Ten—
Sex- and Age-related Variation in Reproductive Effort of Northern Elephant Seals
Charles J. Deutsch, Daniel E. Crocker, Daniel P. Costa, and Burney J. Le Boeuf
ABSTRACT. The aim of this study was to determine how reproductive effort (RE) varies with age and sex in the northern elephant seal, Mirounga angustirostris . RE is an important feature of life history that links the proximate costs with the fitness costs of reproduction. Percentage of mass lost over the breeding season provided an energetic index of RE because both sexes fast on the rookery. We summarize research conducted over an 11-year period at Año Nuevo, California. Seventy-three females ranging in age from 3 to 12 years were chemically immobilized and weighed during the lactation period, providing 22 measurements of maternal mass loss. Males ranging in age from 5 to 13 years were weighed (N = 56), or their mass was estimated using a photogrammetric technique (N = 94), yielding 87 measurements of breeding mass loss.
The principal findings were as follows. 1 . The magnitude of RE was similar between males and females. Adults of both sexes lost slightly more than one-third of their mass, on average, despite large sex differences in reproductive strategy and body size. Adult males were an average of 1.4 times longer and 3 to 4 times heavier (mean ± SD = 1,814 ± 233 kg, range = 1,430–2,550 kg) than adult females (488 ± 80 kg, range = 360–710 kg) at the start of the breeding season. Male mating effort was more variable than female parental effort, and some bulls lost up to half of their arrival mass. The timing and intensity of RE differed between the sexes: males fasted three times longer than females, but they incurred less than one-third the mass-specific mass loss rate of females. Males suffered vastly more external injuries from conspecifics than did females, yet females were probably at greater risk of receiving life-threatening internal injuries. 2 . Female effort during lactation was not correlated with maternal age or mass, but gestation effort (i.e., relative neonatal mass) declined with increasing maternal mass. Absolute measures of investment in offspring—including maternal mass loss, neonatal mass, weanling mass, and pup mass gain—were directly proportional to maternal mass, which increased from 3 to 6 years of age before reaching an asymptote. Maternal investment in sons was similar to that in daughters. 3 . Males delayed serious efforts at mating until age 6, two to three years later than females but still physically
immature, and mean RE was subsequently constant with age. Dominant males devoted a greater proportion of body stores to breeding and obtained higher mating success than did subordinates.
The similarity in RE of growing "subadults" and physically mature breeders for both sexes was contrary to theoretical expectations, considering the poorer reproductive success and higher fitness costs of the former. Possible explanations include the existence of a threshold RE below which fitness costs are minimal, the higher fitness benefit of early-born offspring in an expanding population, and the benefit of experience to future reproductive performance.
One can, in effect, treat the sexes as if they were different species, the opposite sex being a resource relevant to producing maximum surviving offspring.
—R. L. Trivers (1972)
Males and females reproduce in fundamentally different ways, and the resulting divergent selective pressures have led to numerous sex differences in morphology, physiology, life history, and behavior (Trivers 1972, 1985; Glucksman 1974; Clutton-Brock, Guinness, and Albon 1982). A crucial aspect of mammalian life history that has rarely been compared quantitatively across the sexes is reproductive effort (RE). Typically defined as the proportion of available time and energy that an organism allocates to reproduction over a specified period of time (Gadgil and Bossert 1970; Hirshfield and Tinkle 1975; Tuomi, Hakala, and Haukioja 1983), RE also includes a component of risk that is important but more difficult to quantify (Calow 1979; Warner 1980). Reproductive effort can be divided into two categories: mating effort, which involves expenditure of time, energy, or risk to obtain matings (e.g., searching for mates, fighting for dominance or territory necessary for access to mates); and parental effort, which involves such expenditure to produce, nurture, and raise offspring (Low 1978). Herein lies a major difference between the RE of males and of females. In the majority of mammals and in most polygynous species, males exhibit little or no parental care, so that virtually all male RE is mating effort (Alexander and Borgia 1979). In contrast, females invest considerably in their young, especially in mammals, which exhibit extended postnatal care and nurture offspring via the energetically expensive process of lactation (see Loudon and Racey 1987). Consequently, the selective pressures that have shaped the pattern of RE should differ between the sexes. Female RE is thought to have evolved primarily in response to ecological and demographic factors, whereas the evolved pattern of male RE has apparently been strongly influenced by the mating system and the intensity of male-male competition for mates (Trivers 1972; Warner 1980; Thornhill 1981).
The principal reason for the paucity of empirical studies contrasting male and female RE in polygynous vertebrates is that male mating effort has been neglected (Stearns 1976; Warner 1980; Gittleman and Thompson
1988). Furthermore, sex differences in energy intake during the breeding season and in risks associated with reproducing (e.g., Ryan 1985) often make it difficult to measure RE in a comparable way for each sex (Knapton 1984). Ideally, one would study a species in which breeding is temporally separated from foraging and other activities related to growth and survival. Then essentially all activity would serve a reproductive function, all energy expended above maintenance levels would be devoted to reproduction, and energy intake would be nil or absent. Such conditions are found in some phocids, such as elephant seals and gray seals, in which both sexes fast and remain on land during the breeding season, relying on stored energy reserves to fuel their metabolic and reproductive needs. Studies on phocid reproductive energetics were initially conducted on females and pups due to their smaller size, greater site predictability, and relevance of the work to parental investment theory (Fedak and Anderson 1982; Ortiz, Le Boeuf, and Costa 1984; Stewart and Lavigne 1984; Costa et al. 1986; Stewart 1986; Bowen, Boness, and Oftedal 1987; Hill 1987; Tedman and Green 1987; McCann, Fedak, and Harwood 1989; Kovacs, Lavigne, and Innes 1991; Hammill et al. 1991; Bowen, Oftedal, and Boness 1992; Fedak et al., this volume; see review by Oftedal, Boness, and Tedman 1987). Logistic difficulties involving the capture and weighing of large, aggressive, and mobile animals have hindered research on male reproductive energetics in pinnipeds until recently (Anderson and Fedak 1985; Reilly 1989; Deutsch, Haley, and Le Boeuf 1990; Boyd and Duck 1991; Reilly and Fedak 1991; Twiss 1991; Bartsh, Johnston, and Siniff 1992; Fedak et al., this volume; Walker and Bowen, in press).
We studied sex- and age-related variation in reproductive effort of the highly polygynous and sexually dimorphic northern elephant seal, M. angustirostris , in central California. Sex differences in life history and behavior are numerous in this species (see Le Boeuf 1981; Le Boeuf and Reiter 1988; Le Boeuf and Laws, this volume). Age at primiparity ranges from 2 to 6 years, with most females initially giving birth at age 3 (36%) or age 4 (54%) at Año Nuevo (Reiter and Le Boeuf 1991). Females produce one pup annually thereafter, with occasional skipping (Huber 1987), until death at a maximum age of 19 years. Male growth is extended by two to three years, and reproduction is delayed compared to females. Males attain sexual maturity by age 5 and rarely skip breeding seasons subsequently (Clinton 1990); yet due to intrasexual competition, most do not mate until physical maturity at age 8 or 9 (Clinton, this volume), five years later than the age of first mating for most females. The maximum life span recorded for a male is 14 years.
Considerable interest in optimal life history theory has focused on identifying the age-specific pattern of reproduction that maximizes fitness for
a given set of demographic conditions and constraints (see Charlesworth 1980). Assuming that reproduction imposes a cost, measured as a reduction in future fitness (Bell 1980; Bell and Koufopanou 1985), there should be a level of RE at each age that optimizes trade-offs between current and future reproduction (Williams 1966a , 1966b ; Gadgil and Bossert 1970; Pianka and Parker 1975). A frequently asserted prediction is that RE should increase with age as residual reproductive value declines; that is, since older individuals generally have less to lose in terms of future offspring than younger ones, investment should be high late in life (Williams 1966b ; Gadgil and Bossert 1970; Pianka 1976; Caswell 1982). This trend is favored by low population growth rate, low extrinsic adult mortality rate, and continuous increase in body size or reproductive efficiency during adulthood (Charlesworth 1980). The opposite conditions theoretically favor a decline in RE with age. In rapidly expanding populations, for example, breeding should occur as soon as physiologically possible (Lewontin 1965; Cody 1971; Stearns 1976), and young adults should exhibit the greatest effort (Charlesworth and León 1976). Though studies on this point among long-lived birds and mammals are few, there is some empirical support for increasing (Clutton-Brock 1984; Pugesek 1981, 1983, 1984; Hamer and Furness 1991; Pärt, Gustafsson, and Moreno 1992; but see Nur 1984), decreasing (Stewart 1986), and constant (Reid 1988) RE with age; other studies have yielded mixed results (Green 1990).
The expected ontogenetic pattern of resource allocation to reproduction must be considered separately for each sex. Reproductive value of female northern elephant seals declines gradually from primiparity to age 11 and then drops steeply to the end of the life span (Reiter and Le Boeuf 1991), suggesting that the fitness cost of reproduction should also decrease with age, especially among the oldest animals. Weaning success increases with age and experience, particularly from sexual maturity to physical maturity at age 6 (Reiter, Panken, and Le Boeuf 1981; Reiter and Le Boeuf 1991; Sydeman et al. 1991). This combination of rising benefits and declining potential costs of breeding with age suggests that maternal effort should increase with age. However, the current high population growth rate (Cooper and Stewart 1983; Stewart et al., this volume) enhances the fitness value of early-born offspring relative to those born later in life, and this will favor high effort early in life. Given these opposing forces, it is difficult to predict how female RE should change with age, although we expect a high terminal investment when residual reproductive value is low (i.e., > 10 years old). Since natality rate is high and relatively constant with age after the initiation of reproduction (Le Boeuf and Reiter 1988; Huber et al. 1991), our study focused on the RE of parous females and did not consider the frequency of barren years.
For male elephant seals, sexual selection is likely to have shaped the agespecific pattern of RE. Intense intrasexual competition among males in highly polygynous species should favor a lifetime mating strategy involving little investment when young and a high mating effort after attaining a reproductively competitive size (Warner 1980; Maher and Byers 1987). Male elephant seals have a relatively short effective breeding life span (age 8–13), with mating success rising precipitously on reaching physical maturity (Le Boeuf and Reiter 1988; Clinton and Le Boeuf 1993). Since subadult males are growing, any allocation of time and energy to reproduction might reduce their growth rate (e.g., Green and Rothstein 1991), final adult size (e.g., Boyce 1981), and, hence, future fecundity. Therefore, we predicted that male mating effort should be low during the subadult period (age 4–7) when the fitness benefit of reproductive investment is nil and potential costs are high and that effort would increase abruptly at adulthood when bulls are able to fight effectively for high dominance rank and access to mates. Contrary to expectation, C. J. Deutsch, M. P. Haley, and B. J. Le Boeuf (1990) found that older subadults (6–7 years old) expended the same RE (i.e., percent mass loss) as low-ranking adult males.
In this chapter, we compare reproductive effort between male and female northern elephant seals as indicated by mass loss while breeding (energy component), duration of stay on the rookery (time component), and incidence of external injuries (risk component). Our objectives are to: (1) summarize existing data on body mass as a function of age for both sexes, much of which is currently unpublished or scattered across a number of publications; (2) compare the magnitude of male mating effort with female parental effort, in terms of relative mass loss, time commitment, and apparent risk; and (3) describe age-specific patterns of RE for both sexes and relate them to changes in reproductive success and mortality with age.
Methods
General Methods and Study Area
The studies summarized here were conducted from 1981 to 1991 at Año Nuevo State Reserve, 70 km south of San Francisco, California. Most work was done on the expanding mainland colony (see Le Boeuf and Kaza 1981 for a description of the area), which increased over the study period from 300 to 1,500 breeding females distributed among 8 to 16 harems. The annual maximum in number of sexually mature males present on the Año Nuevo mainland during the breeding season (December to March) fluctuated between 300 and 500, of which about one-third were adults. Individuals were identified over the course of the breeding season by applying cream bleach or black hair dye to the pelage. Numbered plastic tags placed
in the webbing of the hind flippers provided information on age and permitted identification of individuals from year to year.
Exact ages were unknown for most males in the study. Males were assigned to one of five age classes based on their overall size and on the development of secondary sexual characters (neck shield and proboscis), both of which correspond with age to within about one year (Le Boeuf 1974; Cox 1983; Clinton 1990): subadult-one (SA1) males = 4 years old; subadult-two (SA2) = 5 years; subadult-three (SA3) = 6 years; subadult-four (SA4) = 7 years; and adult males (AD) = 8–14 years. Most observations concern the last three age classes, since there were few SA1 and SA2 males present during the breeding season. Known-age adult males and those with known histories were further classified as either young adult (first- or second-year adult, approximately 8–9 years of age) or old adult (at least third-year adult, 10 years and older).
Copulations and dominance interactions among males were recorded in the field using standard methods, and the estimated number of females inseminated provided a measure of male mating success (Le Boeuf 1974). Dominance ranks of adult males were determined in each year using the Bradley-Terry method (Boyd and Silk 1983; Haley, Deutsch, and Le Boeuf, in press), and these values were used to assign males to one of three rank classes.
Measurement of Body Mass and Mass Loss
Females
One or more weights were obtained on 73 females and their pups near the beginning and/or the end of the female's lactation period. Sixty-one mothers were of known age, ranging from 3 to 12 years old. The sample includes female-pup pairs weighed during the course of studies on diving behavior (Le Boeuf et al. 1986, 1988, 1989) and reproductive energetics (Costa et al. 1986; Crocker 1992; D. Costa and B. Le Boeuf, unpubl. data). An additional 18 pups were weighed within three days of birth and again within six days of weaning, 17 of whose mothers were of known age (Kretzmann 1990; Kretzmann, Costa, and Le Boeuf 1993). The 91 motherpup pairs included 9 females that were experimental subjects in two or more years, giving a total of 79 different females. After chemically immobilizing a lactating female, she and her pup were weighed with a tripod scale (accurate to ± 2.5 kg) and standard length measurements were taken (Costa et al. 1986). For the energetic studies, 22 different female-pup pairs were weighed 0 to 5 days postpartum, and the procedure was repeated an average of 21.2 ± 2.0 days later (range = 18–25 days), within a few days of weaning. Methods are given in more detail in D. P. Costa et al. (1986) and D. E. Crocker (1992). Data on energy expenditure, body composition, and milk intake are presented elsewhere (Ortiz, Le Boeuf, and Costa 1984; Costa et al. 1986; Crocker 1992; Kretzmann, Costa, and Le Boeuf 1993).
Females that abandoned or lost their pup after experimental manipulation (N = 5), or who regularly nursed more than one pup (N = 2), were excluded from calculations of maternal mass loss, departure mass, lactation duration, pup mass gain, and pup mass and length at weaning.
Males
Body mass was obtained or estimated for 140 different males (n = 247 measurements of mass) ranging in age from 5 to 13 years and in dominance status from low-ranking to alpha male. Estimates of daily and seasonal mass loss are presented for 82 males (n = 87 measurements) and 71 males (n = 75 measurements), respectively. The data on male body mass come from two studies.
1. In 1988 and 1989, 56 males were weighed on a platform scale (accurate to + 0.25%), yielding 95 weights and 30 measures of breeding mass loss over a mean ± SD interval of 32.7 ± 18.7 days (range = 9–83 days). One male was anesthetized; the rest were either lured onto the scale using a model of a female elephant seal and playback of female vocalizations or moved onto the scale using tarpaulins and playback of male threat vocalizations. Details of weighing methods and results appear in Deutsch, Haley, and Le Boeuf (1990).
2. Body mass was estimated for an additional 94 males at the start and/or the end of the 1989 breeding season using a photogrammetric technique that predicts mass with a 95% confidence interval of ± 14% (r2 = .93; Haley, Deutsch, and Le Boeuf 1991). This provided 152 estimates of mass and 57 measures of mass loss over a mean interval of 58.0 ± 13.3 days (range = 42–96 days) (Deutsch 1990).
Since there were no significant differences in daily or seasonal mass loss (absolute and mass-specific) of males between the direct weighing and photogrammetric methods (t-tests for each age class, p > .05), the two data sets were pooled for presentation here. Photogrammetric estimates of adult male body mass at arrival and departure were significantly greater, however, than were the extrapolated figures based on direct measurements (arrival: 1,851 ± 236 vs. 1,710 ± 200 kg, t = 2.09, df = 61, p < .05; departure: 1,227 ± 144 vs. 1,098 ± 115 kg, t = 3.72, df = 75, p < .001). This probably reflects the fact that large, high-ranking males (which were difficult to weigh) comprised a greater proportion of the photographic data set than the direct weight data set. The two methods of measuring mass did not differ significantly for SA3 males (p > .05), but photogrammetric estimates were significantly greater than direct estimates for SA4 males (arrival: 1,539 ± 234 vs. 1,274 ± 140 kg, t = 2.26, df = 11, p < .05; departure: 1,106 ± 158 vs. 870 ± 107 kg, t = 3.81, df = 18, p < .01). This mass difference was apparently not due to a methodological discrepancy, because the
photographic sample of SA4 males was indeed larger in standard length than the weighed sample (p < .05). Standard length was measured photogrammetrically in both studies (Haley, Deutsch, and Le Boeuf 1991).
Incidence of Injuries
Injuries provide one index of risk taken in reproduction because they represent the degree to which the animal was exposed to potentially damaging or lethal interactions while reproducing (Deutsch 1990; Le Boeuf and Mesnick 1991). Wounds can get infected and, if severe, can reduce fecundity (e.g., Clutton-Brock et al. 1979; Le Boeuf, Riedman, and Keyes 1982) or lead to death (e.g., Laws 1953; Wilkinson and Shank 1976). The incidence of external injuries was measured by recording the number, location, and severity of fresh wounds on males (1989) and females (1991) during the main mating period in February. Wounds were considered fresh if they were open, bleeding, or oozing and thus represented recent injuries. This analysis was limited to injuries inflicted by conspecifics and excluded shark-inflicted wounds.
Data Analysis
Values of body mass are presented at arrival (or parturition for females) and departure dates to standardize comparisons across ages and sexes. Only direct measurements of mass and breeding mass loss are used in the sex comparison. Descriptive statistics are presented as mean ± one standard deviation (SD). For nine females and nine males that were sampled in two or more years, mean values of size, mass change, and other variables were calculated for each individual; these values were then used in the calculation of overall means by sex or age class (e.g., table 10.3). For correlations between RE variables and size or age, however, each measurement was treated as an independent data point (e.g., table 10.4). In these analyses, P-values were determined after reducing the degrees of freedom to equal the number of individuals (rather than measurements) in the sample minus the number of estimated parameters (see Sydeman et al. 1991). The level of statistical significance was set at a = .05 for all tests.
Measures of the effort devoted to reproduction (e.g., mass loss, pup mass gain) are presented here in absolute and mass-specific terms as a function of age and sex. RE should be expressed as a fraction of the organism's ability to invest (e.g., proportion of energy stores or body mass). However, since proportions can sometimes be misleading when the dependent variable varies allometrically with body mass (Packard and Boardman 1987), we adjusted for the potentially confounding effect of body mass on indexes of RE using one of three additional methods: (1) analysis of covariance (ANCOVA) for comparison of male mass loss by age class and for comparison of maternal investment by sex of pup; (2) partial correlations between
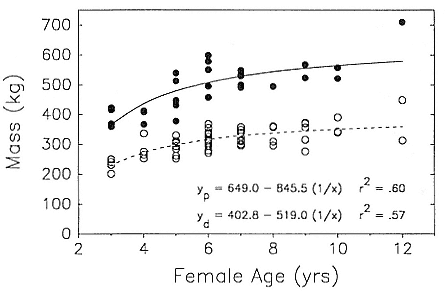
Fig. 10.1
Breeding female mass at parturition (solid circles, N = 28) and at departure from the
rookery (i.e., weaning) (open circles, N = 54) as a function of maternal age. Both
regressions are significant (p < .0001). The heavier departure mass at age 12
is a rough estimate (see footnote c in table 10.3).
maternal investment variables and age, controlling for female mass; and (3) correlation of residuals (from the regression of maternal investment indexes against parturition mass) with maternal age. Plots of residuals were inspected visually for homoscedasticity (i.e., constant variance of residuals around the regression line), and the normality of residuals was checked with the univariate procedure in SAS (SAS Institute 1985). An arcsinesquare-root transformation was applied to percentages before performing parametric statistical tests (Sokal and Rohlf 1981). Descriptions of how mass, mass loss, and other variables were calculated are presented in appendix 10.1.
Results
Sexual Size Dimorphism
The most striking difference between the sexes in elephant seals is their size. At arrival, adult males weighed an average of 3.5 (range = 2–7) times more than adult females (table 10.1; see figs. 10.1 and 10.2). Estimates of arrival mass for adult males varied from 1,430 kg to 2,265 kg for weighed animals and up to 2,550 kg for a photographed animal. Departure mass of
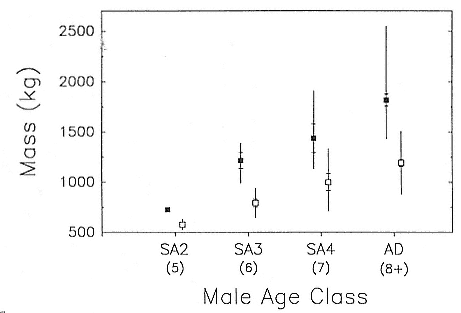
Fig. 10.2
Male mass at arrival (solid squares) and at departure (open squares) from the
breeding rookery as a function of age class. Approximate age in years of each class is
shown in parentheses. Symbols show the mean (squares), 95% confidence interval
of the mean (hatch marks), and range; a 95% confidence interval is not shown
for the SA2 class due to the small sample size. Sample sizes for arrival and
departure mass, respectively, are as follows: SA2 (N = 1,2);
SA3 (N = 12, 18); SA4 (N = 11, 18); Adult (N = 62, 75).
adult males ranged from 895 to 1,500 kg (fig. 10.2). Female mass at parturition (i.e., immediately postpartum) ranged from 360 to 710 kg, and departure mass ranged from 200 kg to at least 400 kg (fig. 10.1; table 10.3). Given that males and females arrive at different times and subsequently lose mass until departure, the mass ratio between an adult male and a lactating female at any given time could range from about 1.5 to 10. However, a three- to sixfold dimorphism in mass would be most common for a typical copulating pair in early February.
Standard length (SL) gives a more straightforward measure of sexual size dimorphism because it does not fluctuate over the annual cycle as does mass. Mean SL of 89 physically mature males measured photogrammetrically in this study was 382 ± 15 cm (range = 350–420 cm), which is similar to the mean of 385 ± 18 cm found by W. L. Clinton (this volume). Physically mature females (i.e., 6+ years of age) measured in this study had a
mean SL of 265 ± 8 cm (range = 248–282 cm, N = 32 individuals). Thus, mature males were an average of 1.44 times longer (range = 1.24–1.69) than mature females.
Both sexes became reproductively active while still growing. Females giving birth for the first time at age 3 had attained approximately 75% of mature mass (fig. 10.1, table 10.3), whereas 6-year-old males (SA3 class) were roughly two-thirds the mass of mature bulls (fig. 10.2, table 10.5). The cross-sectional data on size and a limited amount of longitudinal data indicate that females stopped growing in length and mass at age 6 (parturition mass = 541 ± 58 kg, N = 16, ages 6–12 years) and males reached a physically mature size at 8 to 9 years, growing little or not at all in length thereafter (Clinton, this volume).
Sex Comparison of Reproductive Effort
A comparison of the energetic component (breeding mass loss) and the time component (duration of the breeding fast) of RE for adult male and female elephant seals is presented in table 10.1. The most important finding was that the mean proportion of mass lost over the breeding season was very similar for both sexes (36–37%), suggesting that the energetic component of RE while ashore was also similar. A proximate reason for this outcome was that the greater intensity of the females' parental effort (i.e., percent body mass lost per day) compared to the males' mating effort was balanced by a longer duration of stay for males. Duration of the breeding fast for adult males was about three times longer than that of females (table 10.1). Relative mass loss over the season was significantly more variable for adult males (range for weighed animals = 26–46%) than for females (range = 31–41%) (variance-ratio test, F = 5.78, df = 12, 21, p<.01).
Males clearly incurred a much greater risk of external injury than females. Nearly 90% of males surveyed in February possessed fresh wounds, compared to less than 20% of females (table 10.2). The mean number of fresh wounds per animal was 20 times greater for males than females (table 10.2); those females with open wounds usually had only one to three small cuts. Although most injuries appeared superficial for both sexes, the potential for serious injury was reflected in the percentage of seals with fresh wounds on the head (including face, nose, and eye), which again was significantly higher for males than females.
Female Reproductive Effort in Relation to Maternal Age, Mass, and Pup Sex
Energetic measures of the two components of maternal investment—gestation and lactation—are plotted as a function of maternal age, mass, and pup sex in figure 10.3 and summarized as a function of maternal age in table 10.3.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
Effect of Pup Sex on Maternal Investment
Theoretical predictions (Maynard Smith 1980) and some empirical studies (e.g., Anderson and Fedak 1987; Oftedal, Iverson, and Boness 1987) indicate that females invest more in sons than in daughters in polygynous mammals. Therefore, we initially examined the data, using two-tailed t-tests, for differences in the mother's size, age, and investment or the pup's size and growth rate as a function of pup sex. Of all variables in table 10.3, only percent daily maternal mass loss was significantly different between mothers with sons (1.53 ± 0.10%, N = 10) and mothers with daughters (1.43 ± 0.10%, N = 12; t = 2.43, df = 20, p = .025). Absolute mass loss per day did not vary significantly with sex of the pup (t = 0.71, df = 20, p = .48); after adjusting for the effect of maternal parturition mass, however, mothers of male pups showed a higher mass loss rate than mothers of female pups (ANCOVA: F = 5.34, df = 1, 19, p = .032). This finding is not consistent with our other results because none of the other t-tests revealed a significant effect of pup sex on maternal investment (e.g., total mass loss, pup growth rate); analyses of covariance on the nine other absolute measures of maternal investment in table 10.3, with parturition mass as the covariate, confirmed these results (p > .10 for all tests). Furthermore, recent studies have shown a lack of differential investment in sons versus daughters in both northern and southern elephant seals (McCann, Fedak, and Harwood 1989; Le Boeuf, Condit, and Reiter 1989; Campagna et al. 1992; Kretzmann, Costa, and Le Boeuf 1993; Fedak et al., this volume). Our interpretation is that this one significant result was probably due to chance, since one comparison is expected to be significant at the p = .05 level when over 20 tests are performed simultaneously. None of the slopes defining the linear regressions of maternal investment variables on maternal mass varied significantly with pup sex (general linear models, p > .25), but the correlation coefficients were usually lower for sons than for daughters, as reflected in the greater scatter of points among small mothers of sons (see fig. 10.3e, g, i). T. S. McCann, M. A. Fedak, and J. Harwood (1989) made a similar observation for the southern elephant seal. We con-
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
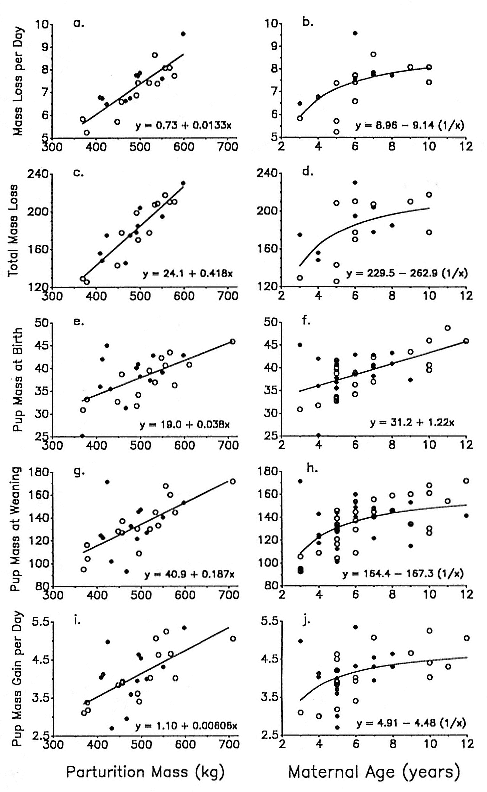
Fig. 10.3
Absolute measures of maternal investment as a function of female parturition mass and
age for male (closed circles) and female (open circles) pups. ( A, B ) Average daily mass loss
of mother. (C, D ) Total mass loss of mother over lactation period. ( E, F ) Pup mass at
birth. (G, H ) Pup mass at weaning. (I, J ) Average daily mass gain of pup. All units of
mass are in kg. See table 10.4 for sample sizes, correlation coefficients,
and levels of significance.
clude that the results do not support the hypothesis that maternal effort varies as a function of pup sex. Therefore, further analyses use the pooled data set.
Gestation Effort:
Pup Mass at Birth
Pup birth mass increased significantly with maternal mass and age (fig. 10.3e, f; table 10.4). Maternal investment during the period of gestation, as indicated by pup birth mass expressed as a fraction of mother's parturition mass, declined significantly with increasing maternal mass (y = 11.44 – 0.0075x) and maternal age (y = 9.36 – 0.234x) (tables 10.3 and 10.4). A similar negative relationship between relative neonatal mass and maternal mass is also found in interspecific comparisons among phocids (Kovacs and Lavigne 1986) and in other mammals (Millar 1977; Robbins and Robbins 1979; Gittleman 1986). Due to their smaller size, young mothers (3–5 years) put forth a greater gestation effort (pup birth mass was 8.64 ± 1.28% of maternal mass, N = 8) than physically mature, older mothers (7.43 ± 0.63%, N = 16; Wilcoxen two-sample test, z = 2.358, p < .05). Maternal age had no significant effect on pup birth mass, however, after accounting for the effect of maternal body mass (table 10.4). Analyses of pup mass at initial treatment (0–5 days postpartum) yielded similar results, although the relationship with age appeared more asymptotic.
Lactation Effort:
Maternal Mass Loss
Daily mass loss of lactating females was strongly and positively correlated with parturition mass and, to a weaker degree, with female age (fig. 10.3a, b; table 10.4). This reflects both increased absolute maintenance costs and increased mass transfer to the pup with larger maternal size (maternal mass loss per day vs. pup mass gain per day: r = .73, N = 22, p < .0001). Independent of maternal mass, age was not significantly correlated with rate of mass loss during lactation (table 10.4).
Total mass loss over the lactation period increased with female mass and age in a similar fashion (fig. 10.3c, d; table 10.4). Total percentage of body mass lost over lactation, our principal index of female reproductive effort, varied between 31 and 41% and showed no significant correlation with maternal mass or age (table 10.4, fig. 10.4). Partial correlation and residual analyses yielded the same results: age had no discernible effect on breeding mass loss after controlling for the effects of maternal mass (table 10.4).
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
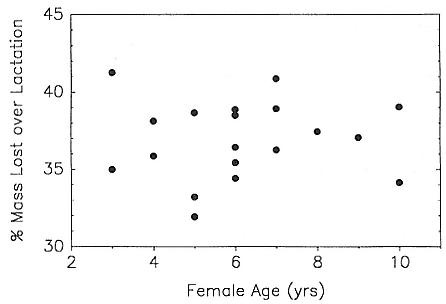
Fig. 10.4
Percentage of female parturition mass lost over the lactation period
as a function of maternal age (r = .04, N = 19, p = .87).
Lactation Effort:
Pup Mass Gain
Mass gain of pups provided another energetic index of maternal investment (e.g., Ortiz, Le Boeuf, and Costa 1984). Pup mass gain per day and over the entire lactation period rose significantly with female mass and age (fig. 10.3i, j; table 10.4). Once again, maternal age had no apparent effect on mass gain of pups after adjusting for maternal mass (table 10.4).
On average, pups gained 57.5% of the mass lost by their mothers (table 10.3). We expected that the efficiency of mass transfer would increase with maternal size since a female's mass-specific energy expenditure for maintenance metabolism (i.e., her "metabolic overhead," Fedak and Anderson 1982) should decline with increasing mass. Thus, a larger mother should be able to allocate more energy to milk production and relatively less to her own maintenance costs. The data did not support this prediction; in fact, there was a nonsignificant tendency in the opposite direction, with younger mothers being somewhat more efficient (tables 10.3, 10.4).
Lactation Duration
Duration of the lactation period averaged 25 to 26 days and was positively correlated with maternal mass (table 10.4). Mean lactation duration increased from age 3 to 5 and then remained approximately constant among older females (table 10.3). Chemical immobilization of the female near the end of lactation sometimes triggered a premature
departure, and so these figures may be biased slightly low (1–2 days at most). The relationship between duration of stay and age was generally similar to that found by J. Reiter, K. J. Panken, and B. J. Le Boeuf (1981). A comparison of experimental and control groups matched for maternal age, year of study, and breeding site showed no significant effects of manipulation on lactation duration or weanling mass (D. Crocker and B. Le Boeuf, unpubl. data).
There was an inverse relationship between the duration and intensity of female RE. Mothers that lost mass at a high daily rate, relative to their body size, nursed their pups for a significantly shorter period compared to those with a less intense maternal effort (partial r = –0.50, N = 22, p < .025, controlling for parturition mass).
Total Maternal Investment:
Weanling Mass
A pup's mass at weaning indicates the mother's total absolute investment in the young, the sum of birth mass plus mass gain during the nursing period. Weaned pup mass increased significantly with maternal mass (fig. 10.3g) and with maternal age up to 6 years (fig. 10.3h, table 10.4). At weaning, pups weighed a mean of 27.2% of mother's parturition mass (range = 20–31%, plus one outlier of 40%), and this was not significantly associated with maternal age (table 10.4). Likewise, controlling for the effect of maternal body mass on weanling mass with partial correlation and residual analyses demonstrated no effect of age independent of maternal mass (table 10.4). The outlier mentioned above may reflect milk stealing by the pup, a behavior that confounds measurements of maternal investment that rely on pup energy gain (see appendix 10.2).
Summary: Effects of Maternal Mass, Age, and Pup Sex on Female RE . Larger mothers invested absolutely but not relatively more in offspring during lactation than smaller mothers. Female RE during gestation, as reflected in relative neonatal mass, was negatively correlated with maternal age and mass. Changes in absolute measures of maternal investment with age generally tracked age-specific changes in body mass, increasing from age 3 to 6 and then reaching a plateau. Age had no discernible effect on any index of female RE (including gestation and lactation components) when the effect of maternal mass was controlled statistically. With the exception of female mass loss rate, all other indexes of maternal investment did not vary significantly with pup sex.
Male Reproductive Effort in Relation to Age, Mass, and Dominance Rank
For all ages combined (N = 71 individuals), male elephant seals lost a mean of 0.37 ± 0.08% of their arrival mass per day and 33.1 ± 7.0% of their
mass over the three-month breeding fast. Daily and seasonal mass loss increased significantly with body mass at arrival (rs = .78, N = 75 measurements, p < .0001; rs = .82, N = 75, p < .0001, respectively), with standard length (rs = .65, N = 87, p < .0001; rs = .61, N = 75, p < .0001, respectively), and with age class (table 10.5), as expected. On a mass-specific basis, however, breeding mass loss was weakly correlated with body mass (daily: rs = .21, N = 75, p = .08; seasonal: rs = .31, N = 75, p < .01) and was not correlated wlth length (daily: rs = .04, N = 75, p = .71; seasonal: rs = .05, N = 75, p = .71) or age (table 10.5). The lack of an age effect for SA3 and older classes was confirmed with an ANCOVA that showed no significant age-related variation in daily mass loss (F = 1.335, df = 2, 67, p = .27) or seasonal mass loss (F = 1.694, df = 2, 67, p = .19) when controlling for the effect of arrival mass; similar results were obtained using length as the covariate. Note that the above positive correlations between measures of mass loss and arrival mass are probably inflated due to the autocorrelation of measurement errors (i.e., an overestimate of arrival mass would cause an overestimate of both absolute and relative mass loss). Length was measured with greater accuracy than mass and showed no correlation with daily or seasonal RE (i.e., percent mass loss).
The mean duration of stay at the Año Nuevo rookery was 91.0 ± 10.9 days (range = 51–109, N = 71, excluding the SA2 male) and did not vary significantly with age among the SA3 to adult classes (table 10.5). This value may be somewhat high because our sample was probably biased against seals that departed early and were therefore less likely to be weighed or photographed a second time. There is no reason to believe that such a bias would vary with age class; Clinton (this volume) showed that average breeding haul-out duration remains fairly constant from age 6 onward.
There is a dramatic increase in male mating effort from 4 to 6 years of age, as demonstrated by the following behavioral measures: increasing duration of stay on the rookery; shifting of the haul-out period so that it overlaps the estrous period; and increasing time spent in proximity to harems (Cox 1983; Clinton 1990; Deutsch 1990; see fig. 9.1, chap. 9). Only one pubescent male (SA2 class) was weighed in this study, and he lost 27% of his mass over a 60-day period (table 10.5). Multiplying the average haul-out duration for the SA2 class (Clinton 1990: a minimum mean of 27 ± 20 days, N = 53; Deutsch 1990: mean of 56 ± 12 days, N = 6) by the mass-specific rate of mass loss for the weighed SA2 male (table 10.5) yields an estimate of 12 to 25% of arrival mass lost over the breeding fast for this age group. This suggests that young subadults devote a smaller proportion of their body stores to reproductive activity, compared to older males, by fasting for a shorter period on the rookery.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The highest levels of RE that we measured were exhibited by some adult males that lost nearly half of their body mass over the breeding season (maximums of 46.3% for a weighed animal and 50.5% for a photographed animal). This was considerably greater than the highest RE shown by younger males (SA3: 38.5%, weight; 35.1%, photograph; SA4: 38.8%, weight; 35.0%, photograph), perhaps because adults had the opportunity to achieve much higher mating success than subadults. The difference in sample size between the age groups provides an alternative explanation that cannot be completely ruled out, but it is unlikely that subadults ever lose much over 40% of their mass.
What can account for the twofold variation in RE among adult males? Total percent mass loss over the breeding season was slightly but not significantly higher for old adult males than for young adults (table 10.6). Age is positively correlated with dominance rank and mating success in males (Le Boeuf 1974; Le Boeuf and Reiter 1988; Clinton and Le Boeuf 1993), and the tendency for older bulls to invest more in reproduction, can be attributed to their higher rank. Although sample sizes were small, table 10.6 shows that the RE of young and old adults was quite similar for a given rank or mating success class. Males in the top third of the dominance hierarchy lost a significantly greater proportion of their arrival mass over the season (36.0 ± 7.8%, N = 23) than did bulls in the middle third (32.2 ± 5.8%, N = 18) and the bottom third of the hierarchy (32.9 ± 6.9%, N = 9) (p < .05, table 10.6). Likewise, males that achieved high mating success (ENFI > 15) devoted a greater effort to reproduction (36.3 ± 6.1% of mass lost, N = 14) compared to adults with moderate sexual success (ENFI of 5–15; 32.6 ± 9.1%, N = 13) or to those who copulated relatively few times (ENFI < 5; 33.8 ± 6.5%, N = 24). The difference in percent mass loss between males with high mating success (median ENFI = 28.8) and those with low to moderate success (median ENFI = 2.0) was not significant, however (p = .098, table 10.6).
Summary:
Effects of Age Class and Dominance Status on Male RE
Error inherent in the photogrammetric measurement of body mass created considerable variation in mass loss variables among individuals, which made it more difficult to detect the real effects of potential factors such as age or dominance status on male mating effort. Nevertheless, the following patterns stood out. 1. The energetic component of male RE increased from age 5 (SA2) to age 6 (SA3), due to increased time spent on the rookery, and then remained constant to adulthood; preliminary data indicate no effect of age on RE of adults. 2. Sexually successful, high-ranking males lost a greater proportion of body mass than did subordinate adults and subadults, but the difference in RE was small compared to the discrepancy in mating success.
| ||||||||||||||||||||||||||||||||||||||||
Discussion
Comparison of Reproductive Effort between the Sexes
This study reveals important similarities and differences in the magnitude and timing of reproductive effort between male and female northern elephant seals. The most striking similarity is in the energetic index of RE, adults of both sexes losing slightly more than one-third of their body mass, on average, while on the breeding rookery. This finding suggests that a common physiological mechanism, perhaps related to changing body composition or increasing protein catabolism (Groscolas 1986; Cherel, Robin, and Le Maho 1988), may underlie the termination of the breeding fast in both sexes.
There is no a priori reason why male and female RE should be equivalent, since the factors affecting reproductive success differ so widely between the sexes in elephant seals. In males, mating success achieved for a given effort depends on the number, competitive ability, and RE of other males on the rookery (Trivers 1972; Warner 1980). Intense male intrasexual com-
petition for mates has been a strong selective force favoring continued growth and delayed maturity, thereby molding the level and developmental timing of mating effort. Consequently, young male elephant seals invest little in reproduction and much in increased growth during the first two or three years that same-age females are pupping, a common phenomenon in polygynous species (Selander 1972; Clutton-Brock, Guinness, and Albon 1982). Compared to males, competitive social interactions exert less influence on female RS (see Reiter, Panken, and Le Boeuf 1981) and, therefore, on the magnitude and ontogeny of maternal effort. Natural selection favors an investment pattern in females that optimizes the opposing effects of energy transfer to the pup on maternal and offspring fitness, relationships that are affected by fasting physiology, foraging ecology, thermoregulatory requirements at sea, and other ecological factors.
Data on mass change for four other phocid species allow comparison of RE between the sexes. The mean percentage of body mass lost over lactation is in the range of 30 to 40% for females of all five species (table 10.7). Breeding mass loss was similar for the two sexes in both species of elephant seals but higher for females than males in gray, Weddell, and harbor seals (table 10.7). The energetic cost of reproduction in the sexually monomorphic harp seal, Phoca groenlandica , appears to be much greater for females than males based on changes in blubber thickness over the breeding season (Sergeant 1973). These data suggest that only in the most polygynous seal species does male mating effort approach the level of female parental effort in terms of energetic costs.
Energy allocation to reproduction (i.e., breeding activity, gonads, gametes, and young) is far greater in female golden-mantled ground squirrels, Spermophilus saturatus , than in males (Kenagy 1987; Kenagy, Sharbaugh, and Nagy 1989), and this is probably the case for most mammals. But is this the best measure of RE? Our use of relative mass loss as an index of RE in fasting pinnipeds included energy devoted to maintenance metabolism while breeding, and so it was not a measure of reproductive energy expenditure per se. Nevertheless, since elephant seals and some other pinniped species must fast in order to breed (see Riedman 1990), it is reasonable to include this maintenance metabolic expenditure as part of the total energy cost of reproduction. Because the mating strategy of males in polygynous species often entails reduced food intake during a period of high activity, males may deplete their energy reserves to a greater extent during the mating period than females do during pregnancy and lactation (e.g., Leader-Williams and Ricketts 1981; Michener and Locklear 1990), even though actual reproductive energy expenditure may be much higher in females. Thus, we consider percent mass loss (an index of percent energy loss) while breeding to be a more appropriate measure of RE in fasting seals than energy allocation to strictly reproductive functions.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Differences in the pattern of reproductive effort between male and female elephant seals are related to their divergent reproductive strategies, as follows:
1. Rate of investment in reproduction, measured as mass-specific daily mass loss, was over three times higher for females than males. This has the effect of reducing the metabolic overhead costs of females relative to males. About 40% of the energy lost by females during lactation is utilized for maintenance and activity metabolism, the rest being transferred to the pup as milk (Costa et al. 1986). On the con-
trary, most of the energy males expend during the breeding season is probably allocated to maintenance metabolism since 85 to 95% of their time is spent resting (Sandegren 1976; Deutsch 1990).
2. Duration of RE was three times longer for males than females, reflecting the importance of early male arrival to obtaining high rank and of a lengthy stay to maximize chances for copulation. A consequence of this longer effort is that males had two fewer months than females to recover lost energy stores and to gain additional mass before the next breeding season. Of course, females invest energy in gestation for eight months of the year, which is not reflected in these comparisons (see Deutsch, Haley, and Le Boeuf 1990).
3. Variation in RE was greater among males than females. Male mating effort was quite flexible, as evidenced by the more than twofold variation in duration of the breeding fast and by great variability in measures of the intensity of effort, such as the proportion of time spent competing near harems (Deutsch 1990) and mass-specific rate of mass loss (Deutsch, Haley, and Le Boeuf 1990). Consequently, some young subordinate males invested considerably less than any female did, and the RE of some high-ranking males soared well above the maximum for females. In contrast, three observations indicate that flexibility in adjusting maternal effort during lactation is limited by energetic considerations. First, mothers that invested at a higher relative rate weaned their pups sooner, suggesting the existence of a cost ceiling. Second, females that lose their pups and therefore invest relatively little energy in lactation remain on the rookery longer than those who rear pups successfully (Reiter, Panken, and Le Boeuf 1981). Third, a 3-year-old mother ("Bat") who nursed two pups simultaneously lost mass at a rate similar to other females of her size (observed = 6.35 kg/day; expected = 6.25 kg/day), and the pups gained mass at a combined rate similar to single pups (observed = 3.46 kg/day; expected = 3.62 kg/day). The minimum RE of parous females would have been lower than 30% mass loss if we had included those that lost their pups and did not nurse. But since 90 to 95% of females pupping on the Año Nuevo mainland are successful in raising their pups to normal weaning age (B. Le Boeuf, unpubl. data), including unsuccessful mothers in our sample would not have altered the above finding.
This sex difference in the variance in RE mirrors the great difference in the variance in reproductive success between the sexes (Le Boeuf and Reiter 1988). Females must invest some minimum RE to produce a healthy offspring, whereas males that invest even less effort have some chance of siring a pup. Extremely high levels of maternal effort (over 40% of body mass
lost) probably benefit a pup relatively little compared to the future costs incurred by the mother, but alpha males can raise their current RS substantially through continued high mating effort. Thus, the sex difference in RE variation reflects the fundamentally different means by which male and female elephant seals maximize their RS.
Reproduction can be a risky activity, sometimes even leading to death (e.g., Wilkinson and Shank 1976; Brunton 1986), so a complete sex comparison must also examine this facet of RE. Frequency of external wounds, the measure of risk used in this study, was many times greater in males than females, reflecting the violent competition among males for high rank and access to females. Similar sex differences in frequency of injury have been found in other polygynous mammals (e.g., Smith 1966; Michener and Locklear 1990). We note, however, that the observed injury rate for females may be an underestimate because they were sampled in harems, but they are most likely to get injured during departure from the rookery. The risk of receiving a lethal injury during agonistic or sexual interactions is relatively small for both sexes at Año Nuevo; female mortality due to male-inflicted injuries (0.1% per year; Le Boeuf and Mesnick 1991) appears to be about twice as high as that for males (Deutsch 1990; C. Deutsch and B. Le Boeuf, unpubl. data). Female elephant seals probably incur a higher incidence of fatal injuries than males because of the large sex difference in mass, the large canines of males, the bites to the female's neck during typical sexual behavior, and the emaciated condition of the female on departure, when she is exposed to mating attempts by aggressive and sexually motivated peripheral bulls (Mesnick and Le Boeuf 1991).
Differences in life history parameters between the sexes may be due to differences in the timing and magnitude of RE. In Richardson's ground squirrels, S. richardsonii , for example, males deplete their fat stores to a greater degree during the mating period than females do during lactation, and this is associated with higher male mortality (Michener and Locklear 1990). The authors conclude that the costs of mating effort for males are greater than the costs of parental effort for females in this species. This pattern is taken to the extreme by the marsupial shrew, Antechinus stuartii , in which all males die soon after the mating season, whereas females are iteroparous (Lee, Wooley, and Braithwaite 1982). In northern elephant seals, the survivorship curves are roughly similar for both sexes (see fig. 22.2 in Le Boeuf and Reiter 1988), although longevity is somewhat greater for females. This is consistent with the similar magnitude of RE for the sexes found in this study.
Age-specific Reproductive Effort and Fitness Consequences
Variation in the energetic component of RE was not associated with age for males 6 years and older or for parous females. This finding, based on mass
loss, is corroborated by complementary data showing no correlation between mass-specific energy expenditure and milk energy output of lactating females and age (Crocker 1992; D. Crocker, unpubl. data) and no age-related variation in male time-activity budgets (Deutsch 1990). Unfortunately, very old females, with the lowest reproductive value and the highest predicted RE, were not included in our sample, but a follow-up study (D. Crocker, unpubl. data) found that three 11- to 12-year-old mothers lost a similar proportion of their body mass over lactation (range = 31–34%) as younger mothers. W. J. Sydeman et al. (1991) suggested that age-specific increases in female aggressiveness, success in dominance interactions, and weaning success could be attributed to increasing maternal effort with age. The energetic data do not support this interpretation; instead we propose that the increase in size and experience with age accounts for the higher dominance status and weaning success of older mothers. Even though the proportion of resources allocated to reproduction did not vary with age, it is still possible that younger, smaller seals incurred a greater risk by depleting their fat reserves to a lower, perhaps marginal, level than larger animals, as R. E. A. Stewart (1986) found for female harp seals. This was apparently not the case in elephant seals, however, since females weaned their pups on reaching 21 to 25% body fat, regardless of maternal age, parturition mass, or initial body composition (Crocker 1992; D. Costa and B. Le Boeuf, unpubl. data). Female investment in offspring was in direct proportion to maternal mass at parturition. This is similar to the pattern of maternal investment in southern elephant seals (McCann, Fedak, and Harwood 1989; Fedak et al., this volume) but unlike harp seals, in which small, young mothers produce weanlings of the same size and lose mass at the same rate as larger, older mothers (Stewart 1986; Kovacs, Lavigne, and Innes 1991).
The fitness costs of reproduction (e.g., reduced survival) should be positively related to the proximate energetic costs (i.e., RE), though probably in a nonlinear fashion, and this relationship should be manifested in the age-specific patterns of RE and mortality. This expectation is partially supported by the fact that age-specific mortality rates rise sharply upon the initiation of reproduction—at primiparity for females (age 3–4; Reiter and Le Boeuf 1991) and at puberty for males (age 5; Clinton, this volume). For females, this survival cost is associated with lactation, since mortality remains low during the year of their first pregnancy. Similarly, Clutton-Brock, Albon, and Guinness (1989) concluded that the fitness costs of reproduction in red deer, Cervus elaphus , are attributable to the energetic demands of lactation and not to gestation.
Two lines of evidence led us to expect the RE of reproductively active but physically immature "subadults" (i.e., 3- to 5-year-old females and 5- to 7-year-old males) to be less than that of physically mature animals. First, RS increases with age in both sexes, especially from puberty to physical
maturity, due largely to the effects of intrasexual competition (see references given on p. 173). Young small females obtain a lower benefit (in terms of weaning success and weanling mass) for the same proximate cost (relative mass loss) as older, larger mothers. Even more striking is the fact that subadult males obtain little immediate benefit for the same mass-specific cost as low-ranking adult males; only 6 to 15% of 6- to 7-year-olds copulate (Clinton 1990). Second, a given RE seems to impose a greater strain on the younger breeders of both sexes, manifested as higher fitness costs. Females giving birth at age 3 suffer reduced survivorship and future fecundity relative to those delaying reproduction to age 4 or 5 (Huber 1987; Reiter and Le Boeuf 1991; but see Sydeman and Nur, this volume). Current mating success is negatively correlated with subsequent survival and fecundity for young males (6–8 years old) but not for older males (Clinton and Le Boeuf 1993; Clinton, this volume). Also, C. R. Cox (1983) found that 6-year-old males that spend a greater amount of time on the breeding beach suffer higher mortality than those exhibiting lower effort. The higher fitness costs incurred by young breeders may result from the simultaneous allocation of energy to growth and reproduction, leaving less available for storage or critical maintenance functions.
Given that benefits of reproductive investment increase and fitness costs decrease with age during the period between sexual and physical maturity, selection should favor a relatively low level of RE for "subadult" animals. This probably explains why most females delay primiparity beyond age 3 (Huber 1987; Reiter and Le Boeuf 1991) and why pubescent males (4–5 years old) invest little time or energy in mating attempts (Clinton 1990; Deutsch 1990), despite the theoretical benefits of early breeding (Sibly and Calow 1986). Still, the pattern of constant mean RE with age for parous females and for males older than 5 years is perplexing. Theoretically, the optimal level of investment at each age is determined by the age-specific functions relating RE to fitness benefits (i.e., current RS) and to fitness costs (i.e., reduction in residual reproductive value). These functions are extremely difficult to determine empirically, and few data are available (Charlesworth 1980). It is noteworthy, however, that a constant age-specific RE is theoretically plausible, at least among adults, as stated by B. Charlesworth (ibid. 246): "It is not difficult, in principle, to find cases in which an optimal life-history exists which has constant reproductive effort among the adult age-classes, and hence constant adult survival and fecundity."
One could reasonably argue that a small, young female must expend a relatively large proportion of her energy stores during lactation to produce a weanling that is large enough to survive well (see Fedak et al., this volume). The point at which survival from weaning to age 2 appears to drop off is at a weaning mass of only 80 to 90 kg (Le Boeuf, Morris, and
Reiter, this volume). A hypothetical young mother of 400 kg would produce a 34-kg newborn (from fig. 10.3) and nurse it for 23 to 26 days with a mass transfer efficiency of 63% (from table 10.3). This female would lose 105 kg, or only 26% of her parturition mass, to produce a 100-kg weanling. Clearly, young mothers invest substantially more (36% of body mass) than is required to produce a viable pup. So this argument can probably be rejected; but the future fecundity of offspring may be enhanced by maternal investment above what is necessary for offspring survival. We offer three other nonexclusive explanations that may account for the lack of age-related variation in RE.
One explanation is that the seals are able to tolerate moderate energy deficiencies up to some threshold level, and only when that threshold is exceeded does a cost of reproduction become apparent (Tuomi, Hakala, and Haukioja 1983). Large fluctuations in body mass (e.g., loss of one-third mass) are a normal part of the elephant seal's annual cycle (Costa et al. 1986; McCann, Fedak, and Harwood 1989; Deutsch, Haley, and Le Boeuf 1990; Kretzmann, Costa, and Le Boeuf 1993; Worthy et al. 1992) and may entail little or no fitness costs in healthy animals. While costs associated with additional increments of investment above a critical level are likely to escalate (Burley 1988), the benefits should approach an asymptote, particularly for females. In fact, the survival benefit of large weanling mass is equivocal (Le Boeuf, Morris, and Reiter, this volume). Crocker (1992) postulated a physiological mechanism, based on changing body composition and rate of protein catabolism, that may account for a threshold relationship between maternal investment and fitness costs. Circumstantial evidence for such a threshold phenomenon includes the fact that female body fat at the end of lactation lies in a narrow range, despite the large variation in initial fat stores, in lactation duration, in absolute rates of mass loss, and in rates of lean and adipose tissue loss (Crocker 1992; see also Gales and Burton 1987). An animal exceeding the hypothesized threshold level of RE might be faced with the choice of either depleting its blubber layer beyond what is necessary for thermoregulation at sea or oxidizing muscle and other lean tissue. While this cost-based explanation is plausible, the trade-off between reproduction and growth (e.g., Robinson and Doyle 1985; Green and Rothstein 1991) would seem difficult to avoid, and so attempting to breed as a growing subadult could reduce size and, hence, fecundity as an adult (Clinton 1990; Haley, Deutsch, and Le Boeuf, in press). Furthermore, the evidence of a trade-off between reproduction and survival for young animals also argues strongly for the existence of substantial benefits, either immediate or delayed, to early reproductive attempts.
The second possibility draws on the idea that in expanding populations the fitness benefit of early-born offspring is greater than that of late-born young, thus favoring early maturity and declining RE with age (Lewontin
1965; Charlesworth and León 1976). This may partially explain why females mature at a relatively young age in M. angustirostris and in exploited populations of M. leonina (Carrick et al. 1962), although the costs of early reproduction would appear to outweigh the benefits under the prevailing demographic conditions (Huber 1987; Reiter and Le Boeuf 1991). The hypothesized effect seems too small, however, to compensate for the extremely low mating success of subadult males, unless we have grossly underestimated their success. Who mates with virgin females and where this occurs is poorly known (Huber et al. 1991) and could potentially affect our interpretation. Overall, we think that this can be a partial explanation at best.
The final explanation for the unexpectedly high RE among physically immature seals is that experience gained when young improves future reproductive performance (Caro 1988; Robinson 1988). The benefits of early maternal experience to subsequent weaning success have been documented in elephant seals (Reiter, Panken, and Le Boeuf 1981; Sydeman et al. 1991). C. J. Deutsch (1990) presents circumstantial evidence for the importance of fighting and sexual experience to males; this includes the observation that subadult males engage in frequent mock-fighting activity in contexts in which no immediate reproductive benefit is possible (e.g., during the molt period). Even male weanlings exhibit behaviors during social interactions that resemble the fighting behavior of adult males (Rasa 1971; Reiter, Stinson, and Le Boeuf 1978), suggesting that early social experience plays an important role in reproducing successfully later in life (Fagen 1981). This raises the interesting question of whether such social play among juvenile males should be considered a form of early RE with delayed benefits that has evolved via sexual selection on adult males.
In conclusion, we found that the magnitude of reproductive effort in northern elephant seals, as estimated by relative mass loss, was similar for males and females but that the temporal patterning of effort differed between the sexes both within a breeding season and over development. The similarity in RE between the sexes was unexpected, given the large size dimorphism, divergent reproductive strategies, and the generally held view that female mammals invest more in reproduction than males. RE did not vary with age in males (older than 5 years) or parous females, despite an increase in reproductive success and a decline in the fitness costs of breeding with age for both sexes. Future studies should investigate whether the substantial interindividual variation in RE is related to the amount of energy stores available at the start of breeding, thus potentially linking foraging success at sea with reproductive success on land.
Acknowledgments
Numerous students and colleagues assisted in fieldwork over the decade covered by this study, and to them we are very grateful. We also thank
M. Haley and J. Williams for their collaboration in the mass loss studies; K. Richer for collecting data on male injuries; M. Kretzmann for contributing data on pup mass and mass change; and the rangers at Año Nuevo State Park for their cooperation in the field. We are grateful to S. Blackwell, M. Bryden, R. Gentry, G. Kooyman, and J. Reiter for making helpful comments on earlier versions of this manuscript. The research was funded in part by the National Science Foundation and by the Biology Board of Studies and the Institute of Marine Sciences at the University of California, Santa Cruz.
Appendix 10.1
Calculation of Body Mass and Mass Loss
Body Mass at Arrival (Males) or Parturition (Females)
Calculated by using measured rates of mass loss for each animal to extrapolate from date of first weighing (initial mass) back to arrival date (males) or parturition date (females). For 14 males weighed (directly or photogrammetrically) only at the start of the breeding fast, initial mass was extrapolated to arrival date using the regression between mass loss per day (y) and length (where x = 0.9* standard length): y = –86.87 + 91.42x – 31.85x2 + 3.84x3 (r2 = .68, N = 28, p < .0001; Deutsch, Haley, and Le Boeuf 1990). For 12 females weighed only at the start of lactation, initial mass was extrapolated to parturition date using the regression between mass loss per day (y) and initial mass (x): y = 1.058 + 0.01306x (r2 = .62, N = 22, p < .0001). All units of mass are given in kg; length is given in meters.
Body Mass at Departure from the Rookery (Both Sexes)
Calculated by using measured rates of mass loss for each animal to extrapolate from date of last weighing (final mass) forward to departure date. For 31 males weighed only at the end of the breeding fast, final mass was extrapolated to departure date using the regression above. For 40 females weighed only near the end of lactation, the corresponding calculation was based on the regression between mass loss per day (y) and final mass (x): y = 1.486 + .01797x (r2 = .52, N = 22, p = .0002). Similar mass estimates could have been obtained for these 40 females (and the 12 above) using standard length to estimate mass loss rate (r2 = .57, N = 22, p < .0001).
Total Mass Loss over the Breeding Season (Males) or the Lactation Period (Females)
Equals estimated arrival/parturition mass minus estimated departure mass for animals weighed two or more times over the season. Females weighed twice over an interval of less than 18 days were excluded from mass loss analyses. Total mass loss expressed as a percentage of arrival/parturition mass was used as an index of the energetic component of RE.
Pup Mass at Birth
Since different pups were initially weighed from 0 to 5 days postpartum, we standardized pup mass by estimating mass at birth. Initial pup mass (kg) was regressed against pup age (days) for 41 pups 0 to 5 days of age, yielding the following linear regression: Initial mass = 36.9 + 2.2 (age); r2 = .29. For pups 0 to 1 day old, birth mass was taken to equal initial mass. For pups 2 to 5 days old, a mass gain of 2.2 kg/day was assumed, starting at day 1; this amount was subtracted from initial mass to obtain estimated birth mass. Since other studies typically report initial mass and since mass gain of pups can be quite variable during the first week (McCann, Fedak, and Harwood 1989), we also report initial mass.
Pup Mass at Weaning
Pups were usually weighed within a few days of weaning. If weighed before weaning, measured mass gain per day for each pup was used to extrapolate from date of final weighing to weaning date. If weighed after weaning, the following equation was used to estimate mass at weaning since weanlings fast and lose mass:
Mass at weaning = Measured mass·(e k·d ),
where k = .00596 and d = number of days between weaning and weighing (modified from Ortiz, Costa, and Le Boeuf 1978; P. M. Morris, pers. comm.). Pups weighed more than 10 days after weaning were excluded from analysis.
Total Pup Mass Gain
Equals estimated mass at weaning minus estimated mass at birth.
Appendix 10.2
Comments on an Outlier for Pup Mass Gain
The outlier was a male pup of a 424 kg, 3-year-old mother ("G22") and deserves special mention because it illustrates some of the problems inherent in interpreting this type of data. Values for this individual lie well outside the expected range for pup mass gain and weaning mass (fig. 10.3g–j). There are at least three possible explanations for this finding: (1) high maternal investment; (2) high efficiency of mass transfer; and/or (3) milk stealing by the pup from other females. G22 gave birth to an exceptionally large pup (10 kg heavier than expected for her size; fig. 10.3e, f), and she lost 41.2% of her parturition mass, the highest value measured for a female. This large mass loss was due primarily to an unusually long lactation period (27 days) for a 3-year-old. Even taking G22's high RE into consideration, however, the pup was weaned about 30 kg heavier than expected. This fact, plus the suspiciously high mass transfer efficiency of 77% (range was 45–67% for the rest), suggests that the pup stole milk from neighbor-
ing females during the nursing period, although this was not observed. For this reason, maternal mass or energy loss is probably a more reliable index of investment than pup mass or energy gain.
References
Alexander, R. D., and G. Borgia. 1979. On the origin and basis of the male-female phenomenon. In Sexual Selection and Reproductive Competition in Insects , ed. M. S. Blum and N. A. Blum, 417–440. New York: Academic Press.
Anderson, S. S., and M. A. Fedak. 1985. Grey seal males: Energetic and behavioural links between size and sexual success. Animal Behaviour 33: 829–838.
———. 1987. Grey seal, Halichoerus grypus , energetics: Females invest more in male offspring. Journal of Zoology, London 211: 667–679.
Bartsh, S. S., S. D. Johnston, and D. B. Siniff. 1992. Territorial behavior and breeding frequency of male Weddell seals (Leptonychotes weddellii ) in relation to age, size, and concentrations of serum testosterone and cortisol. Canadian Journal of Zoology 70: 680–692.
Bell, G. 1980. The costs of reproduction and their consequences. American Naturalist 116: 45–76.
Bell, G., and V. Koufopanou. 1985. The costs of reproduction. Oxford Surveys in Evolutionary Biology 3: 83–131.
Boness, D. J., W. D. Bowen, and O. T. Oftedal. In press. Evidence of a maternal foraging cycle resembling that of otariid seals in a small phocid, the harbor seal. Behavioral Ecology and Sociobiology .
Bowen, W. D., D. J. Boness, and O. T. Oftedal. 1987. Mass transfer from mother to pup and subsequent mass loss by the weaned pup in the hooded seal, Cystophora cristata. Canadian Journal of Zoology 65: 1–8.
Bowen, W. D., O. T. Oftedal, and D. J. Boness. 1992. Mass and energy transfer during lactation in a small phocid, the harbor seal (Phoca vitulina ). Physiological Zoology 65: 844–866.
Bowen, W. D., and W. T. Stobo. 1991. Reproduction in primiparous and young multiparous grey seal females: Is it successful? In Proceedings of the Ninth Biennial Conference on the Biology of Marine Mammals , December 5–9, 1991, Chicago, 9 (Abstract).
Boyce, M. S. 1981. Beaver life-history responses to exploitation. Journal of Applied Ecology 18: 749–753.
Boyd, I. L., and C. D. Duck. 1991. Mass changes and metabolism in territorial male Antarctic fur seals (Arctocephalus gazella). Physiological Zoology 64: 375–392.
Boyd, R., and J. B. Silk. 1983. A method for assigning cardinal dominance ranks. Animal Behaviour 31: 45–58.
Brunton, D. H. 1986. Fatal antipredator behavior of a killdeer. Wilson Bulletin 98: 605–607.
Burley, N. 1988. The differential-allocation hypothesis: An experimental test. American Naturalist 132: 611–628.
Calow, P. 1979. The cost of reproduction—A physiological approach. Biological Review 54: 23–40.
Campagna, C., B. J. Le Boeuf, M. Lewis, and C. Bisioli. 1992. The Fisherian seal: Equal investment in male and female pups in southern elephant seals. Journal of Zoology, London 226: 551–561.
Caro, T. M. 1988. Adaptive significance of play: Are we getting closer? Trends in Ecology and Evolution 3: 50–54.
Carrick, R., S. E. Csordas, S. E. Ingham, and K. Keith. 1962. Studies on the southern elephant seal, Mirounga leonina (L.). IV. Breeding and development. CSIRO Wildlife Research 7: 161–197.
Caswell, H. 1982. Optimal life history and the age-specific costs of reproduction. Journal of Theoretical Biology 98: 519–529.
Charlesworth, B. 1980. Evolution in Age-Structured Populations . Cambridge: Cambridge University Press.
Charlesworth, B., and J. A. León. 1976. The relation of reproductive effort to age. American Naturalist 110: 449–459.
Cherel, Y., J.-P. Robin, and Y. Le Maho. 1988. Physiology and biochemistry of long-term fasting in birds. Canadian Journal of Zoology 66: 159–166.
Clinton, W. L. 1990. Sexual selection and the life-history of male northern elephant seals. Ph.D. dissertation, University of California, Santa Cruz.
Clinton, W. L., and B. J. Le Boeuf. 1993. Sexual selection's effects on male life history and the pattern of male mortality. Ecology 74: 1884–1892.
Clutton-Brock, T. H. 1984. Reproductive effort and terminal investment in iteroparous animals. American Naturalist 123: 212–229.
Clutton-Brock, T. H., S. D. Albon, R. M. Gibson, and F. E. Guinness. 1979. The logical stag: Adaptive aspects of fighting in red deer (Cervus elaphus L.). Animal Behaviour 27: 211–225.
Clutton-Brock, T. H., S. D. Albon, and F. E. Guinness. 1989. Fitness costs of gestation and lactation in wild mammals. Nature 337: 260–262.
Clutton-Brock, T. H., F. E. Guinness, and S. D. Albon. 1982. Red Deer: Behavior and Ecology of Two Sexes . Chicago: University of Chicago Press.
Cody, M. L. 1971. Ecological aspects of reproduction. In Avian Biology , vol. 1, ed. D. S. Farner and J. R. King, 461–512. New York: Academic Press.
Cooper, C. F., and B. S. Stewart. 1983. Demography of northern elephant seals, 1911–1982. Science 219: 969–971.
Costa, D. P., B. J. Le Boeuf, C. L. Ortiz, and A. C. Huntley. 1986. The energetics of lactation in the northern elephant seal. Journal of Zoology, London 209: 21–33.
Cox, C. R. 1983. Reproductive behaviour of sub-adult elephant seals: The cost of breeding. In Behavioral Energetics: The Cost of Survival in Vertebrates , ed. W. P. Aspey and S. I. Lustick, 89–115. Columbus: Ohio State University Press.
Crocker, D. E. 1992. Reproductive effort and age in female northern elephant seals. Master's thesis, University of California, Santa Cruz.
Deutsch, C. J. 1990. Behavioral and energetic aspects of reproductive effort in male northern elephant seals (Mirounga angustirostris ). Ph.D. dissertation, University of California, Santa Cruz.
Deutsch, C. J., M. P. Haley, and B. J. Le Boeuf. 1990. Reproductive effort of male northern elephant seals: Estimates from mass loss. Canadian Journal of Zoology 68: 2580–2593.
Fagen, R. 1981. Animal Play Behavior . London: Oxford University Press.
Fedak, M. A., and S. S. Anderson. 1982. The energetics of lactation: Accurate measurements from a large wild mammal, the grey seal (Halichoerus grypus ). Journal of Zoology, London 198: 473–479.
———. 1987. Estimating the energy requirements of seals from weight changes. In Approaches to Marine Mammal Energetics , ed. A. C. Huntley, D. P. Costa, G. A. J. Worthy, and M. A. Castellini, 205–226. Lawrence, Kan.: Allen Press.
Gadgil, M., and W. H. Bossert. 1970. Life historical consequences of natural selection. American Naturalist 104: 1–24.
Gales, N. J., and H. R. Burton. 1987. Ultrasonic measurement of blubber thickness of the southern elephant seal, Mirounga leonina (Linn.). Australian Journal of Zoology 35: 207–217.
Gittleman, J. L. 1986. Carnivore life-history patterns: Allometric, phylogenetic, and ecological associations. American Naturalist 127: 744–771.
Gittleman, J. L., and S. D. Thompson. 1988. Energy allocation in mammalian reproduction. American Zoologist 28: 863–875.
Glucksman, A. 1974. Sexual dimorphism in mammals. Biological Review of Cambridge Philosophical Society 49: 423–475.
Green, W. C. H. 1990. Reproductive effort and associated costs in bison (Bison bison ): Do older mothers try harder? Behavioral Ecology 1: 148–160.
Green, W. C. H., and A. Rothstein. 1991. Trade-offs between growth and reproduction in female bison. Oecologia 86: 521–527.
Groscolas, R. 1986. Changes in body mass, body temperature and plasma fuel levels during the natural breeding fast in male and female emperor penguins, Aptenodytes forsteri. Journal of Comparative Physiology 156B: 521–527.
Haley, M. P., C. J. Deutsch, and B. J. Le Boeuf. 1991. A method for estimating mass of large pinnipeds. Marine Mammal Science 7: 157–164.
———. In press. Size, dominance and copulatory success in male northern elephant seals, Mirounga angustirostris. Animal Behaviour .
Hamer, K. C., and R. W. Furness. 1991. Age-specific breeding performance and reproductive effort in great skuas, Catharacta skua. Journal of Animal Ecology 60: 693–704.
Hammill, M. O., C. Lydersen, M. Ryg, and T. G. Smith. 1991. Lactation in the ringed seal (Phoca hispida). Canadian Journal of Fisheries and Aquatic Science 48: 2471–2476.
Härkönen, T., and M.-P. Heide-Jørgensen. 1990. Comparative life histories of East Atlantic and other harbour seal populations. Ophelia 32: 211–235.
Hill, S. E. B. 1987. Reproductive ecology of Weddell seals (Leptonychotes weddelli ) in McMurdo Sound, Antarctica. Ph.D. dissertation, University of Minnesota, Minneapolis.
Hirshfield, M. F., and D. W. Tinkle. 1975. Natural selection and the evolution of reproductive effort. Proceedings of the National Academy of Science, USA 72: 2227–2231.
Huber, H. R. 1987. Natality and weaning success in relation to age of first reproduction in northern elephant seals. Canadian Journal of Zoology 65: 1311–1316.
Huber, H. R., A. C. Rovetta, L. A. Fry, and S. Johnston. 1991. Age-specific natality of northern elephant seals at the South Farallon Islands, California. Journal of Mammalogy 72: 525–534.
Iverson, S. J., W. D. Bowen, D. J. Boness, and O. T. Oftedal. 1993. The effect of maternal size and milk energy output on pup growth in grey seals (Halichoerus grypus). Physiological Zoology 66: 61–88.
Kenagy, G. J. 1987. Energy allocation for reproduction in the golden-mantled ground squirrel. Symposium of Zoological Society of London 57: 259–273.
Kenagy, G. J., S. M. Sharbaugh, and K. S. Nagy. 1989. Annual cycle of energy and time expenditure in a golden-mantled ground squirrel population. Oecologia 78: 269–282.
Knapton, R. W. 1984. Parental investment: The problem of currency. Canadian Journal of Zoology 62: 2673–2674.
Kovacs, K. M., and D. M. Lavigne. 1986. Maternal investment and neonatal growth in phocid seals. Journal of Animal Ecology 55: 1035–1051.
Kovacs, K. M., D. M. Lavigne, and S. Innes. 1991. Mass transfer efficiency between harp seal (Phoca groenlandica ) mothers and their pups during lactation. Journal of Zoology, London 223: 213–221.
Kretzmann, M. B. 1990. Maternal investment and the post-weaning fast in northern elephant seals: Evidence for sexual equality. Master's thesis, University of California, Santa Cruz.
Kretzmann, M. B., D. P. Costa, and B. J. Le Boeuf. 1993. Maternal energy investment in elephant seal pups: Evidence for sexual equality? American Naturalist 141: 466–480.
Laws, R. M. 1953. The elephant seal (Mirounga leonina Linn.). I. Growth and age. Falkland Islands Dependencies Survey, Scientific Reports 8: 1–62.
Leader-Williams, N., and C. Ricketts. 1981. Seasonal and sexual patterns of growth and condition of reindeer introduced into South Georgia. Oikos 38: 27–39.
Le Boeuf, B. J. 1974. Male-male competition and reproductive success in elephant seals. American Zoologist 14: 163–176.
———. 1981. Elephant seals. In The Natural History of Año Nuevo , ed. B. J. Le Boeuf and S. Kaza, 326–374. Pacific Grove, Calif.: Boxwood Press.
Le Boeuf, B. J., R. Condit, and J. Reiter. 1989. Parental investment and the secondary sex ratio in northern elephant seals. Behavioral Ecology and Sociobiology 25: 109–117.
Le Boeuf, B. J., D. P. Costa, A. C. Huntley, and S. D. Feldkamp. 1988. Continuous, deep diving in female northern elephant seals, Mirounga angustirostris. Canadian Journal of Zoology 66: 446–458.
Le Boeuf, B. J., D. P. Costa, A. C. Huntley, G. L. Kooyman, and R. W. Davis. 1986. Pattern and depth of dives in northern elephant seals, Mirounga angustirostris. Journal of Zoology, London 208: 1–7.
Le Boeuf, B. J., and S. Kaza, ed. 1981. The Natural History of Año Nuevo . Pacific Grove, Calif.: Boxwood Press.
Le Boeuf, B. J., and S. Mesnick. 1991. Sexual behavior of male northern elephant seals. I. Lethal injuries to adult females. Behaviour 116: 143–162.
Le Boeuf, B. J., Y. Naito, A. C. Huntley, and T. Asaga. 1989. Prolonged, continuous, deep diving by northern elephant seals. Canadian Journal of Zoology 67: 2514–2519.
Le Boeuf, B. J., and J. Reiter. 1988. Lifetime reproductive success in northern
elephant seals. In Reproductive Success: Studies of Individual Variation in Contrasting Breeding Systems , ed. T. H. Clutton-Brock, 344–362. Chicago: University of Chicago Press.
Le Boeuf, B. J., M. Riedman, and R. S. Keyes. 1982. White shark predation on pinnipeds in California coastal waters. Fishery Bulletin 80: 891–895.
Lee, A. K., P. Wooley, and R. W. Braithewaite. 1982. Life history strategies of dasyurid marsupials. In Carnivorous Marsupials , vol. 1, ed. M. Archer, 1–11. Chipping Norton, NSW: Surry Beatty and Sons.
Lewontin, R. C. 1965. Selection for colonizing ability. In The Genetics of Colonizing Species , ed. H. G. Baker and G. L. Stebbins, 77–91. New York: Academic Press.
Loudon, A. S. I., and P. A. Racey, eds. 1987. Reproductive Energetics of Mammals . Symposia of the Zoological Society of London no. 57. Oxford: Oxford University Press.
Low, B. S. 1978. Environmental uncertainties and the parental strategies of marsupials and placentals. American Naturalist 112: 197–213.
McCann, T. S., M. A. Fedak, and J. Harwood. 1989. Parental investment in southern elephant seals, Mirounga leonina. Behavioral Ecology and Sociobiology 25: 81–87.
Maher, C. R., and J. A. Byers. 1987. Age-related changes in reproductive effort of male bison. Behavioral Ecology and Sociobiology 21: 91–96.
Maynard Smith, J. 1980. A new theory of sexual investment. Behavioral Ecology and Sociobiology 7: 247–251.
Mesnick, S., and B. J. Le Boeuf. 1991. Sexual behavior of male northern elephant seals. II. Female responses to potentially fatal mating encounters. Behaviour 117: 262–280.
Michener, G. R., and L. Locklear. 1990. Differential costs of reproductive effort for male and female Richardson's ground squirrels. Ecology 71: 855–868.
Millar, J. S. 1977. Adaptive features of mammalian reproduction. Evolution 31: 370–386.
Nur, N. 1984. Increased reproductive success with age in the California gull: Due to increased effort or improvement of skill? Oikos 43: 407–408.
Oftedal, O. T., D. J. Boness, and R. A. Tedman. 1987. The behavior, physiology, and anatomy of lactation in the Pinnipedia. Current Mammalogy 1: 175–245.
Oftedal, O. T., S. J. Iverson, and D. J. Boness. 1987. Milk and energy intakes of suckling California sea lion, Zalophus californianus , pups in relation to sex, growth, and predicted maintenance requirements. Physiological Zoology 60: 560–575.
Ortiz, C. L., D. P. Costa, and B. J. Le Boeuf. 1978. Water and energy flux in elephant seal pups fasting under natural conditions. Physiological Zoology 51: 166–178.
Ortiz, C. L., B. J. Le Boeuf, and D. P. Costa. 1984. Milk intake: An index of maternal investment in elephant seal pups. American Naturalist 124: 416–422.
Packard, G. C., and T. J. Boardman. 1987. The misuse of ratios to scale physiological data that vary allometrically with body size. In New Directions in Ecological Physiology , ed. M. E. Feder, A. F. Bennett, W. W. Burggren, and R. B. Huey, 216–239. Cambridge: Cambridge University Press.
Pärt, T., L. Gustafsson, and J. Moreno. 1992. "Terminal investment" and a sexual conflict in the collared flycatcher (Ficedula albicollis). American Naturalist 140: 868–882.
Pianka, E. R. 1976. Natural selection of optimal reproductive tactics. American Zoologist 16: 775–784.
Pianka, E. R., and W. S. Parker. 1975. Age-specific reproductive tactics. American Naturalist 109: 453–464.
Pugesek, B. H. 1981. Increased reproductive effort with age in the California gull (Larus californicus). Science 212: 822–823.
———. 1983. The relationship between parental age and reproductive effort in the California gull. Behavioral Ecology and Sociobiology 13: 161–171.
———. 1984. Age-specific reproductive tactics in the California gull. Oikos 43: 409–410.
Rasa, O. A. E. 1971. Social interaction and object manipulation in weaned pups of the northern elephant seal Mirounga angustirostris. Zeitschift Tierpsychologie 29: 82–102.
Reid, W. V. 1988. Age-specific patterns of reproduction in the glaucous-winged gull: Increased effort with age? Ecology 69: 1454–1465.
Reilly, J. J. 1989. The water and energy metabolism of breeding male grey seals (Halichoerus grypus ). In Proceedings of the Eighth Biennial Conference on the Biology of Marine Mammals , December 7–11, 1989, Pacific Grove, Calif., 53 (Abstract).
Reilly, J. J., and M. A. Fedak. 1991. Rates of water turnover and energy expenditure of free-living male common seals (Phoca vitulina). Journal of Zoology, London 223: 461–468.
Reiter, J., and B. J. Le Boeuf. 1991. Life history consequences of variation in age at primiparity in northern elephant seals. Behavioral Ecology and Sociobiology 28: 153–160.
Reiter, J., K. J. Panken, and B. J. Le Boeuf. 1981. Female competition and reproductive success in northern elephant seals. Animal Behaviour 29: 670–687.
Reiter, J., N. L. Stinson, and B. J. Le Boeuf. 1978. Northern elephant seal development: The transition from weaning to nutritional independence. Behavioral Ecology and Sociobiology 3: 337–367.
Rice, W. R., and S. D. Gaines. 1989. One-way analysis of variance with unequal variances. Proceedings of the National Academy of Sciences, USA 86: 8183–8184.
———. 1993. Calculating p-values for ANOVA with unequal variances. Journal of Statistical Computation and Simulation 46: 19–22.
Riedman, M. 1990. The Pinnipeds: Seals, Sea Lions, and Walruses . Berkeley, Los Angeles, and Oxford: University of California Press.
Robbins, C. T., and B. L. Robbins. 1979. Fetal and neonatal growth patterns and maternal reproductive effort in ungulates and subungulates. American Naturalist 114: 101–116.
Robinson, B. W., and R. W. Doyle. 1985. Trade-off between male reproduction (amplexus) and growth in the amphipod Gammarus lawrencianus. Biological Bulletin 168: 482–488.
Robinson, S. K. 1988. Anti-social and social behaviour of adolescent yellow-rumped caciques (Icterinae: Cacicus cela). Animal Behaviour 36: 1482–1495.
Ryan, M. J. 1985. The Túngara Frog: A Study in Sexual Selection and Communication . Chicago: University of Chicago Press.
Sandegren, F. E. 1976. Agonistic behavior in the male northern elephant seal. Behaviour 57: 136–158.
SAS Institute. 1985. SAS User's Guide: Statistics . Ver. 5. Cary, N.C.: SAS Institute.
Selander, R. K. 1972. Sexual selection and dimorphism in birds. In Sexual Selection and the Descent of Man, 1871–1971 , ed. B. G. Campbell, 180–230. Chicago: Aldine.
Sergeant, D. E. 1973. Feeding, growth, and productivity of Northwest Atlantic harp seals (Pagophilus groenlandicus). Journal of Fisheries Research Board of Canada 30: 17–29.
Sibly, R., and P. Calow. 1986. Why breeding earlier is always worthwhile. Journal of Theoretical Biology 123: 311–319.
Smith, M. S. R. 1966. Injuries as an indication of social behaviour in the Weddell seal (Leptonychotes weddelli). Mammalia 30: 241–246.
Sokal, R. R., and F. S. Rohlf. 1981. Biometry . San Francisco: W. H. Freeman and Co.
Stearns, S. C. 1976. Life history tactics: A review of the ideas. Quarterly Review of Biology 51: 3–47.
Stewart, R. E. A. 1986. Energetics of age-specific reproductive effort in female harp seals, Phoca groenlandica. Journal of Zoology, London (A) 208: 503–517.
Stewart, R. E. A., and D. M. Lavigne. 1984. Energy transfer and female condition in nursing harp seals, Phoca groenlandica. Holarctic Ecology 7: 183–194.
Sydeman, W. J., H. R. Huber, S. D. Emslie, C. A. Ribic, and N. Nur. 1991. Age-specific weaning success of northern elephant seals in relation to previous breeding experience. Ecology 72: 2204–2217.
Tedman, R., and B. Green. 1987. Water and sodium fluxes and lactational energetics in suckling pups of Weddell seals (Leptonychotes weddellii). Journal of Zoology, London 212: 29–42.
Testa, J. W., S. E. B. Hill, and D. B. Siniff. 1989. Diving behavior and maternal investment in Weddell seals (Leptonychotes weddellii). Marine Mammal Science 5: 399–405.
Thornhill, R. 1981. Panorpa (Mecoptera: Panorpidae) scorpionflies: Systems for understanding resource-defense polygyny and alternative male reproductive efforts. Annual Review of Ecology and Systematics 12: 355–386.
Trivers, R. L. 1972. Parental investment and sexual selection. In Sexual Selection and the Descent of Man, 1871–1971 , ed. B. Campbell, 136–179. Chicago: Aldine.
———. 1985. Social Evolution . Menlo Park, Calif.: Benjamin/Cummings Publishing Co.
Tuomi, J., T. Hakala, and E. Haukioja. 1983. Alternative concepts of reproductive effort, costs of reproduction, and selection in life-history evolution. American Zoologist 23: 25–34.
Twiss, S. D. 1991. Behavioural and energetic determinants of individual mating success in male grey seals (Halichoerus grypus , Fabricius 1791). Ph.D. thesis, University of Glasgow, Scotland.
Walker, B. G., and W. D. Bowen. In press. Changes in body mass and feeding behaviour in male harbour seals, Phoca vitulina , in relation to female reproductive status. Journal of Zoology, London .
Warner, R. R. 1980. The coevolution of life-history and behavioral characteristics. In Sociobiology: Beyond Nature/Nurture? ed. G. W. Barlow and J. S. Silverberg, 151–188. Boulder, Colo.: Westview Press.
Wilkinson, P. F., and C. C. Shank. 1976. Rutting-fight mortality among musk oxen
on Banks Island, Northwest Territories, Canada. Animal Behaviour 24: 756–758.
Williams, G. C. 1966a . Natural selection, the costs of reproduction, and a refinement of Lack's principle. American Naturalist 100: 687–690.
———. 1966b. Adaptation and Natural Selection . Princeton: Princeton University Press.
Worthy, G. A. J., P. A. Morris, D. P. Costa, and B. J. Le Boeuf. 1992. Moult energetics of the northern elephant seal. Journal of Zoology, London 227: 257–265.