3—
Radiobiology
Mice and Men
By the time the danger from neutron rays was appreciated, high-energy x rays no longer held promise for cancer therapy. The result obtained by Lauritsen and Packard of Columbia's Institute of Cancer Research in 1931—that 550 kV rays had no more deleterious effect on Drosophila eggs or the common mouse tumor "sarcoma 180" than an equal quantity of 50 kV rays—had been substantiated and extended. Lawrence had conceded to Wood that the 1 MV Sloan plants probably would have no greater curative properties than standard x-ray apparatus, and experience at the University's Medical School fully confirmed the concession.[98] Hence the possible medical value of neutron therapy held unusual interest for Lawrence both for itself and as a replacement for a played-out technology. A principal objective of the very first experiments with neutron irradiation was to compare its biological effects with those of x rays.
John Lawrence and his technical advisor, Ernest Lawrence, who in July 1935 became "consulting physicist" to the Medical School, exposed $120 worth of rats near the beryllium target of the 27-inch cyclotron and at the Sloan machine in San Francisco. The neutrons appeared to be about ten times as effective as x rays per roentgen in altering the makeup of rodent blood, or five times as effective per unit of ionization since (they estimated) a roentgen of neutrons made twice the ionization in rat tissue that a roentgen of x rays did. Since the standard tolerable limit of x rays was 0.1 r/day, they recommended prudently that the maximum for n rays be 0.01 r/day.[99] While the Lawrences zapped rats, Aebersold and Raymond E. Zirkle, a medical physicist visiting from the
[98] Packard and Lauritsen, Science, 73 (1931), 321–2; J.H. Lawrence to Ridenour, 6 Feb 1940 (14/5). This was to go beyond Tuve, Radiology, 20 (1933), 289–33, talk of Dec 1931, who showed that the penetrating power of 2,000 kV x rays in water should not much exceed that of 200 kV rays, but would draw "no biologic conclusions."
[99] Lawrence to Chauncey Leake, 10 Jul 1935, and reply, 12 Jul (20/6); Lawrence and Lawrence, NAS, Proc., 22 (Feb 1936), 126–7, 133; Lawrence to Poillon, 2 Mar 1936 (15/17), itemizing the costs of John Lawrence's summer experiments: $120 for mice, $150 for a technician, $312 for travel and expenses.
University of Pennsylvania, tried the effects of the radiations on delaying the growth of wheat seedlings. Here one roentgen of neutrons did the damage of 20 r of x rays. Would neutrons prove ten or twenty times as effective as x rays in other biological contexts? "The general question is of more than theoretical interest, for it bears directly on the possibility of using very fast neutrons in the treatment of tumors."[100]
John Lawrence returned to Berkeley early in February 1936 to take up the general question. With the help of Aebersold, the Lawrence brothers cooked some mice with 84 r/m of neutrons, and other mice with 32 r/m of x rays; neutrons killed with a third the dose (as measured in roentgens) needed for death by x radiation. The numbers fell out differently for sarcoma 180. About four times as large a dose of x rays as n rays was required to prevent pieces of tumor irradiated apart from the mouse from taking after implantation. Call the quantity of x rays needed to kill the tumor (mouse) Xt (Xm ) and the corresponding quantities for neutrons Nt (Nm ). Then Nt = (3/4)N m (Xt /Xm ). That is an exciting equation. It says that although the x radiation required to kill a tumor might exceed that required to kill its host, the lethal neutron dose for the tumor might not. The quantity X t /Xm has only to be less than 1.3. Unfortunately, according to the measurements of Aebersold and the Lawrences, Xt /Xm = 3.5. Still, neutrons appeared to hurt tumors more, and the body less, than x rays.[101]
The doctor paid his next visit in the summer of 1936. "Things are humming," his brother wrote the Chemical Foundation, then eager for news relevant to their big commitment to the medical cyclotron. "The [27-inch] cyclotron is in operation daily, hundreds of mice and hundreds of tumors are being killed by neutron rays." John Lawrence and Aebersold repeated the work on
[100] Zirkle and Aebersold, NAS, Proc., 22 (Feb 1936), 136–7; cf. Zirkle, Aebersold, and Dempster, Am. jl. cancer, 29 (1937), 556–62, on the edge of neutrons over x rays in the destruction of Drosophila eggs; Axelrod, Aebersold, and Lawrence, SEBM, Proc., 48 (1941), 252, give a table of differential effects of neutron and x rays on various biological systems.
[101] Lawrence, Aebersold, and Lawrence, NAS, Proc., 22 (1936), 543–57, rec'd 23 Jul 1936, amplified in J.H. Lawrence and Robert Tennant, Jl. exptl. med., 66 (1937), 667–88; Lawrence to Exner, 4 Feb 1936 (9/21), and to Cork, 22 Feb 1936 (5/1).
sarcoma 180 with another mouse tumor, obtained from Yale. It took 3,600 r of x rays, or 700 r of neutrons, to kill half the tumor particles before implantation; on healthy mice, 400 r of x rays had the same lethality as 120 r of neutrons. Hence neutrons killed Yale tumors 3,600/700 times, and Berkeley mice 400/120 times, as effectively as x rays. In this case, Nt = (2/3)N m (Xt /Xm ).[102] Numbers were moving in the right direction. The addressee was William Crocker. John Lawrence's preliminary, but "highly significant," results had figured in Ernest Lawrence's declaration to Sproul in late February 1936 of the need for a clinical arrangement like Lauritsen's to test the efficacy of neutrons on human cancers. Now John Lawrence's firm comparative data about mouse tumors helped to convince Crocker to give the clinic. At the beginning of September, Sproul pitched effectively, as follows: "The newly [!] discovered neutron ray . . . seems to provide a means of overcoming the handicap which now limits the effectiveness of the x ray in the treatment of cancer. It appears that it can be used to increase the destruction of cancerous tissue without increasing the damage to the normal tissue." Greatly overplaying John Lawrence's results, Sproul suggested that neutrons might be three times more effective—wreak thrice the havoc to tumors for the same damage to the body—as x rays.[103]
With Crocker's gift secured, the problem of staff for the clinic demanded solution. During the spring of 1936, the dean of the Medical School, Langley Porter, pressed by R.S. Stone to tighten ties with the Laboratory, met with Sproul and Ernest Lawrence for dinner at the Bohemian Club. Subsequently, Porter's assistant, Chauncey Leake, sought appointments for John Lawrence and Paul Aebersold in the Medical School. The business went slowly. As Poillon, himself a physician, had warned, "Medical men are extremely jealous of their prerogatives and . . . even to have a physicist suggest what they might do is received with anything but acclaim." By the time John Lawrence's appointment came through, he had decided to return to Yale; he kept up his work at
[102] Lawrence to Buffum, 11 Aug 1936 (3/38), to Exner, 5 Aug 1936 (9/21), and to Cork, 14 Aug 1936 (5/1); Lawrence, Radiology, 29 (1937), 316–8.
[103] Lawrence to Sproul, 20 Feb 1936, addendum; Sproul to D.J. Murphy, 2 Sep 1936; both in UCPF. Lawrence had to press his more cautious brother to publish his more complete data; Lawrence to John Lawrence, 20 May 1936 (11/16).
Berkeley on visits that totalled about six months during the academic year 1936/37.[104] Neither he nor Stone wished to postpone neutron therapy until the Crocker cyclotron started up. Ernest Lawrence, concerned to show quick progress, agreed. The Lawrence brothers may have been especially, though irrelevantly inspired by the dramatic improvement of their mother under the rays of the Sloan tube. She had long complained of abdominal pain. In November 1937, the Mayo Clinic discovered an inoperable uterine tumor and gave her three months to live. John Lawrence brought her to San Francisco; Stone irradiated her several times with supervoltage x rays; the tumor melted away. Although the swelling may not have been a tumor at all, its erasure by x rays could not but have encouraged the Lawrences to press to make available an agent they had reason to believe would be still more powerful and beneficial.[105]
As a preliminary to its clinical use, the diffuse neutron beam from the beryllium target of the 37-inch cyclotron had to be collimated and directed to a treatment port. Aebersold had the job, which he discharged by rearranging the water tanks of the cyclotron shielding and by lining the beam channel with lead (fig. 8.5). That kept the intensity within the channel almost twenty times that outside it at the port 70 cm from the beryllium target, where the patient received about 12 r/m. Carpenters transmuted a window of the Laboratory into a door opening into a demountable treatment room entirely screening the cyclotron; "the patients will hardly know they are next to such a monster."[106] A parade of physicians, including one from the National Advisory Cancer Council, trooped through the Laboratory during the summer and gave their
[104] Stone to Porter, 30 Mar, and Porter to Sproul, 31 Mar 1936 (UCPF, 393/"physics"); Lawrence to Sproul, 12 Apr, and Leake to Lawrence, 13 Apr 1936 (20/16); Poillon to Lawrence, 25 Feb 1936, and Lawrence to Poillon, 5 Oct 1936 (15/17).
[105] Lawrence to Sproul, 9 Sep 1938 (UCPF, 446/416), and to E.R. Crowther, 6 Jan and 4 Feb 1938 (5/5); Childs, Genius , 198–9, 278–9; Lawrence, "Faculty research lecture," 20 Mar 1938 (40/20): "It is evident that . . . the neutron rays . . . have important medical applications in therapy." Cf. John Lawrence to Lawrence, [Mar 1936] (11/16).
[106] Cooksey to Allen, 5 Sep 1938 (1/14); Lawrence to Exner, 22 Sep 1938 (9/21), quote, to Hopwood, 5 Oct 1938 (9/15), and to Cockcroft, same date (4/5); Aebersold, PR, 56 (1939), 714–27; Compton, notes on visit, 16 Sep 1938 (13/29).
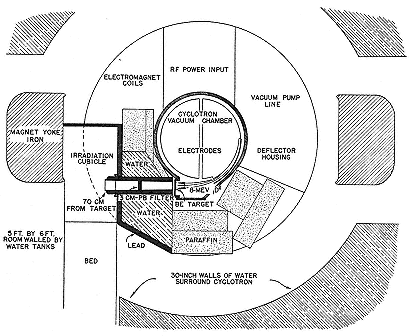
Fig. 8.5
Aebersold's arrangement for neutron therapy. The treatment room is at the
left, within the magnet yoke. Not all the water tanks surrounding the
cyclotron are shown. Aebersold, PR, 56 (1939), 717.
blessings to the general work, if not to the therapeutic intiative. Their good judgment pleased and surprised Cooksey. "My opinion of doctors as a whole has risen tremendously." The first patients were exposed on September 26, 1938, just in time for a visit by the NACC's Arthur Compton. Their skin showed effects no worse than those caused by 12 r/m of x rays. Lawrence informed Sproul. "It gives me great pleasure to report an event of historic interest. . . . I personally believe, and my views are shared by my medical colleagues, that this will be the beginning of a new method of cancer therapy which in a few years will be as widespread as that of x rays and radium." What better time to ask for money? $2,000 for power and supplies, $1,400 for furnishings? "We could, of course, slow down our activities, including bringing to a halt the clinical therapy, but in view of the great immediate importance of this pioneering work it would be no less than tragic to do so."[107]
[107] Cooksey to Lawrence, 10, 14, 15 and 18 (quote) June, 1938 (4/21); Lawrence to Sproul, 12 Oct 1938 (13/29 and UCPF), quote, and to Crowder, 6 Oct1938 (5/5); Lawrence, "Report to the Research Corporation," 3 Jan 1939 (15/17A).
After five months' experience with therapy at the 37-inch, Stone judged that the results of single erythema doses—doses sufficient to redden the skin—gave encouragement for "a complete course of therapy" and hope for "better results than are now being obtained." It would have been difficult to reach a different conclusion as the cyclotroneers began to fish for a beam in the 60-inch cyclotron. The first patient to absorb neutrons from the Crocker cracker, Mr. Robert Penney, received treatment on November 20, 1939 (plate 8.4). A regular clinical program did not begin until the end of January 1940. Then a few tumors vanished. "Dr Stone and John are very enthusiastic about the results." Thus brother Ernest. But John himself would not go beyond the meaningless formulation of the weather forecaster: "[There is] better than a fifty-fifty chance that neutrons are going to be of great value in therapy."[108] He was therefore just better than half wrong when Stone evaluated the program in 1948. Only one of the 24 patients treated at the 37-inch cyclotron in 1938 and 1939 was then alive, and only 17 of the 226 treated at the 60-inch between 1939 and 1943. All but one of the 250 had been considered incurable. The survivors suffered what Stone described as "distressing late effects" that might not have occurred had they undergone x-ray rather than neutron therapy. He judged that he and John Lawrence had overexposed their patients. "Neutron therapy as administered by us has resulted in such bad late sequelae in proportion to the few good results that it should not be continued." Stone's negative evaluation put an end to fast-neutron therapy for two decades.[109]
After much experimentation, treatment of human cancers by neutron rays recommenced around 1970, at, among other places, the Hammersmith Hospital in London, which used a cyclotron constructed in 1952. By 1978 over 3,000 patients had been treated at eleven centers in Europe, Japan, and the United States. The therapy proved effective against advanced, superficially placed
[108] Lawrence to Frank Hinman, 12 Jan (24/18), to Poillon, 31 Jan (15/8), and to Kruger, 14 Mar 1940 (10/20), quote; John Lawrence to Ridenour, 6 Feb 1940 (14/22), quote.
[109] Stone, Am. jl. roent., 59 (June 1948), 771, 775–6, 784. Cf. Sheline et al., ibid., 111 (1971), 31–41.
tumors that could be irradiated with little damage to neighboring normal tissue. The experience at Hammersmith through 1984 was that 70 percent of such tumors regressed after treatment with 7.5 MeV neutrons in comparison with 35 percent after treatment with x rays.[110] Stone and the Lawrences had the right idea but the wrong dosage.
Grand Elixirs
Lawrence had brought the idea of clinical use of Na24 to Poillon (who marked it "basic, with important commercial applications") in his report for 1934. He had in mind, apparently, that the gamma rays of radiosodium might replace radium's in the general treatment of cancer. In the spring of 1935, about the time that he began to worry about excessive exposure of cyclotroneers to neutrons, he also began to plan provisionally for clinical tests of Na24 and P32 . He was confirmed in this intention by a visit in April of Charles Sheard, head of biophysical research at the Mayo Foundation; and also by the Board of Regents, who responded to Sproul's report about the discovery of Na24 by declaring their interest in "the far reaching biological aspects of the discoveries and the new possibilities of radio therapy."[111] In May, Lawrence lectured the board of the Research Corporation on the power of the rays from radiosodiuim; in July, he could make 50 mg of the stuff, enough, he thought, for clinical tests; in August, treatment began, or would have, had the cyclotron not broken down.[112]
Radiosodium did not answer expectations. As advertised in the Research Corporation's patent, it did not cause harmful side effects, to dogs at least, even when given in large doses; but then neither did it do outstanding damage to tumorous tissue. Two leukemia patients received doses of radiosodium in the spring of 1936; one had 147 mCi in all, the largest quantity of active
[110] Field, Curr. top. rad. res. quart., 11 (1976), 1–85; Raju, Heavy particle therapy , 78–169; and Catterel in Acc. dei XL, Memorie, 8:2 (1984), 251–4.
[111] Lawrence, "Report [for 1934]" (RC); Lawrence to Leuschner, 12 Apr 1935 (20/13), and to Rutherford, 17 Apr 1935 (ER); Sproul to Lawrence, 31 May 1935 (20/19).
[112] RC, Board of Directors, "Minutes," 23 May 1935, 1061 (RC); Lawrence to Beams, 20 Jul 1935 (2/26), and to Poillon, 28 Aug 1935 (RC).
material administered that year. Neither benefitted or suffered. Hamilton inferred that he might practice safely on normal people. He fed his subjects from 80 µCi to 200 µCi of patent-pending Na24 while they sat with one hand (and its arm) in a lead cylinder grasping a Geiger counter (fig. 8.6). The counter indicated that active material reached the hand within a few minutes of ingestion. In subsequent, more careful experiments with the same setup, radioisotopes of sodium, chlorine, bromine, and iodine made it from mouth to hand in from three to six minutes.[113] Injected in one arm, the tell-tale tracers arrived in the other in about twenty seconds. Radiosodium accordingly had some employment in studies of circulation and water balance in the body. It became a diagnostic tool for vascular disorders; for a time it stood literally at the cutting edge of research, as an indicator of the best site for amputation of impaired parts.[114]
The most useful of the harmless tracers of Hamilton's experiment was radioiodine because it concentrates very strongly in a particular organ, the thyroid, which has at least as much iodine as all the rest of the body. The first in the field were a group in Cambridge, led by Saul Hertz of the Massachusetts General Hospital and including Robley Evans of MIT. They gave I128 made with neutrons from a Rn-Be source to rabbits, which, when "finely minced," disclosed that they had deposited radioiodine in their thyroids very soon after eating it—which was fortunate, since I128 has the inconveniently short half-life of 25 minutes. Hamilton wanted something with a week's demi-duration, and so informed Seaborg. "I then told him that I would try." A month later, Livingood and Seaborg presented their 8-day iodine, I131 , untangled from the results of irradiating tellurium with deuterons.[115] With this I131 , Hamilton and Myron Soley of the Medical School
[113] Hamilton and Stone, SEBM, Proc., 35 (1937), 595–8, on leukemia; Hamilton, NAS, Proc., 29 (1937), 521–7, and Am. jl. physiol., 124 (1938), 667–78, on radiosodium and radiohalides; Hamilton and Alles, Am. jl. physiol., 125 (1939), 410–3, on dosing dogs with radioalkalis; Lawrence to Hopwood, 28 Jul 1938 (9/15).
[114] A.H. Compton, notes on visit to Berkeley, 16 Sep 1938 (13/29); Hamilton in Wisconsin, Univ., Symposium , 339–40.
[115] Hertz, Roberts, and Evans, SEMB, Proc., 38 (1938), 510–3; Seaborg, Jl., 1 , 332, 340 (30 Mar and 2 May 1938).
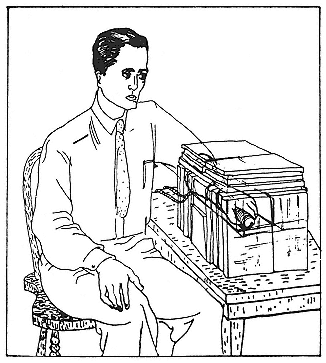
Fig. 8.6
Hamilton's arrangement for detecting the circulation of
radioactive salts. The hand in the lead cylinder grasps a
Geiger counter. Hamilton, Jl appl. phys., 12 (1941), 449.
showed that uptake of iodine by patients suffering from toxic goiter or overactive thyroid exceeded tenfold the uptake by normal persons, and that parts of thyroids invaded or destroyed by cancer cells could not fix iodine at all. (This last information came from autoradiography: sufferers about to have their defective thyroids cut out ingested radioiodine; sections of the removed thyroids were laid against x ray film; and the portions of the sections containing the active material photographed themselves.) It appeared that the rate of manufacture of the hormone issuing from the thyroid, thyroxin, depended upon the organ's ability to take up iodine and that radioiodine would not be a good weapon against thyroid cancer.[116]
[116] Hamilton and Soley, Am. jl. physiol., 127 (1939), 557–72, and 131 (1940),135–43; Hamilton, Soley, and Eichorn, Univ. Calif., Publ. pharm., 1 (1940), 339–67; cf. Hamilton, Jl. appl. phys., 12 (1941), 448–53.
Research and development then split. L.I. Chaikoff of the University's Department of Physiology and several collaborators used cyclotron-produced I131 to study the general biochemistry of iodine; by 1942 they had made important progress in elucidating the process of its fixation in the thyroid.[117] Hamilton and Lawrence, and also Hertz and Roberts, moved on to therapy. The treatment of noncancerous hyperthyroidism began at Berkeley in 1940 and about the same time at the Massachusetts General Hospital, which received I131 from the Laboratory for the purpose. Both groups had much the same experience. In four of five cases treated, the cyclotron-produced I131 (or, rather, combination of I131 with the 12.7-hour I130 , also discovered by Livingood and Seaborg), diminished the goiters, relieved symptoms (some of many years' standing), and increased emotional stability within a week or two of administration. Does ranged from 5 to 28 mCi. No deleterious side effects occurred within six years of commencement of treatment. Still, a cautious physician could not rule out development of thyroid cancer or damage to kidneys or bone marrow. Chemical and surgical treatment improved; and the best judgment when the first clinical experiences were evaluated limited radioiodine to patients intolerant of the new drugs or unable to undergo therapy. Effective treatment of thyroid cancer by I131 dates from after the war.[118]
The work with radioactive species of alkalis and halogens, however promising and stimulating, was but side play in the Laboratory's great drama of radiomedicine. The protagonist there was radiophosphorus—easy to make, of convenient half-life (about 14 days), with a good strong beta ray (maximum of 1.7 MeV), and known to concentrate in bones. In 1935, Hevesy and a colleague
[117] Hamilton and Soley, Am. jl. physiol., 127 (1939), 557–72; Hamilton, Radiology, 39 (1942), 553–63; Chaikoff and Taurog in Wisconsin, Univ., Symposium , 308–24.
[118] John Lawrence to G. Failla, 23 Jan 1940 (5/7); Hertz and Roberts, Jl. clin. inv., 21 (1942), 624, and Hertz, Roberts, Means, and Evans, Am. jl. physiol., 128 (1940), 565, 575; Hamilton and Lawrence, Jl. clin. inv., 21 (1942), 624; Hamilton and Soley, Am. jl. physiol., 131 (1940), 135–43; Hertz in Wisconsin, Univ., Symposium , 379–80, 387–9.
at Bohr's institute (for theoretical physics!) fed rats P32 made from Rn-Be neutrons on S32 , measured the hot phosphorus in the feces, destroyed the animals, and found, among other things, that bone is a dynamic tissue. Further inquiry disclosed that young rats concentrated phosphorus very quickly and substantially in their growing bones.[119] The inquiry continued in San Francisco at the University's Medical School with samples of P32 having activities 100 or 1,000 times Hevesy's and with chicks in place of rats. The researchers, S.F. Cook, K.G. Scott, and Philip Abelson, confirmed that new phosphorus goes primarily to the bones, and also to the musculature; and they found that between 4 and 60 days after ingestion, the labelled phosphorus migrated from muscle and small intestine to bone and bone marrow. The spleen enjoyed high deposits throughout.[120]
These results encouraged the speculation that P32 might help control blood diseases such as polycythemia vera (a multiplication of red blood cells causing nosebleeds and an enlarged spleen) and leukemia. John Lawrence treated a lady suffering from polycythemia vera in 1936; her symptoms remitted (permanently, as it happened), and plans for proceeding to leukemia were made. Early in 1938 John Lawrence took the entire output of P32 from the 37-inch cyclotron. Part he fed to cancerous mice, part, in therapeutic doses, to a leukemic human. The mice confirmed the supposition grounding the therapy: they concentrated the active phosphorus in their fast-growing tumors, especially in tissue invaded by leukemic cells, and did so at the expense of deposition in their bones. Hence the indication: P32 as a weapon against cancers of the bone and bone marrow.[121] The human suffered from chronic leukemia of the bone marrow (myelogenous leukemia). He got 70 mCi of P32 over two months, which made his blood picture normal; a great triumph, which, as Lawrence
[119] Chievitz and Hevesy, Nature, 136 (1935), 754–5, and Dansk. Vidensk. Selsk., Biol. meddelser, 13 (1937), in Hevesy, Selected papers , 60–2, 63–78.
[120] Cook, Scott, and Abelson, NAS, Proc., 23 (1937), 528–33.
[121] J.H. Lawrence, Jl. nucl. med., 20 (1979), 563; Lawrence to DuBridge, 5 Mar 1938 (15/26); J.H. Lawrence, "Present status of the biological investigations," 25 Aug 1938 (13/29); J.H. Lawrence and Scott, SEBM, Proc., 40 (Apr 1939), 694–6.
rightly cautioned in telling Chadwick, "should not be mentioned in public." But in private, he advised, it might be just the thing to mention to people considering building a medical cyclotron. That was certainly the message that Karl Compton took home from his visit to the Laboratory during April 1938, when the patient's blood appeared so nearly normal that it did not allow firm diagnosis of his condition. As MIT's Robley Evans reported Compton's reaction: "Immediately on arriving home from his visit to your laboratory, Compton called me over to describe with unbounded enthusiasm your leukemia work. . . . I am sure Compton's visit to 'cyclotron headquarters' did much to kindle his already enthusiastic support [for a cyclotron for MIT]."[122]
This was to compound Lawrence's optimism with physicists' ignorance of medical matters. John Lawrence was much more circumspect than his brother. He wrote Evans that the remission accorded his patient might have been accomplished by x rays, a point that Ernest Lawrence then kept in mind. In a report on the treatment of two cases of myelogenous leukemia published in a biomedical journal, John Lawrence and his collegues limited themselves to facts about uptake of P32 in the blood and retention in the body, and made no clinical inferences.[123] By June 1939, John Lawrence was treating a dozen patients, who took a total of 20 or 25 mCi a year, and had in consequence a life expectancy that he judged to be similar to that procured with x rays, some two or three years. In July he left for Europe, to bring tidings of the Laboratory's progress in radiobiology and radiomedicine to the British Association for the Advancement of Science. He mentioned Hamilton's work with radioactive alkalis and radioiodine, the indications for leukemic therapy, and the occurrence of remissions under treatment.[124]
[122] Lawrence to Chadwick, 30 Apr 1938 (3/34); Evans to Lawrence, 15 Apr 1938 (7/8); Lawrence to A.H. Compton, 19 Sep 1938 (4/10), same news, same message.
[123] J.H. Lawrence to Evans, 9 and 12 Apr 1938 (7/8); J.H. Lawrence to Hektoen, 22 Sep 1938 (13/39); Tuttle, Scott, and J.H. Lawrence, SEBM, Proc., 41 (May 1939), 20–5.
[124] J.H. Lawrence to L.A. Erf, 2 June 1939 (7/6); Lawrence, Nature, 145 (27 Jan 1940), 125–7; Tuve, "Present technical status of the use of radioactive tracers," 10 May 1939 (MAT, 25/"Hamilton Club"), 4.
The meeting of the British Association ended as Germany invaded Poland. Two days later, on September 3, 1939, Britain declared war. John Lawrence then set sail in the Athenia , which the Germans promptly torpedoed. The physician saved himself, bravely, after tending the wounded. Ernest Lawrence pulled what strings he could to obtain a berth for John on the first American ship sailing from Britain to the United States. The most useful of these strings involved leukemic politics. Early in August the Laboratory had received a visit from F.C. Walcott, an influential Republican senator from Connecticut until the Democratic landslide in 1936, who had come West as a guest of Herbert Hoover. He had a son, Alex, suffering from leukemia. The senator placed his hopes in the cyclotron and P32 and asked that John Lawrence stop to see Alex in New York on his return from Europe. When John seemed stuck after the sinking of the Athenia , Walcott pulled his strings, attached to the American ambassador to Britain and the president of the merchant marine. Within a week John had a berth on the Nieuwamsterdam .[125] He was restored to his leukemia patients, who now included Alex Walcott, in October. Results were mixed. P32 did nothing for Alex. Evaluating the situation in February 1940, a few months before Alex died, John Lawrence could not rate P32 more effective than other therapeutic agents in the control of leukemia. His best hope was that "possibly it will turn out to be slightly better."[126]
The continuing study of the metabolism of phosphorus in mice supported the hope. The physiologists at Berkeley showed that the generation of phospholipids in tumor cells took place as vigorously as in the most active organs, the liver, kidney, and small intestine, and that, in contrast to the normal active organs, tumors retain their P32 for long periods of time.[127] Lawrence and his associates
[125] Childs, Genius , 293; Walcott to Lawrence, 12 Aug 1939, Lawrence to J.H. Lawrence, 19 Sep 1939, and telegrams among the Lawrences and Walcott, Sep 1939 (18/6). In the event, John did not need his brother's influence; he sailed not on the Nieuwamsterdam but on an American ship chartered to bring home the survivors.
[126] J.H. Lawrence to Ridenour, 6 Feb 1940 (14/22); infra, §10.1.
[127] Jones, Chaikoff, and Lawrence, Jl. biol. chem., 128 (1939), 631–44, ibid., 133 (1940), 319–27; and Am. jl. cancer, 40 (1940), 235, 241–2, 250; Lawrence to M.W. Schramm, International Cancer Research Foundation, 29 Jul and 18 Aug 1938 (9/22), requesting $2,100 for this work.
at the Crocker Laboratory confirmed the phosphorophyllic tastes of leukemic tissue.[128] In this respect humans behaved like mice. Lawrence and Lowell Erf, a physician expert in hematology and supported on fellowships, gave tracer doses of P32 to seven patients about to die of various cancers. In their last moments, as autopsy disclosed, they had put as much phosphorus per gram wet weight in their malignant tissues, or in tissues infiltrated by malignant cells, as in their most active organs. These results, according to John Lawrence, had constituted the rationale for the therapeutic use of radiophosphorus. The argument, however, was indirect. The first direct trials against cancerous growth in mice were, in Erf's words, "very disappointing."[129]
On May 18, 1940, Arthur Compton and James Murphy of the National Advisory Cancer Council looked in to see what the council supported at Berkeley. Ernest Lawrence, on John's advice, had gone to Sonoma to recover from a sore throat; but he came down to the Laboratory to meet men so important for his future work. The day did not go well. Murphy, an expert on animal experimentation, graded the Laboratory's procedures and facilities poor or worse. Cooksey later visited Murphy's institute. "[I] was tremendously impressed with the facilities. . . . It was obvious that our set up was terrible in his eyes." The conversation on May 18 switched to politics. Compton complained that an article he had written for a newspaper outlining the duties of scientists toward science and the nation had been misinterpreted. He planned to reply. Lawrence and Cooksey told him, politely no doubt, that he should have kept his mouth shut in the first place. Berkeley reserved its radicalness for technology and medicine. The report of the site visitors helped the NACC decide not to underwrite the expansion that John Lawrence suggested to it: investigation of differential effects of n rays and x rays on animals; metabolic studies of iron, sodium, potassium, sulphur, and iodine on hundreds of animals; methods to improve uptake of potassium and boron in
[128] J.H. Lawrence et al., Jl. clin. inv., 19 (1940), 263–71; Tuttle, Erf, and Lawrence, ibid., 20 (1941), 57–61; Erf and Lawrence, ibid., 567, 575.
[129] Erf and J.H. Lawrence, SEBM, 46 (Apr 1941), 694–5; J.H. Lawrence, Nature, 145 (27 Jan 1940), 127; Erf to Theodore B. Wallace, Smith Kline and French, 6 Mar 1940 (7/6).
tumor tissue. On first consideration, NACC declined support for animal experimentation and cut back that for Stone's clinical application of neutron rays.[130]
Ernest Lawrence was not accustomed to such rebuffs. He accepted that the council might not care to support experiments with animals to refine neutron therapy or clinical use of radioiosotopes. "But it is totally incomprehensible to me that there should be any suggestion of curtailing the clinical program with neutron rays. . . . It seems to me a primary obligation on the part of all of us to see that the program of exploring these possibilities be carried forward full steam ahead. . . . The cancer program simply must go forward as Dr Stone and my brother have planned it." Compton, the recipient of this appeal and demand, allowed that Stone's program would most probably be funded in full.[131] It was. But the council stood its ground on animal experimentation.
The clinical program with radioelements rested on a financial and technical base quite different from that of neutron therapy. Treatment did not require immediate access to a cyclotron or special facilities at the Laboratory. The chief therapeutic agent, P32 , came so plentifully that, in John Lawrence's estimate, he could treat all the chronic leukemia in California without interfering with other obligations of the cyclotron.[132] The cost of machine time for making P32 could be passed on to the patient. The positive signs of phosphorus therapy outweighed the negative and opened the brief flurry of interest in commercial production of radioisotopes by cyclotrons arrested by the war. The psychology of support appears plainly from the initiative of Hans Zimmer, professor at the Harvard Medical School, dying of leukemia, who believed that he had obtained some benefit from P32 . It bothered him that the amount of radiophosphorus available fell far short of the nation's, if not of California's needs. He appealed to the Carnegie Foundation for $12,000 for a cyclotron for his Medical School.[133] Although John Lawrence advised against rushing
[130] Cooksey to Poillon, 25 Jul 1940, and J.H. Lawrence to Dr. Smith, NACC, 1 June 1940 (13/29A). On John Lawrence as Laboratory doctor: Cooksey to Lawrence, 29 Mar 1939 (4/22).
[131] Lawrence to A.H. Compton, 21 Jul, and reply, 26 Jul 1940 (4/10).
[132] J.H. Lawrence to V. Bush, 10 Sep 1940 (15/18).
[133] Zimmer to E.P. Keppel, 31 Aug 1940 (15/18);
commercial production, Poillon pushed it, confident in and counting on the Research Corporation's patents on the cyclotron and the Van de Graaff.[134] Despite the attitude of the NACC, the Laboratory had no trouble expanding the basis of support of its biomedicine in 1940/41. The clinical program in radioisotopes had the support of the Jane Coffin Childs Fund for Medical Research, the Darian Foundation, Merck and Company, the Columbia Foundation, and the Donner Foundation.[135]
In 1948 Byron Hall of the Mayo Clinic evaluated the results of the clinical use of P32 in Berkeley, the Medical School of the University of Rochester, and his own institution. "It is too early [he wrote] to make a final evaluation of this form of therapy." But he allowed that it had brought relief to sufferers from polycythemia vera and the chronic forms of leukemia. The clinic had by then treated 154 cases of the one and 33 of the other. Symptoms in almost all of the 124 cases of polycythemia vera for which adequate follow-up data existed improved or disappeared. In 85 percent of the cases, the blood picture remitted satisfactorily and risk of hemorrhage and thrombosis decreased. Remissions of up to five years were achieved, but most lasted under two years. Complications—drop of white-blood-cell count, severe anemia, acute leukemia—occurred in a total of 30 percent of the cases. In general, P32 raised the life expectancy of sufferers from polycythemia vera as much as vitamin B12 did that of victims of pernicious anemia. Twenty patients with chronic myelogenous leukemia were followed. Many obtained the same sort of remission they would have acquired from x rays. Eleven of the patients died within six years of treatment. Chronic lymphatic leukemia proved more receptive. Six of six patients continued in remission from ten to twenty-six months after treatment. Radiophosphorus conferred no benefit on patients with acute leukemia, Hodgkin's disease of the bone marrow, or multiple myeloma.[136]
[134] Poillon's correspondence with American Cyanamid, General Electric, and Westinghouse, Apr–Oct 1940 (RC, "cyclotron licensing"); Poillon to Lawrence, 26 Sep 1940 (15/26A); RC, Board of Directors, minutes, 29 Oct 1941, 1496.
[135] Correspondence between the donors and recipients, 1940/41 (UCPF, 515/400 and 541/312).
[136] Hall in Wisconsin, Univ., Symposium , 353–76; J.H. Lawrence, Manowitz, and Loeb, Radioisotopes and radiation , 50.
Rosetta Stones
On May 25, 1939, the "University Explorer," reaching deep to advertise the Laboratory, announced the arrival of radioactive fertilizer in Hawaii. A professor at the University there had imported P32 from Berkeley by Pan American clipper to test its power on pineapples. An explorer of the consequences of the innovations of the Laboratory had (and has!) his work cut out: "The influence of the cyclotron has been felt in so many different fields of science that no one can predict its ultimate value to mankind."[137]
The influence began to spread at home, in 1937/38, among members of Berkeley's Chemistry Department. The first practitioners of radioactive tracing were Libby, Seaborg, and, above all, Ruben, through whose efforts University biologists took up the technique. Purely chemical applications, which were not irrelevant to the biologist, included the vast fields of exchange reactions and reaction mechanisms; the nature of the inquiry may be indicated by studies by Libby's students of exchanges between various valence forms of sulphur and of photochemical processes in solution, and by Libby himself of the reactions of recoil nuclei activated by neutrons. Another conspicuous line, detection of impurities by their radioactivity, which had been an unwelcome and misleading annoyance, was practiced for a time by Seaborg and Livingood, who found copper in nickel, iron in cobalt, and phosphorus and sulphur everywhere.[138]
Ruben and P32 inspired a major direction of research in the Physiology Department on the metabolism of phospholipids or phosphatides, which occur in all living tissues in connection with fatty deposits. Ruben, Chaikoff, and their students and colleagues, who included the chemist I. Perlman and, occasionally, John Lawrence, ground up rats and birds fed radiophosphorus under various regimes—fasting, normal diets, fatty diets—to learn the loci of the creation and destruction of phospholipids. Following John
[137] "University Explorer," 25 May 1939 (40/15).
[138] Kamen, Science, 140 (1963), 587, re Ruben; Seaborg, Chem. rev., 27 (1940), 250–73, esp. 256, 263, 266; Voge and Libby, JACS, 59 (1937), 2474; Rollefson and Libby, Jl. chem. phys., 5 (1937), 569–71; Seaborg and Livingood, JACS, 60 (1938), 1784–6.
Lawrence's interests, they also examined cancerous mice. They extended the findings of Segré's associates in Palermo, who, with an Italian touch, had fed olive oil and Berkeley phosphorus to their rats; which, when anatomized, disclosed that the liver is the fastest metabolizer of phospholipids. The Berkeley group further showed that excised bits of liver, kidney, and intestine continued to work at phospholipid metabolism in vitro, and that the rate of its metabolism in tumors transplanted from one animal to another was characteristic of the tumor, not the animal.[139]
Two other sustained programs in radioactive tracing that thrived on the Laboratory's output deserve mention. One grew in the Biochemistry Department around David Greenberg's ongoing investigations of mineral metabolism. Inspired, he said, by the "revolutionary nature and potential importance" of radiotracing, Greenberg and his associates began as others did, feeding radiophosphorus to rats and examining deposits in tissue and feces. They went on to the phosphorus metabolism of rachitic animals. They were the first to use radiocalcium (Ca45 ), a commodity more costly than Fe59 , as a tracer. In 1940 and 1941 they published information on the metabolism, deposition, and elimination of manganese, iron, and cobalt, the last, as they found, a possible cause of polycythemia.[140] The second program, conducted by S.C. Brooks at the Medical School, studied the transport of ions into and within plant cells. Here radioactive indicators brought light where darkness had long prevailed. According to an authoritative reviewer, the results of Brooks and his associates released biologists from "the necessity of postulating mysterious properties for cellular membranes and protoplasm."[141] Further
[139] Artom et al., Nature, 139 (15 May 1937), 836–7, and ibid. (26 June 1937), 1105–6; Perlman, Ruben, Chaikoff, and others, in various combinations, Jl. biol. chem., 122 (1937), 169–82, 123 (1938), 587–93, 124 (1938), 795–802, 126 (1938), 493–500, 127 (1938), 211–20, 128 (1939), 631–44, 735–42. This and similar work from other laboratories is reviewed in Greenberg, Ann. rev. biochem., 8 (1939), 276–7; Hevesy, ibid., 9 (1940), 649–54; and Hamilton, Jl. appl. phys., 12 (1941), 445, and Radiology, 39 (1942), 545–8.
[140] Greenberg reviewed his own work in Ann. rev. biochem., 8 (1939), 269–70, quote, and in Wisconsin, Univ., Symposium (1948), 263–9, 270–3, 279–81. Cf. Kohler, From medical chemistry , 329; Greenberg, "Recollections" (UCA); Hamilton, Radiology, 39 (1942), 566–7.
[141] Loofbourow, RMP, 12 (1940), 275, with references to the literature.
attacks on these mysteries occurred at the School of Agriculture, where Perry Stout and his co-workers made the uptake of alkali and halide salts by growing trees and shrubs their subject of study. They settled the vexed question whether salts rise through the bark as well as through the xylem (the answer is no) and demonstrated by persuasive autoradiography the deposit of P32 in the leaves and fruit of growing plants (plate 8.5).[142]
It did not take much, apart from the material, to set up with radioactive tracers and to get quick, publishable results. There were so many animals and plants, so many elements, so many permutations of experimental circumstances. Hence the Laboratory received many requests for its products, which, if they did not save lives, might improve careers. Lawrence honored requests from outside the University on the basis of merit, other factors being equal. His most generous support went to workers in Rochester and in Copenhagen, the two places where, in the opinion of Merle Tuve, no fan of the Laboratory, work with tracers second only to Berkeley's was being done.[143] In Copenhagen, Hevesy's group continued work with P32 , following it throughout the body and its products, into blood, eggs, milk, across cell walls, to and from the liver, and into the brain. Like Chaikoff's group, they paid much attention to phospholipid metabolism; and they worked out other biochemical pathways, notably the role of phosphorus compounds in the enzymatic breakdown of carbohydrates (glycolisis). Lawrence encouraged Hevesy to ask for "as much [P32 ] as he can use. . . . We are more than eager to help his important work."[144]
Hevesy received the Nobel prize in chemistry in 1944 for his contributions to the tracer method. The leader of the Rochester
[142] Stout and Hoagland, Am. jl. botany, 26 (1939), 320–4; Arnon, Stout, and Sipos, ibid., 27 (1940), 791–8; J.H. Lawrence, Nature, 145 (1940), 125–6, and "Summary of biological and medical investigations," Jan 1940 (22/11); Hamilton, Radiology, 39 (1942), 550–2.
[143] Tuve, "Status of the use of radioactive tracers," 10 May 1939 (MAT, 25/"Hamilton Club"), 2–3.
[144] Hevesy, Selected papers , 111–74, 440–1; Hevesy et al., Acta biol. exp., 12 (1938), 34–44; Lawrence to Bohr, 17 Feb, quote, and 14 Nov 1938, and Bohr to Lawrence, 25 Jul 1939 (3/3); correspondence with Hevesy, 1937–40 (9/7); Hevesy in Frisch et al., Trends , 115.
group, George Whipple, already had a Nobel prize, that of 1934 in physiology and medicine, for work on anemia. Radioiron sent from Berkeley allowed him to wallow more deeply in his favorite subject. His group, in which P.F. Hahn took the lead, showed that anemic dogs accepted iron in any form, collected it rapidly in the bone marrow, and disbursed it rapidly in blood cells. A normal dog would absorb almost no iron at all. It appeared that ingested iron entered the blood stream only if the body's iron store had been depleted; otherwise it passed directly through the intestines. Whipple expected to devise a satisfactory treatment of anemic patients using ordinary iron on the basis of the knowledge labelled iron gave him.[145] The information did not come easily. Most of the time Whipple's group were as deprived of iron as their dogs. The cyclotron could not keep up the supply: the 37-inch made less than 1 mµCi of Fe59 per µAh, the 60-inch only 0.03 µCi. A radioiron with suitably high specific activity, Fe55 (t = 4 years), can be made by (d,n) on manganese, but it was not available before the war.[146]
The Rochester iron men hoped that the radioisotopes furnished by physicists would turn out to be the "'Rosetta Stone' for the undertaking and study of body metabolism." That was to be very optimistic. The Stone then had nothing to say about the largest part of the bodies of plants and animals: no useful radioactive tracer for hydrogen, carbon, oxygen, or nitrogen had yet been found. No one felt this difficulty more than Kamen and Ruben, who had boldly set forth in 1938 to find the way through photosynthesis armed with C11 , which has a half-life of about 21 minutes. An experiment consisted of making the isotope, burning it to carbon dioxide, feeding it to plants, chopping the leaves into a beaker, adding as carrier any substance they guessed might have been labelled with C11 by the plant, and examining the various
[145] Hahn et al., Jl. exp. med., 69 (1939), 739–53, 70 (1939), 443–51, and 71 (1940), 731–6; Whipple's ambition, recorded by Tuve, "Status of the use of radioactive tracers," 10 May 1939 (MAT, 25/"Hamilton Club"), 7; Hamilton, Radiology, 39 (1942), 564–6, and Jl. appl. phys., 12 (1941), 455–6.
[146] Whipple to Lawrence, 23 Nov, and reply, 30 Nov 1937, and Lawrence to DuBridge, same date (15/26); letters to Lawrence from Evans, 6 Mar 1939 (7/8), and from Van Voorhis, 5 Feb 1939 (17/14).
carriers. All in an hour or two. The pace forced out their first collaborator, Zev Hassid, who suffered from high blood pressure. They made three of these hectic runs a week for three years.[147]
They made some progress. A plant fed in the dark attached labelled CO2 to a big molecule RH, R is an unknown radical, making a compound RCOOH. The reaction appeared to be reversible. In the light, however, and the presence of chlorophyll, RCOOH gained water and lost oxygen, irreversibly, to become RCH2 OH. This structure may be considered a molecule R'H, which can fix another CO2 molecule in the same way to become RCH2 OCH2 OH, and so on. R might then break off, leaving a sugar. The scheme was novel and, in keeping with the requirements of philosophers, had an easily testable consequence that distinguished it from older theories. The consequence: that RH be a very big molecule. For evidence, Kamen and Ruben turned to the ultracentrifuge. The best local setup was at Stanford. Ruben stationed himself there, awaiting with counters ready the delivery of the labelled samples Kamen rushed down from Berkeley. Their lives eased with the discovery that Shell Development Company in Emeryville, adjacent to Berkeley, had a similar centrifuge. The machine showed RH to have a molecular weight between 500 and 1,000.[148] To go farther, to identify the heavy molecule and the intermediates in photosynthesis, Kamen and Ruben needed a longer-lived isotope of carbon. A bout with N13 , which has a half-life of 10.5 minutes and which did not quite enable them to decide whether nonleguminous plants can fix nitrogen, further indicated the limits on biochemical research placed by lack of tracers for organic reactions.[149]
Another path lay open. For many years biochemists had been following reactions by tagging compounds with naturally occurring isotopes. They would introduce a substance artificially enriched with deuterium or with C13 , which makes up a little over 1 percent of ordinary carbon. They had then only to take the final
[147] Hahn et al., Jl. exp. med., 69 (1939), 739; Kamen, Radiant science , 84–7.
[148] Ruben, Hassid, and Kamen, JACS, 61 (1939), 661–3, 62 (1940), 3443–55; Kamen and Ruben, Jl. appl. phys., 12 (1941), 326; Kamen, Radiant science , 105–9; Hamilton, Radiology, 39 (1942), 567–9, and Jl. appl. phys., 12 (1941), 457–8.
[149] Ruben, Hassid, and Kamen, Science, 91 (14 June 1940), 578–9.
products of interest and assay them for the relative abundance of the rare isotope in a mass spectrograph. Urey's colleague at Columbia, Rudolf Schoenheimer, the great master of the technique, routinely detected changes of 1 percent in isotopic abundance, which, in the case of carbon, meant one part in ten thousand. With some encouragement from the Research Corporation, Urey had perfected a method to enrich the concentration of C13 , which he turned over to Columbia University to patent at the end of 1939.[150] At about the same time, the Eastman Corporation sought advice about the likely market for the stable isotope N15 as a tracer for nitrogen. Urey formed and did not hide the opinion that any foundation truly wishing to advance biochemistry and biology should assist in making rare natural isotopes available and not throw its money into cyclotrons.
Lawrence was then at the beginning of his negotiations with the Rockefeller Foundation for what became a request for a million dollars. Around October 1, 1939, Lawrence summoned Kamen and ordered him to find a radioactive carbon, nitrogen, and/or oxygen to silence Urey. "He said I could have both the 37-inch and the 60-inch cyclotrons and all the time I needed, as well as help from whomever I requested—Segrè, Seaborg, anyone!" Naturally Kamen chose to center his all-out search on carbon, and all the more after he had demonstrated with Segrè's help that no useful oxygen or nitrogen could be made by irradiating oxygen with alpha particles or deuterons. He pinned his hopes on an internal target of graphite, which he baked with deuterons for 5,700 µAh during the first six weeks of 1940. On February 13, Kamen terminated his exposure and left the hot target for Ruben to analyze.[151]
Kamen recalled that he had turned to deuterons on graphite in "desperation and resignation." The only likely candidate for a useful radiocarbon was C14 made by (d,p) on the rare isotope C13 .
[150] Poillon to Lawrence, 21 Sep, and reply, 28 Sep 1937 (15/17A); Urey to Douglas C. Gibbs, secretary of University Patents, Inc., 22 Nov 1939, 1 Mar 1940 (Urey P, 8). The patent was filed 3 Aug 1940. The agreement, as usual with University Patents, gave 7 percent of gross receipts to the inventor; letters to Urey from Gibbs, 8 Aug, and from Howard S. Neiman, 13 Aug 1940 (Urey P, 8).
[151] Kamen, Radiant science , 127–30, and Science, 140 (1963), 588–9.
But for reasons similar to those that inhibited the discovery of the activity of H3 , C14 did not appear to possess the characteristics desired by radiobiologists. For C14 had already been discovered at the Laboratory. In 1934, in one of his first observations with his cloud chamber, Kurie had seen half a dozen tracks that indicated a new sort of radiation stimulated by neutrons. In addition to the then known (n,a ) process, he saw what he interpreted as (n,p) reactions on air, either N14 (n,p)C14 or O16 (n,p)N16 . His attribution, challenged by the Cavendish, was established by Bonner and Brubaker in 1936.[152] Kurie and Kamen then studied the profusion of (n,p) events made possible by the cyclotron's enlarged neutron flux; they found the recoil tracks of the C14 ions and the liberated protons to be plentiful and conspicuous enough to serve as a measure of the stopping power of the air in the cloud chamber.[153]
From general considerations set forth by Bethe and Bacher, not more than one of a set of isobars can be stable. But N14 is stable. The beta decay of C14 to N14 would depend upon their difference in mass, which Bethe and Bacher estimated at 100 or 200 MeV. "Assuming the b -transitions to be allowed, the lifetimes would be between 1/2 and 20 years." In the same bit of beryllium irradiated by deuterons in which he identified Be10 (in fact H3 ), McMillan had also noted a weak activity of about three months, which he ascribed to C14 made via (d,p) on a carbon impurity in the target. He then tried to make C14 in quantity by (n,p) by exposing ammonium nitrate to neutrons from the 37-inch cyclotron; the experiment ended with the accidental breakage of the salt's container and was not renewed.[154] Livingston and Bethe accepted McMillan's estimate of a half-life of several months and Oppenheimer's student Phillip Morrison refined and confirmed their calculations. But careful search, notably by Ernest Pollard of Yale, who tried (d,p) on C13 and (a ,p) on B11 , found the product
[152] Kurie, PR, 45 (1934), 904, letter of 15 June, and PR, 46 (1934), 330; Chadwick and Goldhaber, Camb. Phil. Soc., Proc., 31 (1935), 612; Bonner and Brubaker, PR, 49 (1936), 778; Livingston and Bethe, RMP, 9 (1937), 344.
[153] Kamen, Radiant science , 123–4, and Science, 140 (1963), 585.
[154] Bethe and Bacher, RMP, 8 (1936), 101–3, 201; McMillan, PR, 49 (1936), 875–6; Kamen, Science, 140 (1963), 586.
protons, and calculated that C14 should yield soft beta rays, disclosed no activity ascribable to any carbon isotope.[155] Hence Kamen's doubt that anything interesting would result from bombarding charcoal with deuterons.
The first tests confirmed his pessimism. Carbon removed from a target of calcium carbonate did not excite a thin-walled Geiger counter. When placed inside a screen-walled counter devised by two of Ruben's colleagues, however, it gave some weak signs, about half the usual background count. That equivocal message marked the discovery of the most important radioactive tracer found at the Laboratory. On leap-year day 1940 the good news was sent for publication.[156] The reason that the decay of C14 had been so difficult to detect is that its period is very long. Ruben and Kamen first estimated "years;" a larger irradiation (some 13,000 µAh) of a better probe target enriched in C13 allowed a much higher and better estimate, thousands of years. That was a puzzle for the theorists, who, however, in the persons of Oppenheimer and L.I. Schiff, helpfully remarked that the nuclei of C14 and N14 must differ enough in angular momentum to retard the decay by the amount observed.[157] In any case, the long-sought long-lived carbon was in hand, and it remained only to discover how to make it in sufficient amounts to satisfy the expected large demand. Kamen and Ruben returned to Kurie's process, N14 (n,p)C14 , irradiating large carboys of concentrated ammonium nitrate with neutrons from the 60-inch cyclotron. Although the yield was greater and the recovery easier than with deuteron-irradiated graphite, Lawrence ordered the process discontinued. He had heard that ammonium nitrate presented a serious hazard of explosion and no weight of chemical opinion could persuade him of its safety in solution.[158]
[155] Livingston and Bethe, RMP, 9 (1937), 344; Pollard, PR, 56 (1939), 1168, letter of 14 Nov; Kamen, Radiant science , 126. Pollard had irradiated his carbon for twelve hours with 0.5 µA, which, he supposed, would not give a detectable activity if the period of C were several years or more.
[156] Ruben and Kamen (1940), 549, letter of 29 Feb; Libby and Lee, PR, 55 (1939), 245.
[157] Kamen and Ruben, PR, 58 (1940), 194, late June 1940; Ruben and Kamen, PR, 59 (1941), 350–1, 354.
[158] Ibid., 351–2; Kamen to Urey, May 1940 (10/10); Kamen, Radiant science , 139–40, and Science, 140 (1963), 589–90.
Lawrence lost no time informing Urey of the miraculous appearance of C14 and in asking him for a sample of purified C13 to serve as a target for Kamen. That, he intimated, would be the true value of Urey's separation process; C14 would not depress the market for C13 ; "quite the contrary." The rules of science are strict. "Needless to say [Urey replied] we will surely send him the material he wants." But then the obvious question arose: why go to the trouble and expense of transmuting C13 into C14 for tracing if C13 will serve au naturel? Calculations by Urey, Kamen, Ruben, and Tuve concurred. As Kamen put it, C14 was "an ace in the hole," something for very special applications, such as the study of photosynthesis, where the big molecules involved might dilute the natural isotope past detection. To make a quantity of C14 useful for the purpose would require a long time on the machine. "It is quite beyond all probability to make more than one or two such strong samples in your or my lifetime as conditions are at present."[159] Urey gave Kodak his apparatus and encouraged them to proceed. They agreed to make N15 and, if that succeeded, to try C13 . Kodak did not like Urey's method of separation, which used deadly hydrogen cyanide gas, and they worried that the more prolific route via N14 would make C13 unnecessary.[160]
Although Lawrence, Kamen, and Ruben joined Urey and others in urging the commercial production of C13 ,[161] Kodak did not move to serve the mass market of biotracers before the war. The frustration of having too little heavy carbon to unlock the secrets of life was eased by war. In 1945 Urey again tried to enlist Kodak. Again the question: if C14 can be made plentifully without separated C13 , why invest in a heavy-carbon plant? But if not,
[159] Lawrence to Urey, 24 Feb 1940; Urey to Lawrence, 29 Feb and 1 Mar 1940; Kamen to Urey, 13 May 1940, quote, all in 17/40; Tuve to Urey, 9 May and 3 June 1940 (MAT, 25/"biophys. 1940").
[160] Urey to C.E.K. Mees (Kodak), 27 Apr, 2 May, 12 Aug 1940; Mees to Urey, 1 and 29 May 1940, all in Urey P, 3/M; Urey to Hevesy, 25 Oct 1940 (Urey P, 2), and to Lawrence, 27 Oct 1940 (17/40).
[161] Lawrence to Urey, 3 Oct 1940 (17/40); Urey to Mees, 6 Nov 1940 (Urey P, 3/M).
Kodak should come to the rescue, lest the country "lose years in applying the tracer technique to chemical and biological problems."[162] Kodak wisely remained out of the picture. The tremendous neutron fluxes in the piles built during the war created C14 in plenty. Thus the sciences of life gained "the most important tracer available among the artificial radioactive elements."[163]
[162] Urey to Otto Beeck, Shell Development Co., 21 Mar, and to Paul H. Emmett, Mellon Institute of Industrial Research, 12 Apr and 9 Jul, and to A. Keith Brewer, National Bureau of Standards, 16 Jul 1945; letters to Urey from W.O. Kenyan, Kodak, 12 Sep, Mees, 11 Jul, and A.O.C. Nier, 20 Aug 1945, quote, all in Urey P, 1/"misc. corresp."
[163] Kamen in Wisconsin, Univ., Symposium , 150.