13.4.5—
Model for Cytokinin Action
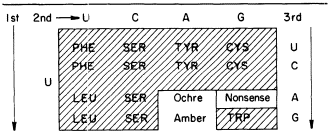
Cytokinins are found in transfer-RNAs of animals, microorganisms and plants (see Cherry, 1973). Furthermore, in those cases where the nucleotide sequence is known the native cytokinin, IPA (isopentenyladenosine), or a modified form, is found adjacent to the anticodon. At the moment it appears that all IPA containing species of tRNA recognize codon triplets which begin with U (Skoog & Armstrong 1970). Thus, the anticodon loop of a cytokinin-containing RNA could be one of at least sixteen codon sequences of the following type: A— —IPA. By eliminating the nonsense terminator and the phenylalanine codons, it seems that IPA-containing tRNAs would be confined to less than ten codons (see Table 13.1).
|
It may be that IPA adjacent to the anticodon of these tRNA species plays some role in messenger-RNA-tRNA-ribosome recognition, and that these tRNA species are required for the translation of some messenger-RNAs into specific proteins. Based on these assumptions it is possible to speculate on the mechanism of action of cytokinins in plants. From the observation that cytokinins appear not to be incorporated into tRNA, as precursor units, but rather the isopentenyl group comes from mevalonic acid (Chen & Hall, 1969), it seems likely that cytokinins have no effect on the synthesis of tRNA containing IPA. Furthermore, a number of chemicals, including various isomers of IPA, kinetin, 6-benzyladenine and even diphenylurea, have cytokinin biological activities, and it appears that these cytokinins do not participate in tRNA synthesis. If this is true, then what is the mechanism of cytokinin action?
A model presented in Fig. 13.6 is based on the speculation that specific
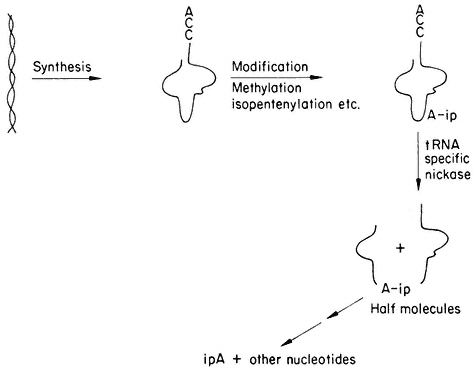
Figure 13.6
A model of cytokinin action. Based on the fact that tRNA specific nucleases
are known, it is suggested that free cytokinins protect cytokinin-containing
tRNAs by inhibiting nuclease activity, specific as well as general.
nucleases break the primary structure of the IPA containing tRNA (Cherry & Anderson, 1971). The IPA would provide a unique attachment for the enzyme to bind to and subsequently to break the phosphodiester bonds. The action of this enzyme would destroy the function of the tRNA and yield free cytokinin. Cytokinins including 6-benzyladenine and diphenylurea added exogenously to plants or tissue cultures would defer senescence by essentially protecting the cytokinin-containing tRNA species. This protection would be mediated by the exogenously added cytokinin binding to the nuclease and competitively inhibiting its action. Alternatively, the cytokinin could bind to other agents which in turn prevent an increase in nuclease activity which would destroy the cytokinin-containing tRNA. Therefore, tissue treated with cytokinin would retain its IPA-containing tRNA species and continue to synthesize essential proteins as long as sufficient free cytokinin was present within the tissue.
Preliminary unpublished work of Locy (1974) provides suggestive evidence of a tRNA-specific nuclease. We have found that chloroplasts of soybeans contain a nuclease which specifically degrades one of the three tyrosyl-tRNAs. At the moment we do not have any evidence to suggest that free cytokinin controls the activity of this tRNA nuclease. In regards to this model it is to be emphasized that it is hypothetical and is presented as a working base upon which to investigate further the mechanism of action of cytokinins at the molecular level.