A. Precis of Modern Structure Theory
Valence refers to the number of bonds an atom can form with its neighbors. Organic chemists normally regard hydrogen and halogen atoms (fluorine, chlorine, bromine, and iodine) as monovalent, oxygen and sulfur as divalent, nitrogen and phosphorus as trivalent, and carbon atoms as tetravalent. These elements provide the building blocks for the large majority of all organic compounds. Following the valence rules, one can schematically construct organic compounds by linking the atoms together. The simplest organic compound is methane, CH4 ; the carbon atom is tetravalent and each of the hydrogen atoms is monovalent, so the rules are satisfied and the compound is stable. The first homolog (that is, next in the same chemical series) of methane is ethane, constructed by removing one of the four hydrogen atoms of methane (which produces a methyl radical, -CH3 , sometimes symbolized Me) and filling the vacant valence with another methyl group: H3 C-CH3 . Note that conditions of carbon tetravalence and hydrogen monovalence are still met.
Taking one hydrogen from each carbon atom of ethane will schematically construct ethylene, the simplest of the class of hydrocarbons known as olefins : H2 C=CH2 . Note that the two unoccupied valences on the two adjacent carbons may be considered to have linked with each other. Olefins by definition have at least one double bond between adjacent carbon atoms, so that each of these atoms has only two remaining valences for attachment to hydrogen atoms. Ethylene chloride results from the addition of a chlorine molecule, Cl2 , to ethylene, with the carbon-carbon double bond thus disappearing:
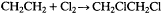
Substances containing only carbon and hydrogen (hydrocarbons ) are mostly fairly unreactive and chemically uninteresting compounds. In general, chemical interest is created by the introduction of heteroatoms , i.e., atoms other than carbon or hydrogen. The most common and important of these is oxygen. Heteroatoms, or any multiple bonds, tend to create chemically reactive sites within an organic molecule and are called functional groups . Most reactions center on such groups of atoms.
Some examples will help. If a hydrogen atom is removed from either carbon atom of ethane (creating ethyl, CH3 CH2 -, often symbolized Et) and is replaced by a hydroxyl radical, -OH, one obtains ethyl alcohol, CH3 CH2 OH. if one then extracts the remaining two hydrogens from the second carbon atom of alcohol and replaces them with one double-bonded oxygen atom, we have the formula for acetic acid, CH3 COOH. The -COOH or CO2 H group (O=C-OH, with one valence still remaining on the carbon atom) is known as carboxyl . An intermediate stage of oxidation between alcohol and acid is
aldehyde, CH3 HC=O; it is carboxyl without the second oxygen atom. Two carboxyl groups connected together (or, equivalently, acetic acid in which the three hydrogens attached to the first carbon atom are replaced by =O and -OH), is the simplest dibasic (i.e., double) organic acid, oxalic acid. It can be obtained by vigorous oxidation of acetic acid or alcohol. If the oxidation is done more carefully or indirectly, other functional groups can be introduced. Glycol has hydroxyl groups on both carbon atoms; it is a double alcohol. Glyoxal has two aldehyde groups on the two carbons, a double aldehyde. Glycolic acid, a hydroxy-acid, is acetic acid in which one of the hydrogens of the methyl group is replaced by hydroxyl, HOCH2 CO2 H. Analogous homologs can be created from the three-carbon hydrocarbon, propane, and from the three-carbon acid, propionic acid, CH3 CH2 CO2 H. Propionic acid with a hydroxyl group on the middle carbon is lactic acid; the trialcohol with a hydroxyl on each carbon atom is glycerin.
This gives only a sample of the richness of organic chemical formulas, but it accurately reflects the sort of schematic manipulations that characterize structural organic chemistry.