PART I—
POPULATION ECOLOGY
Two—
History and Present Status of the Northern Elephant Seal Population
Brent S. Stewart, Pamela K. Yochem, Harriet R. Huber, Robert L. DeLong, Ronald J. Jameson, William J. Sydeman, Sarah G. Allen, and Burney J. Le Boeuf
ABSTRACT. The northern elephant seal, Mirounga angustirostris , was presumed extinct by 1892 owing primarily to commercial harvesting for their blubber oil that began in the early 1800s. A small, residual breeding colony survived, however, and with legal protection from further hunting, it grew rapidly through the early 1900s. Immigrants steadily colonized other island and mainland sites in Baja California and California so that by 1991 seals were breeding on fifteen islands and at three mainland beaches. Sixty-four percent of 28,164 northern elephant seal pups born in 1991 were produced on two southern California Channel Islands, San Miguel and San Nicolas. The entire elephant seal population was estimated to number around 127,000 in 1991 and was apparently still increasing by more than 6% annually. The remarkable demographic vitality and sustained population increase of northern elephant seals has evidently been unaffected by the species' low genetic variability and contrasts with recent declines of some populations of the more genetically polymorphic southern elephant seal, M. leonina .
Few, if any, living species today have been so deeply scored, so driven to the very brink of extermination
—L. M. Huey (1930)
Numerous terrestrial and marine species, like the northern elephant seal, experienced great population reductions in the nineteenth and twentieth centuries. But the single remarkable fact about the history of the northern elephant seal population is that despite only narrowly averting extinction, it rebounded with an unparalleled, century-long period of exponential increase (see, e.g., Reeves, Stewart, and Leatherwood 1992 and McCullough and Barrett 1992 for reviews of trends in pinnipeds and other vertebrates). Here we briefly review the population reduction and document its impressive recovery. We focus on number of births as an index of growth during the past three decades and estimate current population size.
Prehistory
Northern elephant seals lived in California waters by the late Pleistocene, evidently derived from monachine ancestors (Callophoca group) that entered the Pacific Ocean from the Caribbean through the Central American Seaway in the early Pliocene (Hendey 1972; Barnes and Mitchell 1975; Repenning, Ray, and Grigorescu 1979; de Muizon 1982). Little is known about their distribution during the Pleistocene when dynamic eustatic changes (Orr 1967; Vedder and Howell 1980) both greatly increased and decreased shoreline habitat available to pinnipeds, but archaeological remains show that elephant seals were in southern California waters when humans colonized the region over 15,000 years ago (e.g., Walker and Craig 1979; Snethkamp 1987; Bleitz 1993). Relatively large numbers of aboriginals lived on most of the California islands through the early nineteenth century, using the diverse marine resources on and near the islands for food, clothing, and housing; elephant seals and other pinnipeds were particularly important to aboriginal subsistence (Meighan 1959; Reimnan 1964; Glassow 1980; Stewart et al. 1993).
Commercial Exploitation
Elephant seal, sea otter, whale, and fur seal hunters operated on and around the California islands from the early 1800s through the 1860s (Scammon 1870, 1874; Ogden 1933, 1941), but they left few records of northern elephant seal harvests. By 1850, northern elephant seals were scarce (Scammon 1870, 1874); it was not until 1866 that northern and southern elephant seals were scientifically recognized as taxonomically distinct (Gill 1866; but see Stewart and Huber 1993).
What we know to be incontrovertible about northern elephant seals in the early and mid-1800s is the following. Their distribution and abundance prior to 1840 is unknown. A few northern elephant seals were killed by sealers at Islas Los Coronados in 1840 and 1846, at Santa Barbara Island in May 1841, and at Cedros and Guadalupe islands in 1846 (Busch 1985). Scammon made a disappointing sealing expedition along the California coast in 1852; during a 5-month period he collected about 350 barrels of oil (Scammon 1874), probably the equivalent of around 100 to 200 adult elephant seals (see Busch 1985). Another 10-month expedition in 1857 met with even less success. Between 1865 and 1880, only a few elephant seals were reported at Isla de Guadalupe and Islas San Benito. Because all were killed as they were encountered, the species was considered extinct by the late 1870s (Townsend 1885). But in 1880, a small herd was discovered on the Baja California mainland south of Isla Cedros, at Bahia San Cristobal (fig. 2.1). Over the next four years, all 335 seals that were seen were killed by
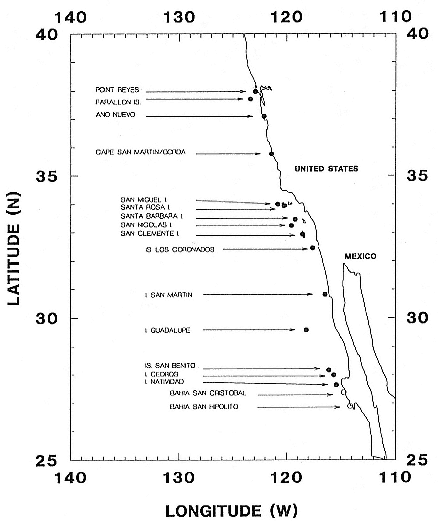
Fig. 2.1
Location of northern elephant seal colonies in 1991 (closed circles) and
other alleged historical rookeries (open circles) in U.S. and Mexican waters.
the crews of six ships that visited the beach regularly, mostly in autumn. Three years later, in 1883, 80 elephant seals were found and killed at Isla de Guadalupe, and 4 were killed there in 1884. The species was again considered extinct, and no elephant seals were seen until May 1892, when C. H. Townsend and A. W. Anthony discovered 9 at Isla de Guadalupe; 7 of them were killed for the Smithsonian's museum collection (Townsend 1912; Anthony 1924). "This action was considered justifiable at the time, as the
species was considered doomed to extinction by way of the sealer's trypot and few if any specimens were to be found in the museums of North America" (Anthony 1924: 146). The species was again presumed extinct, for the third time. But small numbers continued to show up at Isla de Guadalupe through 1911, and museum collectors continued to kill them: 4 in 1904 (Townsend 1912) and 14 of 40 on May 26, 1907. "This was a severe stroke dealt to a struggling species, but the appetite of science must be satisfied" (Huey 1930: 189). Townsend returned to Isla de Guadalupe on March 2, 1911, and killed 10 more seals but this time left 125 alive on the beach; on his return voyage to San Diego he searched for elephant seals at Bahia San Cristobal, Islas San Benito, and Isla Cedros but found none (Townsend 1912).
G. A. Bartholomew and C. L. Hubbs (1960), based on their interpretations of published counts of seals from the early 1900s, estimated that the total population in 1890 numbered fewer than 100 animals and speculated that it may have been as small as 20. The actual number of elephant seals that were present during the population bottleneck (or bottlenecks) in the 1800s and early 1900s is unknown because of the following flaws in sightings reports: (1) in most cases, the dates of sightings were not reported; (2) many sightings for which dates were provided were during the nonbreeding season; and (3) the age and sex composition of the seals observed was not determined. This information is vital because the number of seals on land, as well as the composition with respect to age and sex, varies greatly with time of year (Bartholomew 1951; Le Boeuf and Bonnell 1980; Stewart 1989). For example, when Townsend (1912) visited Isla de Guadalupe on March 2, 1911, and left 125 seals alive, it would have been at the end of the breeding season. At this time, some adult males should have been present, but nearly all females should already have returned to sea, leaving their weaned pups behind. Townsend noted that the herd consisted mostly of large males and immature animals of various sizes but that there were more than 15 adult females and 6 newborn young present. The photographs he took, however, show that most of the other "immature" seals were weaned pups, and it is likely that most of the seals ashore were actually molted pups-of-the-year (i.e., about 2 months old). He, like other early authors, also concluded erroneously that early March was the beginning of the breeding season, rather than the end, which emphasizes just how little was known about the natural history of elephant seals before George Bartholomew began his pioneering work on the species in the 1940s (e.g., Bartholomew 1952).
Regardless of whether the bottleneck population numbered in the tens or perhaps low hundreds, the important point is that the thousands of elephant seals alive today are all descendants of that small remnant herd.
Initial Recovery:
1900–1965
Northern elephant seals bred only at Isla de Guadalupe from the late 1890s through the 1920s. The colony grew steadily, despite sporadic poaching and scientific collecting (Bartholomew and Hubbs 1960). That early period of increase was chronicled by W. Rothschild (1908, 1910), C. M. Harris (1909), C. H. Townsend (1912), A. W. Anthony (1924) and L. M. Huey (1924, 1925, 1927, 1930) and thoroughly reviewed by Bartholomew and Hubbs (1960). On July 12, 1922, when mostly adult males were ashore molting, 264 seals were counted; a few months later, the Mexican government declared Isla de Guadalupe a biological reserve, and the seals were afforded protection from harassment and poaching (Hanna 1925). From that time on, elephant seals expanded their range; K. W. Radford, R. T. Orr, and C. L. Hubbs (1965) reviewed observations of seasonal migrants during the early 1900s along the coast from San Diego to southeastern Alaska. Other sightings were reviewed by Bartholomew and Hubbs (1960); seals were first seen on Islas San Benito in 1918, San Miguel Island in 1925, Los Coronados and Santa Barbara Island in 1948, San Nicolas Island in 1949, and Año Nuevo Island in 1955. Breeding evidently began in the 1930s at Islas San Benito, in the early 1950s at San Miguel, San Nicolas, and Santa Barbara islands (Bartholomew and Boolootian 1960; Odell 1974; Stewart 1989), and in 1961 at Año Nuevo Island (Radford, Orr, and Hubbs 1965).
From published and available unpublished counts, Bartholomew and Hubbs (1960) estimated that the total population numbered approximately 13,000 in 1957 and approximately 15,000 in 1960, with about 91% of the population residing at Isla de Guadalupe, 8% at Islas San Benito, and 1% on the Channel Islands.
Recent Trends and Present Status:
1965–1991
Documentation of the population's recovery improved as more became known of the seasonal patterns of terrestrial abundance in the 1950s and 1960s. Table 2.1 lists births at each rookery from 1958 through 1991. The methods used varied slightly among colonies (see appendix 2.1), but all yielded estimates of births either from combined direct counts of suckling, weaned, and dead pups or derived from corrected counts of adult females made during peak breeding season (late January). Most pup counts were made on foot in February, after most births had occurred but before pups had left the rookeries. Some Mexican beaches with difficult access were surveyed from boats. The data for the three islands of Islas San Benito are combined in table 2.1 because of their closeness to each other; data for Año
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Nuevo Island and Año Nuevo mainland are combined for the same reason. From the data in table 2.1, we conclude the following.
The total elephant seal population, as reflected by births, increased 6.3% annually (= finite rate of increase, l , where l = er ; see appendix 2.2) from 1965 through 1991 (r = .061; R2 , the coefficient of determination = .947; p, the significance of slope ¹ 0, < .001; see appendix 2.2). C. F. Cooper and B. S. Stewart (1983) calculated its increase at 8.3% from 1965 through 1977. The lower rate that we calculated here for the entire period (1965–1991) is evidently due to the lack of any apparent increase in Mexico since 1970. Growth of the total population from 1965 through 1991 was due primarily to growth at California rookeries, where births increased 14.1% annually (r = .132, R2 = .901, p < .001), only slightly less than from 1965 through 1982 (l = 1.145; Cooper and Stewart 1983).
Births increased slightly in Mexico between 1965 and 1970 but have not changed since then (fig. 2.2; slope of regression of births on time = 0, p = .903; see appendix 2.2). D. W. Rice, K. W. Kenyon, and D. Lluch B. (1965) suggested that carrying capacity of the Isla de Guadalupe rookery was reached by 1960. Counts made since then at the largest breeding beaches at Isla de Guadalupe support that conclusion; virtually all breeding space is now occupied and crowded during peak breeding season (J. P. Gallo-Reynoso and A. Figueroa-Carranza, unpubl. data). Because there are few recent counts at Islas San Benito, the trends on these islands are less clear (table 2.1). However, surveys of the central island (the easiest of the three to census and the site at which the data are most complete) show steady growth since 1970 (B. J. Le Boeuf, unpubl. data; B. S. Stewart, unpubl. data; J. P. Gallo-Reynoso and A. Figueroa-Carranza, unpubl. data). Births almost tripled from the early to mid-1970s (table 2.1). The central island accounted for 28.1, 37.2, and 45.1% of births on the entire island group in 1970, 1977, and 1980, respectively. If we assume that the 1,666 pups produced on the central island in 1991 accounted for 37% of the total pup production in that year, then the rookery produced 4,500 pups in 1991 and the colony is evidently still increasing. This is our tentative conclusion, but we must be guarded about the accuracy of the estimate. Some of the increase of central island numbers may have resulted from movements of seals from the west island where tourist and fishing activities have increased during the past two decades. Despite the increases at Isla Cedros and Islas San Benito, the Mexican population has not changed substantially during the past two decades, evidently because births at Isla de Guadalupe have declined, after peaking in the late 1960s (table 2.1).
The rapid increase in births at San Miguel Island, the largest colony in the species' range, accounts for most of the growth in California. Elephant seals bred only at the western tip of the island in 1968 (Le Boeuf and Bon-
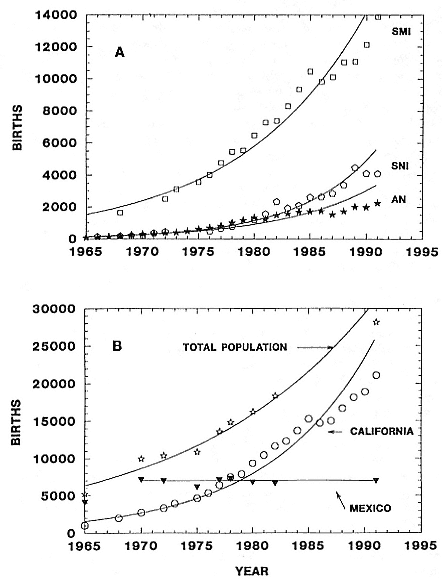
Fig. 2.2
Growth of the northern elephant seal population as reflected by births.
(A) Increases in births at San Miguel Island (SMI), San Nicolas Island (SNI), and
Año Nuevo Island and mainland combined (AN). (B) Growth of the entire
northern elephant seal population, California segment, and Mexican segment.
An intense El Niño affected North Pacific waters from late 1982 through 1983
(see text for details of immediate and delayed effects on northern elephant seals).
nell 1980; R. L. DeLong, pers. observ.). In subsequent years, breeding groups appeared farther east, so that by 1980, seals were breeding along the entire southern coast (Stewart 1989, 1992). Some of the northern beaches. however, are unused still. Births increased 9.3% annually (r = .089, R2 = .963, p < .001) from 1965 through 1991; that growth and the coincident eastward expansion of breeding led to the colonization of Santa Rosa Island in 1985 (Stewart and Yochem 1986). Growth at San Nicolas Island (the second-largest colony), where expansion has followed patterns similar to those at San Miguel Island (Stewart 1989, 1992), has also been rapid (l = 1.158, r = .147, R2 = .976, p < .001). The brief decline in California births in 1985 was evidently due to poor recruitment of pups (owing to poor survival or retarded maturation or both; Huber, Beckham, and Nisbet 1991; Le Boeuf and Reiter 1991; B. S. Stewart, unpubl. data) that were born just before and during the 1982–1983 El Niño Southern Oscillation event. Pregnancy rates declined temporarily at some rookeries in 1984 and 1985 but there is no evidence that adult survival changed as a result of this intense oceanographic perturbation (Huber, Beckham, and Nisbet 1991; Le Boeuf and Reiter 1991).
Many new colonies formed in the last three decades, including at least three in Mexico. Elephant seals have clearly established breeding colonies on Isla Cedros and Islas Los Coronados. Births increased eightfold at Isla Cedros, an island that could sustain many more seals. Breeding space is limited on Islas Los Coronados, so carrying capacity has evidently been reached. Pups have been born on Isla Natividad, but monitoring of this island has been poor. At least two pups were produced on Isla San Martín (not shown in table 2.1) in 1978 (Le Boeuf and Mate 1978), but heavy human traffic on this island may preclude future growth.
California has at least six colonies that were founded since 1960. The San Clemente Island and the Santa Rosa Island colonies are in southern California. The other four colonies are in Central California; Cape San Martín/Gorda and Point Reyes Headlands are on the mainland, the Año Nuevo colony occupies both a small island and the immediate mainland, and the South Farallons colony is on an island (fig. 2.1). Año Nuevo Island reached carrying capacity in the late 1970s with annual production slightly under 1,000 pups. The colonization of Point Reyes Headlands in 1981 (Allen, Peaslee, and Huber 1989) is evidently linked to growth of the Año Nuevo and South Farallon Islands colonies. Births are still increasing at Año Nuevo and at Point Reyes Headlands. The recent explosive increase in births on beaches near Cape San Martín/Gorda is difficult to explain. Pups were first born in the area on a small, steep-backed gravel beach about 1 km north of Cape San Martín in 1981 or perhaps 1980. Breeding was restricted to that exposed site until 1989 when seals abandoned it and be-
gan using a longer gravel beach about 2 km south near Gorda. The better protection of that site against winter storms and surf was evidently more attractive to pregnant females, as indicated by the fourfold increase in births in the past two years (table 2.1).
Colonization Process, Immigration and Emigration
Births increased rapidly following colonization of all sites (table 2.1, fig. 2.2), and several colonies are still in this incipient growth stage. Immigrants from Isla de Guadalupe almost certainly colonized the other islands in Mexico and those in southern California. Our observation of the movement patterns of tagged seals during the past three decades (Condit and Le Boeuf 1984; B. J. Le Boeuf, unpubl. data; B. S. Stewart, unpubl. data) support that idea and also indicate the following: Año Nuevo was colonized by immigrants from San Miguel Island and to a lesser extent, immigrants from San Nicolas Island; the South Farallon Islands were colonized by immigrants from San Miguel, San Nicolas, and Año Nuevo islands (Le Boeuf, Ainley, and Lewis 1974; Huber et al. 1991). Some rookeries established in the 1980s were colonized by seals from neighboring rookeries. For example, Point Reyes Headlands was initially colonized by seals from the South Farallon Islands and Año Nuevo, and only recently have immigrants from San Miguel and San Nicolas islands been observed there (Allen, Peaslee, and Huber 1989; S. G. Allen, unpubl. data).
Some northern rookeries (e.g., Año Nuevo) in the expanding part of the range apparently still owe their growth more to a high immigration rate than to internal recruitment (which fuels most of the growth at rookeries at San Nicolas and San Miguel islands). Reproductive success of females at Año Nuevo has not been sufficient to account for the increases there (Le Boeuf and Reiter 1988). San Miguel Island seems to be the main source of immigrants. Immigration is also the primary cause of growth at the South Farallon Islands colony (Huber et al. 1991), where immigration rates from Año Nuevo, San Miguel Island, and San Nicolas Island were 3.9, 1.9, and 0.6%, respectively, between 1974 and 1986. These immigration rates were positively correlated with proximity to the South Farallon Islands.
Seals began colonizing new areas before carrying capacities were reached at most natal beaches. For example, Channel Islands colonists began breeding at Año Nuevo Island at least 20 years before San Miguel or San Nicolas Island habitats became crowded (see Orr and Poulter 1965; Stewart 1989, 1992). Similarly, Año Nuevo Island colonists began breeding at the South Farallon Islands and at Año Nuevo mainland 6 to 8 years before the island reached carrying capacity (see Le Boeuf, Ainley, and Lewis 1974; Reiter, Panken, and Le Boeuf 1981; Le Boeuf and Reiter 1991).
Total Population Size
The dynamic age structure of the northern elephant seal population (e.g., Huber et al. 1991) hinders accurate predictions of present population size from pup counts or of total seals hauled out at any time. Estimates of births are, however, useful for estimating rate of change in population size, although such calculations have problems (e.g., see Berkson and DeMaster 1985). Despite some obvious shortcomings, we use pup counts as a convenient index of population growth because superior measures of life history parameters are not available for each rookery.
Total population size may be about 3.5 to 4.5 times births (e.g., Hewer 1964; Bonner 1976; Harwood and Prime 1978, Stewart 1989). For comparison with southern elephant seals (Laws, this volume), we use T. S. McCann's formula and multiply births by 3.5 to estimate total population size at the end of the breeding season, exclusive of pups (McCann 1985). From table 2.1, we multiply 3.5 times the 28,164 pups born in 1991 to obtain the estimate of 98,574 elephant seals older than pups in the entire population in 1991. If the young of the year are added to this figure, there were approximately 127,000 elephant seals in existence in early spring 1991.
In 1991, Mexican rookeries contributed 25.5% of all births and California, 74.8%; San Miguel Island alone produced nearly half (49.3%) of all elephant seal pups. The world total of southern elephant seals in 1991 (Laws, this volume) was roughly 6.8 times larger than that of northern elephant seals.
Future Growth
The northern elephant seal has lived in eastern North Pacific waters for at least several hundred thousand years. Their occurrence and apparent vitality in these waters today is remarkable considering their fortuitous emergence in the twentieth century after facing extinction in the nineteenth century. There seem to be few barriers to the species' continued population growth and range expansion. In the immediate future, growth of the population will probably be determined primarily by events on southern California rookeries. Growth at San Nicolas and San Miguel islands will almost certainly slow as the limited remaining habitat becomes occupied and as crowding on those islands constrains reproductive success. The new colony at Santa Rosa Island, however, has substantial breeding beach habitat that could support continued rapid growth in California. Neighboring Santa Cruz Island also offers some additional habitat, although of poorer quality than at Santa Rosa Island. The seals may also continue their northward expansion. They are now hauling out at Cape St. George in northern
California, at Cape Arago in Oregon, and on Vancouver Island in British Columbia. Recent information on seasonal movements and foraging locations of northern elephant seals (see DeLong, Stewart, and Hill 1992; Stewart and DeLong 1993; Stewart and DeLong, this volume; Le Boeuf, this volume) suggests that eventual breeding at these sites is quite plausible.
M. L. Bonnell and R. K. Selander (1974) found that northern elephant seals were homozygous at 23 loci coding for 20 blood allozymes and suggested that this was due to a loss of genetic variability when elephant seals were reduced to small numbers in the 1800s. Recent research on nuclear and mitochondrial DNA (Hoelzel et al. 1991; Lehman, Wayne, and Stewart 1993) reaffirms the earlier findings of low heterozygosity, although these studies revealed greater levels of variability than the electrophoretic analysis of blood allozymes did. Reduced genetic variability may compromise the population viability of some species of mammals (e.g., O'Brien et al. 1985; O'Brien et al. 1987), but many other species have persisted for a long time despite population bottlenecks, founder events, isolation, inbreeding, and low levels of genetic variation (e.g., Gill 1980; Nevo, Beiles, and Ben-Shlomo 1984; Gilbert et al. 1990; Benirshke and Kumamoto 1991; Wayne et al. 1991). A lack of substantial genetic variability has not limited the phenomenal population recovery of northern elephant seals. Indeed, their recovery contrasts ironically with the recent decline of some populations of the closely related southern elephant seal (Laws, this volume), which is genetically more polymorphic (McDermid, Ananthakrishna, and Agar 1972; Hoelzel et al. 1991). The consequences of low genetic variability for future population growth of northern elephant seals are unpredictable.
Appendix 2.1
Field Data Collection Methods
Survey methods differed slightly among rookeries as described below due to differences in colony size, dispersion, and logistical constraints. Nonetheless, our studies produced annual estimates of births and, in most cases, neonatal mortality at each colony.
San Miguel Island
Each year in late February two or three people walked among and counted weaned, suckling, and dead pups at all beaches on SMI. Observers' counts of live pups were compared after each relatively small group (< 100) was counted; counts usually differed by less than 2%, but if the tallies differed by 5% or more, the group was counted again. In this way an entire cohort of pups distributed along approximately 30 km of shoreline could be surveyed in two or three days.
San Nicolas Island
Each breeding season, surveys were made every one to two days at three sites and once each week at all breeding sites along the
35 km of coastline of SNI. Weaned and suckling pups were tallied two or three times during each survey, and dead pups were marked and mapped to ensure that those that died prior to weaning were accounted for but none more than once. The number of births and pup deaths was determined for each breeding site and summed at the end of the season to determine total annual production.
Santa Rosa Island and Santa Barbara Island
The numbers of live pups present on SRI and SBI were determined by photographing them during aerial surveys in late January. These counts were corrected to estimate each year's births according to seasonal phenology of births documented by Stewart (1989). Pup mortality was not determined.
Cape San Martín
Nursing, weaned, and dead pups were counted periodically in January, February, or early March each year at various small beaches within 2 km of Cape San Martín beginning in 1981. Preweaning mortality could not be determined accurately.
Año Nuevo
Most estimates of births were derived from daily or weekly counts during the breeding season of all seals present; those counts included dead, suckling, and weaned pups. In some circumstances births were estimated as follows: (1) counts of females present at ANI and ANML in late January were first adjusted to account for those that had already left the rookeries and for those that had not yet arrived to provide an estimate of the number of females that visited during the breeding season; (2) 98% of the females estimated to have hauled out were assumed to have given birth. Prior to 1980, all or most pups that died were accounted for by removing them from breeding aggregations or marking them with paint or dye. Since 1980, preweaning mortality on the island has been estimated as follows: (1) weaned and suckling pups were counted on March 1 or 2 to yield an estimate of pups weaned for the season; (2) mortality was then derived by subtracting that estimate from an estimate of the number of females that gave birth during the season (as summarized above). Some estimates of births reported here (table 2.1) are corrections of those published earlier.
South Farallon Islands
Prior to 1987, births were determined in several ways depending on breeding location at SFAR. Pup carcasses were removed from the nine breeding sites whenever possible. At sites that were not washed by high tides, all deaths were accounted for because all females that gave birth, and their pups, were marked with hair dye. At other sites, observations of a female's appearance (i.e., blood on her hind quarters) or behavior were used to determine if newborn pups had disappeared (and
presumably drowned) undetected during high tides or storms. Weaned pups were counted at the end of the breeding season and added to estimates of records of dead pups to derive an estimate of each season's births.
Beginning in 1987, births at the primary breeding site were determined as follows: (1) a peak count of females was made in late January; (2) based on reproductive characteristics of tagged females observed during the season, 93.2% of the females present during the peak count were assumed to have given birth; and (3) the estimate was adjusted to account for females that had departed already or had not yet arrived. Pup mortality was estimated indirectly by subtracting the number of weaned pups counted in late February from the estimate of births derived from the peak season female count. Births and pup deaths at the other eight breeding sites were determined by monitoring all females that were present and that were uniquely marked with hair dye.
Point Reyes Headland
Counts of live and dead pups were made at least weekly from bluffs overlooking beaches along the Point Reyes Headland. Estimates of pup deaths are rough minima because some carcasses probably washed out to sea undetected between observations. Estimates of births were made by adding the peak count of live pups to estimates of deaths that occurred prior to that count.
Mexican Islands
Surveys were made opportunistically on foot or from skiffs nearshore from 1968 to 1991 at IG, ISB, IC, and at other small rookeries in Mexico (fig. 2.1, table 2.1). Complete surveys of Isla de Guadalupe's west side were rarely made because of rough island terrain and heavy seas near the coastlines. We report only counts made near the end of the breeding season when nearly all births had occurred but when few pups had departed the rookeries.
Appendix 2.2
Analyses of Rates of Change of Elephant Seal Births
We calculated observed rates of increase (r = intrinsic rate of increase) in births by linear regression (Zar 1974). We examined the fit of an exponential model, Loge number of births regressed on time where r is the slope of the regression line (Caughley and Birch 1971; Caughley 1977). We present the coefficient of determination (= R2 to distinguish it from rate of increase) to describe the proportion of the total variation in births that is accounted for by time. For comparative purposes, we convert exponential rates (r) to finite rates (er = l ; Caughley 1977: 6). When the exponential model fit poorly, we used a linear model of births regressed on time. For both mod-
els, we present the level of significance (p) at which we did (if p < .05) or did not (if p > .05) reject the null hypothesis that the slope of regressions did not differ from zero.
References
Allen, S. G., S. C. Peaslee, and H. R. Huber. 1989. Colonization by northern elephant seals of the Point Reyes Peninsula, California. Marine Mammal Science 5: 298–302.
Anthony, A. W. 1924. Notes on the present status of the northern elephant seal, Mirounga angustirostris. Journal of Mammalogy 5: 145–152.
Antonelis, G. A., S. Leatherwood, and D. K. Odell. 1981. Population growth and censuses of the northern elephant seal, Mirounga angustirostris , on the California Channel Islands. United States Fishery Bulletin 79: 562–567.
Barnes, L. G., and E. D. Mitchell. 1975. Late Cenozoic northeast Pacific phocidae. Rapports et Proces-verbaux des Reunions Conseil International pour L'exploration de la Mer 169: 34–42.
Bartholomew, G. A. 1951. Spring, summer, and fall censuses of the pinnipeds on San Nicolas Island, California. Journal of Mammalogy 32: 15–21.
———. 1952. Reproductive and social behavior of the northern elephant seal. University of California Publications in Zoology 47: 369–427.
Bartholomew, G. A., and R. A. Boolootian. 1960. Numbers and population structure of the pinnipeds on the California Channel Islands. Journal of Mammalogy 41: 366–375.
Bartholomew, G. A., and C. L. Hubbs. 1960. Population growth and seasonal movements of the northern elephant seal, Mirounga angustirostris. Mammalia 24: 313–324.
Benirshke, K., and A. T. Kumamoto. 1991. Mammalian cytogenetics and conservation of species. Journal of Heredity 82: 187–191.
Berkson, J. M., and D. P. DeMaster. 1985. Use of pup counts in indexing population changes in pinnipeds. Canadian Journal of Fisheries and Aquatic Sciences 42: 873–879.
Bleitz, D. 1993. The prehistoric exploitation of marine mammals and birds at San Nicolas Island, California. In Third California Islands Symposium: Recent Advances in Research on the California Islands , ed. F. G. Hochberg, 519–536. Santa Barbara, Calif.: Santa Barbara Museum of Natural History.
Bonnell, M. L., and R. K. Selander. 1974. Elephant seals: Genetic variation and near extinction. Science 184: 908–909.
Bonner, W. N. 1976. Stocks of grey seals and common seals in Great Britain. Natural Environment Research Council Publications, Series C , 16: 1–16.
Busch, B. C. 1985. The War against the Seals . Montreal: McGill-Queen's University Press.
Caughley, G. 1977. Analysis of Vertebrate Populations . London: John Wiley and Sons.
Caughley, G., and L. C. Birch. 1971. Rate of increase. Journal of Wildlife Management 35: 658–663.
Condit, R., and B. J. Le Boeuf. 1984. Feeding habits and feeding grounds of the northern elephant seal. Journal of Mammalogy 65: 281–290.
Cooper, C. F., and B. S. Stewart. 1983. Demography of northern elephant seals, 1911–1982. Science 210: 969–971.
DeLong, R. L., B. S. Stewart, and R. D. Hill. 1992. Documenting migrations of northern elephant seals using daylength. Marine Mammal Science 8: 155–159.
Gilbert, D. A., N. Lehman, S. J. O'Brien, and R. K. Wayne. 1990. Genetic finger-printing reflects population differentiation in the California Channel Island fox. Nature 344: 764–767.
Gill, A. E. 1980. Evolutionary genetics of California Islands Peromyscus . In The California Islands: Proceedings of a Multidisciplinary Symposium , ed. D. M. Power, 719–744. Santa Barbara, Calif.: Santa Barbara Museum of Natural History.
Gill, T. 1866. On a new species of the genus Macrorhinus. Proceedings of the Chicago Academy of Sciences 1: 33–34.
Glassow, M. A. 1980. Recent developments in the archaeology of the Channel Islands. In The California Islands: Proceedings of a Multidisciplinary Symposium , ed. D. M. Power, 79–99. Santa Barbara, Calif.: Santa Barbara Museum of Natural History.
Hanna, G. D. 1925. Expedition to Guadalupe Island, Mexico—general report. Proceedings of the California Academy of Sciences 14: 217–275.
Harris, C. M. 1909. A cruise after sea elephants. Pacific Monthly 21: 331–339.
Harwood, J., and J. H. Prime. 1978. Some factors affecting the size of British grey seal populations. Journal of Applied Ecology 15: 401–411.
Hendey, Q. B. 1972. The evolution and dispersal of the monachinae. Annals of the South African Museum 59: 99–113.
Hewer, H. R. 1964. The determination of age, sexual maturity and a life-table in the grey seal (Halichoerus grypus ). Proceedings of the Zoological Society, London 142: 593–624.
Hoelzel, A. R., J. Halley, C. Campagna, T. Arnbom, and B. J. Le Boeuf. 1991. Molecular genetic variation in elephant seals; the effect of population bottlenecks. In Abstracts of the Ninth Biennial Conference on the Biology of Marine Mammals , Chicago, Ill., 34.
Huber, H. 1987. Natality and weaning success in relation to age of first reproduction in northern elephant seals. Canadian Journal of Zoology 65: 1311–1316.
Huber, H. R., C. Beckham, and J. Nisbet. 1991. Effects of the 1982–83 El Niño on northern elephant seals at the South Farallon Islands, California. In Pinnipeds and El Niño: Responses to Environmental Stress , ed. F. Trillmich and K. Ono, 219–233. Berlin: Springer Verlag.
Huber, H. R., A. C. Rovetta, L. A. Fry, and S. Johnston. 1991. Age specific natality of northern elephant seals at the South Farallon Islands, California. Journal of Mammalogy 72: 525–534.
Huey, L. M. 1924. Recent observations on the northern elephant seal. Journal of Mammalogy 5: 237–242.
———. 1925. Late information on the Guadalupe Island elephant seal herd. Journal of Mammalogy 6: 126–127.
———. 1927. The latest northern elephant seal census. Journal of Mammalogy 8: 160–161.
———. 1930. Past and present status of the northern elephant seal with a note on the Guadalupe fur seal. Journal of Mammalogy 11: 188–194.
Klopfer, P. H., and B. K. Gilbert. 1966. A note on retrieval and recognition of young in the elephant seal, Mirounga angustirostris. Zeitschrift für Tierpsychologie 6: 757–760.
Le Boeuf, B. J., D. G. Ainley, and T. J. Lewis. 1974. Elephant seals on the Farallones: Population structure of an incipient breeding colony. Journal of Mammalogy 55: 370–385.
Le Boeuf, B. J., and M. L. Bonnell. 1980. Pinnipeds of the California Channel Islands: Abundance and distribution. In The California Islands: Proceedings of a Multidisciplinary Symposium , ed. D. M. Power, 475–493. Santa Barbara, Calif.: Santa Barbara Museum of Natural History.
Le Boeuf, B. J., and K. T. Briggs. 1977. The cost of living in a seal harem. Mammalia 41: 167–195.
Le Boeuf, B. J., D. A. Countryman, and C. L. Hubbs. 1975. Records of elephant seals, Mirounga angustirostris , on Los Coronados Islands, Baja California, Mexico, with recent analyses of the breeding population. Transactions of the San Diego Society of Natural History 18: 1–7.
Le Boeuf, B. J., and B. R. Mate. 1978. Elephant seals colonize additional Mexican and California islands. Journal of Mammalogy 59: 621–622.
Le Boeuf, B. J., and K. J. Panken. 1977. Elephant seals breeding on the mainland in California. Proceedings of the California Academy of Sciences 41: 267–280.
Le Boeuf, B. J., and J. Reiter. 1988. Lifetime reproductive success in northern elephant seals. In Reproductive Success , ed. T. H. Clutton-Brock, 344–362. Chicago: University of Chicago Press.
———. 1991. Biological effects associated with El Niño Southern Oscillation, 1982–83, on northern elephant seals breeding at Año Nuevo, California. In Pinnipeds and El Niño: Responses to Environmental Stress , ed. F. Trillmich and K. Ono, 206–218. Berlin: Springer Verlag.
Lehman, N., R. K. Wayne, and B. S. Stewart. 1993. Comparative levels of genetic variability between harbor seals and northern elephant seals using genetic finger-printing. In Marine Mammals: Advances in Behavioural and Population Biology , ed. I. L. Boyd, 49–60. Symposia of the Zoological Society of London no. 66. London: Oxford University Press.
McCann, T. S. 1985. Size, status and demography of southern elephant seal (Mirounga leonina ) populations. In Sea Mammals of South Latitudes: Proceedings of a Symposium of the 52d ANZAAS Congress in Sydney—May 1982 , ed. L. K. Ling and M. M. Bryden, 1–17. Northfield: South Australian Museum.
McCullough, D. R., and R. H. Barrett, eds. 1992. Wildlife 2001: Populations . New York: Elsevier Applied Science.
McDermid, E. M., R. Ananthakrishna, and N. S. Agar. 1972. Electrophoretic investigation of plasma and red cell proteins and enzymes of Macquarie Island elephant seals. Animal Blood Groups and Biochemical Genetics 3: 85–94.
Meighan, C. W. 1959. The Little Harbor site, Catalina Island: An example of ecological interpretation in archaeology. American Antiquities 24: 383–405.
Muizon, C. de. 1982. Phocid phylogeny and dispersal. Annals of the South African Museum 89: 175–213.
Nevo, E., A. Beiles, and R. Ben-Shlomo. 1984. The evolutionary significance of genetic diversity. Lecture Notes in Biomathematics 53: 13–213.
O'Brien, S. J., M. E. Roelke, L. Marker, L. Neuman, C. A. Winkler, D. Meltzer, L. Colly, J. F. Evermann, M. Bush, and D. E. Wildt. 1985. Genetic basis for species vulnerability in the cheetah. Science 227: 1428–1434.
O'Brien, S. J., D. E. Wildt, M. Bush, T. M. Caro, C. Fitzgibbon, I. Aggundey, and R. E. Leakey. 1987. East African cheetahs: Evidence for two population bottlenecks? Proceedings of the National Academy of Sciences 84: 5083–5086.
Odell, D. K. 1971. Censuses of pinnipeds breeding on the California Channel Islands. Journal of Mammalogy 52: 187–190.
———. 1974. Seasonal occurrence of the northern elephant seal, Mirounga angustirostris , on San Nicolas Island, California. Journal of Mammalogy 51: 81–95.
Ogden, A. 1933. Russian sea otter and seal hunting on the California coast, 1803–1841. California Historical Society Quarterly 12: 217–239.
———. 1941. The California sea otter trade, 1784–1848. University of California Publications in History 26: 1–251.
Orr, P. C. 1967. Geochronology of Santa Rosa Island, California. In Proceedings of the Symposium on the Biology of the California Islands , 317–326. Santa Barbara, Calif.: Santa Barbara Botanic Garden.
Orr, R. T., and T. C. Poulter. 1965. The pinniped population of Año Nuevo Island, California. Proceedings of the California Academy of Sciences 32: 377–404.
Peterson, R. S., R. L. Gentry, and B. J. Le Boeuf. 1968. Investigations of pinnipeds on San Miguel and San Nicolas islands. Unpublished manuscript, University of California, Santa Cruz.
Poulter, T. C., and R. Jennings. 1966. Annual Report to the Division of Beaches and Parks, State of California . Menlo Park, Calif.: Stanford Research Institute.
Radford, K. W., R. T. Orr, and C. L. Hubbs. 1965. Reestablishment of the northern elephant seal (Mirounga angustirostris ) off Central California. Proceedings of the California Academy of Sciences 31: 601–612.
Reeves, R. R., B. S. Stewart, and S. Leatherwood. 1992. The Sierra Club Handbook of Seals and Sirenians . San Francisco: Sierra Club Books.
Reinman, F. M. 1964. Maritime adaptation on San Nicolas Island, California: A preliminary and speculative evaluation. In University of California at Los Angeles Archaeological Surveys, Annual Report 1963–1964, 50–84.
Reiter, J., K. J. Panken, and B. J. Le Boeuf. 1981. Female competition and reproductive success in northern elephant seals. Animal Behaviour 29: 670–687.
Repenning, C. A., C. E. Ray, and D. Grigorescu. 1979. Pinniped biogeography. In Historical Biogeography, Plate Tectonics, and the Changing Environment: Proceedings of the Thirty-seventh Annual Biology Colloquium, and Selected Papers , ed. J. Gray and A. Boucot, 359–369. Corvallis: Oregon State University Press.
Rice, D. W., K. W. Kenyon, and D. Lluch B. 1965. Pinniped populations at Islas Guadalupe, San Benito, and Cedros, Baja California, in 1965. Transactions of the San Diego Society of Natural History 14: 73–84.
Rothschild, W. 1908. Mirounga angustirostris (Gill). Novitates Zoologicae 15(2): 393–394.
———. 1910. Notes on sea elephants (Mirounga ). Novitates Zoologicae 17(3): 445–446.
Scammon, C. M. 1870. Sea-elephant hunting. Overland Monthly 4(2): 112–117.
———. 1874. The Marine Mammals of the Northwestern Coast of North America . San Francisco: J. H. Carmany and Co.
Snethkamp, P. E. 1987. Prehistoric subsistence variability on San Miguel Island. In Third California Islands Symposium: Program and Abstracts . Santa Barbara, Calif.
Stewart, B. S. 1989. The ecology and population biology of the northern elephant seal, Mirounga angustirostris (Gill) 1866, on the southern California Channel Islands. Ph.D. dissertation, University of California, Los Angeles.
———. 1992. Population recovery of northern elephant seals on the southern California Channel Islands. In Wildlife 2001: Populations , ed. D. R. McCullough and R. H. Barrett, 1075–1086. London: Elsevier Applied Science.
Stewart, B. S., and R. L. DeLong. 1993. Seasonal dispersion and habitat use of foraging northern elephant seals. In Marine Mammals: Advances in Behavioural and Population Biology , ed. I. L. Boyd, 179–194. Symposia of the Zoological Society of London no. 66. London: Oxford University Press.
Stewart, B. S., and H. R. Huber. 1993. Mammalian species: Mirounga angustirostris. American Journal of Mammalogists 449: 1–10.
Stewart, B. S., and P. K. Yochem. 1984. Seasonal abundance and distribution of pinnipeds on San Nicolas Island, California. Bulletin of the Southern California Academy of Sciences 83: 121–132.
———. 1986. Northern elephant seals breeding at Santa Rosa Island, California. Journal of Mammalogy 67: 402–403.
Stewart, B. S., P. K. Yochem, R. L. DeLong, and G. A. Antonelis. 1993. Trends in abundance and status of pinnipeds on the southern California Channel Islands. In Third California Islands Symposium: Recent Advances in Research on the California Islands , ed. F. G. Hochberg, 501–516. Santa Barbara, Calif.: Santa Barbara Museum of Natural History.
Townsend, C. H. 1885. An account of recent captures of the California sea-elephant and statistics relating to the present abundance of the species. Proceedings of the U.S. National Museum 8: 90–94.
———. 1912. The northern elephant seal Macrorhinus angustirostris (Gill). Zoologica 1: 159–173.
Vedder, J. G., and D. G. Howell. 1980. Topographic evolution of the southern California Borderland during late Cenozoic time. In The California Islands: Proceedings of a Multidisciplinary Symposium , ed. D. M. Power, 7–31. Santa Barbara, Calif.: Santa Barbara Museum of Natural History.
Walker, P. L., and S. Craig. 1979. Archaeological evidence concerning the prehistoric occurrence of sea mammals at Point Bennett, San Miguel Island. California Fish and Game 65: 50–54.
Wayne, R. K., S. B. George, D. Gilbert, P. W. Collins, S. D. Kovach, D. Girman, and N. Lehman. 1991. A morphologic and genetic study of the island fox, Urocyon littoralis. Evolution 45: 1849–1868.
Zar, J. H. 1974. Biostatistical Analysis . Englewood Cliffs, N.J.: Prentice-Hall.
Three—
History and Present Status of Southern Elephant Seal Populations
Richard M. Laws
ABSTRACT. The total world population of southern elephant seals in 1990 was estimated at 664,000. Of the three (or four) main stocks, South Georgia and Peninsula Valdes account for 60% of the total; Isles Kerguelen, 28%; and Macquarie Island, 12%. The species was hunted for its oil in the eighteenth and nineteenth centuries and subsequently recovered under protection. The only large-scale industry this century was a government-licensed one at South Georgia, from 1909 to 1964, which was restricted to adult males. The history and rational basis for this industry is described here, including some effects of sealing, population decline from 1931 to 1951, and subsequent recovery to sustained yield level under a research-based management plan. Pup production was estimated, population models were drawn up, and the size of the South Georgia population in 1951 was calculated. A comprehensive survey in the 1985 breeding season indicated the same annual pup production as for 1951 and a population of 357,000 in 1990. Population models indicate that some 75% of the adult male population is at sea during the breeding season, which has implications for aquatic mating of virgin females and nonpregnant mature females.
A literature review of other stocks examines various estimates of pup production by site and years between 1949 and 1990 and converts these by a raising factor to estimates of total population sizes by years. During this period, different stocks of southern elephant seals have increased (Península Valdes, Argentina, by 144% since 1975); have probably remained stable after rapid recovery from exploitation (South Georgia, 15% due to postexploitation recovery of the male stock); or have decreased dramatically at annual rates varying between 2.1% and 8% at different places and periods. Total percentage decreases since 1949 are estimated at 50% for Heard Island, 84% for Marion Island, 57% for Macquarie Island, 96% for Campbell Island, and 93% for Signy Island.
This chapter sets the scene for the more detailed discussions presented by those studying key colonies. Those discussions deal with the factors responsible for changes in population number and advance hypotheses to account for the precipitous decline of certain populations.
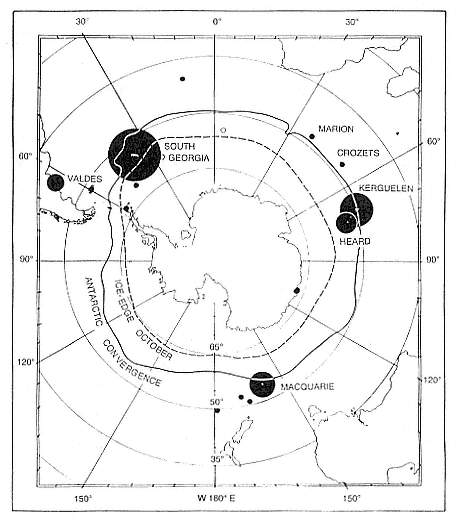
Fig. 3.1
Breeding distribution of southern elephant seals in 1990. Closed circles: known
breeding colonies (area proportional to the estimated population sizes except for
colonies of less than 5,000). Open circles: probable small breeding
populations. Fuller data are presented in table 3.1.
In terms of numbers, the southern elephant seal, M. leonina , is one of the more abundant seal species in the world. There are three main stocks, defined (Laws 1960) as the South Georgia stock, the Kerguelen stock, and the Macquarie Island stock (fig. 3.1). The first is the largest numerically and includes elephant seal breeding colonies in the Scotia arc (South Georgia, South Orkney Islands, South Shetland Islands, South Sandwich Islands) and Gough and Bouvet islands, together with South America and
the Falkland Islands. Although more recent information indicates movements between South American and Falkland Islands elephant seal colonies (Laws, unpubl.), up to now no movements have been reported between them and the remaining colonies; so there may be four stocks. The second stock includes Kerguelen and Heard islands, Marion and Prince Edward islands, and Iles Crozets. Single births have been recorded at Amsterdam and St. Paul islands and South Africa (Laws 1960). Finally, the Macquarie Island Stock includes Macquarie Island, Campbell Island, Auckland Islands, and Antipodes Islands, and one birth has been reported from Tasmania (Laws 1960). A very small number of births have also been reported from the Antarctic continent (Murray 1981). The centers of the three main stocks are roughly 90° and 107° longitude apart, except in the Pacific Ocean sector (163°), which is devoid of suitable islands in the right latitudes and supports no breeding colonies. Genetic studies by N. J. Gales, M. Adams, and H. R. Burton (1989) reported on elephant seal blood samples from Heard and Macquarie islands that were examined for protein variation. They concluded that the two populations "may have diverged genetically, and a very limited gene flow exists between the islands, a finding consistent with limited information from mark-recapture studies" (57). It is likely that a similar division exists between them and the South Georgia stock.
The sealers of the eighteenth and nineteenth centuries came first to hunt the more valuable fur seals for their pelts but did not ignore the larger species, which was hunted for its oil. The right whalers in southern waters also took elephant seals. As fur seals declined, the sealers concentrated more on elephant seals (Bonner 1982; Headland 1989). In his chronological list of Antarctic expeditions, R. Headland (1989) analyzed 930 voyages to the Antarctic between 1786 and 1928, the majority by U.S. (46.4%) and British (19.1%) ships, and showed the frequency of sealing voyages by year. There were progressively lower peaks, separated by periods of partial recovery, about 1820, 1842, 1855, 1875, and 1906. The early voyages primarily took fur seals, which were hunted almost to extinction; the objective of the later voyages was primarily the elephant seal. It is not known how many elephant seals were taken during this period, but it was probably in excess of one million of both sexes, assuming that the original populations totaled at least 600,000 to 750,000, as in this century (Laws 1960; McCann 1985). The sealing industry had virtually ended by 1909, and protection was subsequently conferred on most stocks, which recovered from the overhunting.
The only large-scale elephant sealing during this century was the government-licensed industry at South Georgia between 1909 and 1964 (see Laws 1953a , 1960; Bonner 1958; and Headland 1984), although small numbers were taken in other places, particularly Kerguelen, where 12,000 bulls were taken between 1958 and 1964 (van Aarde 1980). Over the course of sixty-five years, some 260,000 elephant seals, predominantly adult males, were taken at South Georgia (Headland 1984). The industry came to an
end in 1964 as a result of the collapse of the whaling industry at South Georgia; the sealing industry was on a firm sustainable yield basis. As will be shown, the size of the South Georgia elephant seal population in 1951, when government-controlled sealing was at its height, was similar to the size of the population in 1985, some twenty years after sealing had ended. However, the populations in the Kerguelen and Macquarie islands stocks declined steadily over forty years from 1950, even in the absence of sealing. It seems that the elephant seal population in Argentina has been increasing in recent years.
Below I describe the recent history of twentieth-century sealing at South Georgia, because of its relevance to estimating abundance and trends, and what is known about population sizes this century in the three (or four) main stocks, which are considered separately. Estimates, updated to 1990, are given for the total world population of the species.
South Georgia Stock
The Sealing Industry, 1910–1964
The biological basis underpinning rational exploitation of this species is its highly polygynous land-breeding behavior. The sexes are nearly equal in numbers at birth, and a single adult male can serve a large number of cows. In fact, the overwhelming majority of matings are carried out by a very small minority of breeding bulls; according to M. N. Bester and I. S. Wilkinson (this volume), "dominant bulls controlling the harems achieved over 98% of all matings." A large surplus of males can therefore be taken from the beaches without affecting recruitment to the population. Polygyny has resulted in the socially mature male being on average about eight times the weight of the average breeding female (Laws 1984). As the sealing industry sought oil from the blubber, it was most convenient to take the bulls in the earlier part of the breeding season in the spring, when the oil yield is greatest. Because the larger males haul out first on the beaches, the sealers automatically selected the largest animals. Oil yields from autumn sealing are lower, and this practice was discontinued in the 1950s.
To prevent recurrence of the former indiscriminate slaughter, the Falklands Islands government introduced the Seal Fishery Ordinance in 1899 (improved by further ordinances in 1904, 1909, and 1921), with the intention of placing the industry on a rational basis. The coastline of South Georgia was divided into four sealing divisions (and four reserves where no seals were taken). The divisions were generally worked in rotation, each year one of them being closed to sealing. Licenses to take a stipulated number of adult male elephant seals were issued annually to the sealing company. The first license was issued in 1909 to the oldest established whaling company at South Georgia, which held a monopoly until the 1961–1962
season. There was no sealing in 1962–1963, and licenses were issued to a Japanese company for the last two seasons, 1963–1964 and 1964–1965. With the cessation of autumn sealing in the 1950s, sealing began early in September and ended about mid-November. The sealing methods are described in detail by me (Laws 1953a ) and by Bonner (1958). Although attempts were made to utilize carcasses, this was found to be uneconomical and the sole product of the industry continued to be blubber oil.
For many years, the annual quota under license was fixed at 6,000. But in 1948, it was raised to 7,500; in 1949 and 1950, to 9,000; and in 1951, was set at 8,000. Until 1952, the quota was divided equally among the three divisions worked each year, although seal abundances in the four divisions have probably always been unequal. The sealing regulations were altered in 1952 as a result of recommendations made by me (Laws 1960).
Effects of Sealing
Previously, I have presented a variety of evidence that indicated that from the late 1930s onward there were adverse changes in the population, despite the conservation measures in force (Laws 1960). Most significant, it was shown that the average catch of seals per catcher's day's work (CDW) in October had progressively declined by 28% from 1931 to 1951. Over these two decades, there had also been a decline in the oil yield per seal and a lengthening of the season, trends that had begun well before the higher catches in 1948–1951. Consideration of the sex ratios on the breeding beaches, harem sizes (on exploited beaches and in a reserve), and the low average age of males on the breeding beaches supported the indications that the demonstrated decline in the catch per unit effort represented a real decrease in the size of the adult male component of the population.
In Laws 1960, I reported counts of seals on beaches in Divisions I–III and one count in Division IV. These counts were corrected for date and for pup mortality, based on my research, and I concluded that approximately 20,000 pups were born in Division I and 15,000 in Division II. Supplementary information was obtained from the sealers which indicated that seals were much more abundant in Divisions III and IV, and I assumed that 32,500 pups were born in each of these divisions, making an estimated total of approximately 100,000 pups that survived to weaning. Assessing pup mortality from birth to weaning at 2% gave a figure of about 102,000 pups at birth.
I constructed population models to assess the size of the total population in each division, based on my demographic research, on the size of the commercial catches in previous years, and on the age composition of the commercial kill of males in 1951, based on growth layers in the teeth (Laws 1952, 1953b ). Some assumptions were made, and predictions about certain future statistics were advanced, the confirmation of which later appeared to support the assumptions. The results of these calculations were estimates of
the size of the population in each division and of the annual accessions to each age class (see Laws 1960 for details). Other information on breeding behavior suggested that the stock of breeding bulls in each division should be kept equal to one-twelfth of the stock of adult cows, and on this basis surplus accessions of bulls for all divisions combined were calculated at 5,842, close to the average annual catch. But the catches in Divisions I and II for this period were, respectively, about 24% and 65% higher than the estimated surpluses for those divisions, whereas the catches in Divisions III and IV were well below.
Therefore, a new system was introduced in 1952 to control sealing. Most important, divisional quotas were set for the five years 1952–1956, proportional to the estimated size of the available surplus in each division. A safeguard was introduced to monitor the age composition of the catch, by requiring canine teeth to be collected from a 5% sample of the catch which would subsequently be examined to determine age structure of the catch. A minimum length regulation was introduced.
Favorable results were immediately apparent, with a rise in the catch per CDW of 26% over the first 4-year period. In general, sealing operations were conducted earlier (midseason date two weeks earlier) and the average oil yield increased substantially. About 300 teeth were collected annually between 1952 and 1964 for age determination, and W. N. Bonner made control collections from a total of over 400 seals caught. The mean age of the catch sample in 1951 was 6.64 ± 0.34 (SE) years; in 1952, it was 7.20 ± 0.14; and it increased to level off at about 7.71 ± 0.19, 7.70 ± 0.18, and 7.69 ± 0.15 years in 1961, 1963, and 1964, respectively (Laws 1979). This leveling off confirmed that the catch size was sustainable; it corresponds to the estimated average age on attaining social maturity, and the management strategy was to monitor the age distribution of the catch and to adjust annual quotas, as necessary, so as to keep the average age of the catch at 7½ to 8 years. The success of this policy gave confidence in the general accuracy of the estimates of population abundance and life table parameters (see below).
Population Size in 1951
In formulating my recommendations, I had constructed provisional life tables for the two sexes. These life tables adjusted for 102,000 births annually indicated an average midyear population of about 310,000 elephant seals at South Georgia in 1951, that is, about 3.1 times the number of births (see Laws 1960 for details). I reviewed available information on other breeding colonies of the overall South Georgia stock and concluded that its total size was a little more than 315,100.
McCann (1985) revised the life tables, assuming higher female fecundity (88% vs. 82.5%). He found no change in the number of pups born in Cumberland Bay, no evidence for an increase or decrease in the cow
population, and no significant change in the age at first pupping since 1964. He concluded that "a suitable factor by which to convert pup counts to total population of all animals older than pups of the year at the end of the breeding season is 3.5" (14). McCann (1980) also found no difference in body size in 1976–1977 compared to 1948–1951. He reported a three- to fourfold increase since exploitation ended in numbers of bulls ashore, an increase in the mean age of harem bulls, and increased average harem size; he also found that bulls hauled out earlier in the season and stayed ashore longer. There was little change in the number of breeding cows or their age structure. (Comparisons were with results in my papers, based on fieldwork in 1948–1951, listed in Laws 1960.) McCann (1985) concluded that the South Georgia population in 1980 was 350,000 (actually, it should have been 357,000 if calculated from births × 3.5).
McCann's estimate was derived from the 1951 estimate of pups born and his life tables for an unexploited population. In 1951, however, at the height of the sealing period, even assuming a similar pup production, the total population should have been somewhat lower, because the numbers of adult males were reduced in the exploited population. Comparison of Laws (1960) and McCann (1985) shows that the female life tables are really quite similar (perhaps not surprising as McCann adopted some important parameters from me, especially first- and second-year survival); the male life tables differ only in the higher first- and second-year mortality assumed by me and in the reduced survival of older males in the exploited population.
I have therefore recalculated the exploited male life table, by raising the first-year survival to the female level, as McCann did; this gives almost identical survival rates for the two models up to 6 years. Above 6 years the selective removal of older males reduced the adult male population, so that in the exploited 1951 population model there was virtually no survival beyond age 12, compared with age 20 in McCann's (1985) life table. I have calculated the number of adult males estimated by the two models, assuming 54,000 male pups at birth in both exploitation and postexploitation models; McCann reported a sex ratio of 53% males at birth. For the 1951 model there were 17,280 males aged 7 to 12 years (none were older), and for 1985 there were an estimated 36,000 aged 7 to 20 years (the upper limit). The difference is nearly 19,000, suggesting that the 1951 total South Georgia population size was actually about 338,000 (357,000 – 19,000). Sealing ended in 1964, so the recovery in adult male numbers should have been virtually complete by 1970 as full cohorts successively acceded to the adult male population.
Population Size in 1985
The elephant seal population of South Georgia was surveyed comprehensively during the 1985 breeding season (McCann and Rothery 1988). There were 87,711 females and 10,260 adult males counted on shore, and the
female counts were corrected using a model of haul-out distribution over time to adjust for the date of count. The annual pup production was estimated to be about 102,000, the same as the estimate in Laws (1960). I actually counted 26,260 live pups in Divisions I and II and gave an estimate for pup production, corrected for date of count, of 31,075; I rounded up this estimate to 35,000, to allow for the beaches I did not visit. This can be compared with T. S. McCann and P. Rothery's (1988) corrected total of 34,665 in these two divisions, which is very close. McCann and Rothery obtained corrected pup counts of 42,892 and 24,676 for Divisions III and IV, respectively, and suggested that as the numbers in Divisions I and II were approximately the same in 1985 as in 1951, the same should be true of the totals in Divisions III and IV. Thus, there is a good basis for assuming that the number of pups was indeed about 102,000 in both periods, and applying the factor 3.5 gives a population other than pups of 357,000 in 1985. (A possible but small source of error lies in the adoption of my 1960 estimate of pup mortality (2%); e.g., Condy [1978] estimated preweaning mortality at 6%.)
Thus, the total pup production at South Georgia was very similar in two estimates 34 years apart. This suggests that the South Georgia population may have been almost stable since 1951, apart from an increase in adult males after sealing ended in 1964. However, McCann and Rothery (1988) show that substantial annual fluctuations occurred in the number of cows ashore on certain beaches, and their data suggest a possible decline in the 1960s. It seems likely therefore that over the last 40 years, the South Georgia population size has fluctuated around 350,000.
The 1985 count and model presented above indicate that of some 36,000 adult males (aged 7 to 20 years) estimated to be in the South Georgia population, only some 10,000 were counted on land at any one time during the breeding season, and about 26,000 were therefore in the sea, since they have not been seen elsewhere on land. This is relevant to hypotheses about the mating of the virgin females; they are very rarely seen on the breeding beaches in the breeding season, yet the 1985 model (McCann 1985) indicates that some 21,000 virgin females (equivalent to about a sixth of all mature females) mate for the first time at age 3. I observed aquatic matings and believed that newly mature females mate for the first time not on land but in the sea, probably during the usual breeding season of the adult cows (Laws 1956). (With 88% fecundity, the model also indicates that 12% of all adult females—an estimated 14,000—would be nonpregnant in the pupping season and probably also would mate aquatically). Behavior during the aquatic phase of the life cycle may have implications for the causes of the decline in other southern elephant seal populations (SCAR 1991; Bester and Wilkinson, this volume; Hindell, Slip, and Burton, this volume; Fedak et al., this volume).
Other Populations in the South Georgia Stock
Patagonia and the Falkland Islands
I estimated less than 1,000 pups born in the Falkland Islands, representing a population of less than 3,500 (Laws 1960); there is no recent information. For Patagonia, at Península Valdes, I. S. Carrara (1952) estimated a population of approximately 1,000 in 1951; J. A. Scolaro (1976) estimated 3,933 pups born in 1975, implying a population of 13,800 (3.5 × births); D. F. Vergani, M. N. Lewis, and Z. B. Stranganelli (1987) estimated 6,737 pups born there in 1982 (pop. 23,579), increasing at 5.1% annually between 1975 and 1982. C. Campagna and M. Lewis (1992) give the results of eight surveys from 1969 to 1990, corrected for date and area surveyed, concluding that 7,455 pups were born in 1982 (pop. 25,092) and 9,636 pups were born in 1990 (pop. 33,726), having increased at 3.2% annually since 1982.
South Orkney Islands
I estimated the size of the breeding population I studied at Signy Island to be 300 in 1948–1950 (Laws 1960). Subsequently, this has decreased to less than 20 (unpublished British Antarctic Survey records).
King George Island
Vergani (pers. comm.) reported 708 pups born in 1980, declining to 560 by 1990, which represents total populations of approximately 2,500 and 2,000.
Nelson Island
Vergani, Lewis, and Stranganelli (1987) reported 106 pups born in 1985, representing a population of approximately 370.
Avian Island
One birth has been reported from Avian Island (68° S.) (unpublished British Antarctic Survey record).
Gough Island
Bester (1980; pers. comm.) reported 32 pups born in 1977 and 28 in 1990, suggesting a stable population of about 105.
South Sandwich Islands and Bouvet Island
The species probably breeds in small numbers on these islands, but there is no information.
These populations represent a total of about 39,720 in 1990, about 10% of the total South Georgia stock in 1990, which is estimated at about 397,000 compared with McCann's (1985) estimate of some 369,000 based on counts spread over earlier years (table 3.1). The difference is due to a real increase in the Península Valdes population and the upward revision of the earlier South Georgia estimate to allow for the recovery of the adult male stock as discussed above.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The Iles Kerguelen Stock
Iles Kerguelen:
Courbet Peninsula
Here there are difficulties in making comparisons from year to year because of uncertainties about the proportion of the population censused. Almost all breed on the Courbet Peninsula between Point Molloy and Cap Noir, here called the study area. R. J. van Aarde (1980) summarized previous counts of breeding cows plus weaned pups (roughly equal to pups born) in the study area in 1958, 1960, and 1970 and presented his own figures for 1977.
These were, respectively, 41,970, 40,050, 55,252, and 42,420. However, the first two are underestimates because they were uncorrected and so are not comparable. On the basis of the estimates for 1970 and 1977, van Aarde calculated that there had been a decline of 4.6% annually over this period (and, raising by 3.5 times, this corresponds to a decline in total population from 193,382 to 148,470). Bester (1982) counted 36,900 pups in the study area in 1979, representing a population of 129,150; a continued decline of 4.6% from 1977 would have resulted in 38,607 pups (total 135,124). C. Guinet, P. Jouventin, and H. Weimerskirch (1992) obtained a figure of 41,000 pups in 1989 and suggest that the population has stabilized; this would indicate a population of about 143,500 in 1990.
Heard Island
R. Carrick and S. E. Ingham (1962) gave an estimate of 23,000 pups for 1949, representing a total population of 80,500. By 1985, Burton (1986) estimated pup production at 13,000, representing a population of 45,500, which had therefore declined by 2.4% annually, averaged over the period from 1949 to 1985. Continuing decline at this rate would result in a population of 40,355 in 1990, a total decline of 50%.
Marion Island
In 1973 and 1976, total numbers in various age classes were counted in the period November 13–19, when combined pup and weaner counts were at a maximum (Condy 1978); no adjustments to these were therefore necessary except for preweaning mortality (5.99%). A mean of 1,049 pups and weaners were counted, which adjusted for mortality gave an estimated 1,115 births annually in this period. Raised by 3.5, this indicates a total population of 3,903 in 1976. R. W. Rand (1962) had counted the pups, weaners, and dead pups on the island in the second half of November 1951 and obtained a figure of 3,662, corresponding to a total population of 12,817. (Condy calculated a 69.5% decline between 1951 and 1976.) P. R. Condy (1977) analyzed three comparable counts from 1951, 1965, and 1974 and estimated the rate of decrease of the Marion Island breeding population over this period at 4.8% a year.
To study the trend between 1973 and 1982 in more detail, J. D. Skinner and R. J. van Aarde (1983) analyzed results of censuses from a subset of the breeding beaches, representing about a third of the annual pup production, for the period 1973–1982 (excepting 1978); they are not directly comparable to Condy's (1977) figures. This indicated an exponential decrease in pup production over the period 1973–1982 at a mean annual rate of 8%.
The most recent data come from Bester and Wilkinson (this volume) and Wilkinson and Bester (in press). The first relate to a main study area representing about 10% of the coastline, censused from 1974 to 1989 fol-
lowing the methods of Condy (1978). In 1976 and 1986–1989, total pup counts of the whole island were made, which confirmed that numbers in the main study area provide a representative index of the total population. They conclude that the overall decline between 1974 and 1989 was 4.8% annually, but in 1983–1989 it had slowed to 1.9%. Wilkinson and Bester (in press) estimated the total pup production for the island in 1989 at 585; a further 1.9% decline would reduce this to 574 in 1990. Raised by 3.5, this gives an estimated total population of 2,009 in 1990, a decline of 83.7% since 1951 and of 48.5% since 1976. In percentage terms, this population has been most adversely affected of all except Signy Island and Campbell Island (see below).
Prince Edward Island
Condy (1978) also counted 386 pups and weaners on the east coast of Prince Edward Island in 1977; applying Condy's (1978) correction factor for preweaning mortality gives an estimate of 411, raised to a total population of 1,439. If this population also declined on average at 4.8% annually between 1974 and 1989 and 1.9% in 1989–1990, its 1990 population would be 782.
Iles Crozet
A. Barrat and J. L. Mougin (1978) reported about 3,000 pup births in 1976, corresponding to a total population of 10,500, having declined by 5.8% annually between 1966 and 1976 (SCAR 1991). Guinet, Jouventin, and Weimerskirch (1992) reported 612 pups in 1989, representing by inference a total population of 2,142, having declined at 5.7% annually between 1980 and 1989. Extrapolating a further year at this rate of decline implies a population of 2,019 in 1990 (SCAR 1991).
The populations comprising the Iles de Kerguelen stock represent a total of about 189,000 in 1990 (table 3.1), about 28% of the total world population, having declined dramatically over the last 40 years, one population by over 80%. This is in marked contrast to the South Georgia stock, which has increased at South Georgia since 1951 by 20,000 (15.3%) as a result of the postexploitation recovery of the male component and at Península Valdes by 20,000 (144%) since 1975, due to natural increase.
The Macquarie Island Stock
Macquarie Island
M. A. Hindell and H. R. Burton (1987) have recently described the past and recent status of the elephant seal at Macquarie Island. This is one of the most continuously studied populations since observations began in
1949. Until the early 1980s, only adult females and males were counted in the isthmus study area where most of the seals haul out to breed (Carrick and Ingham 1960; Carrick et al. 1962). However, Hindell and Burton (1988) showed that the haul-out pattern of breeding females is so predictable that a census on any day within two weeks of the haul-out maximum can be used to estimate the maximum number accurately. In the 1985 breeding season, daily counts of adult females, weaned pups, dead pups, and breeding males were made on the isthmus study area, and a complete island census was carried out on a single day, a week before the peak number; an estimate of the peak number of females was derived from the haul-out curve modeled from the isthmus data.
Hindell and Burton (1987) estimated the maximum number of females ashore on the isthmus for 21 years between 1949 and 1985. They showed that there was a significant correlation between years and maximum number of females, with an average annual rate of decrease of 2.1%. There was a similar percentage decrease in the numbers of males ashore.
The estimated total island pup production in 1959 was 44,501, compared with 24,713 in 1985. Applying the 3.5 raising factor (McCann 1985) gives a total population of 156,000 in 1959 and 86,500 in 1985, that is, 44.6% less than in 1959. Hindell and Burton (1987) concluded that the population was probably declining at least from 1950, and they estimated that the whole island population in 1949 was about 183,000. Extrapolating from 1985 to 1990, assuming a continued decline at 2.1% per annum, gives a total population of 77,791 in 1990, which represents an overall decline of 57.5% since 1949.
Campbell Island
There is a small population of elephant seals at Campbell Island. J. H. Sorensen (1950) gave estimates from counts of the numbers of pups born in 1941, 1942, 1945, and 1947, increasing from 75 to 100 in 1941 to 191 in 1947. Taking these figures at face value, this suggests an average of about 140 pups born in the 1940s and, applying the raising factor used by McCann (1985), a total population of about 500 at this time. The only recent information appears to be from R. H. Taylor and G. A. Taylor (1989), who give a pup production of only 5, representing a total population numbered in tens of animals, let us say 20. This would represent a decline of 96% since the 1940s.
Antipodes Island
Taylor and Taylor (1989) also give an estimate of 113 for the pup production at Antipodes Island in 1978. There is no information on the trend in this population, but if there has been no change, applying the raising factor gives a population of about 400 in 1990.
Thus the Macquarie Island stock, which is essentially confined to Macquarie Island for breeding, had an estimated total size of about 78,000 in 1990 (table 3.1), representing only 12% of the total world population, having declined dramatically from about 183,000 in 1949, a decrease of 57% over 42 years. This is again in marked contrast to the South Georgia stock and more like the Iles Kerguelen stock.
Conclusions
As the work summarized here indicates, during the period from 1949 to the present, different populations of southern elephant seals have increased (Península Valdes), probably remained stable after rapid recovery from the exploitation that ended in 1964 (South Georgia), or decreased dramatically (Iles Kerguelen, Marion Island, Macquarie Island, Signy Island, Campbell Island).
The population sizes are derived from estimates of pups born, raised by the factor of 3.5 to obtain estimates of the size of populations, excluding pups. Different raising factors should be applied to increasing, stable, or declining populations, and in the absence of adequate demographic knowledge of the different populations, the estimates of annual pup production are more accurate than population abundance estimates. However, it is desirable to have population abundance estimates for other purposes (such as estimating population biomass and food consumption; Boyd, Arnbom, and Fedak, this volume) and since a common factor is applied, the derived population trends are the same as for pup production. Subject to this reservation, table 3.1 shows an estimated total population of the southern elephant seal in 1990 of about 664,000, which compares with an estimated total population of 758,000 derived from data obtained at different times during the period 1949–1980 (McCann 1985), an overall decline of some 12.3%. The decline is not universal, however, and 60% of the total world population is in the South Georgia (and South American) stock, which is stable or increasing.
If we look at the populations that have decreased, currently about 40% of the total world population, they have decreased on average by about 31%, but some have decreased to a much greater extent; for example, the major stock at Macquarie Island has declined by an estimated 57% since 1949. Information about the possible causes of the declines is presented elsewhere in this volume (chaps. 4, 5, and 6). A workshop meeting convened by the Scientific Committee on Antarctic Research (SCAR 1991) also addressed the possible causes of past and present population trends but did not reach any firm conclusion. It seems that the causes of the declines may differ among populations; however, the causes remain speculative and essentially unknown. In any case, the contrast between the population
trends in the northern elephant seal, M. angustirostris (Stewart et al., this volume), and the southern elephant seal is striking and is an urgent topic for further research.
There is no evidence to suggest that the population declines are the result of factors influencing the seals while ashore, indicating that they result from factors operating while the animals are at sea (SCAR 1991). In this respect, the evidence presented here indicating that a large proportion of adult males and newly mature and fully adult females remain in the sea during the breeding season may be relevant. For successful mating, it seems unlikely that these components of the population are widely dispersed at this time. They may be in the general vicinity of the breeding islands, or relatively concentrated in other parts of the species' foraging range. Tracking studies using satellite geolocation equipment should eventually provide an answer to this question.
Much of this chapter describes the government-controlled industry at South Georgia between 1909 and 1964. This is a topic that is not covered elsewhere in this volume and has been included here because of the light it throws on the population dynamics of the species—and because this stock constitutes some 60% of the total population of the species. Despite the removal of some 260,000 bulls during a 65-year period, the South Georgia population appears to have remained fairly stable or to have fluctuated only slightly, a confirmation that the industry was on a firm sustainable yield basis after 1952. This elephant seal stock in fact provides a classic example of rational utilization of a natural renewable resource.
References
Barrat, A., and J. L. Mougin. 1978. L'elephant de mer Mirounga leonina de L'île de la Possession, Archipel Crozet (46°25¢ S, 51°45¢ E). Mammalia 42: 143–174.
Bester, M. N. 1980. The southern elephant seal Mirounga leonina at Gough Island. South African Journal of Zoology 15: 235–239.
———. 1982. An analysis of the southern elephant seal Mirounga leonina breeding population at Kerguelen. South African Journal of Antarctic Research 12: 11–16.
Bonner, W. N. 1958. Exploitation and conservation of seals in South Georgia. Oryx 4: 373–380.
———. 1982. Seals and Man . Seattle: University of Washington Press.
Burton, H. 1986. A substantial decline in numbers of the southern elephant seal at Heard Island. Tasmanian Naturalist 86: 4–8.
Campagna, C., B. J. Le Boeuf, M. Lewis, and C. Bisioli. 1992. Equal investment in male and female offspring in southern elephant seals. Journal of Zoology, London 226: 551–561.
Campagna, C., and M. Lewis. 1992. Growth and distribution of a southern elephant seal colony. Marine Mammal Science 8: 387–396.
Carrara, I. S. 1952. Lobos marinos, pinguinos y guaneras de las costas del littoral
maritimo e islas adyacentes de la Republica Argentina. Universidad Nacional La Plata, Facultad de Sciencias Veterinarias, Catedra de Higiene e Industrias, Publ. Espec.
Carrick, R., S. E. Csordas, S. E. Ingham, and K. Keith. 1962. Studies on the southern elephant seal, Mirounga leonina (L.). III. The annual cycle in relation to age and sex. CSIRO Wildlife Research 7: 119–160.
Carrick, R., and S. E. Ingham. 1960. Ecological studies on the southern elephant seal, Mirounga leonina (L.), at Macquarie Island and Heard Island. Mammalia 24: 325–342.
———. 1962. Studies on the southern elephant seal, Mirounga leonina (L.). V. Population dynamics and utilization. CSIRO Wildlife Research 7: 198–206.
Condy, P. R. 1977. Annual cycle of the southern elephant seal Mirounga leonina (Linn.) at Marion Island. South African Journal of Zoology 14: 95–102.
———. 1978. The distribution and abundance of southern elephant seals Mirounga leonina (Linn.) on the Prince Edward Islands. South African Journal of Antarctic Research 8: 42–48.
Gales, N. J., M. Adams, and H. R. Burton. 1989. Genetic relatedness of two populations of the southern elephant seal, Mirounga leonina. Marine Mammal Science 5: 57–67.
Guinet, C., P. Jouventin, and H. Weimerskirch. 1992. Population changes and movements of southern elephant seals on Crozet and Kerguelen archipelagos in the last decades. Polar Biology 12: 349–356.
Headland, R. 1984. The Island of South Georgia . Cambridge: Cambridge University Press.
———. 1989. Chronological List of Antarctic Expeditions and Related Historical Events . Cambridge: Cambridge University Press.
Hindell, M. A. 1991. Some life-history parameters of a declining population of southern elephant seals, Mirounga leonina. Journal of Animal Ecology 60: 119–134.
Hindell, M. A., and H. R. Burton. 1987. Past and present status of the southern elephant seal (Mirounga leonina ) at Macquarie Island. Journal of Zoology, London 213: 365–380.
———. 1988. The history of the elephant sealing industry at Macquarie Island and an estimate of the pre-sealing numbers. Papers and Proceedings of the Royal Society of Tasmania 122: 159–176.
Laws, R. M. 1952. A new method of age determination for mammals. Nature 169: 172.
———. 1953a . The elephant seal industry at South Georgia. Polar Record 6: 746–754.
———. 1953b . A new method of age determination for mammals with special reference to the elephant seal, Mirounga leonina Linn. Falkland Islands Dependencies Survey, Scientific Reports 2: 1–11.
———. 1956. The elephant seal (Mirounga leonina Linn.). II. General, social, and reproductive behaviour. Falkland Islands Dependencies Survey, Scientific Reports 13: 1–88.
———. 1960. The southern elephant seal (Mirounga leonina Linn.) at South Georgia. Norsk Hvalfangst-Tidende 49: 446–476, 520–542.
———. 1979. Monitoring whale and seal populations. In Monitoring the Marine Environment , ed. D. Nichols, 115–140. New York: Praeger.
———. 1984. Seals. In Antarctic Ecology , vol. 2, ed. R. M. Laws, 621–715. London: Academic Press.
McCann, T. S. 1980. Population structure and social organization of southern elephant seals, Mirounga leonina (L.). Biological Journal of the Linnaean Society 14: 133–150.
———. 1985. Size, status and demography of southern elephant seal (Mirounga leonina ) populations. In Sea Mammals of South Latitudes: Proceedings of a Symposium of the 52d ANZAAS Congress in Sydney—May 1982 , ed. J. K. Ling and M. M. Bryden, 1–17. Northfield: South Australian Museum.
McCann, T. S., and P. Rothery. 1988. Population size and status of the southern elephant seal (Mirounga leonina ) at South Georgia 1951–1985. Polar Biology 8: 305–309.
Murray, M. D. 1981. The breeding of the southern elephant seal, Mirounga leonina L., on the Antarctic continent. Polar Record 20: 370–371.
Rand, R. W. 1962. Elephant seals on Marion Island. African Wildlife 16: 191–198.
SCAR. 1988. Report of a meeting held at Hobart, Tasmania, August 23–25, 1988. Biomass Report Series , no. 59: 1–61.
———. 1991. Report of the workshop on southern elephant seals, Monterey, California, May 22–23, 1991.
Scolaro, J. A. 1976. Censo de elefantes marinos (Mirounga leonina L.) en el territorio continental Argentino. Informes Técnicos 1.4.2, Centro Nacional Patagonico, Puerto Madryn, R. Argentina, 1–12.
Skinner, J. D., and R. J. van Aarde. 1983. Observations on the trend of the breeding population of southern elephant seals, Mirounga leonina , at Marion Island. Journal of Applied Ecology 20: 707–712.
Sorensen, J. H. 1950. Elephant seals of Campbell Island. Cape Expedition Series 6: 1–31.
Taylor, R. H., and G. A. Taylor. 1989. Re-assessment of the status of southern elephant seals (Mirounga leonina ) in New Zealand. New Zealand Journal of Marine and Freshwater Research 23: 201–213.
van Aarde, R. J. 1980. Fluctuations in the population of southern elephant seals Mirounga leonina at Kerguelen Island. South African Journal of Zoology 15: 99–106.
Vergani, D. F., M. N. Lewis, and Z. B. Stranganelli. 1987. Observation on haul-out patterns and trends of the breeding populations of southern elephant seals at Península Valdes (Patagonia) and Stranger Point (King George Island). SC-CCAMLR-VI/BG/36 , Hobart, Tasmania, 1–9.
Vergani, D. F., and Z. B. Stanganelli. 1990. Fluctuations in breeding populations of elephant seals Mirounga leonina at Stranger Point, King George Island 1980–88. In Antarctic Ecosystems , ed. K. R. Kerry and G. N. Hempel, 241–245. Berlin: Springer Verlag.
Wilkinson, I. S., and M. N. Bester. 1990. Duration of postweaning fast and local dispersion in the southern elephant seal, Mirounga leonina , at Marion Island. Journal of Zoology, London 222: 591–600.
———. In press. Population parameters of the southern elephant seal population at Marion Island. Antarctic Science .
Four—
Possible Causes of the Decline of Southern Elephant Seal Populations in the Southern Pacific and Southern Indian Oceans
Mark A. Hindell, David J. Slip, and Harry R. Burton
ABSTRACT. There are several characteristics of declining southern elephant seal populations that provide a basis for the formulation and testing of hypotheses designed to explain the decline. Some southern elephant seal populations seem to be declining independently of other major Southern Ocean vertebrate consumers, but only two of the three distinct stocks have exhibited a decline. At Macquarie Island, the adult male and female components of the population seem to be equally affected. Studies conducted in the 1950s and 1960s indicate that elephant seals from Macquarie Island had lower survivorship and slower growth rates and reached sexual maturity later than those from South Georgia. Survival of first-year seals decreased dramatically during the 1960s and led to the almost total failure of the 1965 cohort; the fate of later cohorts has not been determined.
Attempts to explain the recent behavior of southern elephant seal populations have so far concentrated on finding a single cause to account for the characteristics of all populations, rather than a series of independent explanations. Two main hypotheses are advanced, one involving equilibration processes after intense sealing pressure and the other concerned with fluctuations in the ocean environment. However, while there may be a single driving factor influencing the populations of southern elephant seals in the Indian and Pacific Ocean sectors, there may be additional local factors that also regulate the populations. In some cases, the interaction between these effects may obscure understanding of the main driving force. While the hypotheses have not yet been tested, they help in planning future research. Priorities should be the maintenance of censusing programs, long-term and cross-sectional demographic studies, and investigations into several aspects of the biology of first-year seals, particularly diet and foraging ranges.
Southern elephant seals, M. leonina Linn., have been known to science since the time of Linnaeus. For most of that time, however, their contact with man was purely as an exploitable resource, and they were ruthlessly hunted
at all of their major breeding grounds as a source of high-quality oil. Only since the middle of this century have these animals been the focus of serious scientific attention.
The 1950s and 1960s saw major research programs at both the South Georgia and Macquarie Island breeding sites. The result of this research effort was a new understanding of many important aspects of the basic biology of the species, such as reproductive biology (Laws 1956a ), developmental biology (Laws 1953, Bryden 1968a ), behavior and social structure (Laws 1956b ; Carrick, Csordas, and Ingham 1962; Carrick et al. 1962), and population biology (Laws 1960).
A major shift in research perspectives for southern elephant seals occurred in the mid-1980s, with reports of significant declines in several major populations. These reports coincided with an increasing awareness of the value of the Southern Ocean ecosystem both commercially and scientifically. The decline of a major vertebrate predator was regarded as possible cause for concern that other components of the ecosystem were also changing in as yet undetected ways.
This chapter summarizes what is known about the population declines in southern elephant seals at Macquarie Island and at other breeding sites to compare this to trends in other Southern Ocean vertebrate populations and to review the various explanations that have been advanced to explain the decline so far. A new hypothesis is also advanced, and the most fruitful directions for future research are suggested.
Population Trends
Macquarie Island
In 1985, the population at Macquarie Island was found to have decreased at a rate of about 2% per annum (Hindell and Burton 1987), calculated on the assumption that the decline had been operating since the early 1950s. The nature of the census data was such that there was no good a priori reason for assigning the beginning of the decline to any point during the 30-year time series (fig. 4.1). More recent analysis of demographic data collected at Macquarie Island during the 1950s and 1960s suggested that the decline may have started during the early 1960s (Hindell 1991). However, this has little effect on the estimated rate of decline, which remains at about 2% per annum.
There were no apparent differences in the overall rate of decline of adult males and females at Macquarie Island. And as adult male and female elephant seals occupy largely separate regions of the Southern Ocean during their time at sea (Hindell, Burton, and Slip 1991), whatever factor is causing the decline appears to be operating on the younger age classes, possibly before sexual differences in foraging patterns develop.
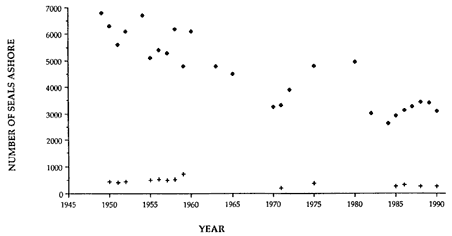
Fig. 4.1
The peak number of breeding females (diamonds) and males (crosses)
on the isthmus beaches at Macquarie Island between 1950 and 1990.
Kerguelen and South Georgia Stocks
The population decline at Macquarie Island should not be considered in isolation, as similar declines are occurring elsewhere in the Southern Ocean. Study of the behavior of all populations of southern elephant seals will help to understand the situation at Macquarie Island.
Southern elephant seal populations can be grouped into three apparently distinct stocks: Macquarie Island, Iles Kerguelen, and South Georgia. The most recent census data on subpopulations in each of these stocks are listed in table 4.1. The relatively inaccessible nature of many of the rookeries combined with the brevity of the breeding season makes accurate population estimates difficult in some cases, and interpopulation comparisons should be made with care. However, one major trend stands out. All the populations from the Kerguelen stock have been declining at least until the mid-1980s, while the populations from the South Georgia stock have been generally stable or increasing. The populations within the Kerguelen stock may be declining asynchronously with each other and with the Macquarie Island population, but this could be partly due to artifacts of the limited data. Nonetheless, these data suggest that there is something different about the South Georgia seals or about some aspect of their environment.
Other Species
Until recently, elephant seals were not regarded as truly Antarctic seals and were seen as ecologically distinct from the "ice seals." Recent studies on the
foraging ranges of elephant seals (see Slip, Hindell, and Burton, this volume) indicate that many adults spend a considerable proportion of their annual cycle in Antarctic waters. Therefore, it is relevant to compare the population trends of other Antarctic vertebrate consumers with those of elephant seals, and as yearling elephant seals may not move far from their natal islands (Carrick et al. 1962), it is also appropriate to examine population trends of other subantarctic species.
The status of other phocid species in the Southern Ocean is somewhat uncertain. Too little is known about the status of Ross seals, Ommatophoca rossi , and leopard seals, Hydrurga leptonyx , to make an assessment of any population trends at the moment (Erikson and Hanson 1990). There is no evidence of long-term changes in Weddell seal, Leptonychotes weddelli , populations at a number of sites around Antarctica (Testa and Siniff 1987; Green, Wong, and Burton 1992), although cycles in reproductive performance have been recorded (Testa et al. 1990). A. W. Erikson and M. B. Hanson (1990) reported a drop in the numbers of crabeater seals, Lobodon carcinophagus , in the Weddell Sea, but it is uncertain whether this represents a real decline in abundance, a change in distribution, or even sampling artifacts (see Green, Wong, and Burton 1992). Both Antarctic fur seal, Arctocephalus gazella , and sub-Antarctic fur seal, Arctocephalus tropicalis , populations are increasing at all of their breeding sites (e.g., Shaughnessy, Shaughnessy, and Keage 1988; Bengtson et al. 1990).
The baleen whale species that were once heavily harvested in the Southern Ocean all seem to be increasing in numbers (Bengtson 1984; Bryden, Kirkwood, and Slade 1990). Penguins constitute over 90% of the Antarctic avian biomass, but to date there have been no reports of major population declines in this important component of the Antarctic marine ecosystem (Woehler and Johnstone 1991). On the contrary, several species have increased (Laws 1985).
Of all the vertebrate consumers breeding on subantarctic islands, only the wandering albatross has declined in numbers (Hindell and Burton 1987), but this has been attributed to interactions with long-line fishing fleets (Croxall et al. 1990). Giant petrel populations have also been reported as declining in recent years (E. Woehler, pers. comm.), but as these animals rely on elephant seal carrion for a significant part of their diet (Hunter 1983), this decline could simply be a consequence of the elephant seal decline. Some rockhopper penguin, Eudyptes chrysocome , populations have also declined in recent years; the causes for these declines are as yet unknown (Moors 1986).
With the possible exception of crabeater seals and giant petrels and the certain exception of rockhopper penguins and wandering albatross, no other Southern Ocean consumer has exhibited large-scale population de-
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
clines. Several, such as the fur seals, baleen whales, and some penguin species, are actually increasing. This suggests that whatever is responsible for the decline in some southern elephant seal populations is acting on some aspect of their life history that is unique to these populations.
Demographic Data
There have been three studies of population biology of southern elephant seals. Two were based at South Georgia, one in the 1950s when sealing was still a commercial enterprise (Laws 1960) and the other during the 1970s, some 20 years after sealing had ceased (McCann 1985). Both of these studies were cross-sectional in nature, with life tables derived from age structure data, which were in turn derived from age estimates made from tooth rings. The population at South Georgia is thought to have been stable over the time between the two studies, and the population had largely recovered from the effects of sealing by the late 1970s (McCann and Rothery 1988). Consequently, the South Georgia demographic data from McCann's (1985) study may be regarded as representing a stable, undisturbed population and a valuable source of data for comparison with the declining populations.
A longitudinal study based on 15 cohorts of seals branded at Macquarie Island between 1950 and 1965 (Hindell 1991) revealed several features of their population biology pertinent to understanding the decline in numbers. First-year survival was essentially stable during the 1950s (fig. 4.2). The mean first-year survival for females was 46% and for males 42%. In the early 1960s, first-year survival declined dramatically, with the almost complete failure of the 1965 cohort (survivorship was less than 2%). However, survival of all other age classes appeared to be stable between the 1950s and 1960s. Unfortunately, it was not possible to analyze the success of later cohorts.
There were several significant differences between the Macquarie Island population parameters and those found in the stable South Georgia population. First-year survival of the Macquarie Island elephant seals during the 1950s was substantially lower than the 60% for both sexes estimated for South Georgia (McCann 1985). Survival in all other age classes was also lower at Macquarie Island than at South Georgia (fig. 4.3), although the differences in methodology make quantitative comparisons difficult. Seals from the South Georgia stock have higher growth rates, both preweaning and postweaning, than those from Macquarie Island (Bryden 1968b ). The slower growth rates of the Macquarie Island seals may be responsible for the 12-month delay in the average age at first breeding compared with South Georgia (Carrick, Csordas, and Ingham 1962).
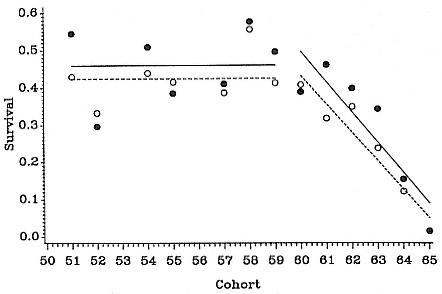
Fig. 4.2
Survival to age = 1 year for males (dashed lines, open circles) and females
(solid lines, solid circles) in 13 cohorts between 1951
and 1965 (from Hindell 1991).
Possible Explanations
An acceptable explanation of population trends in the southern elephant seal will need to account for the known characteristics of the declines. While it is possible that the declines in different populations may have independent causes, it is worthwhile to attempt to find a single explanation that will account for the decrease of all elephant seal populations.
Southern elephant seal declines are characterized by the following:
1. Both the adult male and adult female components of the Macquarie Island population appear to have declined at the same rate.
2. Only populations within the Macquarie and Kerguelen stocks have declined, while those from the South Georgia stock are probably stable. The timing and rates of the declines may also differ between subpopulations.
3. Southern elephant seal populations appear to have declined independently of other mammalian or avian species in the Antarctic or subantarctic sectors of the Southern Ocean.
4. Growth rates were lower at Macquarie Island than at South Georgia
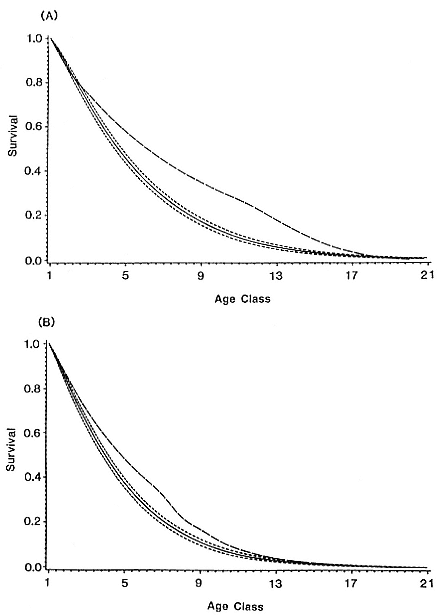
Fig. 4.3
Post-year 1 survival curves for (A) females from Macquarie Island (showing 95%
confidence limits in dotted lines) and South Georgia, and (B) males from
Macquarie Island (showing 95% confidence limits in dotted lines)
and South Georgia (from Hindell 1991).
during the 1950s and 1960s. Age at first breeding was also one year later at Macquarie Island than at South Georgia.
5. Survivorship in all age classes was lower in the Macquarie Island population than in the South Georgia population.
6. There was a marked decline in the first-year survival of Macquarie Island animals between 1960 and 1965 which resulted in the almost complete failure of the 1965 cohort; post-year-one survival did not appear to change at this time (Hindell 1991).
A number of explanations have been advanced to explain these observations with varying degrees of success. The hypotheses can be loosely divided into three groups: intrinsic factors, predation, and the availability of food.
Intrinsic Factors
The most likely intrinsic factor that could influence elephant seal population size is some kind of density-dependent mechanism. Both northern and southern elephant seals can exhibit density-dependent pup mortality, with pup mortality increasing on very crowded beaches (Reiter, Stinson, and Le Boeuf 1978; van Aarde 1980b ). Although it is possible that this mechanism might regulate elephant seal populations, it fails to explain many of the known characteristics of the decline. For example, it cannot account for both the apparent stability of the South Georgia population and the concurrent decline at Macquarie and Kergeulen. Also, as the overcrowding of harems results in increases in preweaning mortality, this mechanism does not account for the sharp decline in first-year survival during the 1960s (the animals used in that study were all branded after they were weaned).
It has been suggested that mass mortalities caused by disease, particularly parasite infestations, may be an important factor in population regulation in marine mammals (Harwood and Hall 1990). However, there is no evidence of widespread disease in southern elephant seal breeding aggregations.
Predation
Killer whales, Orcinus orca , are undoubtedly a major cause of juvenile mortality in southern elephant seals (Condy, van Aarde, and Bester 1978). Increases in the number of killer whales preying on newly weaned elephant seals might produce the changes in juvenile mortality described above and may also help explain the lower adult survival at Macquarie Island. However, there is no evidence for increased abundance of killer whales, and it would not satisfactorily explain the differences in growth rates and attainment of age of first breeding between the Macquarie and South Georgia populations.
Availability of Food
This category can be divided into three subgroups: competition, equilibration processes, and changes in the ocean environment.
Competition
Southern elephant seals could be competing with other Southern Ocean vertebrate consumers for basic food resources, particularly as a number of these species are currently increasing in number. However, adult elephant seals forage on deep-dwelling benthic and pelagic prey, which are generally unavailable to other mammal or bird species (Hindell, Slip, and Burton 1991), so the chances of direct competition must be limited. As we know little about the prey of first-year elephant seals, it is possible that this age class does share a prey species with one of the other increasing species, but recent data from northern elephant seals suggest that juveniles of 2 years are capable of undertaking the same dive patterns as adults (Le Boeuf, this volume). If young animals from the southern species can also make deep dives, they will equally be able to avoid direct competition with shallower-diving species. This hypothesis fails to account for the sudden decline in first-year survival observed in the 1960s, as it is difficult to understand how this could be caused by competition with other populations that were increasing at a steady rate.
It has also been suggested that elephant seals have had their resource base reduced by the recent introduction of commercial fishing operations in various regions of the Southern Ocean. M. Pascal (1986) suggested that the decline in elephant seal numbers at Iles Kerguelen may be linked to the very intense fishing activity in the area which occurred concurrently with the decline. However, there is little or no commercial fishing for deep water fishes in the areas identified as principal foraging grounds for elephant seals from Macquarie Island (Hindell, Burton, and Slip 1991; Slip, Hindell, and Burton, this volume).
Equilibration Processes
One explanation consistent with the known characteristics of the decline is the population "overshoot" hypothesis. This hypothesis states that the current decline in elephant seal numbers at Macquarie Island (and the other populations) is a direct consequence of the heavy exploitation of elephant seals during the eighteenth and early nineteenth century. The sealing industry reduced the elephant seal population at Macquarie Island to far below the original level and continued to suppress it for almost one hundred years (Hindell and Burton 1988a ). During this time, prey species may have increased so that when commercial sealing ended in the early part of this century, an abundant resource was available to the elephant seals, which enabled an increase in the population such that it overshot the presealing population size. Thus, the population
decline described since then may be the result of subsequent overexploitation of the food resource and a decrease toward equilibrium.
This hypothesis accounts for many of the observed characteristics of the decline. The South Georgia population may not have demonstrated an overshoot and decline because it was exploited in a managed fashion (at least for this century), while the differences in timing and rates of the declines in the other populations could be explained by different timing and extents of sealing pressure. The reduced growth rates and lower adult survival at Macquarie Island during the 1950s may have been a result of the population approaching the maximum carrying capacity of an inflated food resource. As the food resources became inadequate, the population began to decrease, principally driven by an increase in juvenile mortality as documented for the early 1960s.
The observation that both the male and female components of the population declined at a similar rate further supports the notion that the decline is a consequence of juvenile mortality. As adult male and female elephant seals use different foraging grounds, it seems most likely that the factor causing the decline has acted on the age classes in which pronounced sexual differences in morphology and behavior have not yet developed.
If the populations in the Kerguelen and Macquarie stocks declined synchronously, then the overshoot hypothesis would be substantially weakened. The hypothesis predicts that the timing and rate of the decline depends on the severity and duration of the sealing at each island (or group of islands where a common food source is used). It is unlikely that these would result in synchronous declines at a large number of populations over a considerable geographic range. Unfortunately, the time series data on abundance are inadequate to solve this problem.
Another problem with the overshoot hypothesis is that it may not account for declines in small populations, like Marion Island or Campbell Island, that may never have been heavily exploited. The hypothesis will only apply if (a) these populations share common feeding grounds with other, exploited populations and are thus exposed to the same "boom and bust" responses of the prey species or (b) such small populations are only splinter groups of the larger populations, perhaps formed when the exploited populations were approaching their maximum predecline densities.
Changes in the Ocean Environment
Another possible explanation is that the decline is due to changes in the ocean environment that have had an impact on the food species used by southern elephant seals (Burton 1986). Recent studies on global climate change have brought an increased awareness of large-scale environmental patterns and correlations between climatic fluctuations and population changes. For example, air pressure dis-
tribution over the Southern Hemisphere is influenced irregularly by the Southern Oscillation, which results in El Niño off Peru (van Loon and Shea 1988), and a scarcity of krill and a drop in breeding success of some land-based predators around South Georgia was observed in years that followed a strong El Niño Southern Oscillation (ENSO) event (Croxall et al. 1988; Priddle et al. 1988). Anomalies in air pressure at sea level affect the generation and movements of cyclones and anticyclones in the area between the subtropical ridge and the Antarctic continent (van Loon and Shea 1988), and these atmospheric processes force changes in the physical environment of the Southern Ocean, with subsequent effects on the biota (Sahrhage 1988).
The foraging grounds of adult southern elephant seals from the Macquarie stock lie off the coast of the Antarctic continent between longitude 135°E and 165°W (see Slip, Hindell, and Burton, this volume), and elephant seals from the Kerguelen stock have been regularly sighted ashore between 38°E and 110°E (Bester 1988; Gales and Burton 1989). Fluctuations in air pressure, affecting the physical environment of this area of the Southern Ocean might influence distribution and abundance of prey of these stocks. ENSO events are poorly correlated with longer-term climatic variability across the western margins of the Australian continent (Allen, Beck, and Mitchell 1990), and there appears to be no relationship between ENSO events and elephant seal numbers at Macquarie Island. However, climatic fluctuations in this area and the southern Indian Ocean appear to be a consequence of other forcings (Allen and Haylock 1993).
Analyses of mean sea level pressure data since 1957 from the wider Indian Ocean region, including coastal Antarctic stations, show that weather patterns in southwestern Australia are associated with fluctuations in the continental anticyclone and the long-wave trough to the south-southeast of Australia. This high latitude trough has shown strong persistence near the Antarctic station of Casey (110.5°W) in winter (June, July, August) and has fluctuated only in intensity, deepening to pressures below 985 hPa between 1966–1971 and 1975–1989 and becoming shallow with pressures above 985 hPa between 1957–1965 and 1972–1974 (Allan and Haylock 1993). This permanent low pressure region centered north of Casey has broadened since the early 1960s to include the Vestfold Hills on the shores of Pridz Bay, a known foraging area for seals of the Kerguelen stock (Bester 1988; Gales and Burton 1989). The deepening of this trough between 1966 and 1971 corresponds to the increase in first-year mortality at Macquarie Island.
Thus, although the causative factors are unknown, the fluctuations in this trough may have forced or have been associated with changes in the ocean environment that resulted in reduced food resources for elephant seal populations of the Macquarie and Kerguelen stocks and led to their
decline. The South Georgia population may have been unaffected by such changes and remained stable because variation in environmental conditions differ from one area of the Southern Ocean to another (Sahrhage 1988). In the absence of hard data, this is very speculative.
Predictions from These Hypotheses
Of the possible explanations presented above, two (the equilibration and the ocean environment hypotheses) are consistent with what we know of the decline of elephant seals at Macquarie Island and provide a possible explanation of the decline of other elephant seal populations. There is an obvious need for more research into many aspects of elephant seal population biology and ecology.
One approach to deciding future research directions is to test the various predictions arising from the alternative hypotheses and perhaps eliminate them. Not all have testable predictions, but the last two can be tested. This offers a framework for further research.
Predictions from the Equilibration Hypothesis
The equilibration or overshoot hypothesis predicts that elephant seals have a very simple predator-prey relationship and that they utilize a resource not exploited by other major predators. This is partly supported by recent studies of adult foraging behavior (see above).
As first-year survival decreased rapidly in advance of adult survival, some factor limited first-year survival. This suggests that first-year animals will be fundamentally different from adults either in their diet, their foraging grounds, or their ability to find and capture prey in competition with adult seals.
The hypothesis also predicts that the population should eventually stabilize at around the presealing population size. As this was estimated to be about 90,000 to 100,000 seals for Macquarie Island (Hindell and Burton 1988a ) and the present population size is approximately 90,000 seals, the decline should slow down or stop in the near future. However, the equilibration process may be expected to continue with a series of "undershoots" and "overshoots" of diminishing magnitude.
The seals would show an increase in growth rate and adult body size coincidental with the stabilization of population size. This would also be accompanied by decreases in the age of first breeding and increases in both adult and first-year survival.
As northern elephant seals are very similar in many ways to their southern congener, and they were also subjected to intense sealing pressure in the last century, they may exhibit population trends similar to southern elephant seals. At present, northern elephant seal populations are increas-
ing rapidly (Stewart et al., this volume). If the equilibration hypothesis holds and the populations are left completely undisturbed, they should eventually level off and then decline as they also fully exploit their major food species.
Predictions from the Environmental Change Hypothesis
The central premise of this hypothesis is that the Southern Ocean presents elephant seals with an environment where their specific food resources are patchily distributed in both space and time, and this patchiness is influenced by ENSO events. Accessibility to these patches directly affects survival of first-year animals (and thus regulates population size) and can change over short periods (less than a decade).
Thus, if it is valid, first-year survivorship should change markedly over quite short time intervals, certainly within a decade and possibly at even shorter intervals; they should be nondirectional, responding directly to variations in the marine environment. In contrast, while the equilibration hypothesis also predicts changes in first-year survival, it predicts them to be directional with a trend for increasing survival as the population returns to its original level.
If changes in weather patterns and ocean circulation have a direct effect on the distribution and abundance of elephant seal food resources (particularly those of first-year seals), then the seals would be expected to exhibit changes in feeding strategies and foraging grounds over quite short time spans, as they attempt to adjust to changes in density and location of prey species. The hypothesis further predicts, as does the equilibration hypothesis, that first-year animals will have different feeding strategies and foraging grounds than older seals.
Directions for Future Research
There are three main lines of investigation that should be pursued to test the predictions from these hypotheses and to promote understanding of the underlying reasons for the observed changes in elephant seal numbers. With most species of large mammals, juvenile survival is a major component of the process of population regulation. The single most striking aspect of the population dynamics of the Macquarie Island elephant seals was the decline in first-year survival that occurred in the early 1960s. Changes in population size since that time may have been largely due to this major perturbation in the recruitment process, although it is unknown whether reduced first-year survival was sustained after 1965. Both the equilibration and the environmental change hypotheses rely heavily on the premise that first-year elephant seals are different from adult seals, particularly with respect to diet and foraging areas or to their ability to exploit them. First-year
animals are also the component of the population that we know least about, and so they are an obvious target for research efforts. Foraging behavior and foraging areas can be studied and contrasted with those of adults, using time-depth and geolocation recorders. Although a considerable number of recorders will inevitably be lost through natural mortality, making it difficult and expensive to obtain acceptable sample sizes, this remains the best method for collecting the relevant data. It may also be possible to study diet directly as these young seals often haul out on sub-Antarctic islands during the winter months (Hindell and Burton 1988b ).
Another priority is the establishment of demographic studies. There are two possible approaches. The first is to renew long-term mark-resighting programs similar to the one at Macquarie Island in the 1950s or the one that recently began at Marion Island (Bester and Wilkinson, this volume). Although these studies may not produce valuable data for a number of years, long-term demographic information is fundamental to testing several of the predictions outlined above and is also central to management decisions that may need to be made in the future. The second approach is to conduct cross-sectional age structure studies similar to those from South Georgia. These have the advantage of supplying some types of demographic data, such as estimates of age-specific survival, quickly. It may be possible to obtain age estimates from incisors painlessly removed under anesthetic (Arnbom et al. 1992), which will greatly increase the practicality of such an approach.
Another research priority is the maintenance of simple censusing programs. In many ways, this should be seen as the primary research priority, as it can be performed cheaply and easily at a large number of locations. Data on any future population fluctuations will be required to test predictions from both the equilibration and environmental change hypotheses and provide ongoing information on the status of the species throughout its range.
References
Allen, R. J., K. Beck, and W. M. Mitchell. 1990. Sea level and rainfall correlations in Australia: Tropical links. Journal of Climate 3: 838–846.
Allen, R. J., and M. Haylock. 1993. Circulation features associated with the winter rainfall decrease in southwestern Australia: Implications for greenhouse and natural variability studies. Journal of Climate 6: 1356–1367.
Arnbom, T. A., N. J. Lunn, I. L. Boyd, and T. Barton. 1992. Ageing live Antarctic fur seals and southern elephant seals. Marine Mammal Science 8: 37–43.
Barrat, A., and J. L. Mougin. 1978. L'elephant de mer Mirounga leonina de L'ile de la Possession, archipel Crozet (46°25¢ S, 51°45¢ E). Mammalia 42: 143–174.
Bengtson, J. L. 1984. Review of Antarctic Marine Fauna. Selected Papers Presented to the Scientific Committee of CCAMLR 1982–1984 , 1–226.
Bengtson, J. L., L. M. Ferm, T. J. Harkonen, and B. S. Stewart. 1990. Abundance of Antarctic fur seals in the South Shetland Islands, Antarctica, during the 1986–87 austral summer. In Antarctic Ecosystems: Ecological Change and Conservation , ed. K. R. Kerry and G. Hempel, 265–270. Berlin and Heidelberg: Springer Verlag.
Bester, M. N. 1988. Marking and monitoring studies of the Kerguelen stock of southern elephant seals Mirounga leonina and their bearing on biological research in the Vestfold Hills. Hydrobiologica 165: 269–277.
Bryden, M. M. 1968a . Development and growth of the southern elephant seal (Mirounga leonina Linn.). Papers and Proceedings of the Royal Society of Tasmania 102: 25–30.
———. 1968b. Control of growth in two populations of elephant seals. Nature 217: 1106–1108.
Bryden, M. M., G. P. Kirkwood, and R. W. Slade. 1990. Humpback whales, Area V. An increase in numbers off Australia's east coast. In Antarctic Ecosystems: Ecological Change and Conservation , ed. K. R. Kerry and G. Hempel, 271–277. Berlin and Heidelberg: Springer Verlag.
Burton, H. R. 1986. A substantial decline in numbers of the southern elephant seal at Heard Island. Tasmanian Naturalist 86: 4–8.
Carrick, R., S. E. Csordas, and S. E. Ingham. 1962. Studies on the southern elephant seal, Mirounga leonina (L.). IV. Breeding and development. CSIRO Wildlife Research 7: 161–197.
Carrick, R., S. E. Csordas, S. E. Ingham, and K. Keith. 1962. Studies on the southern elephant seal, Mirounga leonina (L.). III. The annual cycle in relation to age and sex. CSIRO Wildlife Research 7: 119–160.
Condy, P. R., R. J. van Aarde, and M. N. Bester. 1978. The seasonal occurrence and behaviour of killer whales Orcinus orca , at Marion Island. Journal of Zoology, London 184: 449–464.
Croxall, J. P., T. S. McCann, P. A. Prince, and P. Rothery. 1988. Reproductive performance of seabirds and seals at South Georgia and Signy Island, South Orkney Islands, 1976–1987: Implications for Southern Ocean monitoring studies. In Antarctic Ocean and Resources Variability , ed. D. Sahrhage, 261–285. Berlin and Heidelberg: Springer Verlag.
Croxall, J. P., P. Rothery, S. P. C. Pickering, and P. A. Prince. 1990. Reproductive performance, recruitment and survival of wandering albatrosses Diomedea exulans at Bird Island, South Georgia. Journal of Animal Ecology 59: 775–796.
Erickson, A. W., and M. B. Hanson. 1990. Continental estimates and population trends of Antarctic seals. In Antarctic Ecosystems: Ecological Change and Conservation , ed. K. R. Kerry and G. Hempel, 253–264. Berlin and Heidelberg: Springer Verlag.
Gales, N. J., and H. R. Burton. 1989. The past and present status of the southern elephant seal (Mirounga leonina Linn.) in greater Antarctica. Mammalia 53: 35–47.
Green, K., V. Wong, and H. R. Burton. 1992. A population decline in Weddell seals: Real or sampling artifact? CSIRO Wildlife Research 19: 59–64.
Guinet, C., P. Jouventin, and H. Weimerskirch. 1992. Population changes, movements of southern elephant seals on Crozet and Kerguelen archipelagos in the last decades. Polar Biology 12: 349–356.
Harwood, J., and A. Hall. 1990. Mass mortality in marine mammals: Its implications for population dynamics and genetics. Trends in Evolution and Ecology 5: 254–257.
Hindell, M. A. 1991. Some life history parameters of a declining population of southern elephant seals, Mirounga leonina. Journal of Animal Ecology 60: 119–134.
Hindell, M. A., and H. R. Burton. 1987. Past and present status of the southern elephant seal (Mirounga leonina ) at Macquarie Island. Journal of Zoology, London 213: 365–380.
———. 1988a . The history of the elephant seal industry at Macquarie Island and estimates of the pre-sealing numbers. Papers and Proceedings of the Royal Society of Tasmania 122: 159–176.
———. 1988b . Seasonal haul-out patterns of the southern elephant seal (Mirounga leonina L.) at Macquarie Island. Journal of Mammalogy 69: 81–88.
Hindell, M. A., H. R. Burton, and D. J. Slip. 1991. Foraging areas of southern elephant seals, Mirounga leonina , as inferred from water temperature data. Australian Journal of Marine and Freshwater Research 42: 115–128.
Hindell, M. A., D. J. Slip, and H. R. Burton. 1991. Diving behaviour of adult male and female southern elephant seals. Australian Journal of Zoology 39: 595–619.
Hunter, S. 1983. The food and feeding ecology of the giant petrels Macronectes halli and M. giaganteus at South Georgia. Journal of Zoology, London 200: 521–538.
Laws, R. M. 1953. The elephant seal (Mirounga leonina Linn.). I. Growth and age. Falkland Islands Dependencies Survey, Scientific Reports 8: 1–61.
———. 1956a . The elephant seal (Mirounga leonina Linn.). II. General, social and reproductive behaviour. Falkland Islands Dependencies Survey, Scientific Reports 13: 1–88.
———. 1956b . The elephant seal (Mirounga leonina Linn.). III. The physiology of reproduction. Falkland Islands Dependencies Survey, Scientific Reports 15: 1–66.
———. 1960. The southern elephant seal (Mirounga leonina Linn.) at South Georgia. Norsk Hvalfangst-Tidende 49: 466–476, 520–542.
———. 1985. Ecology of the Southern Ocean. American Scientist 73: 26–40.
McCann, T. S. 1985. Size, status and demography of southern elephant seal Mirounga leonina populations. In Sea Mammals of South Latitudes: Proceedings of a Symposium of the 52d ANZAAS Congress in Sydney —May 1982 , ed. L. K. Ling and M. M. Bryden, 1–17. Northfield: South Australian Museum.
McCann, T. S., and P. Rothery. 1988. Population size and status of the southern elephant seal (Mirounga leonina ) at South Georgia, 1951–1985. Polar Biology 8: 305–309.
Moors, P. J. 1986. Decline in numbers of Rockhopper penguins at Campbell Island. Polar Record 23: 69–73.
Pascal, M. 1986. Numerical changes in the population of elephant seals (Mirounga leonina L.) in the Kerguelen Archipelago during the past 30 years. In Marine Mammal Fishery Interactions , ed. J. R. Beddington, R. J. H. Beverton, and D. M. Lavigne, 170–186. London: George Allen and Unwin.
Priddle, J., J. P. Croxall, I. Everson, R. B. Heywood, E. J. Murphy, P. A. Prince, and C. B. Sear. 1988. Large-scale fluctuations in distribution and abundance of krill—A discussion of possible causes. In Antarctic Ocean and Resources Variability , ed. D. Sahrhage, 261–285. Berlin and Heidelberg: Springer Verlag.
Reiter, J., N. L. Stinson, and B. J. Le Boeuf. 1978. Northern elephant seal development: The transition from weaning to nutritional independence. Behavioral Ecology and Sociobiology 3: 337–367.
Sahrhage, D. 1988. Some indications for environmental and krill resources variability in the Southern Ocean. In Antarctic Ocean and Resources Variability , ed. D. Sahrhage, 33–40. Berlin and Heidelberg: Springer Verlag.
SCAR. 1991. Report of the workshop on southern elephant seals, Monterey, California, May 22–23, 1991.
Shaughnessy, P. D., G. L. Shaughnessy, and P. L. Keage. 1988. Fur-seals at Heard Island: Recovery from past exploitation? In Marine Mammals of Australasia—Field Biology and Captive Management , ed. M. L. Augee, 71–77. Sydney: Royal Zoological Society of New South Wales.
Taylor, R. H., and G. A. Taylor. 1989. Re-assessment of the status of southern elephant seals (Mirounga leonina ) in New Zealand. New Zealand Journal of Marine and Freshwater Research 23: 201–213.
Testa, J. W., G. Oehlert, D. G. Ainley, J. L. Bengtson, D. B. Siniff, R. M. Laws, and D. Rounsevell. 1990. Temporal variability in Antarctic marine ecosystems: Periodic fluctuations in the phocid seals. Canadian Journal of Fisheries and Aquatic Science 48: 631–639.
Testa, J. W., and D. B. Siniff. 1987. Population dynamics of Weddell seals (Leptonychotes weddelli ) in McMurdo Sound, Antarctica. Ecological Monographs 57: 149–165.
van Aarde, R. J. 1980a . Fluctuations in the population of southern elephant seals Mirounga leonina at Kerguelen Island. South African Journal of Zoology 15: 99–106.
———. 1980b . Harem structure of the southern elephant seal Mirounga leonina at Kerguelen Island. Review Ecologie (Terre Vie) 34: 31–44.
van Loon, H., and D. J. Shea. 1988. A survey of atmospheric elements at the ocean's surface south of 40°S. In Antarctic Ocean and Resources Variability , ed. D. Sahrhage, 3–20. Berlin and Heidelberg: Springer Verlag.
Vergani, D. F., M. N. Lewis, and Z. B. Stranganelli. 1987. Observation on haul-out patterns and trends of the breeding populations of southern elephant seals at Península Valdes (Patagonia) and Stranger Point (King George Island). SCCAMLR-VI/BG/ 36: 1–9.
Woehler, E. J., and Johnstone, G. W. 1991. Status and conservation of the seabirds of the Australian Antarctic Territory. In Seabird Status and Conservation: A Supplement , ed. J. P. Croxall, 279–308. Technical Publication No. 11, International Council for Bird Preservation, Cambridge, England.
Five—
Population Ecology of Southern Elephant Seals at Marion Island
Marthan N. Bester and Ian S. Wilkinson
ABSTRACT. Research has highlighted the continued decline in the southern elephant seal population at Marion Island, and life table data implicate recently matured cows as the most vulnerable part of the population. Observation of onshore behavior suggests that the factors producing the elevated mortality rates of these cows operate at sea. Current research is concentrated on the investigation of at-sea behavior of this component of the population.
Declines in the southern elephant seal populations in the southern Indian Ocean, including the population at Marion Island, have been evident since the 1970s (Condy 1978; van Aarde 1980; Skinner and van Aarde 1983; Burton 1985). Notwithstanding a number of hypotheses that have been offered to explain the observed declines (ibid.), the driving forces behind them are, as yet, poorly understood.
Comprehensive tagging studies commenced at Marion Island in 1983 to permit the first assessment of life history parameters such as age-specific survival, age-specific fecundity, and age at maturity for females in the Marion Island population based on data collected at Marion Island. Previous comments on population characteristics for this population (Condy 1977) were based largely on statistics drawn from the populations at Macquarie Island (Carrick and Ingham 1962) and South Georgia (Laws 1960). Such population parameters cannot tell us what the cause of the decline is, but they can highlight weak links within the population at this site, which allows the focusing of future research efforts.
This chapter describes the present status of the Marion Island population and analyzes the population based on parameters derived from the 1983 cohort. In addition, we present the first data on movements of cows from Marion Island during the pelagic phase of their life cycle.
Methods
Counts of pups were conducted in a main study area (MSA) representing ± 10% of the coastline at Marion Island (46°54¢ S, 37°45¢ E) during the breeding seasons from 1974 to 1989 following the methods of P. R. Condy (1978). In 1976 and 1986–1989, the number of births for the whole of Marion Island was determined from counts made at all the breeding beaches at the end of October (Wilkinson and Bester, in press). The number of pups born in the MSA expressed as a percentage (range: 24–31%) of the total island pup production was calculated for 1976 and the years 1986–1989. Given that the proportion of births occurring in the MSA did not change significantly in these years (Wilkinson 1991), the pup population in the MSA is assumed to be representative of the entire population as an index of population size. Rates of population change were calculated using the exponential equation (Caughley 1977)
Nt = No ert
for the periods 1974–1989 and 1983–1989. The years 1983–1989 coincide with the period for which data on population parameters are available. From 1983 onward, weaned pups were tagged and beaches around the whole of Marion Island searched at 7- to 10-day intervals to resight these individuals (Bester 1989; Wilkinson 1991). Age-specific survival rates incorporating tag losses were calculated using Jolly-Seber mark-recapture analysis (see Wilkinson 1991). Rates of loss for individual tags ranged from 0.5% in the first year to 4.5% in the fifth year, which yielded combined loss rates (two tags) of 0.0 to 0.6%. Intercohort differences in first-year survival were calculated according to I. S. Wilkinson (1991). Calculation of age at first reproduction followed the method of A. E. York (1983), and fecundity followed the method of Wilkinson (1991). Sex ratio at birth was taken to be the same as the ratio of male:female weanlings that were tagged between 1983 and 1989 (Wilkinson 1991). The net reproductive rate (Ro ), calculated using the equation
Ro = S lx mx
where S lx mx = the sum of the product of the age-specific survival and fecundity values of females of a cohort (Caughley 1977), of the population at Marion Island and other components of the life table are described in Wilkinson (1991). In calculating the life table, empirical data were used up to age 5, after which the mortality rate was assumed to be constant (Harwood and Prime 1978) until a year or two before death (McCann 1985). Fecundity rates were assumed to remain constant after the age of full recruitment (age 6 in this population) to the breeding population until death, with no reproductive senescence (Hindell and Little 1988). All cows were
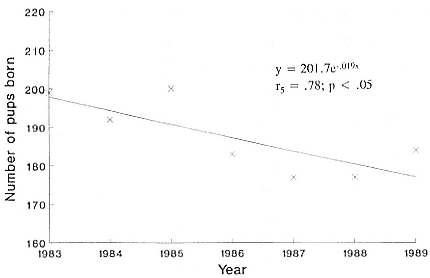
Fig. 5.1
Annual pup production for southern elephant seals within the MSA, Marion
Island, for the years 1983 to 1989. The equation refers to the line fitted
through the data points using least squares regression analysis (Zar 1984).
assumed to produce only one pup (Laws 1956), at a sex ratio found in the present study, and female longevity was 23 years (Hindell and Little 1988).
Data on individual movement of postbreeding cows (n = 3, aged 4, 7, and 7 years) at sea were collected with microprocessor-controlled Time-Depth Recorders (Wildlife Computers, Woodinville, Wash.) using the geolocation option, which included measurement of surface water temperature. The recorders were deployed on cows that were immobilized chemically using a remote injection method (Bester 1988b ) and following procedures detailed in M. N. Bester and H. M. Pansegrouw (1992). The Geolocation analysis software package of Wildlife Computers was used to calculate the daily longitude and latitude from light-level data, the theory and precision of which are discussed by R. D. Hill (1991).
Results and Discussion
Trends in Population Size and Population Parameters
The Marion Island population declined an average of 4.8% per annum between 1974 and 1989; it had slowed to 1.9% per annum between 1983 and 1989 (fig. 5.1). The total island pup production for 1989 was 585 individuals. As immigration and emigration are virtually nonexistent (Burton
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1985; Bester 1989; Gales, Adams, and Burton 1989), the observed decline must be a consequence of an imbalance between births and deaths within the population.
Cows of the 1983 cohort produced pups for the first time at age 3. An estimated 26.2% of cows had produced pups at age 3, 56.5% at age 4, 76.3% at age 5 and 100% of those cows known to still be alive at age 6. The sex ratio of 3,856 pups tagged between 1983 and 1989 was 1.04:1 (male:female), which does not differ significantly from unity (c 2 (1) = 1.82, p >.05). The mean age of these females at first pupping was 4.41 years.
Survival of females was 61.8% to age 1, 45% to age 2, 35.2% to age 3, 25.6% to age 4, and 21.1% to age 5 (table 5.1). The mortality rate
decreased up to age 3, then increased in the fourth year, and further decreased from age 4 to 5 (table 5.1). Intercohort comparison of first-year survival showed no differences for the years 1983 to 1988.
An indication of the viability of a population is shown by its net reproductive rate (Ro ). The Ro represents the number of daughters born to a female during her lifetime, or the rate of increase in the population with each passing generation (Caughley 1977). Therefore, to maintain itself, a population must have an Ro of 1. In the case of the population at Marion Island, the Ro is 0.661, resulting in a loss of 34% of the population with each passing generation. As Ro is a composite of both survival and fecundity, it can be affected by age-specific survival, pregnancy rate, and sex ratio of offspring.
The mean age at first pupping and hence recruitment to the adult component of the population is similar for Marion Island and the stable South Georgia population (McCann 1985; McCann and Rothery 1988) and occurs a year earlier than at Macquarie Island (Hindell 1991). The apparent 100% pupping rates of females age 6 and older are higher than the 85% reported at South Georgia (McCann 1985), and only reports for hooded seals (98%; Øritsland 1964, in Riedman 1990) and northern elephant seals (97.8%; Le Boeuf and Reiter 1988) are similar in magnitude.
Observations of sexual behavior at a single beach at Marion Island over three consecutive summers showed that dominant bulls controlling the harems in these years achieved over 98% of all matings and that the success of these matings was not affected by their timing in the season, or by prior (during the same season) levels of sexual activity of the bull (Wilkinson 1991). In view of the high pupping rates observed and the observed effectiveness of the few bulls that did breed, the "paucity of males" hypothesis (Skinner and van Aarde 1983) can be dismissed as a possible factor in the decline.
Manipulation of Life Table Data
The proportion of female pups at Marion Island (48.9%) is higher than the figure of 47% quoted for South Georgia, which resulted in an Ro of greater than 1 (McCann 1985). The proportion of female pups born would need to increase to 75% to allow the Ro to reach a value of 1 if pupping rates and age-specific survival remained constant (table 5.2).
When considering the age at primiparity, maturity would have to be advanced by two years, with breeding beginning at age 1 (26.2% pupping) and recruitment to the adult population complete by age 4 (100% pupping), to realize an Ro of 1. The present mean age at maturity (4.41) is already lower than previously reported at this site (Condy 1977) and similar to the stable South Georgia population (McCann 1985). Given that the cows are reproducing at an age that is already early for this species and
| ||||||||||||||||||
| ||||||||||||||||||||||
that they are producing offspring at the maximum rate, it would seem that age-specific survival is the key to the decline. Higher survival rates would improve age-specific survival/fecundity values and the resultant Ro value.
Assuming all other parameters are held constant, first-year survival would have to increase to 95% to produce a stable population (table 5.3). The present observed survival to age 1 is already the highest ever reported for this species, and given the major improvement that is required to stabilize the population, it seems unlikely that this is the vulnerable age group. Studies on the reproductive success of cows at Marion Island also showed low preweaning mortality rates (Wilkinson 1991).
The annual adult survival rate of females (82.4%) is too low to maintain
| ||||||||||||||||||||||
the population and needs to be increased to 90% to stabilize the population (table 5.4). The assumption made that survival rate of adults remains constant from age 5 until a year or two before death at age 23 (Hindell and Little 1988) contrasts with the view of T. S. McCann (1985) and R. M. Laws (1960) who assumed survival to remain constant from maturity to age 10 and then decline annually until death at age 20. However, the assumption of constant adult survival agrees with a study on gray seals, Halichoerus grypus , by J. Harwood and J. H. Prime (1978) and provides the highest Ro that is possible. If survival declined after age 10, the Ro would be lower still.
This manipulation of life table data shows the relative importance of the first year and adult components of the population. A 1% change in annual adult survival results in an approximately 5% change in Ro (table 5.4) but only a 1% change in Ro when first-year survival is changed by a similar amount (table 5.3). As mentioned, survival to age 1 is higher than ever reported in this species, and if it is then assumed that adult annual survival rate is not abnormally low, then it may be juvenile survival rates that are the problem, resulting in lowered recruitment to the adult age class.
If the figures for first-year survival and adult annual survival (from age 5 onward) described above are assumed correct and maintained, while replacing juvenile survival (l2 – l4 ; see table 5.1) with the values calculated for South Georgia (McCann 1985), the Ro value exceeds one (table 5.5).
Mortality Rates of Recently Matured Cows
The data for the 1983 cohort show that there is an increase in the mortality of 3-year-old females. This increase in mortality comes at a crucial time for the population. Although this age group is not the most reproductively
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
valuable (evidenced by the lx mx values in table 5.1), a high mortality rate at this age will, combined with high juvenile mortality, reduce the level of recruitment to the adult population and thus affect the survival/fecundity schedule, lowering Ro . The sharp increase in mortality among 3-year-old females comes at a time when animals at Marion Island are maturing sexually and are exposed to greater physiological stress levels resulting from gestation and lactation.
Gestation and the postpartum lactation period impose increased ener-
getic demands on the female, and these costs are relatively higher among young females (Reiter and Le Boeuf 1991) that are still in a more rapid phase of growth and development than their older counterparts (Laws 1953; Reiter, Panken, and Le Boeuf 1981). During the lactation period the cows may also lose up to 43% of their initial prepartum mass (Costa et al., 1986). Cows at Marion Island that were observed leaving the breeding beaches at the end of lactation were noticeably emaciated (Wilkinson 1991), implying a severe drain on their body reserves. In contrast, some cows at South Georgia were observed to leave the beaches with large blubber stores intact (McCann, Fedak, and Harwood 1989), possibly indicating that the nutritional status of the cows at the two sites differs.
Given current knowledge of the terrestrial component of the life cycle at Marion Island, it would appear that factors operating during the pelagic phase of the annual cycle, of subadult and adult cows in particular, hold the key to the decline process.
Cow Movements at Sea
The three cows that were tracked ranged over entirely different areas (fig. 5.2). None were followed for the total time at sea (67, 69, and 78 days, respectively), but two cows appeared to be returning to the island when recordings ceased. The cows spent a large proportion of their time in reasonably well-defined areas at, or near, the limit of their feeding range (between 1,100 and 1,400 km) (Bester and Pansegrouw 1992). The most circumscribed foraging area (that of the 4-year-old cow) lay south of the Antarctic Polar Front at 54°–57°S latitude and 25°–29°E longitude in cold surface water (minimum temperature of –1.7°C). The two other cows moved north to widely separated (by 15°–20° longitude) areas between approximately 40°–45°S latitude (fig. 5.2). The northwest-bound cow encountered warmer surface water (maximum temperature of 14.1°C) consistent with the mean position of the Subtropical Convergence at 41°40¢ S latitude which is recognizable at the sea surface by a mean decrease in temperature from 17.9°C to 10.6°C (Lutjeharms, Walters, and Allanson 1985). The northeast-moving cow appeared to remain in water between 5.3°C to 7.3°C at the Subantarctic Front (central surface temperature 7°C; Lutjeharms 1985). The cows therefore ranged widely without overlap in their feeding areas, the choice of which might have been influenced by biological enhancement at oceanic frontal systems (see Lutjeharms, Walters, and Allanson 1985). It is likely, however, that the small sample size has identified only a small part of the actual feeding range of elephant seal cows from Marion Island during the postbreeding period (Bester and Pansegrouw 1992), since three of five postbreeding cows from Macquarie Island foraged in deep oceanic waters off the Antarctic coast (Hindell, Burton, and Slip 1991).
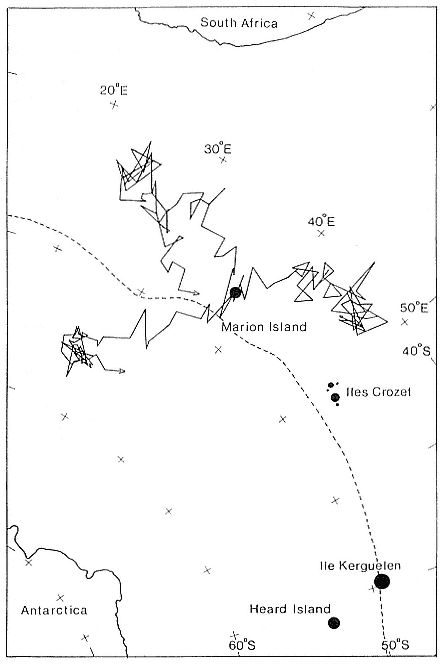
Fig. 5.2
At-sea movements of 3 southern elephant seal cows from Marion Island,
during their postbreeding pelagic period. The mean position of the
Antarctic Polar Front is shown (dashed line).
References
Bester, M. N. 1988a . Marking and monitoring studies of the Kerguelen stock of southern elephant seals, Mirounga leonina , and their bearing on biological research in the Vestfold Hills. Hydrobiologia 165: 269–277.
———. 1988b . Chemical restraint of Antarctic fur seals and southern elephant seals. South African Journal of Wildlife Research 18: 57–60.
———. 1989. Movements of southern elephant seals and subantarctic fur seals in relation to Marion Island. Marine Mammal Science 5: 257–265.
Bester, M. N., and H. M. Pansegrouw. 1992. Ranging behaviour of southern elephant seal cows from Marion Island. South African Journal of Science 88: 574–577.
Burton, H. R. 1985. Tagging studies of male southern elephant seals (Mirounga leonina L.) in the Vestfold Hills area, Antarctica, and some aspects of their behaviour. In Sea Mammals of South Latitudes: Proceedings of a Symposium of the 52d ANZAAS Congress in Sydney—May 1982 , ed. L. K. Ling and M. M. Bryden, 19–30. Northfield: South Australian Museum.
Carrick, R., and S. E. Ingham. 1962. Studies of the southern elephant seal Mirounga leonina (L.). V. Population dynamics and utilization. CSIRO Wildlife Research 7: 198–206.
Caughley, G. 1977. Analysis of Vertebrate Populations . New York: John Wiley and Sons.
Condy, P. R. 1977. The ecology of the southern elephant seal, Mirounga leonina (Linnaeus 1758), at Marion Island. D.Sc. dissertation, University of Pretoria, South Africa.
———. 1978. The distribution and abundance of southern elephant seals Mirounga leonina (Linn.) on the Prince Edward Islands. South African Journal of Antarctic Research 8: 42–48.
Costa, D. P., B. J. Le Boeuf, A. C. Huntley, and C. L. Ortiz. 1986. The energetics of lactation in the northern elephant seal, Mirounga angustirostris. Journal of Zoology, London 209: 21–33.
Gales, N. J., M. Adams, and H. R. Burton. 1989. Genetic relatedness of two populations of the southern elephant seal, M. leonina. Marine Mammal Science 5: 57–67.
Harwood, J., and J. H. Prime. 1978. Some factors affecting the size of British grey seal populations. Journal of Applied Ecology 15: 401–411.
Hill, R. D. 1991. Geolocation by Light-Level Readings . Woodinville, Wash.: Wildlife Computers Geolocation Instruction Manual.
Hindell, M. A. 1991. Some life-history parameters of a declining population of southern elephant seals, Mirounga leonina. Journal of Animal Ecology 60: 119–134.
Hindell, M. A., and H. R. Burton. 1987. Past and present status of the southern elephant seal (Mirounga leonina ) at Macquarie Island. Journal of Zoology, London 213: 365–380.
Hindell, M. A., H. R. Burton, and D. J. Slip. 1991. Foraging areas of southern elephant seals, Mirounga leonina , as inferred from water temperature data. Australian Journal of Marine and Freshwater Research 42: 115–128.
Hindell, M. A., and G. J. Little. 1988. Longevity, fertility and philopatry of two
female southern elephant seals (Mirounga leonina ) at Macquarie Island. Marine Mammal Science 4: 168–171.
Krebs, C. J. 1985. Ecology: The Experimental Analysis of Distribution and Abundance . New York: Harper and Row.
Laws, R. M. 1953. The elephant seal (Mirounga leonina Linn.). I. Growth and age. Falkland Islands Dependencies Survey, Scientific Reports 8: 1–62.
———. 1956. The elephant seal (Mirounga leonina Linn.). II. General, social and reproductive behaviour. Falkland Islands Dependencies Survey, Scientific Reports 13: 1–88.
———. 1960. The southern elephant seal (Mirounga leonina Linn.) at South Georgia. Norsk Hvalfangst-Tidende 49: 466–476, 520–542.
Le Boeuf, B. J., and J. Reiter. 1988. Lifetime reproductive success in northern elephant seals. In Reproductive Success , ed. T. H. Clutton-Brock, 344–362. Chicago: University of Chicago Press.
Lutjeharms, J. R. E. 1985. Location of frontal systems between Africa and Antarctica. Deep-Sea Research 32: 1499–1509.
Lutjeharms, J. R. E., N. M. Walters, and B. R. Allanson. 1985. Oceanic frontal systems and biological enhancement. In Antarctic Nutrient Cycles and Food Webs , ed. W. R. Siegfried, R. M. Laws, and P. R. Condy, 11–31. Berlin: Springer Verlag.
McCann, T. S. 1985. Size, status and demography of southern elephant seal Mirounga leonina populations. In Sea Mammals of South Latitudes: Proceedings of a Symposium of the 52d ANZAAS Congress in Sydney—May 1982 , ed. L. K. Ling and M. M. Bryden, 1–17. Northfield: South Australian Museum.
McCann, T. S., M. A. Fedak, and J. Harwood. 1989. Parental investment in southern elephant seals, Mirounga leonina. Behavioral Ecology and Sociobiology 25: 81–87.
McCann, T. S., and P. Rothery. 1988. Population size and status of the southern elephant seal (Mirounga leonina ) at South Georgia, 1951–1985. Polar Biology 8: 305–309.
Reiter, J., and B. J. Le Boeuf. 1991. Life history consequences of variation in age at primiparity in northern elephant seals. Behavioral Ecology and Sociobiology 28: 153–160.
Reiter, J., K. J. Panken, and B. J. Le Boeuf. 1981. Female competition and reproductive success in northern elephant seals. Animal Behavior 29: 670–687.
Riedman, M. L. 1990. The Pinnipeds: Seals, Sea Lions, and Walruses . Berkeley and Los Angeles: University of California Press.
Skinner, J. D., and R. J. van Aarde. 1983. Observations on the trend of the breeding population of southern elephant seals, Mirounga leonina , at Marion Island. Journal of Applied Ecology 20: 707–712.
Taylor, R. H., and G. A. Taylor. 1989. Reassessment of the status of southern elephant seals (Mirounga leonina ) in New Zealand. New Zealand Journal of Marine and Freshwater Research 23: 201–213.
van Arade, R. J. 1980. Fluctuations in the population of southern elephant seals Mirounga leonina at Kerguelen Island. South African Journal of Zoology 15: 99–106.
Wilkinson, I. S. 1991. Factors affecting reproductive success of southern elephant seals, Mirounga leonina , at Marion Island. Ph.D. dissertation, University of Pretoria, South Africa.
Wilkinson, I. S., and M. N. Bester. In press. Population parameters of the southern elephant seal population at Marion Island. Antarctic Science .
York, A. E. 1983. Average age at first reproduction of the northern fur seal (Callorhinus ursinus). Canadian Journal of Fisheries and Aquatic Science 40: 121–127.
Zar, J. H. 1984. Biostatistical Analysis . 2d ed. Englewood Cliffs, N.J.: Prentice-Hall.
Six—
Biomass and Energy Consumption of the South Georgia Population of Southern Elephant Seals
Ian L. Boyd, Tom A. Arnbom, and Michael A. Fedak
ABSTRACT. The total annual energy expenditure was estimated for different age and sex classes of southern elephant seals, Mirounga leonina , that breed at South Georgia. The estimated energy costs of reproduction, growth, foraging, and molt were used to calculate an annual energy budget for individuals in each age and sex class. This was combined with population size and age structure to estimate population energy requirements. The estimated average metabolic cost of maintenance for adult males and females was 0.17 and 0.39 MJ/year, respectively. Male biomass accounted for 63% of the total population biomass (222,903 metric tonnes), and the metabolic power for the whole population averaged over one year was 190 MWatts. Total energy expenditure of each age class declined during the first two years but then began to increase because of the onset of reproduction in females and because of increased energy costs of foraging and growth in males. Foraging accounted for 63.2% and 68.2% of the annual energy budget in males and females, respectively. The total annual energy expenditure was 6.01 × 109 MJ/year, and 59% of this was accounted for by males. The gross energy requirement was 7.89 × 109 MJ/year. The production efficiency was 8.2% Average daily gross energy intake during potential foraging periods was 77.3 and 43.2 MJ/day for males and females, respectively. This suggested a capture rate of 0.26 and 0.15 kg of fish or muscular squid per dive for males and females, respectively. Biomass of food consumed depended on assumptions about diet composition. If southern elephant seals at South Georgia fed exclusively on squid, the consumption biomass was 2.28 × 106 tonnes/year.
Total food or energy requirements of pinnipeds are important for assessing the impact of their populations on marine resources and the potential effects of changes in the abundance of prey on population size and distribution. Within a pinniped population, demand for food can vary between different age and sex classes because the abundance of individuals is a declining function of age and there are differing nutritional requirements for growth
or reproduction within each age and sex class. Although an estimate of the total energy requirements of a population can be useful, it is often more instructive to estimate energy demand for different age and sex classes because this can help to identify which parts of a population are most vulnerable to food shortage or which have the greatest potential impact on food resources.
This study is concerned exclusively with southern elephant seals, Mirounga leonina , that breed at South Georgia. This population has remained stable since the 1950s (Laws 1960; McCann and Rothery 1988), but this study has clear implications for interpretations of factors leading to declining numbers of southern elephant seals in other populations (van Aarde 1980; Skinner and van Aarde 1983; Hindell and Burton 1987; Hindell 1991). In addition, calculation of the total energy requirements of a population involves the synthesis of data about age structure, growth, and energy costs of reproduction, molt, locomotion, and foraging. Therefore, it is a means of highlighting the strengths and weaknesses of current data sets within a structured model.
Estimates of annual food consumption among phocids have been made for harp, Phoca groenlandica (Lavigne et al. 1985), gray, Halichoerus grypus (Fedak, Anderson, and Harwood 1981), and southern elephant seals (Laws 1977; McCann 1985). The estimates for harp and gray seals were based on empirical and best estimates of the energy budgets of individuals (derived from analyses of carcass composition), energy investment in reproduction, and measured metabolic costs of maintenance, growth, and age-dependent survivorship, combined with diet composition and assimilation efficiency to estimate food consumption. In contrast, estimates for southern elephant seals were largely based on crude estimates of food consumption per unit of biomass, which gives little scope for estimating the effects of changes in prey abundance on the population or of changes in the population parameters on food consumption. More recently, studies using time-depth-temperature recorders have provided significant information about the foraging of southern elephant seals (Boyd and Arnbom 1991; Hindell 1991) in terms of both swimming behavior and the location of prey in the water column. This has been augmented by detailed studies of the diet (Rodhouse et al. 1992) and population size (McCann and Rothery 1988).
The main objectives of this paper were to: (1) bring together recent information about southern elephant seal energetics (Fedak et al., this volume) by considering energy consumption in the broad context of the South Georgia population; (2) show how energy demand changes in relation to age both for individuals and for whole age classes; (3) contrast patterns of energy demand in the two sexes; and (4) provide an indication of what interaction there is likely to be between elephant seals and commercial fisheries in the Atlantic sector of the Southern Ocean.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Methods
Population Parameters, Growth, and Biomass
Annual Survival Rate
T. S. McCann's (1985) revision of R. M. Laws's (1960) life tables for male and female southern elephant seals was used to provide statistics on survival rates from birth to 20 years of age (table 6.1). These were based on cross-sectional age distributions from material collected by Laws and from a collection made in 1978. Comparison was made between these survival rates and those for the Macquarie Island stock (Hindell 1991), where longitudinal data have been collected on survival rates of individuals. Estimates of survivorship differed for the two stocks, especially survival rates up to 1 year and in the 5- to 12-year age classes (ibid.). Given that the Macquarie Island stock has been in decline while the South Georgia stock has been stable in recent years (Hindell and Burton
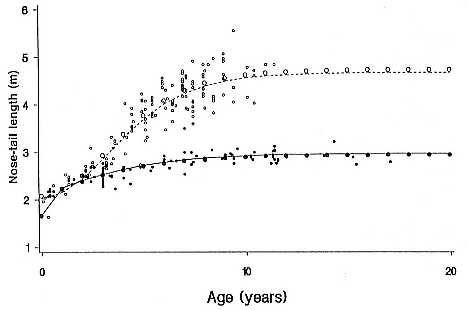
Fig. 6.1
The relationship between nose-tail (N–T) straight length and age for female (dots)
and male (open circles) southern elephant seals. Data were from Laws (1953), and
Gompertz growth models (large dots and solid line for females; large open circles
and dashed line for males) were fitted by least squares regression.
The regression equations were:
female length = 168 + 128 × e(–e – 0.028[age – 4.5])
male length = 202.4 + 264.9 × e(–e – 0.039[age – 39.73])
1987; McCann and Rothery 1988), reduced survival at Macquarie Island would be sufficient to account for this difference. The maximum longevity for a female elephant seal from South Georgia is 21 years (Arnbom et al. 1992), which is close to the 20 years predicted by McCann's life table.
Survival rate estimates for males are less certain than those for females. Males were assumed to become sexually mature at 4 years of age but did not breed until they were > 6 years of age (Laws 1953).
Growth
For the purposes of this analysis, we define the mean size of seals in any age class as the size at the end of the period of reproductive investment each year (the end of the breeding season). For seals < 1 year old, this means from the time of nutritional independence or weaning. Laws (1953) gave the nose-tail curved lengths of male and female elephant seals in relation to age. Curved lengths are about 10% greater than nose-tail straight length, so Laws's data were corrected to the equivalent nose-tail
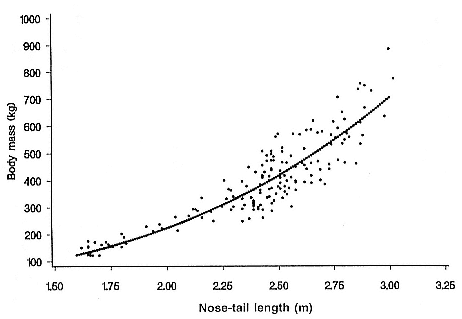
Fig. 6.2
The relationship between nose-tail straight length and mass for 162 female elephant
seals. The line was fitted by least squares regression (length = 22.61 + 25.33 mass3 ).
straight length and asymptotic growth curves were fitted to the data by least squares regression (fig. 6.1). The predicted values for each age were used as the mean length for a given age (table 6.1). The model overestimated weaning mass of both sexes, so the values given by T. S. McCann, M. A. Fedak, and J. Harwood (1989) were used for this age class.
Recent studies in which females were chemically immobilized (Baker et al. 1990; McCann, Fedak, and Harwood 1989; Boyd, Arnbom, and Fedak 1993) have yielded a relationship between mass and nose-tail length for females (fig. 6.2). This was used to estimate mean mass for each age class of elephant seal (table 6.1). The relationship between mass and length in males described by Bryden (1969b , 1972) was used to calculate the equivalent mass-age relationship for males. The pectoral girth of seals was also estimated using the equations in M. M. Bryden (1969b ) which were subsequently corrected (Bryden 1972; see table 6.1).
Biomass
The number of individuals in each age class was calculated from a pup production of 102,000 (McCann and Rothery 1988), assuming a 1:1 sex ratio at birth (McCann 1985) and a stable population age structure. The biomass of each age class was derived from the product of the
number of individuals in each age class and the mean mass of those individuals (table 6.1). The total body gross energy (TBGE) for that biomass was calculated using the equation provided by J. J. Reilly and M. A. Fedak (1990) derived from body composition analyses of gray seals. We assumed a mean water content of 55% derived from estimates of northern elephant seal, M. angustirostris , water content during lactation and molt (Costa et al. 1986; Worthy et al. 1992). The validity of using the Reilly and Fedak equation on different species was discussed in relation to other work on ringed seals, Phoca hispida (Stirling and McEwan 1975), and was broadly confirmed by I. L. Boyd and C. D. Duck (1991). Estimates of biomass are calculated in metric tonnes; 1 tonne equals 1,000 kg.
Energy Expenditure
The annual cycle of southern elephant seals involves three distinct activities: (1) reproduction in adult seals, (2) molt, and (3) foraging at sea (Laws 1960). Each of these will have a characteristic energy cost depending on the mass, reproductive status, and sex of the seal. An additional cost will be incurred in terms of body growth, which will vary with age, although reversible growth, such as seasonal fattening before breeding or molt, is considered as one of the costs of reproduction or molt in this analysis. The total energy expenditure (E) for an individual southern elephant seal can be expressed as
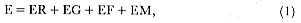
where ER, EG, EF, and EM are the energy costs of reproduction, growth, foraging, and molt, respectively. ER includes the heat increments of lactation, gestation, and, in the case of males, harem defense and competition for mates. EF includes the heat increments of locomotion and feeding the estimates of assimilation efficiency and duration of foraging. EG includes the energy value of tissue growth but excludes seasonal changes in body reserves.
Energy of Reproduction
The metabolic rate, measured by metabolic water production, of female southern elephant seals during lactation was 88 MJ/day (Fedak et al., this volume) or 3.1 times the predicted standard metabolic rate (SMR). The general relationship (SMR = 0.293 W0.75 MJ/d) between SMR and body mass of mammals has been discussed and confirmed for seals by Lavigne et al. (1986). Energy expenditure on metabolism was 46% of total energy expenditure. This shows that the total energy expenditure of lactation is 6 times SMR, which is in close agreement with measurements made in northern elephant seals (Costa et al. 1986). The mean duration of suckling used to calculate the heat increment of lactation was 23 days for mothers with either male or female pups (McCann, Fedak, and
Harwood 1989; Fedak et al., this volume). Thus, the energy of reproduction for an individual female was obtained from 6 × SMR × 23.
The mean mass of elephant seals at birth was 42.5 kg (McCann, Fedak, and Harwood 1989). The energy density of fetal seals at term has been calculated for ringed seals (9.04 MJ/kg; Stirling and McEwan 1975), harp seals (7.24 MJ/kg; Worthy and Lavigne 1983) and gray seals (5.76 MJ/kg; Anderson and Fedak 1987). The variation in these figures probably reflects species differences in the fat content of pups at birth. We do not know which value is the most appropriate for southern elephant seals, but because some of the ringed seal pups in I. Stirling and E. H. McEwan's study (1975) could have been suckled and this figure may be an overestimate of energy density, we used a conservative value of 6.5 MJ/kg in this analysis. The mass of southern elephant seal placentas is 5 kg, and an energy density of 0.46 MJ/kg (Lavigne and Stewart 1979) was used to calculate total energy content.
There is less information about the energy costs of reproduction in males. The only pinniped studies that have measured energy expenditure showed that breeding male northern elephant seals and territorial male Antarctic fur seals, Arctocephalus gazella , both have metabolic rates 3.3 times SMR (Deutsch, Haley, and Le Boeuf 1990; Boyd and Duck 1991). The competitive mating behavior of these species is similar to the southern elephant seal (McCann 1981). Therefore, we used the same value to calculate the metabolic costs of reproduction in males > 6 years of age. According to McCann (1980), the number of bulls ashore at the breeding grounds increases from September 1 to October 1 and remains roughly stable thereafter until November 18 when the number declines rapidly. We have assumed that they are present from September 14 until November 20 (58 days). The duration of the postweaning fast for pups was 45 days.
Energy of Molt
The energy cost of molt in southern elephant seals was estimated to be 2.4 times SMR (Boyd, Arnbom, and Fedak 1993), which is similar to that measured for adult female northern elephant seals (Worthy et al. 1992). Molting lasts 28 and 40 days on average in adult female and male southern elephant seals, respectively (Ling and Bryden 1981). These values were used to calculate the heat increment of molt in both sexes.
Energy of Growth
The body composition of southern elephant seals shows minor changes at different stages of life (Bryden 1969a ). However, because the scale of these changes is small, we have assumed that growth of body components is proportional to growth in total mass. The annual growth increment of southern elephant seals was calculated from the data on body mass (table 6.1), and the energy increment it represents was calculated using the equation for TBGE provided by Reilly and Fedak (1990).
Energy of Foraging
Activity budgets of seals during the aquatic phases of the annual cycle are based on time depth recorder outputs for southern elephant seals (Boyd and Arnbom 1991; Slip, Hindell, and Burton, this volume). While at sea, adult elephant seals spend 88% of their time diving. Velocities of descent and ascent were 1.64 m/s and 1/44 m/s, which makes sense in terms of empirical measurements of the cost of transport in phocids (Davis, Williams, and Kooyman 1985; Williams and Kooyman 1985). Seventy-four percent of dives had a profile showing that the seals remained at a specific depth for 54% of the duration of the dive. A further 10% of dives had a period when the seals remained at a constant depth before the resumption of a descent phase, and 16% of dives had no period spent at constant depth. Mean dive duration was 17 minutes for each type of dive.
The work required to transport a body through seawater is given by
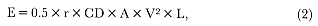
where CD is the coefficient of drag, V is the velocity, r is the average water density (1.027 × 103 kg/m3 for the Southern Ocean, data from British Antarctic Survey), A is the maximum cross-sectional area, and L is the distance. This is an extended form of the equation of drag (Williams 1987). Estimates of CD will depend on the shape and size of the seal. Values of CD measured for seals range from 0.06 to 0.09 (Williams and Kooyman 1985; Feldkamp 1987), but these are for gliding seals, and it is well known that the drag associated with active swimming is greater. Furthermore, an average adult female elephant seal (length 3.0 m) has a Reynolds number (Re) of 4.3 × 106 , which is close to Re = 5 × 106 for turbulent flow at a velocity of 1.5 m/s. This suggests that most elephant seals are close to the boundary of turbulent flow. Put together, this suggests that the CD for southern elephant seals is greater than measured values for other species would suggest. Therefore, a value of 0.12 was used in the calculation.
T. M. Williams, G. L. Kooyman, and D. A. Croll (1990) showed that harbor seals, Phoca vitulina , had a swimming efficiency (power output as a percentage of power input) that varied with velocity but was approximately 10% at 1.5 m/s.
Using the parameters given in table 6.1, we calculated the average cost of transport for the South Georgia elephant seal population. This assumed that most elephant seals forage in a way similar to the small sample for which detailed measurements are available. It also assumed that nonbreeders continued to forage during the breeding season but that all seals molted on land.
For harp seals, the heat increment of digestion was estimated to be 17% of the gross energy intake (Gallivan and Ronald 1981), and for harp seals and northern fur seals, the assimilation efficiency was estimated to be 90%
of gross energy intake (Keiver, Ronald, and Beamish 1984; Fadely, Worthy, and Costa 1990) on a diet of fish. There is no information for elephant seals, so we have used these figures in our calculations of the heat increment of foraging.
Diet Composition and Population Energy Consumption
It has been difficult to obtain an accurate measure of the diet of southern elephant seals at South Georgia mainly because it has not yet proved possible to measure diet at the foraging grounds. We have to rely on stomach samples obtained from elephant seals at the breeding and molting areas, which can be many hundreds of kilometers from the foraging areas (Fedak et al, this volume). Laws (1956) examined 139 stomachs, of which 108 contained no food; of the remainder, 24 contained squid and 9 fish. Laws (1977) therefore suggested that the diet of southern elephant seals consisted of 75% squid and 25% fish by weight. P. G. Rodhouse et al. (1992) have described the cephalopod prey, from samples obtained by stomach flushing, and confirmed that squid made up the greatest proportion of the diet. No fish remains were discovered, but fish are probably underrepresented in stomach flushes because of their rapid rate of digestion. Therefore, we have little justification for departing from Laws's overall figure for diet composition. In addition, we have to assume for the present that diet measured from stomach lavaging is broadly indicative of overall diet, although the analysis presented here also provides an indication of what amounts of different types of prey would be taken if the proportions of squid and fish departed from those suggested by Laws (1977).
Energy densities vary considerably between different species of squid, so the squid diet was divided into three parts: (1) muscular squid, (2) gelatinous squid, and (3) cranchiid squid, which have a leathery mantle. The classification of each species plus its representation by percentage mass of squid in the diet is given in table 6.2. Energy values were assigned following A. Clarke et al. (1985). We assumed that fish taken were a mixture of myctophids and notothenids. Although there is no evidence that southern elephant seals feed on myctophids, they are dominant among species of shoaling fish in the parts of the water column in which elephants seals apparently feed (Hindell 1991; Boyd and Arnbom 1991), which suggests that they are potential prey. Energy densities of myctophids are in the range of 7–8 MJ/kg (Cherel and Ridoux 1992), and we have used a value of 7.5 MJ/kg. The energy density of other fish species was assumed to be 4 MJ/kg (ibid.).
The total biomass of each item consumed in the diet was calculated from the gross energy requirement of the population (GE), so that
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
where I1m , I2m , . . . , lnm denote the mass of each item in the diet and I1e , I2e , . . . , lne denote the energy value (MJ/kg) of each item. This equation was solved for the mass of each item from knowledge of the proportion by mass of each item in the diet. Therefore,
where r1, r2, r3, and so on were the ratios of Inm to the mass of the other items in the diet.
Results
Annual Energy Budget
The pattern of energy demand by each age class reflected the changing number, mass, and reproductive condition with age (fig. 6.3). Total energy expenditure in both sexes declined initially, because of the high rate of juvenile mortality, and then began to increase. Among females, this was due to the introduction of the additional burden of reproduction, while among males, the increase was the result of growth and increased costs of locomotion associated with larger body size.
The energy expenditure of individual males increased to 55 × 103 MJ up to age 10 with a sharp increase in energy expenditure associated with the
Fig. 6.3
Changes in the energy expenditure of different age classes of the
stable southern elephant seal stock at South Georgia.
beginning of reproduction at age 7 (fig. 6.4). In females, the asymptote of annual energy expenditure was reached at a similar age but at about one quarter of the energy expenditure of males.
The total population size was 469,000, with a sex ratio of 1.25 females for each male. The total biomass was 222,903 tonnes, of which male biomass made up 63% and the mean mass was 205 kg. The estimated total annual energy expenditure for the population was 6.01 × 109 MJ, which was equivalent to 190 Mw averaged over the whole year.
Fig. 6.4
Changes in the per capita energy expenditure of female and
male southern elephant seals in relation to age.
The annual energy expenditure associated with foraging (EF) was 63.2% and 68.2% of the total energy costs in males and females, respectively (fig. 6.5), and EF was the largest single form of energy expenditure. Overall, 65.2% of the energy expenditure of the population was spent on foraging. Among males, the energy expenditure of molt (EM) was the next largest energy demand (13.7%), and this was greater than the total energy
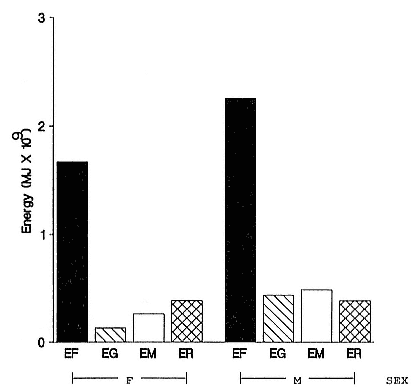
Fig. 6.5
Components of the annual energy budget of southern elephant seals from
South Georgia. EF = foraging energy expenditure; EG = energy expenditure
of growth; EM = energy expenditure of molt; ER = energy
expenditure of reproduction.
expenditure of molt in females (10.8% of female total). Energy expenditure on reproduction (ER) was similar in the two sexes (3.87 × 108 MJ for females and 3.88 × 108 MJ for males). This was 10.9% and 15.8% of the total energy expenditure for males and females, respectively. The energy expenditure on growth was 12.3% and 5.3% of the total expenditure for males and females, respectively.
Food Consumption
The total biomass of squid and fish (assuming 100% myctophids) consumed was 1.71 and 0.26 million metric tonnes per year, respectively (table 6.3). The female section of the population consumed 0.63 and 0.11 million tonnes of squid and fish, while equivalent values for males were 1.08 and 0.15 million tonnes, respectively. The biomass of each squid species consumed suggested that both males and females specialized in the large,
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
muscular species of high energy density (table 6.2) compared with the species of lower energy density (fig. 6.6).
Production Efficiency
The gross energy requirement (GE) of the population was 7.82 × 109 MJ (3.18 × 109 MJ for females and 4.64 × 109 MJ for males), and the production energy was 0.65 × 109 MJ. The production efficiency was therefore 8.2%. On average, gross energy intake during potential foraging time was 77.3 and 43.2 MJ/day for males and females, respectively. This suggests a capture rate of 0.26 and 0.15 kg of muscular squid or fish per dive for males and females, respectively, based on dive rates obtained from Boyd and Arnbom (1991) and D. Slip, M. Hindell, and H. Burton (this volume).
Discussion
It was not possible to provide statistical confidence intervals on estimates of energy expenditure because of the very variable quality of the data used to produce these estimates. Therefore, the results should be interpreted with care, although there is evidence supporting their accuracy. For example, our estimate of the gross energy requirements of the South Georgia elephant seal population compares well with the estimate for harp seals given by D. M. Lavigne et al. (1985). They estimated a per capita energy requirement of 11.3 × 103 MJ/year, while for the larger elephant seal, the value was 16.8 × 103 MJ/year. In addition, W. Sakamoto et al. (1989) esti-
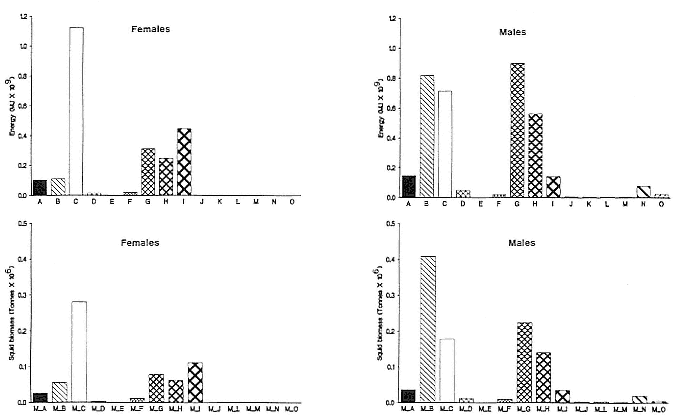
Fig. 6.6
Composition of the squid portion of the diet of southern elephant seals from the South Georgia stock
expressed in terms of energy value and biomass. The squid species are those labeled in table 6.2.
mated the daily gross energy requirement for an average adult female northern elephant seal when foraging as about 40 MJ/day. In this study we estimate a value of 41 MJ/day for the daily energy expenditure of adult females averaged over the whole year.
We have estimated population biomass to be 1.21 times that estimated by McCann (1985). This is because we calculated biomass at the end of the breeding season, whereas McCann did not include the biomass of the 0+ age class. His estimates of increasing mass with age were also more conservative, but our experience of weighing both male and female elephant seals has suggested that the estimates of mass given in table 6.1 are realistic. There are also significant differences between the food consumption of the population estimated in this study compared with the estimate made by McCann (1985). We found a total biomass consumption of 2.1 million tonnes (given the 3:1 numerical ratio of squid to fish in the diet), whereas McCann (1985) estimated this to be 3.68 million tonnes. The difference is largely caused by the figures used by McCann to translate population biomass into an estimate of food consumption. Following Laws (1977), he assumed that the annual food consumption was 20 times body mass. Our estimates suggest that for elephant seals the multiplier should be closer to 10. This is similar to the estimate made for harp seals on a fish diet by Lavigne et al. (1985).
A further reason for the reduced size of the multiplier is the apparently high production efficiency shown by southern elephant seals. W. F. Humphreys (1979) showed that production efficiency for mainly small herbivorous mammals rarely exceeded 5%. Respiratory costs are reduced in elephant seals because of the large body size (Lavigne et al. 1986) and because the cost of transport per unit of mass declines with increased mass. Since the cost of foraging, which is largely associated with the cost of transport, was the major form of energy expenditure, then the respiratory costs of a population of large swimming mammals will be lower than a population with an identical biomass but made up of a greater number of small individuals. Thus, the production efficiency of a harp seal population is about half that of the elephant seal. The long delay in reproductive maturity of males also contributes greatly to the production efficiency because energy normally expended on reproduction is available to be routed into growth.
Apart from the suggestion of Laws (1977), there is no evidence that elephant seals feed with a ratio of three parts squid to one part fish by weight. This highlights one of the greatest deficiencies in our knowledge of southern elephant seal foraging ecology. The diet may contain more or less fish, and it may also contain more benthic and demersal species that will have energy densities more similar to the muscular squid (4 MJ/kg, table 6.2, Cheral and Ridoux 1992) than to myctophids, which are particularly
oily. Table 6.3 compares the biomass of fish and squid consumed with different proportions of fish and squid in the diet. Estimates of total food consumed also depend on the energy values applied to each item of the diet. In this study these are only crude estimates that, in the case of squid, are based on estimates of the energy density of related temperate squid species (Clarke et al. 1985). Little is known about some of the species found in the diet of southern elephant seals, and we cannot be certain that the general classification into "muscular" or "gelatinous" forms is correct. Estimates of total consumption must also be interpreted with care because lavaging animals soon after they arrive on land probably only indicates diet in the immediate surroundings of the landing site or is biased by items, such as squid beaks, that persist in the stomach longer than other food remains.
One of the most significant potential threats to the stability of the South Georgia elephant seal stock is competition for food with commercial fisheries. This study is a synthesis of knowledge about the biology of southern elephant seals at South Georgia that provides an important, albeit imprecise, insight into the potential interactions between the South Georgia southern elephant seal population and its food supply. The metabolic costs of maintenance for fully grown adult female southern elephant seals was 0.39 MJ/kg.75 , and for adult males it was 0.17 MJ/kg.75 . The differences between the sexes is probably caused by differences in the foraging component of energy expenditure because the cost of locomotion increases in direct proportion to cross-sectional area. Large changes in mass are accompanied by relatively small changes in cross-sectional area, resulting in reduced costs of locomotion per unit mass. The total annual energy expenditure for the southern elephant seal population at South Georgia was 6.01 × 109 MJ/year, and 59% of this was accounted for by males. Despite contributing nothing to the energy costs of raising young and having only a small chance of reproducing (Deutsch, Haley, and Le Boeuf 1990), males demand a greater proportion of the total resources than females. They may also require richer foraging grounds because during potential periods of foraging, the rate of energy intake of males has to be almost twice that of females. These differences suggest that male survival, growth, and condition may be more responsive to changes in environmental conditions than that of females. However, the high production efficiency of southern elephant seals also suggests that they may be able to exploit highly dispersed prey resources.
Acknowledgments
This chapter is the result of a collaborative research project between the British Antarctic Survey (Natural Environment Research Council) and the Sea Mammal Research Unit (NERC). We thank T. Barton, C. Chambers, B. J. McConnell, A. Morton, and A. Taylor for assistance in the field.
References
Anderson, S. S., and M. A. Fedak. 1987. Gray seal, Halichoerus grypus , energetics: Females invest more in male offspring. Journal of Zoology, London 211: 667–679.
Arnbom, T. A., N. J. Lunn, I. L. Boyd, and T. Barton. 1992. Ageing live Antarctic fur seals and southern elephant seals. Marine Mammal Science 8: 37–43.
Baker, J. R., M. A. Fedak, S. S. Anderson, T. Arnbom, and J. R. Baker. 1990. Use of tiletamine-zolazepam mixture to immobilise wild gray seals and southern elephant seals. Veterinary Record 126: 75–77.
Boyd, I. L., and T. Arnbom. 1991. Diving behaviour in relation to water temperature in the southern elephant seal: Foraging implications. Polar Biology 11: 259–266.
Boyd, I. L., T. Arnbom, and M. A. Fedak. 1993. Water flux, body composition and metabolic rate during molt in female southern elephant seals (Mirounga leonina ). Physiology Zoology 66: 43–60.
Boyd, I. L., and C. D. Duck. 1991. Mass changes and metabolism in territorial male Antarctic fur seals (Arctocephalus gazella ). Physiological Zoology 64: 375–392.
Bryden, M. M. 1969a . Relative growth of the major body components of the southern elephant seal, Mirounga leonina (L.). Australian Journal of Zoology 17: 153–177.
———. 1969b . Growth of the southern elephant seal, Mirounga leonina (Linn.). Growth 33: 69–82.
———. 1972. Body size and composition of elephant seals (Mirounga leonina ): Absolute measurements and estimates from bone dimensions. Journal of Zoology, London 167: 265–276.
Cherel, Y., and V. Ridoux. 1992. Prey species and nutritive value of food fed during summer to king penguin, Aptenodytes patagonica , chicks at Possession Island, Crozet Archipelago. Ibis 134: 118–127.
Clarke, A., M. R. Clarke, L. J. Holmes, and T. D. Waters. 1985. Calorific values and elemental analysis of eleven species of oceanic squids (Mollusca: Cephalopoda). Journal of the Marine Biological Association 65: 983–986.
Costa, D. P., B. J. Le Boeuf, A. C. Huntley, and C. L. Ortiz. 1986. The energetics of lactation in the northern elephant seal, Mirounga angustirostris. Journal of Zoology, London 209: 21–33.
Davis, R. W., T. M. Williams, and G. L. Kooyman. 1985. Swimming metabolism of yearling and adult harbor seals, Phoca vitulina. Physiological Zoology 58: 590–596.
Deutsch, C. J., M. P. Haley, and B. J. Le Boeuf. 1990. Reproductive effort of male northern elephant seals: Estimates from mass loss. Canadian Journal of Zoology 68: 2580–2593.
Fadely, B. S., G. A. J. Worthy, and D. P. Costa. 1990. Assimilation efficiency of northern fur seals determined using dietary manganese. Journal of Wildlife Management 54: 246–251.
Fedak, M. A., S. S. Anderson, and J. Harwood. 1981. The energetics of the gray seal (Halichoerus grypus ) in European waters: Energy flow and management implications. Final Report to the European Communities on Contract ENV 405-80-UK (B).
Feldkamp, S. D. 1987. Swimming in the California sea lion: Morphometrics, drag, and energetics. Journal of Experimental Biology 131: 117–135.
Gallivan, G. J., and K. Ronald. 1981. Apparent specific dynamics action in the harp seal (Phoca groenlandica ). Comparative Biochemistry and Physiology 69A: 579–581.
Hindell, M. A. 1991. Some life-history parameters of a declining population of southern elephant seals, Mirounga leonina. Journal of Animal Ecology 60: 119–134.
Hindell, M. A., and H. Burton. 1987. Past and present status of the southern elephant seal (Mirounga leonina ) at Macquarie Island. Journal of Zoology, London 213: 365–380.
Humphreys, W. F. 1979. Production and respiration in animal populations. Journal of Animal Ecology 48: 427–453.
Keiver, K. M., K. Ronald, and F. W. H. Beamish. 1984. Metabolizable energy requirements for maintenance and faecal and urinary losses of juvenile harp seals (Phoca groenlandica ). Canadian Journal of Zoology 62: 769–776.
Lavigne, D. M., S. Innes, R. E. A. Stewart, and G. A. J. Worthy. 1985. An annual energy budget for northwest Atlantic harp seals. In Marine Mammals and Fisheries ed. J. R. Beddington, R. J. H. Beverton, and D. M. Lavigne, 319–336. London: George Allen and Unwin.
Lavigne, D. M., S. Innes, G. A. J. Worthy, K. M. Kovacs, O. J. Schmitz, and J. P. Hickie. 1986. Metabolic rates of seals and whales. Canadian Journal of Zoology 64: 279–284.
Lavigne, D. M., and R. E. A. Stewart. 1979. Energy content of harp seal placentas. Journal of Mammalogy 60: 854–856.
Laws, R. M. 1953. The elephant seal (Mirounga leonina Linn.). I. Growth and age. Falkland Islands Dependencies Survey, Scientific Reports 8: 1–62.
———. 1956. The elephant seal (Mirounga leonina Linn.). I. General, social and reproductive behavior. Falkland Islands Dependencies Survey, Scientific Reports 13: 1–88.
———. 1960. The elephant seal (Mirounga leonina Linn.) at South Georgia. Norsk Hvalfangst-Tidende 49: 466–476, 520–542.
———. 1977. Seals and whales of the Southern Ocean. Philosophical Transactions of the Royal Society of London, Series B , 279: 81–96.
Ling, J. K., and M. M. Bryden. 1981. Southern elephant seal, Mirounga leonina Linnaeus, 1758. In Handbook of Marine Mammals . 2. Seals , ed. S. H. Ridgway and R. J. Harrison, 297–327. London: Academic Press.
McCann, T. S. 1980. Population structure and social organization of southern elephant seals, Mirounga leonina (L.). Biological Journal of the Linnaean Society 14: 133–150.
———. 1981. Aggression and sexual activity of male southern elephant seals, Mirounga leonina. Journal of Zoology, London 195: 295–310.
———. 1985. Size, status and demography of southern elephant seal (Mirounga leonina ) populations. In Sea Mammals in South Latitudes: Proceedings of a Symposium of the 52d ANZAAS Congress in Sydney—May 1982 , ed. J. K. Ling and M. M. Bryden, 1–17. Northfield: South Australian Museum.
McCann, T. S., M. A. Fedak, and J. Harwood. 1989. Parental investment in southern elephant seals, Mirounga leonina. Behavioral Ecology and Sociobiology 25: 81–87.
McCann, T. S., and P. Rothery. 1988. Population size and status of the southern elephant seal (Mirounga leonina ) at South Georgia, 1951–1985. Polar Biology 8: 305–309.
Reilly, J. J., and M. A. Fedak. 1990. Measurement of the body composition of living gray seals by hydrogen isotope dilution. Journal of Applied Physiology 69: 885–891.
Rodhouse, P. G., T. A. Arnbom, M. A. Fedak, J. Yeatman, and A. W. A. Murray. 1992. Cephalopod prey of the southern elephant seal Mirounga leonina L. Canadian Journal of Zoology 70: 1007–1015.
Sakamoto, W., Y. Naito, A. C. Huntley, and B. J. Le Boeuf. 1989. Daily gross energy requirements of female northern elephant seal, Mirounga angustirostris , at sea. Nippon Suisan Gakkaishi 55: 2057–2063.
Skinner, J. D., and R. J. van Aarde. 1983. Observations on the trend of the population of southern elephant seals, Mirounga leonina , at Marion Island. Journal of Applied Ecology 20: 707–712.
Stirling, I., and E. H. McEwan. 1975. The caloric value of whole ringed seals (Phoca hispida ) in relation to polar bear (Ursus maritimus ) ecology and hunting behavior. Canadian Journal of Zoology 53: 1021–1027.
van Aarde, R. J. 1980. Fluctuations in the population of southern elephant seals, Mirounga leonina , at Kerguelen Island. South African Journal of Zoology 15: 99–106.
Williams, T. M. 1987. Approaches to the study of exercise physiology and hydrodynamics in marine mammals. In Approaches to Marine Mammal Energetics , ed. A. C. Huntley, D. P. Costa, G. A. J. Worthy, and M. A. Castellini, 127–146. Special Publication no. 1. Lawrence, Kan.: Society for Marine Mammalogy.
Williams, T. M., and G. L. Kooyman. 1985. Swimming performance and hydrodynamic characteristics of harbor seals, Phoca vitulina. Physiological Zoology 58: 576–589.
Williams, T. M., G. L. Kooyman, and D. A. Croll. 1990. The effect of submergence on heart rate and oxygen consumption of swimming seals and sea lions. Journal of Comparative Physiology 160B: 637–644.
Worthy, G. A. J., and D. M. Lavigne. 1983. Changes in the energy stores during postnatal development of the harp seal, Phoca groenlandica. Journal of Mammalogy 64: 89–96.
Worthy, G. A. J., P. A. Morris, D. P. Costa, and B. J. Le Boeuf. 1992. Molt energetics of the northern elephant seal. Journal of Zoology, London 227: 257–265.