PART TWO
THE TREATMENT
GLENDOWER : I can call spirits from the vasty deep.
HOTSPUR : Why, so can I, or so can any man, But will they come when you do call for them?
I Henry IV III.i. 51–53
3
Government Promotes Medical Device Discovery
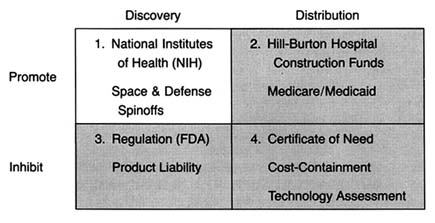
Figure 6. The policy matrix.
After World War II, the federal government was firmly committed to supporting for basic scientific research. Indeed, government came to be seen as a legitimate vehicle for the promotion of innovation. The issue was no longer whether government should provide research and development (R&D) support, but to whom, how much, and in what form. Three primary areas of federal research emerged—defense, space, and civilian, the last dominated by biomedical science. (See figure 6.) There is constant political debate about the overall size of the federal R&D pie and the allocation of funds among these various programs.[1]
Since 1960, federal funds have accounted for from 47 to 66 percent of all R&D spending in the United States. There are three trends in federal support: (1) from the late 1940s to about 1967 there was steady growth in all areas, with 1957 being a starting point for the NIH budget; (2) from 1967 to 1977 there was a general leveling off of investment in space and defense, although life sciences held steady; and (3) from 1977 and throughout the Reagan era defense spending increased at the expense of civilian R&D. Harvey Brooks, "National Science Policy and Technological Innovation," in Landau and Rosenberg, eds., The Positive Sum Strategy. Overall, defense related R&D accounted for about 50 to 60 percent of total federal expenditures throughout the period. See discussion in Richard M. Cyert and David C. Mowery, eds., Technology and Employment: Innovation and Growth in the U.S. Economy (Washington, D.C.: National Academy Press, 1987), 35-38.
Research and development support can take several forms—federal money for basic science research, federal funds directed or targeted toward specific technologies or products, and federal incentives for institutional transfer of technology from basic science to commercialization. The federal government has provided
all three forms to biomedical research, each with the potential to affect the medical device industry. Figure 7 illustrates how the forms of funding related to the innovation continuum.
Federal R&D dollars have affected medical device innovation at various points on the innovation continuum. This chapter discusses the evolution of various policies to support medical device discovery. The first, and most significant, policy involves grants for basic science research through the National Institutes of Health. The second, introduced in the 1960s, targets specific technologies at the point of invention and development. The Artificial Heart Program (AHP) at the NIH illustrates this form of support. Medical device discovery has benefited from spinoffs of targeted programs for space and defense purposes, and these programs are discussed as well. Finally, this chapter describes congressional policies to facilitate technology transfer—from universities, government laboratories, and private firms. The goal of these policies is to rectify perceived institutional barriers to progress along the innovation continuum.
Government Support for Basic Science
When Congress promoted health research after the war, the NIH was the logical federal institution to administer it. Congress both enlarged the authority of the NIH and provided increasingly generous funding for it. The Public Health Service Act of 1944 extended NIH research authority from cancer to health and disease generally.[2]
James A. Shannon, "Advancement of Medical Research: A Twenty-Year View of the Role of the National Institutes of Health," Journal of Medical Education 42 (1967): 97-108, 98.
In 1948, the National Heart Institute and the National Dental Institute were established, and the name of the agency officially changed to the National Institutes of Health to reflect the categorical or disease oriented approach of the multi-institute structure. The total congressional appropriation rose from $7 million in 1947 to $70 million by 1952.[3]Cited in Natalie Davis Spingarn, Heartbeat: The Politics of Health Research (Washington, D.C.: Robert B. Luce, 1976), 28.
By 1955, the NIH awarded 3,300 research grants and 1,900 training grants, accounting for over 70 percent of the NIH budget.[4]Shannon, "Advancement," 100.
President Kennedy, a strong supporter of the NIH, included in his first budget an increase of $40 million, the largest increase ever for medical research and the largest percentage raise since 1955. The rationale for this rapid expansion was "an expanding economy, a favorable political ambience, a consensus stemming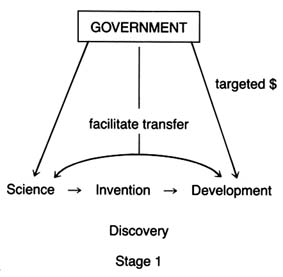
Figure 7. Forms of government support for biomedical R&D.
largely from World War II technological success that scientific research can pay off big, and a set of remarkably effective health leaders in both public and private sectors."[5]
Spingarn, Heartbeat, 25.
The primary form or strategy of support has been called the "boiling soup" concept. The investigator-initiated grant process allowed scientists to work on many projects. Essentially undirected, individual investigators each followed his or her own path of discovery. This philosophy was well articulated in the Senate Appropriations Committee Report on the Labor-HEW Appropriation Bill for 1967.
The committee continues to be convinced that progress of medical knowledge is basically dependent upon full support of undirected basic and applied research effort of scientists working individually or in groups on the ideas, problems, and purposes of their selection and judged by their scientific peers to be scientifically meaningful, excellent, and relevant to extending knowledge of human health and disease.[6]
Cited in Shannon, "Advancement," 101.
Congress maintained a hands-off approach and "tiptoed lightly so as not to disturb genius at work."[7]
Spingarn, Heartbeat, 2.
Certain institutional patterns emerged as a result. Support flowed from the NIH to researchers engaged in basic science at universities and medical schools. The NIH had an enormous influence on the research environment. Research became the major, if not the dominant, feature in academic medicine, and a partnership between universities and government scientists developed. As government came to provide virtually all of the resources for academic research, these institutions thrived on the largesse.[8]
Ruth S. Hanft, "Biomedical Research: Influence on Technology," in R. Southby et al., eds., Health Care Technology Under Financial Constraints (Columbus, Ohio: Battelle Press, 1987), 160-171, 167.
By 1979, the NIH provided over 40 percent of all health R&D funds (see figure 8). This strategy forged a very strong philosophical and institutional link between the NIH and academic medicine.While basic science in the academic research community received the bulk of NIH funds, Congress increased the role of small business in federally sponsored research through the Small Business Innovation Development Act of 1982. The act created the Small Business Innovation and Research Program (SBIR), the goal of which is to support small firms in early stages of research with the expectation that they will ultimately attract private capital and commercialize their results. A small, fixed percentage of all research funds must be directed toward small businesses under the law, and eleven federal agencies are required to participate. The NIH accounts for 92 percent of the SBIR projects within the Department of Health and Human Services and devoted $61.6 million to the program in fiscal year 1987.[9]
U.S. Department of Health and Human Services, Abstracts of Small Business Innovation Research (SBIR) Phase I and Phase II Projects, Fiscal Year 1987. Under the SBIR, phase I awards are generally for $50,000 for a period of about six months and are intended for technical feasibility studies. Phase II, for periods of one to three years, continues the research effort initiated in phase I, and awards do not exceed $500,000. In 1987, NIH awards to the SBIR accounted for $61.6 million.
SBIR proposals are investigator initiated and reviewed through traditional NIH mechanisms. Some successes have emerged from the program. For example, two current commercial devices resulted from SBIR funding—a laser that removes certain birthmarks known as port-wine stains and an electrode that can be swallowed to use in emergency cardiac pacing.[10]
Senate Committee on Appropriations, Subcommittee on Labor, Health and Human Services, Education and Related Agencies, NIH Budget Request for Fiscal Year 1989, cited in testimony of Frank E. Samuel, Jr., president, Health Industry Manufacturers Association (Unpublished document from HIMA, 24 May 1988).
That support for basic scientific research leads to advances in medicine is not in question. In a well-known study, Comroe and Dripps found that basic research provided a critical influence in the long road leading to clinical applications and technology useful in patient care.[11]
Julius H. Comroe, Jr., and Robert D. Dripps, "Scientific Basis for the Support of Biomedical Science," Science 192 (April 1976): 105-111.
While direct links are often difficult to demonstrate, the general consensus can be summed up in the following manner.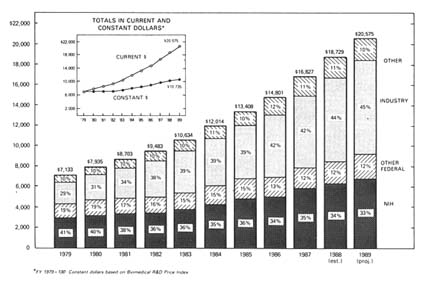
Figure 8. National support for health R&D by source, 1979–1989 (in millions of dollars).
Source: NIH Data Book, no. 90-1261 (December 1989).
Although innovation in pharmaceuticals and medical devices has been largely generated in the private sector by private research and investment, it is doubtful whether much of this would have taken place without the base of knowledge resulting from government-sponsored programs. Much modern medical instrumentation and diagnostics derive from basic advances in the physical sciences, including laboratory instrumentation, which occurred as a result of broad-based government sponsorship of fundamental physics, chemistry, and biology.[12]
Brooks, "National Science Policy," 122, citing Philip Handler, ed., The Life Sciences (Washington, D.C.: National Academy of Sciences, 1970); and Henry G. Grabowski and John M. Vernon, "The Pharmaceutical Industry," in Richard R. Nelson, ed., Government and Technical Progress: A Cross-Industry Analysis (New York: Pergamon Press, 1982), 283-360.
How is the information developed in these laboratories transferred to industry? Academic journals are the traditional outlet for scientific findings. Academic researchers are rewarded for publishing the results of their scientific work. This information is in the public domain, and scientists, engineers, and entrepreneurs interested in product development acquire this information and apply it to their own projects. Thus, federal funds only indirectly support product development, depending on the initiative of private firms.
Scientific journals can be an inefficient means of transferring
technology. Delays in publication and the multiplicity of articles affect the speed and completeness of information acquisition. Efficiency of transfer can be complicated for medical devices, the development of which may depend on information from many disciplines with data contained in a wide variety of journals and specialties.
The focus of NIH support on basic science created institutional limitations for medical device development. First, there tends to be an anti-engineering bias at the NIH. Unlike biochemical research, such as studies of organ function or disease processes, medical device innovation is multidisciplinary and may require a confluence of engineering disciplines and materials sciences as well as biochemistry, medicine, and biology. Indeed, as recently as 1987, a committee of the National Research Council concluded that bioengineering studies accounted for only about 3 percent of the NIH extramural research budget. This low figure may reflect either a lack of applicant interest or, more likely, a lack of receptivity by NIH committees, given that few engineers participate in ranking and funding decisions.[13]
Engineering Research Board, "Bioengineering Systems Research in the United States: An Overview," in Directions in Engineering Research: An Assessment of Opportunities and Needs (Washington, D.C.: National Academy Press, 1987), 77-112, 79. While it is true that the National Science Foundation (NSF) sponsors projects in scientific and engineering research, many of which are in biological engineering, the resources of the NSF are significantly less than those of the NIH. For example, in fiscal year 1988, funding for molecular biosciences at NSF was $44.6 million, for cellular biosciences, $54.24 million, and for instrumentation and resources, $34.15 million. National Science Foundation, Guide to Programs, Fiscal Year 1989 (Washington, D.C.: GPO, 1989). Compare this to the 1986 budget of the National Heart, Lung, and Blood Institute, only one of the institutes within the NIH, which received $821,901,000 for fiscal year 1986. National Heart, Lung, and Blood Institute, Fact Book, Fiscal Year 1986 (Washington, D.C.: GPO, 1986).
Thus, although it may be difficult to pinpoint direct cause and effect, medical device innovation was probably assisted in general by the growing expertise in fundamental scientific understanding of the human body and disease processes that the NIH policies encouraged. However, a government policy biased toward biochemistry may have disadvantaged the engineering based medical device industry, at least relative to other forms of biomedical research.
Direct Targeting of Medical Device Technologies
Several events occurred in the 1960s that fostered changes in the NIH research focus. Increased scientific understanding of disease led to more and better options for medical care. That created public clamor for greater access to these new treatments. In 1966, Congress enacted the Medicare and Medicaid programs for the elderly, disabled, and indigent, which are discussed at greater length in chapter 4. Members of Congress could then point to support for medical services as evidence of
their concern for the public's health. Spending for services became more popular than basic science research, the results of which are long-term and difficult to document.
To the extent that Congress supported R&D, the pressure was for "results not research," as President Johnson so aptly put it.[14]
Cited in Spingarn, Heartbeat, 32.
There was growing public concern in the mid-sixties for the NIH to justify its size and its mission. Congress saw the success of the space program, with its targeted and focused goals, as a model for solving technological challenges in health research. The result was increased political pressure for more federal control of research through targeted programs with specific goals.In the next few years, Congress established NIH programs modeled upon a systems approach to innovation. The Artificial Heart Program (AHP) in the National Heart Institute (NHI), the Artificial Kidney (AK-CU) Program in the National Institute of Arthritis and Metabolic Diseases, and aspects of Nixon's War on Cancer are three of the better-known targeted projects.[15]
This book focuses on the artificial heart program. For more extensive discussion of the War on Cancer, see Rettig, A Cancer Crusade; for kidney dialysis, see Plough, Borrowed Time; and Renee C. Fox and Judith P. Swazey, The Courage to Fail: A Social View of Organ Transplants and Dialysis, 2d ed. (Chicago: University of Chicago Press, 1973). Dialysis is discussed at greater length in chapter 4.
Mission oriented research also included the creation of large centers with groups of investigators performing government designed research and clinical trials. By the 1970s, mission oriented research comprised almost 40 percent of the NHI's budget, although other institutes retained a stronger commitment to the traditional model of investigator-initiated grants.The Artificial Heart Program illustrates the institutional adjustments required for the NIH to manage technology development in a targeted program. It also highlights the politics of federal R&D as Congress tangled with scientists over the creation and continuation of the program.
The Artificial Heart Program
In order to understand the political appeal of developing an artificial heart, one must know something about cardiology. Heart failure is the most common disorder leading to loss of health or life. In theory, an artificial replacement heart can prevent the threat of imminent death from end-stage heart disease. Early estimates of the number of patients who might benefit from an artificial heart were as high as 130,000 per year.[16]
As more was learned about the complexities of the technology, the numbers have been revised dramatically downward. See Working Group on Mechanical Circulatory Support of the National Heart, Lung, and Blood Institute, Artificial Heart and Assist Devices: Directions, Needs, Costs, Societal and Ethical Issues (May 1985), 16.
The heart functions mechanically, rather than through a primarily chemical or electrochemical process as the kidney and liver do. Far more than other vital organs, the heart is inherently suitable for replacement by a mechanical device. Scientific work on the concept had been underway in several laboratories in the 1950s. Researchers knew that they had to design a pump, an engine to drive it, and a power source. The challenge was to find materials to line the pump that would not injure the blood and a substance for the heart itself that was flexible and durable enough to withstand the constant squeezing and relaxing motions. All the parts needed to be synchronized to sustain life.[17]
Ibid., 9-14.
(See figure 9.)Scientists were divided on the feasibility or desirability of artificial hearts; the NIH was unlikely to have funded proposals to work on the technology. The most enthusiastic supporters were some heart surgeons, notably Dr. Michael DeBakey. Congress, unlike the NIH scientists, was receptive because of the political salience of the project. As noted above, many Americans feared death from heart disease, and efforts to eliminate the disease were popular. The idea captured the imagination of Congress and fit the new notion of results oriented research. The successes of the space program were an added impetus. Recently, Dr. DeBakey pointedly recalled the political situation. "Jim Shannon [the NIH director] was opposed to the concept and NIH's involvement in it because he thought there was not enough basic knowledge and that it was not scientifically sound. I went over his head, to Congress."[18]
New York Times, 17 May 1988, B7.
As a result, Congress studied the feasibility of the project. It established the Artificial Heart Program in July 1964 with an initial appropriation of $581,000. The program was housed in the National Heart, Lung, and Blood Institute (NHLBI), the successor to the old NHI.
Targeted programs like the AHP challenged the relationship between academic medicine and the NIH. The program, freed from the traditional concepts of NIH basic research, was much more open to engineering and to industry. Development of the artificial heart required technological expertise not found in medical schools or university science departments; engineers and private firms had to be included.[19]
This involvement was later institutionalized in the Small Business Innovation Development Act of 1982, which increased the role of small businesses in federally supported research and development (Public Law 97-219). This law created the Small Business Innovation Research (SBIR) Program, involving eleven federal agencies, of which the Department of Health and Human Services (HHS) is the second largest participant. The NIH accounts for 92 percent of the SBIR activity in the DHHS. The goal is to support the small business through early stages of research so that it can attract private capital and commercialize the results. U.S. Department of Health and Human Services, Abstracts of SBIR Projects, Fiscal Year 1987.
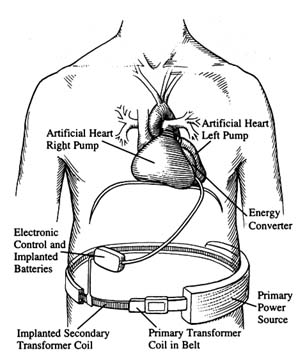
Figure 9. A fully implanted artificial heart system.
Source: Artificial Heart and Assist Devices:
Directions, Needs, Costs, Societal and Ethical Issues,
NIH publication no. 85-2723 (May 1985), 11.
The NIH needed new tools and procedures to implement targeted programs. The National Cancer Institute (NCI), under Dr. Kenneth Endicott, had pioneered the use of the contract, despite considerable objections from more traditional scientists. The contract form allowed the professional staff at the NCI to specify what research it desired, to solicit proposals from interested researchers in business and academics, and to choose who would undertake the proposed project.
The use of the contract also appeared in the early days of the AHP. Dr. John R. Beem, director of the AHP, came from industry. He assembled a small staff familiar with systems approaches, including experts associated with NASA and the air force. Because of the technological requirements, the NIH sought special
permission to consider contracts rather than grants and, in 1964, let nine contracts which were among their first nondrug contracts. They covered such goals as the development of pumps, drive units, mock circulating systems, and blood-compatible materials. The early contracts were with medical engineering teams at various large industrial firms, such as Westinghouse and SRI, none of which were traditional NIH partners. Contracts with small innovators later became the norm.[20]
One of the successful contractors, Novacor, a small, innovative company, is profiled in chapter 9.
These contracts with industry were controversial within the NIH, primarily because they threatened the traditional partnership with universities. Some NIH leaders did not enthusiastically support contracts. They "did not take kindly … to the idea of taking large amounts of research money and funneling them to profit-making industrial firms through the targeted contract mechanism."[21]
Spingarn, Heartbeat, 148.
Conflicts between engineers and medical scientists arose. One chronicler of the program wrote:The two disciplines, medicine and the scientific art, and engineering with its underpinnings in the physical sciences, could not always fulfill each other's expectations. The NHI emphasis was naturally on the medical researcher and the clinical investigator and their needs; the engineers were often treated as "hardware kids" and many marriages between teams at hospital centers and industrial laboratories ended in divorce.[22]
Ibid., 152.
The program has been steeped in politics throughout its twenty-five years. Congressional enthusiasm began to wane in the late 1960s when results were not immediately forthcoming, but support has persisted, albeit at modest levels. Within a few years of its inception, the program expenditures had increased to approximately $10 million. Since 1975, expenditures have stabilized in the range of $10 to $12 million annually. This amount represents only about 1 percent of the NHLBI budget. The total expenditures to 1988 have been about $240 million.
The most recent political conflict over the program occurred in 1988, when a small group of senators forced Dr. Claude Lenfant, director of the NHLBI, to reconsider a proposal to restructure the Artificial Heart Program. Lenfant had suspended financing for research on totally implantable hearts, choosing to
focus instead on development of LVADs, or left ventricle assist devices, which help but do not replace the diseased heart. The NIH capitulated; the New York Times reported that Lenfant's superiors, "fearing that all of their future programs would be in jeopardy, forced him to 'eat a little crow.'"[23]
New York Times, 8 July 1988.
There is a contentious debate about the wisdom of the AHP, with critics questioning both the costs of the technology and the ethics of the research. Twenty-five years after the program began, a viable artificial organ has not yet been produced. Some of the ethical and social issues raised by this program, and its relationship with other sources of public policy, are discussed in chapters 9 and 10. Without engaging in the debate at this point, we can reflect on the benefits of this and other similar programs for medical device innovation.
For companies in the industry, the benefits of targeted device development are clear. The AHP broke down some of the traditional barriers to bioengineering that had developed within the NIH. The agency began to interact with firms as well as university scientists, reducing institutional and disciplinary barriers for applied bioengineering projects. Thus, medical device innovators have been included in, but are clearly only a small part of, the mission of the NIH.
Medical Device Spinoffs from Space and Defense Research
Anyone who has spent time in a hospital is aware of the array of equipment used to monitor a patient's condition. Many patients may not know that the needs of astronauts contributed to this technology. Patients undergoing a noninvasive ultrasound diagnosis are probably unaware that it was the navy's search for tools to detect enemy submarines that generated understanding of this technology. Similarly, the intensive search for military uses for lasers has brought us promising treatments for cataracts and cancerous tumors.
How did these space and defense technologies arrive in our health care system? Obviously, the space and defense programs do not focus on medicine; they have other specific missions to
accomplish. Through a variety of ways, however, medical technologies have developed from research in these nonmedical, government sponsored programs.
Medical Applications of Space Technologies
Sputnik, the USSR's successful satellite launched in late 1957, alarmed Americans who assumed that the United States was losing technological competitiveness to the Soviets. The U.S. space program was a direct response. By early 1958, Congress had introduced numerous bills; President Eisenhower signed the National Aeronautics and Space Act in July 1958.[24]
See discussion in Eugene M. Emme, Aeronautics and Astronautics: An American Chronology of Science and Technology in the Exploration of Space, 1915-1960 (Washington, D.C.: NASA, 1961), 87.
The National Aeronautics and Space Administration (NASA) had to consolidate a number of projects and personnel from government and recast the former National Advisory Committee on Aeronautics (NACA). NACA had engaged in in-house research entirely and had little experience in developing and implementing large-scale projects.[25]
Jane van Nimmen and Leonard C. Bruno, eds., NASA Historical Data Book (Washington, D.C.: NASA, 1988), 6.
NASA became a contracting agency; 90 percent of its annual expenditures by 1962 went for goods and services procured by outside contractors.From the beginning, NASA was a public relations tool for the United States. Not only was it designed to win the space race, but also it let everyone know that the U.S. had restaked its claim to be the world leader in technology. To this end, NASA was to "provide the widest practicable and appropriate dissemination of information concerning its activities and the results thereof."[26]
NASA Center History Series, Adventures in Research (Washington, D.C.: NASA, 1970), 370.
NASA's survival depended on success in its mission and public perception that the program was worthwhile. Thus NASA had to fight to establish and maintain its legitimacy.NASA's first decade was successful in terms of government and popular support. Following the launch of the Apollo and the moon landing in 1969, however, enthusiasm for the program waned. During NASA's second decade, its total budget fell. President Nixon urged NASA to turn its attention to solving practical problems on earth, and funding dedicated to promoting civilian applications of NASA supported technology rose.[27]
Van Nimmen and Bruno, Historical Data Book, 244.
Toward the end of the 1970s, Congress created NASA's Technology Utilization Program, which included regional technology transfer experts. Their job was to monitor R&D contracts toensure that new technology, whether developed in-house or by contractors, would be available for secondary use. In addition, NASA opened a number of user-assistance centers to provide information retrieval services and technical help to industrial and government entities. NASA characterizes itself as a national resource providing "a bank of technical knowledge available for reuse."[28]
NASA, SpinOFF (Washington, D.C.: NASA, 1958), 3. SpinOFF, an annual NASA publication, describes technologies that have been produced using NASA expertise. The examples that appear in the text were derived from a review of SpinOFF stories.
By 1985, NASA claimed an estimated 30,000 secondary applications of aerospace technology.Many of the systems developed for the space program have medical applications. The technology tends to derive from NASA's needs for super-efficient, yet small and light, technologies and its need to monitor the vital signs and overall health of astronauts in space. Technology transfer to medicine has occurred in several ways. NASA contractors form new companies to market products that are based on technology developed for NASA. Sometimes large firms form medically related subsidiaries subsequent to completion of a NASA contract. On occasion, companies with no prior relationship with the agency approach NASA to acquire the technology necessary for the development of a medical product. In all cases, NASA encourages the transfer of technology to civilian uses.
For example, NASA needed new technology to meet the challenges of monitoring the astronauts' vital signs. The commonly used conducting electrode was attached to the body through a paste electrolyte. This method could not be used for long-term monitoring because the paste dries and causes distortions of the data. Other electrodes that made direct contact without use of paste electrolyte failed because body movements caused signal-distorting noise as well. NASA contracted with Texas Technical University scientists who developed an advanced electrocardiographic electrode. It was constructed of a thin dielectric (non-conducting) film applied to a stainless steel surface. It functioned immediately on contact with the skin and was not affected by ambient conditions of temperature, light, or moisture. NASA was assigned the patent and subsequently awarded a license for its use to a California entrepreneur who founded Heart Rate, Inc. The small firm has continued to develop and produce heart rate monitors for medical markets.[29]
NASA, SpinOFF (1984), 61.
The Q-Med firm produced another monitoring product from
electrode technology developed at NASA's Johnson Space Center. Q-Med received an exclusive license from NASA to manufacture and market electrodes in 1984. The firm's monitor assists ambulatory patients who have coronary artery disease and can be worn for days, months, or years to evaluate every heartbeat. It stores information for later review by a physician, who can program it for specific cardiac conditions. The monitor can summon immediate aid if the wearer experiences abnormal heartbeats.[30]
NASA, SpinOFF (1985), 25.
NASA also needed information about spacecraft conditions. For example, McDonnell Douglas Corporation, a firm with many NASA contracts, developed a device to detect bacterial contamination in a space vehicle. In another contract, it developed additional capabilities to detect and identify bacterial infections among the crew of a manned mission to Mars. McDonnell Douglas formed the Vitek subsidiary to manufacture and market a system known as the AutoMicrobic System (AMS). The system, introduced in 1979, offered rapid identification and early treatment of infection. AMS provides results in four to thirteen hours; conventional culture preparations take from two to four days.[31]
NASA, SpinOFF (1987), 76-77.
The confinements of the small spacecraft created a need for miniaturized products. NASA developed a portable X-ray instrument that is now produced as a medical system. The lixiscope, or l ow i ntensity X-ray i maging scope, is a self-contained, battery-powered fluoroscope that produces an instant image through use of a small amount of radioactive isotope. It uses less than 1 percent of the radiation required by conventional X-ray devices and can be used in emergency field situations and in dental and orthopedic surgery.[32]
NASA, SpinOFF (1983), 88.
Lixi acquired an exclusive NASA license to produce one version of the device.NASA awarded a contract to Parker Hannifin Corporation, one of the world's primary suppliers of fluid system components, to develop and produce equipment for controlling the flow of propellants into the engines of the Saturn moonbooster. It subsequently has worked on many other space projects, including miniaturized systems. In 1977, Parker's aerospace group formed a biomedical products division to apply aerospace technology,
particularly miniaturized fluid control technology, to medical devices. Products include a continuous, computer directed system to deliver medication. Parker's key contributions were a tiny pump capable of metering medication to target organs in precise doses, an external programmable medication device for external use, and a plasma filtration system that removes from the blood certain substances believed to contribute to the progression of diseases such as rheumatoid arthritis and lupus.[33]
NASA, SpinOFF (1981), 74-75.
Another application has been an implantable, programmable medication system that meters the flow of drugs. The electronic system delivers programmed medication by wireless telemetry—a space technology—in which command signals are sent to the implanted device by means of a transmitting antenna. Precise monitoring of drugs can be a godsend to a patient. Such a system allows for constant levels of medication, avoiding the highs and lows caused by administering injections at set intervals. Targeting drugs to specific organs makes dosages more accurate and avoids exposing the whole body to toxic therapeutic agents. Some of this research involves cooperative efforts by NASA, universities, and private firms. The Applied Physics Lab at Johns Hopkins University and the Goddard Space Flight Center offer program management and technical expertise. Medical equipment companies, including Pacesetter, provide part of the funding and produce these systems for the commercial market.[34]
NASA, SpinOFF (1981), 88-91.
The NASA experience offers some important lessons. NASA has demonstrated that there is no inherent conflict in government, universities, and industries working together to create useful medical products. It has also shown, on a limited scale and within its particular mission, that government supported R&D can be effectively transferred to the private sector. The benefits to the medical device industry have been important, albeit small relative to NASA's overall expenditures.
Medical Applications of Defense Technologies
Spending on military R&D has been a high priority and accounts for nearly half of all federal R&D. Important medical technologies have emerged from defense research, and the stories of
ultrasound and lasers illustrate the potential, as well as the limitations, of medical spinoffs from defense related R&D.
Ultrasound
Ultrasound is a mechanical vibration at high frequencies above the range of human hearing. In 1880, Pierre and Jacques Curie discovered what is known as the piezoelectric effect, in which an electric charge is produced in response to pressure on such materials as quartz. Conversely, mechanical deformation results from an applied voltage. The impact of sound waves producing this mechanical deformation can be transformed into electrical energy and recorded. Devices to generate and to detect ultrasonic energy are derived from this discovery.[35]
For a complete history of ultrasound, see Barry B. Goldberg and Barbara A. Kimmelman, eds., Medical Diagnostic Ultrasound: A Retrospective on Its Fortieth Anniversary (Rochester, N.Y.: Eastman Kodak Company, 1988), 3; this book also contains an extensive list of references.
World War I led to the first efforts to develop large-scale practical applications of this physical concept. The French government commissioned a physicist to use high-frequency ultrasound to detect submarines underwater. These efforts, conducted in cooperation with Britain and the United States, continued throughout the war. No practical results were achieved at the time, but work continued between the wars. The Naval Research Laboratory refined the basic technology using new electronic techniques and studied the qualities of underwater sound. Scientists also studied industrial applications, including the ability to detect otherwise hidden flaws in industrial materials. There were some medical applications, but these were virtually therapeutic rather than diagnostic, based on the controversial concept of irradiating the body to cure diseases.
World War II accelerated research; military sonar (sound navigation and ranging) and radar techniques were based on the echo principle of ultrasound. Virtually all of the later diagnostic applications of ultrasound involved direct contact and/or collaboration with military and industrial personnel and equipment. The war was crucial to the development of the technology.
Industrial and medical applications began to develop in many nations after the war. Dr. George Ludwig was an early American leader who had spent the years from 1947 to 1949 at the Naval Medical Research Institute. He and his collaborators conducted
experiments for the navy on the diagnostic capacities of ultrasound, concentrating on the detection of gallstones. Ludwig acknowledged military research as well as industrial applications as the sources for his investigations.
Dr. John J. Wild, an Englishman familiar with ultrasonic ranging from his wartime experiences, was another early researcher. He came to the United States after World War II. In conjunction with the Wold Chamberlain Naval Air Station, he began experiments to measure tissue thickness. In collaboration with navy engineers, Wild discovered that echoes from tumor-invaded tissue were distinguishable from those produced by normal tissue, establishing the potential for diagnostic applications. Wild later set up research facilities at the University of Minnesota.
Another early pioneer was William J. Fry, a physicist who worked on the design of piezoelectric transducers at the Naval Research Laboratory Underwater Sound Division during the war. He left in 1946, taking his expertise to the University of Illinois where he founded the Bioacoustics Laboratory. In the 1950s, he recognized that high-intensity ultrasound could eventually provide unique advantages to investigating brain mechanisms. In pursuit of these goals, the Office of Naval Research granted him a contract to develop equipment that would pinpoint lesions within the central nervous system of animals.
By the end of the 1950s, ultrasound diagnosis had been introduced into many medical specialties, including neurology, cardiology, gynecology, and ophthalmology. Engineers and physicists, in private industry and in universities, provided the technical design skills to the medical practitioners who understood clinical needs and had access to patients. This fruitful symbiotic relationship continued through the decades as the benefits of ultrasound became more widely recognized. The NIH supported many of the academic research programs from which commercial instruments emerged.[36]
Frost & Sullivan, Ultrasonic Medical Market (New York: June 1975); Frost & Sullivan, Government Sponsored Medical Instrumentation, Device and Diagnostics Research and Development (New York: March 1978). Cited and discussed in William G. Mitchell, "Dynamic Commercialization: An Organizational Economic Analysis of Innovation in the Medical Diagnostic Imaging Industry" (Unpublished dissertation, School of Business Administration, University of California, Berkeley, 1988).
The value of the medical technology was quickly recognized. The procedure was significantly safer than X-rays and could detect certain problems more effectively. The first commercial sales occurred in 1963, and sales held steady at about $1 million
a year until the late sixties. They skyrocketed in the 1970s, rising from $10 million in 1973, to $77 million in 1980, to $145 million in 1987.[37]
Mitchell, "Dynamic Commercialization," fig. 4.3.
Lasers
The medical applications for lasers have had a much more complicated development than ultrasound. Laser is an acronym for l ight a mplification by s timulated e mission of r adiation. The theoretical knowledge to create a laser has been available since the 1920s, but the technical ingredients were not assembled until the 1950s. Pioneers in laser technology include Professors Gordon Gould and Charles Townes, and Arthur Schawlow from Bell Labs. Laser research quickly became the focus of physics, engineering, and optical sciences. Development followed no simple linear sequence. The scientific inquiry, device development, commercialization, and application of system components were parallel occurrences and influenced each other. Lasers represent a family of devices that have each developed and matured at different rates for a wide variety of applications.[38]
U.S. Congress, Office of Technology Assessment, The Maturation of Laser Technology: Social and Technical Factors, prepared under contract to the Laser Institute of America by Joan Lisa Bromberg, Contract No. H3-5210, January 1988.
A laser requires a lasing medium, or the substance to which energy is applied to create laser light. It must also have an excitation source, or a source of energy, and an optical resonator, which is the chamber where light is held, amplified, and released in a controlled manner. There are a number of types of laser light—it can be continuous or pulsed and have several visible colors or only ultraviolet—and a range of power levels. Types of lasers are named for the lasing medium. For example, there are chemical lasers, lasers with a fiber-optic light source, gas dynamic lasers, such as helium-neon with low-energy beams or carbon dioxide with low- to high-energy beams, and excimer lasers, which produce a high-energy ultraviolet output of gases combined under pressure.
In 1960, between twenty-five and fifty organizations worldwide worked on lasers. Within three years, the number increased to more than five hundred. There were anticipated industrial, military, and medical uses for the technology, and the military provided the most lucrative funds for R&D. In 1963, military funding was $15 million while industry invested only $5 to $10
million, and laser sales at that time were a mere $1 million. Clearly the private market could not sustain the costs of research. Many of the early researchers formed small companies and gravitated to the military. Herbert Dwight, an engineer and founder of Spectra-Physics, commented that "it was relatively easy on an unsolicited proposal to go out and get a relatively nominal contract with somebody like the Naval Research Laboratory … a well-known researcher with a good idea could sit down with a top representative of the NRL and pretty much on a word of mouth commitment get money to do work in promising areas."[39]
Bromberg, "Lasers," 28.
The Department of Defense, while quite enthusiastic about the potential of lasers, was unsophisticated in early contracts and got very little for its money in some instances. One company, TRG, received about $2 million of federal money and produced comparatively few discoveries. In 1965, a Battelle Memorial Institute report stated that "[the] very high [Department of Defense] budget for [research and development, testing, and evaluation] funds … encouraged many small companies to be forced to serve extremely specialized defense markets."[40]
The Implications of Reduced Defense Demand for the Electronics Industry, U.S. Arms Control, and Disarmament Agency (Columbus, Ohio: Battelle Memorial Institute, September 1965), cited in Bromberg, "Lasers," 33.
Military funding diverted research from the civilian markets. Consumer oriented firms did not have the resources to invest in long-term R&D. Laser applications in civilian areas stagnated as the Department of Defense directed R&D money to strategic and battlefield weapons, especially during the height of the Vietnam War. The military liked lasers because of their performance superiority over microwave systems. Military R&D focused on very high-powered lasers in the search for "death ray" weapons. Nonmilitary applications required low-powered beams that would not harm human tissue. It can be argued that industrial and medical applications were seriously disadvantaged by the diversion of laser research to military applications.
Thus, many civilian oriented firms floundered. As late as 1987, U.S. manufacturers, as well as European and Japanese firms, struggled to show a profit. These problems are not caused solely by a skewed focus on military applications. The technology has many variations and has advanced rapidly, leaving some companies with outdated systems. There are a wide variety of laser uses, and for any given type there are only a limited number of units produced, so manufacturing costs remain high.[41]
Biomedical Business International 10: 12 (14 July 1987): 113-115.
The maturity of the clinical applications varies across medical fields (see figure 10).
However, the civilian laser market, despite its rocky start, still looks promising for medicine in the long term. The most advanced, or mature, area of clinical application is ophthalmic surgery, and many other medical specialties are beginning to use lasers as well. There are promising new applications in cardiovascular surgery, in which research sponsored by the military has played a role. Excimer lasers, developed for the Defense Department at the Jet Propulsion Laboratory,[42]
"Now Lasers Are Taking Aim at Heart Disease," Business Week, 19 December 1988, 98.
generate short pulses of ultraviolet light that break down the molecules in plaque. Doctors can thread a tiny optical fiber through the artery. The laser probe vaporizes the fatty deposits without damaging surrounding tissue. Before this development, the laser beam sometimes burned a hole in the artery wall or made an opening through the blockage that allowed a clot to form or fatty deposits to rebuild. In early 1987, the FDA approved a laser probe developed by Trimadyne Company.It has been argued that while defense related R&D has created important spillovers to civilian uses, these occur only in the early stages of development, when "technologies appear to display greater commonality between military and civilian design and performance characteristics. Over time, military and civilian requirements typically diverge, resulting in declining commercial payoffs from military R&D."[43]
Cyert and Mowery, Technology, 37, citing Richard Nelson and R. Langlois, "Industrial Innovation Policy: Lessons from American History," Science 217 (February 1983): 814-818.
Indeed, some have suggested that defense R&D may actually interfere with the competitive abilities of firms, citing among other reasons the erosion of some firms' cost discipline as a result of operating in the more insulated competitive environment of military procurement.[44]Ibid., citing Leslie Brueckner and Michael Borrus, "Assessing the Commercial Impact of the VHSIC Program" (Paper delivered at the Berkeley Roundtable on the International Economy, University of California, Berkeley, 1984).
In laser research, there have clearly been benefits from military research to the civilian producers. However, the evidence supports the analysis that industrial and medical uses were delayed or disrupted because of diversion of research to military purposes.
Both the space and the defense R&D programs have had spin-off effects for medical devices. However, these benefits have been modest, particularly in relation to the expenditures of the space program, and, in the case of defense research, unpredictable. Without devaluing the benefits, medical science
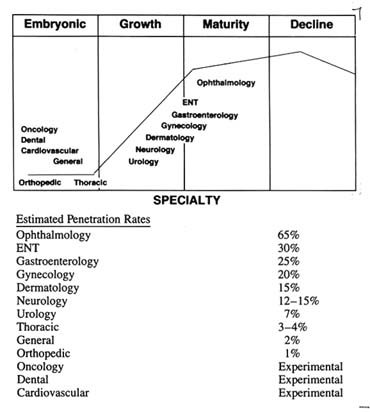
Figure 10. Surgical lasers: maturity of clinical applications.
Source: "Surgical Lasers: Market, Applications, and Trends,"
Biomedical Business International 10:12 (14 July 1978), 114.
clearly cannot rely on these programs for systematic and sustained technological growth. As the case of laser research demonstrates, defense spending can obstruct the evolution and the development of civilian technologies. Nevertheless, government efforts can facilitate collaboration among universities, firms, and government scientists to produce technologies desired by the government.
Encouraging Technology Transfer
As we have seen, federal policies influenced the relationships among institutions in the research community. The dominance
of NIH investigator-initiated grants solidified the strong connection between universities and the NIH. Significant research also occurred within the NIH in conjunction with its intramural research program. Innovative work often languished in universities and government laboratories without transfer to the private sector for commercialization.
Figure 11 captures these institutional barriers to the transfer of technology in the area of biomedical research. The link between the NIH and universities was very strong; the links between universities and government laboratories and the private sector were weak. Consequently, many innovative ideas were developed in academic and government laboratories, but they were never commercialized. This section explores the reasons for these problems and describes the public policies that were designed to help move technology along the innovation continuum.
University-Firm Relationships
In the early twentieth century, university researchers and industrial firms had little in common. Industrial research was not organized, and industrialists generally believed that the role of manufacturers was to make products. Contacts between firms and universities generally only occurred when university graduates entered firms as employees. Studies of the pharmaceutical industry between the wars revealed that researchers based in universities tended to denigrate the atmosphere in industry, which did not place a high value on research. As firms saw the benefits of research on the bottom line, however, they initiated modest interaction with universities.
In the 1940s and the early 1950s, contact between these two sectors was strengthened through fellowships, scholarships, and direct grants in aid from industry to institutions of higher learning. Individual consulting relationships developed between professors and firms. In that period, 300 firms engaged in forms of university support; 50 of them subsidized 270 biomedical projects at 70 different universities.[45]
John P. Swarm, American Scientists and the Pharmaceutical Industry (Baltimore: Johns Hopkins University Press, 1988), 170.
Federal funding for scientific investigators burgeoned after
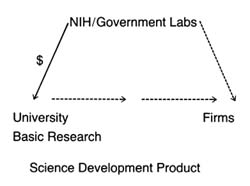
Figure 11.
Institutional relationships in biomedical research.
World War II. The presence of federal money for biomedical research decreased incentives for individuals in universities to work with industry. It was much easier to go hat in hand to the NIH for support. Business-academic interactions reached their nadir in 1970, reflecting the high and consistent level in NIH support at the time. Academic institutions have typically received little direct payment from companies to which technology was transferred; most of the benefits have been indirect. The transfer has not generally occurred through licenses, but only when university trained students seek jobs in firms, when firms review academic journals, when individual professors are hired as consultants, or when researchers set up their own companies.[46]
Mitchell, "Dynamic Commercialization," 107.
Indeed, the general trend before 1980 was that most commercial technological advances did not pass through either government or university patent offices. Some start-up companies did negotiate licenses with the institutions where the founders had worked; others did not.
Universities had little incentive to push for patent rights. This lack of incentive is tied to government policy. Under the terms of federal grants, the federal agencies that paid for the research held the patents. Indeed, few universities even had formal patent or licensing offices; many had contract relationships with off-campus patent agents.[47]
Adeline B. Hale and Arthur B. Hale, eds., Medical and Healthcare Marketplace Guide (Miami: International Biomedical Information Service, 1986).
Many researchers failed to disclosetheir research because they either did not want to share revenue with the university or did not want to bother with the headaches of dealing with off-campus agents.
Because the NIH supported so much basic research, few researchers could claim patent rights in any case. The federal agencies were allowed to assign rights to the universities or to individual researchers, but intensive negotiation was required and rarely undertaken. Only institutions with extremely active research units bothered to establish patent offices. A study of the field of diagnostic imaging showed that institutions with patent offices tended to have more academic imaging products licensed.[48]
The term diagnostic imaging refers to medical technologies such as X-ray and magnetic resonance imaging (MRI and ultrasound, among others).
Institutions with no patent office had few licenses even when the researchers were actively contributing to imaging innovation.[49]Mitchell, "Dynamic Commercialization," chap. 5.
By the late 1970s, shifts in government policy triggered changes in these institutional arrangements. The federal government began to reduce its commitment to federal research support. These cuts in federal biomedical research funding were exacerbated by the impact of inflation on research costs. Universities responded by encouraging contact with private industry, a potential source of research funds. One index of interaction is the flow of resources from firms to universities. The National Science Foundation estimated that corporate expenditures on university research would reach $670 million in 1987, up from $235 million in 1980.[50]
Cited in Calvin Sims, "Business-Campus Ventures Grow," New York Times, 14 December 1987, 25, 27. The top university recipients in 1986 were Massachusetts Institute of Technology, Georgia Institute of Technology, Carnegie Mellon University, Pennsylvania State University, and University of Washington.
Corporate funds generally fall into three categories: gifts, research awards, and awards for instruction, equipment, and facilities. In fiscal year 1988 at the University of Michigan, for example, industry provided $104 million in gifts and grants (15.3 percent of total gift income) and $20.5 million in contract research expenditures (8.7 percent of total research expenditures). Research funds from industry rose 82 percent between 1983 and 1988, paralleling the growth in total university research expenditures. These figures do not include individual consulting relationships between faculty researchers and firms.[51]
Judith Nowak, "The University of Michigan Policy Environment for University-Industry Interaction" (Paper delivered at Institute of Medicine Workshop on Government-Industry Collaboration in Biomedical Research and Education, Washington, D.C., 26-29 February, 1989).
Universities also developed significant formal institutional relationships with business, such as the creation of research parks and research consortia on or near campuses. Nearly four dozen universities have or are seriously considering the establishment
of research parks, including Stanford, North Carolina, Duke, Yale, and Wisconsin. By 1988, Johns Hopkins University Medical School had over two hundred separate agreements with industry.[52]
David Blake, remarks at the Institute of Medicine, Forum on Drug Development and Regulation, Washington, D.C., 3 March 1989.
Congress also initiated some affirmative policy changes. In 1980, it passed the Bayh-Dole Patent and Trademark Amendments.[53]
Public Law 96-517 (12 December 1980).
This law gave nonprofit organizations, notably universities, rights to inventions made under federal grants and contracts. The new policy led to increased efforts by universities to report, license, and develop inventions. In 1984, the policy was extended to federal laboratories operated by universities and nonprofit corporations.[54]Public Law 98-620 (9 October 1984).
Many academic institutions have responded by creating patent, licensing, and industry liaison offices or by increasing the activity of existing offices. In 1985, for example, the total royalty income received by Stanford, MIT, and the University of California had risen from less than $5 million per year during the early 1980s to about $12.5 million annually. University of California revenue grew from $3.4 million to $5.4 million between 1985 and 1987 alone.[55]Mitchell, "Dynamic Commercialization," 110.
Holding a patent will encourage technology transfer because licensing patent rights can be profitable. Universities have worked out elaborate policies for royalty sharing. Individual professors and the universities themselves stand to profit handsomely. The act of licensing transfers these innovative ideas to the private sector, a transfer that is consistent with the promotion of private sector initiatives and the downplaying of federal agencies that characterize the 1980s. Unlike the targeting approach of the 1970s, whereby a federal agency like the NIH decided what technologies to procure and paid for them to be developed, this new technology policy provides financial incentives in the private sector to promote technology transfer. The early data indicate that the incentives are working and that relationships between firms and universities have strengthened as a result.
Business-Government Relationships
Basic research also occurs in federal laboratories. The federal government spent approximately $18 billion in 1986 on research
and development at over seven hundred federal laboratories. Although the NIH devotes only about 10 percent of its funds for in-house or intramural research, the research productivity of NIH scientists has grown steadily. Despite the fact that government laboratories have produced over 28,000 patents, only 5 percent have ever been licensed. Indeed, the NIH held few patents and practically gave away licenses. After all, the government scientists worked for salaries and were committed to research; the NIH had ample funds from Congress without supplementing income with licensing arrangements.
In 1980, Congress enacted the Stevenson-Wydler Technology Innovation Act to encourage the transfer of federal technology to industry.[56]
Public Law 96-480 (October 1980).
Technology transfer from federal laboratories to industry became a national policy. The law created government offices to evaluate new technologies and to promote transfer, but it was not fully implemented or funded. Many of the federal laboratories lacked clear legal authority to enter into cooperative research projects.In 1986, Congress passed the Federal Technology Transfer Act, amending Stevenson-Wydler to allow federal laboratories to enter into cooperative research with private industry, universities, and others.[57]
Public Law 99-953 (1986).
It established a dual employee award system of sharing royalties between the agency and the individual researcher and making cash awards as well. Specifically, it provided for at least a 15 percent royalty payment to laboratory employees from the income received by the agency from the licensing of an invention. It also established a Consortium for Technology Transfer.President Reagan issued an executive order in 1987 that called for enforcement and compliance with the law.[58]
Executive Order 12591, Facilitating Access to Science and Technology (April 1987).
This order required that federal agencies "identify and encourage persons to act as conduits between and among federal laboratories, universities and the private sector for the transfer of technology, and to implement, as expeditiously as practicable, royalty sharing programs with inventors who were employees of the agency at the time their inventions were made, and cash award programs." Federal agencies in general, and the NIH in particular, struggled to define the parameters of relationships between its own scientists and commercial organizations. The NIH Officeof Invention Development reported in 1989 that it reviewed four to five cooperative research and development agreements (CREDAS) each month.
Federal policies clearly have promoted innovation in biomedical sciences. How well have medical devices fared under these policies? The relationship between NIH and universities for promotion of basic scientific research benefits all scientific progress, which includes medical devices along with other medical technologies. However, the traditional bias of the NIH against engineering and other physical sciences has undoubtedly meant that some device technologies were overlooked. Most complex devices, particularly implantables, require a multidisciplinary approach to innovation. An unduly narrow focus on biochemistry and pharmacology at the expense of physics, engineering, and biomaterials science would not promote development in those fields.
The medical device industry stands to benefit from policy changes that have promoted government links with private industry. Congressional efforts to direct the NIH toward targeted development have supported some medical device innovation. The support provided to small innovators pursuing long-term investigations undoubtedly has fostered technology that would otherwise be abandoned. The Artificial Heart Program illustrates both the strengths and the weaknesses of the approach. Clearly, some innovative firms, and some technology, would not exist without NIH support.
Similarly, the recent efforts to strengthen the relationship of universities and government labs to private firms will also benefit the device industry. As with targeted development, this approach erodes the traditional focus on basic science, emphasizing instead the commercial potential of new ideas. Collaboration with universities presents positive opportunities for the device industry. Because of the multidisciplinary nature of device development, the more alternative routes available to an innovator the better.
These changes raise significant social issues that are often overlooked. Is government targeting of medical technology a wise use of public funds? Are these products different from other goods, justifying public expenditures for product development?
Such a view "assumes that if a potential capability exists to cure a life-threatening disease, there exists a moral obligation to develop that capability. It is a kind of extension of the philosophy underlying the Hippocratic oath to the development of new technologies."[59]
Brooks, "National Science Policy," 155.
Other issues arise when taxpayers' money is used to develop products. Should these beneficial products be turned over to the private firms who will make a profit on them? What if the products developed with government funds are so expensive that some citizens will not be able to afford them? The artificial heart will be an extraordinarily expensive technology. Does it make any difference whether the source of funds was public or private or whether the product is widely available? Is it equally immoral for government to fail to promote a lifesaving or life-enhancing technology if there is the basic knowledge to develop it but no source of private funds?
How well does government pick technologies to promote? There is contentious debate over the wisdom of industrial policy, which refers to deliberate government programs that channel resources to particular industrial sectors to promote or protect them. Does government have the skill to pick winners in the medical marketplace?
Additional issues arise in relation to the institutional changes discussed above. Some have called the federal policies to strengthen the ties of university and government laboratories to industry misguided because of the institutional effects. They ask, for example, whether the profit motive will subvert the scientific missions of universities and government researchers, who may be lured by profits from the private sector and ignore the pursuit of knowledge for its own sake. Will conflicts of interest between public employees and the private sector arise as a result? Some fear that the NIH will become a private procurement lab for industry. Who will engage in the long-term scientific investigations with no immediate commercial potential if professors are busy collaborating with industry? Will academic researchers hesitate to disclose their findings until the patents are filed, thereby restricting the free flow of information at scientific meetings? Secrecy is antithetical to scientific progress, but it is essential to profitmaking in a competitive environment.
These tantalizing questions are not easy to answer. However, they illustrate the complex issues raised by government promotion of medical discoveries, questions we will explore further in the concluding chapter. Now we turn to government promotion of medical device distribution—the other end of the innovation continuum.
4
Government Promotes Medical Device Distribution
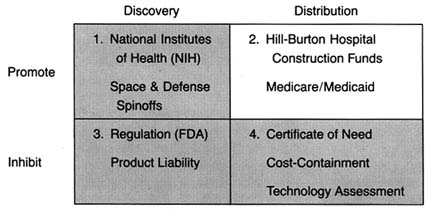
Figure 12. The policy matrix.
Throughout the 1950s and 1960s, the federal government remained firmly committed to promoting biomedical research. Public belief in the benefits of technology characterized this period. As technological improvements in medical treatment emerged after World War II, many people were priced out of the increasingly sophisticated and desirable medical market. At the same time, there was growing belief that it was immoral not to provide some level of health care to everyone who needed it.
Demand for access to health care increased. The economics of health soon became a political issue. In a variety of ways, government accepted greater social responsibility for the structure of health care delivery and access for excluded groups. Indeed, the public sector moved from a refusal to become involved in health
care to the nation's largest single purchaser of services in a relatively brief period. By the mid-1980s, public funds accounted for 20 percent of health care spending and 40 percent of payments to hospitals.[1]
For data on spending, see R. M. Gibson et al., "National Health Expenditures, 1982," Health Care Financing Review 9 (Fall 1987): 23-24. See also Daniel R. Waldo et al., "National Health Expenditures, 1985," Health Care Financing Review 8 (Fall 1986).
This chapter examines how the government commitment to health care has influenced the medical device industry from the postwar period until 1983. Our examination breaks at that juncture because massive changes in the structure of the federal payment system that year ushered in the era of cost containment. In the period before 1983, government policy promoted distribution of medical device technology. The entry of government led to unrestrained growth in the demand for medical devices. Government policy encouraged acquisition regardless of cost and was biased toward growth of hospital based technology. Government affected both the size and the composition of the medical device market. (See figure 12.)
As a third-party payer, rather than a provider of services, government refrained from direct involvement in treatment decisions. The result was only tenuous control over total program costs. These policies provide the background for the cost-containment efforts of the late 1970s and 1980s that will be discussed in chapter 7.
This chapter illustrates the impact of government policy through three case studies. The story of the artificial kidney shows how government spending literally created the market for this expensive, lifesaving technology. The introduction of the CT scanner, a computer assisted X-ray device that revolutionized diagnostic imaging, illustrates the effect of government spending policy on the diffusion of high-cost capital equipment. Finally, the rapid growth of the cardiac pacemaker market reveals how unrestrained payment can lead to market abuse.
A brief caveat before proceeding: Medicare and Medicaid are extremely complex public policies, and the discussion here is inevitably cursory. This chapter examines the impact of the government payment systems on medical devices. Many less pertinent, but otherwise important, aspects of these payment schemes are not discussed. Interested readers should consult the notes for more detailed studies.
Policy Overview, 1950–1983
Hill-Burton Promotes Hospital Growth
Very little hospital construction took place during the depression and World War II. After the war, however, there was both a general belief that hospital beds were in short supply and much concern about the uneven distribution of beds among the states and between rural and urban areas.[2]
Commission on Hospital Care, Hospital Care in the United States: A Study of the Function of the General Hospital, Its Role in the Care of All Types of Illnesses, and the Conduct of Activities Related to Patient Service with Recommendations for Its Extension and Integration for More Adequate Care of the American Public (New York: The Commonwealth Fund, 1947).
Congress passed the Hospital Survey and Construction Act of 1946, which has come to be known as Hill-Burton, the names of its congressional sponsors.[3]Public Law 79-725. For a complete description of the history of the Hill-Burton Program, see Judith R. Lave and Lester B. Lave, The Hospital Construction Act: An Evaluation of the Hill-Burton Program, 1948-1973 (Washington, D.C.: American Enterprise Institute, 1974).
Hill-Burton represented an unprecedented involvement of the federal government in facilitating access to health care. The objectives of the new legislation were to survey the need for construction and to aid in the building of public and other nonprofit hospitals.Consistent with ongoing concerns about the propriety of federal involvement in health services, the program was set up as a federal and state partnership. An agency in each state was designated as the state approved Hill-Burton organization and was given an initial grant to survey hospital needs. The state then received funds to carry out the construction program, subject to federal approval. Priority was given to states where shortages were the greatest. The ultimate allotment formula was based on the state's relative population and its per capita income. The poorer and the more rural the state, the greater the level of federal funds available to it.
In the period of 1946–1971, short-term acute or general hospitals received the largest share of Hill-Burton support, averaging over 71 percent of program funds. While Hill-Burton funds did not dominate spending on hospital facilities, their impact on hospitals was high. Between 1949 and 1962, the federal government paid directly about 10 percent of the annual costs of all hospital construction under the program. In other words, about 30 percent of all hospital construction projects received some form of federal assistance.[4]
Lave and Lave, Hospital Construction, chap. 1.
The number of available hospital beds grew accordingly. In 1948, there were 469,398 short-term beds; by 1969, the number had almost doubled to 826,711. Of these, 40 percent had been
partially supported by Hill-Burton monies.[5]
Ibid., 25.
Studies indicate that the program had a generally significant effect on the change in hospital beds per capita between 1947 and 1970.[6]Ibid., 37.
In particular, it increased the number of hospital beds in smaller cities and targeted low-income states.The impact clearly favored the growth of short-term acute care facilities. Some years after the program began, there was a recognition of the bias in favor of these institutions. In 1954, Congress amended the law to provide grants to assist with out-patient facilities and long-term care facilities. In 1964, additional changes earmarked funds specifically for modernization of older facilities rather than for a further increase of beds. Despite these amendments, the thrust of the program was expansion of acute care facilities. Government funds essentially established the mix of facilities in the marketplace. The result was growth of the potential market for medical technology appropriately designed for these settings. The beds were available; the problem then became access to this costly and sophisticated hospital care.
The Pressure for Access Grows
Expansion of hospitals inevitably led to pressure to provide more services as hospitals sought to fill their beds. During the prewar period, particularly during the depression, families denied themselves medical care. However, medicine now offered more benefits than ever before, particularly in the modern hospital setting. In response, interest groups began to press policies that would increase access to these new and expensive therapies. Some turned to government as the logical source of funds for health care services. However, reformers confronted the traditional long-standing objection to federal involvement in health care services.
This opposition to federal entry into health care was intense. In stark contrast to the expansion of Social Security during the postwar period, there was a political deadlock over state supported health insurance proposals.[7]
Starr, Transformation, 286.
Physicians, represented by the American Medical Association (AMA), and many business groups strongly opposed all forms of national health insurance.The AMA denounced disability insurance as "another step toward wholesale nationalization of medical care and the socialization of the practice of medicine."[8]
Arthur J. Altmeyer, The Formative Years of Social Security (Madison: University of Wisconsin Press, 1968), 185-186, cited in Starr, Transformation, 286 n. 151.
The debate has been characterized as partly ideological, partly social, and partly material. For all these reasons, compulsory national health insurance was not forthcoming in the 1950s. Only American veterans received extensive, federally supported medical care through Veterans Administration hospitals that were greatly expanded during the postwar period. "The AMA opposed the extension of the veterans' program to nonservice connected illness, but the veterans were one lobby even the medical profession could not overcome."[9]
Starr, Transformation, 289.
There is real irony in this physician-led opposition to federal health programs given both the subsequent expansion of the patient base through Medicare and Medicaid and the flow of millions of dollars to physicians from government coffers.Although the government remained intransigent, there were options in the private sector for some groups. The middle class continued to seek forms of private insurance coverage; unions began to look for health benefits in collective bargaining agreements. By the 1950s, there was a stable pattern of growth in private insurance coverage, expanding the market for health care to the employed and the middle class. Much of the insurance was available to working people as fringe benefits; labor managed to bargain successfully for health insurance. By mid-1958, nearly two-thirds of the population had some insurance coverage for hospital costs. The higher the family income, the more likely that it had insurance. In 1948, 72 percent of patients paid directly for health care and only 6 percent had any form of private third-party insurance. By 1966, 52 percent of patients paid directly for health care and 25 percent had private insurance (see table 2). The numbers that received care from public funds remained relatively stable—19 percent in 1948 and only 21 percent by 1966. The poor received welfare and charity care when they could. The retired, unemployed, and disabled were often virtually excluded from the benefits of hospital based care.
The availability of insurance provided stability to the market and increased market size. The financing mechanisms through
| ||||||||||||||||||||||||||||
payroll withholding kept spending stable during recessions and reduced market uncertainty for providers and suppliers. Although causality is difficult to document, the growth in private health care expenditures during this period did expand the market for medical products, particularly in the hospital sector. The value of medical product shipments, based on data in the SIC codes, began to climb, and sales in the five relevant SIC categories rose at an average annual rate of 6 percent, which is three times the growth rate immediately before World War II but less than half of the wartime rate of increase.[10]
Foote, "Crutches to CT Scans," 10.
Table 3 captures the boom in sales during this period.Despite the growth of private insurance, pressure to expand access to health care from those outside the medical care system continued. Some favored a compulsory and contributory health insurance system. Although legislation had been introduced as early as 1958, the real impetus came after the Democratic sweep of the presidency and the Congress in 1964. In 1965, President Johnson signed the Medicare Amendments to the Social Security Act in which the federal government definitively entered the marketplace. The new law's intention was to open the health care system to the elderly. The president declared: "Every citizen will be able, in his productive years when he is earning, to insure
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
himself against the ravages of illness in his old age…. No longer will illness crush and destroy the savings that they have so carefully put away over a lifetime."[11]
Quoted in Andrew Stein, "Medicare's Broken Promises," New York Times Magazine, 17 February 1985, 44, 84. For a detailed discussion of the politics of Medicare, see Starr, Transformation, book. 2, chap. 1; see also Rashi Fein, Medical Care, Medical Costs: The Search for a Health Insurance Policy (Cambridge: Harvard University Press, 1986).
Medicare in Brief
This section briefly examines the key attributes of the Medicare insurance program through 1983, at which time occurred a massive restructuring to contain costs.[12]
These changes, which altered the thrust of the program, are discussed in chapter 7 along with other cost-containment policies.
Medicare's hospital insurance program, Part A, covered specific hospital inpatient services for the elderly and some other extended care. Part B, Medicare's supplementary medical insurance program, covered costs associated with physicians and hospital outpatient services and various other kinds of limited ambulatory care. Part A is supported by the Medicare trust fund and is available to all elderly citizens. Part B is a voluntary program, supported by subscriber payments and congressional appropriations. In 1972, Medicare eligibility was extended to disabled persons and most persons with end-stage renal disease (ESRD), those with kidney failure (see table 4).[13]Social Security Amendments of 1972, Public Law 92-603. The specific impact of this legislation on kidney dialysis equipment is discussed in the next section of this chapter.
Moreover, the Medicare-eligible population has greater health needs than the average citizen. While the elderly constitute about 11.2 percent of the population, they account for 31.4 percent of the health care costs.[14]
U.S. Congress, Office of Technology Assessment, Medical Technology and the Costs of the Medicare Program (Washington, D.C.: GPO, July 1984), 3.
By the mid-1980s, Medicare became the largest single payer for hospital services, accounting for 28 percent of the nation's hospital bills. Medicare also accounted for a significant portion of funds for physician payments under Part B.[15]See Waldo et al., "National Health Expenditures."
The Medicare program had an immediate and significant impact on medical devices. Medicare costs are tied to the dollars paid by the government for services provided under the programs. The method of reimbursement was a cost-plus system that retroactively compensated providers for all "necessary and proper" expenses associated with treatment for the covered individuals. This system encouraged the purchase and use of medical technology.
Reimbursement rates for Medicare patients included a capital cost pass-through, which meant that hospitals could receive reimbursement for capital expenditures to the extent that those
| ||||||||||||||||||||||||||||||||||||||||
capital costs were part of Medicare services. (Capital expenditures generally include durable medical equipment, such as beds, operating room machinery, and diagnostic equipment.) Hospital administrators had little reason to resist pressure from physicians and others to buy new, specialized, and perhaps underutilized equipment. Indeed, the growing prevalence of third-party financing, particularly in the public sector, is considered one of the major causes of inflation in hospital costs.[16]
Lave and Lave, Hospital Construction, 54.
Much of those costs were associated with spending on medical devices.The Medicare system is administered by the Health Care Financing Administration (HCFA). The HCFA contracts with private organizations, such as Blue Cross and Blue Shield, to process the claims. Private insurers, called fiscal intermediaries , handle claims under Part A of the program; insurers for Part B
are called carriers . Figure 13 illustrates the complicated process for Medicare claims.
Claims processing is an enormous undertaking. In 1987, HCFA processed approximately 366 million Medicare claims.[17]
U.S. Department of Health and Human Services, 1987 budget request, 5 February 1986.
In addition, HCFA also handles disputes about whether Medicare covers a particular procedure or technology. To be eligible for Medicare payment, there must be a determination that a new technology or device will be covered. Indeed, advisors to the Department of Health and Human Services concluded in a recent report that "Medicare coverage policy involves so large a portion of U.S. health care delivery that it can significantly affect the diffusion of a technology as well as the environment for technological innovation."[18]National Advisory Council on Health Care Technology Assessment, The Medicare Coverage Process (14 September 1988). This report reviews and then critiques HCFA's coverage process.
The Medicare Act prohibited payment for any items or service not considered reasonable and necessary for patient care. However, the law did not include a comprehensive list of items or services considered "reasonable and necessary" under the program. Medicare coverage policy continuously evolved and was implemented in a decentralized manner. Some coverage decisions were made at a national level by HCFA's central office, under advice from the federal Office of Health Technology Assessment (OHTA).[19]
For detailed discussion of OHTA, see Committee for Evaluating Medical Technologies in Clinical Use, Assessing Medical Technologies (Washington, D.C.: National Academy Press, 1985), particularly 355-363.
Most decisions were made by Medicare contractors who processed claims. The decentralized nature of the process can create an uncertain marketplace for newly introduced technologies. However, in the early years of the federal program, coverage decisions were consistently favorable for devices and the complex process was little threat to the industry.Medicaid in Brief
The 1965 law also established the Medicaid program, a silent partner to Medicare that received much more publicity at the time. The goals and structure of Medicaid are quite different than Medicare. Its purpose is to provide payment for medical care for certain low-income families defined by law as medically needy. The goal is to increase access of the poor to health care services.[20]
For a complete description of the Medicaid program, see Allen D. Spiegel, ed., The Medicaid Experience (Germantown, Md.: Aspen Systems, 1979). See also Thomas W. Grannemann and Mark V. Pauly, Controlling Medicaid Costs: Federalism, Competition, and Choice (Washington, D.C.: American Enterprise Institute, 1982); and Robert Stevens and Rosemary Stevens, Welfare Medicine in America: A Case of Medicaid (New York: Free Press, 1974).
Unlike Medicare, which is a wholly federal program, Medicaid uses a combination of federal and state funds but states
Figure 13. Model of Medicare's coverage process for individual medical technologies.
Source: Medical Technology and Costs of the Medicare Program (Washington, D.C.:
Office of Technology Assessment, July 1984), 76.
control and administer them. Medicare is tied to Social Security and has uniform national standards for eligibility and benefits. Medicaid, however, defers to the states on many aspects of its programs for the poor and places more restrictions on physician participation. States are not required to participate in Medicaid, but elaborate financial incentives virtually guarantee participation. By 1977, all states had a Medicaid program in place. States may impose complicated eligibility requirements and benefits vary significantly from state to state. States set the definition of income limits for an individual or a family. These limits differ considerably among the states. Many families below the federally defined poverty line are not eligible for Medicaid in some states.[21]
Stephen F. Loebs, "Medicaid: A Survey of Indicators and Issues," in Spiegel, The Medicaid Experience, 5-19.
The federal government matches the state expenditures in the program based on a formula tied to each state's per capita income. The federal contribution to the program ranges from approximately 50 to 78 percent of the state's total costs.[22]
Charles N. Oberg and Cynthia Longseth Polich, "Medicaid: Entering the Third Decade," Health Affairs 7 (Fall 1988): 83-96, 85.
When Medicaid was passed, supporters argued that it would add only $250 million to the health care expenditures of the federal government. In the first year of the program, the outlays of the federal and state governments were $1.5 billion. By 1975, spending rose to $14.2 billion, and in 1987 the expenditures exceeded $47 billion (see figure 14).The number of people enrolled in the programs has increased as well. There were 4.5 million recipients in 1968 and 24 million in 1977, at Medicaid's peak; the figure dropped to 23.2 million in 1987. Medicaid accounted for over 10 percent of America's total health care expenses in the 1980s. At that time the distribution of recipients included dependent children under twenty-one years of age, adults in families with dependent children, persons over sixty-five, the permanently and totally disabled, and the blind. The types of services covered include inpatient, acute care, skilled nursing homes, mental hospitals, physicians services, and outpatient and clinic services. However, inpatient services (including hospitals and nursing homes) constitute about 70 percent of Medicaid payments.[23]
Loebs, "Medicaid," 6-8.
The program has been tremendously controversial. It has been criticized for rapidly rising costs, well-documented claims of fraud and abuse by providers, and questions about management,
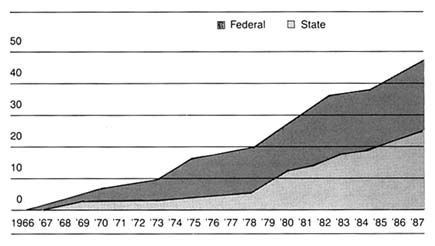
Figure 14. Federal and state Medicaid expenditures, 1966–1987 (in billions of dollars).
Source: Health Care Financing Administration. Reprinted from Oberg and Polichj,
"Medicaid: Entering the Third Decade," Health Affairs (Fall 1988), 85.
quality, and equity. But Medicaid remains the primary vehicle for access to health care for the nation's poor.
Impact on Medical Device Sales
These two major health initiatives led to greatly increased spending on health. National health care expenditures rose from $40.46 billion in 1965, which was 5.9 percent of the GNP, to $322.3 billion, or 10.5 percent of the GNP, by 1982. Per capita expenditures increased more than fivefold, from $207 in 1965 to $1,337 in 1982. The public share of coverage rose from only 21 percent in 1966 to 41 percent in 1982.
It is clear that private insurance programs helped to increase and to stabilize the health care market generally. However, the infusion of capital from federal and state governments brought millions of heretofore excluded individuals into the system.
Without question, government spending significantly expanded the marketplace for health care services and, inevitably, for medical devices associated with treatment. In general, hospitals benefited the most by federal and state spending programs,
but device sales increased in all relevant categories. Examination of the individual SIC categories of medical devices supports the conclusion that federal spending expanded industry sales. In 1982, hospitals purchased $7 billion of the $16.8 billion in sales of products in the five SIC codes, and this total does not include some infrequently purchased larger items (see table 5).
Three SIC categories—3693: X-ray, electromedical, and electrotherapeutic apparatus; 3841: surgical and medical supplies; and 3842: surgical appliances and supplies—are particularly closely tied to federal Medicare and Medicaid payments because of their strong association with a hospital base. Dental and ophthalmic supplies (SIC 3843 and 3851 respectively) are less likely to be covered by Medicare payments. Before Medicare, four of the five SIC categories had similar growth patterns from 1945 to 1965; the fifth, surgical and medical instruments, grew faster because of demand stimulated by hospital construction. After 1965, however, sales in the three Medicare affected categories were much higher (14 to 22 percent) than the other two (8 to 11 percent).[24]
U.S. Bureau of the Census, Census of Manufactures: Industry Series (Washington, D.C.: GPO, 1963, 1982), table 6c.
The following case studies illustrate more specifically the powerful impact of federal spending on medical device growth.
Government Policy and Medical Device Distribution
Government programs dominated segments of the medical device market and had an enormous impact on the size and composition of those market segments. Government policies for payment can create or eliminate a product market or can force the product to move from one market segment to another. As the leading force in paying for medical services, the government indirectly shaped the market for medical device innovations.
Government Influences Market Size: The Case of Kidney Dialysis
The kidneys maintain the equilibrium of dozens of chemicals in the body, control the pressure, acidity, and volume of blood, and filter the blood to remove excess fluid and waste products.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
Kidneys can be damaged by diseases, infections, obstructions, toxins, or shock, any of which can lead to end-stage renal disease (ESRD). When the kidneys fail, the body swells with water and accumulates wastes and poisons. The individual may become comatose and may ultimately die.
Hemodialysis is the term used to describe the kidney's filtration of blood. The artificial kidney accomplishes hemodialysis through diffusion, which rids the body of toxins, and ultrafiltration, which removes excess fluid.[25]
Janice M. Cauwels, The Body Shop: Bionic Revolutions in Medicine (St. Louis: C. V. Mosby, 1986), chap. 12. For additional discussion of dialysis, see B. D. Colen, Hard Choices: Mixed Blessings of Modern Medical Technology (New York: Putnam, 1986).
The developmental history of hemodialysis dates back to the early 1900s. Early experiments confirmed the conceptual basis for dialysis, but there remained several barriers—particularly the lack of anticoagulants and an effective filtration membrane. The development of compound heparin solved the first problem; commercial production of cellophane solved the second.[26]Rettig, "Lessons Learned," 154.
Willem J. Kolff developed the first artificial kidney machine in Holland in the 1940s. The first American machine was built in 1947, in collaboration with researchers at Peter Bent Brigham Hospital. This equipment worked well on patients suffering acute kidney failure. However, patients with chronic kidney failure could survive only if they remained connected to the machine.
The problem was that of access to the veins; each connection required surgical operations. With the invention of the Quinton-Scribner shunt in 1959, a device that permitted repeated connections, the machine could be used for patients with chronic kidney failure. It is interesting to note that the critical material in the shunt was Teflon, an inert fluorocarbon resin that the body did not reject.[27]
Ibid., 154-156.
By the early 1960s, patients were receiving dialysis experimentally in Seattle, and the country began to be aware of this new lifesaving technology. Hemodialysis costs were high because of the hospital space required, the expensive machinery, and the need for trained personnel. In 1967, for example, the Veterans Administration Hospital in Los Angeles estimated the cost of dialysis at $28,000 per year per patient. Because of these high costs, the number of people receiving dialysis in the 1960s remained small. There were approximately 800 dialysis patients in 121 centers in 1967, many of whom were subsidized by funds from voluntary agencies.[28]
David Sanders and Jesse Dukeninier, Jr., "Medical Advance and Legal Lag: Hemodialysis and Kidney Transplantation," UCLA Law Review 15 (1968): 357-413, 366.
The small size of the dialysis market operated as a disincentive to innovation and production.There were always equipment vendors ready to sell artificial kidneys to prospective buyers, even prior to the Quinton-Scribner shunt. The scarce resource was never machines, but money. But the few companies that did supply both machines and disposables for dialysis supported very little R&D directed to developing a better artificial kidney. Unit costs for kidney machines were high, the buyers were few, and the financial means for paying for treatment costs for the potential patient pool were uncertain in all but a few cases. There were simply no market incentives for private investment in R&D in building better artificial kidneys.[29]
Rettig, "Lessons Learned," 161-162, citing discussion in Biomedical Engineering Development and Production, a report by the Biomedical Engineering Resource Corporation, State of Illinois, to the National Institute of General Medical Sciences, National Institutes of Health, Washington, D.C. (July 1969), 12-21. See also National Academy of Engineering, Committee on the Interplay of Engineering with Biology and Medicine, Government Patent Policy (Washington, D.C.: National Academy of Engineering, 1970).
Access to the limited number of kidney dialysis units became a highly charged public issue. Patients turned away from the treatment would die within weeks. Centers began to create priorities for patients, sometimes based on concepts of the relative social worth of individuals. Government policy in the past had approached medical problems through disease specific interventions (such as the Heart Disease, Cancer, and Stroke Act), but the dominant policy was to support basic research and evaluate the products of research.[30]
See, generally, Plough, Borrowed Time.
The delivery of services was left to theprivate market, except in the cases of the elderly under Medicare and the indigent under Medicaid. For kidney failure, there was an available technology that worked. The problem for patients was how to pay for it.
Congress responded in 1972 by amending the Social Security Act. The new law provided federal reimbursement for nearly all the costs of dialysis for virtually every person with ESRD. The impact was immediate. Government policy dramatically increased the size of the market for dialysis equipment and supplies. In 1972, forty patients per million in population were receiving long-term dialysis in the United States, primarily supported by nonprofit organizations.[31]
U.S. Congress, Office of Technology Assessment, "Medical Technology," 34-36.
By 1977 there were 895 approved facilities, providing 7,306 dialysis stations and serving over 27,000 patients. By 1980 there were 50,000 long-term hemodialysis patients; by 1982 there were 58,391.In addition to increasing the number of patients, the federal policies favored hospital based or community based dialysis over home dialysis. Nearly 40 percent of the patients were being dialyzed in their homes at the time of the 1972 law. The percentage declined significantly, to about 13 percent, by 1980. The shift to the costlier treatment centers occurred because home dialysis was reimbursed under Part B of Medicare, which meant that fewer items were covered than in hospital and community dialysis centers under Part A. There were, of course, other relevant factors in addition to reimbursement, including the stress on family life of home dialysis and the increased age and morbidity of dialysis patients as the treatment became more common. Despite subsequent amendments to realign the incentives to encourage home dialysis, the costs of the ESRD program mounted. The actual costs of the program in 1974 were $242.5 million. By 1983, the program cost Medicare $2.2 billion.[32]
John C. Moskop, "The Moral Limits to Federal Funding for Kidney Disease," Hastings Center Report (April 1987): 11-15.
It is clear that the dialysis equipment industry thrived in this growing, publicly financed marketplace. Indeed, it has been said that "governmental intervention in the marketplace has been the single most important factor influencing both supply and demand" for the ESRD related equipment industry.[33]
Plough, Borrowed Time, 128-129.
Within five years of the establishment of the ESRD program, yearly sales in the dialysis industry were nearly $300 million.The renal dialysis equipment industry includes firms that
manufacture and distribute dialyzers or artificial kidneys, delivery and monitoring equipment, and disposable equipment such as blood tubing, connectors, and needles and syringes.[34]
Ibid., 130-154.
The industry is highly concentrated, with the three largest firms sharing 72.1 percent of the kidney machine marketplace in the mid-1970s. Suppliers of hemodialysis related products were slightly less concentrated. Baxter Travenol was the clear industry leader, with 40 percent of the total market by the mid-1970s. Its sales and earnings records were outstanding during the 1960s and 1970s, with "ongoing market expansion and new kidney dialysis products" as a key to the firm's success.[35]Ibid., 137.
Baxter flourished in an environment where price competition was rather limited. The role of government as the primary payer has been found to intensify nonprice competition. The prosperity of the industry was linked to federal dollars. By the late 1970s, large corporate firms such as Johnson & Johnson and Eli Lilly entered the ESRD market by acquiring smaller existing firms. Baxter continued to lead the field, with 38 percent of the disposable market and 34 percent of the equipment market in 1981.
There is controversy about the government's role in kidney dialysis, particularly in regard to the costs of the program. Indeed, ESRD patients represent only one-quarter of 1 percent of Medicare beneficiaries, but they consume about 4 percent of total Medicare benefit payments. There is concern that patients with poor prognoses are given treatment that cannot improve the quality of their lives. Some argue that perverse incentives in the payment structure have directed dialysis patients to more expensive centers rather than to lower-cost home dialysis. The debate illustrates the hard questions of allocation of medical resources. Advocates of universal dialysis say the United States can afford it; others question the wisdom of this expensive program, rejecting the accusation of lack of compassion for kidney patients.
I do not believe we can satisfy all the health care needs of our citizens. If that is the case, we cannot solve the problem by showing more compassion since compassionately providing care for some
will require depriving others of the care they need. In this context, then, the question of how best to use our limited health care funds becomes crucially important.[36]
Moskop, responding to letters criticizing his article "Moral Limits," in Hastings Center Report (December 1987): 43-44. See also the letters of Gerald H. Dessner and Carole Robbins Myers on pp. 42-43 of the same issue.
Government Influences Distribution: The Case of CT Scanners
The invention of the X-ray greatly enhanced physicians' ability to diagnose medical problems. Computed tomography (CT) represents a significant technological advance over earlier technologies that produce images to aid in diagnosis of disease.[37]
For a discussion of the comparative benefits of imaging technologies, see Mitchell, "Dynamic Commercialization," chap. 4, 42-56.
CT scanners are more sensitive to variations in bone and tissue density than X-rays are, and they produce images with greater resolution and speed, thereby reducing the patient's exposure to radiation.Computed tomography relies on X-ray images. However, CT emits X-rays from multiple sources, and multiple electronic receptors surrounding the patient receive them. The information from the multiple views is processed by a computer that reconstructs the "slice" of the patient's body. The CT scan has a superior ability to differentiate among soft tissues (such as the liver, spleen, or kidney) and provides a much better depiction of the anatomy and the diseases affecting these tissues than do previous technologies. Indeed, the range of density recorded increases from about 20 with conventional X-rays, or as little as 1 percent of the information in the ray, to more than 2,000 with CT scans.[38]
Bruce J. Hillman, "Government Health Policy and the Diffusion of New Medical Devices," Health Services Research 21 (December 1986): 681-711, 689.
Radon, a German researcher, worked out the early mathematical basis for the reconstruction of images from projections in 1917. Research continued during the 1950s, and in 1961, neurologist William Oldendorf constructed a tomographic device at UCLA and received the first patent in 1962. Despite subsequent work, corporations and physicians showed no interest in commercial development.[39]
U.S. Congress, Office of Technology Assessment, Policy Implications of the Computed Tomography (CT) Scanner (Washington, D.C.: GPO, August 1978).
The first commercial interest in CT occurred in Britain. Godfrey Hounsfield, an engineer at the labs of EMI, a British electronics firm, developed a CT instrument in 1967. No X-ray companies wanted to license CT technology. However, the British Department of Health supported the construction of a prototype
head scanner in the early 1970s. The Mayo Clinic installed the first X-ray scanner in the United States in June 1973.[40]
Earl P. Steinberg, Jane E. Sisk, and Katherine E. Locke, "X-ray CT and Magnetic Resonance Imagers: Diffusion Patterns and Policy Issues," New England Journal of Medicine 313 (3 October 1985): 859-864, 860.
By the end of the year, there were six EMI head scanners in the United States. Orders poured in following the showing of EMI's first-generation head scanner at the November 1973 meetings of the Radiological Society of North America. The price of the original scanners was about $400,000. There was widespread diffusion within four years, despite the high cost and the high annual operating expenses (approximately $400,000). During 1974, American sales were almost $20 million; in 1975, EMI shipped 120 units and reached sales of $60 million. The market continued to grow in the 1970s; corporate entry matched sales growth as competitors entered the American market.[41]Data on sales in the industry are available from Diagnostic Imaging (San Francisco: Miller-Freeman Publishing, 1979-1990).
Technical improvements followed, and the units moved through four generations of operating methods within four years. The first generation was the EMI head scanner that used one X-ray generator to produce a single beam of radiation that was captured in one detector. By 1975 the second generation used two or more beams and two or more detectors, producing images significantly faster. In 1976 the third-generation head scanners used a single fan beam and hundreds of contiguous detectors. The NIH had become interested by this time and contracted with American Science and Engineering (AS&E) to develop a scanner. In 1976, AS&E introduced a commercial fourth-generation body scanner that produced images in under five seconds using a fan beam source and rotating detectors.
Numerous firms entered the fast-growing field. General Electric became the leading manufacturer with a market share of 60 percent by 1981.[42]
Diagnostic Imaging (1981), 2. Annual sales data for 1981.
It achieved success by acquiring the CT operations of several competitors, and its design dominated the CT market. Because of GE's reputation and size, buyers trusted that it would remain in business and shied away from new, potentially unstable companies.By 1983 the CT market reached $750 million. The rate of adoption and the diffusion of this expensive piece of equipment was extremely rapid in its first ten years. Government money is not the only factor relevant to rapid diffusion. CT represented a major clinical breakthrough, and there were no real uncertainties
during the first few years regarding the possible outdating of the core technology. For clinical reasons alone, acquisition made sense.
However, the price was very high. The availability of federal reimbursement dollars made the acquisition decision easier for hospitals. The diffusion of CT occurred entirely during the era of cost-based hospital reimbursement that promoted technology acquisition. Medicare had developed a complicated capital-cost pass-through program that permitted hospitals to submit capital-cost reports to Medicare to recoup a percentage of capital investments based on the share of Medicare participation in designated hospital departments. All operating costs of the technology were billed on the basis of the "reasonable and necessary" test.[43]
Alan L. Hillman and J. Sanford Schwartz, "The Adoption and Diffusion of CT and MRI in the United States: A Comparative Analysis," Medical Care 23 (November 1985): 1283-1294, 1288.
Although there were some efforts to control technology diffusion by the mid-1970s, most states did not have viable regulations affecting hospital acquisition at this time.[44]
The early cost-containment policies, including Certificate of Need (CON) and state based efforts to limit diffusion, will be discussed in detail in chapter 7. It is worth noting that we have begun to see the impact of policy proliferation as conflicting policies arise. At this point, however, cost-containment plans were relatively ineffective and diffusion proceeded apace.
Where there was control on hospital purchasing, however, the response was an increase in the number of outpatient CT scanners. About 19 percent were placed outside the hospital in the first four years of availability overall, but outpatient siting was much higher in states such as New York and Massachusetts because they had some constraints in place.[45]Hillman, "Government Health Policy," 691-692.
Many studies have compared CT diffusion with the subsequent introduction of magnetic resonance imaging (MRI), a newer, competitive imaging technology. MRI was introduced in the 1980s, when cost-containment policies were more mature.[46]
See Hillman, "Government Health Policy"; Hillman and Schwartz, "Adoption and Diffusion"; and Steinberg et al., "X-ray CT."
The studies reveal that federal cost controls have played a major role in slowing diffusion rates for MRI. The conclusions underscore the theme that government payment policies in the 1960s and 1970s promoted widespread diffusion of medical technologies regardless of cost.Government Policies and Fraud: The Case of Pacemakers
Given the incentive structure of the Medicare system, it is not surprising that there were abuses. The market for cardiac pacemakers, sold and implanted almost exclusively in elderly Medicare recipients, was rife with fraud. The technology itself is
lifesaving; it was unnecessary implantation and excessive competition in the industry that caused problems. The development of the technology illustrates the best in creative innovation; the subsequent misuse of the product represents the shameful side of the medical technology market.
The adult heart beats as many as one hundred thousand times a day. The steady beating is regulated by a natural pacemaker, called the sinus node, which is located in the atrium, or upper right chamber of the heart. An electrical impulse travels to the bottom heart chambers, the ventricles, through a midway junction, the A-V node. This sequence causes the heart to contract. If the electrical signals are sent too slowly, or if the junction is blocked, the individual will experience shortness of breath, dizziness, fainting, convulsions, and death.
The concept of heart stimulation by external means dates back to the 1800s. However, the development of a totally implantable device that could stimulate the regular beating of the heart required the confluence of numerous technological advancements. Once these technologies were available, someone had to have the vision to recognize the medical need and develop the product.[47]
Battelle Columbus Laboratories, "Interactions of Science and Technology in the Innovative Process: Some Case Studies," report prepared for the National Science Foundation, Contract NSF—C 667 (Columbus, Ohio: 19 March 1973), see sec. 5, 1-14.
The pacemaker depended on innovation in semiconductor and electronics technology, principally the transistor, which became available in 1948. In addition, sophisticated battery technology was necessary for circuitry operation. World War II had stimulated the development of improved sealed alkaline dry-cell batteries that allowed the pacemaker system to be encapsulated in a resin and implanted. The pacing system, which comes in contact with cardiac tissue, required lead wires that were mechanically strong, relatively low-resistant, and well insulated against leakage. Finally, the product required silicone rubber and epoxy resins—biomaterials compatible with human tissue.
The market for such a heart-pacing device became apparent to some physicians with the first open-heart surgery in the late 1950s. To stimulate the heart after surgery, physicians had used bulky units that plugged into wall outlets and applied strong currents to the patient's heart. Wilson Greatbatch, an electrical engineer, began work on an implantable permanent cardiac pacemaker in the mid-1950s while he was at the University of
Buffalo.[48]
Wilson Greatbatch, "Vignette 8: The First Successful Implantable Cardiac Pacemaker," in U.S. Office of Technology Assessment, Inventors' Vignettes: Success and Failure in the Development of Medical Devices, Contractors' Documents, Health Program (Washington, D.C., October 1986), 8:1-15.
He found little interest in his pacemaker concept among cardiologists at the time. Through a professional organization that brought together doctors and engineers, he met William Chardack, a surgeon at the local Veterans Administration hospital. Chardack encouraged development and in April 1958 the first model cardiac pacemaker was implanted in a dog.[49]Ibid., 8:5.
Greatbatch worked full time on the device, using his own personal funds to finance the development. In the next two years, working alone in his wood-heated barn, he made fifty pacemakers, ten of which were implanted in human beings.In 1961, Chardack and Greatbatch collaborated with another electrical engineer, Earl Bakken, who was president of Medtronic, a small Minneapolis company. Medtronic had been formed in 1949 as an outgrowth of the electronic hospital equipment repair business that Bakken began as a graduate student. The selling and servicing of hospital equipment led to requests from physicians to modify equipment or to design and produce products needed for special tests. Among those physician customers was C. Walton Lillehie, a pioneer open-heart surgeon at the University of Minnesota. Lillehie turned to Medtronic to develop a reliable power source for heart stimulation. Bakken developed the first wearable, battery operated external pacemaker in 1958. These external pacemakers, however, were cumbersome, the lead wires to the heart snagged and dislodged, and there was risk of infection where the wires passed through the skin. By late 1960, the team of Bakken and Greatbatch was formed. Medtronic secured exclusive rights to produce and market the Chardack-Greatbatch implantable pacemaker device.
The first clinically successful, self-contained, battery powered pacemaker was implanted in a human being in 1960. Within a decade of their introduction, pacemakers became a useful tool for cardiac patients. But problems with many of the early devices arose, and considerable incremental innovation and development followed. Battery technology improved, in part as a result of NASA supported technology on hermetically sealed, nickel cadmium batteries that could function for years in orbiting spacecraft. The inventor of the first rechargeable cardiac pacemaker formed Pacesetter Systems to manufacture and market the rechargeable battery developed in 1968. Greatbatch worked
on the development of lithium batteries, which had considerably greater longevity than earlier ones. Cardiac Pacemakers (CPI) introduced them in 1971. Intermedics entered the market in 1973 with a second lithium battery. Additional problems arose with the lead wire technology, including fracturing and dislodgement. New materials, processes, and designs have been introduced. Medtronic has been the industry leader in electrode tip and lead wire design.[50]
Biomedical Business International 10 (14 September 1987): 136-137.
Since its inception, the pacemaker industry has been both highly competitive and highly innovative. The industry is fairly stable; while a number of companies entered early, five companies have dominated the marketplace. Medtronic, the pioneer in pacemakers, has remained the industry leader, holding 42 percent of market share in 1988. Pacesetter Systems was founded in 1970 in a joint effort with the Applied Physics Laboratory of the Johns Hopkins University. In 1985 the company was acquired by Siemens-Elema AB, then a world leader in pacemakers, though it had only a small share of the U.S. market. The buyout gave Siemens-Pacesetter the second position in the current U.S. market.
Another early entrant was CPI, founded in 1971 by a management group formerly associated with Medtronic. The firm was an innovator in lithium iodide battery powered pacemakers, and was acquired by Eli Lilly in 1978. Its market share is about 5 percent. Albert Beutel, a young pacemaker salesman, founded Intermedics in 1974. It started operations as the nation's second largest producer of lithium iodine battery powered pacers and holds a 14 percent market share.[51]
Ibid. See also Robert McGough, "Everybody's Money," Forbes, 27 February 1984, 149, 152.
Cordis Corporation, which pioneered the programmable pacemakers, recently sold its floundering pacemaker division to Telectronics, a U.S. subsidiary of Australia's Nucleus Limited and one of the world's largest producers of implantable pacemakers. Telectronics took over the manufacturing facility of General Electric in 1976, when that firm withdrew from pacemaker production. Telectronics/Cordis holds a 13 percent market share.Over the course of the last twelve years, the industry was highly innovative. The earliest devices were single-chamber products with pulse generators permanently preset at the time
of implantation. Technological advances included the development of multiprogrammable units that allow the physician to modify the parameters, such as the rate of beats and the level of electrical stimulation, without surgery. Another important innovation was the dual-chamber unit. Dual-chamber devices benefit patients who have a loss of synchrony between the two chambers of the heart. Dual-chamber units represented only 5 percent of implants in 1981 and 23 percent of implants by 1984.[52]
Biomedical Business International 10 (14 September 1987): 137.
The most recent innovation was the introduction of the rate-responsive pacemaker. Medtronic's Activitrax is the first single-chamber pacemaker that detects body movement and automatically adjusts paced heart rates based on activity. This is accomplished by means of an activity sensing crystal bonded to the inside of the pacemaker's titanium shell. When the crystal is stressed by pressure produced from body activity, it creates a tiny electrical current that signals the pacemaker to change rates. Medtronic received FDA approval in June 1986. Within the first year on the market, rate-responsive pacemakers captured 22 percent of the American market and account for Medtronic's continuing success.[53]
Ibid.
(See figure 15.)There has been additional innovation in lead wire technology. Lead wires link the power source to the heart muscle. Medtronic has been the leader in electrode tip design. Advances in software, sensors to detect and respond to physiologic demands, and microelectronics and batteries to reduce the size and weight of pacemakers continue to advance the usefulness of this medical device for more and more patients.
By the time pacemakers were sophisticated enough to enter the medical marketplace, the Medicare program was well underway. Because the medical conditions that respond to pacemaking generally afflict older people, innovators in this field had a federally subsidized market for their products.
The pacemaker market grew steadily with sales increasing from 60,000 units in 1974, to 80,000 in 1976, and to 114,000 in 1984. Throughout the 1980s, about 85 percent of all pacemaker surgeries were eligible for Medicare reimbursement. In 1984, Medicare paid $775 million to hospitals for pacemaker surgeries, including $400 million for hospital purchases of the

Figure 15.
The Medtronic Legend™ pacemaker. Photo courtesy of Medtronic, Inc.
pacemaker devices themselves. Given the role of Medicare, the pacemaker industry is inextricably linked to the government policy that essentially supports the marketplace.[54]
Medicare payments in 1984 totaled $42 billion to 6,000 hospitals. Sales apparently fell off slightly in response to reimbursement and pricing pressures in the next two years. By 1986 the market resumed growth, with forecasts of 6 percent per unit and 10 percent revenue growth per year between 1987 and 1990.
While innovative and competitive, this industry is hardly a model of corporate responsibility. The structure of the federally subsidized market fostered some of the high-pressure sales tactics that led to significant fraud and abuse. Under the Medicare cost-plus system, there was little incentive for hospitals to seek discounts and no incentive to get warranty credits when a pacemaker under warranty failed. Companies began rapacious competition on nonprice attributes. A 1981 report stated that "the absence of price competition as a significant factor in the domestic market has, of course, spawned the kind of competitive environment that exists today. The cardiologist or surgeon who makes the product decision is insensitive to price since the pacemaker … is reimbursable by various health insurance programs."[55]
Kidder, Peabody, "Cardiac Pacemaker Industry Analysis, 22 January 1981," cited in the Office of Inspector General, Draft Audit Report, More Efficient Procurement of Heart Pacemakers Could Result in Medicare Savings of Over $64 Million Annually, ACN 08-22608, submitted to the Senate Special Committee on Aging, Hearings on Fraud, Waste, and Abuse in the Medicare Pacemaker Industry, 97th Cong., 2d sess. (Washington, D.C.: GPO, 1982), 139-140.
There were roughly 500–550 salespeople for only 1,500 physicians
that implanted pacemakers. An article in Medical World News in September 1982 reported that companies were offering physicians free vacations, stock options at reduced prices, cash kickbacks, and consulting jobs with liberal compensation to persuade them to use the products.[56]
Hearings on Fraud, Waste, and Abuse, app. 2, item 6, Draft Audit Report, HHS, 2 September 1982, from Richard P. Kusserow, inspector general, to Carolyne Davis, administrator, HCFA.
Companies instituted sales incentive programs to encourage unnecessary implantations, as well as unnecessary explantations and reinsertions of new products.The Senate Special Committee on Aging held hearings in 1982 to investigate the industry. One disgruntled former salesman summed up the situation: "In all my twenty years experience in the medical sales field, I have never seen a business so dirty, so immensely profitable, and so absent normal competitive price controls as this one."[57]
Hearings on Fraud, Waste, and Abuse, testimony of Howard Hofferman, 30-50.
The General Accounting Office undertook a study of the situation as a result of the 1982 hearings, and Congress held follow-up hearings in 1985.[58]Comptroller General, Report to the Chair, Senate Special Committee on Aging, Medicare's Policies and Prospective Payment Rates for Cardiac Pacemaker Surgeries Need Review and Revision, GAO-HRD-8539, 26 February 1985.
By then, however, Medicare cost controls had been instituted, and the hospital purchasing environment had changed. In an effort to control costs generally, the HCFA instituted oversight and price-setting controls. The GAO found that PPS had given hospitals financial incentives to be more cost-conscious purchasers. Clearly the pacemaker producers would feel the pinch as price became an important attribute in pacemaker purchasing decisions.Other companies encountered criminal problems. On 12 October 1983, Pacesetter Systems was indicted on four counts of offering to pay and paying kickbacks and one count of conspiracy. Company officials pleaded guilty to charges, and its former president pleaded nolo contendere. On 6 July 1984, Telectronics pleaded guilty to four counts of having paid kickbacks to a cardiologist. The FBI also uncovered more sophisticated kickback schemes.[59]
Senate Special Committee on Aging, Pacemakers Revisited: A Saga of Benign Neglect, 99th Cong., 1st sess. (Washington, D.C.: GPO, 1985), 99-104, 129.
Cordis, unsullied in the 1982 congressional investigations, pleaded guilty in 1988 to multiple federal charges that it sold pacemakers it knew were faulty.[60]Michael Allen, "Cordis Admits It Hid Defects of Pacemakers," Wall Street Journal, 1 September 1988, 6.
A federal judge rejected the plea agreement with the government in which Cordis would have paid a $123,000 fine, saying that "the … fine simply is not commensurate with the crime." In April 1989 Cordis pleaded guilty to twenty-five criminal violations, including thirteen felony counts. Cordis agreed to pay the maximum fine of $623,000, plus $141,000 to reimburse the government for its investigativecosts, and to pay $5 million for the civil fraud of selling defective pacemakers to the VA and the DHHS. Four former executives also faced trial in 1989 on forty-three counts.[61]
Washington Post, 13 August 1989, 41.
Medtronic settled for $3 million with the government to reimburse Medicare for replacement of defective pacemaker leads.[62]"Medtronic Inc. Expects Record Sales and Profit in Current Fiscal Year," Wall Street Journal, 21 August 1987, 6.
It is clear that the Medicare program, per se, did not cause the abusive practices in some pacemaker companies. However, the availability of reimbursement dollars promoted an atmosphere of non-price competition that encouraged less scrupulous companies. More recent efforts to establish oversight and financial incentive for hospitals have begun to change the environment. Medicare's lack of controls must take some responsibility for the abuses.
Conclusion
The three technologies presented here represent important medical products. Countless lives have been saved by kidney dialysis. More accurate diagnosis through the CT scanner benefits all patients. The quality of life for heart patients is improved by implanted pacemakers. These cases are representative of many medical devices appearing on the market in the 1960s and 1970s. Public policy promoted the distribution of medical devices. In an era when costs were relatively unimportant and few restraints on acquisition existed, devices had potentially large markets. From an industry perspective, this was a golden age for device innovation. Until the 1970s, the prescriptions for our patient—the medical device industry—fueled growth and development at the discovery and the distribution stage.
However, we have seen that unrestrained industry growth is not necessarily socially desirable. Abuses occurred in both Medicare and Medicaid programs. This golden age for device producers could not and did not last. Early efforts to contain costs and slow diffusion appeared by the mid-1970s. Concern about safety led to expansion of FDA regulation. New prescriptions were needed to keep the patient in its place. We now turn to these new efforts to control and inhibit device technology.
5
Government Inhibits Medical Device Discovery: Regulation
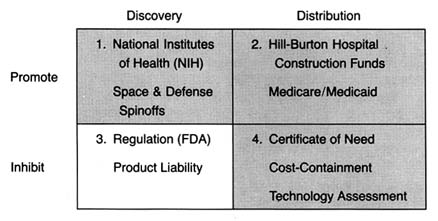
Figure 16. The policy matrix.
The policies discussed in chapters 3 and 4 changed American medicine. Neither was designed specifically to promote the medical device industry; federal R&D was primarily directed at bio-medical research, and federal and state payment programs were designed to increase access to health care. Nevertheless, the medical device industry benefited. Increasingly sophisticated devices became available to large numbers of people. Devices began to lose their association with quack products and were frequently linked to therapeutic breakthroughs.
However, many of these sophisticated new technologies, such as pacemakers, kidney dialysis equipment, and diagnostic instruments, presented risks along with their potential benefits. Adverse effects associated with cardiac pacemakers, intrauterine
devices (IUDs), and implanted interocular lenses, in particular, raised public concern.
The ground was fertile for federal regulation of these products. The late 1960s and the 1970s were a time of growing consumer activism and power. Product safety became a desirable value that the federal government was expected to protect. The government was expanding safety regulation in many new areas, including consumer products, the workplace, and the environment. In many instances, Congress created new regulatory agencies to address safety issues, including the Environmental Protection Agency (1969), the Occupational Safety and Health Administration (1972), and the Consumer Product Safety Commission (1972). In the case of medical devices, the FDA was already in place with expertise and some preexisting jurisdiction over the industry.
This chapter describes the political process leading to the expanded regulation of medical devices, the first major legislation directed exclusively at the device industry. (See figure 16.) Issues related to the structure and implementation of the Medical Device Amendments of 1976 are discussed, illustrated by cases such as IUDs, lithotripsy equipment, pacemaker components, and tampons.
Once again, a brief caveat before proceeding. By the 1970s, the effects of policy proliferation became apparent. For example, the impact of some regulation was blunted by the Medicare policies that encouraged purchases regardless of cost. Thus costs associated with regulation could be passed along without concern. For other products, the interaction with regulation and product liability increased environmental threats. Issues relating to product liability are discussed fully in chapter 6.
Medical Device Regulation Comes of Age
The Limitations of FDA Authority
As we saw in chapter 2, the FDA had very limited powers over medical devices under the 1938 Food, Drug, and Cosmetic Act. Its primary authority was seizure of individual devices found to be adulterated or misbranded. Most of the FDA's early enforcement
activity was directed toward controlling obvious quack devices. However, even this limited regulatory activity declined during World War II. Because of the scarcity of metals and other critical materials, production of nonessential devices was restricted, and consequently the pace of seizures and prosecutions dropped to less than six per year.[1]
House Committee on Government Operations, Hearings on Regulation of Medical Devices (Intrauterine Contraceptive Devices), 93rd Cong., 1st sess. (Washington, D.C.: GPO, 1973), 180.
After World War II, there was an increase in device quackery because of the cheap availability of war surplus electrical and electronic equipment.[2]
Davidson, "Preventive 'Medicine' for Medical Devices: Is Further Regulation Required?" Marquette Law Review 55 (Fall 1972): 423-424.
A variety of products that used dangerous gases (such as ozone and chlorine), radio waves, heat, and massage were marketed for the treatment of almost every disease known. Among the most dangerous were quack devices that used radium, uranium ore, and other radioactive substances and that purported to cure common problems such as sinus infections and arthritis.The pace of FDA seizures picked up in response. In a report in 1963, the Bureau of Enforcement of the FDA's Device Division stated that from 1961 to 1963, the FDA seized 111 different types of misbranded or worthless devices, involving 15,070 individual units. Fifty-four diagnostic and treatment devices were taken in 358 seizure actions from June 1962 to June 1963.[3]
Milstead, 1963 Congress on Quackery, 30.
Some states tried to tackle the problem with their own legislation. For example, California had passed a state Pure Foods and Drug Act in 1907, one year after the first federal act. The law prohibited any claim for a food, drug, or device that was false or misleading in any particular. California brought over sixty court actions from 1948 to 1957. The focus of this early activity was on fraudulent devices; concern about the safety of clearly therapeutic products came later.[4]Ibid.
Congressional legislative activity focused on drug risks in the late 1960s. Congress increased FDA power to regulate drugs in 1962 in response to controversial evidence linking birth defects and the drug thalidomide.[5]
Temin, Taking Your Medicine, 123-126.
The 1962 amendments to the Food, Drug, and Cosmetic Act greatly strengthened federal power over drugs by requiring proof that the product was both safe and efficacious before it received FDA marketing approval. Because of the previous distinction between drugs and devices made in the 1938 law, these expanded powers did not apply to new medical devices.[6]See discussion in chapter 2.
By the late 1960s, problems associated with legitimate, therapeutically desirable medical devices that were flooding the marketplace began to surface. In 1970, Dr. Theodore Cooper, then director of the NIH Heart and Lung Institute, completed a survey of the previous ten years that revealed 10,000 injuries from medical devices, including 731 deaths. Defective heart valves caused 512 of the deaths.[7]
U.S. Department of Health, Education, and Welfare, Cooper Committee, Medical Devices: A Legislative Plan, Study Group on Medical Devices (Washington, D.C.: GPO, 1970). Cited and discussed in Medical Device Amendments of 1975, Hearings on H.R. 5545, H.R. 974, and S. 510 Before the Subcommittee on Health and the Environment of the Committee on Interstate and Foreign Commerce, statement of Rep. Fred B. Rooney, 94th Cong., 1st sess., 199. See also Theodore Cooper, "Device Legislation," Food, Drug, Cosmetic Law Journal 26 (April 1971): 165-172. There have been challenges to the data in the Cooper report, but the public attention the study received made the issue of device safety politically salient.
In particular, problems had arisen involving defective pacemakers[8]U.S. Comptroller General, Food and Drug Administration's Investigation of Defective Cardiac Pacemakers Recalled by the General Electric Company 21 (1975). GE decided to voluntarily recall over 22,000 pacemakers because some malfunctioned due to moisture that seeped into the pacemaker circuitry, probably due to faulty seals.
and intrauterine devices.[9]See discussion in this section.
Congress, the FDA, and the public became concerned.Creative Regulation by the FDA
The increase in legitimate medical devices complicated the FDA's regulatory efforts. The sophisticated new technologies, such as pacemakers, kidney dialysis units, cardiac, renal, and other catheters, surgical implants, and diagnostic instruments, challenged the FDA's expertise. The agency's regulatory power was limited to seizure of products already on the market. In order to bring a seizure action under the law, the FDA had to consult experts, sponsor research, and gather data to meet its statutory burden of proof in court. The real problem was that seizures were simply not a reasonable response to devices that had benefits as well as risks. The goal was not to remove these products from the market as much as to ensure that these innovations were safe.
In the absence of additional authority, the FDA began to implement the law more aggressively. One tactic was to construe the statutory definition of drug broadly enough to include products that were clearly medical devices and thus would allow the agency to regulate devices much as it regulated drugs.
Device companies challenged this effort to impose drug regulation on devices. Two important court decisions in the late 1960s upheld the FDA's broad reading of the term drug . In AMP v. Gardner , the court reviewed the FDA's classification as a "new drug" a nylon binding device used to tie off severed blood vessels during surgery.[10]
389 F.2d 825 (2d Cir. 1968).
The court broadly construed the purpose of the 1938 act and the 1962 amendments, holding that the goal was to keep inadequately tested and potentially harmful "medical products" out of interstate commerce. Emphasizing the protectivepurposes of the law enabled the government to regulate as a drug any product not generally recognized as safe.
The next year, the Supreme Court followed similar reasoning in United States v. An Article of Drug … Bacto-Unidisk .[11]
394 U.S. 784 (1969).
In 1960, after the product had been in use for four years, the secretary of HEW classified an antibiotic disk as a drug. The product, which never came into contact with the human body and was therefore not metabolized, was used as a screening test in a laboratory to determine the proper antibiotic to administer. Its classification as a drug came after the agency received numerous complaints from the medical profession, hospitals, and laboratory technicians that the statements of potency for the disks were unreliable. The FDA found it "vital for the protection of public health" to adopt the regulations.[12]25 Federal Register 9370 (30 September 1960).
The Court, acknowledging that the FDA was an expert agency charged with the enforcement of remedial regulation, deferred to the secretary's medical judgment. It concluded that the term drug was a legal term of art for purposes of the law, which was a broader interpretation than the strict medical definition. The Court determined that the parallel definitions of drugs and devices, discussed previously, was "semantic." Concluding that there was no "practical significance to the distinction" until subsequent amendments to the 1938 Act, the Court gave the term "a liberal construction consistent with the act's overriding purpose to protect public health."[13]
394 U.S. 784, 798 (1969).
IUDs
The FDA's response to problems associated with implanted intrauterine devices illustrates its efforts to regulate creatively to protect the public. IUDs to prevent pregnancy had been available since the turn of the century. Although they had been generally dismissed as dangerous by respectable practitioners, the technology began to be reevaluated in the late 1950s. The renewed interest in contraceptives, the availability of inert plastics that caused fewer tissue reactions, and the growing controversies about the safety of the new contraceptive pills all encouraged research on IUDs.
IUD devices began to enter the market in the mid-1960s.
From 1969 to the early 1970s, IUD use skyrocketed. By the end of 1970, three million women in the United States had been fitted with a variety of IUD devices. Marketing was aggressive, and the competition among firms was keen. The top sellers included Ortho Pharmaceutical's Lippes Loop, the Saf-T-Coil produced by Schmidt Labs, and the now infamous Dalkon Shield introduced by A. H. Robins in January 1971.[14]
The Dalkon Shield claimed to be superior to other products because of its unique shape. The nature of the product and the harms related to its design are discussed more fully in chapter 6.
G. D. Searle entered the market in the same year with the Cu-7, a device shaped like the number 7 with a small thread of copper wound around the vertical arm, which the company claimed increased the product's efficacy. The data regarding unit sales testify to the early success of these products (see table 6).Despite burgeoning sales, product safety remained a concern. As early as 1968, an FDA advisory committee on obstetrics and gynecology cited significant injuries and some deaths associated with IUDs.[15]
House Hearings on Medical Devices, Advisory Committee on Obstetrics and Gynecology of the Food and Drug Administration, Report on Intrauterine Contraceptive Devices (1968), 441.
The devices generated numerous complaints, and there were reports of infections, sterility, and, on some occasions, death.Under the device provisions of the 1938 law, the FDA had only the limited power to seize individual products, an impractical remedy in this situation. An internal FDA memorandum recommended that the AMP v. Gardner precedent be used to designate all products intended for prolonged internal use to be considered "drugs" for purposes of premarket approval.[16]
House Hearings on Regulation of Medical Devices, memorandum of William Goodrich, assistant general counsel of the FDA, 19 March 1968, 205-206.
If this recommendation had been adopted, all implanted devices, including pacemakers and IUDs, would have been officially considered drugs under the law.The agency did not go so far. In 1971 it considered a proposed rule on the classification of IUDs. At that time, G. D. Searle began to market the Cu-7 IUD. Because this device contained a noninert substance, the FDA's final rule in 1973 distinguished between device-type IUDs and drug IUDs. The agency treated as a regulated drug any IUD that contained heavy metals or any substance that might be biologically active in the body. An IUD was a device, hence exempt from premarket approval requirements, if it was fabricated entirely from inactive materials or if substances "added to improve the physical characteristics [did] not contribute to contraception through chemical action on or within the body."[17]
44 Federal Register 6173 (31 January 1979).
Thus, Searle's Cu-7 was subjected
| ||||||||||||||||||||||||
to premarket approval, as was Progestasert, an IUD with a timed-release contraceptive hormone that entered the market in 1976.
FDA officials later admitted the frustration of using the drug provisions as a substitute for adequate device regulation. Despite efforts to increase the staff for the Medical Devices Program, the agency had limited resources for the regulation of devices as drugs.[18]
House Hearings on Regulation of Medical Devices, 183.
Other important jurisdictional issues arose as well. In 1972, Peter Barton Hutt, general counsel for the FDA, said that "The administrative burden of handling all devices under the new drug provisions of the act would be overwhelming…. If we were to reclassify all devices as new drugs, difficult legal issues would be raised about our authority to allow them to remain on the market pending approval of an NDA [new drug approval]. Wholesale removal of marketed products would, of course, not be medically warranted."[19]House Hearings on Regulation of Medical Devices, statement of Peter Barton Hutt, 209.
Without clear legislative authority, the FDA was unwilling to regulate devices through use of the provisions intended to apply only to drugs.As might be expected, problems related to devices continued to arise. When reports of severe adverse reactions were specifically associated with the Dalkon Shield, an advisor to the FDA recommended that it be removed from the market. The big question was how to do so under the law. The only power the FDA had was to get a court injunction to halt interstate shipments of adulterated and misbranded products and to proceed to seize them one at a time. Of course, for implanted products, safety would clearly be better served by preventing these products from entering the market in the first place. A. H. Robins finally admitted the inevitability of some government action,
and, in the wake of significant adverse publicity, the company voluntarily suspended sales, pending a hearing of FDA advisory bodies. The product never returned to the market.[20]
Enter policy proliferation. The company's action may well have been motivated primarily by the fear of lawsuits, not the fear of FDA action. In any event, the FDA had few powers to invoke. Issues relating to product liability will be discussed in chapter 6.
The FDA ultimately got the outcome it desired, but its formal regulatory impotence did not go unnoticed by Congress or the public.Congress Takes Action
The limited powers of the FDA had been graphically demonstrated during the Dalkon Shield controversy. Congress not only was aware of the publicity concerning harmful devices but also had held hearings on several products during this period.[21]
Pacemaker hearings, medical device (IUD) hearings.
Bills to expand the FDA's authority over devices had been introduced every year from 1969 to 1975. The likelihood of congressional action was increased by the fact that consumer activism was at its peak. The controversies surrounding IUDs mobilized the nascent women's movement, and defective cardiac pacemakers caused concern among the elderly. Ralph Nader's Health Research Group vigorously lobbied government to protect consumers in the areas of medicine and health products.On the other side, the medical device industry was not well organized. Until this time, government had been either neutral or a benefactor, not a threat to the industry's well-being. In fact, there was no trade association until the mid-1970s. Unlike the drug industry, which was represented by the old and powerful Pharmaceutical Manufacturers Association (PMA), the device producers were a disparate group with no clearly identifiable or shared issues. Many were small innovators with little or no experience with the political process.
The prospect of regulation spurred organizing efforts. The Health Industry Manufacturers Association (HIMA) formed in 1976, but it was too late to stop Congress from regulating the industry. Indeed, the organization was established in direct response to the new regulatory threat. Some larger device companies had their own Washington offices that handled government relations; for smaller companies, HIMA was the only representation. HIMA has since become larger and more active, but in the 1970s members of the industry were reactive, not proactive. Regulation was only a matter of time.
The Medical Device Amendments of 1976
The Medical Device Amendments of 1976 sought to provide "reasonable assurance of safety and effectiveness' for all devices.[22]
Public Law 94-295, 90 Stat. 539 (1976) codified at 21 United States Code secs. 360c-360k (1982), (a)(1-3). For detailed discussion of the Medical Device Amendments, see Foote, "Loops and Loopholes"; David A. Kessler, Stuart M. Pape, and David N. Sundwall, "The Federal Regulation of Medical Devices," New England Journal of Medicine 317 (6 August 1987): 357-366; and Jonathan S. Kahan, "The Evolution of FDA Regulation of New Medical Device Technology and Product Applications," Food, Drug, Cosmetic Law Journal 41 (1986): 207-214.
The FDA was to determine whether such assurance existed by "weighing any probable benefit to health from the use of the device against any probable risk of injury or illness from such use." The law conferred powers upon the FDA to regulate medical devices during all phases of development, testing, production, distribution, and use.In order to accomplish these goals, Congress devised a complicated regulatory scheme. This complexity arose from both the diversity of the products to be regulated and the lack of trust between Congress and the FDA at that time. The diversity of devices dictated a regulatory system that would provide levels of government scrutiny appropriate to the nature of each device. The lack of trust meant that Congress did not give the agency discretion to implement the law; instead, detailed provisions were intended to force the agency to regulate with vigor.[23]
See letter from Representative Paul Rogers, one of the authors of the legislation to Alexander Schmidt, commissioner of the FDA, 21 June 1976 (cited in Foote, "Administrative Preemption," 1446, n. 74).
In the law, Congress used two different methods to group medical devices: first, devices were divided into three classes on the basis of risk, with increasing rigor from Class I to Class III; and second, they were divided into seven categories (preamendment, postamendment, substantially equivalent, implant, custom, investigational, and transitional). It is not surprising that a complicated system emerged from these numerous divisions.
In brief, Class I, general controls, is the least regulated class, and it requires producers to comply with regulations on registration, premarketing notice, record keeping, labeling, reporting of adverse experiences, and good manufacturing processes. These controls apply to all three classes of devices. Manufacturers of Class I devices must register their establishments and list their devices with the FDA and notify it at least ninety days before they intend to market a device. Tongue depressors are an example of a Class I device. Class II devices are those for which general controls are considered insufficient to ensure safety and effectiveness and for which information exists to establish performance standards. Well over half of the devices on the market are in Class II.
Class III consists of those devices for which general controls alone are insufficient to ensure safety and efficacy and for which information does not exist to establish a performance standard and the device supports life, prevents health impairment, or presents a potentially unreasonable risk of illness or injury. Only those devices placed in Class III receive premarket reviews similar to those conducted on drugs. The manufacturer must submit a premarket approval application (PMA) that provides sufficient data to assure the FDA that the device is safe and efficacious. Only a small fraction (about 8 percent) of all devices are placed in Class III, including heart valves and other implanted products.
The categories set forth in the law established guidelines for classification. For example, implanted devices are assumed to require a Class III placement, and custom and investigational devices can be exempt from premarket testing and performance standards. Examples of implants include cardiac pacemakers and artificial hips; custom devices include dentures and orthopedic shoes; and at present investigational devices include the artificial heart and positron emission tomography (PET) imaging machines. Transitional devices are those regulated as drugs (such as copper-based IUDs) before the passage of the law, and they are automatically assigned to Class III. Devices on the market at the time the law was passed are referred to as preamendment or preenactment devices . These products are assumed to be in Class I unless their safety and efficacy cannot be ensured without more regulation. Manufacturers can petition for reclassification under certain circumstances.
One provision has assumed a greater significance in practice than was perceived when the law was drafted. New devices that are shown to be "substantially equivalent" to a device on the market before the law was passed are assigned to the same class as their earlier counterparts, and manufacturers have to provide information on testing and approval only if the earlier products required it. To receive the designation of substantial equivalence, section 510k requires producers to notify the FDA at least ninety days before marketing. This premarket notification must contain enough information for the FDA to determine whether the device is substantially equivalent to a device already being
marketed.[24]
The process is referred to as a 510k after the number of the provision in the bill.
A product need not be identical, but it cannot differ markedly in design or materials. If a device meets the equivalence requirement, it can go directly to market without further scrutiny. The benefits to manufacturers of "a 510k" are enormous, as their products can enter the market quickly and without great effort.This complex regulatory framework invites maneuvering on the part of producers. Unlike the drug law that treats all new chemical entities (NCEs) alike, the medical device amendments present a large number of options and opportunities to manipulate the system. The FDA's management of the device law has been very controversial. Frequent hearings and investigations by Congress have tended to conclude that the FDA has not measured up.[25]
For example, Report of the House Subcommittee on Oversight and Investigations, Committee on Energy and Commerce, MedicalDevice Regulation: The FDA's Neglected Child (Washington, D.C.: GPO, 1983); Comptroller General Report to Congress, Federal Regulation of Medical Devices—Problems Still to Be Overcome, GAO-HRD-83-53 (Washington, D.C.: General Accounting Office, September 1983); United States General Accounting Office, Report to the Chairman, Senate Committee on Governmental Affairs, Early Warning of Problems Is Hampered by Severe Underreporting, GAO-PEMD-87-1 (Washington, D.C.: General Accounting Office, December 1986); and General Accounting Office, Briefing Report to the Chairman, House Subcommittee on Health and the Environment, Committee on Energy and Commerce, Medical Device Recalls: An Overview and Analysis 1983-1988, GAO-PEMD-89-15r (Washington, D.C.: General Accounting Office, August 1989).
On the other hand, some in industry have accused the FDA of overregulation, inefficiency, and harassment. For its part, the FDA claims that limited resources and expanding demands hamper enforcement.Implementation of the FDA's New Powers
FDA authority over the device industry falls into two general categories—barriers to market entry (premarket controls) and the power to oversee production of a marketed product or to remove it from the marketplace (postmarket controls). Issues of implementation have arisen in both categories.
Premarket Controls: Problems of Classification and Categorization
The classification of the device determines the level of scrutiny it receives. The ability of the law to reduce risks depends upon a rational classification process. If barriers are too high, desirable innovations will be discouraged. If they are too low, the public will not receive the protection the law intended. Given the number of vastly different devices subject to regulation and the limited resources and energy of the agency, there are many problems regarding classifications.
Because the degree of regulation varies significantly depending on the classification of a device, it is not surprising that there
have been disputes over how the FDA evaluates industry petitions to reclassify a device. An important set of judicial opinions clarified the FDAs authority to deny reclassification petitions.
Two appellate court decisions in the D.C. Circuit Court of Appeals affirmed the FDA's discretion to deny reclassification petitions if it finds insufficient scientific evidence to do so. In Contact Lens Manufacturers Association v. FDA,[26]
766 F.2d 592 (1985).
the trade association challenged the FDA's refusal to reclassify rigid gas-permeable lenses (RGP) from Class III to Class I. Hard contact lenses (polymethylmethacrylate, or PMMA) have been marketed in the United States since the early 1950s. Soft lenses (hydroxyethylmethacrylate, or HEMA) lenses are a more recent development. In September 1975, citing their "novelty," the FDA announced that all HEMA lenses would be regarded as "new drugs" and regulated as such. Upon passage of the Medical Device Amendments, all devices regulated as drugs were automatically in Class III under a "transitional" device provision.[27]21 U.S.C. sec. 360j(1)(1)(E).
In 1981, the FDA considered reclassification of RGP lenses, a type of soft lens, into Class I, which would greatly have reduced regulatory oversight. However, after receiving extensive comments, the FDA withdrew its proposed reclassification,[28]48 Federal Register 56, 778 (1983).
and the industry association petitioned the court for review of the FDA's power. The court upheld the FDA's authority to withdraw its proposal. In General Medical v. FDA[29]General Medical v. FDA, 770 F.2d 214 (1985).
the court upheld the FDA's decision to deny a petition for reclassification of the Drionic device, a product used to prevent excessive perspiration.Additional problems have arisen regarding devices on the market before the passage of the law. Many of these devices are considered Class III, but they have been largely ignored. The first premarket approval application (PMA) for a preenactment device was not required until June 1984, a full eight years after the law had been passed. The FDA stated that the implanted cerebellar stimulator was chosen because of contradictory information about its effectiveness for some indications.[30]
The device is used to electrically stimulate the cerebellar cortex of a patient's brain in treatment of intractable epilepsy and some movement disorders.
In 1983, the FDA published a notice of intent to require premarketing approval of twelve other preenactment devices.[31]Medical Device Bulletin (Washington, D.C.: FDA, August 1984). See also Kessler et al., "Federal Regulation," 362, nn. 5, 12.
After years of controversy, the FDA finally required PMAs for preenactment heart valves in June 1987.The FDA has also been criticized for its failure to implement
the Class II requirements. Class II devices are supposed to meet performance standards to ensure safety and efficacy. The statutory provisions for the selection of a standard-setting body and the drafting of standards are exceedingly detailed.[32]
The excessive detail in the law derives from a congressional desire to limit FDA discretion by carefully spelling out procedures to be followed. This strategy is ineffective, as it has hampered the implementation of many provisions.
The process itself would be costly and slow, arguably locking in the state of the art at the time a standard was set. More than 50 percent of the 1,700 classified types of devices are in Class II, but not one performance standard had been issued by 1988.[33]Kessler et al., "Federal Regulation," 362.
Given that performance standards are the only distinction between Class II and Class I, this situation makes a mockery of the classification system.Premarket Notification: Pacemaker Leads
Problems related to pacemaker leads illustrate the controversy surrounding 510k, the provision for premarket notification as a substitute for FDA review. Pacemaker leads connect a pacemaker's power source to the heart muscle itself. Innovators have had many problems with lead design—leads tend to become dislodged and render the pacing device ineffective. Many new designs emerged as the pacemaker industry developed, and there were a significant number of lead failures.
Congress held hearings in 1984 on the Medtronic polyurethane pacemaker leads. The congressional inquiry was prompted by reports that certain Medtronic pacemaker leads failed at abnormally high rates—about 10 percent or greater by the third year after implantation. There was much concern about the history and status of Medtronic's premarket notification (510k) submissions for polyurethane leads; major manufacturing and design changes that could affect the safety and effectiveness of their leads occurred without any FDA premarket scrutiny. Lead innovations had been designated "substantially equivalent" because leads performed the same function as the earlier products, but these were clearly very different in design, materials, and structure from their predecessors.
The FDA subsequently modified its procedures for reviewing premarket notification applications because of its experiences with Medtronic. By the mid-1980s, FDA required more evidence of comparable safety and effectiveness to support substantial
equivalence decisions: results of all types of testing, more elaborate statistical analyses of test data, and, for cardiovascular devices that are life-supporting, life-sustaining, or implanted, summaries of equivalence similar to summaries of safety and effectiveness required for premarket approval.[34]
Food and Drug Administration, Guidance on the Center for Devices and Radiological Health's Premarket Notification and Review Program (Department of Health and Human Services, 1986).
The underlying premise for 510k procedures was that a product was substantially similar to one on the market, and presumably its safety and efficacy had already been determined. It is fundamentally inconsistent to have innovative design and manufacturing changes enter the market in this fashion. After over a decade, the FDA finally began to rectify the problem.The Burdens of Class III: The Case of Extracorporeal Shock-wave Lithotripsy
Only a very small percentage of devices are placed in Class III and therefore are subject to the full premarket review similar to drug evaluations. This process can be extremely time-consuming and expensive for the producer. Of course, the purpose is to produce sufficient safety and efficacy data to ensure that the product meets the statutory standards before entering the market. The introduction of extracorporeal shock-wave lithotripsy (ESWL) in 1984 illustrates dynamic innovation in the private sector and its interrelationship with regulation.
Kidney stones in the urinary tract (urolithiasis) develop when minerals, primarily calcium and oxalate, form crystals rather than being diluted and passed out of the body. More than 300,000 patients a year (70 percent of them young to middle-aged males) develop kidney stones. For many, treatment with fluids and painkillers is sufficient; for 20 to 40 percent, the stones cause infections, impaired kidney function, or severe pain and warrant more aggressive intervention. Until the last decade, surgery to remove the stones was the only form of medical help for severe kidney stone problems.[35]
Deborah B. Citrin, "Extracorporeal Shock-wave Lithotripsy," Spectrum (Arthur D. Little Decision Resources, August 1987): 2:85-88.
The first major advance was in the early 1980s. Percutaneous endoscopic techniques permitted a physician to make a small incision and attempt stone extraction or disintegration using a special scope. The second major advance was ESWL. Its most exciting feature was that it offered a noninvasive way to treat kidney
stones. The first ESWL devices required the patient to be placed in a water bath. After X-ray monitors positioned the patient, a high-voltage underwater spark generated intense sound waves. The resultant waves disintegrated the stone into fine bits of sand that could easily pass out of the body. (The term lithotripsy comes from classical Greek and means "stone crushing."[36]
Alan N. G. Barkun and Thierry Ponchon, "Extracorporeal Biliary Lithotripsy: Review of Experimental Studies and a Clinical Update," Annals of Internal Medicine 112 (15 January 1990): 126-137, 126.
) Subsequent technological modifications eliminated the need for the water bath, and mobile units were developed. Devices that use optical fibers as conduits for laser light pulses that fragment the stones are currently in experimental stages of development.[37]Gary M. Stephenson and Greg Freiherr, "High-Tech Attack: How Lithotripters Chip Away Stones," Healthweek, 4 December 1989, 25.
While ESWL is an exciting innovation, several factors might have led to skepticism about its likely commercial success. The equipment was very expensive (early models cost at least $1.5 million), there was a viable surgical alternative, and the patient base was small and likely to remain so. And because the device was in Class III, it was subject to the highest level of premarket scrutiny.[38]
Federal payment policies were not critically important here because only a small percentage of kidney stone patients are covered by Medicare. Thus, the regulatory issues can be seen clearly.
The product took thirteen months to receive FDA approval, slightly longer than the average of one year. Despite this delay to market, it diffused rapidly once available. There were over two hundred lithotripters in operation within two years of introduction (see figure 17). The market now includes 220 devices and is basically saturated. Of ten firms in the market, only four have received FDA approval; the others have devices in investigational stages (see table 7). The market leader is Dornier Medical Systems, the first to receive a PMA; the others include Medstone International, Diasonics, Technomed International, and Northgate Research.[39]
For data on the industry, see Biomedical Business International 11 (15 July 1988): 99-101.
The next generation of machines is already in development. In a relatively short time, there have been major improvements in the original device; other designs, such as those that use laser technology, are on the horizon. There have been a number of creative marketing solutions to the problems of high cost and low patient volume. Entrepreneurs have put together joint ventures with physicians and hospitals that ensure a broad patient base, lower the unit cost of treatment, and amortize the cost of the device. Some free-standing centers have developed relationships with providers of other forms of kidney stone treatment so
Figure 17.
Treating kidney stones with shock waves. Adapted from Ron Winslow,
"Costly Shock-wave Machines Fare Poorly on Gallstones," Wall Street
Journal , 9 February 1991, B1.
that comprehensive services and alternative treatments to lithotripsy are all available in one location.[40]
Miles Weiss and Greg Freiherr, "Romancing the Market for Stones," Healthweek, 4 December 1989, 18-20.
What lessons can we learn from this case about the nature of innovation in the device industry? How can we explain the success of this expensive, highly regulated technology? One possible explanation is that promising and truly useful technologies usually succeed despite the barriers placed in their paths. However, it may be that the dynamism and creativity are based on the expectation of enormous market expansion through the application of this technology to patients with gallstones, a much more prevalent clinical condition than kidney stones. There are 20 million gallstone patients in the United States, with 487,000 gall bladder removals in hospitals every year. Medicare plays an important role because gallstone disease affects many elderly people. The treatment of gallbladder disease is a $5 billion market. If lithotripsy could be applied to some of these patients, hospitals could avoid many of the surgical costs and the firms could compete for this greatly expanded market.[41]
Tim Brightbill, "Gallstone Lithotripsy Suffers FDA Setback," Healthweek, 4 December 1989, 25-26. For a more scientific discussion of gallstone, or biliary, lithotripsy, see Michael Sackmann et al., "Shock-wave Lithotripsy of Gallbladder Stones: The First 175 Patients," New England Journal of Medicine 318 (18 February 1988): 393-397. See also Barkun and Ponchon, "Extracorporeal Biliary."
(See figure 18.)Whether that expansion will occur is now in doubt, and here is where the policy process reenters. In October 1989, an FDA
advisory panel recommended that the agency disapprove the PMAs filed by Dornier Medical Systems and Medstone International for gallstone (biliary) lithotripters. The panel members expressed concern about the safety data in the PMAs. Questions were also raised about the effectiveness of lithotripsy for destroying all gallstones. Preliminary evaluations revealed that only a small percentage of patients with gallstones may benefit from EWSL.[42]
Brightbill, 25-26.
The delay (or possible denial) in marketing approval may allow competitors to catch up with the two leaders, although the ultimate clinical usefulness of biliary lithotripsy remains uncertain. Manufacturers have been slow to gather sufficient data because the lack of any third-party reimbursement for this new procedure has limited the number of patients who have received it. In addition, because the drugs used in conjunction with the treatment work slowly, studies are often time-consuming. In the meantime, alternative treatments are developing, including a laser that views and snips off the gallbladder and pulls it out through a small incision. Other experiments include a rotary device that whips gallstones until they liquefy and then draws out the resulting "soup."[43]
Ron Winslow, "Costly Shock-wave Machines Fare Poorly on Gallstones, Disappointing Hospitals," Wall Street Journal, 9 February 1990, B1, B6.
The failure of biliary lithotripters to receive FDA approval may only be a temporary and minor delay. It may also indicate that the technology is inappropriate for the proposed use, and the FDA is sagely valuing safety concerns over the desires of the innovative firms to rush to market. Or we may be seeing a regulatory failure in which the FDA is inappropriately obstructing a valuable innovation from the marketplace. The FDA's decision delays reimbursement from third-party payers, including Medicare, which will rarely pay for unapproved technologies, further burdening the innovators. The FDA approval does not necessarily guarantee Medicare's coverage of the procedure. The Health Care Financing Administration (HCFA), Medicare's payment authority, makes its own assessments of new technologies for coverage and payment decisions, often independently of FDA findings.[44]
For a complete discussion of the post-1983 Medicare coverage process, see chapter 7.
The lithotripsy industry remains dynamic, highly innovative, and very competitive. However, the market for kidney stone treatment is saturated and not expanding. No improved
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Figure 18. Electrohydraulic shock-wave lithotripter.
Source: Healthweek , 4 December 1989, 25.
technology to date has left competitors outmoded. Whether the expansion for use in gallstone treatment will occur depends upon the public sector—the FDA and Medicare—as well as private third-party payers. The layering effect becomes important here because if the FDA has not approved a treatment, then the HCFA will not cover it. And, even if the procedure has been FDA approved, approval does not ensure private or public sector third-party payment.
Postmarket Controls: Reporting Failures
The postmarket surveillance system has four main components: (1) voluntary reporting of problems from users, such as doctors or hospitals to the FDA, manufacturers, and others; (2) mandatory reporting of known problems by manufacturers to the FDA; (3) monitoring and analysis of problems by the FDA; and
(4) a recall process to correct products or remove them from the market.[45]
House Subcommittee on Health and the Environment, statement of Charles A. Bowsher, comptroller general, Medical Devices: The Public Health at Risk, 6 November 1989.
Significant controversy has surrounded the reporting requirements. Until 1984, the reporting of adverse effects associated with medical devices was voluntary. The FDA received reports from physicians, hospitals, and manufacturers, and these data were entered into the FDA's Device Experience Network (DEN). Investigations revealed that adverse reactions were seriously underreported.[46]
Senate Committee on Governmental Affairs, Report to the Chairman: Early Warning of Problems Is Hampered by Severe Underreporting (Washington, D.C.: General Accounting Office, December 1986).
The FDA promulgated a mandatory medical device reporting rule (MDR) that went into effect in December 1984. The key element in the rule is that manufacturers and importers must report to the FDA when they receive or otherwise become aware of information that reasonably suggests that a product has caused or contributed to serious injury or death, or has malfunctioned and is likely to cause harm if the malfunction recurs. There are tight time frames for reporting. In general, an injury is considered serious if it is life-threatening or results in permanent impairment of a bodily function or permanent damage to body structure. Users such as hospitals and doctors can report voluntarily, but they are not required to do so.[47]
Final Rule, 49 Federal Register 36326-36351 (14 September 1984).
Serious problems that came to light through MDR were burns related to the misuse of apnea monitors and early depletion of batteries for portable defibrillators.From the FDA's perspective, MDR also serves as a barometer of trends of adverse product performance. The FDA has received 18,000 MDR reports since the regulation became effective. Eighteen cardiovascular, anesthesiology, and general hospital devices have accounted for 70 percent of the reports.
There has been a great deal of criticism of MDR from the industry, which has argued that the system forces overreporting because of the breadth of the definitions and the short time frame.[48]
Office of Management and Budget Symposium, March 1986.
On the other hand, some health advocates maintain that the reporting system is hampered by the lack of FDA jurisdiction over hospitals and physicians, neither of which can be ordered to report malfunctions. Recent GAO studies of the FDA device recalls (removal from the market of a product that violates FDA laws) found that only half of all recalls had an MDRreport associated with them. The FDA became aware of the majority of device problems in ways other than through the required reports, and it did not have reports available in the majority of cases when decisions about health hazards were made. The GAO concluded that "this suggests that the reports have not served as an effective 'early warning' of device problems serious enough to warrant a recall."[49]
Medical Device Recalls (Washington, D.C.: General Accounting Office, August 1989).
Congress conducted further investigations into reporting failures in late 1989. At a hearing held by the House Subcommittee on Health and the Environment, Congressman Sikorski excoriated manufacturers who failed to report hazards associated with their products. Citing GAO statistics, he noted that 48 percent of high-risk products that had been recalled had never reported problems to the FDA. Grieving parents also explained that their son died because an infant monitoring system failed to notify them that he was not breathing.[50]
House Subcommittee on Health and the Environment, Committee on Energy and Commerce, statement of Gerald Sikorski and testimony of Michael B. Davis, Sr., and Cory J. Davis, 6 November 1989.
The comptroller general testified that the GAO had investigated all major components of the postmarket surveillance system since 1986. It found that the FDA was receiving more information than it previously had, but it also noted that the degree of compliance with MDR could not be established, that the FDA's data-processing system was not adequate to handle the reports it did receive, and that the results of the analyses were often not definitive.[51]
House Subcommittee on Health and the Environment, testimony of Charles Bowsher, Medical Devices, 13-15.
Thus the controversy over the FDA's ability to perform its duties under the law continued into the 1990s.Informal Powers
It is important to remember, however, that a federal agency like the FDA need not always initiate formal action to get results. Although there have been substantial problems with reporting requirements, the FDA has exercised its other postmarketing surveillance powers effectively—and often behind the scenes. The case of toxic shock syndrome illustrates this point. It is particularly striking to compare the FDA's informal power in this case to the Dalkon Shield recall in 1976, before the passage of the device law. When problems arose relating to the Dalkon Shield, the FDA had no clear authority to order a product recall.
When the toxic shock crisis arose in the early 1980s, a very different FDA, with a larger stock of potential tools, took charge.
Tampons were introduced in the 1930s and the Tampax brand dominated the market for decades. In the 1970s, Playtex, Johnson & Johnson, and Kimberly Clark marketed tampon varieties. Procter & Gamble, the large consumer product company, entered the market in late 1979. After a $75 million massive media and direct marketing campaign in late 1979, its Rely brand had acquired a 20 percent share of the billion-dollar industry.[52]
For a discussion of this case in greater detail, see Susan Bartlett Foote, "Corporate Responsibility in a Changing Legal Environment," California Management Review 26 (Spring 1984): 217-228, 221.
Toxic shock syndrome (TSS) is a rare and mysterious disease characterized by high fever, rash, nervous disorders, and potentially fatal physiologic shock. TSS was not initially associated with tampon use. In January 1980, epidemiologists from Minnesota and Wisconsin reported a total of twelve TSS cases to the federal Centers for Disease Control (CDC). The data revealed surprising patterns—all patients were women using tampons. Through that spring, the CDC received additional reports from many states. Following a retrospective case study in May 1980, tampon manufacturers were made aware of the hazards tentatively associated with their products. There were many unanswered medical questions, but because millions of women used tampons, there was fear of potential widespread injuries. In a study of fifty women who had TSS in September 1980, the CDC revealed that 71 percent of those surveyed had used the Rely brand. Procter & Gamble officials immediately defended the product. Within a week, however, they suspended sales of Rely and signed a comprehensive consent decree with the FDA.
The speed of this action can be attributed to several factors, including the specter of product liability suits and the fear of general rejection of Procter & Gamble products. The FDA played an important part in the response. It was under significant public and media pressure to protect the public from TSS, despite the unanswered medical questions and the inconclusive findings in the small CDC studies. Procter & Gamble's timing was clearly determined by the FDA, who had called a meeting one day after the release of the damaging CDC study in September. The agency gave the company one week to generate evidence that the product was safe.
Despite enormous effort, Procter & Gamble could not rebut the CDC's scientific findings in that time, and it feared that overt refusal to cooperate with the FDA would have damaged the company's reputation. The result was a voluntary withdrawal of the product. This action occurred in the shadow of the FDA's powers; the decree itself states that the FDA was "contemplating the possibility of invoking the provision of [medical device law] to compel the firm" to recall Rely.[53]
Consent decree signed by Procter & Gamble and the FDA (22 September 1980).
Assessing FDA Impact on Medical Device Innovation
The goal of FDA regulation is to establish a threshold of safety and efficacy for medical devices. The regulatory process does not intend to destroy innovation or drive away "good" technology. The challenge is to establish a balance between safety and innovation. Has the balance been achieved?
Opinions are strong on all sides. Congress has generally approached the evaluation from a consumer perspective. It has sharply and regularly criticized the FDA for underregulation and failure to enforce regulatory standards. On the other hand, industry representatives have complained about unnecessary and cumbersome regulatory burdens. Who is right?
The data are inconclusive as to the effects of regulation on innovation. Generally, however, studies have indicated that, in the aggregate, device regulation has not inhibited the introduction of new goods.[54]
U.S. Department of Health and Human Services, A Survey of Medical Device Manufacturers, prepared for the Bureau of Medical Devices, Food and Drug Administration by Louis Harris and Associates, no. 802005 (Washington, D.C., July 1982). This comprehensive, but early, survey concluded that the impacts were minor, although smaller firms might feel regulatory effects more strongly than larger ones.
Although few negative effects on equipment development have been found, small manufacturers may bear a greater burden than larger ones. Some researchers found that smaller firms were less likely to introduce Class III devices after device regulation was in place.[55]Oscar Hauptman and Edward B. Roberts, "FDA Regulation of Product Risk and the Growth of Young Biomedical Firms" (Working paper, Sloan School of Management, Massachusetts Institute of Technology, 1986).
In a study of new product introductions of diagnostic imaging devices, however, the results offered no evidence of bias against small firms.[56]Mitchell, "Dynamic Commercialization." Mitchell tried to measure regulatory effects by comparing the types of firms that introduced computed tomography (CT) scanners and nuclear magnetic resonance (NMR) imaging devices. CT diffusion preceded the 1976 law and NMR was introduced subsequently. He hypothesizes that if there were more start-up companies in computer homographics than in magnetic resonance imaging, then one could conclude that there was a regulation induced bias. His data indicates there was no evidence of small-firm liability.
The Medical Device Amendments of 1976 present a watershed for medical device innovators. The passage of the law forced all producers to consider the potential impact of federal regulation. This possible federal intervention, both as a barrier to and a manipulator of the marketplace, inextricably links the private producer to the FDA. The law attempted to deal with the
complexity and the diversity of medical devices; it is these characteristics of the industry that have led to problems of effective regulation. Even at the lowest levels of scrutiny, compliance with FDA requirements involves time and expense to the producer. For devices in Class III, the delays and the costs are much higher.
It is obvious, however, that to firms that must meet regulatory requirements, the barriers may seem high. The impact, one can conclude, is spotty across various manufacturers and types of products. Clearly the FDA has not fully implemented all the provisions of the law, and there are powerful critics of both the FDA and the industry in Congress. If the law were fully implemented or made more stringent, then greater impacts on more firms would be inevitable. The policy question that remains, then, is whether we need more safety; if so, at what cost to the producers? Can we balance the interests of innovation and safety?[57]
These issues will be pursued further in chapters 9 and 10.
Another policy designed to inhibit discovery of devices was emerging alongside the regulatory arena. Rules relating to product liability also had the potential to influence the health of our patient. It is to these legal constraints that we now turn.
6
Government Inhibits Medical Device Discovery: Product Liability
Personal injury law allocates responsibility for the costs of accidents.[1]
I use the term personal injury law to refer to both product liability and negligence—two different theories of liability. Technically, product liability refers to the legal theory of liability derived from strict liability, which assesses responsibility for defective products without concern for fault. Negligence, on the other hand, is a separate legal theory where liability is assessed on the basis of fault. Medical device producers, indeed all product producers, can be held liable under either theory. Both are discussed in greater detail in this chapter.
In product related cases, injured individuals (plaintiffs) seek to shift the costs to the producers (defendants).[2]Plaintiffs generally sue everyone in the chain of distribution—manufacturers, wholesalers, retailers, and others. This discussion focuses on manufacturers.
This area of law expanded dramatically during the 1970s. The rules of liability changed so that more injured consumers had the opportunity to prevail, and the size of the awards to victorious plaintiffs tended to rise as well. The increased risk of being sued introduced new uncertainties for manufacturers and created additional potential burdens for innovators.This increase in liability exposure occurred at the same time that medical device regulation emerged. Regulation and liability share a common goal—to deter the production of products that do not meet a standard of safety. However, these two institutions accomplish the goal in vastly different ways. Federal regulation is uniform and national. Much of the FDA's device regulation is prospective, that is, the rule is known before the manufacturer begins to market the product. (Of course, if problems arise in the marketplace, the FDA does have postmarketing surveillance power.)[3]
See discussion of the FDA in chapter 5.
In contrast, liability laws vary from state to state, subjecting producers to fifty different possible sets of rules. Liability is retrospective, in that the process begins after the harm occurs. Finally, the institutions use different standards and mechanisms to determine safety. The FDA imposes primarily scientific evaluation; judges and juries apply principles rooted in law and experience rather than in science. In addition to deterrence, liability law seeks to compensate victims for the costs of the injury, leading to damage awards. Product liability also has a
punitive component—the system can impose punitive damages for behavior that is particularly reprehensible.
This chapter describes the evolution of product liability law in the 1970s and the impact of those changes on medical devices. The cases of the A. H. Robins Dalkon Shield and the Pfizer-Shiley heart valve illustrate some important issues raised by liability litigation. Once again, a caveat is in order. Assessment of the impact of liability on producers is hampered by the lack of reliable data.[4]
The lack of reliable data is substantial, a problem noted and discussed in two major studies of product liability trends. The Rand Corporation, Institute for Civil Justice issued a study by Terry Dungworth, Product Liability and the Business Sector: Litigation Trends in Federal Courts, R-3668-ICI (1989) and the General Accounting Office, Briefing Report to the Chairman, House Subcommittee on Commerce, Consumer Protection, and Competitiveness, Committee on Energy and Commerce, Product Liability: Extent of 'Litigation Explosion' in Federal Courts Questions, GAO-HRD-88-36BR (January 1988). Both studies focus on federal court filings because the data are more accessible than in state courts, where records are not uniform and are difficult to acquire. In addition, corporate and insurance company records are confidential, and data about litigation costs or settlement amounts are not disclosed.
Information on settlements and litigation costs are confidential; court records are inconsistent in different states and are difficult to obtain. In addition, because of the diversity in legal requirements from state to state, generalizations about legal trends are limited. Finally, because liability rules can change with each court decision and can be altered by state or federal legislation or by voter initiative,[5]Traditionally, this area of law was dominated by common-law principles, that is, law that evolves through the judicial interpretation of precedents or previous court decisions. In state court, a state legislature can pass statutes that supercede the common-law principles; these statutes are then enforced by the court. In some states, such as California, there are also ballot initiatives that are voted on by the electorate. If the initiative passes, it becomes law and must be enforced by the court. Frequently, the courts are called upon to interpret unclear provisions in the statutes, which they must do in order to enforce the law.
the target is a moving one. Nevertheless, trends can be identified and some conclusions can be drawn.Breaking Legal Barriers
Changing Theories of Liability
Until the early 1960s, there were substantial barriers to successful legal claims for injuries related to products. Several legal trends broke down those barriers so that injured individuals could more readily prevail against producers.
Negligence
To win a negligence case, the plaintiff must prove that a defendant's behavior failed to meet a legal standard of conduct and that the behavior caused the plaintiff's harm.[6]
These are called the elements of the case. The plaintiff must plead all the required elements in the complaint filed in the court and must prove them all to win. For further reading of tort law, see G. Edward White, Tort Law in America: An Intellectual History (New York: Oxford University Press, 1985). For a law and economics perspective, see Guido Calabresi, The Costs of Accidents: A Legal and Economic Analysis (New Haven: Yale University Press, 1970). For a basic primer on the rules of tort law, see Edward J. Kionka, Torts in a Nutshell: Injuries to Persons and Property (St. Paul, Minn.: West, 1977).
Until the mid-nineteenth century, negligence "was the merest dot on the law."[7]Jethro K. Lieberman, The Litigious Society (New York: Basic Books, 1981), 35.
Negligence is a neutral concept, one that need not give advantage to a corporate defendant or an individual plaintiff. However, as negligence law evolved in the late nineteenth and early twentieth centuries, it supported the risk-taking behavior of industrial entrepreneurs and fit nicely within the ethic of individualism and laissez-faire.[8]For an interesting discussion of the history of American law, see Morton J. Horwitz, The Transformation of American Law, 1780-1860 (Cambridge: Harvard University Press, 1977), especially chaps. 6-7. See also Grant Gilmore, The Ages of American Law (New Haven: Yale University Press, 1977); and Lawrence M. Friedman, A History of American Law (New York: Simon and Schuster, 1973).
Judges considered the values of economic progress; they often carefully circumscribed the defendant'sduty of care and provided nearly impenetrable defenses.[9]
With a circumscribed standard of care, it was difficult for the plaintiff to show that the defendant's conduct fell below the legally imposed standard of behavior. Defenses that the defendant could raise included contributory negligence (if the plaintiff contributed even in a minor way to his own injury, he would automatically lose) and assumption of risk (certain activities are assumed to be risky, and the plaintiff must bear the consequences of those risks he undertakes). A good defense protected defendants from liability.
With negligence law so limited, most cases involving product injuries relied on the rules of contract, which were quite limited in their own right.[10]For example, the law required that the plaintiff be a party to the contract in order to sue, thus spouses or children of the person who signed the contract for product purchase could not bring an action. This is called privity of contract. Contract remedies, or the amount of money that can be claimed, also were narrowly defined by contemporary personal injury standards.
Yet, there were pressures to protect individuals injured by industrial progress. These forces increased as accidents involving railroads rose. One change in direction came in 1916, when Benjamin Cardozo, chief judge of the New York Court of Appeals, overthrew the doctrine of privity in the famous case of MacPherson v. Buick . A wooden automobile wheel collapsed, injuring the driver. MacPherson, the driver, sued Buick Motor Company, which had negligently failed to inspect and discover the defect. Buick's defense was that the company had no contractual relationship with MacPherson, that is, no privity of contract, because he had bought the car from a dealer, not directly from Buick. Cardozo held the manufacturer's duty of care was owed to all those who might ultimately use the product. This was a first step in the process that broke down contract law barriers placed in the way of injured persons.
A host of other doctrines, too numerous to describe here, began over the next fifty years to shift the balance in favor of the plaintiffs. By the 1960s, many state courts had greatly expanded the scope of duties owed to others, had abolished defenses available to defendants, and had begun to assess significantly higher damage awards.[11]
For example, California eliminated the defense of contributory negligence in Li v. Yellow Cab, 13C. 3d 804 (1970). Now plaintiffs can prevail even if they contributed to their own harm, but their damages will be reduced by the percentage share that is attributed to their own behavior.
Product Liability
Courts began to reflect frustration with the limitations on plaintiffs under traditional contract and negligence principles. These frustrations help to explain the development of the concept of strict liability and its applicability to producers.
Strict liability (called product liability when applied to product cases) differs from negligence in that it is not premised on fault. The doctrine looks to the nature of the product, not the behavior of the producer. If a product is found to be defective when placed in the stream of commerce, the producer may be liable for the harm that it causes, regardless of fault. Determining the parameters of the concept of defect is pivotal in these
cases.[12]
Although the principles vary from state to state, a product can be considered defective if its design leads to harm or if there is a failure to adequately warn the user of its risks.
Although strict liability had existed in the law since the nineteenth century, the theory became crucial in relation to product cases in the 1960s.The California Supreme Court led the way when it announced the standard of strict tort liability for personal injuries caused by products in Greenman v. Yuba Power Products . The court held the defendant manufacturer liable for injuries caused by the defective design and construction of a home power tool. Liability was imposed irrespective of the traditional limits on warranties derived from contract law. The court stated that "[a] manufacturer is strictly liable in tort when an article he places in the market, knowing that it is to be used without inspection for defects, proves to have a defect that causes injury to a human being."[13]
59 Cal. 2d 57, at 62. A few years earlier, in a dissenting opinion in Escola v. Coca Cola Bottling Co., Justice Traynor of the California Supreme Court set forth the grounds for the strict liability standard for product defects that was adopted by a large majority of jurisdictions nearly two decades later. While this dissent was largely ignored, it planted the seeds for the subsequent revolution. 24 Cal. 2d 453, 461 (1944).
Virtually every state subsequently adopted these principles of liability. Indeed, only one year after the Greenman case, the American Law Institute (ALI), which represents a prestigious body of legal scholars, adopted section 402A of the Restatement (Second) of Torts , which sets forth the strict liability standard.[14]
The American Law Institute (ALI) does not make law. However, distinguished scholars have traditionally analyzed and evaluated trends in the law and compiled them in books known as Restatements. The Restatements are not binding on courts. However, they are frequently consulted by judges in the course of drafting opinions, are very influential, and are often cited by judges for support in altering common-law principles.
The theory of strict liability has been elaborated and refined in various jurisdictions, including the definition of defect, the extension of the concept of defect to product design, the notion of defective warnings, and the restrictions on defenses available to the manufacturers and the duties of manufacturers when consumers misuse the product.[15]For a comprehensive discussion of the theory of product liability, see George Priest, "The Invention of Enterprise Liability: A Critical History of the Intellectual Foundations of Modern Tort Law," Journal of Legal Studies 14 (1985): 461-527. See also James A. Henderson and Theodore Eisenberg, "The Quiet Revolution in Products Liability: An Empirical Study of Legal Change," UCLA Law Review 37 (1990): 479-553.
In general, courts have held that a showing of a defect which caused[16]
What constitutes legal causation is another area fraught with controversy but is beyond the scope of our discussion. For those who want to understand the debate, see Steven Shavell, "An Analysis of Causation and the Scope of Liability in the Law of Torts," Journal of Legal Studies 9 (June 1980): 463-516.
injury is sufficient to justify strict liability. There are three basic categories of product defects. The first is a flaw in manufacturing that causes a product to differ from the intended result of the producer. The second is a design defect that causes a product to fail to perform as safely as a consumer would expect or that creates risks that outweigh the benefits of the intended design. The third arises when a product is dangerous because it lacks adequate instructions or warnings. Cases may be brought under one or more of these categories.Damage Awards
If a plaintiff wins the case, the next step is to assess the amount of damages to which he or she is entitled. The jury can award
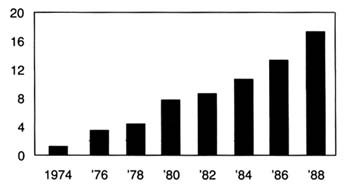
Figure 19.
Product liability suits filed in federal district courts (in thousands).
Source: Administrative Office of U.S. Courts. Reprinted from
Wall Street Journal, 22 August 1989, A 16.
damages for actual out-of-pocket losses, such as medical expenses, lost wages, and property damages. In addition, juries can include noneconomic damages, such as the value of the pain and suffering of the plaintiff, a subjective judgment that can add significantly to the total award amount. The amount awarded in jury verdicts has been increasing steadily since the 1960s, and much of this increase can be attributed to medical malpractice and product liability cases.[17]
Once again, a caveat on the reliability of data. Of the millions of insurance claims filed each year, only 2 percent are resolved through lawsuits. Less than 5 percent of the cases that are tried reach a verdict; the rest are settled. See Ivy E. Broder, "Characteristics of Million Dollar Awards: Jury Verdicts and Final Disbursements," Justice System Journal 11 (Winter 1986): 353. Many jury verdicts do not reflect what the plaintiff actually receives because these awards can be reduced on appeal. Jury awards are used as a benchmark for settlement amounts, however, and do reflect broader trends. See discussion in Robert Litan, Peter Swire, and Clifford Winston, "The U.S. Liability System: Backgrounds and Trends," in Robert Litan and Clifford Winston, eds., Liability: Perspectives and Policy (Washington, D.C.: The Brookings Institution, 1988).
(See figure 19 and table 8.)Punitive damages are another controversial area of liability law. Theoretically, punitive damages are intended to punish wrongdoers whose conduct is particularly reprehensible. They are added on top of the award of actual damages, which are intended to fully compensate the plaintiff for losses incurred. The standards for assessing conduct are relatively vague and require few limits.[18]
For a review of the principles of punitive damages, see Jane Mallor and Barry Roberts, "Punitive Damages: Toward a Principled Approach," Hastings Law Journal 31 (1980): 641-670. See also Richard J. Mahoney and Stephen P. Littlejohn, "Innovation on Trial: Punitive Damages Versus New Products," Science 246 (15 December 1989): 1395-1399.
For many years, punitive damages played a minor role in American law. As late as 1955, the largest punitive damage verdict in the history of California was only $75,000.[19]See discussion in Andrew L. Frey, "Do Punitives Fit the Crime?" National Law Journal, 9 October 1989, 13-14.
By the 1970s, however, punitive damages were frequently awarded, with many verdicts well over one million dollars.[20]See "Punitive Damages: How Much Is Too Much?" Business Week, 27 March 1989, 54-55.
There is considerable debate about why the liability laws changed in this way. Edward H. Levi of the University of Chicago identifies forces within the social experience of America. He believes that holding producers responsible for injuries
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
reflects views of the 1930s, when government control was increasing generally and greater government responsibility for individual welfare was thought proper.[21]
Edward H. Levi, "An Introduction to Legal Reasoning," University of Chicago Law Review 15 (1948): 501-574.
Others have argued that the change was tied to the growing complexity of products, which made consumers less able to evaluate them on an individual basis, and to the rise of the U.S. welfare state, particularly after World War II.[22]Lester W. Feezer, "Tort Liability of Manufacturers and Vendors," Minnesota Law Review 10 (1925): 1-27.
Economists Landes and Posner have an economic explanation: shifting responsibility to producers was an efficient response to urbanization and a reflection of "internalizing" costs to efficiency in manufacturing.[23]William M. Landes and Richard A. Posner, The Economic Structure of Tort Law (Cambridge: Harvard University Press, 1987).
Yale law professor George Priest takes the view that modern tort law reflects a consensus of the best methods for controlling the sources of injuries related to products. Under what he calls the "theory of enterprise liability," businesses are held responsible for losses resulting from products they introduce into commerce, reflecting the perceived appropriate relationship between product manufacturers and consumers as well as the role of internalizing costs to affect accident levels and how to distribute risk.[24]Priest, "Critical History," 463.
Clearly the reasons for these changes in the legal environment are complex and multiple. The extent of their impact is also controversial. Personal injury and product liability relate to all consumer products. However, pharmaceutical products have been singled out for special treatment under the law. Medical devices have not received similar consideration, although there are conflicting trends in recent court decisions.
Special Protection for Drugs, Not Devices
A special exception to strict liability has been carved out for drugs and vaccines because of their unique status in society. During debates at the American Law Institute regarding product liability, members proposed that drugs should be exempted from strict liability because it would be "against the public interest" because of the law's "very serious tendency to stifle medical research and testing." A comment (known as "comment k") following the relevant section in the Restatement provides that the producer of a properly manufactured prescription drug may be held liable for injuries caused by the product only if it was not accompanied by a warning of the dangers that the manufacturer
knew or should have known. The comment balances basic tort law considerations of deterrence, incentives for safety, and compensation by recognizing that drugs and vaccines are unavoidably unsafe. Comment k has been adopted in virtually all jurisdictions that have considered the matter.[25]
For detailed discussion of comment k, see Victor E. Schwartz, "Unavoidably Unsafe Products: Clarifying the Meaning and Policy Behind Comment K," Washington and Lee Law Review 42 (1985): 1139-1148, and Joseph A. Page, "Generic Product Risks: The Case Against Comment K and for Strict Tort Liability," New York University Law Review 58 (1983): 853-891. The California Supreme Court recently disapproved the holding in a prior case which would have conditioned the application of the exemption under certain circumstances. California came down squarely on the side of comment k. In holding that a drug manufacturer's liability for a design defect in a drug should not be measured by strict liability, the court reasoned that "because of the public interest in the development, availability, and reasonable price of drugs, the appropriate test for determining responsibility is comment k.... Public policy favors the development and marketing of beneficial new drugs, even though some risks, perhaps serious ones, might accompany their introduction, because drugs can save lives and reduce pain and suffering." Brown v. Superior Court, 44C 3d 1049, 245 Cal. Rptr. 412 (31 March 1988) at 1059.
Of course, as pharmaceutical manufacturers would be the first to say, the comment k exemption does not eliminate liability exposure. Drugs and vaccines may be exempt from design defect claims, but producers may still be held liable for failure to warn and for negligence. Because pharmaceutical products account for many of the liability actions, this exemption is quite limited in practice.[26]
The GAO report found that drug products, including bendectin, a morning sickness drug, DES, a synthetic hormone used to prevent miscarriage, and Oraflex, an arthritis medicine, accounted for significant amounts of product liability litigation. GAO, "Product Liability," 12.
Generally speaking, medical devices have been treated like all other consumer products with regard to both negligence and strict liability in most jurisdictions. A handful of cases from scattered courts, however, have grappled with the relationship of the special exemption for drugs under comment k to other medical products like medical devices. Three cases involve injuries from IUDs. While these cases do not presage any major shifts in the case law, they do provide some insight on the thorny problem of distinguishing drugs from devices in the policymaking process.
In Terhune v. A. H. Robins,[ 27]
90 Wash. 2d 9 (1978).
the plaintiff suffered injuries from a Dalkon Shield. She argued that A. H. Robins had failed to warn her of the risks associated with the product. The Washington State Supreme Court held that because the IUD is a prescription device, comment k applies. (The case was brought before the Medical Device Amendments had been implemented; the court said that the fact that there was no FDA approval before marketing was irrelevant.) Precedents held that the duty to warn of risks associated with prescription drugs ran only from manufacturer to physician. Prescription devices, which cannot be legally sold except to physicians, or under the prescription of a physician, are classified the same as prescription drugs for purposes of warning.The Oklahoma Supreme Court decided a similar case several years later. In McKee v. Moore,[28]
648 P.2d 21 (Okla. 1982).
the plaintiff was injured by a Lippes Loop, the IUD manufactured by Ortho Pharmaceutical. As in the Terhune case, the plaintiff alleged that the companyfailed to warn of side effects. The Oklahoma Supreme Court equated prescription drugs and devices: "[U]nlike most other products, however, prescription drugs and devices may cause unwanted side effects even though they have been carefully and properly manufactured."[29]
Ibid., 23.
The issue is somewhat different in the context of design defects. A California appellate court recently grappled with the differences between drugs and devices in the area of design. In Collins v. Ortho Pharmaceutical,[ 30]
231 Cal. Rptr. 396 (1986).
the plaintiff alleged that the Lippes Loop that injured her was defectively designed. The court, citing the Terhune and McKee cases, equated prescription drugs and prescription devices. These products have been determined to be unavoidably unsafe, said the court, because they are reviewed by the FDA and contain warnings about use. The discussion in the case seems to confuse the concept of a prescription product with the process of premarket approval. Of course, all drugs must undergo premarket approval by the FDA before marketing. However, as we know, the Medical Device Amendments do not require premarket screening for all devices. Indeed, the device IUDs, including the Lippes Loop, did not require PMAs when they entered the market in the early 1970s.[31]For discussion of the Medical Device Amendments, see chapter 4. All Class I and Class II devices, as well as those entering the market under the 510k provision, do not undergo safety and efficacy screening similar to drugs. Some of these products may be limited to prescriptions or have labeling requirements imposed, however.
To the extent that the court is assuming that the FDA's premarket approval confers the unavoidably unsafe status, the analogy to devices is inapt. However, the analogy does seem appropriate for Class III devices.Many unanswered questions remain. How much meaning does prescription confer? What about hospital equipment that is not prescribed per se, such as resuscitation equipment or monitoring apparatus? The answers are unclear, though there are important distinctions that the courts have not begun to consider.
Manufacturing defects are most frequently cited as the cause of injury in product-related suits arising from the use of medical devices. While it is increasingly common for strict liability claims to be brought in medical device cases, negligence continues to be the most common theory of recovery. The struggle in the courts on how to characterize medical devices for purposes of liability underscores the diversity of the products in the industry and the limited understanding of the relationship of devices to medical
care. It also recalls the thorny drug/device distinctions that FDA and Congress grappled with in crafting regulatory principles.
The Relationship of Device Liability to Medical Malpractice
Malpractice cases are brought under negligence theory. In a medical context, the plaintiff must show that the health professional's performance fell below the standard of care in the community. The impact of medical malpractice on the practice of medicine has been the subject of much debate, which is beyond the scope of this inquiry.[32]
For a discussion of medical malpractice, see Patricia Danzon, Medical Malpractice: Theory, Evidence, and Public Policy (Cambridge: Harvard University Press, 1985).
However, there is interaction between product liability and medical practice, and that interaction has consequences for medical device producers. Fear of malpractice claims has encouraged what is known as defensive medicine: the practitioner is cautious, often ordering batteries of tests that may not be medically necessary in order to protect against future claims. This behavior has led to overuse of some medical technology, including medical devices.Malpractice and product liability cases often are filed simultaneously. For example, in many IUD cases, women sue both their doctors and the product manufacturer. The manufacturer's liability is based on failure to warn of dangerous side effects or on production of a defectively designed product. A doctor's failure to inform the patient of risks associated with IUD use, failure to perform a thorough examination, negligent insertion or removal of an IUD, failure to warn of the risks of pregnancy when the device is in place, and failure to monitor the patient for adverse reactions can all establish claims.[33]
Guerry R. Thornton, Jr., "Intrauterine Devices: IUD Cases May Be Product Liability or Medical Negligence," Trial (November 1986): 44-48, 44.
Device manufacturers have replaced physicians as the most frequently named defendants in cases involving medical device use.[34]
Duane Gingerich, ed., Medical Product Liability: A Comprehensive Guide and Sourcebook (New York: F & S Press, 1981), 57.
In some instances, the physicians have allied with plaintiffs' attorneys against the manufacturer. In Airco v. Simmons First National Bank,[35]638 S.W. 2d 660 (Ark. 1982).
one of the largest medical device cases to date, the plaintiff's attorney was encouraged by the doctors whom he had charged with malpractice to sue the manufacturer of the anesthesiology equipment used in the surgery. The court held the manufacturer primarily liable for the death in the case. Airco and the physicians' partnership admitted liability for compensatorydamages shortly before trial. The jury assessed $1.07 million in damages against both defendants and $3 million in punitive damages against Airco. Airco's appeal of the punitive damages award was rejected by the Arkansas Supreme Court, which found a sufficient record to support the jury's findings of a design defect in the ventilator component of Airco's breathing apparatus.
A number of states have placed caps on malpractice awards available in the courts. Legislatively imposed caps on medical malpractice may increase the likelihood that medical device manufacturers will bear additional costs to compensate injured individuals.
The Impact on Device Innovation
There is no consensus on the size or extent of the liability crisis. Without entering that debate, it is possible to speculate on its impact on innovation in the device industry.
There is no question that the expansion of product liability has affected medical device producers. There is a greater likelihood of successful lawsuits against manufacturers and inevitably higher insurance premiums have resulted for all producers. Recently one defense attorney noted: "I'd be willing to bet that ten years ago there weren't five cases in the United States against medical device manufacturers. Now there are that many every day."[36]
David Lauter, "A New Rx for Liability," National Law Journal (15 August 1983), 1, 10.
While data are hard to come by, this comment captures the trend. The consensus is that device producers face significant liability exposure. In general, claims are on the rise, losses have increased, and recovery rates for plaintiffs have gone up.MEDMARC, an industry-owned insurance company for medical device manufacturers which has 440 members, may reflect the liability situation of the industry. The president of MEDMARC reported that claims rose 42 percent between 1986 and 1987. On average, the plaintiff recovery rate for hospital equipment cases is about 71 percent and for IUDs about 78 percent.[37]
Kathleen Doheny, "Liability Claims," Medical Device and Diagnostic Industry (June 1988): 58-61, quoting Jaxon White, President of Medmarc Insurance, Fairfax, Virginia.
Many producers and service providers have experienced exceptionally high increases in insurance premiums or have been denied coverage altogether. The industries most seriously affected include manufacturers of pharmaceutical and medicaldevices, hospitals, physicians, and those dealing with hazardous materials.[38]
Priest, "Critical History," 1582.
For example, Puritan-Bennett, a leading manufacturer of hospital equipment such as anesthesia devices, faced a 750 percent increase in insurance premiums in 1986, with less coverage and higher deductibles.[39]Michael Brody, "When Products Turn into Liabilities," Fortune, 3 March 1986, 20-24.
Both G. D. Searle, the manufacturer of the Copper 7 IUD, and Ortho Pharmaceutical, the producer of the Lippes Loop, claim that insurance and liability exposure caused them to withdraw their product.[40]Luigi Mastroianni, Jr., Peter J. Donaldson, and Thomas T. Kane, eds., Developing New Contraceptives: Obstacles and Opportunities (Washington D.C.: National Academy Press, 1990).
As one might expect, the device industry asserts that innovation is threatened by this legal environment. HIMA surveyed its membership to determine its views on product liability.[41]
Health Industry Manufacturers Association, "Product Liability Question Results" (Unpublished document, 14 October 1987). Forty-nine companies received the questionnaire, 39 responded. HIMA has two hundred member companies.
Of the respondents, most reported soaring insurance premiums, and 25 percent reported that product liability deterred them from pursuing new products, including products that would fall into FDA Class III or that require highly skilled practitioners. Other medical organizations support this view. The American Medical Association has concluded that product liability inhibits innovative research in the development of new medical technologies.[42]See Report BB (A-88), "Impact of Product Liability on the Development of New Medical Technologies" (Unpublished document of the American Medical Association, undated).
Although the data are incomplete, there is no question that the threat of product liability creates uncertainty. Except in instances of fraud or deception, producers may not know what long-term risks their products present. They may be underinsured or, in the present liability environment, unable to acquire insurance. Laws vary from state to state, laws change over time, and outcomes depend on a variety of factors unique to each case. The size of awards also varies greatly, even in instances where the actions of defendants are the same. Product liability can destroy a company or a product. However, even the most conscientious producer faces an uncertain liability future. The potential of liability policies to disrupt company operations is high. The following case studies illustrate the impact of liability laws on producers.
The Dalkon Shield
IUDs are probably the most controversial medical device in the United States. Chapter 4 discussed their entry and rapid distribution into the market in the early 1970s.[43]
See discussion in chapter 4.
By the mid-1980s, the technology had practically disappeared. The story of theDalkon Shield has been told elsewhere in great detail.[44]
See Morton Mintz, At Any Cost: Corporate Greed, Women, and the Dalkon Shield (New York: Pantheon, 1985); Susan Perry and Jim Dawson, Nightmare: Women and the Dalkon Shield (New York: Macmillan, 1985); and Sheldon Engelmayer and Robert Wagman, Lord's Justice: One Judge's Battle to Expose the Deadly Dalkon Shield IUD (New York: Doubleday, 1985).
It is discussed here to illustrate the impact of mass tort litigation on a firm.Few defend the actions of A. H. Robins Company, either in the marketing of the IUD or in its subsequent behavior after the product was withdrawn. It is generally agreed that Robins entered the contraceptive market without knowledge or experience. It relied on erroneous research data, ignored warnings of product risks, and denied the existence of evidence to the contrary. The court found serious wrongdoing on the part of the company, and an official court document affirmed "a strong prima facie case that [the] defendant, with the knowledge and participation of in-house counsel, has engaged in an ongoing fraud by knowingly misrepresenting the nature, quality, safety, and efficacy of the Dalkon Shield from 1970–1984."[45]
Hewitt v. A. H. Robins Co., No. 3-83-1291 (3rd Div. Minn., 21 February 1985).
The problem with the product has been traced to its multifilament tail, a string attached to the plastic shield (see figure 20). The string allowed women to check that the product was in place and facilitated removal by a physician. This string was not an impervious strand but was composed of many strands that allowed bacteria to be drawn from body fluids into the uterus. The result was inflammation and infection, leading to illness, sterility, and, in some instances, death.[46]
Subrata N. Chakravarty, "Tunnel Vision," Forbes, 21 May 1984, 214-215.
The product was removed from the market in 1974, after several deaths and 110 cases of septic abortion (miscarriage caused by infection in the uterus). Then the lawsuits began. By June 1985, there were 9,230 claims settled and 5,100 pending; Robins had paid out $378 million at that time.
The cases continued to pour in. The company filed for Chapter 11 bankruptcy protection in August 1985.[47]
New York Times, 22 August 1985, 36.
As part of the proceedings, a reorganization plan had to be approved, and it could not go into effect until it was clear that no legal challenges to it survived. A plan was finally approved at the end of 1989, more than four years after the initial bankruptcy filing.[48]Wall Street Journal, 7 November 1989, A3.
This action cleared the way for the acquisition of the company by American Home Products and the establishment of a trust fund of $2.3 billion to compensate women who had not yet settled their claims with Robins.The plan transferred all responsibility for the Dalkon Shield
Figure 20. The Dalkon Shield.
Source: Washington Post National Weekly, 6 May 1985, 6.
claims to the trust. The trust funds were available to resolve the remaining 112,814 claims pending in 1989.[49]
Alan Cooper, "Way Is Cleared for Robins Trust," National Law Journal, 20 November 1989, 3, 30.
Twenty years after the product was marketed and sixteen years after it had been removed, injured claimants still awaited compensation. In late 1989, a federal grand jury began a criminal investigation into allegations that Robins concealed information and obstructed civil litigation.[50]Ibid., 30.
The course of this litigation raises questions about the efficiency of the tort system to accomplish its goal to compensate for
injuries. It also raises issues of deterrence. Who has been deterred? Ideally, of course, only unscrupulous companies or producers of unsafe products should be deterred by liability law. However, it appears that bona fide contraception innovators have generally abandoned the market in the wake of these lawsuits. It is difficult to justify a liability system when its primary goals—compensation and deterrence—are not met.
Heart Valves
Another medical device controversy arose in 1990. This case involved the Bjork-Shiley Convexo-Concave heart valve. Heart valves regulate blood flow and are essential to an efficiently functioning heart. Defective valves can lead to constant fatigue, periodic congestive heart failure, and other ailments. The development of artificial replacement valves became a challenge to medical device producers.
In 1968, Dr. Viking O. Bjork, a Swedish professor, began working on mechanical heart valves to replace defective ones. Bjork provided the design, and the Shiley Company engineered and manufactured the valves. The design, a curved, quarter-size disk that tilted back and forth inside a metal ring, was intended to reduce the risk of blood clots, a significant problem with previous implants.[51]
Barry Meier, "Designer of Faulty Heart Valve Seeks Redemption in New Device," New York Times Science, 17 April 1990, B5-6.
Approximately 394 of the 85,000 valves of this design that were sold worldwide between 1978 and 1986 have failed. The problem involves fractures in the struts that are welded on the inside of the valve that controls blood flow through the heart (see figure 21).[52]By 1980, it was clear that some of the original valves that opened 60 degrees were malfunctioning. Bjork convinced Shiley that it should produce a 70 degree valve, which would offer improved flow. Shiley apparently remilled many of the 60 degree valves; these products were even more hazardous than those they replaced. None were sold in the United States, and they were removed from the world market in 1983. Approximately 4,000 overseas patients received the valves. By 1990, seventy had died. See discussion in Greg Rushford, "Pfizer's Telltale Heart Valve," Legal Times, 26 February 1990, 1, 10-13.
The engineering flaw that led to strut fracture in the valve caused 252 reported deaths.[53]Michael Waldholz, "Pfizer Inc. Says Its Reserves, Insurance Are Adequate to Cover Heart Valve Suits," Wall Street Journal, 26 February 1990.
Shiley stopped selling the valve in 1986. Over two hundred lawsuits have been filed; many more are expected.Several tentative conclusions can be drawn from this case, although many of the legal issues were still pending in 1990. First, policy proliferation contributed to the problem. The FDA had jurisdiction over the valves. Congress began investigating the FDA's role in 1990 and accused Shiley of continuing to market the valves even after officials became aware of the manufacturing problems. Apparently as early as 1980, Shiley urged FDA officials not to notify the public because of the anxiety it
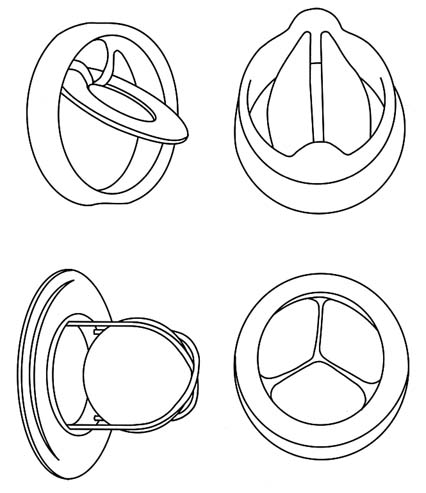
Figure 21. Examples of artificial heart valves.
Source: Marti Asner, "Artificial Valves: A Heart-Rending Story,"
FDA Consumer 15:8 (October 1981), 5.
might cause patients with implanted valves. A congressional report criticized the FDA, asserting that it was too slow in removing the valves from the market and did not properly inform the public of the risks.[54]
Greg Rushford, "Pfizer Fires Opening Salvo in Its Public Defense," Legal Times, 5 March 1990, 6.
The allegations raise important questions about the ability of the FDA to oversee the marketplace.Another issue involves innovation. Will the legal liability facing Shiley deter others from entering the heart valve market or force current producers to withdraw from the market? Will the
heart valve market soon follow the decline of the IUD industry? Will important incremental improvements in a valuable lifesaving technology be lost? For example, 76,000 units of an improved Shiley monostrut (one-strut) valve have been used without failure in Europe since 1983. The FDA, claiming it needs more clinical data, has not yet approved this innovation. Is the FDA being overcautious because of the current controversy? Will this stance aggravate the deteriorating conditions for innovators?
Finally, one can ask whether litigation can resolve the dilemmas faced by patients. What about the individuals who have defective valves already implanted? They face life-threatening surgery to remove them or daily fear that the valve might fail. Does it matter that they might have died without access to the innovation in the first place? Do we have unrealistic expectations about the medical products that we use? Is the newfound anxiety legally recognizable? Some heart valve recipients have sued Pfizer on the grounds that they have increased "anxiety" knowing they are living with a defective valve. Some courts have recognized the viability of an anxiety claim, but only if Shiley engaged in fraudulent, rather than merely negligent, behavior.[55]
A fraud claim differs from negligence. Fraud requires that the defendant misrepresented the product, knowingly with intent to induce the plaintiff to enter into the transaction. When fraud is involved, rather than simple negligence, the court has held that claims for anxiety can be heard. See Khan v. Shiley, Inc., 226 Cal. Rptr. 106 (30 January 1990).
Assessing the Fate of Two Technologies: Pacemakers and IUDs Compared
The motivations for greater government involvement and the manner in which that involvement occurred can be illustrated by two postwar technologies—the cardiac pacemaker (see chapter 5) and the IUD. Both products had antecedents stretching back many decades, but the arrival of these modern implanted devices occurred in the 1970s and 1980s. In both cases, the products diffused rapidly and widely, so that several million women used the IUD by the mid-1970s and tens of thousands of cardiac patients had the early pacemakers implanted. There is a danger that a focus on these two technologies might skew our perceptions of the field because both generated much controversy, while thousands of other new medical devices received little or no public attention. However, comparison of these two products provides useful insights into the evolution of public policies that
potentially inhibit device discovery. The public debates these devices generated led to political pressure for device regulation and illustrate the impact of the new product liability system.
It is intriguing to note that the IUD and pacemaker industries evolved quite differently by the mid-1980s. In 1986, there was only one small manufacturer of IUDs and one new entrant on the horizon. All the other major producers had withdrawn from the market, and sales were only a fraction of what they had been ten years before. The market for cardiac pacemakers, on the other hand, has continued to boom. There have been many important technological improvements. While some producers generated controversy, primarily in regard to sales tactics, early entrants prospered and many new companies thrived.
The contrast between these two innovations helps us ask important questions about the role of regulation and product liability in device innovation. The contrasts raise tantalizing questions as to why technologies succeed or fail, providing insight into the future of device innovation.
The advent of FDA regulation in the mid-1970s and the simultaneous expansion of product liability in the state courts substantially altered the interaction of the private sector and government. Inventors and developers of products could not afford either to ignore regulatory intervention before marketing a product or to ignore the regulators and the courts if subsequent risks occurred.
Regulation alone did not significantly disrupt the industry as a whole, although smaller firms bore a disproportionate share of regulatory costs. Product liability exposure presented a more general threat, particularly for evolving complex technologies, including implanted devices. The threat of liability and adverse legal outcomes work to shift costs of injuries through insurance from the consumers to the producers of products.
Pacemakers and IUDs can provide insights into the impact of these two pervasive regulatory and liability policies on innovation. There are many similarities between these two devices. Both are innovative implanted products, although the cardiac pacemaker is more complex because of its need of a power source and the need for lead wires to the heart. Both products were produced by a range of competitors for what were believed
to be large markets. Both markets included large reputable firms, and innovative smaller companies. Both had unscrupulous firms. There is evidence, for example, that A. H. Robins intentionally falsified research data; Cordis Corporation has been accused of selling defective pacemakers with faulty batteries even after knowing of the defects. Four former Cordis officers have also been indicted for fraud.[56]
New York Times, 20 October 1988.
Both technologies were subjected to significant regulation through the Class III mechanism after the law was passed. Both products gave rise to thousands of lawsuits.Yet, by the mid-1980s, only the Alza Corporation remained in the IUD business, dominating a very small market that represented less than one-tenth the number of total sales in the IUD heyday of 1974. By contrast, the pacemaker market was booming. Medtronic, the company that pioneered the device, remained the industry leader, but many other companies maintained innovative and lucrative positions in the field.
How can we explain this disparity? What can we learn from these cases? First, the vulnerability of an innovation to adverse regulatory or product liability effects may depend on the nature of the risks the product presents. Adverse reactions to IUDs included death and sterility in young women who were otherwise healthy. Pacemakers, even if they malfunction, do not generally cause death, only a return of the symptoms.
Second, the risks presented by a product may relate not to the technology per se, but to its use by inappropriate candidates. Women for whom IUDs were inappropriate experienced severe reactions. Pacemakers implanted unnecessarily do not present greater risks than those implanted in patients who benefited from them. Also, pacemakers are used by individuals with serious preexisting medical conditions. IUDs are used by healthy young women. Adverse reactions in this population seem more unnecessary and devastating than reactions in elderly cardiac patients.
Third, both IUDs and pacemakers suffered from adverse publicity brought about by regulation and product liability cases. However, negative publicity may affect a product more if there are alternatives available for the consumers. Because there were
alternatives to IUDs, it was easier for users to abandon them and switch to contraceptive pills or other barrier forms of contraception. Pacemakers continued to fulfill a critical function for which no alternative existed.
Fourth, there may be contributory effects from other public policies. The widespread availability of public Medicare funds for pacemaker implantation could have played a role in keeping the market healthy, leading to greater incremental innovation and product improvement. The market issues relevant to pacemakers will be discussed in the following chapter. The IUD did not benefit from substantial third-party payment support.
Fifth, both regulation and liability are crude tools for the prevention of product risks. Regulation attempts to operate proactively by eliminating potential risks before marketing. Arguably, this process would screen out inappropriate products, eliminating the need for product liability. Clearly the regulatory process is imperfect, because not all risks are eliminated. And the more burdensome the regulation, the more likely that desirable innovations are deterred or deflected. Product liability, a retrospective risk-reduction tool, can seriously inhibit a product or a company that produces a subsequently discovered high-risk device. Product liability may deter legitimate innovators from entering important fields of research.
It is clear that the full force of regulation and liability does not inevitably eliminate innovations. The pacemaker has remained a viable product, even in the face of controversy, and incremental innovative improvements have been continuously produced. Although the IUD did not flourish, the technology remains viable. Indeed, the efforts of two small companies, Alza Corporation and GynoPharma, which are discussed in Chapter 7, illustrate how firms can adapt controversial technologies to the current regulatory and liability environment.
Indeed, as policies have proliferated, their effects on the industry can only be understood in relationship to one another. It seems clear that the introduction of policies to inhibit device discovery—regulation and product liability—have negative effects on some products. However, the whole free-spending environment generated by government payment policies tended
to blunt the impact of regulation and liability. This environment, however, began to change when cost control became the theme of the 1980s. When efforts to inhibit device distribution began in earnest, the potential for serious impacts on innovators emerged.
7
Government Inhibits Medical Device Distribution
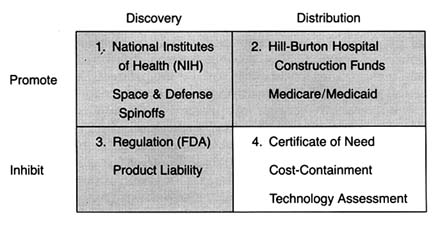
Figure 22. The policy matrix.
The federal and state payment programs described in chapter 4 provided little incentive for hospitals, providers, or eligible patients to consider costs when making decisions about health care. As a result, there were few economic barriers to the use of any new and apparently safe technology. Even the advent of regulation and the expansion of product liability in the 1970s did not appear to slow the growth of the health care technology market, at least in the aggregate.
However, concerns about the escalating costs of health care generated new attitudes toward medical delivery systems. The belief that more is better gave way to assertions that the system was too large, unwieldy, and wasteful. From the early 1970s through the mid-1980s, at least one dozen major federal laws
were enacted in response to spiraling medical costs.[1]
Lawrence D. Brown, "Introduction to a Decade of Transition," Journal of Health Politics, Policy and Law 11 (1986): 569-580, 571.
The dominant statutory and regulatory theme was cost containment. These new laws did not constitute revolutionary change. They did presage a conceptual shift, however, toward two potentially conflicting goals—promotion of competition in health care and greater government regulation and control.[2]Detailed discussion of these major and complex institutional reorganizations, such as HMOs (health maintenance organizations), capitation plans, and other forms of managed care, is beyond my scope here. For further reading, see Judith A. Hale and Mary M. Hunter, From HMO Movement to Managed Care Industry: The Future of HMOs in a Volatile Healthcare Market (Excelsior, Minn.: InterStudy Center for Managed Care Research, 1988); and Peter Boland, Making Managed Healthcare Work: A Practical Guide to Strategies and Solutions (New York: McGraw-Hill, 1991).
This chapter reviews the impact of government cost-control measures on the distribution of medical devices. Many considered medical technology a key culprit in the rising costs of health care. One widely cited 1979 study estimated that the contribution of new technology to hospital cost increases ran as high as 50 percent.[3]
Stuart H. Altman and Richard Blendon, Medical Technologies: The Culprit Behind Health Care Costs? Proceedings of the 1977 Sun Valley Forum on National Health (Washington, D.C., 1979), cited in Gloria Ruby, H. David Banta, and Anne K. Burns, "Medicare Coverage, Medicare Costs and Medical Technology," Journal of Health Politics, Policy and Law 10 (1985): 141-155, n.2.
There is no question that the cost-containment policies intended to slow the introduction and the diffusion of new technologies, particularly cost-raising products. The justification was that effective controls would not only reduce costs but also improve care because unconstrained diffusion led to excessive tests, treatments, and risks as well. (See figure 22.)There are three types of cost-containment strategies: behavioral, budgetary, and informational. Behavioral regulation tries to inhibit diffusion by modifying the behavior of medical decision makers. These policies influence decisions about use, expansion, and acquisition of technology. Prime examples of this policy strategy are the state based certificate-of-need programs (CON), described in more detail below. Budgetary regulation sets rates or expenditures, leaving administrators free to manage within the cost constraints established by the payers. Budgetary controls are epitomized by Medicare's prospective payment system (PPS). Informational regulation inhibits the adoption and diffusion of new medical technologies through prospective evaluation techniques, including technology assessment and the newer outcomes, or effectiveness, research.
Case studies illustrating the potentially powerful impact of these policies on the adoption and diffusion of medical devices include the intraocular lens (IOLs), artificial replacement lenses implanted after cataract surgery, and cochlear implants, permanently implanted devices that mitigate severe hearing impairment. Both of these cases demonstrate the impact of payment policies on the introduction of new devices. Also, they highlight the collective impact of a broad range of policies—from NIH, to
FDA, to HCFA—on the entire innovation process. By the 1970s and 1980s, policy proliferation was in full swing.
Policy Overview
Regulation of Market Behavior
The early cost-containment programs of the 1970s have been characterized as behavioral in that they tried to block decisions by providers about use, expansion, and acquisition of technology.[4]
See Brown, "Introduction to a Decade," in which he establishes these helpful categorizations of cost-containment policies.
The goal was to eliminate waste and unnecessary expenditure, in order to save the cost-based reimbursement system.One such effort was Title XVI of the National Health Planning and Resources Development Act of 1974, which supplanted the Hill-Burton program.[5]
David S. Salkever and Thomas W. Bice, Hospital Certificate-of-Need Controls: Impact on Investment, Costs, and Use (Washington, D.C.: American Enterprise Institute, 1979), 3.
These new controls stipulated that federal subsidies to eligible institutions must be made in compliance with statewide cost-control plans. In the 1970s, government subsidies became less important than other sources of funds, and the influence of these controls diminished considerably.In 1972, Congress also authorized establishment of federally funded, private Professional Standards Review Organizations (PSROs) to conduct independent quality and utilization reviews of hospital services under Medicare and Medicaid.[6]
Social Security Amendments of 1972, sec. 259F(b).
These efforts were highly controversial. Congress formally terminated the program ten years later, replacing it with an alternative peer-review approach. The Professional Review Organizations (PROs) established under the 1983 Medicare reform, discussed below, intended to monitor hospitals to determine if Medicare payments were appropriate.The statewide plans referred to in the health planning legislation generally meant certificate-of-need programs. New York state's passage of a certificate-of-need law in 1964 was the first government sponsored investment control. Concern about the inflation in health care costs led to the rapid diffusion of these types of controls. By 1978, thirty-eight states had adopted similar programs. State programs varied widely in terms of the types of institutions covered by the controls, thresholds for review, and legal sanctions for failure to comply.[7]
Clark C. Havighurst, "Regulation of Health Facilities and Services by Certificate-of-Need," Virginia Law Review 59 (October 1973): 1143-1232.
The goals of these laws generally were to eliminate "unnecessary" expansion of hospitals and to encourage less costly alternatives to hospital care. The easiest measure of these goals was excess bed capacity. Certificate-of-need agencies were predisposed to disapprove a hospital's proposal for new beds and spent considerable time and energy reviewing them. However, it is argued that controls were less successful when reviewing new services and equipment proposals, for several reasons: the costs of acquiring information about services and equipment are high, denials open the agency to charges of withholding from citizens the benefits of medical progress, and denials are likely to raise the ire of physician groups who want access to the same equipment that their colleagues have at other hospitals.[8]
Salkever and Bice, Hospital Certificate-of-Need, 20.
The evidence indicates that certificate-of-need programs resulted in no significant effect on total investments among hospitals but did alter the composition of those investments. Specifically, there was lower growth of bed supplies and higher growth of plant assets per bed. Thus, for most areas of medical device technology, these particular cost-containment strategies probably had little or no impact.Regulation through Budgetary Constraints
An alternative means to control hospital inputs is to put caps on reimbursements paid to hospitals from the federal and state governments. In September 1982, Congress directed the Department of Health and Human Services (HHS) to propose a plan to revise the Medicare payment system. That December, the Health Care Financing Administration (HCFA), the agency responsible for processing Medicare claims, proposed a prospective payment plan. Medicare had been building empirical data on this type of reimbursement scheme with demonstration projects in several states. Congress passed the Social Security Amendments of 1983 the following March, with most of the new provisions to be gradually phased in over a three-year period.[9]
Much has been written on the prospective payment system. See U.S. Congress, Office of Technology Assessment, Medicare's Prospective Payment System: Strategies for Evaluating Cost, Quality, and Medical Technology, OTA-H-262 (Washington, D.C.: GPO, October 1985). See also Louise B. Russell, Medicare's New Hospital Payment System: Is It Working? (Washington, D.C.: The Brookings Institution, 1989).
In brief, the plan created a complex prospective payment system, known as PPS. Medicare now bases its prices for Medicare hospital cases on a comprehensive classification system comprised of about 470 mutually exclusive categories called
"diagnostic related groups" (DRGs). The basic assumption is that all illnesses can be grouped according to disease system, intensity of resources consumed, and length of stay, among other categories, and that such groups reflect the average cost of providing services to all patients with diseases in that DRG. The price is then determined by calculating an average price per case for all Medicare cases plus the weight of the DRG assigned to the particular patient's case. The hospital is reimbursed at a price set in advance for each DRG rather than the actual cost of treatment. As of 1988, DRG reimbursement applied only to inpatient care. Physicians' services continued to be paid out on the old "reasonable cost" basis, but Congress passed legislation to extend a form of prospective payment to physicians in the 1990s.[10]
In 1986, Congress created the Physician Payment Review Commission (PPRC) to advise it on reforms of the methods used to pay physicians under Medicare. Its guiding principle has been that payment reform should provide equitable payment to doctors, protect beneficiaries, and slow the rate of increase of Medicare expenditures. As part of Omnibus Budget Reconciliation Act of 1989 (OBRA), Congress enacted comprehensive reform of Medicare physician payments. For a detailed discussion of Medicare physician payment issues, see Physician Payment Review Commission, Annual Report to Congress, 1990 (Washington, D.C.). The new fee schedule will not be in place until 1992 at the earliest. Its impact on medical device markets cannot be predicted at this time.
Congress understood that the prospective rate-setting process needed the flexibility to respond to advances in all health care technology. Congress created the Prospective Payment Assessment Commission (ProPAC) to participate in the process of updating the hospital payment rates in an independent and public fashion. ProPAC members are appointed by the Office of Technology Assessment (OTA), which is a congressional advisory body, and ProPAC's seventeen members must represent a wide range of constituencies. Its responsibilities under the law are to make annual recommendations to HHS on the appropriate percentage change in Medicare payments for hospital services and to make recommendations on necessary changes in the DRGs, including the establishment of new groups, modification of existing groups, and changing their relative weights when appropriate. HHS is not bound to adopt the recommendations of ProPAC and, to date, has not done so in many cases.[11]
The law requires that ProPAC submit annual reports to Congress that contain its recommendations on updating the Medicare prospective payments and modifying the diagnosis related group (DRG) classification and weighing factors. Public Law 98-21, sec. 1886(e)(4). See, for example, Prospective Payment Assessment Commission, Medicare Prospective Payment and the American Health Care System: Report to the Congress (April 1985) and annual reports thereafter.
The intended effect of this dramatically new payment structure was to encourage efficient delivery of health care services in the hospital sector, ultimately reducing federal Medicare expenditures. The intention to stabilize or reduce the market size as well as to inhibit unnecessary expenditures on underutilized equipment was an important goal. Consistent reductions in aggregate equipment expenditures have not, apparently, occurred, although sales in some SIC categories leveled off for a time because of uncertainty about the future.
The true impact of the new program on medical device producers
has been mitigated somewhat because it has not been fully implemented. At the end of the 1980s, one major issue relating to medical equipment had not been resolved. Congress deferred a decision on how the system should treat major capital expenditures by hospitals. Many medical devices, such as diagnostic equipment and monitoring and anesthesia products, fall into this category.
Under the cost-plus system of reimbursement before PPS, hospitals prepared capital-cost reports for Medicare. Medicare paid 100 percent of the reasonable costs of capital equipment (defined as land, buildings, and movable equipment) that were attributed to the care of Medicare patients in each hospital cost center. This procedure was known as the capital-cost pass-through , and it provided incentives for hospitals to expand and improve their capital base. The payment program, along with the growth of private insurance that provided stable cash flow, improved the borrowing power of hospitals and encouraged them to finance construction and equipment acquisition through debt. Hospital capital pass-throughs accounted for about 8 percent of the total Medicare spending in fiscal 1984 (with 14 percent of that attributable to depreciation of movable assets—that is, devices), a modest but important aspect of the financial picture for hospitals.[12]
Senate Committee on Finance, Hospital Capital Cost Reimbursement Under the Medicare Program, prepared for the Congressional Research Service of the Library of Congress by Julian Pettengill, 5 November 1985. See also Ross M. Mullner, "Trends in Hospital Capital and the Prospective Payment System: Issues and Implications," in Henry P. Brehm and Ross M. Mullner, eds., Health Care, Technology, and the Competitive Environment (New York: Praeger, 1989).
When PPS passed in 1983, Congress initially excluded capital-related costs from the prospective payment system, deferring a decision on the issue until October 1986. At the time, Congress sought more information because of the complexity of hospital capital spending and because of the variations in investment cycles that might give arbitrary advantages to hospitals with recently completed capital expansion if controls were imposed on a specific date.[13]
Brehm and Mullner, Health Care.
Congress proposed several capital-cost plans in 1985, but voted in 1986 and 1987 simply to reduce the percentage of capital-cost pass-through payments (phased in from a 3.5 percent reduction in 1987 to a 15 percent reduction in 1988), without tackling the more difficult task of folding capital costs into the prospective payment system. These congressional efforts prevented HCFA from imposing its own rules.[14]
52 Federal Register 33168-33199 (1 September 1987).
By the end of the 1980s, then, there was a two-tiered payment
system for equipment, allowing pass-throughs for capital but controlling all other hospital expenditures. The system has benefited equipment producers, at least in the short run. The expectation was that if costs for labor and services were controlled and capital equipment costs could be passed through, then hospitals would channel funds into capital projects and the purchase of labor-saving equipment. Indeed, a 1988 study found that spending for major movable equipment, in particular, increased after 1983.[15]
Frank A. Sloan, Michael A. Morrisey, and Joseph Valvona, "Effects of the Medicare Prospective Payment System on Hospital Cost Containment: An Early Appraisal," Milbank Quarterly 66 (1988): 191-220.
Debates on how to finally structure capital costs continued through the 1980s. HCFA urged merging capital costs into PPS. ProPAC, however, changed course in 1990 and opposed any change in the capital-cost program. Capital spending continued to rise, jumping 28 percent in 1989 to $15 billion. HCFA approved one-third of that amount, or about $5 billion in Medicare capital spending. This amount represented about 9 percent of the program's $58 billion, part A budget. Estimates for 1990 were $19.3 billion, up another 27 percent.[16]
Stephen K. Cooper, "ProPAC Nixes Capital Expenditure Reform Scheme," Healthweek, 7 May 1990, 15, 36. Any decision on the pass-through was delayed during 1990, and, regardless of the change, it would not take effect until 1992 or 1993. Erich Kirshner, "HCFA, AHA Maneuver on Medicare Capital Reimbursement," Healthweek, 30 July 1990, 9.
PPS could have had a much greater impact on innovative medical technology if the original plan had been fully implemented. Through the 1980s, the capital-cost pass-through operated as a modest safety valve on some innovative devices that are considered capital equipment. Yet, continuation of the debate into the 1990s underscores the pervasive market uncertainty facing medical device producers.
Information through Technology Assessment
Another technique to control the adoption and diffusion of technology is to assess it prospectively. If a new technology does not pass the evaluation screen, a market barrier can be imposed. Ongoing comparative assessment can promote the abandonment of outdated technologies and thereby affect the rate of diffusion.
Prospective assessment is not limited to evaluation on the basis of cost. Indeed, the concept, as originally conceived, was quite broad. The idea was formally developed by Congressman Emilio Daddario, chair of the House Subcommittee on Science, Research, and Development in 1965.[17]
H. David Banta and Clyde J. Beheny, "Policy Formulation and Technology Assessment," Milbank Memorial Fund Quarterly 59 (1981): 445-479.
His work recognized thatscientific and technological developments present potential social consequences. The goal of technology assessment was to examine the safety, efficacy, indications for use, cost, and cost-effectiveness of a particular technology, including the social, economic, and ethical consequences to improve health care decisions.[18]
For a thorough overview of all the forms of technology assessment and the variety of institutions engaged in the enterprise, see the Institute of Medicine, Assessing Medical Technologies (Washington, D.C.: National Academy Press, 1985).
While the concept of medical technology assessment sounds high-minded, its implementation raised a number of very complex issues. Assessment involves three levels of information: gathering of data, evaluating data, and imposing decisions through regulation based upon the evaluation. In addition, assessments can apply to a broad range of technologies—drugs, devices, procedures, and systems—or to just one. Finally, assessment can cover many attributes of a technology, including safety or cost, or it can be limited to only one attribute. Thus, for example, the Food and Drug Administration can be considered a technology assessment agency. Its jurisdiction extends to drugs and devices (not procedures); it evaluates the attributes of safety and efficacy only (not cost or cost-effectiveness); it can require data gathering from the producer; and it regulates based on its evaluation of the data.
It is clear that those who control technology assessments become pivotal gatekeepers for adoption and diffusion of that technology. Thus the history of medical technology assessment is inextricably linked to the tensions among interest groups to influence assessment results. Much of the battle has also involved private sector efforts to prevent government from increasing its share of the gatekeeper function. This concern is especially acute when the goals of the government are to contain costs and arguably to prevent innovations from entering the marketplace, particularly if they are perceived to increase costs.
Government agencies entered the technology assessment process at an early stage to accomplish a variety of goals. As might be expected, government efforts were piecemeal and underfunded. The market does not generate necessary and complete information on medical technologies because clinical testing is time-consuming and expensive. It is easy for competitors to become free riders by observing the technological choices of others. Government plunged ahead. In addition to the FDA, new
programs were created in both the administrative and the legislative branches of government, including the Health Program at the Office of Technology Assessment (legislative branch), the National Center for Health Services Research (NCHSR), the NIH's Office of Medical Applications of Research (OMAR), and, for a short time, the National Center for Health Care Technology (NCHCT). HCFA uses the services of the Offices of Health Technology Assessment (OHTA) within HHS to provide assessment data. OHTA has a very small budget and is limited to safety and efficacy review.[19]
For a discussion of the institutional issues in technology assessment, see Foote, "Assessing Medical Technology."
Both producer and physician groups in the private sector resented government efforts to control technology. Their opposition has been particularly intense when a government agency has the power to regulate decision making rather than the ability just to gather information. For example, in 1978 the American Medical Association (AMA) opposed the creation of the NCHCT, which had regulatory power, alleging that it would interfere with medical practice. HIMA argued that the new assessment agency was a threat to innovation. Both groups strongly and successfully advocated the dismantling of the agency in 1982.[20]
Ibid., 69.
When PPS passed, cost control became an important federal goal. Despite their concerns, technology producers understood that information about benefits as well as costs was now essential for government. A cost-conscious government payer (HCFA) accounted for the direct and the indirect purchasers of over 40 percent of all medical technology. HCFA would gather information regardless of whether the industry or professions cooperated. Government clearly assumed an important and indisputable role. Indeed, as the 1980s progressed, cost dominated the technology assessment debate, and HCFA established itself in a leadership position. Many private payers followed its lead.
Many believed, although HCFA vehemently denied it, that the agency made coverage decisions based predominantly on cost issues, at least since the inception of PPS. The debate about HCFA's jurisdiction and authority to evaluate a technology's cost-effectiveness in order to make coverage determinations illustrates the nature of the debate.
The legislative mandate of HCFA is to decide whether a medical
service is "reasonable and necessary" in order to provide Medicare coverage. Many coverage decisions are made by insurers that contract with Medicare, and there is much regional variation. However, some major coverage decisions are made by the national agency.[21]
See discussion in chapter 4.
In a rule proposed in 1989, HCFA attempted to clarify the meaning of "reasonable and necessary" under the Medicare program. It proposed to add the criterion of cost-effectiveness to considerations of safety and effectiveness; it also proposed to consider whether a technology was experimental or investigational. It justified its proposal thus: "HCFA is including cost-effectiveness as a criterion because we believe considerations of cost are relevant in deciding whether to expand or continue coverage of technologies, particularly in the context of the current explosion of high-cost technologies."[22]54 Federal Register 4302-4318, 4308-4309 (30 January 1989).
Both the AMA and HIMA opposed the addition of cost-effectiveness as a coverage criterion, arguing that it raised substantial legal, methodological, and policy questions. The AMA also argued that HCFA lacked the statutory authority to make the decision and that Medicare's purpose is to meet medical needs, not to make evaluations and comparisons among technologies that amount to the practice of medicine. It also argued that cost-effectiveness analysis is impractical, time-consuming, and inherently subjective.[23]
William McGivney, "AMA Responds to HCFA's Proposed Coverage Criteria and Procedures," American Medical Association Tech 2 (May 1989): 4-6.
HIMA had similar concerns, and added that cost should be a factor only in reimbursement decisions, not for coverage policy. HIMA stated that the proposal would provide a major barrier to entry for new technology. These concerns of organized medicine and industry echo their complaints against the now-defunct NCHCT. No action had been taken on the proposal by 1990. Regardless of the outcome, however, it is likely that HCFA will take cost into consideration, either overtly with clarified authority or somewhat more indirectly.Cost-effectiveness considerations have also crept into other technology assessments. A recent example involves the deliberations of the FDA Advisory Panel on Obstetrics and Gynecology Devices. The FDA has no statutory authority to consider costs in its deliberations; it is limited by law to considerations of safety and efficacy. However, the advisory panel voted unanimously against approval of Healthdyne's home uterine monitoring systems
for premature labor.[24]
Cited in James G. Dickinson, ed., Dickinson's FDA, March 15, 1989, 9.
The device reads the uterine activity of a pregnant woman and transmits it by modem to a professional in a clinical setting. The advisory panel was concerned about the absence of direct clinical proof that product use reduced morbidity or mortality, although others argued that in vitro diagnostics have never been required to produce such data. Also apparent in the advisory panel deliberations were concerns that the technology would become a costly new standard of care. The American College of Obstetricians and Gynecologists (ACOG) issued a statement that the device would cost about $80 a day, averaging $5,616 per patient and potentially costing $5.6 billion a year. Observers commented that costs appeared to have influenced the panel's decision to disapprove the device: "FDA advisory panels are intended to guide the agency on product safety and efficacy and risk-benefit. Increasingly, especially in the area of devices, panels have come under pressure from … [HCFA] … to factor in cost-benefit considerations as well."[25]Ibid.
The trend for the 1990s is movement from traditional technology assessment to the measurement of outcomes. Broadly conceived, outcomes research measures the patient's quality of life as the result of medical treatment. Purchasers are seeking value for their money, and providers are interested in how to ensure the best quality care. While the thrust of outcomes research is slightly different from technology assessment, the barriers to its attainment are similar. There is no consensus on how to measure outcomes or who should pay for it.
Despite these problems, Congress demonstrated its support for the concept of outcomes research with the 1989 creation of the Agency for Health Care Policy and Research (AHCPR) to "enhance the quality, appropriateness, and effectiveness of health care services and to improve access to services."[26]
Public Law 101-239. See also Ron Geigle and Stanley B. Jones, "Outcomes Measurement: A Report from the Front," Inquiry 7 (1990): 7-13. For details on the new agency, see U.S. Department of Health and Human Services, AHCPR: Purpose and Programs (Washington, D.C.: September 1990).
Congress appropriated $568 million over the next five years to fund its activities. Among its goals are the facilitation of practice guidelines to assist physicians, a treatment effectiveness program to assess the effects of variations of treatment on outcomes, and support for programs in health services research and training of researchers.Many express optimism about the potential for outcomes research. But even in its earliest stages, there is mutual distrust
between payers and providers and a belief that payers will assess technology on the basis of cost, not quality. As one observer put it, "[W]hat looks like effectiveness research from one perspective looks like cost-cutting from another. Providers continue to distrust the long reach of government; government looks with narrowed eyes on the amount spent for providers' services."[27]
Janet Ochs Wiener, ed., Medicine and Health: Perspectives, 9 October 1989, 1.
Case Studies: The Impact of Government Cost Containment on Distribution
The introduction of cost containment into government payment policies has had and will continue to have a powerful impact on medical device producers. HCFA is now as important a barrier as the FDA. Medicare coverage and payment policies can delay and limit access of an innovation to the marketplace. Most importantly, market access becomes problematical. There are no clear policy guidelines for producers as HCFA continually experiments with regulatory approaches, and Congress periodically intervenes as a watchdog and accomplice in the search for ways to control government spending. Additionally, there are short-run market distortions based on the idiosyncrasies of the Medicare system. For example, imposing DRGs on the hospitals only opened opportunities in the less regulated outpatient segment of Medicare. How long that differential will last is not known, as extensions of PPS to physicians' fees and outpatient settings were on the horizon in 1990. Once again, the future is uncertain, hampering long-term strategic planning in the industry.
The following case studies illustrate the powerful influence of government payment policies on a new technology. They also introduce the realities of policy proliferation. Cost containment appeared in an already complex, regulated market. In the cases of the intraocular lens and the cochlear implant, the interrelationships of the multiple policies play a role in the potential success or failure of the technologies.
Intraocular Lenses
Millions of Americans suffer from eye diseases that impair vision. Cataracts, opacities of the lens of the eye, often result from
degenerative changes in old age or from diseases such as diabetes. The symptoms include gradual loss of vision, and treatment most commonly involves removal of the diseased lens and the implantation of an intraocular lens (IOL) to restore sight.[28]
Eileen McCarthy, Robert Pokras, and Mary Moien, "National Trends in Lens Extraction: 1965-1984," Journal of the American Optometric Association 59 (January 1988): 31-35.
Ophthalmology in general, and IOLs in particular, represent one of the largest and most dynamic health care markets. The FDA regulates IOLs, and, because most of the implant candidates are elderly, the market is strongly tied to Medicare payment policy. This policy, as well as the interaction between regulation and reimbursement, has the potential for significant impact on the industry.
IOLs are one of the few ophthalmic products that the FDA has placed in Class III.[29]
See chapter 4 for a detailed discussion of FDA regulation.
Regulated since 1979, IOLs are subject to a special requirement imposed by Congress and enforced by the FDA.[30]David M. Worthen et al., "Update Report on Intraocular Lenses," American Academy of Ophthalmology 88 (May 1981): 381-385; and Walter J. Stark et al., "The Role of the Food and Drug Administration in Ophthalmology: An Editorial," Archives Ophthalmology 104 (August 1986): 1145-1147.
As with all Class III devices, the FDA reviews data on safety and efficacy in the premarket approval (PMA) application. During the experimental stage, Class III products may receive an IDE, or investigational device exemption, that allows them to be used in controlled studies while the manufacturer gathers and evaluates the data about their safety and efficacy. The collection of data supporting a PMA is expensive and time-consuming and may represent a significant barrier to entry for smaller innovative firms. For IOLs, however, a special exception was made whereby the producers could charge for the costs of the implanted lenses while still in the investigational (IDE) stage. This exemption facilitated the development of IOLs during the two or more years of FDA-required device testing in clinical settings.Indeed, the availability of Medicare payment for this primarily elderly patient population essentially guaranteed a large, stable market for lens removal and IOL implantation. The average cataract patient is sixty-eight years old; Medicare is the sole payer for almost all cataract surgery. The frequency of IOL implants has grown rapidly in the 1980s. In 1979, there were 177,000 implants; by 1986, the number was 888,000. By 1988, there were 1.2 million implants in the United States and another one million internationally. Annual IOL sales have been estimated at $360 million in the United States alone (see table 9).[31]
Biomedical Business International 12 (17 May 1989): 70-71.
Medicare pays close to $1.5 billion annually for cataract operations,
| ||||||||||||||||||||||||||||||||||||
making them the largest item in the Medicare program in 1988.[32]
Milt Freudenheim, "Medicare's Curbs on Cataract Fees," New York Times, 15 November 1988, C2.
During the 1980s much of the treatment shifted from hospital to outpatient surgery centers or physicians' offices, which are covered under Part B of Medicare and thus are not under the DRG system. The growth can be attributed to advances in the technology, including cataract management, anesthesia, surgical technique, and postoperative care. New IOL technology includes soft lenses that can be implanted with smaller incisions (the one-stitch lens is a recent innovation), and bifocal implants and other specialty lenses have been developed recently.The industry is dynamic and competitive. There are a number of companies in the IOL field, ranging from very large firms such as Johnson & Johnson (IOLAB), CooperVision (recently acquired by Alcon/Nestle), and Allergan (purchased by SmithKline Beecham in 1989). Smaller firms include IOPTEX Research, a privately held industry leader, and Chiron Ophthalmics, a subsidiary of Chiron Corporation, a biotechnology firm. There are also foreign IOL makers from West Germany, France, Belgium, Israel, and Japan.
Changes in both Medicare policy and FDA regulations present threats to the IOL market. Congress has reduced federal
Medicare payments for cataract surgery twice since 1986. HCFA has lowered the amount of payment to physicians for the procedure. These changes have come in the wake of allegations of market abuse by cataract surgeons. Congress investigated the situation as early as 1985;[33]
House Ways and Means Committee, Medicare Reimbursement for Cataract Surgery 99-37, 99th Cong. (Washington, D.C.: GPO, 1 August 1985).
additional hearings were held in 1990, prompted by reports that surgeons employed abusive marketing techniques to round up elderly patients, and that many earned more than $1 million a year performing surgery covered by Medicare.[34]Dwight E. M. Angell, "Cataract Surgeons Face a Critical Eye," Healthweek, 23 April 1990, 1, 8-9.
In 1990, HCFA reduced the payment rate for an IOL implanted during cataract extraction. The revised rate of $200 meant an average reduction of at least $100 from the former IOL rate.[35]
Freudenheim, "Medicare's Curbs."
This lower rate was based on an audit by the Office of the Inspector General to determine how much was actually paid for lenses after subtracting various rebates and discounts often associated with lens purchases. However, many of the newer specialty lenses average over $300 each.The FDA has imposed new requirements for data collection as well. For new bifocal and multifocal products, fifty implants have to be studied for a year, and then the studies can be expanded to five hundred implants. Overall, it is likely to take nearly four years for a new lens to receive premarket approval. The longer period for approval is less onerous if the innovator receives at least partial payment for the experimental lens implants. Rumors have been circulating that the exemption allowing payment for IOLs under IDEs will soon be rescinded. HCFA officials state that this move will encourage producers to progress from the IDE to the PMA stage. They assert that companies have been allowing products to languish under IDEs because the economic incentive to go to market is reduced by the exception. The device is paid for in either case. However, the longer testing requirements and the threatened withdrawal of payment during the investigational period potentially will have a significant impact on newer, less well-capitalized entrants.
Cochlear Implants
Cochlear implants, a technology that permits individuals with profound hearing loss to receive auditory cues, have had a very
different reception than IOLs. Unlike implanted lenses that diffused rapidly to millions of elderly, cochlear implants have not fared well. Indeed, Medicare reimbursement was made for only sixty-nine such implants in fiscal 1987, despite estimates that sixty thousand to two hundred thousand Americans could benefit from the device. Many industrial competitors never entered the field or have since abandoned it, leaving only three firms still in the market in 1990. This medical device has met resistance throughout its history, and the collective impact of policy hurdles has been profound.
The cochlea, a structure in the inner ear, translates sounds from mechanical vibrations to electrical signals. These signals are produced by cells in the cochlea. The cells have a fringe of tiny hairs that bend in response to vibrations in the outer and middle ear. Those responses produce electrical signals that stimulate the auditory nerve and send messages that the brain interprets as sound.[36]
Nancy M. Kane and Paul D. Manoukian, "The Effect of the Medicare Prospective Payment System on the Adoption of New Technology: The Case of Cochlear Implants," New England Journal of Medicine 321 (16 November 1989): 1378-1383.
This natural system of hearing is versatile enough to transmit the full range of sounds. If the hair cells in the cochlea are damaged by injury or disease, the individual is condemned to deafness. Over two hundred thousand Americans suffer this profound hearing loss, and conventional hearing aids are useless for them. Cochlear implants, at least at this stage of development, cannot restore the world of sound. They do allow for reception of sounds such as sirens and voices, auditory cues that are vital for safety and for some social interactions. But the recipient of the implant cannot hear normal conversation.
The possibility of producing useful hearing by electrical stimulation of the cochlea occurred by accident.[37]
Robin P. Michelson, "Cochlear Implants: Personal Perspectives," in Robert A. Schindler and Michael M. Merzenich, eds., Cochlear Implants (New York: Raven Press, 1985), 9-11.
When an amplifier used in the operating room to monitor the cochlear response oscillated, the patient heard a very high-pitched tone. Some early work on this type of induced stimulation was published in 1955 and 1956.[38]William F. House, "A Personal Perspective on Cochlear Implants," in Schindler and Merzenich, Cochlear Implants, 13.
Researchers undertook additional work during the early 1960s, but they encountered significant problems. Among the barriers were included adverse patient reactions to the insulating silicone rubber used in the first primitive devices and concern about the effects of long-term stimulation on all auditory sensation. In 1965, the results of studies were submittedto the American Otological Society but were rejected as too controversial for presentation.
As implant technology improved in the late 1960s, some of the problems were resolved, but concerns over ethics and long-term effects still dogged the technology. The National Institutes of Health did not provide any funding for the scientific research, a refusal that some scientists in the field attributed to the bias against biomedical engineering among NIH peer review groups.[39]
See the discussion in chapter 3 on an antiengineering bias at the NIH.
Several policy breakthroughs occurred in the 1970s, when the NIH focus began to shift toward goal oriented, or targeted, programs that would produce identifiable results. The NIH established an intramural program to investigate cortical and subcortical stimulation, primarily for blindness but also for other neurological disorders. While hearing stimulation was not an important part of the original program, it became of greater interest when the research on cortical visual implants appeared clearly unsuccessful. In 1977, the NIH instituted an independent assessment of patients with cochlear implants. The Bilger Report, produced at the University of Pittsburgh, concluded that these products were a definite aid to communication.[40]
R. C. Bilger et al., "Evaluation of Subjects Presently Fitted with Implanted Auditory Prostheses," Annals of Otolaryngology, Rhinology and Laryngology, supplement 38 (1977) 86: 3-10. Discussed in F. Blair Simmons, "History of Cochlear Implants in the United States: A Personal Perspective," in Schindler and Merzenich, Cochlear Implants, 1-7.
Seven years later, in November 1984, the FDA approved the design of a single-channel device for cochlear implantation. The 3M Company produced the device in conjunction with Dr. William House, an early researcher and chief inventor. The device consisted of a receiver similar to that of a hearing aid, a speech-processing minicomputer that transforms the sound signals into electrical signals, another receiver for those electrical signals that is implanted under the skin above and behind the ear, and a thin wire inserted surgically through the mastoid bone into the cochlea to transmit the signals (see figure 23).
By 1985 a handful of companies had entered the market. The 3M Company was the clear leader with the only approved product. Others included the Nucleus Group, an Australian company and parent of the U.S. Cochlear Corporation, Biostim, and Symbion, an outgrowth of research at the University of Utah.[41]
Biomedical Business International 8 (29 March 1985): 47-48.
A private industry group reported that leading hearing-aid manufacturers did not enter the marketplace because "the FDA-related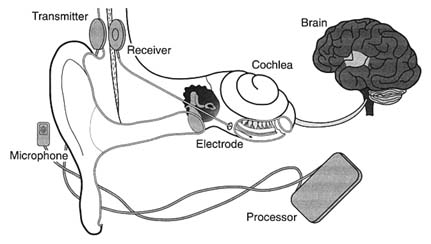
Figure 23. The cochlear implant.
Source: The 3M Company, Cochlear Implant System, n.d.
expense of developing and testing such devices is prohibitive." It was reported that 3M had budgeted over $15 million for cochlear implant development.
Once approved by the FDA, the device faced the hurdle of Medicare's coverage and payment decision. After several years of deliberation, and following endorsement of the device by the AMA in 1983 and the American Academy of Otolaryngology in 1985, Medicare issued a favorable coverage ruling for both single-channel and multichannel devices in September 1986. The next step was for HCFA to assign the technology to a code which would then provide the means for establishing appropriate levels of payment for procedures.[42]
Kane and Manoukian, "The Effect of Medicare," 1379.
HCFA has considerable discretion in the placement of a new procedure into the DRG system. If the device is assigned to a DRG that does not cover the cost during the diffusion period, hospitals implanting the devices will lose money. Hospitals that increase the proportion of cases involving these devices lower their operating margins in those DRGs. ProPAC recommended in 1987 that the cochlear implant be assigned to a device specific, temporary DRG. HCFA did not follow ProPAC's recommendation. Instead, in May 1988 the agency announced a DRG placement
that would not pay the full estimated cost of $14,000 for the implantation of the device.[43]
Ibid., 1380.
Evidence accumulated that hospitals had a strong disincentive to provide cochlear implantation. Ten percent of the 170 hospitals involved openly acknowledged to researchers that they restricted implantations because of the loss of $3,000 to $5,000 for each Medicare case.[44]
Ibid., citing Cochlear Corporation personal communication.
These policies limited the size of the market and deeply affected the private sector producers. The 3M Company stopped marketing the single-channel model actively and halted research on multichannel devices because of the low use rate of both models. The small market discouraged additional investment. There were five firms that developed cochlear implants for the U.S. market from 1978 through 1985. By 1990, three had left and there were no new entrants with FDA approved devices.[45]
Ibid.
It was undisputed that this new technology had limitations, but it also was recognized as useful and beneficial to certain classes of patients. The policy environment was relatively unresponsive to early development. The quest for FDA approval was difficult, time-consuming, and costly. The response of HCFA to the technology was definitely obstructionist. The future of the research and development of this area of technology is now in doubt.
The primary effect of the payment policy has been uncertainty in the marketplace. Firms can no longer count upon growth in their particular market segment. Incremental policy changes are frequent and can have catastrophic effects on the markets for some products. In addition, cost-containment policies have introduced new hurdles to market access, which cause higher costs and delays even for successful new entrants.
Cost control has become an important value in the distribution of medical devices. It presents significant problems because the costs of a new technology are difficult to predict before distribution. Some products may have cost-reducing potential that is not known in the early stages or additional beneficial applications that will emerge during use. It is legitimate to ask how much cost should matter and who should decide that issue.
In addition, the case studies illustrate how complex the policy environment was in 1990. There are significant hurdles at virtually
every stage of innovation. Even promoters such as NIH can place barriers in the paths of the innovators. NIH disapproval can act as a deterrent, as the early years of cochlear implant development reveal. HCFA, once a source of nearly unlimited funds, can significantly delay or even bar technology from the marketplace.
Our medical device patient is now a confirmed recipient of polypharmacy, as the prescriptions have proliferated over time. Before we turn to the prognosis, however, it is necessary to look at the international marketplace. Does the world market provide an outlet for manufacturers constrained by cost controls in the United States? Or are international firms a competitive threat both in the United States and abroad?
8
The International Marketplace: Safety Valve or Competitive Threat?
By the end of the 1980s, the United States medical device market was fraught with uncertainties, particularly as cost-containment pressures grew. International sales opportunities offered a safety valve for American device producers. Foreign competitors, on the other hand, tried to dominate the markets both in their own countries and in the United States.
This chapter provides an overview of the international medical device market. American device firms must compete in an increasingly international marketplace. Penetration of these markets depends on not only trade and tariff issues but also an understanding of government policies on health care regulation and delivery. Three case studies illustrate specific international challenges. The discussion shows how regulation and the structure of health care delivery in Japan affect foreign producers; how efforts at harmonization dominate European concerns; and how China represents the challenges of international competitors in a large and developing marketplace.
Overview of the International Market
Throughout the 1980s, the United States dominated the $36.1 billion worldwide market for medical devices and equipment. America produced 62.3 percent of the world market share and consumed 59.3 percent, or over $20 billion in sales. Japan was a distant second, producing nearly 16 percent of medical equipment consumed worldwide and spending 12.3 percent, or $5 billion annually. West Germany ranked third, with 9.1 percent production and 6.9 percent consumption. Other western European
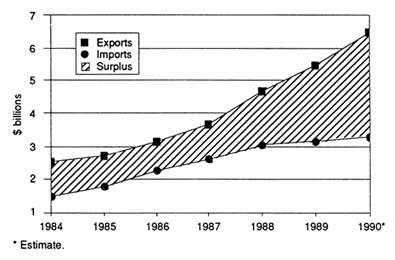
Figure 24.
United States exports and imports of health care technology products,
1984–1990. Source: U.S. Department of Commerce. Reprinted from
Health Industry Manufacturers Association, Competitiveness of the
U.S. Health Care Technology Industry (1991), 13.
countries consumed 9.5 percent and produced 6.7 percent, and all other countries combined accounted for only 6 percent production and 12 percent consumption.[1]
Biomedical Business International 11 (12 December 1988): 185.
Despite these positive statistics, there are mixed signals for U.S. medical device producers. The favorable balance of U.S. trade worldwide has been sliding since 1981. The trend was downward from 1983 to 1987 (see figures 24–27). The slight improvement in 1987 has been generally attributed to the favorable U.S. exchange rates.[2]
HIMA Focus, November 1988, 6, citing data from U.S. Department of Commerce. (Newsletter of the Health Industry Manufacturers Association.)
Department of Commerce data show that for medical device products between 1983 and 1987 the average annual growth rate for exports was 8.9 percent, while imports were more than twice that at 20.4 percent.The trade balance with Japan shows a more precipitous decline for the United States, with a fall to a trade deficit in 1984 (see figure 28). This deficit occurred because exports to Japan did not rise as fast as did imports of Japanese medical device products to America. American and Japanese consumption and trade patterns since 1968 are shown in table 10.
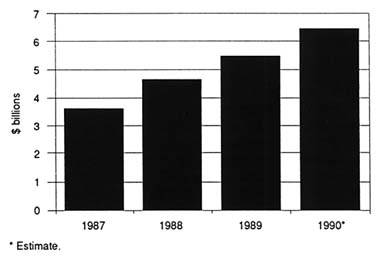
Figure 25. United States medical products exports, 1987–90.
Source: U.S. Department of Commerce. Reprinted from Health
Industry Manufacturers Association, Competitiveness of the
U.S. Health Care Technology Industry (1991), 13.
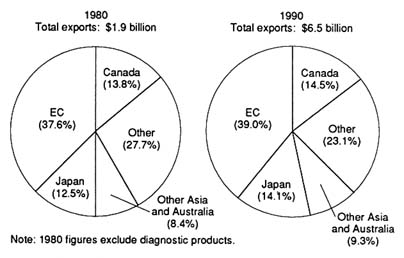
Figure 26.
Major purchasers of United States medical products exports, 1980 and 1990.
Source: U.S. Department of Commerce. Reprinted from Health Industry
Manufacturers Association, Competitiveness of the U.S. Health
Care Technology Industry (1991), 15.
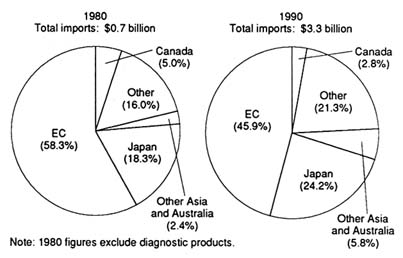
Figure 27.
Major suppliers of United States medical products imports, 1980 and 1990.
Source: U.S. Department of Commerce. Reprinted from Health Industry
Manufacturers Association, Competitiveness of the U.S. Health
Care Technology Industry (1991), 15.
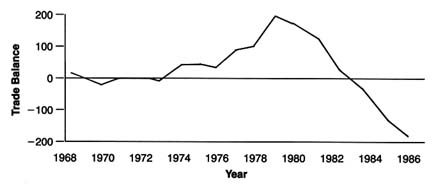
Figure 28.
United States and Japan medical equipment trade balance (based on SIC categories).
Source: Susan Bartlett Foote and Will Mitchell, "Selling American Medical
Equipment in Japan," California Management Review 31:4 (Summer 1989), 147.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Selling American Medical Equipment in Japan
Some have argued that both trade barriers and exchange rates explain our declining balance of trade with Japan, but these are not the complete or even the primary explanations underlying medical equipment trade patterns between the two countries. The data indicate that sales of American equipment have been increasing in Japan. Japan imports at least as great a proportion of its medical equipment as does the United States (about 20 percent, compared to 15 percent in the United States). Despite adverse exchange rates, the American share of medical equipment imports to Japan grew from 30 percent in the early 1970s to over 60 percent in the mid-1980s.[3]
Pacific Projects, "IMR Survey: The Medical Equipment and Health Care Services Market in Japan" (Tokyo, 1987). Pacific Projects, "Survey of Japanese Markets for Medical Equipment," vol. 1 (Tokyo, 1979).
The deficit has occurred because American purchases of Japanese manufactured goods have risen faster than American sales in Japan.Obviously American firms need to preserve their competitive positions in their own marketplace, the largest in the world. However, American companies can also improve device sales in the Japanese market. The following discussion describes the key government policies in the Japanese medical market and then briefly analyzes why certain American products and firms have been successful in the Japanese market.
Safety Regulation as a Nontariff Barrier
Medical devices are subject to extensive government safety regulations in Japan. These regulations may have explicit or implicit effects on foreign producers.[4]
Our discussion is limited to trade issues involving medical devices. For in-depth analysis of trade issues generally, see Clyde V. Prestowitz, Jr., Trading Places: How We Allowed Japan to Take the Lead (New York: Basic Books, 1988). For discussion of Japanese industrial organization see Chalmers Johnson, MITI and the Japanese Miracle: The Growth of Industrial Policy 1925-1975 (Stanford: Stanford University Press, 1982); and James C. Abegglen and George Stalk, Jr., Kaisha: The Japanese Corporation (New York: Basic Books, 1985).
There are no overt tariff barriers to the import of medical equipment into Japan. Ever since the Japanese government suspended the "Buy Japanese" program in the early 1970s, the most significant barrier to entry for medical equipment has been safety regulation. It has been argued that the Japanese Ministry of Health and Welfare (MHW) imposes nontariff trade barriers that discriminate against foreign producers in a protectionist manner.[5]
Vincent A. Bucci, "Japanese Import Restrictions on Medical Devices—An Overview of Recent Statutory Changes," Food, Drug, Cosmetic Law Journal 39 (1984): 405-410.
While there is no evidence of explicit discrimination against foreigners, there has been concern about implicit barriers.In 1961, Japan enacted the Pharmaceutical Affairs Law to control the marketing of medical devices and pharmaceutical products. To bring a product to market, all domestic and foreign producers must obtain either manufacturing or import approvals (shonin ) of the product itself. The MHW issues shonin after consultation with the Central Pharmaceutical Affairs Council, which reviews the scientific data on safety and efficacy submitted by the applicant.
In addition to shonin, every producer must obtain a license to manufacture or import (kyoka ) by presenting documentation that appropriate safety and manufacturing standards have been met. After the shonin and the kyoka have been obtained, the next step is to receive a price listing. The MHW sets prices for drugs and procedures involving medical equipment based on rules established by the Social Insurance Medical Affairs Council (Chuikyo).[6]
See discussion in Susan Bartlett Foote and William Mitchell, "Selling American Medical Equipment in Japan," California Management Review 31 (1989): 146-161, 149. See also M. H. Piscatelli, "American-Japanese Trade Impasse: The Regulation of Medical Device Imports into Japan," Syracuse Journal of International Law and Commerce 12 (1985): 157-169.
Until the late 1970s, Japanese medical equipment purchases were low. Many American firms had little interest in the Japanese market. As Japanese consumption of medical equipment increased in the 1980s, however, American exporters began to look seriously at the Japanese marketplace. In the process, they expressed frustration with the regulatory system. The two governments held trade meetings periodically from 1982 to 1985.[7]
Piscatelli, "American-Japanese," 159-160.
On 2 January 1985, President Reagan and Prime Minister Nakasone specifically identified pharmaceutical and medical equipment trade as one of four important sectors in the market-oriented, sector-selective (MOSS) talks that followed.In January 1986, the U.S. and Japan MOSS negotiating teams issued a final report.[8]
U.S. and Japan MOSS Negotiating Teams, Report on Medical Equipment and Pharmaceutical Market-Oriented, Sector-Selective (MOSS) Discussions (Washington, D.C.: GPO, 1986).
It addressed many issues related to uncertaintiesand delays in the regulatory process and the limited opportunities for producers to communicate with regulatory authorities. Even the Japanese industry supported proposals to streamline the regulatory bureaucracy. According to Koichi Ichikawa, President of the Japan Medical Equipment Manufacturers Association, "[T]he [Japanese] industry has nothing against the U.S. demands which are, for the most part, legitimate concerns regarding the approval-obtaining procedure. Considering that the procedural problem is shared by domestic manufacturers and importers, the industry recommends that the U.S. demands be accepted."[9]
Koichi Ichikawa, "Outlook for the Medical Equipment Industry Bleak in the Short Run: Brighter in the Long Run," Business Japan, June 1985, 71.
Other issues raised in the MOSS negotiations were primarily of concern to foreign producers. The most important was the acceptance of foreign clinical test data as evidence of safety and effectiveness. Some American companies argued that the requirement that all clinical tests be done in Japan on resident Japanese citizens was designed to discriminate against foreign companies, requiring duplication of clinical testing and leading to delay in market entry.[10]
Bucci, "Japanese Import Restrictions," 407. The Japanese are not the only nation skeptical of foreign clinical test data. Indeed, the United States has its own reservations. The FDA dismisses many foreign tests as scientifically flawed or poorly inspected. Problems in reviewing foreign studies arise due to different research traditions and language barriers. See Louis Lasagna, "On Reducing Waste in Foreign Clinical Trials and Postregulation Experience," Clinical Pharmacology and Therapeutics 40 (1986): 369-372.
The Japanese, however, believe strongly in their unique racial makeup. The government argued that these racial differences require validation of safety on Japanese people before marketing. Japanese negotiators in the 1983 talks refused to accept the arguments that foreign data provide sufficient safety information. In 1986, however, they made significant compromises. Under the MOSS agreement, except where there are demonstrable immunological and ethnic differences, foreign clinical data will be accepted for all examination and testing requirements but not for implantable devices, such as heart valves and pacemakers, or those products affecting organic adaptability.[11]MOSS Negotiating Teams, Report.
American firms also objected to restrictions on transfers of shonin from one business entity to another. American companies are considerably less stable than Japanese firms,[12]
William G. Mitchell and Susan Bartlett Foote, "Staying the Course: Comparative Stability of American and Japanese Firms in International Medical Equipment Markets" (Unpublished working paper, University of California, Berkeley, 1988).
often changing ownership. Consequently, they expect flexibility in business arrangements, such as shifts in licensee or in place of operation or manufacture. The rules for transfer of shonin were quite rigid; any change in name or ownership required producers to obtain new approvals and prices with requisite delays and costs. The Japanese agreed to consider changes in these procedures.Americans expressed guarded optimism that the MOSS negotiations would improve the situation for their firms.
Understanding the Medical Marketplace
A fundamental understanding of the health care system has been essential to successful penetration of the Japanese medical equipment marketplace. Japan has a combination of a public commitment to health and a fragmented private sector delivery system. Article 25 of Japan's constitution declares that promotion and improvement of public health, together with social security and social welfare, are the responsibilities of the nation.[13]
M. Hashimoto, "Health Services in Japan," in Michael W. Raffel, ed., Comparative Health Systems (University Park, Penn.: Pennsylvania State University Press, 1984).
The Japanese medical system provides health coverage for virtually all of the population. The private sector delivers the services and is reimbursed by the government. In essence, payment is centralized, but delivery is fragmented. Thousands of autonomous hospitals and other medical institutions purchase equipment and supplies. In 1984 there were 181,000 doctors. By law, the head of a hospital must be a doctor; 76,000 physicians owned their own hospitals or clinics. Solo practice is the norm; hospital chains are rare. Relationships between hospitals are not close, and joint purchase of equipment is not common.[14]
Pacific Projects, "IMR Survey."
Direct government control of purchasing decisions would be difficult or impossible in this system, but it can influence purchase and use decisions through the reimbursement system. The MHW has supplied generous resources to pay for technology, whether domestic or imported. For example, Japan, with half the population of the United States, has about the same number of CT scanners.[15]
R. Niki, "The Wide Distribution of CT Scanners in Japan," Social Science Medicine 21 (1985): 1131-1137.
There are restrictions on purchases. The MHW sets prices for all drugs and diagnostic procedures. In a system dominated by private practitioners, the incentives to provide these products are related to their profitability. The government sets the reimbursement price. Physician and laboratory profits are then determined by the difference between the government's rate and the price paid to purchase the drug or the device or to deliver the service, such as a diagnosis, using the device. By the late 1980s, price issues were highly controversial because the government began to contain costs by reducing reimbursement rates.[16]
Pacific Projects, "IMR Survey"; and "Drug Manufacturers Seek Greater Pricing Flexibility," Japan Economic Journal 30 (May 1987): 21.
MHW cost-control policies have restricted the ability of American and European firms to sell equipment to Japanese purchasers. However, the restriction has not occurred by direct fiat or even by an unstated preference in favor of Japanese manufacturers. Instead, the foreign manufacturers have usually charged more than Japanese companies for medical equipment and are not price competitive. The barrier is primarily the result of a foreign firm's inability or unwillingness to meet prices rather than an intended consequence of Japanese public medical care payment policy.
American Success in Japan
In addition to the Japanese government policies that present barriers to foreign firms, there are significant cultural differences in approaches to health and illness and in expectations about medical equipment. Language barriers can also be a problem.[17]
For interesting discussions of these issues, see John Steslicke, Doctors in Politics: The Political Life of the Japan Medical Association (New York: Praeger, 1973); and Emiko Ohnuki-Tierney, Illness and Culture in Japan: An Anthropological View (Cambridge: Cambridge University Press, 1984).
In order to succeed in this fragmented but quality-and price-conscious market, foreign producers need innovative, cost-competitive products with efficient distribution systems. Japanese physicians respect innovation and often associate that attribute with American goods.[18]U.S. Department of Commerce, International Trade Administration, U.S. Imports of Medical Equipment, 1983-1986 (Unpublished report, 1987).
They have been willing to seek out foreign suppliers of innovative products even when the manufacturer's distribution system was weak.Innovative American companies, such as SmithKline Beecham and Abbott Laboratories (through Dainabott, a joint venture with Dainippon Pharmaceuticals), have established strong leadership positions in clinical chemistry products (for example, blood analyzers, immunochemistry, and reagents).
American firms have an important edge in implant technology. Medtronic dominates the implanted pacemaker technology in Japan, with Cardiac Pacemakers a strong force also. Indeed, in pacemaker technology, several major Japanese firms, notably Toshiba, NEC, and Silver Seiko, tried to enter the field but have abandoned it. Knowledgeable observers speculate that these companies retreated because they lagged in technological and clinical know-how.[19]
Japanese firms fear that their corporate images would be damaged if patient deaths were to be associated with products bearing their names. There is also a cultural inhibition against introducing artificial devices into the body. American companies have been more willing to advocate implants than have Japanese firms; doctors have been receptive because of the medical and financial benefits of implants.
Market success, therefore, requires both a product that is mechanically reliable and a staff that understands its use.Many of the new medical technologies that have diffused through the Japanese medical system have been simpler or cheaper than the best-selling versions in the United States. CT scanners, for example, were introduced into Japan in 1976, about three years after they were first sold in the United States, and are now in almost all hospitals. Most of the scanners, however, are head units, rather than the more expensive whole-body machines common in the United States. Magnetic resonance imagers, too, have diffused through the Japanese medical system. Most have been low-powered units selling for about $500,000 rather than the $2 million helium-cooled superconducting units that have sold well to American institutions. Japanese domestic firms may succeed because of lower production and capital costs.[20]
Michael Rappa, "Capital Financing Strategies of the Japanese Semiconductor Industry," California Management Review 27 (Winter 1985): 85-99.
In addition to the product attributes described above, American firms must master marketing in the fragmented Japanese health care system. Sellers must possess sophisticated and well-developed distribution systems. Language barriers and cultural norms require a Japanese sales force. In turn, sales personnel need to develop stable, long-term relationships with the thousands of physicians who make the purchase decisions. Most foreign companies have been only partly successful in meeting these institutional demands. Direct investment in Japan is expensive and requires a long-term marketing commitment. Japanese purchasers, as well as potential employees, are skeptical of American firms because of the frequent mergers, acquisitions, and corporate reorganizations that characterized American industry in the 1980s.[21]
For a detailed discussion of successful organizational strategies in Japan, see Foote and Mitchell, "Selling American Medical Equipment," 153-158.
Some Japanese regulatory procedures continue to frustrate foreigners and have implicitly discriminatory effects. Government payment policies favor low-cost devices, often produced domestically. There is no substitute for familiarity with the language and the nuances of culture and tradition.
However, the barriers are not insurmountable. Sales of U.S. medical equipment in Japan have been increasing as the Japanese market has grown, from $128 million in 1975 to $355 million in 1986.[22]
Ibid.
New medical equipment can ameliorate social ills and transcend ethnocentric trade concerns. Thus, while the Japanese regulatory system and the medical marketplace mustbe reckoned with, it does not create an impenetrable barrier to foreign medical devices.
The European Economic Community: Uncertainties for American Producers
European Community Structure and Goals
Western Europe represents a substantial share of the global marketplace for American device producers. The twelve nations that comprise the European Economic Community (EC) include a population of over 320 million, most of whom are accustomed to fairly high-quality and highly technical medical care.[23]
Current European Community members include the United Kingdom, Greece, Ireland, the Netherlands, Belgium, Luxembourg, Denmark, Germany, Italy, Spain, Portugal, and France.
Collectively, the EC nations consume 16.4 percent of the world's medical equipment, ranking second in consumption, and purchase 39 percent of U.S. exports. The EC is also an important global competitor, producing 15.8 percent of all medical equipment.[24]Biomedical Business International 9 (12 December 1988): 185.
Many changes in this region were on the horizon in 1990. Indeed, the goal of the EC is unification by 1992. Unification includes the establishment of a common market with no barriers to trade for goods, services, and capital, including the free circulation of labor, the abolition of customs between member states, and the harmonization of regulatory policies among the states.[25]
Article 155, Treaty of Rome (25 March 1957).
In pursuit of unification, all industrial product tariffs were abolished in 1977. Many nontariff trade barriers remained, such as conflicting national product regulations. During the 1970s, domestic economic problems in member states and increased competition in world markets reduced political momentum to achieve a unified European market. A new initiative began in 1985 with a detailed program of action that set 1992 as the deadline for a unified internal European market. Indeed, 1992 has become a rallying point for producers concerned about the future of the European marketplace.The European Community is governed by a quadripartite institutional system consisting of the European Commission with executive powers, the European Council with legislative authority, the European Parliament (also called the Assembly), and the European Court of Justice. Policy-making functions are shared
principally by the Commission and the Council, with the Assembly relegated almost entirely to an advisory role.[26]
For an excellent summary of the organization of the EC, see Oppenheimer, Wolff, & Donnelly, "An Overview of the European Community's 1992 Program" (Prepared for the Health Industry Manufacturers Association, 9 November 1988).
Legislation emanating from these various institutions includes regulations, directives, and court decisions as well as advisory opinions and recommendations. Directives are the most relevant source of law for medical device producers and are formulated within the Commission and adopted by the Council. A directive is binding as to the result it prescribes, but it leaves national authorities the choice of how to achieve the required result. The private sector has some influence on the formulation of EC directives. Interested parties, such as manufacturers' associations, trade associations, and scientific groups, can assert the need for harmonized European legislation.
During the late 1970s and early 1980s, political differences between member states resulted in lengthy delays in the progress of integration. Reforms were passed to facilitate harmonizing legislation. One major change was that directives became mandatory. The council originally had adopted a concept of "optional directives," which permitted producers to choose freely between national standards and community standards if they had been developed. Another change permitted directives to set more general "essential safety requirements" instead of detailed technical specifications of production. Thus, individual countries could implement "technical standards," also referred to as "harmonized standards," to supplement mandated safety requirements as long as state standards conformed to the more general requirements.[27]
International Business Communications, "European Regulation of Medical Devices and Surgical Products, including Electromedical Devices" (Conference materials, New Orleans, 14-15 November 1988), 13.
The Commission's "white paper," published in June 1985, contained three hundred legislative proposals to achieve economic integration. The white paper set out the general objectives, strategy, and philosophy for creation of the unified market.[28]
European Confederation of Medical Suppliers Associations (EUCOMED), "Regulation of Medical Devices and Surgical Products in Europe: How to Achieve Harmonisation" (Conference proceedings, Brussels, 9-10 March 1987), 293.
It declared that there must be mutual recognition of product marketing and manufacturing standards among the states and that legislation harmonizing divergent standards must be enacted to ensure mutual recognition.[29]White paper from the Commission to the European Council, 14 June 1985.
To comply with the white paper, thousands of laws and regulations governing the production and the sale of goods and services had to be either abolished or unified and differences in
technical standards and regulations had to be harmonized. In order to establish uniformity for technical standards, two European standardizing committees were established to elaborate technical standards for the operation and evaluation of testing laboratories and certification bodies. If they elaborate a specific technical standard, that standard must replace the relevant national standards. These committees—CEN (European Committee for Standardization) and CENELEC (European Committee for Electrotechnical Standardization)—have elaborated several specific standards, but many more will be required before harmonization is complete.
Harmonizing Medical Device Policies
The European Commission identified medical products as one of the sectors requiring action to complete the internal market by 1992. As in other industries, however, the creation of a common technical framework for medical devices has been difficult. As of 1990, four directives concerning medical devices had been proposed and were at various stages of the legislative process. The proposed directives would harmonize major differences between member nations regarding technical design specifications for medical devices and the administrative procedures for examinations, tests, and inspections and authorization required for their marketing and use.
Each directive addresses different sectors of the device industry. Four trade groups, corresponding to the types of devices in each category, have participated in drafting the proposed laws. The categories, types of devices included, and participating organizations follow.
|
|
In May 1990, the European Parliament approved the active implantable electromedical devices directive, which was sent to the European Council for a final vote. The nonactive medical devices directive is currently before the European Commission, where the main issue is product classification. The two other designated categories had directives in the early draft stages in 1990.
The directive for active implantable electromedical equipment provides some indication of the contours of medical device regulation. Member nations are directed to comply with the essential safety requirements that are quite broadly stated. In general, patients are to be "adequately protected" from risks related to sterilization, design, manufacturing, and other processes.[30]
Kshitij Mohan, "EC Directives Don't Spell Harmony Yet," Medical Device and Diagnostic Industry 12 (June 1990): 10-12.
They will be protected through the development of harmonized standards, or relevant national standards should no harmonized standards exist, that are consistent with these broadly stated community goals. Manufacturers that comply can affix the EC (European Community) mark to their products, enabling devices to circulate anywhere in the internal market of 1992. Determination on compliance is left to national inspection bodies, which are required by the directive to be independent, expert assessment entities. Many details about these "notified bodies" are left to the individual nations.The drafts of other directives seem substantially similar in form. The goals are identical; the differences relate to the variety of devices covered. For example, the draft on nonactive
devices further divides the products by risk levels, in a manner reminiscent of the FDAs three-tiered classification system.
It is clear that the EC is struggling to develop a coherent, flexible, yet protective, medical device environment. However, a number of important questions are raised by these draft directives, some of which are discussed below.
"Safety" is a Value Judgment
As we saw in chapter 4, the design of a regulatory structure for medical devices presented a difficult challenge for the United States. The European Community's efforts to harmonize regulations present an even greater challenge. In addition to accommodating the diversity of the devices themselves, the EC must harmonize the regulations among all member nations.
Many individual European countries have established safety regulations relating to drugs and medical devices. To a large extent, safety regulations reflect the values of a nation; how much it is willing to pay for a certain level of safety. As we have seen in our discussion of U.S. medical device regulation, there is no such thing as absolute safety—each nation's policies reflect an "acceptable" level of risk.[31]
William W. Lowrance, Of Acceptable Risk: Science and the Determination of Safety (Los Altos, Calif.: William Kauffman, 1976).
Some economists have noted that the "marketing of health care products, equipment, and services tends to closely follow national lines, due largely to national differences in attitudes toward drugs and traditions of medical practice."[32]Economists Advisory Group, "The 'Cost of Non-Europe' in the Pharmaceutical Industry," executive summary, January 1988, cited in Oppenheimer, Wolff, and Donnelly, "An Overview," 15.
Indeed, this social value or cultural aspect of health-related products may explain why the EC's efforts to harmonize pharmaceutical regulations had met with little success as late as 1988.[33]
Ibid.
While drugs and devices may not be the only products with safety values embedded in government policy—automobiles and other vehicles may also reflect different concepts of safety—the value issues cannot be ignored. Indeed, it is precisely these value issues that led the individual nations to impose different comprehensive pre- and postmarketing product requirements and that now make efforts to harmonize more challenging. Once the actual harmonization begins in earnest, how the general "essential safety requirements" will "adequately protect" patients may become considerably more controversial among member nations.Standards and American Producers
The American experience would also suggest that a focus on standards might stultify innovation within the EC. Recall that although the FDA can require standards for all Class II devices, after thirteen years no detailed standards have been drafted. This dearth of regulatory standards is, in part, due to the cumbersome nature of the law's standard development process. However, there is no great push to develop standards because they reflect the state of the art only at the time they are written and are soon rendered obsolete in a dynamic industry.
American industry has tended to rely on voluntary, independent standard-setting organizations, such as the American National Standards Institute (ANSI) and the Association for the Advancement of Medical Instrumentation (AAMI), among others.[34]
For an interesting discussion of voluntary and regulatory standard setting in the United States, see Ross Cheit, Setting Safety Standards: Regulation in the Public and Private Sectors (Berkeley: University of California Press, 1990).
American firms have recognized that they must cooperate with the U.S. government to participate in the international standard-setting process. This voluntary, pluralistic approach is out of synchrony with the harmonized European system, which is leaning toward the imposition of technical standards as its primary regulatory mechanism.[35]Gary Stephenson, "International Standards: Harmonizing to a European Tune," Medical Device and Diagnostic Industry 12 (June 1990): 97-99.
If the EC adopts standards that are substantially different from those in the United States, producers could face extensive barriers to marketing their goods in Europe.Will the Procedures Ensure Uniformity?
Achieving uniformity under the scheme as currently outlined in the directives will be very difficult. If EC standards take years to develop, devices will be subjected to national standards that comply with the vague "essential safety requirements" in the directives. Because member nations vary in their individual commitment to safety, uniformity may be an elusive goal.
Will these Directives Create Nontariff Trade Barriers?
Interested observers have many concerns about the consequences of European unity. Some fear that this large market will erect protectionist barriers that will disadvantage all non-European
Community competitors. European business and industry have pressured for the creation of a pan-European market, and they clearly hope to reap advantages from it. Some predict that as EC nations eliminate trade restrictions among themselves, they may transfer them to foreign market players.[36]
"Reshaping Europe: 1992 and Beyond," Business Week, 12 December 1988, 48-73.
Drawing on this large and protected internal market, European companies will drive out smaller, less competitive European companies and will seek to establish strength in the global marketplace. Requirements for local (EC) content, import quotas, and other tariff barriers could accomplish these protectionist aims.There is also concern that internal efforts to harmonize regulations among the various nations will serve as nontariff trade barriers to outsiders. For example, product design standards could be constructed that are different from American or other international standards. The creation of an "EC" standard would benefit those whose primary market is internal. Observers fear that the considerable influence of European manufacturing interests in developing harmonized standards through the directive process will solidify the strength of insiders at the expense of outsiders. It seems fair to ask who is designing the internal requirements and standards. Some fear that the EC is farming out rule writing to standard-setting bodies that are overly influenced by European product designs rather than by U.S. or international designs.[37]
Stephenson, "International Standards," 98.
One early strategy of outsiders has been to participate in the development of harmonized product standards. Many large multinationals, including American companies such as General Electric, already have large European operations and have been active in the trade groups. However, smaller firms and latecomers may be disadvantaged if "fortress Europe" is realized. Some contradict the widespread view that established U.S. corporations would automatically have status equal to European companies.
The size of the U.S. market and the reputation of its medical device industry both operate in its favor. It would contradict any European global strategy if EC products did not comply with FDA requirements. The United States consumes more than half of all medical technology, a percentage that global strategies
cannot ignore. Furthermore, the general European perception is that U.S. medical products are of high quality and reliability; FDA approval carries weight throughout the world.
There are additional reasons why Europe might not exclude foreign medical devices. Demand for medical products often transcends national, or in this case regional, lines. Inevitably, tensions will arise between the general economic goals of nations and the pressure for access to low-cost health care technology. Europeans are used to high-quality and comprehensive care. And the national governments are often the purchasers of health care products, particularly in some EC nations with comprehensive government-run health care systems, such as the British National Health Service. If EC regulations discriminate against foreign medical technology, then they would pose barriers to U.S. innovations that may be better and/or cheaper than European products. Once again a potential conflict emerges between an economic goal of supporting an industrial sector and the social and political goal of low-cost and effective health care services. Will member nations tolerate limits on choices in health care purchasing in the interests of economic growth?
The Chinese Medical Device Market
Chinese Health Policies
The Chinese medical marketplace illustrates how a government shapes both supply and demand for medical products. China also provides an opportunity to observe the interplay among the three major global competitors in the medical device market—the United States, Japan, and western Europe.
Chinese government policies determine the supply of imported medical technology and the size of the demand for it. Before 1978 the government strictly controlled imports and followed a policy of favoring domestic production. Hospitals and medical schools used little foreign medical equipment.[38]
Teh-wei Hu, "Diffusion of Western Medical Technology in China Since the Economic Reform," International Journal of Technology Assessment in Health Care (1988): 345-358. This excellent article provides the background for the discussion that follows.
Government policy changes in 1978 affected both supply and demand. On the supply side, a relaxation of import restrictions, a favorable climate for medical technology exhibits, and other forms of product promotion increased sales by foreign companies.
On the demand side, more foreign exchange was made available for importation of western medical equipment. After the open-door policy of 1978, China acquired greater reserves of foreign exchange to implement the goal of improving available medical technology.
China's market structure makes it a complicated place to sell medical technology. The medical care system is substantially decentralized in terms of both high-level policy-making and delivery of services. A number of government agencies have authority over the diffusion of medical equipment, and disputes among them occur over tension between economic growth and health care services.
From the early 1950s until 1961, production and distribution of medical technology were overseen by several ministries, all of which were organized to promote economic growth (for example, the Ministry of Light Industry, the Ministry of Chemical Industry, and the Ministry of Mechanical Engineering). Between 1961 and 1978, the Ministry of Health oversaw medical technology. This ministry had a number of departments in charge of medical services, including administration, prevention, education, and financing. Unlike the other ministries, this one did not promote economic growth. The key medical schools and forty affiliated hospitals were directly under its supervision. The ministry controlled the procurement of medical equipment and provided medical technology information.
In 1979 the National Bureau of Medical and Pharmaceutical Management was established under the direct supervision of the State Economic Council. Within the bureau, the China Medical Instrument Corporation is in charge of policy, regulation, and guidelines on research, production, imports, and exports of medical instruments. Although they have substantially different missions, the Ministry of Health and the Bureau of Medical and Pharmaceutical Management must work together. The ministry, for example, must obtain a concurrence from the bureau for the importation of medical equipment to ensure that domestic products have not been overlooked. The Chinese government bureaucracy has tried to balance the goals of domestic economic growth with the growing demand for more sophisticated and technological western medical equipment.
Medical equipment sales depend on a very decentralized purchasing base. The government subsidizes hospitals in fixed amounts according to size. Hospitals rely on income generated from fees for services to make up the difference between government subsidies and expenditures. The local Price Bureau, an independent institution under the supervision of the State Economic Commission, sets the fees. Charges for new diagnostic tests with new equipment can be negotiated with the Price Bureau—a strong incentive to acquire new equipment and charge higher fees.
Each of the twenty-one provinces, five autonomous regions, and three metropolitan areas has its own health department that operates under guidelines from the Ministry of Health. These departments oversee the medical schools, the provincial hospitals, and the activities of local units but do not directly manage local hospitals and other health facilities.
Foreign Competition in China
Foreign competitors must operate within this labyrinthine marketplace. Overall consumption of medical and pharmaceutical equipment has risen dramatically in recent years, from $517 million in 1984 to $740 million in 1986, to nearly $1 billion in 1990.[39]
U.S. Department of Commerce, Coopers & Lybrand Report, cited in Biomedical Business International 12 (16 January 1989): 12-13.
This rise is attributed to the low level of medical technology at the beginning of 1984, the growing demand for medical care, the expansion of the economy, and the state policy to improve service at the provincial level. China's total budget for health care is expected to increase from $2.03 billion in 1987 to $2.49 billion in 1990. There has been a dramatic increase in imports, which grew at an 18 percent average annual rate between 1984 and 1986 and which account for approximately 57 percent of annual consumption. The area of largest growth appears to be in electromedical, radiological, and clinical lab equipment. The total import market is expected to be $530 million by 1990.Who will get the largest share of this important market? In 1988, Japan had a market share of 33 percent, the United States had 24 percent, and West Germany had 9 percent. Equipment cost appears to be a major consideration. Japanese CT scanners
are considerably cheaper than those of U.S. and German competitors and tend to dominate the market. Other important product attributes include the availability of training, service, and replacement parts. Some users indicated that more Japanese medical instruments have been adopted by Chinese medical institutions because the Japanese companies offer superior service.[40]
Hu, "Diffusion," 353.
Chinese government policy plays an important role in shaping the market for medical device technology. Foreign competitors must confront domestic protectionist policies that have economic, rather than social, motivations. It is difficult to do business in this decentralized marketplace. Both domestic and foreign competition are strong in many market segments. Companies that understand the economic and social needs of the Chinese will succeed in this rapidly growing market.
American medical device producers once dominated the world market, and they cannot afford to be complacent about the future. Powerful competitors challenge innovative American firms at home and abroad. Major political, social, and economic changes threaten America's leadership in foreign markets. Successful exporters must understand complex governmental health and regulatory policies as well as the intricacies of tariffs and trade regulations. The world market contains as many uncertainties as the domestic market, and trends of the 1980s show losses for American producers abroad.