13.5—
Actions of Ethylene
13.5.1—
Introduction
Ethylene is the simplest hormone which regulates plant growth (Fig. 13.7). It is a natural constituent of plants and affects a wide array of physiological processes. For many years, however, investigations of ethylene physiology were concerned primarily with fruit ripening. With the advent of gas chromatography many experimenters have begun research on the biochemical and physiological action of ethylene. The production of ethylene by germinating seeds and seedlings suggests that the hormone may be involved in the normal regulation of growth and development. Evidence for such a proposal includes the fact that low concentrations of applied ethylene blocks photo-induced apical bud expansion and hook opening in etiolated pea seedlings. It was shown that apical tissues of
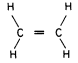
Figure 13.7
Structure of ethylene.
etiolated seedlings are the major site of ethylene production. Light has been found to decrease the tendency of pea stem segments to produce ethylene in response to high concentration of auxin.
On the other hand, red light appears to stimulate ethylene production in dormancy. Another important role of endogenous ethylene in etiolated seedlings is the regulation of radial expansion of the pea epicotyl in a region below the apical hook. The exposure of the plant to red light or to CO2 inhibits an ethylene-mediated increase in the diameter of the epicotyl.
Growth, flowering, abscission and fruit ripening all are affected by ethylene. It is currently popular to speculate that the mode of action of ethylene involves a mechanism which regulates some aspect of the transcription of DNA or the translation of RNA. Studies of the effects of ethylene on abscission and growth indicate sizeable changes in RNA and protein contents. There is indeed evidence of changes in the activities of catalase, peroxidase, and other hydrolases. Furthermore a study showed that exposure of soybean plants to ethylene significantly altered RNA polymerase activity associated with chromatin. As judged by nearest neighbour analysis the RNA produced from ethylene treated plants has a different base composition than that from the control. Even though ethylene at very low concentrations affects a wide array of physiological and biochemical processes in plants, much more work is required to know the mechanism of action.
13.5.2—
Effect of Ethylene on Enzymes
A number of investigators have examined the possibility that ethylene has a direct effect on enzyme activity (Abeles, 1973). However, investigations with b -glucosidase, emulsin, a -amylase, invertase, peroxidase, and adenosine triphosphatase have shown that ethylene has no effect on these enzymes. Results reported by Abeles (1973) have similarly shown that ethylene has no effect on cellulase and carbonic anhydrase. Carbonic anhydrase was chosen because it appeared to be a likely candidate to show a positive response. Carbonic anhydrase contains zinc and has the ability to combine with CO2 , both features which suggest potential sensitivities for ethylene action. Nelson (1939) reported that ethylene increased the activity of trypsin. However, this effect was thought to be due to the removal of O2 since H2 had the same effect.
13.5.3—
Actions on Membranes
Since ethylene is more soluble in oil than in water and since membranes contain large amounts of lipid material, a number of investigators have tested the idea that ethylene affects some aspect of membrane permeability. However, proponents of this idea have failed to note that CO, an ethylene analogue, does not share the same lipid solubilities as ethylene but nevertheless has similar physiological activities. The idea that ethylene has a disruptive effect on the membrane
causing a change in permeability and an alteration in compartmentalization does not appear valid. Ripening fruits exhibit obvious changes in terms of permeability and retention of soluble components and it seems natural to suggest that ethylene leads to changes in membrane permeability which in turn causes softening and increased respiration. However, current evidence suggests that a change in membrane properties is an effect of ripening rather than a cause.
A similar situation exists in flowers. Nichols (1968) pointed out that solute leakage increased from carnations during senescence. Senescence and leakage were promoted by ethylene and reversed by CO2 . Ethylene has no influence on membrane permeability of potato, pea, avocado, banana, and bean. However, on the other hand, von Guttenberg and Beythien (1951) reported that ethylene increased the rate of deplasmolysis of
13.5.4—
Enzyme Induction by Ethylene
Regeimbal and Harvey (1927) were the first to report that ethylene-treated tissues contained higher levels of particular enzymes than the control. They found that ethylene increased the level of protease and invertase extracted from pineapple fruits. Since that time, reports (see Abeles, 1973) have appeared which show effects of ethylene on a number of enzymes. This list includes acid phosphatase, ATPase, a -amylase, cellulase, chitinase, chlorophyllase, cinnamic acid 4-hydroxylase, cytochrome c reductase, diaphorase, b -1,3-glucanase, invertase, malic enzyme, pectin esterase, peroxidase, phenylalanine ammonia-lyase, polygalacturonase, polyphenyloxidase, protease and pyruvic carboxylase.
Enzyme induction does not always depend entirely on the action of ethylene. In some cases, cutting the tissue causes an increase in enzyme activity. The function of ethylene is to reduce the lag time or increase the rate of increase in enzyme activity. Examples of enzymes whose increased activity does not strictly depend on ethylene action include b -1,3-glucanase, malic enzyme, phenylalanine
ammonia-lyase and peroxidase (Abeles, 1973). Excising tissue can cause wound ethylene production and it is possible that this source of ethylene plays some role in enzyme induction in tissue slices. On the other hand, enzyme induction during abscission was dependent on ethylene and for a reasonable period of time no cellulase synthesis or abscission occurred following excision unless ethylene was added to the gaseous phase.
13.5.5—
Effects of Ethylene on RNA Synthesis
The first report of ethylene effects on RNA synthesis was by Turkova et al. (1965) who reported an increase in RNA synthesis during epinasty of tomato leaves. Whether or not RNA synthesis was required for epinasty was not shown, although the idea is intriguing. Studies with actinomycin D indicated that RNA synthesis occurred during abscission and was required for the process to occur (Abeles & Holm, 1966). Support for this interpretation stems from the work of a number of investigators (see Abeles, 1973). It is known that the increase in RNA synthesis precedes that in protein synthesis and is localized at or near the separation layer. The increase in RNA occurred in all fractions including messenger-RNA, ribosomal-RNA and transfer-RNA. Differential extraction of the nucleic acids indicated that the ethylene-stimulated fraction was confined to that portion of RNA extracted by sodium lauryl sulphate with the increase found in ribosomal- and messenger-RNAs. The inhibitor, 5-fluorouracil, which blocks 50% of the ethylene-enhanced incorporation of 32 P into RNA did not inhibit abscission. The greatest inhibition occurred in transfer-RNA and ribosomal-RNA which indicates that the synthesis of all fractions of RNA is not required for abscission. Presumably as long as messenger-RNA was being synthesized, enough ribosomal- and transfer-RNAs were already available within the cell to permit abscission to take place. When all RNA synthesis was blocked by actinomycin D, abscission stopped (Holm & Abeles, 1967). Ethylene has also been found to promote RNA synthesis in preclimacteric fruit. Marei and Romani (1971) reported, as in the case of abscission, that the synthesis of all classes of RNA in fig fruit was stimulated. Holm et al. (1971) found that ethylene increased RNA synthesis in apples and that the increase in RNA was followed by an increase in protein synthesis. However, Sacher and Salminen (1969) reported that they failed to find an increase in RNA synthesis when preclimacteric bananas or avocados were treated with ethylene.
13.5.6—
Effects of Ethylene on Chromatin Activity
Holm et al. (1970) have reported that ethylene inhibited the growth of the apical part of soybean seedlings and caused an increase in the elongating and basal portions of the stem. At the same time, RNA levels in the apical zone were reduced while the levels were enhanced in the elongating and basal regions. Chromatin from the various parts of the seedling were studied to determine if the
capacity for RNA synthesis was also modified. They found that the activity in the apex was reduced while the activity in the elongating and basal regions were promoted. The rate of the response was rapid since the increase in chromatin activity was apparent after 3 hours. Nearest-neighbour analysis of the RNA synthesized demonstrated that there was a qualitative difference between RNA synthesized from chromatins of normal and ethylene-treated tissue. It was concluded that ethylene can regulate RNA synthesis as manifested by a change in quantity and kind of RNA.
13.5.7—
Effects of Ethylene on DNA Metabolism
Plant growth is either promoted, inhibited or unaffected by ethylene depending upon the tissue involved. Examples of growth promotion are swelling, epinasty, hook closure, seedling elongation and seed germination (Abeles, 1973). Bud break is probably a special case. Here no growth takes place as long as ethylene is present. However, after the gas is removed growth of the buds ensues. Growth inhibition is seen as arrested development of buds, leaves or apical meristems. Mature tissue, such as stems and leaves. do not undergo any change of size or weight, although premature senescence usually occurs. Since growth, or the lack of it, may be associated with cytokinesis, it is of interest to learn that DNA synthesis is controlled by ethylene. Holm and Abeles (1967) reported that DNA synthesis or DNA content in bean leaf tissue was not affected by seven hours exposure to ethylene, although abscission was promoted. Later they found that in soybean seedlings treated with ethylene the synthesis of DNA was inhibited in the apex where growth was inhibited but was promoted in the subapical part where swelling took place. Burg et al. (1971) found a similar situation in pea seedlings in which inhibition of cell division, measured as a reduction in metaphase figures, occurred within two hours after ethylene was added. However, it is not clear whether the change in DNA synthesis was the cause or the result of inhibited growth. Ethylene slows the growth of pea seedlings very quickly; for example, Warner and Leopold (1971) found growth slowed six minutes after ethylene was introduced into the gas phase and returned to normal sixteen minutes after the ethylene was removed. Kinetic studies on changes in DNA or other postulated sites of action are thus required to establish the relationship between cause and effect. Burg et al. (1971) have suggested that ethylene regulates DNA synthesis by some action on microtubule structure essential for spindle fibre formation during mitosis. If the action of ethylene was directed toward microtubules, this might also explain the reorientation of cellulose microfibril deposition that occurs in swelling.
13.5.8—
Summary
Even though little is known about the binding site(s) of ethylene, in a few cases such as ripening, abscission, swelling and senescence there is reason to believe
that the combination of ethylene with a site results in the regulation of protein or enzyme synthesis which in turn accounts for the observed responses. In these cases RNA synthesis, presumably messenger-RNA, with the accompanying support of soluble- and ribosomal-RNA, is an essential or early step suggesting regulation of gene action. However, in other cases, especially those associated with the physiology of excised tissue, control is probably not exerted at the level of RNA or protein synthesis. In the case of the inhibition of elongation, the action is apparently directed toward blockage of DNA metabolism. The site of action of ethylene in epinasty, root initiation, intumescence formation and floral initiation is even more poorly understood. The only valid conclusion appears to be that a number of essential features of plant growth and development are susceptible to ethylene action. In the final analysis it is concluded that there may be as many mechanisms of ethylene action as there are modes of ethylene operation.