Chapter 11—
The Genetic Information of Organelles and Its Expression
11.1—
Introduction:
The Concept of Organelle Autonomy
The most notable event in biology at the dawn of the present century was the rediscovery of the Mendelian laws of inheritance by de Vries, Correns, and Tschermak. The realization by Sutton that the results of Mendel's experiments with garden peas could be explained in terms of the visible behaviour of chromosomes during meiosis led to the nucleus being regarded as the sole carrier of the hereditary material. As often happens in science, this splendidly simple view did not survive for long. The results of experiments by Baur (1909) and Correns (1909) on the inheritance of plastid defects in variegated plants were difficult to explain on the basis that the genes concerned were located in the nucleus. In certain cases, the inheritance of such defects occurred only through the maternal line, i.e. it was uniparental. In other cases the inheritance was biparental, but did not obey the rules of Mendel. For a time it appeared that, far from being 'nuclear', some aspects of inheritance were 'unclear'. It was Baur who pointed out that these results were explicable on the assumption of the genetic continuity of an extrachromosomal entity located in the plastid. Much more extensive evidence for this concept was later provided by the work of Renner (1929) using the genus Oenothera; he suggested the term 'plastome' to describe the genetic system in the plastid. Maternal inheritance is thus explicable in terms of the lack of plastids in the pollen tube of some species; the plastome of the new generation is consequently derived entirely from the mother.
These studies of variegated plants provided the first firm evidence for the existence of extrachromosomal inheritance. Since then, further evidence has accumulated with examples known in representatives of most major groups of organisms. Today we realize that organelle genetic systems are a fundamental feature of the organization of eukaryotic cells. The most detailed work has concentrated on plastids and mitochondria, but there are indications that genetic determinants may occur in other organelles found in eukaryotic cells, especially centrioles, basal granules and kinetoplasts. There is no evidence that either peroxisomes or glyoxysomes contain any genetic material. In this chapter, the biochemical evidence relating to the concept of the genetic autonomy of plastids and mitochondria only will be considered.
The concept of chloroplast autonomy was founded on the observation that, in the algae, chloroplasts can be seen to divide and to be passed to the new cells
in cell division (Strasburger, 1882; Green, 1964). This cytological evidence led to the view, first proposed by Schimper (1885) and Meyer (1893), that plastids do not arise de novo, but are formed by the division of pre-existing plastids; this view was supported by the genetic experiments of Baur and Correns carried out in the following two decades. The discovery in 1962 that chloroplasts contain both DNA and ribosomes brought the idea of chloroplast autonomy back into vogue, and this notion has so dominated our concepts of chloroplast development in recent years that several attempts have been made to grow isolated chloroplasts in culture. It is now clear that such attempts are ill-founded.
Our current dogmas maintain that for any biological system to be autonomous it must contain four components; (a) DNA to code for its entire structure; (b) DNA polymerase to replicate the DNA; (c) RNA polymerase to transcribe the DNA; (d) protein-synthesizing machinery to translate the messenger RNAs into all the necessary proteins. Intermediary metabolism is not necessary in principle since a supply of small molecules could be taken up from the environment. An extensive literature establishes that both chloroplasts and mitochondria do in fact contain these four components; the properties and functions of these are discussed in the rest of this chapter. It is equally clear that the DNA does not code for all the organelle proteins nor does the protein-synthesizing system make all the organelle proteins. For example, many genes concerned with chloroplast and mitochondrial structure and function are inherited in a Medelian fashion, and are therefore presumed to be located in the nucleus, while there is increasing evidence that the majority of chloroplast and mitochondrial proteins are synthesized on cytoplasmic ribosomes. It is now realized that the demonstration of the cytological continuity of plastids from generation to generation is not sufficient to establish that they replicate independently of nuclear control. How, then, are we to regard the concept of organelle autonomy ?
It is my contention that chloroplasts and mitochondria are not autonomous in any rigorous sense; the term is useful only as a quick way to describe the fact that these organelles contain some genes and make some proteins. We must regard the formation of these organelles as resulting from a complex interplay between the genomes of the organelles and the genome of the nucleus, and the fascination of this subject lies in unravelling the details of this interplay at the molecular level. Much more information is available about the autonomy of chloroplasts than about the autonomy of plant mitochondria; research on mitochondrial autonomy has concentrated almost entirely on animal and fungal cells. This imbalance is reflected in this chapter, which is concerned largely with chloroplast autonomy.
The current state of knowledge can be summarized by saying that, while some information has been accumulated about the properties of chloroplast nucleic acids and protein synthesis, only recently has any hard evidence emerged about their biological function. Developments at the genetic level in higher plants, and the establishment of isolated chloroplast systems which synthesize
specific protein and RNA molecules, promise to provide increasing understanding of the precise functions of chloroplast nucleic acids and protein synthesis.
11.2—
Chloroplast Autonomy
11.2.1—
Chloroplast DNA
11.2.1.1—
Discovery
The existence of DNA in chloroplasts has been established by both cytological and biochemical criteria. Ris and Plaut (1962) provided the first convincing evidence that chloroplasts contain DNA. Electron microscopy of chloroplasts in sections of both algal and higher plant cells revealed areas of low electron density which contained fibrils 2.5–3.0 nm in diameter. These fibrils were not seen if the sections were treated with deoxyribonuclease. There are earlier reports that chloroplasts contain regions which can be stained with the Feulgen reagent for DNA, but in general these reports have not proved reproducible. It is now clear that in most species the concentration of DNA inside the chloroplast is below the limit of detection by the Feulgen method. It can however be detected by autoradiography after exposure of cells to -H-thymidine; the report of Rose et al. (1974) on the distribution of DNA in dividing spinach chloroplasts contains an excellent example of this method. It is important when evaluating such studies to consider the controls that are used. If the incorporated label is all present in DNA, it should be removed by treatment of sections with deoxyribonuclease, but not by treatment with ribonuclease. This control works well for tobacco and spinach leaves and several algae, but not or maize leaves, where deoxyribonuclease fails to remove the label from chloroplasts.
Most studies on chloroplast DNA since 1962 have been carried out using isolated chloroplasts. This approach suffers from the difficulty of distinguishing chloroplast DNA from DNA originating from nuclei, mitochondria, and microorganisms which may contaminate the chloroplast pellet. The resolution of these problems is still not complete, and depends mainly on the differing properties of the DNA from the various sources. There are four characteristic properties of chloroplast DNA that have been used to identify it: namely buoyant density, ease of renaturation, lack of histones, and the absence of 5-methylcytosine. These properties are discussed below. It must be remembered that there is a possibility some chloroplast DNA may not meet these criteria. If, for example, chloroplasts in some species contain a minor DNA component which is a nuclear transcript, contains 5-methylcytosine and renatures poorly, it is doubtful whether present techniques could identify it as chloroplast in location. In one species the problem of contamination by nuclei and micro-
organisms can be entirely avoided; DNA has been identified in chloroplasts isolated from sterile enucleated plants of Acetabularia (Gibor & Izawa, 1963; Baltus & Brachet, 1963).
11.2.1.2—
Buoyant Density
It is now clear that chloroplast DNA from the algae Euglena, Chlamydomonas, and Chlorella has a buoyant density sufficiently different from that of nuclear DNA to allow resolution by analytical ultracentrifugation, but that in many higher plants the densities of the two DNA types are often, but not always, too close to permit this. This information has been hard-won; the story of the way in which the interpretation of band patterns in neutral caesium chloride density gradients has changed since 1963 has been told by Kirk (1971) and Tewari (1971). A summary is given here since it illustrates the very real problem of establishing the identity of chloroplast DNA from higher plants by biochemical methods.
The first report of the chemical characterization of higher plant chloroplast DNA was provided by Kirk (1963). He found that chloroplast DNA from Vicia faba has a GC content (37.4 %) which is slightly but significantly different from that of nuclear DNA (39.4%). In the same year a contrasting report was published by Chun et al., (1963), who found that chloroplast preparations from Spinacia oleracea and Beta vulgaris contained two components of much higher densities than the nuclear DNA as judged by caesium chloride equilibrium density gradient centrifugation. However the bulk of the DNA in these chloroplast preparations had a density similar to that of nuclear DNA; this band was attributed to contamination of the chloroplast pellet by nuclear fragments, and thus the chloroplast DNA was regarded as the two high density components. There followed a spate of papers which supported the claim that, in higher plants, chloroplast DNA has an appreciably higher density than nuclear DNA. This picture changed when re-examination by more rigorous methods supported the earlier view of Kirk, and the higher density components are now attributed to contamination by mitochondria and bacteria.
The present position can be summarized by saying that, in all the higher plants examined, chloroplast DNA had a base composition of 37.5 ± 1% GC and a buoyant density of 1.697 ± 0.001 g cm–3 . Table 11.1 lists some values for the buoyant densities of chloroplast and nuclear DNA which have been agreed by Kirk and Tewari. Nuclear DNA is seen to have a smaller, larger, or similar density to chloroplast DNA from the same plant, depending on the species. The GC contents in Table 11.1 have been calculated from the buoyant densities, but a more accurate method has been devised by Kirk (1967). This method releases the purine bases by gentle acid hydrolysis and separates the adenine and guanine by ion-exchange chromatography.
The moral to be drawn from this story is that the purity of subcellular fractions from higher plant tissues must be established by positive methods if
meaningful interpretations are to be made. Microbial contamination can be reduced by surface sterilization of fresh tissues and by the use of sterile solutions, while mitochondrial contamination can be assayed by succinic dehydrogenase or cytochrome oxidase activities. Nuclear contamination can be reduced by incubation of intact chloroplasts with deoxyribonuclease and phosphodiesterase. The extent of nuclear contamination is difficult to measure, but the ease of renaturation and the absence of 5-methylcytosine serve to distinguish chloroplast from nuclear DNA in all cases so far studied.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
11.2.1.3—
Ease of Renaturation
A more useful criterion than the buoyant density for detecting chloroplast DNA from higher plants is the ease with which it will renature after heat or alkali denaturation. Nuclear DNA renatures only to a slight extent in a few hours,
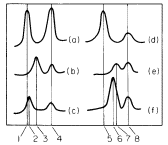
Figure 11.1
Densitometer tracings of ultraviolet absorption photographs of Vicia faba DNA banded
in caesium chloride gradients. (a) Native chloroplast DNA; (b) heat-denatured chloroplast
DNA; (c) renatured chloroplast DNA; (d) native nuclear DNA; (e) heat-denatured nuclear
DNA; (f) renatured nuclear DNA. The numbers refer to densities in g cm-3 as follows: (1)
1.696; (2) 1.699; (3) 1.712; (4) 1.728; (5) 1.696; (6) 1.709; (7) 1.712; (8) 1.728. The
peaks at 1.728 g cm–3 represent marker DNA from Micrococcus radiodurans.
(From Kung & Williams, 1968, by courtesy of Elsevier.)
depending on its content of reiterated sequences. Figure 11.1 illustrates this difference in the case of Vicia faba. The rapid renaturation of chloroplast DNA has encouraged attempts to estimate its genome size from measurements of kinetic complexity. The kinetic complexity of a DNA sample is a measure of the size of the unique set of nucleotide sequences it contains, as judged from the rate at which the DNA renatures. A rapid rate of renaturation implies that like sequences are present in high concentration, and therefore the number of unique sequences, or kinetic complexity, is small. Table 11.2 lists the kinetic complexity, in terms of molecular weight, for the chloroplast DNA from some algae and higher plant species. Some of these values have been corrected from the published figures since the estimate of the molecular weight of the bacteriophage T4 DNA, used as standard, has been revised from 1.3 × 108 to 1.06 × 108 (Dubin et al., 1970). It is striking that the corrected values for the kinetic complexity of chloroplast DNA from the few algae and higher plants examined so far are all in the range 0.9–1.0 × 108 . This may be a coincidence, or it could mean the information content of chloroplast DNA is basically similar throughout the plant kingdom. All the species so far examined belong to the Chlorophyta, with the exception of Euglena. It would be interesting to make such measurements of chloroplast DNA from other plant groups, especially from algae with unusually shaped chloroplasts. Table 11.2 also lists the analytical complexities of chloroplast DNA, i.e. the amount of DNA per chloroplast. Since the kinetic complexities are always much less than the analytical complexities, there must be between 20 and 60 copies of the DNA sequences in each chloroplast. These renaturation studies cannot rule out the possibility of microheterogeneity in nucleotide sequence between the copies but, if it does exist, such heterogeneity is beyond detection by current techniques.
| ||||||||||||||||||||||||||||||||||||
11.2.1.4—
Absence of Histones and 5-Methylcytosine
When released from intact isolated chloroplasts by gentle lysis, chloroplast DNA is not combined with basic proteins. This finding confirms the microscopic observations of Ris and Plaut (1962) that chloroplasts contain DNA fibrils in the same form as they appear in the nucleoplasms of prokaryotic cells. It is also characteristic of chloroplast DNA from both higher plants and algae that it contains no detectable 5-methylcytosine, whereas nuclear DNA invariably does. Whitfeld and Spencer (1968) regard the absence of this base as the most reliable criterion for establishing the purity of chloroplast DNA.
11.2.1.5—
Circularity
A recent discovery is that, when precautions are taken to minimize shearing, a proportion of chloroplast DNA can be isolated as circles. Circular DNA has been reported from chloroplasts of Euglena (Manning et al., 1971), Pisum sativum (Kolodner & Tewari, 1972), Spinacia oleracea (Manning et al., 1972), Antirrhinum majus, Oenothera hookeri, and Beta vulgaris (Hermann et al., 1975). Figure 11.2 shows a molecule of chloroplast DNA from Spinacia oleracea. The significance of circularity in DNA is not known, but it is a useful property since it implies the molecule has not been degraded on isolation. The most interesting aspect of this finding is that in all the species listed the contour length of the majority of the circles is in the range 37–45 µm; this length of double-stranded DNA has a calculated molecular weight of 0.85–1.0 × 108 daltons, which is in the same range as the kinetic complexity of chloroplast DNA (Table 11.2). This correspondence of length and kinetic complexity suggests that the genetic information carried by the chloroplast DNA is accommodated by the length of the circular molecule.

Figure 11.2
Open circular DNA molecule, contour length
44.7 µm, from chloroplasts of Spinacia oleracea.
(Reprinted from Hermann et al., (1975) by courtesy of Elsevier.)
11.2.1.6—
Ploidy
It is clear from the data in Table 11.2, and from the evidence for circular DNA, that each chloroplast may contain many copies of a circular genome, and thus may be genetically polyploid. It has been pointed out by Kirk (1972) that this
multiplicity increases the probability that a mutation will appear in a chloroplast in a given time, but there is likely to be a long delay before the mutation can be expressed, since a mutation in one copy will be swamped by all the other wild-type copies. It is therefore not surprising that known non-Mendelian mutations affecting chloroplasts are small in number, and difficult to induce with mutagens. In Chlamydomonas reinhardi a number of mutations are known which alter the sensitivity of chloroplast ribosomes to inhibition by antibiotics such as erythromycin and carbomycin; some of these mutations show uniparental inheritance (Sager, 1972; Mets & Bogorad, 1972). Those genes which are inherited in a uniparental fashion have been shown by recombinational analysis to form one linkage group which behaves as if it were diploid in vegetative cells. It is difficult to reconcile this diploid behaviour with the evidence which suggests that there are many copies of the genome in each chloroplast. It may be that the uniparental linkage group does not reside in chloroplast DNA, or if it does, that a reduction in the number of genomes in each chloroplast occurs at some stage in the cell cycle, so that only a 'master copy' is passed during sexual reproduction. There is as yet no evidence to resolve these questions.
11.2.1.7—
Functions
A 40 µm circle of double-stranded DNA of unique base sequence is sufficient in principle to code for about 125 proteins each of molecular weight 50,000. Since our knowledge depends ultimately on the validity of the techniques which can be used, the evidence for the functions of chloroplast DNA will be considered in terms of the four methods tried so far:
The Selective Inhibition of Chloroplast DNA Transcription
The antibiotic rifampicin is a potent inhibitor of the initiation of RNA synthesis in bacteria. This compound has been reported to inhibit the incorporation of labelled precursors into chloroplast ribosomal-RNA, but not into cytoplasmic ribosomal-RNA, in several unicellular algae, namely Chlamydomonas, Chlorella, and Acetabularia. Rifampicin has also been reported to inhibit chloroplast RNA polymerase activity in extracts of Chlamydomonas. These results suggest that the functional genes for chloroplast ribosomal-RNA are located in chloroplast DNA and are transcribed by the chloroplast RNA polymerase.
The effect of rifampicin in higher plant systems is controversial. There have been some reports that it specifically inhibits chloroplast ribosomal-RNA synthesis, but other workers could not reproduce this result (Bottomley et al., 1971). There now seems general agreement that chloroplast RNA polymerase from higher plants, as normally prepared and assayed, is insensitive to rifampicin, but this may mean only that the preparations are not initiating RNA synthesis.
Genetic Analysis of Mutants
Many mutations are known which affect chloroplast components, but the vast majority are inherited in a Mendelian fashion and are therefore presumed, to be located in nuclear genes. For example, seven different genes are known which control steps in the chlorophyll biosynthetic pathway, and nuclear genes have been shown to be involved in the synthesis of phosphoribulokinase and at least five components of the photosynthetic electron transport chain (Kirk, 1972; Kirk & Tilney-Bassett, 1967; Levine & Goodenough, 1970).
In Chlamydomonas many mutations affecting chloroplast ribosomes and photosynthetic capacity are inherited as a single linkage group in a uniparental fashion (Sager, 1972; Schlanger & Sager, 1974). In this alga, like gametes fuse completely, and the physical basis for uniparental inheritance has been suggested to be the destruction of the chloroplast DNA of one of the gametes by a modification-restriction system of the type known in bacteria. As pointed out earlier, there are difficulties in concluding that this uniparental class of mutations resides in chloroplast DNA, but it is tempting to believe that it does. A simple interpretation suggests that some of the proteins of the chloroplast ribosomes are encoded in chloroplast DNA; there is also evidence that at least one other protein of the chloroplast ribosome is encoded in a nuclear gene (Mets & Bogorad, 1972).
Rigorous proof that a mutation inherited in a maternal or uniparental fashion actually alters the amino acid sequence of a protein has been obtained in only one case. Chan and Wildman (1972) studied the inheritance of a mutation in the large subunit of Fraction I protein in Nicotiana tabacum at the tryptic peptide level; this mutation was inherited via the maternal line only. By contrast, mutations in the small subunit of Fraction I protein are inherited in a Mendelian fashion (Kawashima & Wildman, 1972). This type of combined biochemical-genetic approach is very promising and deserves further use, especially with cultivated plants where many varieties are available. It must be emphasized that this approach must be conducted at the level of the tryptic peptide analysis of a purified protein. It is not sufficient to show that particular proteins are absent in a mutant variety, since absence of a protein might result from a mutation in a chloroplast gene which controls the formation of a protein encoded in the nucleus.
DNA-RNA Hybridization Studies
If RNA isolated from chloroplasts can be shown to hybridize to chloroplast DNA but not to nuclear DNA, this is good grounds for believing that such RNA is both encoded in and transcribed from chloroplast DNA. A number of hybridization studies have been carried out with chloroplast ribosomal-RNA, which hybridizes to 0.5–6.0% of chloroplast DNA (Ellis & Hartley, 1974). The
most recent study is that of Thomas and Tewari (1974); they found that in a number of higher plants each circle of chloroplast DNA contains two genes for chloroplast ribosomal-RNA. There are several reports that chloroplast ribosomal-RNA will also hybridize to nuclear DNA. The significance of these reports awaits further study, but the possibility is raised that there are two types of chloroplast ribosomal-RNA, one encoded in chloroplast DNA and the other in nuclear DNA. This arrangement could be a means whereby the nucleus exerts control over events in the chloroplasts, especially if it is further postulated that chloroplast ribosomes containing nuclear RNA translate only messengers originating in the nucleus.
There is evidence that some of the transfer RNA species found in chloroplasts will also hybridize to chloroplast DNA. Tewari and Wildman (1970) found that tRNA from tobacco chloroplasts would hybridize to 0.4–0.7% of chloroplast DNA. This amount of DNA would be enough to code for 20 to 30 transfer RNA molecules, each of molecular weight 25,000. This work used unfractionated tRNA however, and more studies need to be done to establish the site of encoding of individual tRNA species.
Identification of RNA and Protein Molecules Synthesized by Isolated Chloroplasts
This is the most direct method; if transcription coupled to translation can be obtained in isolated chloroplasts, identification of the products would simultaneously determine both the structural genes present in chloroplast DNA and the function of chloroplast ribosomes. Work in the author's laboratory has shown that isolated intact chloroplasts from Pisum sativum and Spinacia oleracea will synthesize discrete protein and RNA molecules, but transcription and translation are not coupled (Hartley & Ellis, 1973; Ellis, 1974; Ellis & Hartley, 1974). It is therefore not possible to infer that the proteins synthesized by isolated chloroplasts are encoded in chloroplast DNA. The RNA synthesized by isolated chloroplasts has been analyzed by polyacrylamide gel electrophoresis (Fig. 11.3). The chief product is a species of molecular weight about 2.7 × 106 ; this has been shown by competitive hybridization to chloroplast DNA to be a precursor to chloroplast ribosomal-RNA. Isolated chloroplasts have not been shown convincingly to synthesize ribosomal-RNA; presumably the processing system which trims the precursor molecules does not survive the trauma of isolation (see also chapter 9).
The known genes in chloroplast DNA can be summarized as follows: chloroplast transfer-RNA, chloroplast ribosomal-RNA, several chloroplast ribosomal proteins, and the large subunit of Fraction I protein. These genes account for less than 10% of the total potential coding capacity of a 40 µm circle of DNA. The elucidation of the function of the remaining 90% of chloroplast DNA is the most important problem in this field.
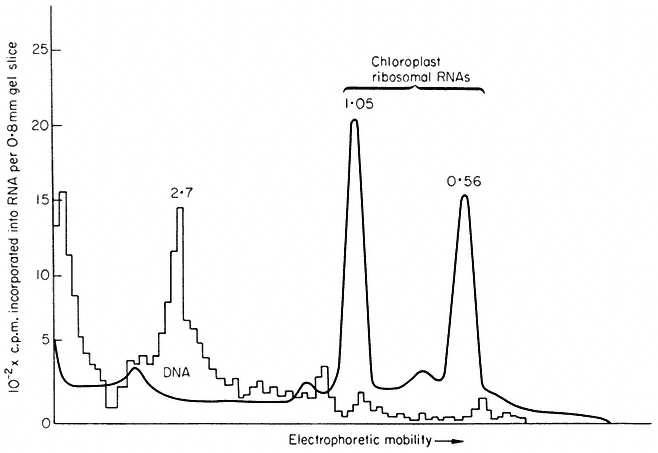
Figure 11.3
Analysis by polyacrylamide gel electrophoresis of RNA synthesized by
chloroplasts isolated from Spinacia oleracea. Isolated chloroplasts were incubated
with 3 H-uridine and 15,000 lux of red light for 20 min at 20°C. Nucleic acid was extracted
and run on polyacrylamide gels. The solid line represents the A260 , while the histogram
shows the radioactivity. The figures are the molecular weights × 10-6 of the RNA components.
(Reprinted from Hartley & Ellis, 1973, by courtesy of the Biochemical Journal).
11.2.2—
Chloroplast DNA Polymerase
DNA polymerase activity has been detected in chloroplast suspensions prepared from Spinacia oleracea. Nicotiana tabacum, and Euglena gracilis. The product renatures readily after heat denaturation, and hybridizes to a much larger extent with chloroplast DNA than with nuclear DNA. It has not been established whether the chloroplast polymerase is identical with any nuclear polymerase or whether it carries out either a replicase or a repair function. The enzyme is bound to the chloroplast membranes, but in the case of Euglena, it has been solubilized by treatment with high concentrations of salt and highly purified; the purified enzyme is inhibited by ethidium bromide (Keller et al., 1973). Flechtner and Sager (1973) have made the interesting observation that treatment of Chlamydomonas cells with ethidium bromide induces a selective and reversible inhibition of chloroplast DNA replication. Thus nuclear DNA synthesis proceeds normally while replication of chloroplast DNA is impaired; the preexisting chloroplast DNA decreases by at least 80% in one cell generation, but this loss is reversible if the drug is removed within 12 hours. This result suggests that one or a few of the chloroplast DNA copies may be protected in some way,
perhaps by close attachment to chloroplast membranes. Such sequestered DNA could act as a 'master' copy, and might account for the diploid behaviour of the uniparental linkage group in this organism. The chloroplast DNA of Euglena and Chlamydomonas has been shown to replicate in a semi-conservative fashion at a different time in the growth cycle from nuclear DNA, but the factors controlling the time and rate of synthesis of chloroplast DNA are unknown.
11.2.3—
Chloroplast RNA Polymerase
DNA-dependent RNA polymerase activity has been demonstrated in chloroplast preparations from several species of algae and higher plants. Most workers study lysed chloroplast preparations because the nucleotide triphosphates used as substrates penetrate the chloroplast envelope at slow rates. Unlike intact
Figure 11.4
Distribution of chloroplast RNA polymerase activity in leaves of
young shoots of Pisum sativum. Pea seedlings were grown for 14 days
and the leaves homogenized. Total RNA polymerase activity was measured
in the low-speed pellet containing nuclei and chloroplasts, and chloroplast
RNA polymerase activity in purified chloroplast membranes. The figures are the
activities of the chloroplast RNA polymerase as percentages of the total activity.
(Unpublished work of J. Bennett.)
chloroplasts, which use light to phosphorylate added 3 H-uridine (Fig. 11.3), lysed preparations do not synthesize discrete species of RNA; instead a polydisperse pattern of products ranging in size from 5s to 23s is obtained (Spencer & Whitfeld, 1967). This difference between the products from intact and lysed chloroplasts may result from the dilution of controlling factors on lysis.
The RNA polymerase activity of chloroplast preparations can represent a high proportion of the total RNA polymerase activity measurable in leaf extracts. Figure 11.4 illustrates this point in the case of the growing shoot of Pisum sativum; the chloroplast polymerase activity can account for half of the total activity in the youngest leaves at the stem apex, but accounts for progressively less in the older leaves.
There are two key questions about chloroplast RNA polymerase which need to be answered: firstly, how similar is it to any nuclear polymerase, and secondly, how is its activity regulated ? The enzyme is bound to the chloroplast lamellae, but a technique for its quantitative removal has been devised (Bennett & Ellis, 1973). The solubilized chloroplast polymerase has been purified, and appears very similar to a nuclear polymerase (Bogorad et al., 1973), but a detailed characterization has yet to be reported. It has been shown that the synthesis of chloroplast ribosomal-RNA in dark-grown plants is phytochrome-mediated (Scott et al., 1971), but the nature of the mechanism which links phytochrome to RNA polymerase is unknown.
11.2.4—
Chloroplast Protein Synthesis
Chloroplast preparations capable of incorporating labelled amino acids into protein have been isolated from higher plants and algae. Table 11.3 illustrates some results obtained with preparations from young bean and tobacco leaves; preparations from mature leaves have little activity. These results are typical of those reported in many studies with many species (Ellis et al., 1973). In such work it is vital to establish that the incorporation is due to chloroplasts and not to microorganisms, intact leaf cells, or cytoplasmic ribosomes. The best criterion is the dependence of incorporation on an added energy source; this is ATP in the case of lysed chloroplasts, or light in the case of intact chloroplasts which can carry out photophosphorylation. Dependence on an added energy source eliminates intact leaf cells and microorganisms as the agents of incorporation. Activity by contaminating cytoplasmic ribosomes can be ruled out by the different sensitivity of the two types of ribosome to antibiotic inhibitors (see 11.2.4.5). Other criteria are the sensitivity of incorporation to added ribonuclease, and to variation in the concentration of added Mg2+ ions; these criteria are useful when lysed chloroplasts or isolated ribosomes are being tested, but not when the incorporation is due to intact chloroplasts.
Some of the characteristics and functions of protein synthesis by chloroplasts will now be considered.
| ||||||||||||||||||||||||||||||
11.2.4.1—
Ribosomes
Lyttleton (1962) was the first to show that green plant cells contain two classes of ribosome which differ in their sedimentation coefficients. Chloroplasts contain 70s ribosomes while the cytoplasm contains 80s ribosomes. Figure 11.5 shows a density gradient analysis of an homogenate of spinach leaves, from which it can be seen that chloroplast ribosomes constitute a high proportion of the total cellular complement of ribosomes; values as high as 60% have been reported.
Chloroplast ribosomes resemble those from prokaryote cells in their s value. Their RNA components are also of the same size as those found in prokaryote ribosomes, i.e. 16s and 23s. The 80s cytoplasmic ribosomes by contrast, contain 18s and 25s RNA molecules. These similarities between chloroplast and prokaryote ribosomes have been much stressed in the past, but it is now clear that these similarities are of size only, and not in the primary structure of the RNA and protein components. For example, ribosomal-RNA from Escherichia coli does not compete with chloroplast ribosomal-RNA from Euglena for hybridization to chloroplast DNA. The protein complement of prokaryote ribosomes is also quite different from that of chloroplast ribosomes as judged by gel electrophoresis and immunological tests. Similarly, the proteins of chloroplast and cytoplasmic ribosomes from the same plant differ distinctly in the patterns they give on gel electrophoresis.
Some of the chloroplast ribosomes are bound to the internal membranes. Electron microscopy has revealed whorl-like polyribosomes attached to the granal and intergranal thylakoid membranes, while analysis of isolated chloroplasts shows that up to 50% of the ribosomes cannot be removed from the membranes by washing with hypotonic buffer. Tao and Jagendorf (1973) have
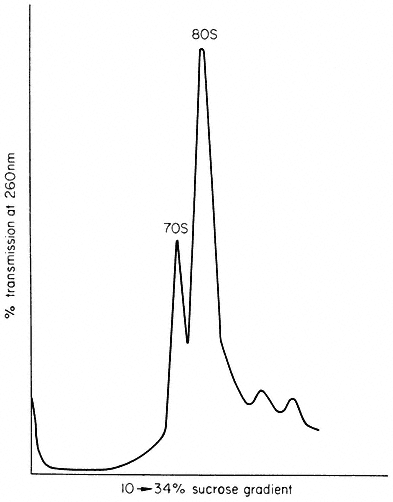
Figure 11.5
Proportion of chloroplast ribosomes in leaves of Spinacia
oleracea. Extracts of spinach leaves were treated with
detergent to release membrane-bound ribosomes, and all the
ribosomes collected by centrifugation. The ribosomes
were analysed on a linear sucrose density gradient.
(Unpublished work of M. R. Hartley.)
shown that some free ribosomes are lost during chloroplast isolation without the chloroplasts lysing irreversibly; when this loss is taken into account, they estimate that in chloroplasts from Pisum sativum, about 20% of the ribosomes are membrane-bound while the remainder are free in the stroma. There is evidence that the two classes of chloroplast ribosome synthesize different types of protein (see 11.2.4.6).
11.2.4.2—
Amino Acid Activation
Chloroplasts isolated from several algae and higher plants have been found to contain aminoacyl-transfer-RNAs and the corresponding synthetases (Ellis et al., 1973). These transfer RNA and synthetase molecules can often, but not always, be distinguished from those involved in the activation of the same amino acids in the cytoplasm. Studies of the compatibility between transfer-RNA and synthetases from the two cellular compartments show that the specificity of interaction ranges from. none to absolute. For example, the cytoplasm of leaves of Phaseolus vulgaris contains a leucyl-transfer-RNA synthetase which can add leucine only to the two leucyl-transfer-RNA species common to the cytoplasm and chloroplasts, but is not able to recognize the three leucyl-transfer-RNA
species found only in the chloroplasts. The chloroplast enzyme, on the other hand, can aminoacylate all five leucyl-transfer-RNA species. It is difficult to attach any biological significance to these variations in specificity. The simplest presumption is that in the intact cell the chloroplast synthetases aminoacylate only the transfer-RNA species located in the chloroplast and these are sufficient for chloroplast protein synthesis to proceed, while the cytoplasmic synthetases aminoacylate only the transfer-RNA species located in the cytoplasm. A more interesting possibility is that some of the transfer RNA species required to translate chloroplast messenger-RNA are acylated in the cytoplasm; such a requirement would provide one way in which protein synthesis in the cytoplasm and chloroplast could be integrated, but there is no direct evidence to support this suggestion. Experiments with Euglena suggest that at least some of the chloroplast aminoacyl-transfer-RNA synthetases are encoded in nuclear DNA and are synthesized on cytoplasmic ribosomes (Hecker et al., 1974).
11.2.4.3—
Initiation of Chloroplast Protein Synthesis
Protein synthesis in bacteria is initiated by N-formyl-methionyl transfer RNA; there is evidence that this is also true in chloroplasts, but not in the cytoplasm of eukaryotic cells. Schwarz et al., (1967) found that chloroplast ribosomes from Euglena would translate RNA from bacteriophage f2 into viral coat protein with N-formylmethionine at the amino terminus. Detailed studies of initiating methionyl-transfer-RNA species have since been reported for several higher plants. For example, there are two methionyl-transfer-RNA species in wheat chloroplasts. One of these can be formylated by a chloroplast transformylase, whereas the other cannot, and may serve to direct methionine into internal positions in the polypeptide chains (Leis & Keller, 1971). Two other methionyl-transfer-RNA species are found in the cytoplasmic fraction, neither of which can be formylated. It is probable from this type of evidence that the initiation of protein synthesis by chloroplasts is similar to that in prokaryotes in that it uses a formylated methionyl-transfer-RNA, but distinct from that in the cytoplasm which uses an unformylated methionyl-transfer-RNA. The significance of this distinction is not clear; it may reflect the evolutionary origin of the compartments in eukaryotic cells, and have no contemporary functional value.
11.2.4.4—
Energy Source
In most studies of protein synthesis by isolated chloroplasts, added ATP has been used as an energy source (see Table 11.3). Since isolated chloroplasts can generate ATP by photophosphorylation, it should be possible to drive protein synthesis in chloroplasts with light. Spencer (1965) found that spinach chloroplasts would use light to incorporate amino acids into protein provided that ADP, inorganic phosphate and pyocyanine were added. The necessity for
pyocyanine as catalyst indicates that the chloroplasts were broken, and had lost their natural catalyst, ferredoxin, by dilution. If precautions are taken to isolate chloroplasts which have their outer envelopes intact, protein synthesis is stimulated twenty-fold by light in the absence of either cofactors or catalysts of photophosphorylation (Fig. 11.6). The rates of light-driven protein synthesis
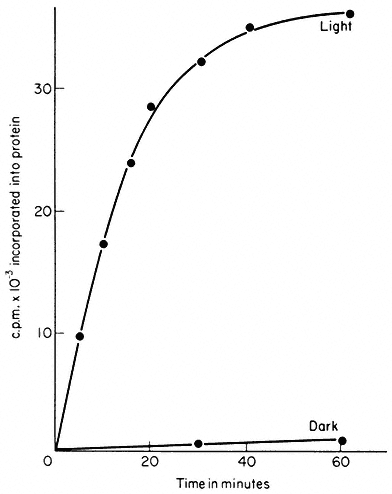
Figure 11.6
Light-driven protein synthesis by chloroplasts isolated from Pisum sativum.
Intact isolated chloroplasts were incubated with 14 C-leucine and 4,000 lux red
light at 20°C. Total protein was then extracted and its radioactivity measured.
(Reprinted from Blair & Ellis, 1973, by courtesy of Elsevier.)
by such chloroplasts are the highest yet recorded for any isolated chloroplast system. The use of intact chloroplasts for studies of protein synthesis has a major advantage over the use of broken chloroplasts in that conditions around the polysomes are more normal with respect to controlling factors. It is thus more likely that correct termination and release of polypeptide chains occurs in intact chloroplasts than in lysed preparations; such intact chloroplasts make discrete protein molecules rather than incomplete polypeptide chains (see 11.2.4.6). A further advantage of using light as the energy source is that the broken chloroplasts, which inevitably contaminate the preparation of intact chloroplasts, cannot contribute to the incorporation in the absence of added cofactors.
It is possible that protein synthesis by chloroplasts in vivo uses ATP provided by the chloroplast itself. However, this is not the case in developing
chloroplasts, since organisms such as Chlorella, Chlamydomonas, and Pinus do not require light to form chloroplasts. The formation of chloroplasts must therefore depend on ATP supplied by the rest of the cell. This conclusion is confirmed by the ability of intact isolated etioplasts from Pisum sativum to use ATP, but not light, as their source of energy for protein synthesis (Siddell & Ellis, 1975).
11.2.4.5—
Inhibitors
It is well established that protein synthesis by isolated chloroplast ribosomes is inhibited by the same antibiotics which inhibit protein synthesis by prokaryote ribosomes. The best studied example is chloramphenicol, which inhibits protein synthesis by isolated chloroplasts from all the species so far tested; inhibition is shown only by the D-threo isomer of chloramphenicol, and not by the other stereo isomers. Three other unrelated antibiotics, spectinomycin, lincomycin, and erythromycin also inhibit protein synthesis by chloroplast ribosomes. Light-driven protein synthesis by chloroplasts from Pisum sativum is especially sensitive to lincomycin, 50% inhibition being given by 0.2 µg ml–1 (Ellis & Hartley, 1971). Protein synthesis by cytoplasmic ribosomes from plants is not inhibited by any of these antibiotics. On the other hand, cytoplasmic ribosomes are inhibited by cycloheximide, which does not affect the activity of chloroplast ribosomes. It must be emphasized that all these findings relate only to the activity of isolated sub-cellular systems; it cannot be assumed from these data alone that it is valid to use these compounds on intact cells with the expectation of inhibiting protein synthesis in one compartment but not in the other.
The protein complements of chloroplast and bacterial ribosomes are different, and thus the above similarity between them must reside in their antibioticbinding sites, and not in the properties of the proteins as revealed by gel electrophoresis or immunological tests. There is no evidence that the mechanism of action of bacterial antibiotics on chloroplast ribosomes is similar to that on bacterial ribosomes; nor is there any understanding of the selective pressure which maintains the sensitivity of chloroplast ribosomes to these antibiotics in the face of mutations to resistance, which can, for example, be found in Chlamydomonas (see 11.2.1.6).
11.2.4.6—
Functions
The high proportion of plant ribosomes located inside chloroplasts raises the question as to their function. Are they required in such quantities because they make a large number of different chloroplast proteins, or because they make a small number of different chloroplast proteins in large amounts? The available evidence suggests that the latter is the case (Ellis et al., 1973; Ellis, 1974).
The problem of identifying which proteins are synthesized by chloroplast ribosomes has been tackled in two ways. The first is to supply antibiotic inhibitors, especially chloramphenicol and cycloheximide, to intact cells making chloroplasts, and determining which proteins are no longer synthesized. The validity of results from experiments with any inhibitor depends absolutely on the specificity of its action in intact cells, and there is evidence that both chloramphenicol and cycloheximide have effects on activities other than protein synthesis in some higher plants (Ellis & MacDonald, 1970). Processes such as ion uptake, oxidative phosphorylation and photophosphorylation are inhibited by all four stereoisomers of chloramphenicol, whereas the inhibition of protein synthesis by isolated chloroplast ribosomes is specific for the D -threo isomer (Ellis, 1969). This stereospecificity provides a means of establishing for any particular tissue whether chloramphenicol is inhibiting protein synthesis directly at the ribosomal level, or in addition, is affecting some other process such as energy supply. It is strongly recommended that only if an inhibition is produced specifically by the D -threo isomer should an interpretation directly involving protein synthesis be invoked. Another problem is that in most of the inhibitor experiments on chloroplast ribosomal function, increases in specific proteins have been measured as enzymic activities rather than as amounts of protein. Failure to observe an effect by a particular inhibitor might therefore mean that the increase in enzymic activity is due to activation of a precursor protein, rather than to de novo synthesis by either chloroplast or cytoplasmic ribosomes.
Bearing these difficulties in mind, this author interprets the bulk of the published inhibitor experiments as suggesting that most of the chloroplast proteins are synthesized, on cytoplasmic ribosomes (Ellis et al., 1973; Ellis, 1975). In all the studies reported on several algae and higher plants, the synthesis of Fraction I protein was found to be inhibited by 70s ribosomal inhibitors. In most cases, the synthesis of the other soluble enzymes of the photosynthetic carbon dioxide reduction cycle appears to occur on cytoplasmic ribosomes; the same is true for ferredoxin and the chloroplast RNA polymerase. Besides Fraction I protein, the only other proteins which appear to be synthesized by chloroplast ribosomes are some of the chloroplast ribosomal and lamellar proteins, including the photosynthetic cytochromes. It must be emphasized, however, that these inhibitor experiments are never more than suggestive. Strictly interpreted, they never say more than that the activity of a particular group of ribosomes is required for a particular protein to accumulate in the chloroplast. This is not the same as saying that these ribosomes actually synthesize that protein, because it is possible that the apoenzyme is synthesized by one class of ribosomes but requires for its appearance in the chloroplast in an active state, additional protein(s) which are synthesized by another class of ribosomes.
That this is true for Fraction I protein has been shown by the second approach to the problem of chloroplast ribosomal function, namely, the
synthesis of specific proteins by isolated sub-cellular systems. This is the most direct approach to this problem but it has been successful only recently because of the difficulty of isolating sub-cellular systems which will carry out complete protein synthesis with fidelity; algal systems are especially difficult in this respect. Isolated intact chloroplasts from Pisum sativum, however, synthesize at least six discrete proteins (Fig. 11.7); similar results have been obtained with
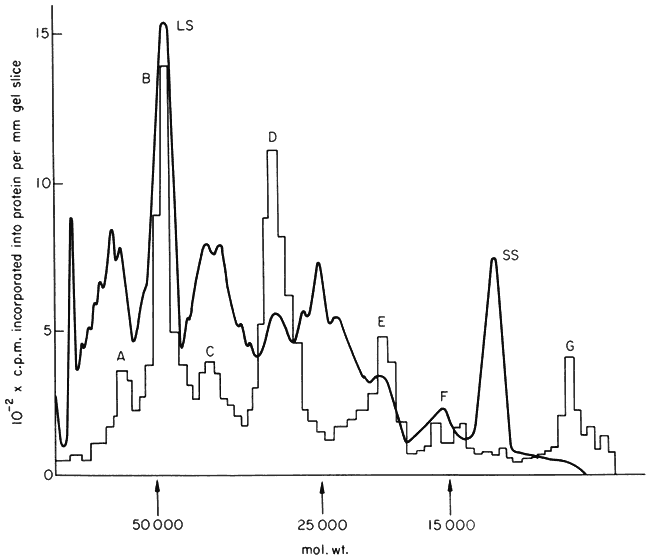
Figure 11.7
Products of protein synthesis by chloroplasts isolated from Pisum sativum.
Chloroplasts were incubated as in Fig. 11.6, and then dissolved in sodium
dodecylsulphate. The solubilized extract was electrophoresed on gels containing
15% polyacrylamide. The smooth line represents the absorbance of stained protein
bands, and the histogram is radioactivity. The letters A–G represent the discrete
radioactive peaks. LS and SS mark the large and small subunit of Fraction I
protein respectively. (From Eaglesham & Ellis, 1974, by courtesy of Elsevier.)
chloroplasts from Hordeum vulgare and Spinacia oleracea. Only one of these proteins (peak B in Fig. 11.7) is soluble; it has been identified as the large subunit of Fraction I protein by tryptic peptide analysis (Blair & Ellis, 1973). Recent work has shown this large subunit to be made by the free ribosomes of the chloroplast, but not by the membrane-bound ribosomes. The high proportion of chloroplast ribosomes found in leaves may thus be required because Fraction I protein is one of the most abundant proteins (Ellis, 1973). The small
subunit of Fraction I protein is not synthesized by isolated chloroplasts, but it has been detected as the product of protein synthesis by isolated cytoplasmic ribosomes from Phaseolus vulgaris leaves (Kekwick & Gray, 1973). The other proteins made by isolated chloroplasts are all membrane-bound, and have so far resisted identification; it is clear they are minor components of the thylakoids. Two proteins associated with the chloroplast envelope are also labelled in isolated pea chloroplasts (Joy & Ellis, 1975). The same pattern of proteins synthesized by intact pea chloroplasts has been found in studies of protein synthesis by isolated pea etioplasts (Siddell & Ellis, 1975).
This in vitro approach to the study of chloroplast ribosomal function gives direct and unambiguous results, and it should be extended to species lower on the evolutionary scale to see how far the pattern found in angiosperms extends. In view of the uniformity of the size of the chloroplast genome in algae and higher plants (Table 11.2), it seems likely that the pattern seen in isolated pea chloroplasts will be universal. However, this assumes that proteins synthesized by chloroplast ribosomes are also encoded by chloroplast DNA; this may not be true if messenger-RNA can cross the chloroplast envelope, but so far this is only a theoretical possibility.
Another area which may repay attention is the study of protein synthesis by plastids other than chloroplasts, especially proplastids and amyloplasts. It seems probable that these plastids synthesize a different range of proteins from chloroplasts. If these proteins are encoded in plastid DNA, they would account for some of the functions of the 90% of this genome which have not been identified (see 11.2.1.7).
11.2.5—
Co-Operation between Chloroplast and Nuclear Genomes
It is clear from the available evidence that the chloroplast genome requires for its expression the co-operation of the nuclear genome. The best information about the details of this co-operation concerns the synthesis of Fraction I protein. By combining the results of Wildman's genetic studies with those of the in vitro studies of protein synthesis, a model for the synthesis of Fraction I protein can be constructed (Fig. 11.8). In this model, the large subunit is both encoded and synthesized within the chloroplast, while the small subunit is both encoded and synthesized outside the chloroplast. This model is tidy, and requires protein, but not nucleic acid, to cross the chloroplast envelope. This transport of protein must be on a large scale because it involves not only the small subunit of Fraction I protein but all the other proteins which, from the inhibitor evidence, are made on cytoplasmic ribosomes. The mechanism of this transport is unknown, but it must be able to distinguish between different proteins. One suggestion is that a protein exists in the outer envelope of the chloroplast which recognizes a site common to all those proteins made on cytoplasmic ribosomes, but destined to function in the chloroplast.
Besides structural and enzymic proteins, regulatory proteins probably also cross the chloroplast envelope. How light regulates chloroplast development is not known, but it would be reasonable to speculate in terms of proteins entering the organelle to trigger nucleic acid and protein synthesis. There is the possibility of proteins also passing out of the organelle, since recent work in Chlamydomonas has implicated chloroplast protein synthesis in the regulation of nuclear DNA replication (Blamire et al., 1974). This question of protein transport into and out of the chloroplast is a crucial one to study if we are to understand how the chloroplast and nuclear genomes interact; almost no research has been carried out in this area. The movement of nucleic acids across the envelope remains a possibility, but no compelling evidence to support it has been published.
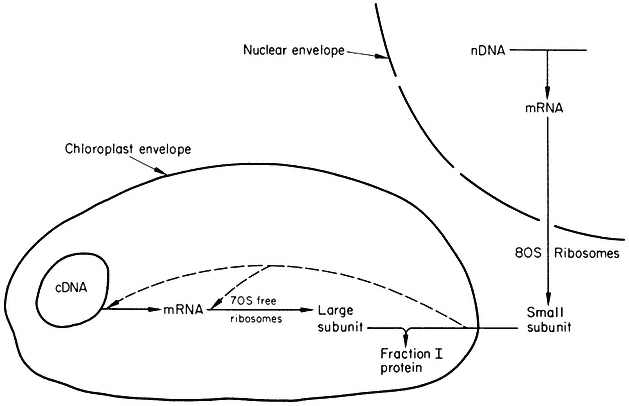
Figure 11.8
Model for the co-operation of nuclear and chloroplast genomes in the
synthesis of Fraction I protein. cDNA and nDNA stand for chloroplast and
nuclear DNA respectively. The dashed lines indicate possible sites at
which small subunits may control the synthesis of large subunits.
(Reprinted from Ellis, 1975, by courtesy of Pergamon Press.)
Another problem needing study is the nature of the mechanism which integrates the synthesis of the two subunits of Fraction I protein in different cellular compartments. The dashed lines in Fig. 11.8 illustrate one possibility; the small subunit is postulated to be required as a positive factor for the initiation of either transcription or translation of the large subunit messenger (Ellis, 1975). The recent detection of the large subunit messenger should allow this possibility to be tested (Hartley et al., 1975).
11.3—
Mitochondrial Autonomy
Research on the biogenesis of plant mitochondria has lagged far behind that on mitochondria from animal and fungal cells. A summary of the position is given here in the hope of stimulating readers to undertake work in this field; recent reviews have appeared on the biogenesis of both animal and fungal mitochondria (Borst, 1971, 1972), and of those in plants (Boulter et al., 1972; Leaver & Harmey, 1973; Leaver, 1975).
The first point to emphasize is that mitochondria constitute a much smaller compartment in plant cells compared to chloroplasts. The amount of mitochondrial DNA and ribosomes is correspondingly much smaller as a percentage of the total cellular complement. The technical difficulties of extracting small quantities of intact, uncontaminated mitochondria from plant tissues make studies on their genetic system more difficult than in the case of chloroplasts. Another difference from chloroplasts is that the DNA and ribosomes of mitochondria are more variable in their properties between species, so the extrapolation of results must be done with caution.
In a range of higher plants the density of mitochondrial DNA is constant at 1.706–1.707 g cm–3 , whereas the nuclear DNA from the same species varies in density between 1.691–1.702 g cm––3 . In many organisms mitochondrial DNA has been isolated in circular form; in contrast to the situation for chloroplasts (see 11.2.1.5), the contour length of the circles varies with the species. Thus, animals have mitochondrial DNA circles of about 5 µm contour length; this corresponds to a molecular weight of about 107 , or 15,000 base pairs. In Ascomycetes the contour length of circular mitochondrial DNA is in the range 20–26 µm, while in higher plants lengths of 30 µm have been reported. The small size of the mitochondrial DNA in animals means that it can code for only a few components. Hybridization studies in Xenopus indicate that mitochondrial ribosomal RNA and at least some mitochondrial transfer RNA are encoded in mitochondrial DNA. The remaining mitochondrial DNA is sufficient to code for only about ten proteins each of molecular weight 50,000. However, kinetic complexity measurements on mitochondrial DNA from higher plants indicate that it has between six and ten times the coding potential of animal mitochondrial DNA. This observation raises the possibility that mitochondria from plants possess a higher degree of autonomy than those from animals, and this reinforces the plea that more research be carried out with plant cells.
The sedimentation coefficients of mitochondrial ribosomes and their component ribosomal-RNAs vary greatly with the species. Animal mitochondria contain so-called 'miniribosomes' which sediment between 55s and 60s, while fungal mitochondria contain 70–74s ribosomes. Leaver and Harmey (1972) have shown that mitochondrial ribosomes from several higher plants sediment at 77s–78s, while their major RNA components sediment at 25s and 18s. Although mitochondrial ribosomes differ in size in different species, they are all similar
in that protein synthesis by them is sensitive to the same set of antibiotics which inhibit prokaryote and chloroplast ribosomes.
Protein synthesis by isolated mitochondria has been studied extensively in animals and fungi, but hardly at all in higher plants. Several proteins of the inner, but not the outer, mitochondrial membrane are synthesized by animal and fungal mitochondria. These proteins have been identified as subunits of the ATPase complex, cytochrome b, and cytochrome c oxidase; the other subunits of these insoluble complexes are synthesized by cytoplasmic ribosomes. This evidence suggests the synthesis of mitochondrial membranes requires the co-operative activity of both mitochondrial and nuclear genomes. This idea of the co-operation between the several genetic systems in eukaryotic cells is already familiar to the reader from section 11.2.5. The same problem of the transport of proteins across the bounding membranes applies to mitochondria as well as to chloroplasts. In contrast to chloroplasts, mitochondria from animal and fungal cells synthesize no soluble proteins; whether higher plant mitochondria synthesize any soluble proteins is unknown.
No protein has so far been rigorously identified as being encoded in mitochondrial DNA in any organism. A simple presumption is that those proteins synthesized by isolated mitochondria are encoded in mitochondrial DNA. It is likely that the integrative controls between nuclear and mitochondrial genomes in higher plants differ from those in animals which contain less than one sixth as much DNA. Why do plant mitochondria apparently contain so much more genetic information to perform the same functions as animal mitochondria? Answering this question is a challenge which must be taken up if our understanding of the origin of this organelle in plants is to improve.
Further Reading
Borst P. (1971) Size, structure and information content of mitochondrial DNA. In Autonomy and Biogenesis of Mitochondria and Chloroplasts, (eds N.K. Boardman, A.W. Linnane & R.M. Smillie), pp. 260–6. North Holland, Amsterdam.
Borst P. (1972) Mitochondrial nucleic acids. Ann. Rev. Biochem.41, 333–76.
Boulter D., Ellis R.J. & Yarwood A. (1972) Biochemistry of protein synthesis in plants. Biol. Rev.47, 113–75.
Ellis R.J. (1975a) The synthesis of chloroplast membranes in Pisum sativum. In Membrane Biogenesis: Mitochondria, Chloroplasts & Bacteria, (ed. A. Tzagoloff). Plenum Publishing Co., New York.
Ellis R.J., Blair G.E. & Hartley M.R. (1973) The nature and function of chloroplast protein synthesis. Biochem. Soc. Symp.38, 137–62.
Ellis R.J. & Hartley M.R. (1974) Nucleic acids of chloroplasts. In Biochemistry of Nucleic Acids, (ed. K. Burton). MTP International Review of Science Biochemistry Series One, Vol. 6, 323–48. Butterworths, London & University Park Press, Baltimore.
Kirk J.T.O. (1972) The genetic control of plastid formation: recent advances and strategies for the future. Sub-Cell. Biochem.1, 333–61.
Leaver C.J. (1975) The biogenesis of plant mitochondria. In The Chemistry and Biochemistry of Plant Proteins, (ed. J. Harborne). pp. 137–65. Academic Press, New York & London.
Leaver C.J. & Harmey M.A. (1973) Plant mitochondrial nucleic acids. Biochem. Soc. Symp.38, 175–93.
Levine R.F. & Goodenough U.W. (1970) The genetics of photosynthesis and of the chloroplast in Chlamydomonas reinhardi. Ann. Rev. Genet,4, 397–408.
Sager R. (1972) Cytoplasmic Genes and Organelles. Academic Press, New York & London.
Tewari K.K. & Wildman S.G. (1970) Information content in the chloroplast DNA. In Control of Organelle Development, (ed. P.L. Miller), pp. 147–79. Symposium 24 of the Society for Experimental Biology, Cambridge University Press, Cambridge.