3) Male length 5 in (13 cm), female length 5 in (12.75 cm); male weight 1/2 oz (15.1 g), female weight 3/5 oz (16 g). Common resident in the White-Inyo Range, from the base of the ranges to the summit of White Mountain Peak, at 14,246 ft (4,342 m).
Rock Wrens occur through an exceptionally wide elevational range in the region, on generally arid canyon slopes or flats wherever rocky outcrops or surfaces occur. They even persist in areas inhabited by few other species, such as the high-elevation, windswept wastes covered sparsely by low scrub, grass, and rocks. Insects are removed from rock crevices and exposed rock surfaces. This species also uses such crevices for nesting, although it may nest in tunnels in earth banks. Rock Wrens sing from prominent rocks, their buzzes and trills ringing and echoing across canyon walls. References: Kroodsma (1975), Weathers (1983).
Canyon Wren,Catherpes mexicanus. (Plate 11.3) Male length 5 in (12.5 cm), female length 4 3/4 in (12.25 cm); male weight 2/5 oz (10.8 g), female weight 3/8 oz (9.7 g). Rare to locally relatively common resident in the White-Inyo Range, with records between 5,100 and 7,400 ft (1,555 and 2,256 m).
Canyon Wrens occur as very widely scattered pairs in the region, their local presence dictated by the availability of either steep, rocky canyon walls, with shady overhangs near pools, or boulder piles with deep, shady crevices. The surrounding vegetation can be varied, ranging from brush or woods to open forest. Males announce their presence by a cascading series of clear, mournful whistles that reverberate through the habitat during the nesting period. The adults sneak through shady cracks among the boulders, apparently using their stark white throats to reflect light into the dark recesses, revealing prey of insects and spiders. Nests are also hidden in such crannies. Reference: Grinnell and Storer (1924).
Bewick's Wren,Thryomanes bewickii. (Fig. 11.33) Male length 4 3/4 in (12 cm), female length 4 3/4 in (12 cm); male weight 3/8 oz (9.3 g), female weight 3/8 oz (9.7 g). Common permanent resident in the White-Inyo Range; recorded from 6,750 to 9,500 ft (2,060 to 2,900 m).
This species frequents mountain slopes and canyons grown to Pinyon-juniper Woodland and mixed brush, especially sagebrush. Nearby Mountain Mahogany will occasionally be entered, but it is not preferred. There is some evidence for a partial winter exodus of the population into riparian willows at lower elevations in canyons and valleys. Bewick's Wrens are active near the ground, where they are commonly out of sight, but foraging birds visit the upper and outer foliage of pinyons and junipers. A variety of insects are gleaned from needle surfaces, twigs, and cracks in bark or trunks. The male sings from exposed perches 5 to 15 ft (1.5 to 4.6 m) above the ground. Members of a pair typically forage within several yards of each other. Nests are hidden in or near the ground in natural hollows or crannies in pinyons and junipers as well as in cavities or protected nooks in human-made structures such as cabins and woodpiles. References: Miller and Stebbins (1964), E. Miller (1941).
House Wren,Troglodytes aedon. (Fig. 11.34) Male length 4 1/2 in (11.5 cm), female length 4 2/5 in (11.25 cm); male weight 3/8 oz (9.8 g), female weight 2/5 oz (10.4 g). Common resident in the White Mountains; recorded from 6,750 to 9,950 ft (2,060 to 3,030 m).
Figure 11.33
Bewick's Wren.
Figure 11.34
House Wren.
House Wrens live in riparian settings, where they nest in natural cavities or in abandoned woodpecker holes in aspens. Preferred nest sites are commonly within 5 ft (1.5 m) of the ground. However, in areas of human settlement, they may also nest under the eaves or in cracks in walls of cabins. Foraging similarly takes place at low levels, in brush, willows, or other such growth around fallen trees and rocks. A diversity of insects, especially those of small size, make up their diet. The bubbling song of male House Wrens is a characteristic summer sound of the aspen zone in the range. References: Kendeigh (1941), Marshall (1957).
Cinclidae (Dippers)
American Dipper,Cinclus mexicanus. (Fig. 11.35) Male length 7 in (17.5 cm), female length 6 3/4 in (17 cm); male weight 2 1/8 oz (60 g), female weight 1 3/4 oz (49 g). Small numbers of this species occur year-round in the deep, wet canyons of the White Mountains, up to elevations of approximately 10,000 ft (3,050 m).
The American Dipper occurs only along permanently flowing streams characterized by cool, swift, clear water. Aquatic insect larvae compose the bulk of their diet, but small fish and other invertebrates are occasionally eaten. Dippers get most of their prey directly from the fast-moving waters. They also flycatch and pick insect larvae from streamside rocks. The ability of dippers to forage in mountain streams is truly remarkable. With their strong feet they grasp the rubble on the bottom and wade
Figure 11.35
American Dipper.
underwater in search of food; in deeper, faster water, they typically use their wings while diving and swimming. The American Dipper usually stands on emerged rocks at the water's edge while foraging. Flight between such perches is rapid and low over the water. This species also breeds in the dampness of the stream environment. Preferred nest substrates are overhanging cliff ledges in areas protected from predators and inclement weather but exposed to water spray. Large rocks, tree roots, and undercut banks are commonly used. The mossy covering of the nest is moist and springy. Birds tend to breed in early spring, an adaptation that allows them to take advantage of an ample insect supply before late spring runoff decreases the availability of food. References: Morse (1979), Price and Bock (1983).
Muscicapidae (Muscicapids). Sylviinae (Kinglets and Gnatcatchers)
Ruby-crowned Kinglet,Regula calendula . (Fig. 11.36) Male length 4 in (10.25 cm), female length 4 in (10 cm); male weight 1/5 oz (6.2 g), female weight 1/5 oz (6 g). Relatively common summer resident locally in the White-Inyo Range; occurs between approximately 9,500 and 10,600 ft (2,900 and 3,230 m) elevation.
This species is most common in coniferous forest of Bristlecone, Limber, and Lodgepole pines. Aspen groves are visited occasionally. Foraging is concentrated on the terminal needle clusters at low to middle heights in the canopy. Here, this kinglet picks small insects from the foliage while nervously flicking its wings. Perches
Figure 11.36
Ruby-crowned Kinglet.
are changed frequently. Occasional sorties after flying insects and hovering flights near foliage are additional common foraging maneuvers. References: Franzreb (1984), Grinnell and Storer (1924).
Blue-gray Gnatcatcher,Polioptila caerulea . (Fig. 11.37) Male length 4 1/4 in (10.75 cm), female length 4 in (10.5 cm); male weight 1/5 oz (5.4 g), female weight 1/5 oz (5.7 g). Common summer resident in the White-Inyo Range, from 5,100 to 9,400 ft (1,555 to 2,870 m).
Blue-gray Gnatcatchers frequent arid Oak Woodland and Chaparral throughout most of their breeding range west of the White-Inyo mountain region. However, in these desert mountains, the species switches to a more xeric habitat of Pinyon-juniper Woodland interspersed with sagebrush and, in some areas, Mountain Mahogany. Nearby streamside thickets of willows and tall sagebrush are used less commonly. Gnatcatchers are conspicuous where they occur, uttering a series of high, thin, wheezy notes while actively foraging among the dense foliage of trees and shrubs. Alighting momentarily on a branch, the bird quickly scans the area for food before flying to the next perch. Large insects are usually picked from leaves and twigs, but some may also be taken on the wing. Nests are small, compact cups attached to upright branch
Figure 11.37
Blue-gray Gnatcatcher.
forks in a tree or bush. Although sometimes placed in the open, they are nonetheless well hidden by virtue of their camouflaging outer material. Blue-gray Gnatcatchers respond readily to human squeaks and imitated owl calls. References: Root (1967), Root (1969).
Turdinae (Thrushes)
Mountain Bluebird,Sialia currucoides . (Plate 11.2) Male length 6 3/5 in (16.75 cm), female length 6 1/2 in (16.5 cm); male weight 1 oz (28.7 g), female weight 1 oz (28.8 g). Common summer resident in the White-Inyo Range, from 7,200 to 10,500 ft (2,200 to 3,200 m).
This species lives in exposed places: meadows, sagebrush flats, sparsely vegetated slopes, and open coniferous forest. There they perch on bush tops, prominent rocks, fences, or dead treetops. Insects are taken from either the air, the ground, or the foliage and woody parts of trees and shrubs. The Mountain Bluebird commonly hovers buoyantly before securing its prey. Nests are mostly placed in cavities in dead conifers or aspens. In late summer, postbreeding family groups forage widely over open country. The Mountain Bluebird winters in flocks at lower elevations, either in agricultural regions in valleys, in the desert, or on mountain slopes grown to juniper, the berries of which are often a staple food during that season. References: Power (1966), Power (1980).
Townsend's Solitaire , Myadestes townsendi . (Fig. 11.38) Male length 7 9/10 in (20 cm), female length 8 1/10 in (20.5 cm); male weight 1 1/8 oz (31.9 g), female weight 1 1/4 oz (35.6 g). Rare summer resident in the White-Inyo Range, between 9,300 and 10,500 ft (2,840 and 3,200 m) elevation. The solitaire also occurs in winter on the lower slopes of these ranges, although these birds are not necessarily from the local summering population.
As a nesting species, solitaires occur only in the best stands of subalpine Limber and Bristlecone Pine forest. Males sing a far-reaching, jumbled series of warbles from spires in the treetops. A more commonly heard vocalization, however, is the brief, haunting whistle that simulates a creaky hinge. Townsend's Solitaires commonly take shady perches near the trunk, below the main foliage canopy of pines. They obtain insects in summer, occasionally by flycatching; winter birds commonly feed on juniper berries. Nests are built in protected crevices in banks, stumps, and exposed tree roots. Reference: Salomonson and Balda (1977).
Hermit Thrush,Catharus guttatus . (Plate 11.4) Male length 6 3/4 in (17.25 cm), female length 6 1/4 in (16 cm); male weight 1 oz (28.5 g), female weight 1 oz (28.8 g). Common summer resident in the White-Inyo Range; recorded from 7,700 to 10,500 ft (2,350 to 3,200 m).
This species lives in the shadiest habitats of the White-Inyo Range: aspen woodland, willow thickets, and coniferous forest of Limber, Bristlecone and Lodgepole pines. Dense groves of Mountain Mahogany, especially on northeast slopes or in
Figure 11.38
Townsend's Solitaire.
ravines, are also suitable; these provide good shade and a ground layer of plant debris in which the thrushes forage. In the morning and again at dusk, males deliver their enchanting, fluty song from prominent perches. The environment occupied by the Hermit Thrush in these mountains seems more open and arid than places where the species occurs in the Sierra Nevada. References: Dilger (1956), Morse (1971).
American Robin,Turdus migratorius . (Fig. 11.39) Male length 9 1/8 in (23.25 cm), female length 9 1/4 in (23.5 cm); male weight 2 5/8 oz (74 g), female weight 2 3/4 oz (78 g). Common summer resident in the White Mountains, from 6,750 to 10,500 ft (2,060 to 3,200 m). Scarce in the Inyo Mountains; there is one June record from Waucoba Canyon, at 7,200 ft (2,200 m).
In the White Mountains region, this familiar species breeds in moist canyon bottoms where meadows and dense thickets of willow, rose, and birch prevail. Such habitats provide soft, open ground for foraging, where worms and other ground invertebrates may be taken. Mud for nest construction is also available in these places. The American Robin builds its nest in many kinds of trees situated near wet areas. Typically, the cup-shaped nest is placed on a branch or in a fork at moderate heights. Males begin to sing on their breeding territories in early spring. They sing most vigorously at dawn and during the early morning hours, and their caroling
Figure 11.39
American Robin.
song can often be heard continuously along streamcourses throughout the summer. References: Howell (1942), James and Shugart (1974), Shedd (1982).
Mimidae (Mockingbirds and Thrashers)
Sage Thrasher,Oreoscoptes montanus . (Fig. 11.40) Male length 7 7/8 in (20 cm), female length 7 7/10 in (19.5 cm); male weight 1 2/5 oz (40 g), female weight 1 1/2 oz (44 g). Present in both ranges during the spring and summer months, from approximately 6,000 to 11,000 ft (1,830 to 3,350 m) elevation in the White Mountains.
Although never numerous, Sage Thrashers are most common in the tall, dense sagebrush present on the alluvial fans in the northern White Mountains. Populations at high elevations, where the brush is of short stature, are sparse. In those areas, however, the birds typically seek out the scattered, thicker patches of brush and use the tallest available sages for both song posts and nest sites. This species inhabits no other plant assemblage in the region. Foraging concentrates on the ground between and below the shrubs, but insects in the foliage are also taken; preferred prey items include grasshoppers, cicadas, beetles, and other large species. The Sage Thrasher builds its bulky nest in a well-concealed position in the top of a sage bush. Four or five spotted blue eggs are laid. Males also sing their rich, warbled song from bush

Figure 11.40
Sage Thrasher.
tops and may commonly be heard across the open terrain for at least 0.5 mi (0.8 km). These birds can also be detected by their strong and direct flight just above the sagebrush tops. Reference: Reynolds and Rich (1978).
Vireonidae (Vireos)
Solitary Vireo,Vireo solitarius . (Fig. 11.41) Male length 5 4/5 in (14.3 cm), female length 5 2/5 in (13.8 cm); male weight 3/5 oz (16.2 g), female weight 3/5 oz (16.8 g). Relatively common summer resident in the White-Inyo Range, between 6,200 and 8,200 ft (1,890 and 2,500 m).
In these mountains, Solitary Vireos inhabit wooded canyon slopes, draws, and flats dominated by large pinyons, junipers, and, to a lesser extent, Mountain Mahogany. Foraging activity ranges from the tree crowns to near the ground. There birds forage for adult and larval insects by moving deliberately through clumps of foliage. The nest is a cup constructed of fine plant fibers, delicate inner bark strips, and grass tops. It is camouflaged externally with grayish or greenish lichens or dried leaves, and it is suspended by its rim from the horizontal fork of a pinyon or juniper branch. Solitary Vireos can be detected from a distance of over 0.25
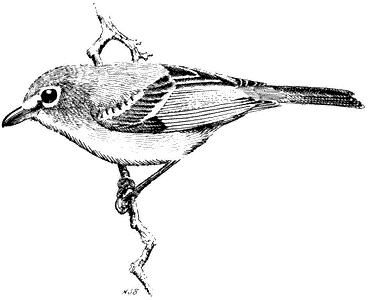
Figure 11.41
Solitary Vireo.
mi (0.4 km) by their song of rich, inflected slurs or burred phrases, often uttered in a question-and-answer pattern. Occasionally, the male may sing from the nest while covering the eggs in the absence of the female. Pairs of these vireos are generally widely spaced, so that four or five territories at most are encountered in a hike of several miles through favorable habitat. The form V. solitarius plumbeus , which inhabits the White-Inyo Range, is a clean, grayish white bird, in contrast to the yellowish green form, V. solitarius cassinii , that breeds on the west side of the Sierra Nevada. V. s. plumbeus is a relative newcomer to the region, unrecorded there prior to the 1960s. Reference: James (1981).
Warbling Vireo,Vireo gilvus . (Fig. 11.42) Male length 4 3/4 in (12 cm), female length 4 3/4 in (12 cm); male weight 3/8 oz (10.8 g), female weight 2/5 oz (11.8 g). Common summer resident in the White-Inyo Range, between 6,200 and 10,500 ft (1,890 and 3,200 m).
Warbling Vireos dwell in the canopy foliage of riparian aspen, willows, and Water Birch. They take insects from their leafy surroundings by deliberate foraging motions. This species can be difficult to find in the treetops, but its presence is revealed by incessant singing, which commonly continues to midday or even into the afternoon, when other birds are silent. In the early morning, especially in spring soon after their arrival, chases of male vireos in territorial encounters, and males and females in pairing flights, are announced by silvery white flashes through the foliage. The nest is a neatly woven cup suspended from a horizontal fork and placed 8–25 ft (2.4–7.6 m) above the ground in deciduous foliage. Brown-headed Cowbirds commonly
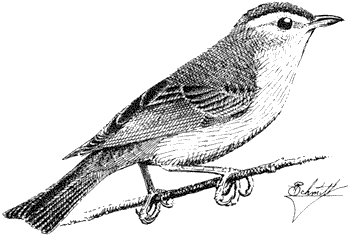
Figure 11.42
Warbling Vireo.
parasitize Warbling Vireo nests in the Sierra Nevada, and possibly also do so in the White Mountains. In favorable habitat, the linear territories of the species occur in uninterrupted succession along mountain streams. References: Grinnell and Storer (1924), Walsberg (1981).
Emberizidae (Emberizids). Parulinae (Wood Warblers)
Orange-crowned Warbler,Vermivora celata . (Fig. 11.43) Male length 4 2/5 in (11.25 cm), female length 4 5/8 in (11.75 cm); male weight 2/5 oz (9.2 g), female weight 2/5 oz (9.1 g). Relatively common summer resident in the White Mountains and locally in the Inyo Mountains, between 6,650 and 9,500 ft (2,030 and 2,900 m).
The interior form of this species lives only in moist canyon bottoms, in the cool riparian strip of aspen, Water Birch, and associated thickets of willow and Wild Rose. Such habitat contrasts greatly with the warm Oak-Chaparral growth at low elevations occupied by the form on the west side of the Sierra Nevada. Like most other species in the genus Vermivora , the Orange-crowned Warbler builds its nest in a concealed place on the ground. The adults are well hidden as they search for insects and spiders in the foliage of willow, rose, and aspen saplings. Most challenging of all is to pinpoint the male as he sings from the crowns of aspen, birch, or willow along the stream. His song — a continuous, insectlike trill of two or three parts, each on a slightly different pitch — is usually delivered in long series from a concealed perch. Although exposed briefly while flying between song perches, he quickly vanishes again in the leafy treetops. References: Mengel (1964), Morrison (1981).
Virginia's Warbler,Vermivora virginiae . (Fig. 11.44) Male length 4 1/4 in (11 cm), female length 4 1/4 in (11 cm); male weight 2/5 oz (8.4 g), female weight 1/4 oz (7.5
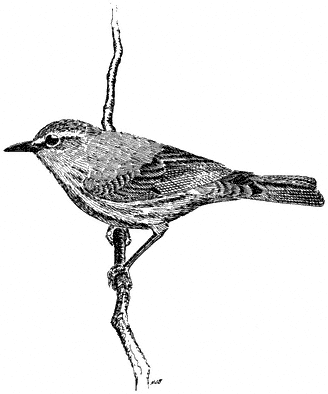
Figure 11.43
Orange-crowned Warbler.
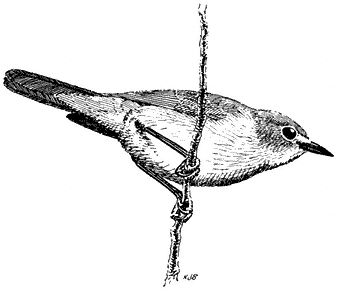
Figure 11.44
Virginia's Warbler.
g). Relatively common summer resident locally in the White Mountains, between 6,750 and 9,500 ft (2,060 and 2,900 m).
This warbler is most abundant on upper slopes in groves of Mountain Mahogany, but it also occurs sparingly in riparian willows and around clumps of seviceberry in the pinyon zone. The plant growth these birds occupy is often clumped among boulder piles or near rock slides. A secretive species, it perches in the leaves just below the foliage top and can be difficult to find even when it is singing. Nonetheless, a squeak or hissing note will usually bring the bird into view. Then it characteristically flicks its tail while uttering chip notes in alarm. Virginia's Warblers take insect food from twigs and leaves of deciduous brush; thus, their sphere of activity is focused within several feet of the ground. Reference: Johnson (1976).
Yellow Warbler,Dendroica petechia . (Fig. 11.45) Male length 4 1/2 in (11.25 cm), female length 4 1/4 in (10.5 cm); male weight 3/10 oz (9.3 g), female weight 3/10 oz (8.5 g). Uncommon local summer resident in the White Mountains, between 6,750 and 8,200 ft (2,060 and 2,500 m).
This species dwells in the canopy of riparian vegetation, mainly in canyons on the east side of the range. It also breeds at scattered oases in the valleys on each side of the mountains. Yellow Warblers prefer tree willows and cottonwoods but will venture sparingly into aspen at higher elevations. Low willow and alder growth along streams is used for foraging, although much insect food is also taken well up in the foliage of large trees. Nests too are placed in these trees from near the ground to middle heights. Elsewhere this species is heavily parasitized by Brown-headed Cowbirds, and
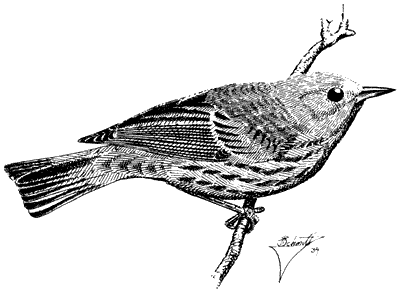
Figure 11.45
Yellow Warbler.
this activity is suspected to contribute to the uncommonness of the Yellow Warbler in the White Mountains. References: Biermann and Sealy (1982), Griscom and Sprunt (1957).
Yellow-rumped Warbler,Dendroica coronata. (Plate 11.4) Male length 5 in (13 cm), female length 5 in (13 cm); male weight 2/5 oz (12.3 g), female weight 2/5 oz (12.5 g). Common summer resident in the White-Inyo Range, occurring from 8,200 to 10,500 ft (2,499 to 3,200 m).
This species summers in Subalpine Forest of Bristlecone Pine, Limber Pine, or Lodgepole Pine, and in associated riparian trees such as aspen. One of the most generalized foragers of all warblers, it takes insects from the foliage by gleaning and hovering and from the air by flycatching. All levels of the habitat are visited, from the ground to the tops of tall conifers. During the nonbreeding season, berries are often eaten. The Yellow-rumped Warbler also varies its nest sites from near the ground to high in the conifers. Reference: Hubbard (1969).
Black-throated Gray Warbler,Dendroica nigrescens. (Plate 11.2) Male length 4 5/8 in (11.8 cm), female length 4 1/2 in (11.5 cm); male weight 1/4 oz (8.1 g), female weight 3/10 oz (8.9 g). Very common summer resident in the White-Inyo Range, from 6,750 to 9,500 ft (2,060 to 2,900 m).
This warbler's preference for sunny and warm environments is reflected in its increasing abundance from north to south in the woodlands of the White-Inyo mountains. Arid portions of Pinyon-juniper Woodland are favored, with occasional use of Mountain Mahogany. The Black-throated Gray Warbler must occur in huge numbers, as it is one of the most commonly encountered species in the region. Its "see-saw," buzzy song, rising in pitch near the end, is a characteristic sound of the pinyon zone through the spring and summer. Adult Black-throated Grays hunt for insects and spiders in dense terminal needle clusters of pinyon. Nests are built in pinyons or in nearby brush. References: Marshall (1957), Morrison (1982).
MacGillivray's Warbler,Oporornis tolmiei. (Fig. 11.46) Male length 4 4/5 in (12.25 cm), female length 4 3/4 in (12 cm); male weight 3/8 oz (10.8 g), female weight 3/8 oz (10.7 g). Common summer resident in the White Mountains, between 6,750 and 9,500 ft (2,060 and 2,900 m).
This warbler inhabits thick undergrowth along streams, attaining its greatest numbers between 8,200 and 9,000 ft (2,500 and 2,740 m) in willows, alder, Water Birch, nettles, and aspen saplings. Shade and dampness are prominent features of the species' habitat. MacGillivray's Warblers forage for insects and nest near the ground in dense foliage and branchwork; thus, they can be very difficult to observe. When singing, however, males are commonly exposed in bush tops from 5 to 15 ft (1.5 to 4.6 m) above the ground. During migration, this species occurs much more widely and can be found even in very open country near damp spots, as long as there is relatively thick brush nearby with shady hiding places. References: Grinnell and Storer (1924), Hutto (1981).
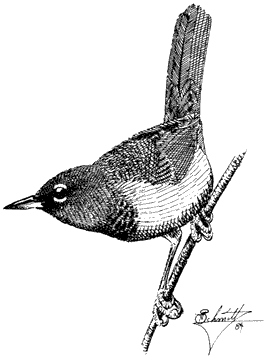
Figure 11.46
MacGillivray's Warbler.
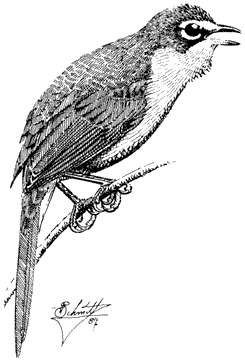
Figure 11.47
Yellow-breasted Chat.
Yellow-breasted Chat,Icteria virens. (Fig. 11.47) Male length 6 7/8 in (17.5 cm), female length 7 in (17.75 cm); male weight 7/8 oz (24.9 g), female weight 1 oz (27.8 g). Local and rare summer resident in the White Mountains, with records at 6,750 ft (2,060 m), an unusually high elevation for the species.
The Yellow-breasted Chat nests only in the densest riparian thickets in foothill canyons on the east side of the range. Total numbers in the region are probably very small. Lush growth of willows, Wild Rose, nettles, and cottonwood saplings are used for all activities. Because of these habitat preferences, chats are usually very difficult to see in their impenetrable haunts. Their presence is typically revealed by an unusual song, a mixture of harsh and varied rattles interspersed with clear whistles. The singing male is commonly obscured by foliage, but patience will be rewarded by a spectacular song flight occurring between thickets, in which the legs are dangled and wings slowly fluttered. Food consists of insects, spiders, and small fruits. References: Petrides (1938), Thompson (1977).
Thraupinae (Tanagers)
Western Tanager,Piranga ludoviciana. (Plate 11.4) Male length 6 1/4 in (16 cm), female length 6 1/4 in (16 cm); male weight 1 oz (27.5 g), female weight 1 oz (27.8 g). Relatively common summer resident in the White-Inyo Range, from 6,750 to 9,800 ft (2,060 to 2,990 m).
This species shuns open Singleleaf Pinyon as breeding habitat in favor of the larger conifers occurring at higher elevations. Occasionally, however, tall pinyon trees intermixed with Mountain Mahogany, as well as aspen, may be used for nesting. Pinyon Woodland is visited much more extensively when the birds are migrating, which may occur well into June. Western Tanagers forage for wasps, ants, beetles, and other insects in the pine canopies, either by picking the insects from the branches and foliage or by snapping them from the air during short jaunts from their perches. Other foods, particularly fruits, are eaten by postbreeding or migrating individuals. Nests of twigs and fine roots are placed on the outer ends of large limbs, from middle heights to the top of the canopy. Although this species prefers the security afforded by dense conifer foliage, dead limbs or trees are known to be used. Brightly colored males are easily spotted by flashes of yellow as they traverse openings in the forest. The short, melodious, languid warble given by the male, or the short pit-er-ic call uttered by both sexes, also announces the presence of the species. Reference: Grinnell and Storer (1924).
Cardinalinae (Cardinals, Grosbeaks, and Allies)
Black-headed Grosbeak,Pheucticus melanocephalus. (Fig. 11.48) Male length 7 in (18 cm), female length 7 1/4 in (18.25 cm); male weight 1 1/2 oz (41 g), female weight 1 1/2 oz (44 g). Very common summer resident in the White-Inyo Range, between 6,750 and 8,900 ft (2,060 and 2,710 m).
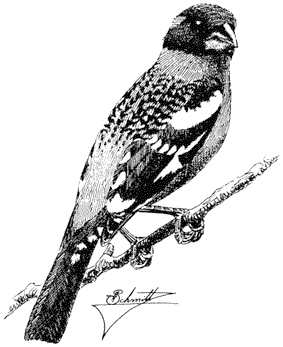
Figure 11.48
Black-headed Grosbeak.
This species is a characteristic mid-elevation bird of the region in diverse woodlands such as pinyon, aspen-willow, and Mountain Mahogany. Between approximately 7,000 and 8,000 ft (2,130 and 2,440 m) elevation, it is very prominent and is almost constantly within sight or sound. The male's rich, warbled song is so loud that it virtually drowns out the voices of most other singing birds in its neighborhood. Male Black-headed Grosbeaks even sing loudly from the nest as they cover the eggs during the absence of the female. Extensive edges and diverse plant mixtures are preferred, these providing the species with foliage insects, buds, and fruits for consumption. References: Weston (1947), Bent (1968).
Lazuli Bunting,Passerina amoena. (Fig. 11.49) Male length 5 in (12.75 cm), female length 4 9/10 in (12.5 cm); male weight 1/2 oz (14.7 g), female weight 1/2 oz (13.6 g). Fairly common summer resident in the White Mountains, recorded between 6,750 and 9,000 ft (2,060 and 2,740 m), but rare above 8,200 ft (2,500 m) except as a postbreeding vagrant.
Lazuli Buntings are restricted in the White Mountains to streamside thickets and are most common in the middle and lower reaches of canyons. Dense tangles of willows, Wild Rose, and Water Birch are chosen for both foraging and nesting, especially where the plant growth is mixed in terms of species composition and stature. The colorful male commonly sings from the tops of tall bushes or small
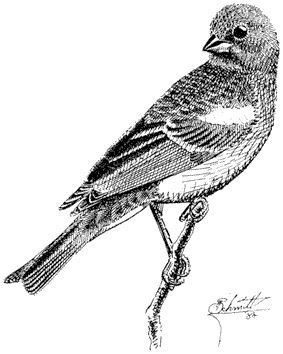
Figure 11.49
Lazuli Bunting.
trees. Occasionally, however, he ascends to the top of an aspen, pinyon, or other conifer and sings from 30 to 40 ft (9 to 12 m) above the ground. The song is characteristically variable, with phrases and short trills at different pitches and often in couplets. The closely related Indigo Bunting (P. cyanea ), with which the Lazuli Bunting is known to hybridize, has been expanding its range southwestward in recent years, and males have been recorded in the White-Inyo mountains. References: Emlen, Rising, and Thompson (1975); Thompson (1976).
Emberizinae (Towhees, Sparrows, and Allies)
Green-tailed Towhee,Pipilo chlorurus. (Fig. 11.50) Male length 6 3/4 in (17 cm), female length 6 1/2 in (16.75 cm); male weight 1 oz (28.9 g), female weight 7/8 oz (24.9 g). Locally common summer resident in the White-Inyo Range, from 6,200 to 10,000 ft (1,890 to 3,050 m).
Green-tailed Towhees breed in brushland in this region, occurring in greatest numbers in open pinyon with mixed brushland. High-elevation habitats of sagebrush mixed with willows and birch are visited sparingly. A particularly favorable site is sagebrush interspersed with serviceberry, currant, and scattered pinyons in a canyon bottom. Arid flats of pure sagebrush are avoided. The adults are skilled runners, moving while crouched in alleyways between the shrubs. They forage on the ground
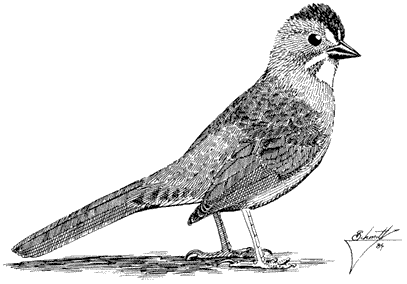
Figure 11.50
Green-tailed Towhee.
for insects and fruit in leaf litter. Nests are placed near the ground in bushes. Adult Green-tailed Towhees decoy readily to a squeak and commonly respond with a catlike mew . Males sing from open perches in pinyon tops at approximately 20 ft (6 m) above the ground. Reference: Grinnell and Storer (1924).
Rufous-sided Towhee,Pipilo erythrophthalmus. (Fig. 11.51) Male length 7 3/4 in (20 cm), female length 7 1/2 in (19.5 cm); male weight 1 3/10 oz (37.4 g), female weight 1 3/10 oz (37.6 g). Common resident in the White-Inyo Range, recorded from 6,000 to 9,000 ft (1,830 to 2,740 m).
This species frequents dense brushy habitats along slopes and canyon bottoms in the pinyon-juniper-mahogany belt. Sagebrush and streamside growth of willow-rose-birch are particularly favored. A ground cover of litter is essential to the presence of Rufous-sided Towhees; there the species rakes up seed of herbaceous vegetation and insects by simultaneously scratching with both feet. While foraging, it stays well hidden in the dense cover of brush or riparian thickets. Nests are usually placed on or near the ground at the base of a shrub or in an otherwise concealed place. During the breeding season, males commonly sing from more exposed areas, such as branches of small pinyons, junipers, or Mountain Mahogany. Although this species is difficult to see except when singing, it will readily announce its presence by a buzzy trill, which is subordinated in this region by one or more harsh introductory notes. References: Kroodsma (1971), Davis (1960).
Chipping Sparrow,Spizella passerina. (Fig. 11.52) Male length 5 1/4 in (13 cm), female length 5 in (12.5 cm); male weight 1/2 oz (12.6 g), female weight 2/5 oz (12.1
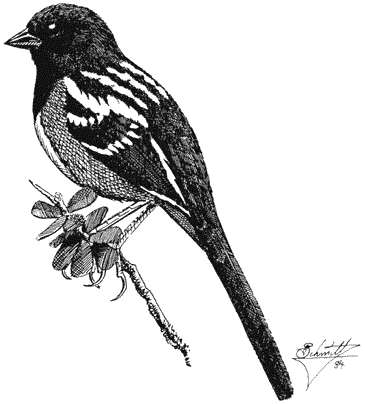
Figure 11.51
Rufous-sided Towhee.
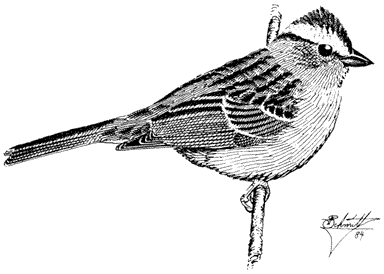
Figure 11.52
Chipping Sparrow.
g). Very common summer resident in the White-Inyo Range, from 7,200 to 10,400 ft (2,200 to 3,170 m).
This sparrow is very numerous in open woods of pinyon, juniper, and Mountain Mahogany. Territories are defended in areas of scattered trees, open brush, rocks and grass; heavy brush is avoided. Chipping Sparrows search the ground for small seeds and insects but will also take buds in bushes and trees, especially during the spring. The dry, harsh trill of the male is a common summertime song in this area. References: Grinnell and Storer (1924), Reynolds and Knapton (1984).
Brewer's Sparrow,Spizella breweri. (Fig. 11.53) Male length 5 1/4 in (13 cm), female length 5 in (12.5 cm); male weight 3/8 oz (11 g), female weight 3/8 oz (10.4 g). Very common summer resident in the White-Inyo region, from 6,750 to 10,300 ft (2,060 to 3,140 m).
Although the Brewer's Sparrow nests over an impressively wide elevational range in these mountains, only sagebrush is occupied. Extensive stands, such as those on the alluvial fans below the pinyon zone or those on benches and flats at high elevations, are used most commonly. Small numbers also nest in sagebrush growth in local openings of otherwise narrow canyons. This sparrow compensates for its plain plumage by emitting an elaborate song — a series of buzzes and trills given at different pitches, one of the longest vocalizations of any species in the region. Brewer's Sparrows forage
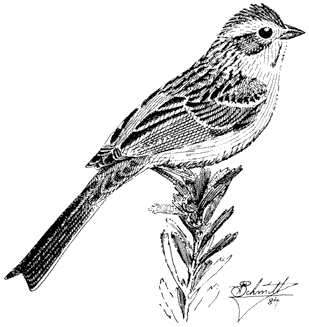
Figure 11.53
Brewer's Sparrow.
on the ground or in bushes for insects and seeds. Nests are well hidden in sagebrush tops. References: Reynolds (1981), Weathers (1983).
Black-chinned Sparrow,Spizella atrogularis. (Fig. 11.54) Male length 5 1/4 in (13 cm), female length 5 1/4 in (13 cm); male weight 3/8 oz (10.6 g), female weight 3/8 oz (10.9 g). Rare and local summer resident in the White Mountains; recorded between 6,750 and 7,000 ft (2,060 and 2,130 m).
The Black-chinned Sparrow occurs sparingly in the White Mountains, at the northern edge of the species' distribution, where it lives in a rather distinctive and characteristic habitat. Although sagebrush is usually prevalent where this sparrow breeds in eastern California, it typically requires a diverse mixture of shrubs in addition to the sage, including Rabbitbrush, Bitterbrush, and Mormon Tea. Furthermore, most records of the species are from brushland sites in varied, sloping terrain, often with jumbled rock outcrops and a scattering of pinyon and/or juniper. Males sing from either the tops of these small trees or from the tops of prominent shrubs and usually fly substantial distances between song perches. Thus, territories
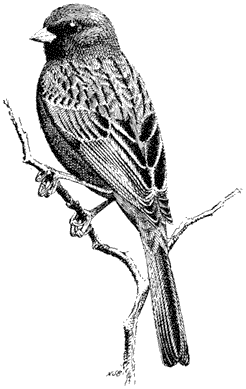
Figure 11.54
Black-chinned Sparrow.
are large, and pairs are widely spaced. The easiest way to detect this trim, handsome sparrow is through its distinctive song — a rapid trill introduced by three clear, sweet notes and increasing in cadence toward the end. Reference: Johnson, Bryant, and Miller (1948).
Vesper Sparrow,Poocetes gramineus. (Fig. 11.55) Male length 6 in (15 cm); female length 5 3/4 in (14.75 cm); male weight 7/8 oz (24.9 g), female weight 4/5 oz (22.7 g). Uncommon summer resident in the White-Inyo Range, from approximately 7,800 to 10,500 ft (2,380 to 3,200 m).
This species occurs in the sagebrush-grassland habitat both below and above the pinyon belt. There only small numbers can be found. Vesper Sparrows are shy birds and commonly remain undetected even when present because of their inconspicuous habits. They forage mostly on the ground, but males usually perch on prominent sage tops when delivering their advertising song. References: Kroodsma (1972), Grinnell and Storer (1924).
Black-throated Sparrow,Amphispiza bilineata. (Fig. 11.56) Male length 5 1/4 in (13 cm), female length 5 in (12.5 cm); male weight 1/2 oz (13.5 g), female weight 1/2 oz (13.2 g). Fairly common summer resident in the White-Inyo Range, occurring from the valley bottoms up to approximately 6,800 ft (2,070 m) elevation.
Black-throated Sparrows occur only in brushland on the alluvial fans on both slopes of the mountains. They generally prefer the warmer, drier areas to the south. Consequently, greatest densities are attained in the southern Shadscale zone, with the species becoming relatively scarce on the cooler sagebrush slopes to the north. The sparrows favor areas that are sparingly vegetated and where an exposed hard rock or gravel surface is evident. Seeds taken from the ground between shrubs constitute the primary food. Nests are placed on the ground in a thick cover of brush. During
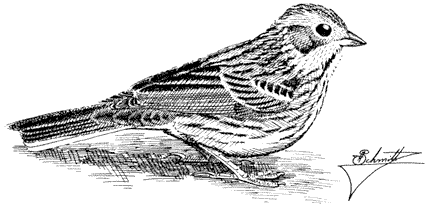
Figure 11.55
Vesper Sparrow.
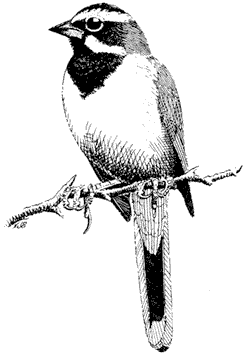
Figure 11.56
Black-throated Sparrow.
the breeding season, these birds can be seen or heard singing from bush tops, and after June, postbreeding family groups filter through the brushland. This strikingly patterned species is often easy to watch and seems tamer than its somewhat plainer relative, the Sage Sparrow. References: Heckenlively (1970), Weathers (1983).
Sage Sparrow,Amphispiza belli. (Fig. 11.57) Male length 5 3/4 in (14.5 cm), female length 5 1/2 in (14 cm); male weight 3/5 oz (16.5 g), female weight 1/2 oz (14.7 g). Very common summer resident in the White-Inyo Range; occurs from the valley floors to approximately 7,500 ft (2,290 m) elevation. Postbreeding birds occur higher, to 8,500 ft (2,590 m), even in early June.
This species occurs continuously along the west-facing slope from the north end of the White Mountains to the southern portion of the Inyos. Curiously, it is rare or lacking on the east side of the mountains south of Trail Canyon. Flats and alluvial fans between 3,500 and 6,500 ft (1,067 and 1,981 m) support the greatest numbers; very high altitudes are avoided. Like its close relative the Black-throated Sparrow, the Sage Sparrow lives only in brushland, and both species commonly occur side by side in the White-Inyo Range. In the north the Sage Sparrow occupies essentially pure sagebrush, while in the south Shadscale (saltbush) is the predominant habitat. Seeds of annual plants provide the primary food source. This sparrow also uses the tops of
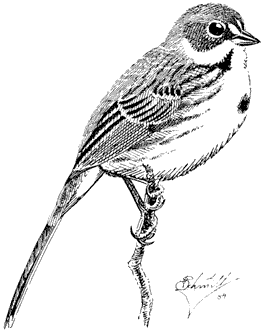
Figure 11.57
Sage Sparrow.
bushes for song posts as well as for concealed nest sites. Occasionally, several males can be heard singing from very close range. The tinkling song carries for several hundred yards unless the bird is downwind. Nesting begins in April in the south, so that fully grown juveniles abound in the saltbush scrub by June, when family groups are commonly encountered. Northern populations breed several weeks later. These birds are typically shy and difficult to approach in the open brushland; when pressed, they will fly long distances and may drop to the ground and run among the shadows below well-spaced shrubs. References: Moldenhauer and Wiens (1970), Wiens (1982).
Fox Sparrow,Passerella iliaca. (Fig. 11.58) Male length 6 1/2 in (16.75 cm), female length 6 1/2 in (16.75 cm); male weight 1 oz (29.7 g), female weight 1 oz (28.1 g). Locally common summer resident in the White Mountains, between 6,750 and 9,600 ft (2,060 and 2,930 m) elevation. Known to occur locally in the Inyo Range (Lead Canyon, 9,600 ft [2,930 m]).
Fox Sparrows breed in dense riparian thickets of willows, Water Birch, aspen saplings, and Wild Rose, especially in the deep canyons on the east side of the mountains. This habitat, shared by all Great Basin and Rocky Mountain subspecies, contrasts strikingly with the chaparral used primarily by all forms in the Sierra Nevada. In favorable streamside growth, Fox Sparrow territories are placed in a
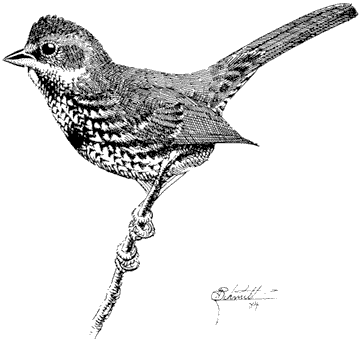
Figure 11.58
Fox Sparrow.
continuous linear sequence, and two or more males are commonly heard at once. Song perches, from 5 to 15 ft (1.5 to 4.6 m) high, are typically in bush tops, which offer partial concealment by sprays of foliage. This species forages principally on the ground in leaf litter, which is kicked away by vigorous backward strokes of the legs. Insects exposed in this way are fed to the nestlings. References: Martin (1977), Martin (1979).
Song Sparrow,Melospiza melodia. (Fig. 11.59). Male length 5 3/4 oz (14.75 cm), female length 5 1/2 in (14.25 cm); male weight 3/4 oz (21.7 g), female weight 3/4 oz (20.4 g). Locally common summer resident in the White Mountains with records up to 9,600 ft (2,930 m); more numerous in Owens Valley to the west of the White-Inyo Range.
The highest elevational record for the form M. m. fisherella occurs in Cottonwood Basin in the White Mountains. There, and throughout this generally arid region, the species is restricted to canyon bottoms with thick, low cover near water. Willow and Wild Rose thickets along streams are commonly occupied. Song Sparrows forage near the water's edge or on damp ground below the willows. Males sing from exposed bush tops, usually no more than several feet above the ground. Food consists of insects and seeds taken from the ground. References: Nice (1937); Smith, Yom-Toy, and Moses (1982).
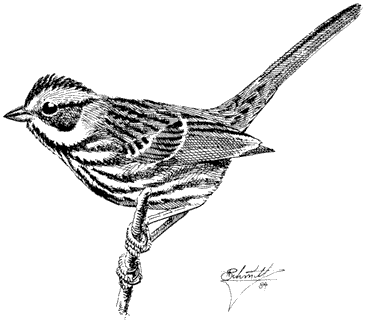
Figure 11.59
Song Sparrow.
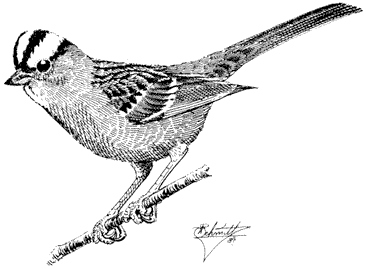
Figure 11.60
White-crowned Sparrow.
White-crowned Sparrow,Zonotrichia leucophrys. (Fig. 11.60) Male length 6 1/2 in (16.5 cm), female length 6 in. (15.5 cm); male weight 1 oz (30 g), female weight 9/10 oz (26.1 g). Common summer resident in the White Mountains, between 8,200 and 10,500 ft (2,500 and 3,200 m).
White-crowned Sparrows frequent high-altitude meadows where willow thickets, young or stunted pines, scattered aspen saplings, and wildflowers are clumped on the boggy ground. Because they favor places with running water, the headwaters of mountain streams are a good place to find the species. The adults are sleek and appear clean-cut. During the summer, they forage for insects on the damp ground, never far from cover. Nests are placed either on the ground under willows or other bushes, or a few feet above the ground in willows or small conifers. In the Subalpine Zone of the high Sierra Nevada, the volume of winter and spring snowfall drastically influences both the timing of summer breeding and the amount of space available for nesting. When the snowpack is heavy, fewer birds breed, and these individuals nest two weeks later than during normal years. Populations in the White Mountains presumably respond in a similar fashion. References: Morton (1978); Morton, Horstman, and Osborn (1972).
Dark-eyed (Oregon) Junco,Junco hyemalis. (Fig. 11.61) Male length 5 1/2 in (14.25 cm), female length 5 1/2 in (14 cm); male weight 3/5 oz (17 g), female weight 5/8 oz (18.3 g). Common summer resident in the White-Inyo Range, between 7,400 and 10,500 ft (2,260 and 3,200 m).
Gray-headed Junco,Junco caniceps. (Fig. 11.62) Male length 6 in (15.25 cm), female length 6 in (15 cm); male weight 5/8 oz (18.4 g), female weight 5/8 oz (18.8 g). Rare to relatively common summer resident in the White-Inyo Range, between 7,400 and 10,500 ft (2,260 and 3,200 m).
Because these two forms hybridize in the region, some authorities combine them into one species (Dark-eyed Junco, Junco hyemalis ). However, most individuals encountered in this area appear to be either pure parental types or very close to one species or the other; thus, we prefer to consider them as two full species. Furthermore, studies from several areas where both forms coexist have shown that each kind prefers to pair with a mate of similar appearance, further evidence that two distinct species are involved.
Juncos prefer cool and shady places where grass or forb cover provides a substrate for foraging and nesting. Thus, woodland of large pinyons on north- or east-facing slopes, riparian growth of willows and aspen, shady groves of large Mountain Mahogany, and Subalpine Forest of Bristlecone, Limber, and Lodgepole pines are all used. Openings in these habitats are favored. Most activities are concentrated on or near the ground. There juncos take insects and seeds and commonly conceal their nests under overhanging plants or turf. However, exposed twigs or tree tops at heights ranging from 10 to 50 ft (3 to 15 m) are chosen by males for song posts. The song is a ringing, musical trill. Juncos give their distinctive popping note when an intruder is
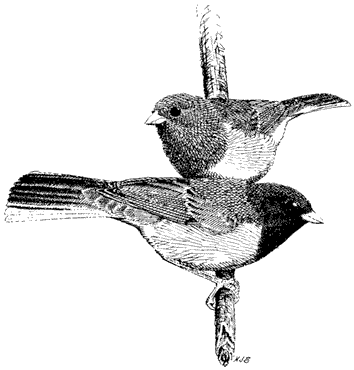
Figure 11.61
Dark-eyed (Oregon) Junco
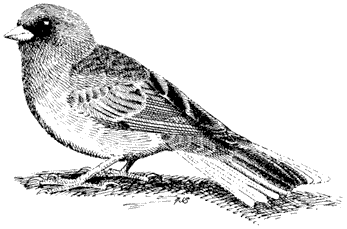
Figure 11.62
Gray-headed Junco.
near the nest, especially after the eggs have hatched. References: Balph (1977), Hadley (1969), A. Miller (1941).
Icterinae (Blackbirds, Orioles, and Allies)
Brewer's Blackbird,Euphagus cyanocephalus. (Plate 11.1) Male length 9 1/4 in (23.5 cm), female length 8 1/4 in (21 cm); male weight 2 5/8 oz (76 g), female weight 2 1/10 oz (59 g). Common summer visitant and local summer resident in the White-Inyo mountain region; recorded up to 9,500 ft (2,900 m) elevation.
This blackbird is a common resident in the vicinity of towns, ranches, and other centers of human activity. It nests in parks, hedgerows, windbreaks of ornamental trees, and riparian growth. In the White Mountains, the Brewer's Blackbird is most likely to be seen as a visitant near meadows and along streams. Wandering individuals may turn up in any kind of habitat and terrain, even at high elevations. References: La Rivers (1944), Williams (1952).
Brown-headed Cowbird,Molothrus ater . (Plate 11.1) Male length 6 3/4 in (17 cm), female length 6 1/4 in (15.75 cm); male weight 1 1/2 oz (41 g), female weight 1 3/10 oz (37 g). Fairly common summer resident in the White-Inyo Range; recorded from the lowland valleys up to 11,600 ft (3,540 m) elevation.
Being nest parasites with no fixed breeding territory, Brown-headed Cowbirds are most commonly seen on the move. The males wander widely when advertising for mates, stopping for several minutes in a treetop or on a power pole to display before departing. This display consists of a gurgling call uttered while the chest is expanded and the tail is spread. A similar liquid note given in flight usually announces the presence of this species. The Brown-headed Cowbird is typically recorded overhead in areas of Pinyon-juniper Woodland, riparian willows, and tall sagebrush. Streamside habitats are especially frequented; thus, the small birds of these environments suffer most from cowbird parasitism. Wandering birds commonly mingle with blackbirds around agricultural areas and pack stations, where they forage on the ground for insects, seeds, grass leaves, and gravel. They feed near piles of manure, prying it forward with their bills to uncover food items. Cowbirds drink water daily in arid regions. References: Scott and Ankney (1983), Verner and Ritter (1983).
Scott's Oriole,Icterus parisorum. (Fig. 11.63) Male length 7 1/2 in (19 cm), female length 7 1/4 in (18.5 cm); male weight 1 3/8 oz (38 g), female weight 1 1/2 oz (41 g). Summer resident in the White-Inyo Range; rare in the north, uncommon in the south.
This species occupies arid woodlands of pinyon, juniper, and, in the northern Mojave Desert, Joshua Tree. Because of their huge territories, the population densities of Scott's Orioles in the region are low. Males announce their positions with a far-reaching song of great beauty — rich, clear whistles reminiscent of the Western Meadowlark. Insect food is taken from the foliage of pinyon or yucca. Nests of these
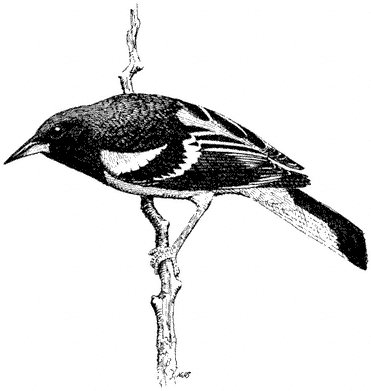
Figure 11.63
Scott's Oriole.
northern populations are commonly built in pinyons; more southerly populations nest in Joshua trees. References: Marshall (1957), Weathers (1983).
Fringillidae (Finches and Allies)
Rosy Finch,Leucosticte arctoa. (Fig. 11.64) Male length 6 in (15.5 cm), female length 6 in (15 cm); male weight 9/10 oz (26 g), female weight 7/8 oz (25 g). Fairly common summer resident in the White Mountains, from above timberline to the summit, at 14,246 ft (4,340 m) elevation. The species does not nest in the Inyo Mountains, where breeding habitat is lacking.
The Rosy Finch is the only species of bird strictly confined to the Alpine Zone in these mountains during the summer. These birds prefer to feed at the edges of snowfields and cirques, and in alpine rocklands or other areas with much exposed bare ground. Seeds and insects, even those frozen in snow, are taken. Strong fliers, Rosy Finches are highly mobile and forage over wide reaches of the Alpine Zone. Nests are placed in damp cracks below cliffs or in rock piles. In winter, flocks have been encountered in the pinyon-sagebrush zone near old mines. There they roost for the
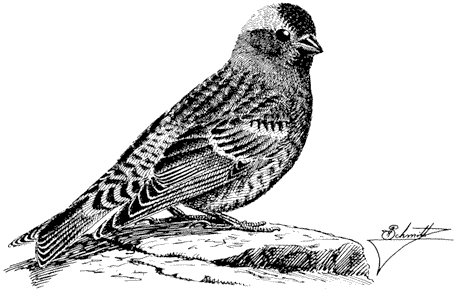
Figure 11.64
Gray-crowned Rosy Finch.
night in protected niches in the walls of abandoned vertical shafts. References: Grinnell and Storer (1924), Johnson (1965).
Cassin's Finch,Carpodacus cassinii . (Fig. 11.65) Male length 5 3/4 in (14.75 cm), female length 5 3/4 in (14.5 cm); male weight 1 oz (27.3 g), female weight 1 oz (26.6 g). Common summer resident in the White-Inyo Range, between 6,750 and 10,500 ft (2,060 and 3,200 m).
In the White and Inyo mountains, Cassin's Finches have been recorded most commonly in the aspen-willow association and in both Bristlecone and Lodgepole pines. They prefer open forest growth in cool, dry places. Locally, they may nest in cool portions of the pinyon zone, particularly where trees are large and mixed with Mountain Mahogany. This finch takes seeds from the ground and nips buds of aspen and conifers. Females of all ages and males up to approximately 14 months old are similar in appearance, with a streaked gray and brown plumage. Older males contrast strikingly, with their vivid hues of pinkish and rose-red. Studies in other areas have shown that the population size of Cassin's Finches fluctuates annually, presumably in response to the abundance of tree buds. Furthermore, males usually outnumber females. Like most true finches, this species demonstrates no real territorial system. Instead, the male defends the space around his mate wherever she goes rather than the area of the nest site. References: Mewaldt and King (1985), Samson (1976).
House Finch,Carpodacus mexicanus. (Fig. 11.66) Male length 5 1/4 in (13.25 cm), female length 5 in (13 cm); male weight 5/8 oz (18.4 g), female weight 5/8 oz
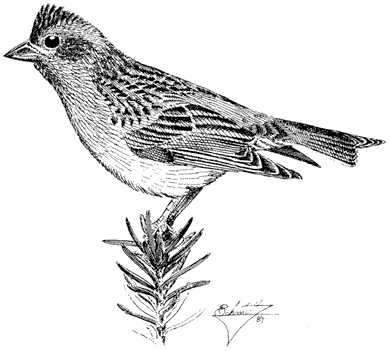
Figure 11.65
Cassin's Finch.
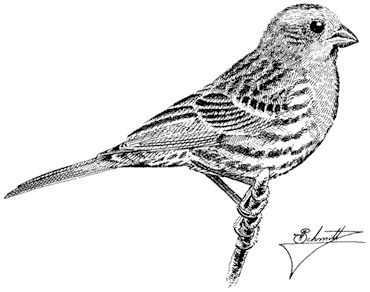
Figure 11.66
House Finch.
(19 g). Uncommon and local summer resident in the White-Inyo Range; occurs from the lowlands to 9,000 ft (2,740 m) elevation.
Although this species is common around lowland ranches and urban plantings, it is sparse in the mountains proper. Most records are from the pinyon-juniper belt. Springs are especially favored because they can fulfill the species' daily water requirements. Desert cactus fruits, when available, also provide some moisture. House Finches feed on a variety of seeds and small fruits. Nests are commonly placed in steep canyon walls or bluffs. Because they prefer warm and sunny environments, there is minimal overlap in the region between this species and its congener, the Cassin's Finch. References: Thompson (1960), Weathers (1983).
Red Crossbill,Loxia curvirostra. (Fig. 11.67) Male length 53/4 in (14.75 cm), female length 5 3/4 in (14.5 cm); male weight 1 1/8 oz (33 g), female weight 1 1/5 oz (34 g). Irregular permanent resident in the White-Inyo Range; recorded from 6,750 to 10,500 ft (2,060 to 3,200 m). Numbers fluctuate annually; usually the species is either uncommon or relatively common.
This erratic species has been recorded uncommonly from the ranges, where its presence and numbers depend on conifer seed production, which also varies annually.
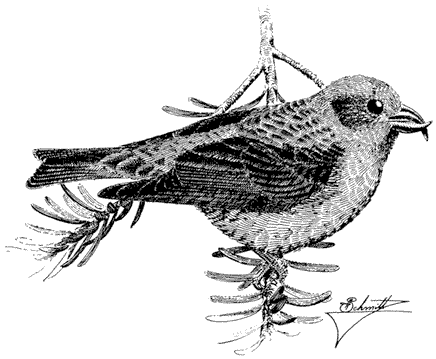
Figure 11.67
Red Crossbill.
Trees producing requisite seeds include Singleleaf Pinyon, Limber Pine, Bristlecone Pine, and Lodgepole Pine. Lodgepole Pine, an important food source for crossbills in the Sierra Nevada and the Cascade Mountains, is presumably of slight importance in the White-Inyo Range because of its very local occurrence there. This finch is usually first detected overhead by its kip-kip or jip-jip call notes. With good fortune, one may encounter a feeding group in a conifer top. The birds quietly work over terminal cones but then burst away without notice in a flurry of call notes. Breeding may occur in any season if seeds are abundant. In the White Mountains, family groups were recorded in the pinyon zone in early June. By the third week of June, these groups had coalesced into larger flocks. References: Beedy and Granholm (1985), Grinnell and Storer (1924).
References
American Ornithologists' Union. 1983. Check-list of North American birds , 6th ed. Allen Press, Lawrence, Kansas.
Anderson, Stanley H. 1976. Comparative food habits of Oregon Nuthatches. Northwest Science 50:213–221.
Armstrong, Joseph T. 1965. Breeding home range in the nighthawk and other birds; its evolutionary and ecological significance. Ecology 16:619–629.
Balda, Russell P., and Gary C. Bateman. 1973. The breeding biology of the Pinyon Jay. Living Bird 11:5–12.
Balgooyen, Thomas G. 1976. Behavior and ecology of the American Kestrel (Falco sparverius L.) in the Sierra Nevada of California. University of California in Zoology 103:1–83.
Balph, Martha H. 1977. Winter social behavior of Dark-eyed Juncos. Communication, social organization, and ecological implications. Animal Behaviour 25:859–884.
Beedy, Edward C., and Stephen L. Granholm. 1985. Discovering Sierra birds . Yosemite Natural History Association and Sequoia Natural History Association.
Behle, William H. 1942. Distribution and variation of the Horned Larks (Otocoris alpestris ) of western North America. University of California Publications in Zoology 46:205–316.
Bent, Arthur C. 1968. Life histories of North American cardinals, grosbeaks, buntings, towhees, finches, sparrows, and allies . U.S. National Museum Bulletin 237, Smithsonian Institution, Washington, D.C.
Biermann, Gloria C., and Spencer G. Sealy. 1982. Parental feeding of nestling Yellow Warblers in relation to brood size and prey availability: Auk 99:332–341.
Brown, Jerram L. 1964. The integration of agonistic behavior in the Steller's Jay, Cyanocitta stelleri (Gmelin). University of California Publications in Zoology 60:223–328.
Brown, Leslie, and Dean Amadon. 1968. Eagles. hawks, and falcons of the world . 2 vols. McGraw-Hill, New York.
Burton, John A. (ed.). 1973. Owls of the world . E. P. Dutton Inc., New York.
Caccamise, Donald. 1974. Competitive relationships of the Common and Lesser Nighthawks. Condor 76.1–20.
Cade, Thomas J. 1982. Falcons of the world . Cornell University Press, Ithaca, N.Y.
Christensen, Glen C. 1954. The Chukar Partridge in Nevada . Nevada fish and Game Commission Biological Bulletin no. 1, State Printing Office, Carson City, Nevada.
Davis, Cheyleen M. 1979. A nesting study of the Brown Creeper. Living Bird 17:237–263.
Davis, Deborah. 1979. Morning and evening roosts of Turkey Vultures at Malheur Refuge, Oregon. Western Birds 10:125–130.
Davis, John. 1960. Nesting behavior of the Rufous-sided Towhee in coastal California. Condor 62:434–456.
Dilger, William C. 1956. Hostile behavior and reproductive isolating mechanisms in the avian genera Catharus and Hylccichla . Auk 73:313–353.
Dixon, Keith L. 1949. Behavior of the Plato Titmouse. Condor 51:110–136.
Dixon, Keith L. 1965. Dominance-subordination relationships in Mountain Chickadees. Condor 67:291–299.
Earhart, Caroline M., and Ned K. Johnson. 1970. Size dimorphism and food habits of North American owls. Condor 72:251–264.
Emlen, Stephen T., James D. Rising, and William L. Thompson. 1975. A behavioral and morphological study of sympatry in the Indigo and Lazuli Buntings of the Great Plains. Wilson Bulletin 87:145–179.
Ervin, Stephen. 1977. Flock size, composition, and behavior in a population of Bushtits. Bird-Banding 48:97–109.
Fitch, Henry S. 1947. Predation by owls in the Sierran foothills of California. Condor 49:137–151.
Fitch, Henry S., Freeman Swenson, and Daniel F. Tillotson. 1946. Behavior and food habits of the Red-tailed Hawk. Condor 48:205–237.
Franzreb, Kathleen E. 1984. Foraging habits of Ruby-crowned and Golden-crowned Kinglets in an Arizona montane forest. Condor 86:139–145.
Garrett, Kimball, and Jon Dunn. 1981. The birds of southern California . Los Angeles Audubon Society, Los Angeles.
Goodwin, Derek. 1976. Crows of the world . Cornell University Press, Ithaca, N.Y.
Grinnell, Joseph, and Alden H. Miller. 1944. The distribution of the birds of California . Pacific Coast Avifauna no. 27, Cooper Ornithological Club, Berkeley, California.
Grinnell, Joseph, and Tracy I. Storer. 1924. Animal life in the Yosemite . University of California Press, Berkeley.
Griscom, Ludlow, and Alexander Sprunt, Jr. 1957. The warblers of America . Devin-Adair, New York.
Gutiérrez, Ralph J. 1980. Comparative ecology of the Mountain and California Quail in the Carmel Valley, California. Living Bird 18:71–93.
Hadley, N. F. 1969. Breeding biology of the Gray-headed Junco, Junco caniceps (Woodhouse) in the Colorado Front Range. Colorado Field Ornithologist 5:15–21.
Heckenlively, Donald B. 1970. Song in a population of Black-throated Sparrows. Condor 72:24–36.
Howell, J. C. 1942. Notes on the nesting habits of the American Robin. American Midland Naturalist 28:529–603.
Hubbard, John P. 1969. The relationships and evolution of the Dendroica coronata complex. Auk 86:393–432.
Hutto, Richard L. 1981. Seasonal variation in the foraging behavior of some migratory western wood warblers. Auk 93:765–777.
Jaeger, Edmund C. 1948. Does the Poor-will "hibernate"? Condor 50:45–46.
James, Frances C., and Hank H. Shugart, Jr. 1974. The phenology of the nesting season of the American Robin (Turdus migratorius ) in the United States. Condor 76: 159–168.
James, Ross D. 1981. Factors affecting variation in the primary song of North American Solitary Vireos (Aves: Vireonidae). Canadian Journal of Zoology 59:2001–2009.
Johnsgard, Paul A. 1973. Grouse and quails of North America . University of Nebraska Press, Lincoln.
Johnsgard, Paul A. 1975. North American game birds of upland and shoreline . University of Nebraska Press, Lincoln.
Johnsgard, Paul A. 1983. The hummingbirds of North America . Smithsonian Institution Press, Washington, D.C.
Johnson, David H., Monroe D. Bryant, and Alden H. Miller. 1948. Vertebrate animals of the Providence Mountains area of California. University of California Publications in Zoology 48:221–376.
Johnson, Ned K. 1963. Biosystematics of sibling species of flycatchers in the Empidonax hammondii-oberholseri-wrightii complex. University of California Publications in Zoology 66:79–238.
Johnson, Ned K. 1966. Bill size and the question of competition in allopatric and sympatric populations of Dusky and Gray Flycatchers. Systematic Zoology 15:70–87.
Johnson, Ned K. 1976. Breeding distribution of Nashville and Virginia's Warblers. Auk 93:219–230.
Johnson, Ned K., and Carla Cicero. 1986. Richness and distribution of montane avifaunas in the White-Inyo region. California. In C. A. Hall, Jr. and D. J. Young (eds.). Natural history of the White-Inyo Range, eastern California and Western Nevada, and high altitude physiology , pp. 137–159. University of California White Mountain Research Station Symposium, August 23–25, 1985, Bishop, Calif., vol. 1.
Johnson, Ned K., and Carla B. Johnson. 1985. Speciation in sapsuckers (Sphyrapicus ): II. Sympatry, hybridization, and mate preference in S. ruber daggetti and S. nuchalis . Auk 102:1–15.
Johnson, Richard E. 1965. Reproductive activities of Rosy Finches, with special reference to Montana. Auk 82:190–205.
Kendeigh, S. Charles. 1941. Territorial and mating behavior of the House Wren . Illinios Biological Monograph no. 18, University of Illinois Press, Urbana.
Kilham, Lawrence. 1972. Reproductive behavior of White-breasted Nuthatches. II. Courtship Auk 89:115–129.
Kilham, Lawrence. 1973. Reproductive behavior of the Red-breasted Nuthatch. I. Courtship. Auk 90:597–609.
Kroodsma, Donald E. 1971. Song variations and staging behavior in the Rufous-sided Towhee, Pipilo erythrophthalmus oregonus. Condor 73:303–308.
Kroodsma, Donald E. 1972. Variations in songs of Vesper Sparrows in Oregon. Wilson Bulletin 84:173–178.
Kroodsma, Donald E. 1975. Song patterning in the Rock Wren. Condor 77:294–303.
La Rivers, Ira. 1944. Observations on the nesting mortality of the Brewer Blackbird, Euphagus cyanocephalus. American Midland Naturalist 32:417–437.
Leopold, A. Starker. 1977. The California Quail . University of California Press, Berkeley.
Marshall, Joe T., Jr. 1957. Birds of Pine-oak Woodland in southern Arizona and adjacent Mexico . Pacific Coast Avifauna no. 32, Cooper Ornithological Society, Berkeley, California.
Marti, Carl D. 1976. A review of prey selection by the Long-eared Owl. Condor 78:331–336.
Martin, Dennis J. 1977. Songs of the Fox Sparrow. I. Structure of song and its comparison with song in other Embenzidae. Condor 79:209–221.
Martin, Dennis J. 1979. Songs of the Fox Sparrow. II. Intra- and interpopulation variation. Condor 81:173–184.
McEllin, Shaun M. 1979. Nest sites and population demographics of White-breasted and Pygmy Nuthatches in Colorado. Condor 81:348–352.
Mengel, Robert M. 1964. The probable history of species formation in some northern wood warblers (Parulidae). Living Bird 3:9–43.
Mewaldt, L. Richard, and James R. King. 1985. Breeding site faithfulness, reproductive biology, and adult survivorship in an isolated population of Cassin's Finches. Condor 87:494–510.
Miller, Alden H. in 1941. Speciation in the avian genus Junco. University of California Publications in Zoology 44:173–434.
Miller, Alden H. 1951. An analysis of the distribution of the birds of California. University of California Publications in Zoology 50:531–644.
Miller, Alden H., and Robert C. Stebbins. 1964. The lives of desert animals in Joshua Tree National Monument . University of California Press, Berkeley.
Miller, Edwin V. 1941. Behavior of the Bewick Wren. Condor 43:81–99.
Moldenhauer, Ralph R., and John A. Wiens. 1970. The water economy of the Sage Sparrow, Amphispiza belli nevadensis. Condor 72:265–275.
Morrison, Michael L. 1981. The structure of western warbler assemblages: Analysis of foraging behavior and habitat selection in Oregon. Auk 98:578–588.
Morrison, Michael L. 1982. The structure of western warbler assemblages: Ecomorphological analysis of the Black-throated Gray and Hermit Warblers. Auk 99:503–513.
Morse, Douglass H. 1971. Effects of the arrival of a new species upon habitat utilization by two forest thrushes in Maine. Wilson Bulletin 83:57–65.
Morse, Pamela J. 1979. Pairing and courtship in the North American dipper. Bird-Banding 50:62–65.
Morton, Martin L. 1978. Snow conditions and the onset of breeding in the Mountain White-crowned Sparrow. Condor 80:285–289.
Morton, Martin L., Judith L. Horstman, and Janet M. Osborn. 1972. Reproductive cycle and nesting success of the Mountain White-crowned Sparrow (Zonotrichia leucophrys oriantha ) in the central Sierra Nevada. Condor 74:152–163.
Nice, Margaret M. 1937 (reprinted 1964). Studies in the life history of the Song Sparrow . 2 vols. Dover, New York.
Norris, Robert A. 1958. Comparative biosystematics and life history of the nuthatches Sitta pygmaea and Sitta pusilla . University of California Publications in Zoology 56:119–300.
Olendorff, Richard R. 1975. Golden Eagle Country . Knopf, New York.
Petrides, George A. 1938. A life history of the Yellow-breasted Chat. Wilson Bulletin 50: 184–189.
Power, Harry W. 1966. Biology of the Mountain Bluebird in Montana. Condor 68:351–371.
Power, Harry W. 1980. The foraging behavior of Mountain Bluebirds with emphasis on sexual foraging differences . Ornithological Monographs no. 28, American Ornithologists' Union, Washington, D.C.
Price, Frank E., and Carl E. Bock. 1983. Population ecology of the Dipper (Cinclus mexicanus) in the Front Range of Colorado . Studies in Avian Biology no. 7, Allen Press, Lawrence, Kansas.
Rea, Amadeo M. 1983. Once a river . University of Arizona Press, Tucson.
Reynolds, John D., and Richard W. Knapton. 1984. Nest site selection and breeding biology of the Chipping Sparrow. Wilson Bulletin 96:488–493.
Reynolds, Richard T., and E. Charles Meslow. 1984. Partitioning of food and niche characteristics of coexisting Accipiter during breeding. Auk 101:761–779.
Reynolds, Timothy D. 1981. Nesting of the Sage Thrasher, Sage Sparrow, and Brewer's Sparrow in southeastern Idaho. Condor 83:61–64.
Reynolds, Timothy D., and Terrell D. Rich. 1978. Reproductive ecology of the Sage Thrasher (Oreoscoptes montanus ) on the Snake River Plain in south-central Idaho. Auk 95:580–582.
Ritter, Lyman V. 1983. Nesting ecology of Scrub Jays in Chico, California. Western Birds 14:147–158.
Root, Richard B. 1967. The niche exploitation pattern of the Blue-gray Gnatcatcher. Ecological Monographs 37:317–350.
Root, Richard B. 1969. The behavior and reproductive success of the Blue-gray Gnatcatcher. Condor 71:16–31.
Salomonson, Michael G., and Russell P. Balda. 1977. Winter territoriality of Townsend's Solitaires (Myasdestes townsendi ) in a Pinyon-Juniper-Ponderosa Pine ecotone. Condor 79:148–161.
Samson, Fred B. 1976. Territory, breeding density, and fall departure in Cassin's Finch. Auk 93:477–497.
Scott David M., and C. Davison Ankney. 1983. The laying cycle of Brown-headed Cowbirds: Passerine chickens? Auk 100:583–592.
Shedd, Douglas H. 1982. Seasonal variation and function of mobbing and related antipredator behaviors of the American Robin (Turdus migratorius ). Auk 99:342–346.
Short, Lester L. 1965. Hybridazation in the flickers (Colaptes ) of North America. Bulletin of the American Museum of Natural History 129:307–428.
Short, Lester L. 1971. Systematics and behavior of some North American woodpeckers, genus Picoides (Aves). Bulletin of the American Museum of Natural History 145:1–118.
Short, Lester L. 1982. Woodpeckers of the world . Monographs, Delaware Museum of Natural History, Greenville.
Smith James N. M., Yoram Yom-Tov, and Richard Moses. 1982. Polygyny, male parental care, and sex ratio in Song Sparrows: An experimental study. Auk 99:555–564.
Snyder, Noel F. R., and James W. Wiley. 1976. Sexual size dimorphism in hawks and owls of North America . Ornithological Monographs no. 20, American Ornithologists Union, Washington, D.C.
Thompson, Charles F. 1977. Experimental removal and replacement of territorial male Yellow-breasted Chats. Auk 94:107–113.
Thompson, William L. 1960. Agonistic behavior in the House Finch. Part II: Factors in aggressiveness and sociality. Condor 62:378–402.
Thompson, William L. 1976. Vocalizations of the Lazuli Bunting. Condor 78:195–207.
Tomback, Diana F. 1978a. Foraging strategies of Clark's Nutcracker. Living Bird 16:123–161.
Tomback, Diana F. 1978b. Pre-roosting flight of the Clark's Nutcracker. Auk 95:554–562.
Verbeek, Nicolaas A. M. 1967. Breeding biology and ecology of the Horned Lark in alpine tundra. Wilson Bulletin 79:208–218.
Verner, Jared, and Lyman V. Ritter. 1983. Current status of the Brown-headed Cowbird in the Sierra National Forest. Auk 100:355–368.
Walsberg, Glenn E. 1981. Nest-site selection and the radiative environment of the Warbling Vireo. Condor 83:86–88.
Weathers, Wesley W. 1983. Birds of Southern California's Deep Canyon . University of California Press, Berkeley.
Weston, Henry G., Jr. 1947. Breeding behavior of the Black-headed Grosbeak. Condor 49:54–73.
Wiens, John A. 1982. Song pattern variation in the Sage Sparrow (Amphispiza belli ): Dialects or epiphenomena? Auk 99:208–229.
Wiley, R. Haven. 1973. Territoriality and non-random mating in Sage Grouse, Centrocercus urophasianus. Animal Behaviour Monographs 6:87–169.
Williams, Laidlaw. 1952. Breeding behavior of the Brewer Blackbird. Condor 54:3–47.
12—
Mammals
Hannah V. Carey and John D. Wehausen
Approximately 60 species of mammals occur in the White Mountains. The diversity of mammalian species in this mountain range is low compared with lower-elevation areas in the temperate zone. This paucity of species reflects the island-like conditions characteristic of montane regions. The dry valleys on either side of the range present major barriers to colonization for many species, particularly those adapted to high-elevation habitats.
The distinguishing feature of this group of vertebrates is the presence of mammary tissue in females, which provides milk to help the young grow quickly. High metabolic rates enable mammals to maintain high and relatively invariant body temperatures. This allows them to inhabit a wide range of environmental conditions but is costly in terms of energy requirements. Such energy requirements are difficult or impossible to meet at higher elevations during the cold season. Mammals inhabiting these elevations in the White Mountains have evolved a variety of solutions to this problem. Some abandon their high body temperatures by hibernating (e.g., marmots and ground squirrels), thus regulating body temperature at a lower setpoint. Some subsist entirely on stored food through the winter, such as the Pika, or use it as a supplement to what they obtain through foraging in winter, as is the case with some mice. Some, such as deer and Mountain Sheep, migrate to lower elevations. Others simply endure the harsh conditions by subsisting on the limited available food. Such species include the White-tailed Hare, which feeds mostly where wind or sun has cleared snow, and shrews and mice, which can forage beneath the snow.
Insectivores (Order Insectivora)
Shrews,Sorex. Although shrews are active both day and night and are relatively abundant in some areas, they are rarely seen. Their high metabolic rate and small size require continual foraging, especially in cold weather, when body temperature is more difficult to maintain. Their diet includes insects, snails, earthworms, and spiders. Owls and Stellar Jays are known to feed on shrews, but many predators appear to avoid them. The White Mountains are home to three species of shrews.
Water Shrew,S. palustris. (Fig. 12.1) Head and body about 3.3 in (8.4 cm), tail 2.5–3 in (6.3–7.6 cm); black, frosted with gray-tipped hairs dorsally, underparts whitish and tinged with brown; tail bicolored, 70–105% of total body length; hind feet with a conspicuous fringe of hairs along the sides and toes. Water Shrews live near edges of clear, cold streams up to 11,600 ft (3,540 m). The stiff, thick hairs
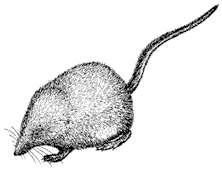
Figure 12.1
Water Shrew.
on the hind feet are effectively used as paddles to propel this relatively large shrew through rushing streams. Thick body fur helps protect this shrew from icy waters as it swims below the surface in search of tadpoles, minnows, and aquatic insects.
Merriam Shrew,S. merriami. Head and body 2.2–2.5 in (5.7–6.3 cm); tail 1.5 in (3.8cm); upper parts light brownish grey, underparts white. The Merriam Shrew has been reported up to 9,500 ft (2,896 m) in the Pinyon-juniper Woodland and in Cottonwood Creek basin. This is the only species of Sorex to occur regularly in arid habitats, far from streams or meadows.
Inyo Shrew,S. tennellus. Body length 2.4 in (6.1 cm), tail about 1.6 in (4 cm); upper surface dark gray, underparts pale, smoky gray. This shrew occurs along streams up to 9,500 ft (2,896 m).
Bats (Order Chiroptera)
Bats are the only mammals capable of true flight. This is accomplished by modification of the long bones of the arm and hand into a wing. Also, the hind limbs are rotated such that the knee joint bends backward instead of forward. The small eyes of the bat contribute little to visual orientation; rather, bats have well-developed ears that allow them to use echolocation for orientation as well as for hunting insects in flight. All species of bats that occur in the White Mountains are insectivorous. Two survival mechanisms are exhibited by bats during the winter, when insects are absent. Some species migrate to more favorable regions, returning months later to their summer ranges. Other species hibernate during the winter to minimize energy expenditure when food is scarce. The following bat species are known from records to occur in the White Mountains: Long-eared Myotis (Myotis evotis) , Hairy-winged Myotis (Myotis volans) , Small-footed Myotis (Myotis subulatus) , Western Pipistrelle (Pipistrellus hesperus) , and Big Brown Bat (Eptesicus fuscus) . Species that are likely to occur at least seasonally in the White Mountains include: Little Brown Myotis (Myotis lucifugus) (Fig. 12.2), Yuma Myotis (Myotis yumanensis) , Fringed Myotis (Myotis thysanoides) , California Myotis (Myotis californicus) , Leib's Myotis (Myotis Leibii) , Hoary
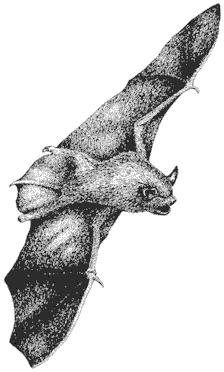
Figure 12.2
Bat: Little Brown Myotis.
Bat (Lasiurus cinereus) , Silvery-haired Bat (Lasionycteris noctivagans) , Pallid Bat (Antrozous pallidus) , Brazilian Free-tailed Bat (Tadarida brasiliensis) , and Western Big-eared Bat (Plecotus townsendii) .
Pikas, Rabbits, and Hares (Order Lagomorpha)
Pika,Ochotona princeps. (Fig. 12.3) Body length 6.2–8.5 in (15.8–21.6 cm); no visible tail; ears round, much shorter than head, with white edges; fur grayish buff to dark brown. Pikas range from about 8,200 ft (2,500 m) to White Mountain Peak summit, at 14,246 ft (4,340 m), but are most common in the upper elevations above timberline. Like the Yellow-bellied Marmot, they prefer to live on talus slopes bordering alpine meadows, where they forage on grasses and forbs. Pikas may be seen carrying food in their mouths from feeding areas to their burrows among the talus; there they stack the food into "haypiles" for subsistence during the winter. Pikas and their relatives, rabbits and hares, are active year-round. Pikas can be located by listening for their distinctive eenk-eenk call. Pika calls may signal approaching predators or represent territorial and mating displays. An annual molt produces a mottled coloration of the fur. Pikas are eaten by weasels, hawks, falcons, Coyotes,

Figure 12.3
Pika carrying vegetation for haypiles.
and other predators. Litter size ranges from one to five, and young are born naked with eyes closed in May or June.
Black-tailed Hare,Lepus californicus . (Fig. 12.4) Head and body 18–25 in (46–63 cm), tail 2–4.4 in (5.0–11.2 cm); ears 3.9–5.7 in (10–14.5 cm); weight 3–7 lb (1.4–3.2 kg); upper surface gray, nearly white beneath; ears with blackish tips; upper side of tail black, running up onto rump, buff gray below. The Black-tailed Hare, also known as the Black-tailed Jackrabbit, ranges from the floor of Owens Valley to about 12,500 ft (3,811 m) but is most common in open, brushy vegetation below
Figure 12.4
Black-tailed Hare.
the Pinyon-juniper Woodland. Black-tailed Hares eat a variety of herbs and shrubs and have wide habitat preferences. Predators include Coyotes, eagles, hawks, owls, and snakes. An annual molt takes place in late summer or early fall. The new coat is dark grayish brown and thick; with time the fur lightens and becomes thinner, which aids in heat dissipation in hot weather. Black-tailed Hares can be distinguished from White-tailed Hares in areas where they co-occur by their smaller size and the brownish underside of the tail (the tail of the White-tailed Hare is white). As many as seven young can be born to a litter.
White-tailed Hare,Lepus townsendii . (Fig. 12.5) Head and body 22–26 in (56–65 cm), tail 2.6–4.3 in (6.6–10.9 cm); ears 3.7–4.8 in (9.5–12.2 cm); weight 5–10 lb (2.3–4.5 kg); grayish brown in summer, white in winter; tail white. White-tailed Hares (or Jackrabbits) range from about 10,000 ft (3,050 m) up to the summit of White Mountain Peak (14,246 ft, 4,340 m). They are common among sagebrush in open areas and feed on grasses and shrubs. This hare undergoes two annual molts, turning white in winter. This most likely aids in making it less conspicuous to its predators, which include Coyotes, Bobcats, owls, and eagles. One may approach a White-tailed Hare quite easily during the summer months without being aware of it, for its gray-brown coat blends in well with the surrounding rocks and shrubs of the Alpine Zone. As with all hares, the young are born fully furred with the eyes open. Litters can contain eight young and are produced once per year.
Nuttall Cottontail,Sylvilagus nuttalli . (Fig. 12.6) Head and body 13.8–15.4 in (35–39 cm), tail 1.6–2 in (4–5 cm); ears 2.1–2.5 in (5.4–6.4 cm); weight 1.2–1.8 lb (0.5–0.8 kg); grayish brown above, white below; tail hairs white to roots; feet densely covered with long hairs. Also known as the Mountain Cottontail, this rabbit frequents the dense cover of brush in the Pinyon-juniper Woodland and in Sagebrush Scrub. It occurs up to 11,000 ft (3,354 m) and feeds on currants, willows, junipers, sagebrush, and grasses. Predators include Bobcats, hawks, owls, and Coyotes. In
Figure 12.5
White-tailed Hare.

Figure 12.6
Nuttall Cottontail.
contrast to the hares, the young of rabbits are born naked with the eyes closed. Litter size is about eight.
Rodents (Order Rodentia)
Marmots, Ground Squirrels, and Chipmunks (Family Sciuridae)
Yellow-bellied Marmot,Marmota flaviventris. (Fig. 12.7) Head and body 14–19 in (36–48 cm); tail 4.5–9 in (11–23 cm); weight 5–10 lb (2.3–4.6 kg); upper surface yellowish brown with white-tipped hairs, underside yellow; white band below eyes. Marmots occur from 9,000 ft (2,744 m) to 13,500 ft (4,115 m) elevation. They inhabit rocky terrain near vegetated areas, preferably alpine meadows bordering talus slopes. White Mountain marmots hibernate from September to late April. During the active season they forage on grasses, sedges, and forbs, storing body fat for overwinter sustenance. When not feeding, marmots usually bask in the sun on rocks, which also serve as refuges from predators. Sharp alarm whistles are given when a predator, such as a Coyote or Golden Eagle, is suspected to be nearby. Yellow-bellied Marmots live in colonies of related adult females and their young. Juveniles are born in early June and first emerge aboveground in early July. Litter size averages five to six.
Golden-mantled Ground Squirrel,Spermophilus lateralis. (Fig. 12.8) Head and body 6–8 in (15–20 cm), tail 2.5–4.8 in (6–12 cm); head and shoulders golden; one white stripe bordered by two long black stripes on each side of back. Golden-
Figure 12.7
Yellow-bellied Marmot.

Figure 12.8
Golden-mantled Ground Squirrel.
mantled Ground Squirrels occur from about 7,000 ft (2,130 m) to 14,000 ft (4,270 m) in most habitats within this range. Their diet consists primarily of seeds, nuts, and fruits. In winter these hibernators subsist on body fat stored during the summer months, and may also eat food cached in their burrows during periodic arousals. Hawks, eagles, Coyotes, and weasels are their main predators. Young appear above-ground in July; litter size is two to six.
Antelope Ground Squirrel,Ammospermophilus leucurus. (Fig. 12.9) Head and body 5.5–6.5 in (14–16.5 cm), tail 2–3 in (5–7.6 cm); grayish brown with one stripe on each side and no dark stripes; tail usually held closely over the back while running, exposing white underside. This fast-moving desert ground squirrel occurs from the floor of Owens Valley to 8,300 ft (2,530 m) in the White Mountains. Whereas most other rodents that live in such hot and arid environments are nocturnal, the Antelope Ground Squirrel is day-active and avoids overheating by allowing its body temperature to rise up to 109°F (43°C) — higher than that of any other nonsweating mammal. Once it reaches this high body temperature, the Antelope Ground Squirrel cools itself by going into an underground burrow, where it dissipates the excess body heat. Highly efficient kidneys and evaporative cooling by fur licking also help this rodent maintain body temperature and water balance in a hot environment. Its diet consists mainly of seeds and fruits, but some animal matter is also eaten. Litter size is six to eight.

Figure 12.9
Antelope Ground Squirrel.
Chipmunks,Eutamias . The chipmunk has alternate dark and light stripes on the body that extend to the head, in contrast to ground squirrels, which lack head stripes. Sides are yellowish to reddish brown, with the undersurface whitish. There are three species of chipmunks in the White Mountains. Identification of individual species requires careful observation of physical characteristics as well as locality and habitat type.
Sagebrush Chipmunk, E. minimus . Head and body 3.7–4.5 in (9.3–11.4 cm), tail 3–4.5 in (7.6–11.4 cm); short, grayish fur with contrasting stripes — dorsal stripe black, outer light stripe white, all stripes extending to base of tail; tail held vertically when moving, yellowish underneath. Occurs from 7,000 ft (2,134 m) to at least 11,800 ft (3,598 m) in sagebrush, mixed Pinyon-juniper Woodland, and Alpine Fell-field habitats.
Panamint Chipmunk, E. panamintinus . Head and body 4.5–4.7 in (11.4–11.9 cm), tail 3.5–4 in (8.9–10.2 cm); crown of head noticeably gray; shoulders and back reddish; outer dark stripes, below the outer white stripes, are inconspicuous; other dorsal dark stripes are reddish or grayish. Occurs above 5,900 ft (1,800 m) to 9,500 ft (2,900 m) in Pinyon-juniper Woodland.
Uinta Chipmunk, E. umbrinus . (Fig. 12.10) Head and body 4.5–5 in (11.4–12.7 cm), tail 3.5–4.6 in (8.9–11.7 cm); back of neck and crown grayish; outer light stripes pure white, dorsal stripes well demarcated, outer dark stripes indistinct. Distribution restricted in the White Mountains to the Bristlecone Pine Forest, 9,500–11,500 ft (2,900–3,510 m).
Chipmunks store large caches of seeds and fruits in their burrows for sustenance during the winter. They may hibernate in some years, but their periods of torpor are not as predictable as those of ground squirrels and marmots. Chipmunks also eat insects and fungi and are themselves eaten by hawks, weasels, Coyotes, snakes, and other carnivores. Litter size is three to six, and young are generally active aboveground by June or July.

Figure 12.10
Uinta Chipmunk.
Pocket Gophers (Family Geomyidae)
Pocket Gophers,Thomomys. Head and body 4.8–7 in (12–18 cm), tail 2–3.8 in (5–9.5 cm); yellowish brown-gray fur; head small and flattened; neck short; shoulders and forearms broad and muscular; eyes small; fur-lined internal cheek pouches on either side of mouth. Two species of pocket gophers inhabit the White Mountains: the Northern Pocket Gopher (T. talpoides ), which ranges from the floor of Owens Valley to 8,200 ft (2,500 m); and the Valley Pocket Gopher (T. bottae ) (Fig. 12.11), occurring from the valley floor to about 10,500 ft (3,200 m). Preferred habitat for both is around meadows in soft soil, rarely in densely wooded or rocky areas. Pocket Gophers are strictly herbivorous, feeding on a wide range of foods including roots and succulent vegetation. The long, curved claws and chisel-like incisors are used to excavate tunnels below the ground surface. Gopher activity is apparent from the characteristic fan-shaped pile of soil marked by concentric rings where soil has been pushed up from below. Predators include owls, hawks, Badgers, Coyotes, and weasels. Young are born in the spring, and number three to six to a litter.
Pocket Mice, and Kangaroo Rats and Mice (Family Heteromyidae)
Pocket Mice,Perognathus. Head and body 2.5–4.8 in (6.4–12.2 cm), tail 3.2–4.8 in (8.1–12.2 cm); upper surface yellowish brown, white below; ears small; an external fur-lined pouch at each side of mouth; forelegs and feet small, hind feet and tail long; hind feet without hair. Two species of these nocturnal rodents are present in the White Mountains. The Long-tailed Pocket Mouse (P. formosus ) (Fig. 12.12) ranges up to 7,100 ft (2,165 m) and is common in rocky areas. It is distinguished by its bicolored tail that is heavily crested with a tuft at its end and darker than the rest of the body. The Great Basin Pocket Mouse (P. parvus ) occurs in sagebrush and Pinyon-juniper Woodland up to about 8,300 ft (2,530 m). Pocket Mice are quadrupedal except for isolated bipedal leaps away from danger. Their cheek pouches serve to transport seeds to underground food caches. During times of extreme weather conditions or food scarcity, Pocket Mice go into torpor, (i.e., lower their body temperature, heart

Figure 12.11
Valley Pocket Gopher.

Figure 12.12
Long-tailed Pocket Mouse.
rate, and metabolic rate to conserve energy). Their main predators include owls and Coyotes. Litter size is two to six, and young are born in June or July.
Kangaroo Rats,Dipodomys. Head and body 4–5 in (10.2–12.7 cm), tail 5–7.3 in (12.7–18.5 cm); body yellowish brown above, whitish below; ears small; external cheek pouches; hind legs large; tail always longer than head and body, has dark dorsal and ventral longitudinal bands with lateral longitudinal stripes and is tufted with long hairs. The White Mountains contain three species of kangaroo rats: Ord's Kangaroo Rat (D. ordii ) (Fig. 12.13) ranges up to 6,500 ft (1,982 m); Merriam's Kangaroo Rat (D. merriami ) occurs up to 7,200 ft (2,195 m); and the largest of the three species, the Great Basin Kangaroo Rat (D. microps) , has been reported up to 7,100 ft (2,165 m). Kangroo Rats are bipedal, foraging at night mostly on seeds. The Great Basin Kangroo Rat is also known as Chisel-tooth, because it uses its flattened incisors to shave off the salty outer layers of saltbush (Atriplex ) leaves before consumption. Kangaroo Rats are noted for their highly developed water conservation ability. Water is derived solely from oxidation of carbohydrates, fats, and protein and is conserved by kidneys that are able to dispose of wastes with an extremely small output of water. Their large auditory chambers help to alert them to attack by owls and Coyotes, their main predators.

Figure 12.13
Ord's Kangaroo Rat.
Mice, Rats, Lemmings, and Voles (Family Cricetidae)
Western Harvest Mouse,Reithrodontomys megalotis. Head and body 2.8–3 in (7.1–7.6 cm), tail 2.3–3.2 in (5.8–8.1 cm); upper parts and sides buffy, underparts grayish brown; tail indistinctly bicolored; no external cheek pouches. Western Harvest Mice have been reported up to 6,700 ft (2,040 m) in the White Mountains, inhabiting damp grassy meadows and marshy areas, but they may occur in other habitats during population peaks. They eat mainly seeds and fruits, with some insects, and they are preyed upon by owls, snakes, and a number of mammalian predators. They commonly use the runways of meadow mice (Microtus spp.) during their nightly foraging periods. Litters are produced several times per year and average four to six young.
Deer Mice,Peromyscus. There are three species of Deer Mice present in the White Mountains. They can be distinguished from other mice by several characteristics, including their large, membranous ears, lack of external cheek pouches, and a tail exceeding 70% of the head and body length. Their diets consist of seeds, fruits, and insects, particularly butterfly and moth larvae. They are active year-round and store caches of food for winter consumption. Deer Mice are preyed upon by many avian and mammalian predators.
Pinyon Mouse, P. truei . Head and body 3.6–4 in (9.1–10.2 cm), tail 3.4–4.8 in (8.6–12.2 cm); brown to dark brown above, creamy white underneath; feet whitish; tail bicolored, whitish below, length exceeds 90% of head and body length; ears very large. Pinyon Mice have been documented up to 9,500 ft (2,896 m) and are common in the Pinyon-juniper Woodland, where they feed on juniper berries and Pinyon Pine nuts. Much of their time is spent in trees.
Deer Mouse, P. maniculatis . (Fig. 12.14) Head and body 2.8–4 in (7.1–10.2 cm), tail 2–5 in (5–12.7 cm); yellowish brown to grayish above, pure white below; feet white; bicolored tail usually less than 90% of head and body length. The Deer Mouse, probably the most common mammal in North America, has the greatest elevational range of any mammal in California. The same subspecies of Deer Mouse that lives on the floor of Death Valley also occurs on White Mountain Peak (14,246 ft, 4,340 m). Deer Mice live in nearly all habitat types.

Figure 12.14
Deer Mouse.
Canyon Mouse, P. crinitus . Head and body 3–3.4 in (7.6–8.6 cm), tail 3.5–4.3 in (8.9–10.9 cm). Fur long and soft, pale yellowish buff above and creamy white below; long hairs on tip of tail; feet white; tail usually longer than the head and body, with a broad, light brown dorsal stripe, creamy white below. Canyon Mice frequent rocky areas up to 8,250 ft (2,515 m) in the White Mountains.
Southern Grasshopper Mouse,Onychomys torridus. Head and body 3.5–4 in (8.9–10.2 cm), tail 1.6–2 in (4–5 cm); light cinnamon above, white below. The Grasshopper Mouse has been found up to 5,000 ft (1,524 m), most commonly in canyons. The diet is 90% animal matter, most of which is arthropods. These nocturnal rodents emit high–pitched calls and usually nest in burrows dug by other mammals. Predators include snakes, weasels, owls, Bobcats, and other carnivores.
Wood Rats,Neotoma. The scaly tail of the largely nocturnal Wood Rat is covered by overlying hairs. Wood Rats eat mostly vegetation and are preyed upon by Coyotes, foxes, owls and large snakes. Litter size is three to four, and young are born in the spring. There are two species of wood rats present in the White Mountains.
Desert Wood Rat, N. lepida . Head and body 5.8–7 in (14.7–17.8 cm), tail 4.3–6.4 in (10.9–16.3 cm); fur grayish, mixed with black hairs above, underparts white or buffy; tail hairs short. Also known as the Pack Rat, this species occurs up to 8,000 ft (2,440 m) in Sagebrush Scrub and Pinyon-juniper Woodland and frequents rocky areas in these regions. It uses cactus for water and nesting material.
Bushy-tailed Wood Rat, N. cinerea . (Fig. 12.15) Head and body 7–9.4 in (17.8–23.9 cm), tail 5.2–7.4 in (13.2–18.8 cm); fur cinnamon brown to buffy, some tipped with black above; white or grayish below; tail with hairs over 2 cm long. Bushy-tailed Wood Rats have been recorded up to 10,300 ft (3,140 m) in the White Mountains; however, a single individual was collected from the summit hut on White Mountain Peak (14,246 ft, 4,340 m) during the summer of 1983. Whether this is an accurate representation of their usual range in these mountains is uncertain.
Figure 12.15
Bushy-tailed Wood Rat.
Sagebrush Vole,Lagurus curtatus. Head and body 3.8–4.5 in (9.6–11.4 cm), tail 0.6–1.1 in (1.5–2.8 cm); fur long and fine, light grayish above and almost white below. Sagebrush Voles occur up to 12,500 ft (3,810 m) in Sagebrush Scrub and Pinyon-juniper Woodland. Similar to meadow voles, they are active both day and night. They feed on nearly anything green and do not require additional drinking water. This species is endemic to the Great Basin. Litters average five young.
Meadow voles,Microtus. There are three species of meadow mice or voles in the White Mountains. All are active both day and night and are preyed upon by hawks, owls, falcons, Coyotes, weasels, and snakes. They feed on stems and leaves of forbs and grasses. The litter size usually ranges from five to eight young.
Montane Vole, M. montanus . Head and body 4–5.5 in. (10.2–14 cm), tail 1.2–2.6 in (3–6.6 cm); fur dark brown, some reddish tinged; undersurface dark gray; tail usually less than one-third of total body length, nearly unicolored. The Montane Vole occurs up to at least 12,500 ft (3,810 m), commonly in alpine meadows or other high-elevation grassy areas. It builds conspicuous runways through grasses and sedges, and it tunnels beneath the snow during the winter. It feeds chiefly on succulent stems and leaves of forbs, rarely grasses.
Long-tailed Meadow Vole, M. longicaudus . (Fig. 12.16) Head and body 4.5–5.3 in (11.4–13.5 cm), tail 2–3.5 in (5–8.9 cm); dorsal band of reddish brown fur with grayish sides and bluish gray underparts; tail usually more than one-third of total body length and more or less bicolored. The Long-tailed Meadow Vole is common along streams and in wet meadows in higher elevations up to 11,500 ft (3,510 m). Unlike the Montane Vole, it does not construct extensive runways.
California Meadow Vole, M. californicus . Head and body 4.6–5.7 in (11.7–14.5 cm), tail 1.6–2.8 in (4–7.1 cm); buffy or dark brown above, commonly with a reddish tinge on the middle of the back; undersurface blue-gray to white; ears project well above fur. This species has been recorded up to 4,500 ft (1,370 m) in Silver Canyon, but occurs mostly along the Owens River.

Figure 12.16
Long-tailed Meadow Vole.
Beavers (Family Castoridae)
Beaver,Castor canadensis. Beavers were introduced to the east side of the White Mountains in the Cottonwood Creek area about 1950. Environmental damage caused by beavers led to an apparently successful program to remove them in the mid-1970s. Remnants of their dams may still be visible on some creeks.
Porcupines (Family Erethizontidae)
Porcupine,Erethizon dorsatum. (Fig. 12.17) Head and body 18–22 in (46–56 cm), tail 7–9 in (18–23 cm); weight usually 9–15 lb (4–7 kg); yellowish to blackish above; stiff quills and spines, especially on rump and tail. Porcupines exhibit a distinct habitat and diet shift with season. In winter they inhabit wooded areas, where they consume tree bark; a variety of habitats and foods are used in summer. During the summer they may range up to 13,500 ft (4,115 m), where they forage on flowers and leaves of alpine forbs. The spines of these slow-moving rodents are actually modified hairs that are loosely attached to the body. When under attack by a Mountain Lion, Bobcat, or Coyote, a slap of the tail is enough to drive the quills into the predator.
Carnivores (Order Carnivora)
Coyote,Canis latrans. (Fig. 12.18) As large as a medium-sized dog, with long, bushy tail; 3–5 ft (1–1.5 m) long, including tail; adults 20–30 lb (9–14 kg); pelage nonuniform in color due to color banding of the hairs, usually appearing grayish with reddish tint, the red commonly more noticeable around the erect ears and neck. Coyotes are distributed throughout the White Mountains at all seasons. Although they are primarily predators of rodents, hares, and rabbits, their diet may include berries, grasshoppers, and carrion. Coyotes generally pose little threat to adult Mule Deer, Mountain Sheep, Pronghorn Antelope, and Tule Elk, but they can prey on the young of these species. Most Coyotes in the White Mountains occur singly or in family groups of a mother with young pups. Most hunting occurs at dawn and dusk,

Figure 12.17
Porcupine.
Figure 12.18
Coyote.
but it is nevertheless common to observe Coyotes during the day. Young are born in the spring.
Gray Fox,Urocyon cinereoargenteus. (Fig. 12.19) As large as a small dog, head and body about 2 ft (60 cm), tail about 1 ft (30 cm) long; weight 9–11 lb (4–5 kg); pelage pepper and salt gray with black on tip of long, bushy tail and tips of prominent, erect, pointed ears. The Gray Fox is uncommon in the White Mountains, probably distributed mostly at middle to low elevations on both sides of the range. Its diet
Figure 12.19
Gray Fox.
includes rodents, hares, rabbits, birds, insects, and fruit, depending on availability. Young are born in spring, usually three to five in a litter.
Ringtail,Bassariscus astutus. (Fig. 12.20) Head and body length 14–17 in (36–41 cm), tail 15 in (38 cm); weight 2–2.5 lb (0.9–1.1 kg); body pelage tan, somewhat lighter beneath, with black-tipped hairs on back; dark eyes conspicuously ringed with white; light spot in front of each ear. A small, very agile relative of the raccoon, with a long, bushy, banded tail, pointed face, and prominent, erect ears, Ringtails commonly have been called the Miner's Cat (although not a cat at all) due to their propensity to take up residence in and around cabins. Ringtails occur in canyons of the White Mountains, living mostly near water. They are very nocturnal, and thus rarely seen by people. Their diet is highly varied, including small mammals, birds, reptiles, carrion, insects, spiders, and fruit. Litters are usually born in late spring.
Marten,Martes americana. About the size of a house cat, but longer and narrower, with bushy tail and sharply pointed face; head and body 14–17 in (36–43 cm), tail 7–9 in (18–23 cm); weight 1–2.5 lb (0.4–1.1 kg); pelage golden brown with yellowish patch on throat and chest. The Marten is apparently a rare inhabitant of timberline coniferous forests, probably occcuring mostly in Limber Pine on the east side of the White Mountains. An opportunistic predator that can climb trees, it preys on birds and small mammals as large as hares but also eats berries and carrion when available. The Marten commonly hunts beneath the snow in winter and is largely solitary. Young are born in spring and litter size is generally two to four.
Wolverine,Gulo luscus. A large, stocky member of the weasel family; head and body 29–32 in (74–81 cm), tail 7–9 in (18–23 cm); weight 35–60 lb (16–27 kg); shaggy, blackish brown pelage, with pale stripe from shoulder to rump on each side, joining at the base of bushy tail; pale patch from eye to ear on either side of head. The Wolverine is a rare inhabitant of the White Mountains, reported on only three

Figure 12.20
Ringtail.

Figure 12.21
Badger.
occasions in over 30 years. Its range is probably the crest and east side of the range. Wolverines are known to eat virtually any animal matter, including carrion, and will eat berries in summer. Young are born in late winter or spring, usually numbering two to four.
Badger,Taxidea taxus. (Fig. 12.21) Head and body 18–22 in (46–56 cm), tail 4–6 in (10–15 cm); weight 13–25 lb (6–11 kg); stature broad and low such that the body fur appears to drag on the ground; pelage yellowish brown to silver gray, with white stripe down middle beginning at nose and sometimes extending to base of the tail; face with upturned snout and black and white patterning. Badgers are distributed throughout the White Mountains, up to at least 12,000 ft (3,660 m). Their feet and legs are adapted for digging out burrowing animals, which are its main food (especially ground squirrels). Insects and other terrestrial animals are eaten opportunistically, but vegetative matter is virtually absent from the diet. Litters of one to five are born in spring.
Long-tailed Weasel,Mustela frenata . (Fig. 12.22) Head and body 8–10.5 in (20–27 cm), tail 4–6 in (10–15 cm); weight less than 0.75 lb (0.34 kg); pelage whitish underneath and brown above, with black on tip of tail; upper pelage usually changes to white in winter in snowy environments. A small, voracious carnivore, whose long and slender body is adapted for hunting small animals in burrows and rocks, this weasel is distributed throughout the White Mountains. Long-tailed Weasels have been recorded as high as 12,500 ft (3,810 m) but probably occur higher. Their diet

Figure 12.22
Long-tailed Weasel.
consists mostly of small mammals but may include insects, reptiles, birds, and eggs. Litters of six to nine are born in spring.
Skunks. Two skunk species inhabit the White Mountains. The Striped Skunk (Mephitis mephitis ) (Fig. 12.23) is about the size of a house cat, with a conspicuous bushy tail, weighing 6–14 lb (2.7–6.4 kg). It is glossy black with two conspicuous white stripes on either side of its back that meet at the neck. The Spotted Skunk (Spilogale putorius) (Fig. 12.24) is considerably smaller, weighing 0.8–2.5 lb (0.4–1.1 kg). Its black pelage is broken by numerous white stripes and spots, including a white tip on the tail. Striped Skunks occur up to about 7,000 ft (2,130 m) and Spotted Skunks to 10,500 ft (3,200 m) in the White Mountains. Both species consume a highly varied diet consisting of invertebrates, small vertebrates, bird eggs, and some fruit, nuts, grains, and roots. Young are generally born during May for both species, with litters of three to six for Spotted Skunks and four to eight for Striped Skunks.
Mountain Lion,Felis concolor. (Fig. 12.25) A large cat with a long tail; head and body 42–54 in (107–137 cm), tail 30–36 in (76–91 cm); adults 80–150 lb (36–68 kg); pelage tawny with white underside, black on back of ears and on tip of long, prominent (but not bushy) tail; kittens spotted with banded tail. The primary food of Mountain Lions is deer, which they hunt by stalking and ambushing. Hares

Figure 12.23
Striped Skunk.
Figure 12.24
Spotted Skunk.
may be eaten if locally abundant, and other ungulates, such as Mountain Sheep and Tule Elk, are generally hunted only where their ranges overlap the deer range. Mountain Lions occur throughout the White Mountains where Mule Deer are present. Their dependence on concealment for hunting keeps them mostly in canyons, where vegetation and rocks meet this need. They are extremely shy of humans and hence rarely seen. They usually occur singly or as a mother with young. Young can be born any month of the year, and litter size is generally two to four.
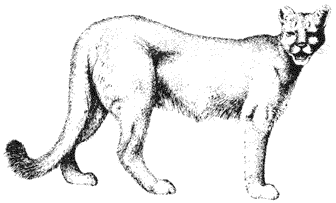
Figure 12.25
Mountain Lion.
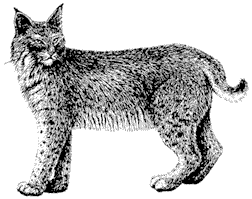
Figure 12.26
Bobcat.
Bobcat,Lynx rufus. (Fig. 12.26) About twice the size of a house cat with proportionately longer legs, especially rear legs, and a short tail; head and body 25–30 in (63–76 cm), tail 5 in (13 cm); weight 15–35 lb (7–16 kg); pelage yellowish to reddish brown and variously streaked and spotted; tail with dark bands toward the tip; prominent ears pointed with tufts of black hair at the tips. Like Coyotes, Bobcats feed mostly on rodents, hares, and rabbits, occasionally catching larger prey. They differ from Coyotes in their method of hunting, using only stealth to stalk and ambush, never running down prey. Consequently, like their relatives the Mountain Lions, Bobcats mostly inhabit areas where they can hide. They occur throughout the White Mountains but rarely use the high, open slopes where the visitor might hope to see one. Bobcats are generally solitary, except when a female is accompanied by kittens. Litter size is usually three to five. Young are generally born in spring and summer.
Even-Toed Ungulates (Order Artiodactyla)
Tule Elk,Cervus elaphus. (Fig. 12.27) Large, standing well over 3 ft (1 m) at the shoulders; adult males up to about 700 lb (320 kg), females about three-quarters the weight of males; pelage light reddish brown with dark brown neck and large, cream-colored rump patch; males carry antlers that are cast and regrown each year and may become very massive with numerous branches in prime adults. Tule Elk are native only west of the crest of the Sierra Nevada in the foothills, Great Valley, and Coast Ranges. They were introduced to Owens Valley in 1933 and 1934 in an attempt to establish a large, free-roaming population. At the time, this subspecies (nannodes ) numbered fewer than 300, and most were in enclosures. The Owens Valley population now extends from Owens Lake to Laws and numbers about 500. The west slope of the White Mountains is used by Tule Elk up to about 8,000 ft (2,440 m) in the region from the Westgard Pass Road to just south of Silver Canyon. This area is used largely in winter and spring and includes calving grounds.
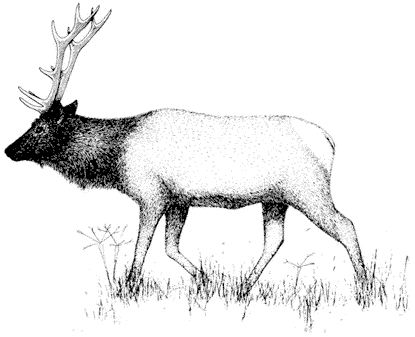
Figure 12.27
Tule Elk.
Mule Deer,Odocoileus hemionus. (Fig. 12.28) Shoulder height about 3 ft (91 cm); weight about 100 lb (45 kg) for females and 130 lb (59 kg) for males; gray-brown pelage with small white rump patch covered by tail; conspicuous, large ears; males carry antlers that increase in length and degree of branching with body size and are cast and regrown each year. Mule deer are widely distributed in the White Mountains. Similar to Mountain Sheep, they migrate seasonally up and down the mountain to obtain the best yearly diet, occurring as high as 14,000 ft. (4,270 m) in summer. They are long-legged and fleet and escape predators by outrunning them. Habitats used by Mule Deer include the open Desert Scrub and alpine communities, as well as the more closed Pinyon Pine, Limber Pine, Bristlecone Pine, and Mountain Mohogany belts in the middle elevations. Common to all habitats is topography that allows fleetness in escaping predators. Consequently, Mule Deer rarely are present on the steep, rocky slopes preferred by Mountain Sheep.
Pronghorn Antelope,Antilocapra americana. (Fig. 12.29) Shoulder height about 33 in (85 cm): weight about 75 lb (34 kg) for females and 110 lb (50 kg) for males; pelage rusty brown on back, with conspicuous white belly, rump patch, and neck stripes; females with very short, inconspicuous (some lacking) horns; males with black horns rarely exceeding 16 in (40 cm) with a short, forward-protruding branch in the middle and hooked tips; horn sheaths are cast and regrown each year in males, unlike
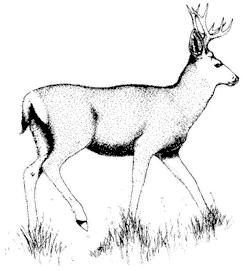
Figure 12.28
Mule Deer.
Figure 12.29
Pronghorn Antelope.
any other horned mammal in the world. Pronghorn Antelopes were once common residents of valleys surrounding the White Mountains. They probably used the lower slopes of the range in spring and migrated to high sagebrush flats in summer. An attempt beginning in 1982 to reintroduce these antelopes to Adobe Valley northwest of Benton has resulted in populations that winter at the base of the White Mountains from Hammil Valley to Queen Valley; occasionally they have been observed high in the range in summer.
Mountain Sheep,Ovis canadensis. (Fig. 12.30) Shoulder height about 36 in. (91 cm); maximum weight about 140 lb (64 kg) for females and 200 lb (91 kg) for males; gray pelage with large, conspicuous white rump patch; females with short, narrow horns that curve back and outward toward tips; adult males with massive horns that grow back and then curve forward to form an arc. Mountain (Bighorn) Sheep are usually found in rugged topography due to their need for steep rocks to escape predators. They also use less rocky terrain if visibility is good and safe rocks are within running distance. Consequently, Mountain Sheep range is usually discontinuous. Where possible, Mountain Sheep exhibit seasonal altitudinal migration in order to

Figure 12.30
Mountain Sheep.
feed on the greenest, most nutritious forage. In the White Mountains they range in elevation from 5,700 ft (1,740 m) to 14,000 ft (4,270 m). Their main predator in the White Mountains is the Mountain Lion, with Coyotes and Bobcats possible predators on lambs. Historically, Mountain Sheep occurred in scattered localities in the southern White Mountains, including Wyman Canyon and the Cottonwood Creek basin, and all west-side canyons from White Mountain Peak to Montgomery Peak in the northern half of the range. Loss of part of this range was probably due to diseases introduced from domestic livestock. Today, Mountain Sheep remain only in the most rugged and inaccessible portion of their original range, from White Mountain Peak to Montgomery Peak. These canyons become less steep and rocky as they approach the crest of the range. Consequently, Mountain Sheep are uneasy and very flighty when feeding high in the mountains in summer. They usually flee into the canyons at such great distances from people that few untrained observers ever see them. There is concern that increased human use north of White Mountain Peak in summer might cause serious displacement of this species from its nutritious summer range.
Odd-Toed Ungulates (Order Perissodactyla)
Wild Horses,Equus caballus. Like Tule Elk, Wild Horses are a nonnative species. Their origin, however, is unintentional, stemming from escaped domestic horses. Their range encompasses the canyons on the east side of the White Mountains from around Cottonwood Creek to the northern end of the range. They range in elevation from the alluvial fans of Fish Lake Valley to high alpine flats, such as Pellisier Flats. A U.S. Forest Service policy to regulate their numbers has been only partially implemented. There is concern over the potentially detrimental impact these large animals may have on fragile alpine ecosystems. Wild Horses live in year-round harems, with bachelor males living in separate groups until dominant enough to obtain a harem.